Abstract
Acid-sensing ion channels (ASICs) in sensory nerves are responsive to increases in the levels of protons in the extracellular medium. Prior studies suggest that the muscle metabolite, lactic acid, plays a role in reflex sympathetic and cardiovascular responses via stimulation of thin muscle afferent nerves. Also, femoral artery occlusion augments the reflex sympathetic nerve response in rats. ASIC3 is a main subtype to appear in sensory nerves in mediating the response induced by increases in protons in the interstitial space of contracting muscles. Thus, in this article, we hypothesized that femoral occlusion increases the expression of ASIC3 in primary afferent neurons innervating muscles, and this contributes to the exaggerated reflex sympathetic responses. Femoral occlusion/vascular insufficiency of the hindlimb muscles was induced by the femoral artery ligation in rats. First, Western blot analysis shows that 24–72 h of femoral artery ligation significantly increased the expression of ASIC3 protein in dorsal root ganglion (optical density, 1.0 ± 0.07 in control vs. 1.65 ± 0.1 after 24 h of occlusion, P < 0.05; n = 6 in each group). There were no significant differences for increases in ASIC3 24 and 72 h postocclusion. Second, experiments using fluorescent immunohistochemistry and retrograde-labeling technique show that a greater percentage of ASIC3 staining neurons are localized in muscle-innervating dorsal root ganglion neurons after the arterial occlusion (78 ± 3% in 24 h post occlusion vs. 59 ± 5% in control, P < 0.05; n = 6 in each group). Third, the reflex responses in renal sympathetic nerve and arterial blood pressure induced by the stimulation of ASIC were examined after an injection of lactic acid into the arterial blood supply of hindlimb muscles of control rats and ligated rats. The results demonstrate that the sympathetic and pressor responses to lactic acid were significantly augmented after femoral occlusion compared with those in the control group. The data of this study suggest that enhanced ASIC3 expression in muscle afferent nerves contributes to the exaggerated reflex sympathetic and pressor responses to lactic acid as seen in arterial occlusion.
Keywords: sensory neuron, acid-sensing ion channel-3, muscle afferents, peripheral arterial disease
during static exercise, arterial blood pressure and sympathetic nerve activity are increased to maintain an adequate perfusion of metabolically active muscles (21, 26, 42, 47, 49). The exercise pressor reflex is believed to partly contribute to the pressor and sympathetic responses to static exercise (35, 38). The afferent arm of the reflex consists of group III and IV thin fiber nerves (20, 22–24). Metabolite-sensitive receptors on thin fiber muscle afferent nerves are stimulated when the concentrations of interstitial metabolites (such as lactic acid and ATP) increase during muscle contraction or ischemia (21, 47). Cardiovascular diseases can alter the reflex responses in the processing of muscle afferent signals via the receptors of sensory nerves (42, 47, 49, 57).
Peripheral arterial disease (PAD) affects lifestyles in 20% of adults who are older than 55 years (6, 40, 43, 44). The narrowing of blood vessels of the lower limbs, mainly due to atherosclerotic vascular disease, is a main cause of PAD (1). Intermittent claudication is the most common symptom of this disease, and it regularly occurs during physical activity but is relieved promptly by rest (44).
A rat model of femoral arterial occlusion is well established to study occlusive PAD (56), because the ligated rats exhibit nearly normal flows at rest but impaired limb blood flow reserve capacity with exercise. A prior study demonstrates that femoral artery occlusion increases the reflex sympathetic nerve and pressor responses to stimulation of metabolically sensitive transient receptor potential vanilloid type 1 (TRPV1) (57); however, the mechanisms at the levels of the receptors of sensory nerves remain largely unknown. Published work suggests that muscle metabolite, lactic acid, and acid-sensing ion channels (ASICs) play a role in muscle afferent-mediated reflex cardiovascular responses to static muscle contraction (9, 13, 14, 36, 37).
ASICs are members of a family of amiloride-sensitive sodium channels and considered as molecular sensors in afferent neurons (16, 29, 30, 39, 41, 50, 60). They are almost ubiquitous in the mammalian nervous system and are activated as pH drops below 7.0. There are six different proteins of ASICs, ASIC1a, -1b, -2a, -2b, -3, and -4, encoded by four genes (ASIC1, -2, -3, and -4). The ASIC3 protein, however, is mostly found in dorsal root ganglia (DRG) where it forms functional channels (29, 53–55) that are opened by proton concentrations found during exercise.
On the basis of those data, we tested the hypothesis that the levels of ASIC3 protein are increased in primary afferent DRG neurons innervating the hindlimb muscles of rats with femoral artery occlusion. We further speculated that alterations in ASIC3 contribute to the exaggerated reflex sympathetic and pressor responses to injection of lactic acid into the arterial blood supply of the hindlimb muscles.
METHODS
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Pennsylvania State College of Medicine and complied with the National Institutes of Health (NIH) guidelines.
Animal model of femoral artery occlusion.
Arterial occlusion was induced by femoral artery ligation in male Sprague-Dawley rats (5–7 wk old) as described previously (57, 58). In brief, after rats were anesthetized by inhalation of an isoflurane-oxygen mixture (2–5% isoflurane in 100% oxygen), the femoral artery was surgically exposed, dissected, and ligated ∼3 mm distal to the inguinal ligament. Sham-operated control limbs underwent the same procedure as described above except that a suture was placed below the femoral artery but was not tied. In experiments using the Western blot analysis and immunocytochemistry, the ligature was performed on one leg and the sham-operated control procedure was performed on another leg. The rats were allowed to recover 6, 24, and 72 h before the experiments were started.
Western blot analysis.
First, lumbar (L4–L6) and cervical DRGs of control and occluded limbs of 20 rats were removed. All DRGs tissues from individual animals were then sampled for the Western blot analysis. Total protein extracts were then prepared by homogenizing DRGs in ice-cold radioimmunoprecipitation assay buffer containing 25 mM Tris·HCl (pH 7.6), 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS) with protease inhibitor cocktail kit (Sigma-Aldrich, St. Louis, MO). After being vortexed, the lysates were centrifuged at 15,000 g for 15 min at 4°C, and the supernatants were collected and stored in −80°C before being used.
Protein concentrations of the DRG samples were determined using the bicinchoninic acid assay reagent kit (Pierce Biotechnology, Rockford, IL) with bovine serum albumin as a standard. Accordingly, at each time, 20 μg of protein was denatured by heating at 95°C for 5 min in an SDS sample buffer (Cell Signaling Technology, Danvers, MA), loaded onto 10–12% SDS-polyacrylamide gels and then transferred electrically to a polyvinylidene fluoride membrane. The membrane was blocked in 5% (wt/vol) nonfat milk in 0.1% Tween-TBS buffer for 1 h and was then incubated overnight with primary antibody: rabbit anti-ASIC3 and mouse anti-β-actin (Sigma-Aldrich). Anti-β-actin antibody was used to show equal loading of the protein in the Western blot analysis.
The membranes were incubated with horseradish peroxidase-linked anti-rabbit and anti-mouse secondary antibody at 1:1,000 dilutions and visualized for immunoreactivity using an enhanced chemiluminescence system (Cell Signaling Technology). According to prior studies (5, 31), a modified method was used for the reading of an arbitrary densitometry unit. The optical density was first obtained using the NIH Scion image Software, and values for densities of ASIC3 immunoreactive bands/β-actin band densities from the same lane were determined. Each of the values was then normalized to a control sample.
Retrograde labeling of DRG neurons.
DRG neurons innervating muscle were labeled by injecting a fluorescent retrograde axonal tracer, Fluoro-Gold (FG; Fluorochrome, Denver, CO), into the gastrocnemius muscle of both control and occluded limbs of six rats (39, 48). FG (4% in saline) was injected into the muscle at 10 different locations. The volume of each injection was 1 μl. The injection needle was placed in the muscle for 5–10 min to prevent the leakage of the tracer. A week was allowed for FG to be transported. In this experiment, the ligation surgery was performed 24 h before the removal of DRG tissues.
Tissue preparation.
The rats was anesthetized by inhalation of an isoflurane-oxygen mixture and then transcardially perfused with 200 ml of ice-cold saline containing 1,000 units heparin followed by 500 ml of 4% freshly prepared ice-cold paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4). L4–6 DRGs of control and occluded limbs were immediately dissected out and immersed in the same fixative at 4°C for 2 h. The tissues were then stored in PBS containing 30% sucrose overnight for cryoprotection and embedded in Tissue-Tek (Sakura Finetek, Torrance, CA) onto dry ice. A cryostat was used to obtain 10 μm of DRG sections. The sections were then mounted onto microscope slides and dried at room temperature.
Immunohistochemistry.
DRG sections were fixed in 4% of paraformaldehyde in PBS for 10 min at room temperature. After being washed with PBS, the tissue were permeabilized, blocked in 0.3% Triton X-100 in PBS supplemented with 5% goat serum for 1 h, and then incubated with guinea pig polyclonal anti-ASIC3 (1:250, Neruomics, Edina, MN) antibody overnight at 4°C. After being washed in PBS (3 × 5 min), the sections were incubated with goat anti-guinea pig fluorescein isothiocyanate (FITC)-labeled secondary antibody (1:200) for 2 h at room temperature. The sections were washed in PBS and coverslipped in Vectashield-mounting medium (Vector, Burlingame CA). Immunostaining was then examined under a Nikon Eclipse 80i microscope equipped with a digital camera (Nikon, Tokyo, Japan).
FG and FITC-labeled DRG neurons were examined using a Nikon Eclipse 80i microscope with appropriate filters, and the images were stored digitally on a computer. At least five sections containing L4–6 DRGs per rat were randomly chosen for analysis of FG and FITC staining intensity. A threshold value of staining intensity was set according to the mean staining intensity of background using the Nis-Elements software (Nikon,). Cells with >1.75 times of background intensity were considered to be positive (10, 57). The number of total ASIC3 immunostaining and FG-positive neurons was counted in each section. The percentages of double (FG and FITC)-labeled neurons were calculated: total number of double-labeled cells × 100/total number of FG-positive cells. Given that thin fiber afferent nerves are engaged in the reflex pressor response (20, 22–24) and that the majority of small-diameter and a proportion of large-diameter DRG neurons have axons that conduct in the group III and IV range (12, 15), the DRG neurons with <35 μm of diameter were selected and counted for analysis of double labeling. Note that the majority of DRG neurons showed a clear nucleus and perimeter and were counted (5, 48). To minimize the possibility of counting a single DRG neuron more than once, DRG sections were collected on five glass slides in series, and the tissues from one of the slides were processed for immunocytochemical analysis.
Recording of reflex cardiovascular and sympathetic responses.
The eight sham-operated control rats and six rats with 24 h of the femoral artery occlusion were anesthetized by inhalation of an isoflurane-oxygen mixture. As described previously, the animals were artificially ventilated (10, 28, 57). Catheters were inserted into an external jugular vein and the carotid arteries for the purposes of drug administration and measurement of arterial blood pressure. Catheters were inserted into the femoral arteries for injection of drugs into the arterial blood supply of the hindlimb muscles. A bundle of the renal nerves were carefully dissected from other connective tissues. A piece of laboratory film was placed under the isolated nerves, and two tips of a bipolar electrode to record neural activity were placed between the nerves and the film. These were embedded in a silicone gel fixed to the surrounding tissue. The renal sympathetic nerve activity (RSNA) signal was amplified with an amplifier (P511, Grass Instruments) and recorded. During the experiments, a continuous infusion of physiological saline was established to maintain fluid balance and basal blood pressure. Body temperature was carefully maintained at 37.5–38.5°C by a heating pad and external heating lamps.
To avoid the confounding effects of anesthesia on the reflex pressor response, decerebration was performed as previously described (10, 28, 57). A transverse section was made anterior to the superior colliculus and extending ventrally to the mammillary bodies. The brain rostral to the section was then removed. Once the decerebration was completed, anesthesia was removed from the inhaled mixture. A recovery period of 60 min was allowed after the decerebration was completed.
The purpose of this experiment was to determine the sympathetic and pressor responses to activation of the proton receptor in sham-operated control rats and occluded rats. Lactic acid (1, 2, and 4 μmol/kg body wt) was injected into the blood supply of the tricep's surae muscle. The injected amount was adjusted by adding 5–20 mM of lactic acid into 0.1–0.2 ml of saline (injected volume) according to the animal's body weight. The concentrations of lactic acid were selected on the basis of the results of a prior study (28, 59). The duration of the injections was 1 min. At least 20 min were allowed between injections.
Experimental data and statistical analysis.
All measured variables were continuously recorded and stored on a computer that used the PowerLab system (ADInstruments, Castle Hill, Australia). Arterial blood pressure was measured by connecting the carotid arterial catheter to a pressure transducer. Mean arterial pressure (MAP) was obtained by integrating the arterial signal with a time constant of 4 s. Heart rate (HR) was determined from the arterial pressure pulse. The peak responses of MAP and HR were determined by the peak change from the control value. RSNA signals were transformed into absolute values, integrated over 1-s intervals, and subtracted by the 1-s integrated background noise. To quantify the sympathetic responses to experimental interventions, basal values were obtained by taking the mean value for the 30 s immediately before each intervention and by ascribing the mean value of 100%, and relative changes from baseline during and after the intervention were then evaluated.
Experimental data were analyzed using SAS 9.13 for Windows (SAS Institute, Cary, NC). Comparisons of variables for ASIC optical density, MAP, and RSNA responses were performed using one-way ANOVA, followed by Tukey post hoc test as appropriate. Comparisons of variables for percentage of double-labeled neurons were made using Student's t-test. A value of P < 0.05 was considered significant.
RESULTS
ASIC3 expression in DRG neurons.
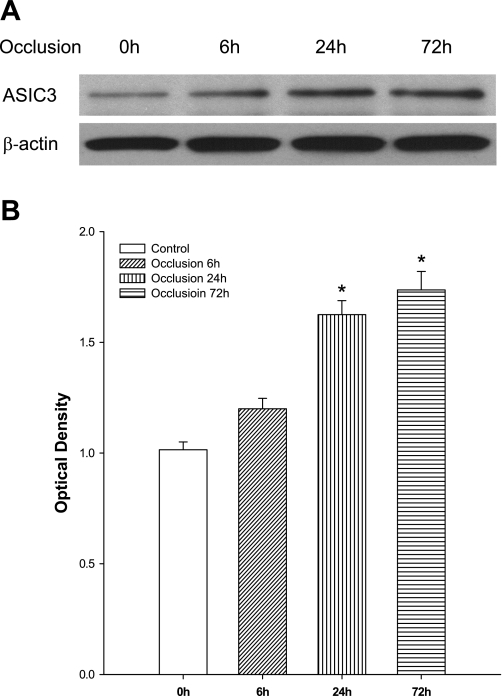
The expression of ASIC3 proteins in L4–L6 DRG neurons of sham-operated control and occluded rats was examined using Western blot analysis. Figure 1 shows that ASIC3 expression was increased 6 h (n = 3) after femoral artery ligation, but this was not significantly different than that in the control (n = 6). Figure 1 further shows that 24 (n = 6) and 72 h (n = 5) of femoral occlusion significantly elevated ASIC3 expression compared with that in the control. However, there were insignificant differences in the levels of ASIC3 proteins 24 and 72 h after the ligation. Thus 24 h of ligation was used for other groups of experiments in this report.
Fig. 1.
Effects of femoral artery occlusion on acid-sensing ion channel-3 (ASIC3) expression in dorsal root ganglion (DRG) neurons at different time courses. Western blot assay was employed to examine ASIC3 proteins in L4–L6 DRG at time points of 6, 24 and 72 h following femoral artery occlusion. The ligation was performed on the one hindlimb. The sham-operated procedure was performed on the contralateral limb of the same rats, and this served as control. A: representative bands of ASIC3 expression. Bands of β-actin are used as control for an equal protein loading. B: average data. The optical density is expressed in arbitrary units normalized against a control sample. Data in histograms represent means ± SE; n = 3–6 in each group. *P < 0.05 compared with control (0 h).
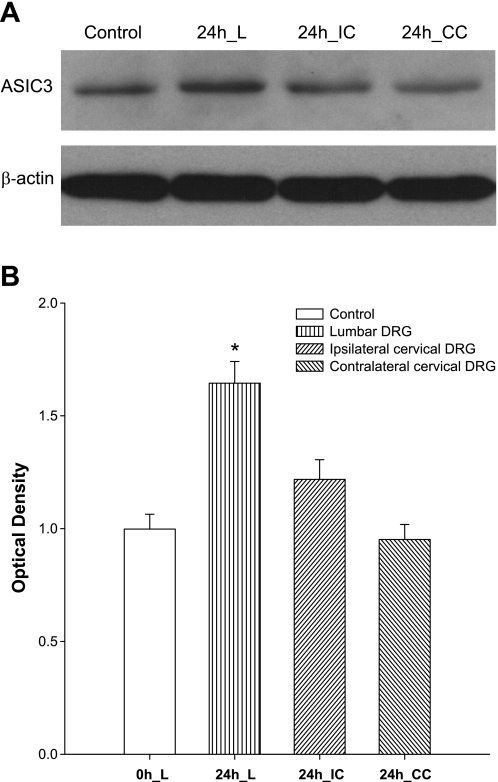
Figure 2 demonstrates typical blots of ASIC3 expression and average data in bilateral L4–L6 and cervical DRG neurons 24 h after femoral occlusion. Arterial occlusion significantly increased the expression of ASIC3 in lumber DRG neurons of occluded leg but not in lumber DRG of control leg and cervical DRG of both legs. After 24 h of ligation, the intensity of the ASIC3 signal in lumbar DRG neurons was ∼1.6-fold greater than that in sham-operated control (optical density, 1.65 ± 0.1 after occlusion vs. 1.0 ± 0.07 in control, P < 0.05; n = 6 in each group).
Fig. 2.
Upregulation of ASIC3 induced by femoral artery occlusion is localized in the lumbar level of DRG. ASIC3 proteins were examined in L4–6 and cervical DRG neurons of occluded limb and control limb using Western blot analysis. Femoral artery ligation (24 h) was performed. The sham-operated procedures were performed on contralateral limb as control, 24 h lumbar (L) DRG, 24 h ipsilateral cervical (IC) DRG, and 24 h contralateral cervical (CC) DRG. A: representative Western blots. B: densitometrical analysis was used to show average data; n = 6 for each group. The optical density is expressed in arbitrary units normalized against a control sample. *P < 0.05 compared with control (0 h).
Colocalization of ASIC3 and retrograde tracer FG.
In this experiment, we further determined whether an upregulation of ASIC3 induced by femoral artery occlusion appears in the DRG neurons' innervating hindlimb muscles. Retrograde tracing and immunofluorescence techniques were combined to examine the DRG neurons' colocalization of fluorescent ASIC3 immunoreactivity and FG previously injected into the hindlimb muscles.
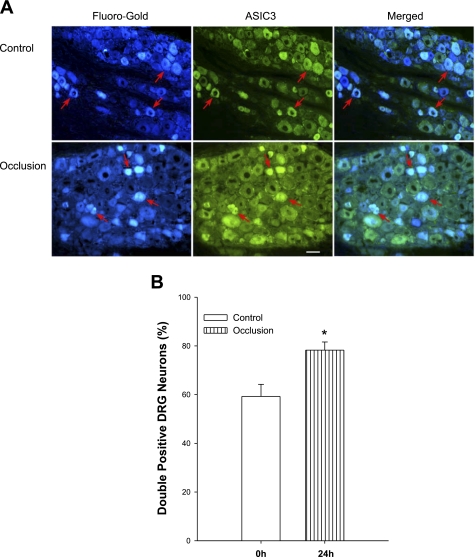
The appearance of the retrogradely transported label FG and ASIC3 within DRG neurons is characterized by a fluorescent blue and green color, respectively (Fig. 3A). The photomicrographs (Fig. 3A) also show that ASIC3 staining predominantly appears in small- and medium-size cells and that only a small portion of large-diameter DRG neurons were ASIC3 positive. The double labels were restrained within small- and medium-diameter DRG neurons.
Fig. 3.
Localization of ASIC3 in muscle-innervating DRG neurons. Fluorescence immunohistochemistry was employed to examine double labeling of ASIC3 and Fluoro-Gold (FG) in DRG neurons of control limb and occluded limb. FG was injected into the hindlimb muscles a week before DRGs were dissected. A: representative photomicrographs. Arrows indicate cells positive for FG and ASIC3. Scale bar = 50 μM. B: histograms show that percentage of double-labeling neurons against total FG-positive cells is greater in DRG neurons' femoral artery occlusion (n = 6) than that in control (n = 6). *P < 0.05 compared with control.
Figure 3A further shows that FG labels appear in ASIC3-positive DRG neurons in control and experimental groups. The number of double-labeled neurons/section is 14 ± 2 in six control limbs and 19 ± 1 in six occluded limbs (P < 0.05 vs. control). Our results further show that the number of FG-positive neurons/section is 24 ± 2 and 25 ± 4 (P > 0.05 vs. control, n = 6 for each group) and that the number of ASIC3-positive neurons/section is 35 ± 4 and 48 ± 3 (P < 0.05 vs. control; n = 6 for each group). Nevertheless, the percentage of double-labeled neurons with FG and ASIC3 was significantly greater in the occluded limbs than that in controls. They were 59 ± 5% in controls (n = 6) and 78 ± 3% (P < 0.05 vs. control) in the ligation group (n = 6). Figure 3B presents the percentage of double-labeled neurons in both experimental groups.
Cardiovascular and sympathetic responses to arterial injection of lactic acid.
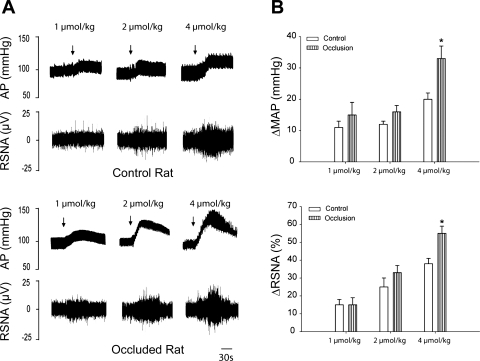
Baseline values for MAP and HR before arterial injections of lactic acid are 123 ± 8 mmHg and 400 ± 22 beats/min in control rats (n = 8), respectively, and 115 ± 10 mmHg and 413 ± 20 beats/min in occluded rats (n = 6), respectively. There were no significant differences in basal MAP and HR before injections. Figure 4, A (typical recordings) and B (average data), shows that 24 h of vascular insufficiency of the hindlimb muscle enhances RSNA and blood pressure responses evoked by lactic acid. The RSNA and MAP responses to arterial injection of lactic acid (4 μmol/kg body wt) were 38 ± 3% and 20 ± 2 mmHg in eight control rats, respectively, and 55 ± 4% and 33 ± 4 mmHg in six occluded rats (P < 0.05 occlusion vs. control), respectively. Note that femoral occlusion did not significantly alter HR responses evoked by an arterial injection of lactic acid. In a subset of experiments, a prior injection of amiloride (6 μg/kg) significantly attenuated the pressor response to 4 μmol/kg of lactic acid. Without and with prior amiloride the MAP response to lactic acid was 18 ± 5 and 8 ± 3 mmHg (P < 0.05, in 3 control rats), respectively, and 29 ± 5 and 12 ± 4 mmHg (P < 0.05, in 4 occluded rats), respectively.
Fig. 4.
Changes in renal sympathetic nerve activity (RSNA) and mean arterial pressure (MAP) in response to stimulation of ASIC3 receptors with arterial injection of lactic acid. Lactic acid (1–4 μmol/kg body wt) was injected into arterial blood supply of hindlimb muscles of control rats (n = 8) and rats with femoral artery occlusion (n = 6). A: typical recordings of RSNA and arterial pressure (AP) responses. Arrows indicate a start of injections. B: average data of MAP and RSNA. Note that baseline MAP is 123 ± 8 mmHg in control rats and 115 ± 10 mmHg in occluded rats (P < 0.05 vs. control). Values are means ± SE. *P < 0.05 compared with sham-operated control.
DISCUSSION
The purpose of our experiment was to determine whether ASIC3 on muscle afferents plays a role in the augmented sympathetic responsiveness evoked by femoral artery ligation. We first examined the total expression of ASIC3 protein in the DRG of sham-operated control rats and rats with the femoral artery occlusion using a Western blot assay. Femoral artery occlusion (24 to 72 h) significantly increased the levels of ASIC3 in lumbar DRG neurons. The densitometrical analyses demonstrate that the expression of ASIC3 proteins was elevated ∼1.6-fold higher after 24 h of occlusion than that in the freely perfused (control) group. Note that the occlusion significantly increased the expression of ASIC3 in lumbar DRG neurons of occluded leg, but not in lumbar DRG of control leg and cervical DRG of both legs. Our results suggest that arterial occlusion was unlikely to affect the expression of ASIC3 outside the lumbar DRG of the occluded limb.
We next examined whether ASIC3 immunostaining appears in the DRG neurons' innervating the hindlimb muscles using retrograde labeling and fluorescence immunohistochemical techniques. The data show that a greater percentage of ASIC3-positive staining was localized in muscle-innervating DRG neurons in rats with femoral artery occlusion than in control rats. Thus it is likely that the levels of ASIC3 proteins are increased by femoral occlusion in DRG neurons that receive sensory inputs of the hindlimb muscles.
Furthermore, we examined the effects of occlusion on RSNA and blood pressure responses evoked by injecting lactic acid into the arterial blood supply of hindlimb muscles. Our data demonstrate that as acid receptors were stimulated, the reflex responses were augmented in rats following the femoral artery occlusion compared with a control group. Note that a blockade of ASIC receptors attenuates lactic acid and muscle contraction-mediated pressor response (9, 13, 14, 36, 37). In addition, prior studies have reported that ASIC3 is a dominant subunit in forming functional H+-gated channels in DRG (53–55). Taken together, our findings suggest that ASIC3 is likely to contribute to femoral occlusion-augmented sympathetic responsiveness to the activation muscle afferent nerves. Nevertheless, we cannot exclusively rule out an engagement of other ASIC subunits in arterial occlusion-evoked enhancement in the muscle reflex.
The activation of thin fiber muscle afferents increases blood pressure and HR via a reflex mechanism (20, 35, 38). The afferent arm of the reflex is composed of mechanically sensitive group III and metabolically sensitive IV afferents (20, 22–24). As the endings of afferents are activated by both mechanical and metabolic stimuli arising in contracting muscles, the exercise pressor reflex is activated (22, 23). Metabolic by-products of muscle contraction including lactic acid accumulate in the interstitium of muscles which, in turn, activate receptors on muscle afferent nerves and evoke the exercise pressor reflex (21, 47). Under conditions of hindlimb ischemia, the reflex responses to contraction are amplified. For example, 72 h of femoral occlusion augments the blood pressure response to static muscle contraction in rats (52). The stimulation of TRPV1 receptors induced by an injection of capsaicin into the arterial blood supply of the hindlimb muscles also increases sympathetic and pressor responses to a greater degree in occluded rats than in control rats (57).
Patients with PAD suffer a disruption in the blood flow in exercising limbs (40, 44). This decreases the transport of nutrients and oxygen into metabolizing tissues as well as slows the removal of waste products from the exercising muscles. In this disease, when compensatory mechanisms such as vasodilation, development of collateral vessels, and anaerobic metabolism (40, 44) cannot meet the oxygen demand of tissue, ischemia develops. Prior studies reported that autonomic responses are enhanced during exercise in PAD patients (2, 3).
The accumulation of lactic acid and the activation of acid-sensitive afferent nerves in skeletal muscle contribute to the reflex cardiovascular responses to static muscle contraction (32, 45, 46). Under the circumstances of inadequate blood supply, an increase in lactic acid in muscles leads to acidification of the extracellular space. Acidosis appears in tissues with ischemia (51, 60). Of note, capsaicin-induced reflex pressor response is greater as the muscle interstitium is made more acidic (pH<6.6) by an infusion of lower pH solution into the femoral artery of rats (9). This prior study also suggests that the effects of lower pH solutions are mediated via ASICs (9). Blocking ASICs receptors using amiloride and more selective antagonist A-317567 (8, 27) also attenuated the pressor response evoked by static exercise and by arterial injection of lactic acid into the hindlimb muscles (13, 28, 37). Accordingly, acid sensing has been considered as an important nature of sensory neurons with thin fiber afferents and plays an important role in mediating metabolic responses to muscle contraction (37, 39). The results of our present study further confirm the role played by ASICs in engagement of the pressor response to lactic acid. Given that ASIC3 specifically appears in the DRG neurons and responsive to proton (29), it is well reasoned that as the femoral artery is occluded, enhanced sympathetic responsiveness to lactic acid is related to greater levels of ASIC3 proteins in the DRG.
Recent studies suggest that ASICs participate in the metabolic component but not the mechanoreceptor component of the exercise pressor reflex in cats (36, 37). Also, a prior investigation supports the idea that ASIC3s are unlikely to contribute to mechanically activated currents in mammalian sensory neurons (7). Based on the experimental results of the ionic property and distribution of ASIC3 receptors, ASIC3 is likely to play a role in engagement in the exercise pressor reflex when muscles are ischemic. First, the ASIC3 receptors are activated with pH ranges that are seen in ischemic muscles (19, 60). Second, lactate can enhance ASIC3 sensitivity to protons (17, 18). Thus ASIC3 is a suitable sensor for lactic acidosis as muscles undergo anaerobic metabolism. Third, ASIC3 is a dominant ASIC subunit, preferentially localized in DRG neurons of thin fiber afferent nerves (53–55). Finally, the data of our present study demonstrate that more ASIC3-positive immunostaining appears in small diameter of DRG neurons innervating the hindlimb muscles after femoral occlusion.
Our present study revealed that the time courses of enhanced ASIC3 and TRPV1 responses are similar following femoral occlusion. Also, the expression of both ASIC3 and TRPV1 receptors largely appear in small diameter of DRG neurons, supporting the idea that ASIC and TRPV1 coexist in the processing of muscle afferent signals. A blockade of ASIC attenuates the pressor response induced by an arterial injection of capsaicin into the hindlimb muscles with acidic interstitium (9). These data also provided evidence that ASICs contribute to the muscle metaboreflex, and as the extracellular milieu is more acid, TRPV1 may play a role. Alterations in ASIC3 and TRPV1 expression and their responsiveness are likely cooperatively engaged in the exaggerated sympathetic and cardiovascular responses during exercise seen in an animal model of femoral artery occlusion and in PAD patients.
It has been reported that rats with the femoral artery ligation exhibit nearly normal flows at rest but impaired limb blood flow reserve capacity with exercise (56). An additional study further demonstrates that no significant differences are seen in the resting levels of intramuscular pH in sham-operated control limb and ligated limb of rats 12 h and 4, 7, and 14 days after the surgery (4). This result is in agreement with the findings in PAD patients, suggesting that muscle pH is not altered in symptomatic legs (11, 25). The same rat model was employed to study sensory neurons' ASIC3 expression in our current report. We postulate that at rest, muscle pH in the occluded limb and systemic pH were unlikely to be altered 24–72 h after the ligation surgery. However, our results demonstrate that 24 and 72 h of the ligation increases ASIC3 expression in DRG neurons, suggesting that there might be lack of a direct correlation between resting muscle pH and femoral occlusion-enhanced ASIC3. The mechanisms by which ASIC3 is promoted need to be determined. It should be noted that during exercise, blood flow directed to the ligated limb of rats is attenuated (56) and intramuscular pH of the ligated limb and symptomatic leg of PAD patients is decreased to a greater degree (4). Thus we speculate that the exaggerated pH response is likely to upregulate ASIC3 expression after femoral artery occlusion. Moreover, prior studies suggest that nerve growth factor (NGF) is responsible for basal ASIC3 expression of DRG neurons via TrkA-activated phospholipase C/protein kinase C pathway (33, 34). This process may contribute to inflammation-induced sensitization to pain (33). Our prior data have shown that arterial occlusion significantly elevates the levels of NGF in DRG neurons 24 and 48 h after the ligation surgery (58). The time courses of enhanced ASIC3 and NGF responses are similar following femoral occlusion. Also, we speculate that the relationship between NGF and ASIC3 with arterial occlusion is similar to what is noted in the presence of inflammation. It is likely that arterial occlusion-induced NGF increase upregulates the expression of ASIC3 in DRG neurons. In addition, our results show that arterial occlusion significantly increases the expression of ASIC3 in lumber DRG neurons of the ligated leg but not in lumber DRG of the control leg and cervical DRG of both legs of the same rats. This suggests that that the effects of ligation are unlikely to be systemic but likely to be localized in the occluded leg.
One limitation of this study is that we cannot exclude a possibility that there was leakage of FG to the joints, skin, or other nonskeletal muscle tissue. The DRG cells identified as having the ASIC3 receptor protein in this experiment may innervate skin and joints in addition to hindlimb skeletal muscle. Given that there is considerable thin fiber innervation of the joints and skin, those afferents are likely to be affected by femoral occlusion and involved in the augmented sympathetic response to lactic acid. To minimize the sensory response from cutaneous afferents in the hindlimb, the skin covering the triceps surae muscle and femoral region was surgically separated from the muscles below in the reflex experiment. Nevertheless, it cannot be ruled out that the injection of lactic acid stimulated afferents from joints and other tissue in the hindlimb in contribution to the sympathetic and pressor responses.
In addition, prior studies suggest that the majority of small-diameter and a proportion of large-diameter DRG neurons have axons that conduct in the group III and IV range (12, 15). We selectively counted the DRG neurons with <35 μm of diameter for analysis of double-labeling cell bodies in this study. However, there is a lack of strong evidence to support that there is a powerful correlation between cell body size and afferent fiber type (i.e., group III and IV) (15). ASIC3 is expressed in small- and large-diameter DRG cells (39). Thus it should be noted that DRG cells analyzed in this study might not exclusively represent the cells that innervate thin fiber afferents. Nonetheless, in a cat study, the discharge of group IV and blood pressure are significantly increased after arterial injection of lactic acid and the effects are attenuated after blocking ASICs (14). This result suggests that stimulation of ASICs on thin fiber afferent nerves contributes to the reflex pressor response to lactic acid.
In conclusion, the results of the present study demonstrate that ASIC3 receptors are upregulated in DRG neurons following femoral artery ligation and that elevated ASIC3 proteins are localized in muscle-innervating afferent nerves, especially in small to medium diameter of neurons. Stimulation of afferent's ASICs receptors with lactic acid augments responses of sympathetic nerve activity and arterial blood pressure in animals with femoral occlusion. These findings suggest that ASIC3 plays an important role in augmented sympathetic responsiveness, as blood supply to the hindlimb muscle is insufficient.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-090720 and P01-HL-096570 and American Heart Association Established Investigator Award 0840130N.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEGMENTS
We express gratitude to Dr. Marc Kaufman for reading the manuscript and thank Chunying Yang for technical assistance.
REFERENCES
- 1.Aslam F, Haque A, Foody J, Lee LV. Peripheral arterial disease: current perspectives and new trends in management. South Med J 102: 1141–1149, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Bakke EF, Hisdal J, Jorgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg 33: 20–25, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Challiss RA, Hayes DJ, Petty RF, Radda GK. An investigation of arterial insufficiency in rat hindlimb. A combined 31P-n.m.r. and bloodflow study. Biochem J 236: 461–467, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho HJ, Staikopoulos V, Furness JB, Jennings EA. Inflammation-induced increase in hyperpolarization-activated, cyclic nucleotide-gated channel protein in trigeminal ganglion neurons and the effect of buprenorphine. Neuroscience 162: 453–461, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 290: 86–97, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol 556: 691–710, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain 117: 88–96, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Gao Z, Li JD, Sinoway LI, Li J. Effect of muscle interstitial pH on P2X and TRPV1 receptor-mediated pressor response. J Appl Physiol 102: 2288–2293, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Gao Z, Xing J, Sinoway L, Li J. P2X receptor-mediated muscle pressor reflex in myocardial infarction. Am J Physiol Heart Circ Physiol 292: H939–H945, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Greiner A, Esterhammer R, Messner H, Biebl M, Mühlthaler H, Fraedrich G, Jaschke WR, Schocke MF. High-energy phosphate metabolism during incremental calf exercise in patients with unilaterally symptomatic peripheral arterial disease measured by phosphor 31 magnetic resonance spectroscopy. J Vasc Surg 43: 978–986, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol 359: 31–46, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol 581: 1271–1282, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes SG, McCord JL, Rainier J, Liu Z, Kaufman MP. Role played by acid-sensitive ion channels in evoking the exercise pressor reflex. Am J Physiol Heart Circ Physiol 295: H1720–H1725, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoheisel U, Mense S. Observations on the morphology of axons and somata of slowly conducting dorsal root ganglion cells in the cat. Brain Res 423: 269–278, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Holzer P. Acid-sensitive ion channels and receptors. Handb Exp Pharmacol 194: 283–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci 4: 869–870, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron 37: 75–84, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neurosci Lett 208: 191–194, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 10, p. 381–447 [Google Scholar]
- 21.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 23.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 24.Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res 18: 663–668, 1984 [DOI] [PubMed] [Google Scholar]
- 25.Kemp GJ, Roberts N, Bimson WE, Bakran A, Harris PL, Gilling-Smith GL, Brennan J, Rankin A, Frostick SP. Mitochondrial function and oxygen supply in normal and in chronically ischemic muscle: a combined 31P magnetic resonance spectroscopy and near infrared spectroscopy study in vivo. J Vasc Surg 34: 1103–1110, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Khan MH, Sinoway LI. Muscle reflex control of sympathetic nerve activity in heart failure: the role of exercise conditioning. Heart Fail Rev 5: 87–100, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Kuduk SD, Di Marco CN, Chang RK, Dipardo RM, Cook SP, Cato MJ, Jovanovska A, Urban MO, Leitl M, Spencer RH, Kane SA, Bilodeau MT, Hartman GD, Bock MG. Amiloride derived inhibitors of acid-sensing ion channel-3 (ASIC3). Bioorg Med Chem Lett 19: 2514–2518, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Li J, Maile MD, Sinoway AN, Sinoway LI. Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol 97: 1709–1714, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem 282: 17325–17329, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Mao W, Ding B, Liang CS. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am J Physiol Heart Circ Physiol 295: H1956–H1965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLean DA, LaNoue KF, Gray KS, Sinoway LI. Effects of hindlimb contraction on pressor and muscle interstitial metabolite responses in the cat. J Appl Physiol 85: 1583–1592, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci 22: 10662–10670, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mamet J, Lazdunski M, Voilley N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J Biol Chem 278: 48907–48913, 2003 [DOI] [PubMed] [Google Scholar]
- 35.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCord JL, Hayes SG, Kaufman MP. Acid-sensing ion and epithelial sodium channels do not contribute to the mechanoreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol 295: H1017–H1024, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCord JL, Tsuchimochi H, Kaufman MP. Acid-sensing ion channels contribute to the metaboreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol 297: H443–H449, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain 1: 35, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muir RL. Peripheral arterial disease: pathophysiology, risk factors, diagnosis, treatment, and prevention. J Vasc Nurs 27: 26–30, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Naves LA, McCleskey EW. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res 38: 1561–1569, 2005 [DOI] [PubMed] [Google Scholar]
- 42.O'Leary DS. Altered reflex cardiovascular control during exercise in heart failure: animal studies. Exp Physiol 91: 73–77, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Ouriel K. Peripheral arterial disease. Lancet 358: 1257–1264, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Rejeski WJ, Tian L, Liao Y, McDermott MM. Social cognitive constructs and the promotion of physical activity in patients with peripheral artery disease. J Cardiopulm Rehabil Prev 28: 65–72, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988 [DOI] [PubMed] [Google Scholar]
- 46.Rotto DM, Stebbins CL, Kaufman MP. Reflex cardiovascular and ventilatory responses to increasing H+ activity in cat hindlimb muscle. J Appl Physiol 67: 256–263, 1989 [DOI] [PubMed] [Google Scholar]
- 47.Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol 99: 5–22, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106: 229–239, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci 25: 2617–2627, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci USA 98: 711–716, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol. 299: H106–H113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem 272: 20975–20978, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177, 1997 [DOI] [PubMed] [Google Scholar]
- 55.Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H+-gated cation channels. Ann NY Acad Sci 868: 67–76, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol 97: 773–780, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Xing J, Gao Z, Lu J, Sinoway LI, Li J. Femoral artery occlusion augments TRPV1-mediated sympathetic responsiveness. Am J Physiol Heart Circ Physiol 295: H1262–H1269, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol Heart Circ Physiol 296: H1380–H1387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xing J, Sinoway L, Li J. Differential responses of sensory neurones innervating glycolytic and oxidative muscle to protons and capsaicin. J Physiol 586: 3245–3252, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res 99: 501–509, 2006 [DOI] [PubMed] [Google Scholar]