Abstract
Doxorubicin (Dox) is an antitumor agent used in cancer treatment, but its clinical use is limited due to cardiotoxicity. Although exercise training can defend against Dox-mediated cardiac damage, the means for this cardioprotection remain unknown. To investigate the mechanism(s) responsible for exercise training-induced cardioprotection against Dox-mediated cardiotoxicity, we tested a two-pronged hypothesis: 1) exercise training protects against Dox-induced cardiotoxicity by preventing Dox-mediated mitochondrial damage/dysfunction and increased oxidative stress and 2) exercise training-induced cardiac expression of the inducible isoform of the 70-kDa heat shock protein 72 (HSP72) is essential to achieve exercise training-induced cardioprotection against Dox toxicity. Animals were randomly assigned to sedentary or exercise groups and paired with either placebo or Dox treatment (i.e., 20 mg/kg body wt ip Dox hydrochloride 24 h before euthanasia). Dox administration resulted in cardiac mitochondrial dysfunction, activation of proteases, and apoptosis. Exercise training increased cardiac antioxidant enzymes and HSP72 protein abundance and protected cardiac myocytes against Dox-induced mitochondrial damage, protease activation, and apoptosis. To determine whether exercise-induced expression of HSP72 in the heart is required for this cardioprotection, we utilized an innovative experimental strategy that successfully prevented exercise-induced increases in myocardial HSP72 levels. However, prevention of exercise-induced increases in myocardial HSP72 did not eliminate the exercise-induced cardioprotective phenotype that is resistant to Dox-mediated injury. Our results indicate that exercise training protects against the detrimental side effects of Dox in cardiac myocytes, in part, by protecting mitochondria against Dox-mediated damage. However, this exercise-induced cardioprotection is independent of myocardial HSP72 levels. Finally, our data are consistent with the concept that increases in cardiac mitochondrial antioxidant enzymes may contribute to exercise-induced cardioprotection.
Keywords: oxidative damage, redox balance, antioxidants, heat shock protein
doxorubicin (Dox) is an anthracycline that is used to treat many human neoplasms, including acute leukemias; lymphomas; and stomach, breast, and ovarian cancers; along with Kaposi's Sarcoma and bone tumors (28). Unfortunately, the clinical use of this effective anticancer drug is limited because of cardiotoxicity (42). Indeed, the negative side effects of Dox are irreversible and include the development of cardiomyopathy and ultimately congestive heart failure (19, 43). Therefore, knowing the biochemical changes that occur in the cardiomyocytes following Dox administration could provide the knowledge required to develop therapeutic countermeasures.
In regard to cardioprotection, exercise training has been shown to be an effective intervention that provides a safeguard against a variety of acute and chronic myocardial insults including ischemia-reperfusion injury (5, 11, 16, 26, 31, 32). Furthermore, it has been demonstrated that exercise training can also protect against Dox-induced cardiotoxicity (1, 2, 4, 6–8, 20). Although the mechanism(s) by which exercise training protects cardiomyocytes against these myocardial insults remains unknown, it has been hypothesized that one of the health benefits of exercise training is due, at least in part, to mitochondrial adaptations (3, 37). Specifically, data from our laboratory reveal that exercise training promotes biochemical alterations in cardiac mitochondria, resulting in a phenotype that resists apoptotic stimuli (i.e., treatment with exogenous oxidants) (24). However, to date, it is unknown whether these exercise training-induced mitochondrial adaptations can protect cardiac mitochondria against Dox-mediated damage.
In addition to mitochondrial adaptations, induction of the inducible isoform of the 70-kDa heat shock protein 72 (HSP72) is known to occur in cardiac myocytes with exercise training, and increased cardiac levels of HSP72 are associated with preservation of cardiac function during periods of oxidative stress (27, 35, 45). The putative mechanisms by which HSP72 can protect cardiomyocytes include control of protein folding, prevention of denaturation and aggregation of intracellular proteins, acceleration of the breakdown of damaged proteins, and by acting as a molecular chaperone (35). A recent study also predicted that upregulation of HSP72 can contribute to the integrity and activity of cardiac mitochondrial complexes by facilitating nuclear-encoded protein importation and assembly in the mitochondrial matrix and via the facilitation of protein folding within the mitochondria (4). Because of these protective benefits of HSP72, some authors have speculated that an exercise training-induced increase in HSP72 is a potential mechanism to explain the exercise training-induced cardioprotection against Dox toxicity (7). However, whether an increase in myocardial HSP72 is essential for exercise-induced cardioprotection against Dox-induced cardiac damage remains unclear since Chicco and collaborators (6–8) have published evidence both for and against the role of HSP72; hence, additional work is required to resolve this issue.
Therefore, to investigate the mechanism(s) responsible for exercise training-induced cardioprotection against Dox-mediated cardiotoxicity, we tested a two-pronged central hypothesis: 1) exercise training protects against Dox-induced cardiotoxicity by prevention of Dox-mediated mitochondrial oxidative damage and bioenergetic dysfunction and 2) exercise training-induced expression of HSP72 is required to achieve exercise-induced cardioprotection against Dox toxicity. Our results support our first hypothesis that exercise training protects cardiac mitochondria following Dox administration. However, our findings do not support the second postulate and reveal that upregulation of cardiac HSP72 levels is not required for exercise training-induced cardioprotection against Dox-induced cardiotoxicity. Finally, our data are consistent with the concept that increases in cardiac mitochondrial antioxidant enzymes may contribute to exercise training-induced cardioprotection following Dox administration.
METHODS
Ethical Approval
All experiments were approved by the local Institutional Animal Care and Use Committees before their initiation and followed guidelines established by the American Physiological Society for the use of animals in research.
Experiment 1
Experiment 1 was designed to test the hypothesis that exercise training protects against Dox-induced cardiotoxicity by preventing Dox-mediated mitochondrial damage and dysfunction.
Experimental design.
Adult Sprague-Dawley (male) rats (4–6 mo old) were randomly assigned to one of four groups: sedentary (n = 8; sedentary), exercise training (n = 7; exercise), sedentary + Dox (n = 6; sedentary-Dox), and exercise training + Dox (n = 7; exercise-Dox). Throughout the experimental period, all animals were housed on a 12-h:12-h light-dark cycle (20–22°C) and provided food (AIN93 diet) and water ad libitum.
Exercise training protocol and Dox administration.
Animals were habituated to treadmill running for 5 days (10, 20, 30, 40, and 50 min exercise/day from day 1 to day 5) followed by 5 consecutive days of treadmill exercise for 60 min/day at 30 m/min, 0% grade (estimated work rate of 70% maximum O2 consumption) (10). Exercise-Dox animals received Dox hydrochloride (20 mg/kg body wt ip) (9, 21, 47) immediately after the final exercise session and were euthanized 24 h later. Sedentary-Dox animals received Dox hydrochloride (20 mg/kg body wt ip) 24 h before euthanasia. These doses of Dox are human clinical doses of this drug that are pharmacologically scaled for use in rats (9, 21, 47). Saline was used as both the vehicle and the placebo. The hearts of exercise animals were excised 24 h after the final exercise bout.
Tissue preparation.
About 500 mg of cardiac ventricular wall were immediately used for mitochondria isolation as described below. Following removal of the heart from the animal, the heart was dissected and flushed free of blood in ice-cold saline. One section (∼30 mg) from the left ventricular wall was immediately frozen in Tissue-tek imbedding medium (Sakura Finetek, Torrance, CA) and stored at −80°C for TUNEL analysis. The remaining cardiac tissue was immediately frozen in liquid nitrogen and subsequently stored at −80°C for biochemical analyses. Cardiac tissue was homogenized 1:10 (wt-vol) in 5 mM Tris (pH 7.5) and 5 mM EDTA (pH 8.0) with a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and centrifuged at 1,500 g for 10 min at 4°C. Resulting supernatant (cytosolic) and pellet (noncytosolic) were collected. The pellet was resuspended 1:10 in the homogenization buffer. The noncytosolic fraction was used to measure ubiquitin-protein conjugates, whereas the cytosolic fraction was used for the remaining biochemical measurements. Cardiac protein content was assessed by the method of Bradford (Sigma).
Mitochondrial isolation and respiration.
Differential centrifugation was used to fractionate cardiac mitochondria as described previously (23, 24, 30). Mitochondrial oxygen consumption (Jo) was measured as described by Messer et al. (29) in a respiration chamber maintained at 37°C (Hansatech Instruments). Isolated mitochondria were incubated with 1 ml of respiration buffer containing (in mM) 100 KCl, 5 KH2PO4, 1 EGTA, 50 MOPS, 10 MgCl2, and 0.2% BSA at 37°C in a water-jacketed respiratory chamber with continuous stirring. Flux through complex I was measured using 2 mM pyruvate and 2 mM malate, whereas flux through complex II was measured using 5 mM succinate. Rotenone (5 μM) was added to prevent electron backflow to complex I in the succinate-driven experiments. The maximal respiration (state 3), defined as the rate of respiration in the presence of ADP, was initiated by adding 0.25 mM ADP to the respiration chamber containing mitochondria and respiratory substrates. State 4 respiration was recorded following the phosphorylation of ADP. The respiratory control ratio (RCR) was calculated by dividing state 3 by state 4 respiration.
Mitochondrial reactive oxygen species production.
Cardiac mitochondrial reactive oxygen species (ROS) production was determined using Amplex Red (Molecular Probes, Eugene, OR). The assay was performed at 37°C in 96-well plates using succinate as the substrate. Specifically, this assay was developed on the concept that horseradish peroxidase catalyzes the H2O2-dependent oxidation of nonfluorescent Amplex Red to fluorescent Resorufin Red, and it is used to measure H2O2 as an indicator of superoxide production. Superoxide dismutase (SOD) was added at 40 units/ml to convert all superoxide into H2O2. We monitored Resorufin formation (Amplex Red oxidation by H2O2) at an excitation wavelength of 545 nm and an emission wavelength of 590 nm using a multiwell plate reader flurometer (SpectraMax; Molecular Devices, Sunnyvale, CA). We recorded the level of Resorufin formation every 5 min for 15 min, and H2O2 production was calculated with a standard curve.
4-Hydroxynonenal-modified proteins and measurement of reactive carbonyl derivatives.
4-Hydroxynonenal (4-HNE) was analyzed as an indicator of cardiac oxidative stress via Western blotting as previously described (25). Oxidized proteins in cardiac tissue were quantified by Western blot by using a commercially available kit (Intergen, Oxy-Blot protein oxidation detection kit; Purchase, NY). The carbonyl groups in the protein side chains of both soluble and insoluble proteins were derivatized to 2,4-dinitrophyenylhydrazone (DNP). These DNP-derivatized protein samples were separated by using SDS-PAGE. After electrophoresis, the proteins were transferred to nitrocellulose membranes and incubated with primary antibody specific to the DNP moiety of the proteins. Membranes were then incubated with the appropriate secondary antibody, and following exposure to chemiluminescent reagents, the membranes were exposed to light-sensitive film. Images of these films were captured and analyzed.
Immunoblotting for antioxidant enzymes, HSP72, calpain, and ubiquitin-protein conjugate content.
Cardiac protein extracts were separated using SDS-PAGE and subsequently electroblotted onto nitrocellulose membranes. The resulting membranes were then stained with Ponceau S and analyzed to verify equal protein loading and transfer. Membranes were incubated with antibody directed against copper zinc superoxide dismutase (SOD1, sc11407; Santa Cruz Biotechnology, Santa Cruz, CA), manganese superoxide dismutase (SOD2, sc30080; Santa Cruz Biotechnology), catalase (sc34281; Santa Cruz Biotechnology), glutathione peroxidase 1 (GPX1, ab22604; Abcam), HSP72 (HSP01, EMD Chemicals, Gibbstown, NJ), ubiquitin-protein conjugates (A100; Boston Biochem, Cambridge, MA), and the active and proteolytic band of calpain protein (2556; Cell Signaling Technology). After incubation with the appropriate secondary antibody coupled to horseradish peroxidase, the membranes were then treated with chemiluminescent reagents and exposed to light-sensitive film. Images of these films were captured and analyzed.
Myocardial apoptosis/necrosis.
Myocardial apoptosis/necrosis was determined by TUNEL (Roche Applied Scientific, Indianapolis, IN). Briefly, 10-μm tissue sections were cut in a Shandon Cryotome cryostat (Life Sciences International) from frozen heart tissue stored in Tissue-tek imbedding medium. Sections were fixed using a 10% Formalin solution, washed, and permiabilized with Triton X-100 in 1% sodium citrate solution. Samples were then air-dried, and positive controls were treated with DNAse I at that time. Samples were blocked using 3% BSA and 20% goat serum. For identification of cell membranes, tissues were incubated with a rabbit anti-laminin antibody and secondary conjugated to Texas red fluorescent tag. Tissue sections were then incubated with the TUNEL enzyme, label solution, and sealed with a DAPI mounting medium for detection of nuclei. TUNEL-stained tissue sections were imaged (about 600 cells) using a Zeiss fluorescent microscope, and TUNEL-positive nuclei were counted under blinded conditions and normalized to tissue cross-sectional area.
Experiment 2
The objective of experiment 2 was to determine whether upregulation of the inducible isoform of 70-kDa HSP72 is required to achieve cardioprotection following Dox administration.
Experimental design.
Adult Sprague-Dawley (male) rats (4–6 mo old) were randomly assigned to one of three groups: sedentary (n = 7), exercise training in ambient temperature of +4°C to prevent exercise-induced body temperature increase (n = 6; exercise-cold), and exercise training in cold + Dox (n = 6; exercise-cold-Dox). Animals were housed on a 12-h:12-h light-dark cycle (20–22°C) and provided food (AIN93 diet) and water ad libitum.
Exercise training protocol and Dox administration.
Animals assigned to the endurance exercise trained groups performed the same training protocol as in experiment 1. However, ambient temperature in which training occurred was 4°C. The decision to exercise animals in this ambient temperature (4°C) was based on previous work from others and our laboratory indicating that endurance exercise in the cold (4°C) environment did not result in a significant increase in cardiac HSP72 (15, 17, 38, 46). Exercise-cold-Dox animals received Dox hydrochloride (20 mg/kg body wt ip) immediately after the final exercise bout, and these animals were euthanized 24 h later. Saline was used as both the vehicle in both the Dox-treated animals and the placebo. The hearts of the exercise-cold animals were excised 24 h after the final exercise bout.
Mitochondrial isolation, respiration, and ROS production.
Identical procedures described above were used to isolate mitochondria from hearts of animals used in experiment 2. Upon mitochondria isolation, oxidative phosphorylation and ROS production were determined.
Western Blots
We utilized Western blots to compare abundance of several proteins in the cardiac tissue of our experimental groups (i.e., HSP72, 4-HNE, SOD1, SOD2, catalase, GPX1, caspase-3, calpain, and ubiquitin-protein conjugates).
Data Analyses
Data are presented as means ± SD. Comparisons between groups from both experiment 1 and experiment 2 for each dependent variable were made by a one-way ANOVA, and, when appropriate, Tukey Honestly Significantly Different (HSD) tests were performed post hoc. Significance was established at P < 0.05.
RESULTS
Experiment 1
Mitochondrial oxidative phosphorylation.
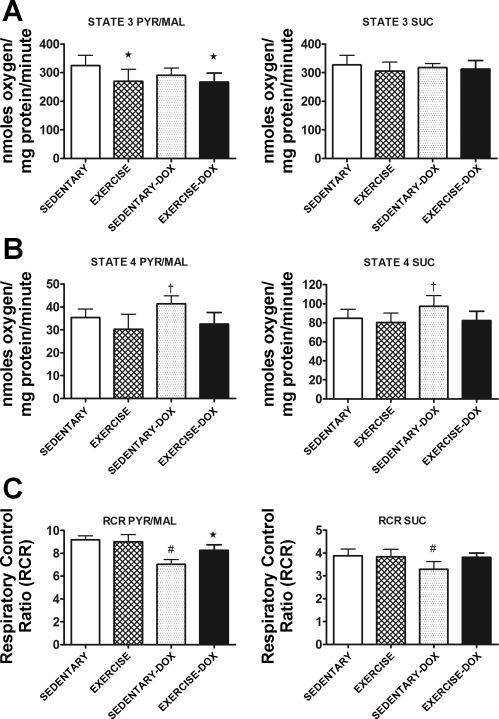
Respiratory control ratio (RCR) data from control animals indicate that our isolation procedure yielded well-coupled cardiac mitochondria. The RCR of cardiac mitochondria from sedentary animals was significantly reduced after Dox treatment when both pyruvate/malate and succinate were used as substrates (Fig. 1). Importantly, coupling was maintained in the exercised trained animals treated with Dox.
Fig. 1.
State 3 respiration (A), state 4 respiration (B), and respiratory control ratio (RCR; C) in mitochondria isolated from cardiac tissue of sedentary, exercise, sedentary + doxorubicin (sedentary-Dox), and exercise + Dox (exercise-Dox) animals. The left column illustrates data obtained using pyruvate/malate (Pyr/Mal) as substrate, whereas the right column shows data obtained using succinate (Suc) as substrate. Data are presented as means ± SD. Sedentary, n = 8; exercise, n = 7; sedentary-Dox, n = 6; and exercise-Dox, n = 7. *P < 0.05 compared with sedentary; †P < 0.05 compared with exercise and exercise-Dox; #P < 0.05 compared with sedentary, exercise, and exercise-Dox.
Mitochondrial ROS production, lipid hydroperoxides, and reactive carbonyl derivatives.
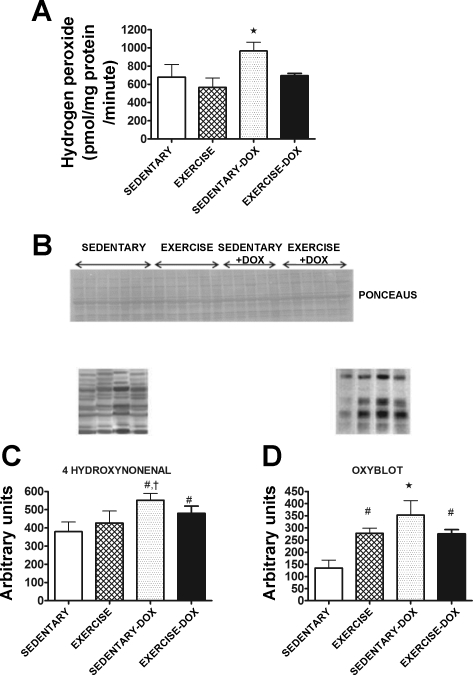
In cardiac mitochondria, Dox treatment significantly increased (P < 0.05) ROS production compared with all other groups (Fig. 2A). Importantly, exercise training before Dox treatment blunted ROS production from cardiac mitochondria. The development of lipid peroxidation in cardiac tissue was determined by measuring β-unsaturated 4-HNE-modified proteins. Our analysis revealed that Dox treatment resulted in significant increases (P < 0.05) in cardiac 4-HNE (Fig. 2C). In addition to 4-HNE, reactive carbonyl derivatives were measured as a marker of protein oxidative damage in cardiac tissue. Dox treatment resulted in significant increases (P < 0.05) in protein oxidative damage in cardiac tissue (Fig. 2D), and protein oxidative damage of cardiac tissue was attenuated when animals were exercise trained before Dox treatment (P < 0.05).
Fig. 2.
A: mitochondrial reactive oxygen species (ROS) production from cardiac tissue of sedentary, exercise, sedentary-Dox, and exercise-Dox animals. B: representative Ponceau S membrane used to verify equal protein loading and transfer for Western blots are shown above the graphs. C: the levels of 4-hydroxynonenal in cardiac tissue were analyzed as an indicator of oxidative stress via Western blotting. D: oxidized proteins in cardiac tissue were quantified by Western blot using the Oxy-Blot technique. Data are presented as means ± SD. Sedentary, n = 7 to 8; exercise, n = 6 to 7; sedentary-Dox, n = 5 to 6; and exercise-Dox, n = 6 to 7. *P < 0.05 compared with sedentary, exercise, and exercise-Dox; #P < 0.05 compared with sedentary; †P < 0.05 compared with exercise.
Cardiac antioxidant enzymes and HSP72.
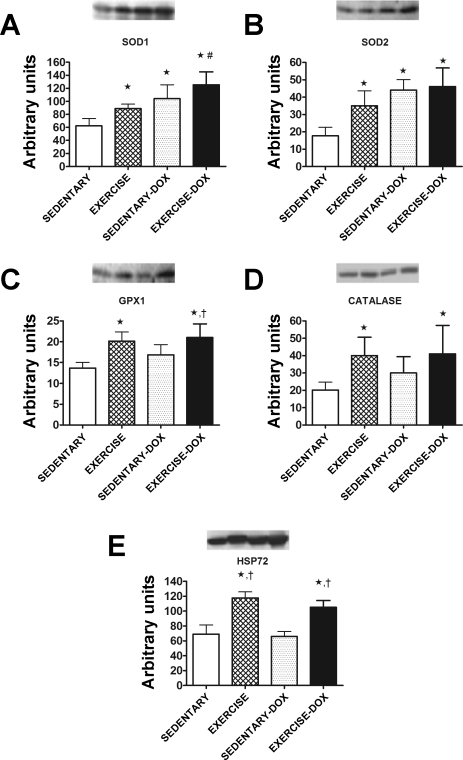
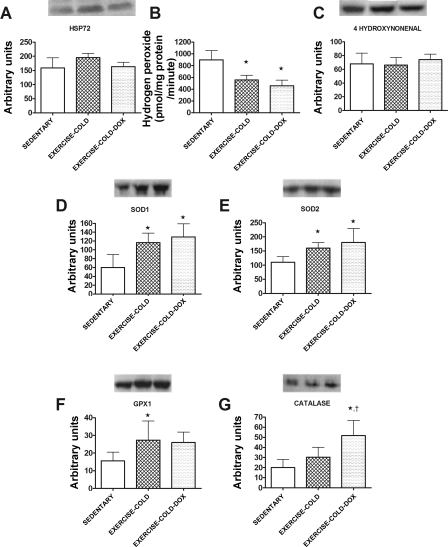
To assess the effects of exercise training on the antioxidant capacity of the cardiac muscle, the protein levels of important antioxidant enzymes were evaluated. Exercise training significantly increased (P < 0.05) the protein levels of both SOD1 (Fig. 3A) and SOD2 (Fig. 3B) in cardiac tissue. In addition, the levels of GPX1 and catalase were also elevated in cardiac tissue following exercise training (Fig. 3, C and D). Furthermore, the protein levels of HSP72 were significantly increased (P < 0.05) in cardiac tissue following exercise training and Dox treatment did not have any effect on cardiac HSP72 levels in either sedentary or exercise trained animals (Fig. 3E).
Fig. 3.
Copper zinc superoxide dismutase (SOD1; A), manganese superoxide dismutase (SOD2; B), glutathione peroxidase 1 (GPX1; C), catalase (D), and heat shock protein 72 (HSP72; E) protein levels in cardiac tissue of sedentary, exercise, sedentary-Dox, and exercise-Dox animals. Representative Western blots are shown above the graphs. Data are presented as means ± SD. Sedentary, n = 7; exercise, n = 6; sedentary-Dox, n = 5; and exercise-Dox, n = 6. *P < 0.05 compared with sedentary; #P < 0.05 compared with exercise; †P < 0.05 compared with sedentary-Dox.
Analysis of myocardial apoptosis/necrosis, ubiquitin-protein conjugates, and calpain.
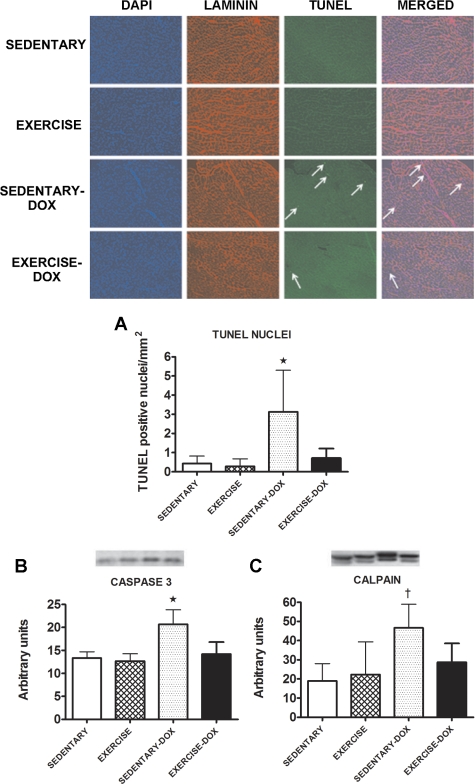
Tissue sections from cardiac muscle were analyzed for indexes of myocardial apoptosis/necrosis. Significantly higher (P < 0.05) numbers of TUNEL-positive nuclei were present in hearts from animals treated with Dox. In contrast, hearts from all other groups exhibited significantly fewer TUNEL-positive nuclei (Fig. 4A). Moreover, compared with hearts from sedentary animals, Dox treatment resulted in a significant rise (P < 0.05) in the cleaved (i.e., active) form of caspase-3 (Fig. 4B). In contrast, caspase-3 activity in hearts from exercise trained animals was not elevated following Dox treatment. Figure 4C shows that following Dox treatment, the active form of calpain is significantly higher (P < 0.05) than other groups. In addition, when compared with that of all other experimental groups, the total level of ubiquitin-protein conjugates was significantly higher (P < 0.05) in cardiac tissue obtained from sedentary animals treated with Dox (sedentary = 12.17 ± 4.40, exercise = 15.50 ± 4.85, sedentary-Dox = 23.33 ± 5.01, and exercise-Dox = 14.67 ± 2.34 arbitrary units).
Fig. 4.
TUNEL-positive nuclei (A) and levels of the active form of caspase-3 (B) and calpain (C) proteins in cardiac tissue of sedentary, exercise, sedentary-Dox, and exercise-Dox animals. Representative images of immunostained cryosections from cardiac tissue are shown. Sections were stained for nuclei (blue), laminin (red), and TUNEL-positive nuclei (green). Western blots of the active band of caspase-3 and calpain are shown above the graphs. Data are presented as means ± SD. Sedentary, n = 7; exercise, n = 6; sedentary-Dox, n = 5; and exercise + Dox, n = 6. *P < 0.05 compared with sedentary, exercise, and exercise-Dox; †P < 0.05 compared with sedentary and exercise.
Experiment 2
Cardiac antioxidant enzymes, HSP72, mitochondrial ROS production, and lipid hydroperoxides.
To prevent an exercise training-induced increase in cardiac HSP72, we exercise trained animals in a cold (+4°C) environment. Indeed, this exercise training protocol prevented the upregulation of cardiac HSP72 (Fig. 5A), which is in agreement with previous data (15, 16, 40). However, exercise training in a cold environment did result in the protein upregulation of important cardiac primary antioxidant enzymes. These results are in agreement with the data obtained when animals were exercise trained in a warm environment. Specifically, SOD1, SOD2, and GPX1 were significantly increased (P < 0.05) in the cold exercise trained groups (Fig. 5, D–G). Furthermore, our data indicate that exercise training in the cold resulted in significantly lower (P < 0.05) ROS production from cardiac mitochondria in both the Dox-treated and nontreated animals (Fig. 5B). Similar to experiment 1, exercise training in the cold before Dox treatment prevented an increase in 4-HNE content in cardiac tissue (Fig. 5C).
Fig. 5.
Cardiac HSP72 protein levels (A), mitochondrial ROS production (B), and 4-hydroxynonenal (C) of sedentary, exercise in 4°C (exercise-cold), and exercise in 4°C + Dox (exercise-cold-Dox) animals. SOD1 (D), SOD2 (E), GPX1 (F), and catalase (G) are also shown. Representative Western blots are shown above the graph. Data are presented as means ± SD. Sedentary, n = 7; exercise-cold, n = 6; and exercise-cold-Dox, n = 6. *P < 0.05 compared with sedentary; †P < 0.05 compared with exercise-cold.
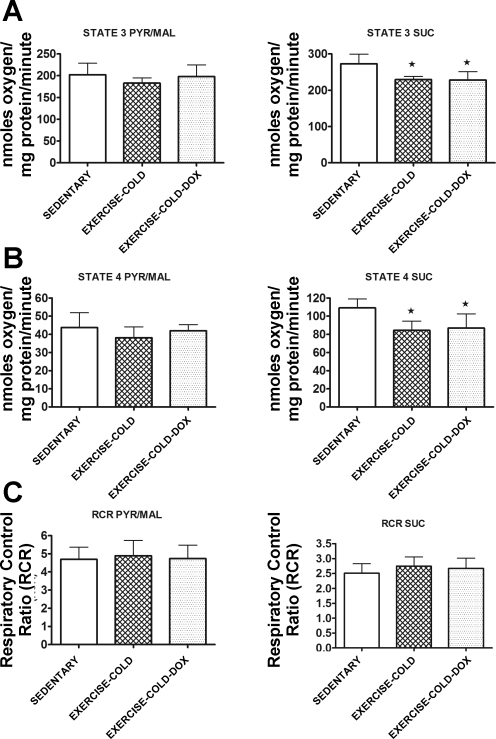
Mitochondrial oxidative phosphorylation.
Exercise in a cold environment did not alter cardiac mitochondrial oxidative phosphorylation (Fig. 6). Specifically, mitochondria isolated from animals that exercise trained in the cold exhibited similar RCR to that of sedentary animals. Importantly, exercise training in the cold was sufficient to maintain mitochondria coupling in animals treated with Dox.
Fig. 6.
State 3 respiration (A), state 4 respiration (B), and RCR (C) in mitochondria isolated from cardiac tissue of sedentary, exercise-cold, and exercise-cold-Dox animals. The left column illustrates data obtained using Pyr/Mal as substrate, whereas the right column shows data obtained using Suc as substrate. Data are presented as means ± SD. Sedentary, n = 7; exercise-cold, n = 6; and exercise-cold-Dox, n = 6. *P < 0.05 compared with sedentary.
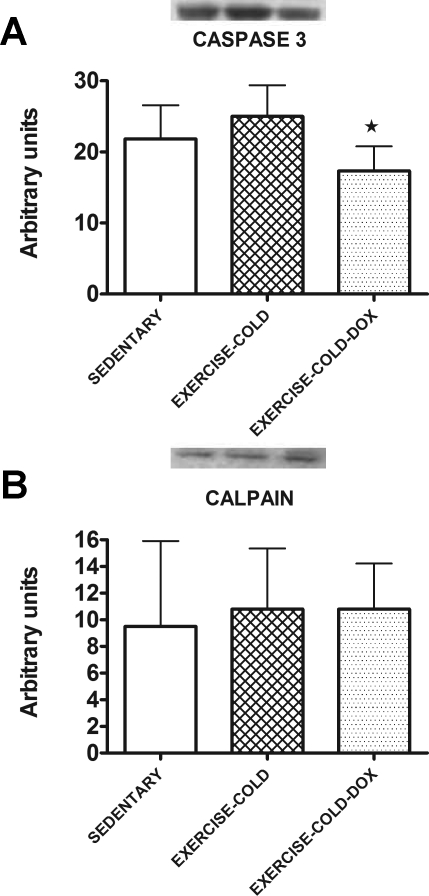
Caspase-3, calpain, and ubiquitin-protein conjugates.
Similar to the results from experiment 1, exercise training in the cold before Dox treatment prevented the cleavage of caspase-3 into its active form (Fig. 7A). Furthermore, the active form of calpain-1 (Fig. 7B) and the total level of ubiquitin-protein conjugates (sedentary = 72.29 ± 21.78, exercise-cold = 68.17 ± 19.35, and exercise-cold-Dox = 94.50 ± 9.33 arbitrary units) did not differ in cardiac tissue from all three groups.
Fig. 7.
Levels of the active form of caspase-3 (A) and calpain (B) proteins in cardiac tissue of sedentary, exercise-cold, and exercise-cold-Dox animals. Data are presented as means ± SD. Sedentary, n = 7; exercise-cold, n = 6; and exercise-cold-Dox, n = 6. *P < 0.05 between exercise-cold and exercise-cold-Dox.
DISCUSSION
Overview of Principal Findings
These experiments provide new and important information regarding the mechanism(s) responsible for exercise training-induced protection against Dox-induced cardiac toxicity. We hypothesized that exercise training prevents Dox-induced cardiac mitochondrial oxidative damage and dysfunction, and we also postulated that exercise training-induced expression of myocardial HSP72 is required to achieve exercise-induced cardioprotection against Dox toxicity. Our results support our first hypothesis that exercise training protects cardiac mitochondria following Dox-induced oxidative damage and dysfunction. In contrast, our results do not support the second hypothesis, and we conclude that upregulation of cardiac HSP72 is not required for exercise-induced protection against Dox-induced cardiotoxicity. However, our data suggest that increases in cardiac mitochondrial antioxidant enzymes could contribute to exercise training-induced cardioprotection following Dox administration. A detailed discussion of these findings follows.
Exercise Training Protects Against Dox-Induced Cardiac Mitochondrial Damage, Oxidative Damage, and Protease Activation
Currently, the clinical use of the highly effective anticancer drug Dox is limited because of cardiotoxicity (42). Indeed, the side effects of Dox are irreversible and include the development of cardiomyopathy and ultimately congestive heart failure (19, 43). Although current data demonstrate that exercise training protects the heart against Dox-induced damage (1, 2, 4, 6–8, 20), the mechanism(s) by which exercise training protects cardiomyocytes remain unclear. At present, the principal mechanism of Dox-induced cardiotoxicity is believed to be increased oxidant production by the mitochondria, leading to protease activation and the induction of apoptosis (1, 2, 4, 6–9, 14, 18). Our data indicate that Dox administration increased ROS production from cardiac mitochondria. Importantly, our results reveal that exercise training blunts this Dox-induced ROS emission from cardiac mitochondria. Furthermore, cardiac protein oxidative damage and 4-HNE formation were increased in animals treated with Dox, but exercise training attenuated this Dox-induced cardiac protein oxidative damage and 4-HNE formation.
Dox-induced damage to mitochondria is significant because mitochondria are vital organelles that play multifaceted roles in cells. Despite their primary role in energy production, mitochondria also generate ROS that can influence numerous cellular functions. It is well established that in tightly coupled mitochondria, the rate of electron flow through the respiratory chain is limited by the rate of ATP synthesis and that the rate of electron flow through the respiratory chain complex is constrained by the rate of proton pumping at the specific coupling sites (reviewed in Ref. 41). Our experiments reveal that mitochondria isolated from hearts of animals treated with Dox exhibit impaired coupling (i.e., lower RCR). Importantly, this Dox-induced cardiac mitochondrial uncoupling was prevented by exercise training before treatment with Dox.
Our technique for assessing mitochondrial superoxide production does not detect superoxide directly, but measures hydrogen peroxide release from mitochondria and, therefore, provides a means of estimating total superoxide production. It follows that differences in cardiac hydrogen peroxide scavenging capacity between our groups would influence the magnitude of hydrogen peroxide release in our system. Therefore, to compare the cardiac ROS scavenging capacity between the experimental groups, we measured the protein abundance of four primary antioxidant enzymes (e.g., SOD1, SOD2, catalase, and GPX1). One of these enzymes (SOD2) is found exclusively in the mitochondria, whereas the other three are found in both mitochondria and cytosol. In our experiments, we determined the protein levels of these antioxidant enzymes in cardiac muscle homogenates to determine the full effects of exercise training and Dox treatment on the expression of these enzymes. In this regard, SOD can dismutate superoxide anions to hydrogen peroxide and catalase can provide protection against oxidative injury by converting hydrogen peroxide to water and oxygen. Similarly, GPX1 utilizes reduced glutathione as a reducing equivalent to reduce hydrogen peroxide to form oxidized glutathione and water. Our findings revealed that Dox treatment alone increased SOD1 and SOD2 protein expression but did not increase cardiac levels of GPX1 or catalase. Therefore, it is feasible that Dox treatment alone increased the production of superoxide anions by the mitochondria inducing an endogenous signal within cardiac tissue to upregulate the expression of both SOD1 and SOD2. This effect may indicate an exacerbation of oxidative damage since GPX1 and catalase were not upregulated, and thus hydrogen peroxide removal was not increased in Dox-treated animals. In contrast, exercise training increased SOD1, SOD2, GPX1, and catalase. This is significant because the increased levels of GPX1 and catalase can convert hydrogen peroxide to water and oxygen and provide additional protection against Dox-induced cardiac oxidative injury.
Dox-induced cardiac apoptosis can result from the activation of endonucleases (e.g., caspase-3-mediated activation of nucleases) that cleave double-stranded DNA between nucleosomes. Caspase-3 is a protease that plays a major role in mitochondrial dependent apoptosis, and caspase-3 can also degrade contractile proteins in the cardiac myocyte (12). In the current experiments, Dox administration resulted in increased levels of the active form of caspase-3 along with an increased number of apoptotic myonuclei (i.e., TUNEL-positive nuclei). In addition, Dox resulted in calpain activation, which has been shown to play a critical role in myocardial apoptosis and necrosis. In addition, the ubiquitin-proteasome pathway is another major proteolytic system responsible for the degradation of cellular proteins. Protein degradation by this multicomponent pathway is accomplished primarily through a two-step process that involves substrate recognition (i.e., ubiquitin conjugation cascade) and subsequent degradation by the proteasome. Protein oxidative damage is significant because oxidized proteins are sensitive to proteolytic degradation (13). Therefore, increased protein ubiquitination in cardiac tissue following Dox administration may reflect a greater degree of protein turnover. Importantly, caspase-3 activation, the number of TUNEL-positive nuclei, calpain activation, and the level of protein ubiquitination were significantly attenuated in animals that exercised before Dox administration. Control of caspase-3, calpain, and ubiquitin-proteasome activation has been linked to oxidative stress, where an increase in oxidant production leads to protease activation (33, 34). Hence, it is feasible that the exercise training-induced increase in cardiac mitochondrial antioxidant enzymes could play a significant role in the prevention of Dox-induced mitochondrial ROS emission and, therefore, prevent the Dox-induced activation of myocardial proteases leading to cardiac damage and apoptosis.
HSP72 as a Putative Protective Mechanism Against Dox-induced Cardiotoxicity
Induction of HSP72 is known to occur in myocardial tissue following exercise training and is associated with the exercise-induced preservation of cardiac function during states of oxidative stress (22, 27, 35, 45). Primary functions of HSPs include: 1) control of protein folding, 2) prevention of denaturation and aggregation of intracellular proteins during stress, 3) acceleration of the breakdown of damaged proteins, and 4) serving as a molecular chaperone (35). Other putative effects of HSP72 include protection against apoptosis, protection against oxidative damage, maintenance of cellular calcium handling, and preservation of mitochondrial integrity in cardiac tissue exposed to a variety of oxidative stressors (27, 35). In reference to mitochondrial protection, upregulation of HSP72 can participate in protecting the integrity and activity of mitochondrial complexes (4). This can be achieved by facilitation of nuclear-encoded protein importation and assembly in the mitochondrial matrix along with an improvement in the folding of proteins within the mitochondria (4). Hence, given the vast protective properties of HSP72, we hypothesized that exercise training-induced increases in myocardial HSP72 levels play a required role in exercise training-induced cardioprotection against Dox-mediated cardiac injury (7).
Exercise training at room temperature (e.g., 22°C) results in increase in core temperature and an increased expression of HSP72 in the heart (15, 16, 27, 35, 44). In the current experiments, we took advantage of the fact that an increase in body temperature is required to achieve exercise training-induced increases of cardiac levels of HSP72 (39, 44). Indeed, to demonstrate cause and effect, separate experimental groups of animals were exercise trained in warm and cold environments to respectively induce and prevent the myocardial HSP72 accumulation associated with exercise training. Importantly, avoiding the exercise training-induced increase in body temperature (via exercise in a cold environment) successfully prevented the exercise training-induced increase in myocardial HSP72. In contrast, exercise training in a warm environment promoted a significant increase in cardiac HSP72 levels. Nonetheless, animals exercise trained in the cold environment exhibited cardiac protection against Dox-induced mitochondrial dysfunction and oxidative damage similar to animals exercise trained in the warm environment. However, animals that exercise trained in the cold environment exhibited similar increases in antioxidant enzymes compared with animals exercise trained in the warm environment. Collectively, these findings indicate that exercise training-induced cardioprotection against Dox-induced damage can be achieved without an increase in myocardial HSP72 content. Therefore, even though the overexpression of HSP72 has the potential to protect cells against Dox injury, our results do not support the hypothesis that elevated cardiac levels of HSP72 are essential to achieve exercise training-induced cardioprotection against Dox-induced damage.
As mentioned previously, our animals were exercised in a cold environment (i.e., ambient temperature ∼4°C) to prevent an exercise-induced increase in body core temperature. This treatment was not intended to induce cold stress in the animals but was designed to accelerate body heat loss during endurance exercise (i.e., preventing large amounts of heat storage). In this regard, a previous study has reported that an acute bout of exercise at this ambient temperature does not result in frank cold stress in rats as evidenced by the observation that body core does not decrease in the exercising animals (15). Nonetheless, it seems likely that the long-term exposure of nonexercising animals to cold stress could potentially affect Dox-induced toxicity and mitochondrial bioenergetics.
Conclusions
Dox-induced cardiotoxicity occurs due to several interrelated factors including radical production, calcium overload, and protease activation. Mitochondria produce radicals, which contribute significantly to Dox-induced mitochondrial oxidative injury. Dox-induced mitochondrial injury is significant because mitochondria can serve as mediators of life or death because these organelles can trigger cell death via both necrosis and apoptosis. The results from these experiments indicate that cardiac mitochondria are susceptible to Dox-induced toxicity and that exercise training for 5 consecutive days can provide protection to mitochondria against Dox-induced damage. Interestingly, several studies performed in the 1990s suggest that as few as 1 to 5 consecutive days of exercise training are sufficient to promote a similar cardioprotective phenotype as long-term exercise training (reviewed in Ref. 36). Importantly, our novel results demonstrate that upregulation of cardiac HSP72 is not required for exercise training-induced protection against Dox-induced cardiotoxicity. Furthermore, our results are consistent with the concept that exercise training-induced increases in cardiac mitochondrial antioxidant enzymes can be a potential mechanism to explain exercise training-induced protection against Dox-induced mitochondrial damage in the heart. These findings provide the basis for future translation studies that could be used to develop therapeutic countermeasures (i.e., mitochondria-targeted antioxidants) to retard Dox-induced cardiotoxicity.
GRANTS
This research was supported by an award from the American Heart Association (to A. N. Kavazis), National Institutes of Health Grant R01HL-067855 (to S. K. Powers), and Research Service of the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Ascensao A, Ferreira R, Oliveira PJ, Magalhaes J. Effects of endurance training and acute Doxorubicin treatment on rat heart mitochondrial alterations induced by in vitro anoxia-reoxygenation. Cardiovasc Toxicol 6: 159– 172, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Ascensao A, Magalhaes J, Soares J, Ferreira R, Neuparth M, Marques F, Oliveira J, Duarte J. Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int J Cardiol 100: 451– 460, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Ascensao A, Magalhaes J, Soares JM, Ferreira R, Neuparth MJ, Marques F, Oliveira PJ, Duarte JA. Endurance training limits the functional alterations of rat heart mitochondria submitted to in vitro anoxia-reoxygenation. Int J Cardiol 109: 169– 178, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Ascensao A, Magalhaes J, Soares JM, Ferreira R, Neuparth MJ, Marques F, Oliveira PJ, Duarte JA. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am J Physiol Heart Circ Physiol 289: H722– H731, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, Kittleson MM, Minhas KM, Berkowitz DE, Wei C, Hare JM. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res 98: 119– 124, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chicco AJ, Hydock DS, Schneider CM, Hayward R. Low-intensity exercise training during doxorubicin treatment protects against cardiotoxicity. J Appl Physiol 100: 519– 527, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Chicco AJ, Schneider CM, Hayward R. Exercise training attenuates acute doxorubicin-induced cardiac dysfunction. J Cardiovasc Pharmacol 47: 182– 189, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Chicco AJ, Schneider CM, Hayward R. Voluntary exercise protects against acute doxorubicin cardiotoxicity in the isolated perfused rat heart. Am J Physiol Regul Integr Comp Physiol 289: R424– R431, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res 62: 4592– 4598, 2002 [PubMed] [Google Scholar]
- 10.Criswell D, Powers S, Dodd S, Lawler J, Edwards W, Renshler K, Grinton S. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Med Sci Sports Exerc 25: 1135– 1140, 1993 [PubMed] [Google Scholar]
- 11.Demirel HA, Powers SK, Zergeroglu MA, Shanely RA, Hamilton K, Coombes J, Naito H. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol 91: 2205– 2212, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113: 115– 123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes-Marcondes MC, Tisdale MJ. Induction of protein catabolism and the ubiquitin-proteasome pathway by mild oxidative stress. Cancer Lett 180: 69– 74, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Green PS, Leeuwenburgh C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim Biophys Acta 1588: 94– 101, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Hamilton KL, Powers SK, Sugiura T, Kim S, Lennon S, Tumer N, Mehta JL. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am J Physiol Heart Circ Physiol 281: H1346– H1352, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Hamilton KL, Staib JL, Phillips T, Hess A, Lennon SL, Powers SK. Exercise, antioxidants, and HSP72: protection against myocardial ischemia/reperfusion. Free Radic Biol Med 34: 800– 809, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Harris MB, Starnes JW. Effects of body temperature during exercise training on myocardial adaptations. Am J Physiol Heart Circ Physiol 280: H2271– H2280, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Jang YM, Kendaiah S, Drew B, Phillips T, Selman C, Julian D, Leeuwenburgh C. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett 577: 483– 490, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Jeyaseelan R, Poizat C, Wu HY, Kedes L. Molecular mechanisms of doxorubicin-induced cardiomyopathy. Selective suppression of Reiske iron-sulfur protein, ADP/ATP translocase, and phosphofructokinase genes is associated with ATP depletion in rat cardiomyocytes. J Biol Chem 272: 5828– 5832, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Ji LL, Mitchell EW. Effects of Adriamycin on heart mitochondrial function in rested and exercised rats. Biochem Pharmacol 47: 877– 885, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Kang YJ, Chen Y, Yu A, Voss-McCowan M, Epstein PN. Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest 100: 1501– 1506, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kavazis AN. Exercise preconditioning of the myocardium. Sports Med 39: 923– 935, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Kavazis AN, Alvarez S, Talbert E, Lee Y, Powers SK. Exercise training induces a cardioprotective phenotype and alterations in cardiac subsarcolemmal and intermyofibrillar mitochondrial proteins. Am J Physiol Heart Circ Physiol 297: H144– H152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kavazis AN, McClung JM, Hood DA, Powers SK. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol 294: H928– H935, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Kavazis AN, Talbert EE, Smuder AJ, Hudson MB, Nelson WB, Powers SK. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med 46: 842– 850, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lennon SL, Quindry JC, French JP, Kim S, Mehta JL, Powers SK. Exercise and myocardial tolerance to ischaemia-reperfusion. Acta Physiol Scand 182: 161– 169, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Locke M, Noble EG, Tanguay RM, Feild MR, Ianuzzo SE, Ianuzzo CD. Activation of heat-shock transcription factor in rat heart after heat shock and exercise. Am J Physiol Cell Physiol 268: C1387– C1394, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Lu P. Monitoring cardiac function in patients receiving doxorubicin. Semin Nucl Med 35: 197– 201, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Messer JI, Jackman MR, Willis WT. Pyruvate and citric acid cycle carbon requirements in isolated skeletal muscle mitochondria. Am J Physiol Cell Physiol 286: C565– C572, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252: 8731– 8739, 1977 [PubMed] [Google Scholar]
- 31.Powers SK, Criswell D, Lawler J, Martin D, Lieu FK, Ji LL, Herb RA. Rigorous exercise training increases superoxide dismutase activity in ventricular myocardium. Am J Physiol Heart Circ Physiol 265: H2094– H2098, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Powers SK, Demirel HA, Vincent HK, Coombes JS, Naito H, Hamilton KL, Shanely RA, Jessup J. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol Regul Integr Comp Physiol 275: R1468– R1477, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 288: R337– R344, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol 102: 2389– 2397, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Powers SK, Locke AM, Demirel HA. Exercise, heat shock proteins, and myocardial protection from I-R injury. Med Sci Sports Exerc 33: 386– 392, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Powers SK, Quindry JC, Kavazis AN. Exercise-induced cardioprotection against myocardial ischemia-reperfusion injury. Free Radic Biol Med 44: 193– 201, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Primeau AJ, Adhihetty PJ, Hood DA. Apoptosis in heart and skeletal muscle. Can J Appl Physiol 27: 349– 395, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Quindry J, French J, Hamilton K, Lee Y, Mehta JL, Powers S. Exercise training provides cardioprotection against ischemia-reperfusion induced apoptosis in young and old animals. Exp Gerontol 40: 416– 425, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Quindry JC, Hamilton KL, French JP, Lee Y, Murlasits Z, Tumer N, Powers SK. Exercise-induced HSP-72 elevation and cardioprotection against infarct and apoptosis. J Appl Physiol 103: 1056– 1062, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Quindry JC, Hamilton KL, French JP, Lee Y, Murlasits Z, Tumer N, Powers SK. Heat shock protein 72 expression is not essential for exercise induced protection against infarction and apoptosis following ischemia-reperfusion (Abstract). FASEB J 19: 2006 [Google Scholar]
- 41.Schonfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med 45: 231– 241, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med 339: 900– 905, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem 207: 77– 86, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Staib JL, Quindry JC, French JP, Criswell DS, Powers SK. Increased temperature, not cardiac load, activates heat shock transcription factor 1 and heat shock protein 72 expression in the heart. Am J Physiol Regul Integr Comp Physiol 292: R432– R439, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Taylor RP, Ciccolo JT, Starnes JW. Effect of exercise training on the ability of the rat heart to tolerate hydrogen peroxide. Cardiovasc Res 58: 575– 581, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Taylor RP, Harris MB, Starnes JW. Acute exercise can improve cardioprotection without increasing heat shock protein content. Am J Physiol Heart Circ Physiol 276: H1098– H1102, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Yen HC, Oberley TD, Vichitbandha S, Ho YS, St Clair DK. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest 98: 1253– 1260, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]