Abstract
Low-salt (LS) diet has been considered to be beneficial in the prevention and treatment of hypertension; however, it also increases plasma angiotensin (ANG) II and may cause adverse cardiovascular effects, such as endothelial dysfunction. We assessed endothelial function of coronary arterioles and vascular superoxide production, as a function of LS diet. Dogs were fed with LS (0.05% NaCl) or a normal-salt (NS, 0.65% NaCl) diet for 2 wk. There were threefold increases in plasma ANG II, associated with a 60% reduction in flow-induced dilation (FID) in coronary arterioles of LS compared with NS dogs. In vessels of NS dogs, FID was primarily mediated by nitric oxide (NO), as indicated by an eliminated FID by Nω-nitro-l-arginine methyl ester (l-NAME). In vessels of LS dogs, however, FID was eliminated. Administration of apocynin, a NAD(P)H oxidase inhibitor, partially restored FID and additional l-NAME eliminated FID. Generation of superoxide, measured with dihydroethidium, was significantly greater in vessels of LS than in NS dogs, which was further increased in response to ANG II or phorbol 12,13-dibutyrate, an agonist of protein kinase C (PKC). The enhanced superoxide was normalized by apocynin, losartan (a blocker of angiotensin type 1 receptor), and chelerythrine chloride (an antagonist of PKC). Western blotting indicated an upregulation of gp91phox and p47phox, associated with increased expression of phosphorylated PKC in vessels of LS dogs. In separate experiments, dogs were fed simultaneously with LS and losartan (LS + Losa) for 2 wk. There was a significant increase in plasma ANG II in LS + Losa dogs, which, however, was associated with normal FID and gp91phox expression in coronary arterioles. In conclusion, LS led to endothelial dysfunction, as indicated by an impaired flow-induced dilation caused by decreasing NO bioavailibility, a response that involves angiotensin-induced activation of PKC that, in turn, activates vascular NAD(P)H oxidase to produce superoxide.
Keywords: low-salt diet, angiotensin, nicotinamide adenine dinucleotide phosphate oxidase, superoxide, flow-induced dilation
excessive activation of the renin-angiotensin-aldosterone system (RAAS), as a major player in the pathogenesis of cardiovascular diseases, has been extensively investigated. The question of whether restriction of sodium intake reduces the incidence of cardiovascular events is still a major issue of controversy (2). Experimental studies and clinical trials have yielded considerable heterogeneity concerning the effects of low-salt diet on blood pressure, as well as cardiovascular morbidity and mortality (3, 10, 14, 16). Sodium restriction, along with a reduction in blood pressure, is associated with increases of other, potentially adverse, cardiovascular effects, such as increases in sympathetic and RAAS activities (14). Given the direct and indirect adverse effects of excess angiotensin (ANG) II on vascular, myocardial, and renal tissues (27), the activation of RAAS may compromise the beneficial effects of sodium restriction on blood pressure and consequently increase cardiovascular risk (2).
An imbalance between the generation of vasoactive mediators, such as increased superoxide formation and decreased nitric oxide (NO) production promotes vascular damage, including endothelial dysfunction, inflammation, and atherosclerosis, in all of which stimulation of vascular NAD(P)H oxidase-derived superoxide by ANG II may be a pathogenetic factor (15, 38, 44). Increased superoxide production contributes to endothelial dysfunction via inactivating NO and transforming it into the prooxidant peroxynitrite, which may further cause eNOS uncoupling and reduced NO bioavailability (41, 42). In this context, it was reported that, as a consequence of sodium restriction, there was an accelerated development of atherosclerosis in aorta of apolipoprotein E-deficient mice (19). Also, the loss of the counterregulatory effects of ANG-(1–7) toward ANG II were proposed to be involved in the detrimental effects of sodium restriction (33). Additionally, vascular dysfunction, as evidenced by reduced acetylcholine-induced vasodilation (6) and enhanced phenylephrine-induced vasoconstriction (13), in aorta of rats fed a LS diet was also reported. These studies, however, provided no mechanistic insight and, moreover, were conducted on conduit vessels that are not primarily responsible for the regulation of peripheral vascular resistance and tissue perfusion. Thus, in the present study, we aimed to evaluate endothelial function of coronary arterioles by determination of flow/shear stress-induced responses to elucidate the mechanism underlying endothelial dysfunction in dogs fed a low-salt diet.
MATERIALS AND METHODS
Animals
Male mongrel dogs (25–28 kg) were fed with a normal-sodium diet (NS, 0.65% of NaCl; n = 7), a low-sodium diet (LS, 0.05% of NaCl; n = 8), or LS plus losartan (LS + Losa; 2 mg·kg−1·day−1 orally, n = 2) for 2 wk after a thoracotomy for implantation of instruments, for measurements of pressure and flow, and for blood sampling (37). Dogs were anesthetized with pentobarbital sodium (25 mg/kg iv). The heart was removed, and the left ventricular free walls were obtained and placed in cold MOPS-buffered (pH 7.4) physiological salt solution (PSS). Hemodynamics reported and the tissues used here were also used in another study, previously published (35). Experimental protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conform to the current guidelines of the National Institutes of Health and the American Physiological Society for the care and use of laboratory animals.
Measurement of Plasma ANG II
Plasma concentration of ANG II was measured with peptide enzyme immunoassay (Peninsula Laboratories).
Isolation of Arterioles
Isolation of arteriolar branches of the left anterior descending coronary artery was performed with the use of microscissors and an operating microscope (Olympus, Lake Success, NY) (37). Segments of subepicardial arterioles, ∼1 mm in length, were separated from the adhering cardiac muscle by careful dissection and were transferred to a vessel chamber containing Krebs bicarbonate-buffered PSS at room temperature. The vessel chamber contained two glass microcannulas, which were connected to two pressure-servo syringe systems (Living Systems, Burlington, VT). The vessel chamber was connected to a reservoir through a suffusion pump. The isolated vessels were incubated in PSS (37°C and pH 7.4) for at least 30 min before experiments.
Experimental Protocols
Flow-induced dilation.
The intravascular pressure of cannulated arterioles was maintained at 60 mmHg. After vessels developed spontaneous tone, flow-diameter relationships were obtained in control conditions and in the presence of apocynin (10−5 M), an inhibitor of NAD(P)H oxidase, or Nω-nitro-l-arginine methyl ester (l-NAME, 3 × 10−4 M), an inhibitor of nitric oxide synthase. Perfusate flow was increased from 0 to 20 μl/min (maximal ∼20 dyne/cm2 shear stress) in steps of 5 μl/min. Each flow step was maintained for 3–5 min to allow the vessels to reach steady-state conditions before their diameter was measured. Inhibitors were added to the vessel chamber and incubated with vessels for at least 30 min before flow-induced dilation was reassessed.
Adenosine- or sodium nitroprusside-induced dilation.
Vasodilation induced by the endothelium-independent agent adenosine (ADO, 10−9 to 10−4 M) was recorded at 60 mmHg of perfusion pressure in no-flow conditions. The agent was added to the vessel chamber, and final concentrations are reported. In separate experiments, sodium nitroprusside (SNP, 10−10 to 10−5 M)-induced dilation was also assessed. At the conclusion of the experiments, passive diameter (PD) of the arterioles, at 60 mmHg, was obtained in calcium-free PSS containing EGTA (1 mM).
Superoxide Detection
Superoxide production in coronary arterioles of NS and LS dogs was determined by two assays.
Assay 1.
Superoxide formation in the endothelium and smooth muscle cells of isolated coronary arterioles was assessed by using dihydroethidium (DHE) staining with confocal fluorescent imaging that was described in detail in our previous publication (20). Briefly, DHE (10−5 M) was administered intra- and extraluminally to cannulated vessels at 60-mmHg pressures for 30 min. After excess DHE was washed out, the vessels were cut longitudinally and fixed in 4% paraformaldehyde for 20 min. The vessel segment was adhered to glass slides with the endothelium facing up and covered with a cover slip with antifading solution for fluorescent confocal microscopy (Bio-Rad MRC 1024ES/Olympus 1 × 70). Three images of the endothelial and smooth muscle layers were consistently obtained per vessel segment. All images were taken with an UPlanFI ×40 objective and identical program settings. A histogram of full-sized fluorescent images was then created to measure the total number and average intensity of red pixels. The product of these two gives a total fluorescent intensity of the image measured, which corresponds to the level of superoxide.
Assay 2.
Quantitative superoxide formation in coronary arterioles was assessed by using DHE and an HPLC/fluorescence detector-based assay to determine 2-hydroxyethidium (2-EOH), a superoxide-induced oxidative product of DHE (11, 43). Briefly, coronary arterioles were isolated and perfused with MOPS-buffered PSS. Intravascular pressure was maintained constant at 60 mmHg. DHE (10−5 M) was then administered intraluminally to control vessels and to those that had been pretreated with apocynin (10−5 M) for 30 min. After a 1-h incubation, excess DHE was washed from the vessels. Vessels were then removed, pulverized in liquid nitrogen, and homogenized in a 1:1 mixture of acetonitrile and water. After centrifugation, the supernatant was collected for HPLC analysis; the remaining tissues were dissolved in 1 N NaOH for the protein measurement with the Bio-Red Protein Assay. In separate experiments, superoxide formation in vessels was determined in control and after incubation of vessels with ANG II (10−7 M) for 30 min, with or without apocynin, losartan [blocker of angiotensin type 1 receptor (AT1R), 2 × 10−6 M], or chelerythrine chloride [CLT, a protein kinase C (PKC) antagonist, at 2 × 10−6 M]. Superoxide formation in the vessels was also determined in control and after incubation of vessels with phorbol 12,13-dibutyrate (PDBu) for 45 min (PKC agonist, 10−5 M), or PDBu plus CLT. In the last protocol, superoxide formation in coronary arteries isolated from LS + Losa dogs was determined in control and after incubation of vessels with PDBu (45 min), or PDBu plus losartan or CLT.
Twenty-microliter samples or 2-EOH standards were separated by a HPLC system (PU-2080 Plus; Jasco) with a C-18 reverse-phase column (5 μm, 4.6 × 250 mm, Ultrasphere ODS; Beckman). The mobile phase was composed of 37% acetonitrile and 0.1% trifluoroacetic acid and run at a flow rate of 1 ml/min. The fluorescent signal of 2-EOH was detected at 480 nm (excitation) and 580 nm (emission) with a fluorescence detector (FP2020 Plus; Jasco). 2-EOH standards were synthesized from potassium nitrosodisulfonate as previously described (45). Standard curves of 2-EOH (0.3–10 pmol) were generated and used to calculate vascular superoxide production as picomoles per milligram protein (pmol/mg) in response to 1 h of incubation with 10 μM DHE.
Western Blot Analysis
Four to six single coronary arterioles were pooled as one sample. Equal amounts of total protein from samples were loaded on a 10% SDS-PAGE and transferred to a PVDF membrane. Membranes were probed with primary antibodies of gp91phox, p47phox, AT1R, and PKC (A-9; detection of all PKC family members) (all from Santa Cruz), and phospho-PKC (p-PKC-βII/Ser660; Cell Signaling), as well as β-actin (Sigma-Aldrich, St. Louis, MO) or β-tubulin (Santa Cruz). Immunoreactive bands were detected with an appropriate second antibody and visualized with a chemiluminescence kit (Pierce, Rockford, IL). Specific bands were normalized to glyceraldehyde-3-phosphate dehydrogenase, β-actin, or β-tubulin.
Statistical Analysis
Data are expressed as means ± SE. Statistical significance was calculated by Student's t-test and by repeated measures of two-way ANOVA, followed by Tukey-Kramer multiple-comparison test. Significance level was taken at P < 0.05.
RESULTS
The characteristics of coronary arterioles of NS, LS, and LS + Losa dogs are shown in Table 1. Active diameter and PD of arterioles in the three groups of dogs were comparable. As a consequence, the basal tone of vessels, expressed as a percentage of their PD, was not different in these three groups.
Table 1.
Characteristics of coronary arterioles of dogs
| Low Salt (n = 7/14) | Low Salt + Losartan (n = 2/8) | Normal Salt (n = 5/10) | |
|---|---|---|---|
| AD, μm | 83.7 ± 5.9 | 102.1 ± 8.7 | 80.5 ± 4.7 |
| PD, μm | 133.7 ± 5.2 | 158.1 ± 14.4 | 130.3 ± 4.7 |
| Basal tone, %PD | 62.9 ± 4.4 | 64.5 ± 3.6 | 61.8 ± 2.7 |
| MAP, mmHg | 97.6 ± 4.3* | 110.8 ± 3.1 |
Values are means ± SE; n, no. of dogs/no. of isolated arterioles. AD, active diameter; PD, passive diameter; MAP, mean aortic pressure.
Significant difference from dogs fed a normal-salt diet.
Plasma ANG II and Mean Aortic Pressure
Plasma ANG II was 3.7 ± 0.9 pg/ml in NS and 14.7 ± 2.8 pg/ml in LS dogs. The increase in circulating ANG II started after the 1st wk and was maintained during the entire period of LS diet. Mean aortic pressure (MAP) was significantly reduced in LS compared with NS dogs (97.6 ± 4.3 vs. 110.8 ± 3.1 mmHg) at the end of LS treatment.
Arteriolar Dilations in Response to LS Diet
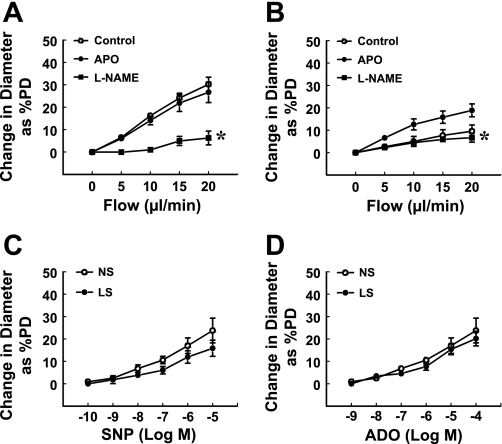
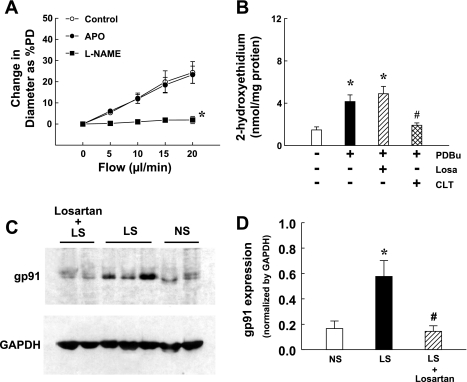
Endothelium-dependent (flow/shear stress-induced) and -independent (ADO- and SNP-induced) responses were assessed (Fig. 1) in coronary arterioles of LS and NS dogs. Flow-induced dilation was significantly attenuated in vessels of LS (10% of PD, Fig. 1B) compared with those of NS (30% of PD, Fig. 1A) dogs. The endothelial mediator responsible for the flow-induced dilation in vessels of NS dogs was NO, as indicated by the elimination of the responses by l-NAME. To clarify the possible role of superoxide, flow-induced dilation was performed in the presence of apocynin. We found that apocynin had no significant effect on flow-induced dilation of NS vessels but significantly increased the response in LS vessels (19% of PD); the increased portion of the responses was then abolished by l-NAME, indicating that inactivation of NO by superoxide most likely accounts for the impaired flow-induced dilation in vessels of LS dogs. l-NAME or apocynin did not significantly affect the basal tone of arterioles in the two groups of dogs. In addition, arteriolar response to SNP or ADO was unchanged between the vessels of the two groups of dogs (Fig. 1, C and D), suggesting a comparable smooth muscle function.
Fig. 1.
Flow-induced dilation of coronary arterioles of normal-salt (NS, n = 5; A) and low-salt (LS, n = 7; B) diet dogs in the control condition and after incubating vessels with apocynin (APO, 10−5 M), or apocynin plus Nω-nitro-l-arginine methyl ester (l-NAME, 3 × 10−4 M) for 30 min. Changes in diameter were normalized to passive diameter (PD) of the vessels. *Significant difference from the curve of APO. C and D: sodium nitroprusside (SNP) and adenosine (ADO)-induced dilations in coronary arterioles of NS and LS dogs.
Superoxide, NAD(P)H, AT1R, and PKC
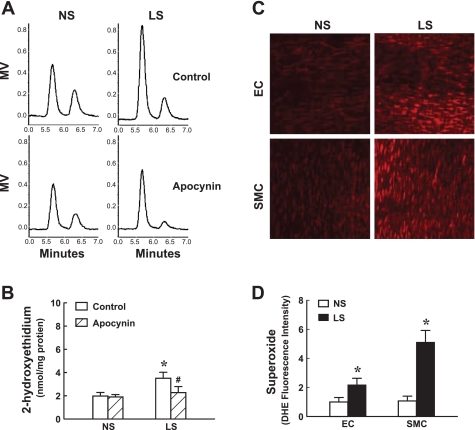
Based on the results showing an apocynin-dependent restoration of flow-induced dilation of LS vessels, we measured superoxide production in coronary arterioles of both groups of dogs. By using an HPLC/fluorescence detector of 2-EOH (Fig. 2, A and B), we demonstrated that, in line with the functional results shown in Fig. 1, the increased superoxide in LS vessels was prevented by apocynin, whereas apocynin did not affect superoxide levels of NS vessels. These results were further confirmed by confocal fluorescent images (Fig. 2, C and D), showing a significantly greater staining for superoxide in both endothelial and smooth muscle layers of LS compared with those of NS vessels.
Fig. 2.
Generation of superoxide in coronary arterioles of dogs using high-performance liquid chromatography (HPLC)/fluorescence detector of 2-hydroxyethidium (A and B) and confocal fluorescent image of superoxide by dihydroethidium (C and D). A and B: HPLC traces and summary data in control and in the presence of apocynin in NS (n = 5) and LS (n = 7) dogs. MV, millivolt. *Significant difference from NS dogs. #Significant difference from the corresponding control. C and D: original and summary data of confocal fluorescent images for superoxide production (red color) in the endothelium and smooth muscle layers in NS (n = 5) and LS (n = 5) dogs. EC, endothelial cells; SMC, smooth muscle cells. *Significant difference from NS dogs.
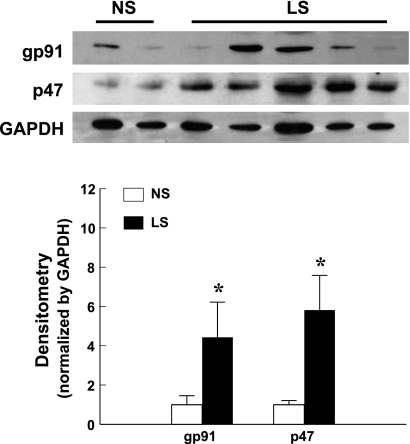
As a major source of vascular superoxide, expression of key components of the NAD(P)H oxidase, gp91phox and p47phox, was determined. Western blot analysis (Fig. 3) shows a significant upregulation of gp91phox and p47phox in coronary arterioles of LS dogs.
Fig. 3.
Protein expression of gp91phox and p47phox in coronary arterioles of dogs. Densitometry data were summarized from two blots. *Significant difference from NS dogs. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
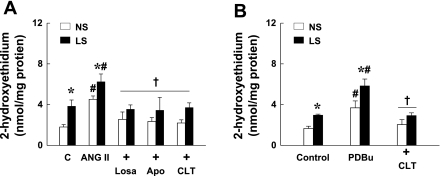
To clarify the specific mechanism underlying LS-induced ANG II to increase vascular superoxide, we determined superoxide formation in coronary vessels of NS and LS dogs treated with ANG II in control conditions and after inhibition of NAD(P)H oxidase, AT1R, or PKC with apocynin, losartan, and CLT, respectively (Fig. 4A). We found that, compared with untreated vessels, superoxide was further increased in ANG II-treated vessels. The increase was significantly greater in vessels of LS than that of NS. This ANG II-induced increase in superoxide was prevented by apocynin, losartan, or CLT, indicating that ANG II, through its interaction with the AT1R, activates NAD(P)H oxidase to produce superoxide via a PKC-dependent mechanism. To further evaluate the specific role of PKC, superoxide was measured in NS and LS vessels that were treated with PDBu, an agonist of PKC. Similar to ANG II, PDBu significantly increased superoxide production, with a significantly greater increase in LS than in NS, which was then prevented by CLT (Fig. 4B).
Fig. 4.
Superoxide production assessed by HPLC/fluorescence detector of 2-hydroxyethidium in coronary arterioles of NS and LS dogs in control (C) and in response to ANG II (10−7 M) and ANG II plus apocynin (10−5 M), losartan (Losa, 2 × 10−6 M), or chelerythrine chloride (CLT, 2 × 10−6 M) (A); and in control and in response to phorbol 12,13-dibutyrate (PDBu, 10−5 M) and PDBu plus CLT (B) (n = 5–7 dogs/group). *Significant difference from NS dogs. #Significant difference from corresponding controls. †Significant difference from those treated with ANG II alone or PDBu alone.
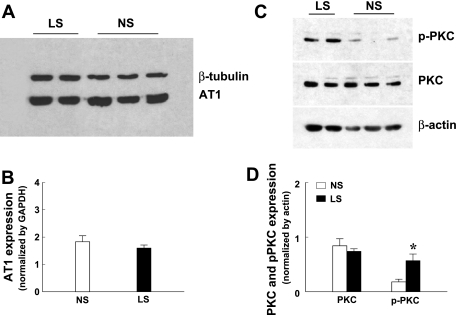
Western blot analysis revealed that the expression of AT1R (Fig. 5, A and B) and PKC (Fig. 5, C and D) was similar between the vessels of two groups of dogs, but the expression of phosphorylated PKC (Fig. 5, C and D) was significantly greater in LS than in NS vessels. These data suggest that ANG II-stimulated activation of vascular PKC is responsible for the upregulation and activation of NAD(P)H oxide and increase of superoxide in coronary arterioles, as a result of the LS.
Fig. 5.
Protein expression of angiotensin type 1 (AT1) receptor (A and B) and protein kinase C (PKC) and phosphorylated PKC (p-PKC-βII/Ser660) (C and D) in coronary arterioles of dogs. Densitometry data were summarized from three blots. *Significant difference from NS dogs.
Role of In Vivo Treatment with Losartan in LS-Induced Endothelial Dysfunction
In separate experiments, dogs were fed simultaneously with LS diet and losartan for 2 wk to clarify whether in vivo treatment with losartan is able to prevent LS-activated signaling in ANG II-induced oxidative stress and endothelial dysfunction. Similar to dogs fed with LS alone, plasma concentration of ANG II in losartan-treated LS dogs increased from 5.08 ± 0.2 to 16.54 ± 0.08 pg/ml. However, flow-induced dilation (25% of PD) was similar to that in NS dogs (Fig. 6A). Superoxide formation in coronary arteries (1.5 ± 0.3 nmol/mg protein) was not different in that of NS dogs (1.8 ± 0.2 nmol/mg protein) but increased significantly in response to PDBu (Fig. 6B). Additional losartan did not, but CLT did, inhibit PDBu-induced superoxide in the vessels. The expression of gp91phox in losartan-treated dogs was similar to that in NS dogs (Fig. 6, B and C). These results confirm the role of ANG II in the mediation of LS-induced endothelial dysfunction.
Fig. 6.
A: flow-induced dilation of coronary arterioles of LS diet dogs treated with losartan (Losa, 2 mg·kg−1·day−1 orally) (n = 2 dogs/8 arterioles) in the control condition and after incubating vessels with apocynin (10−5 M) or apocynin plus l-NAME (3 × 10−4 M) for 30 min. Changes in diameter were normalized to passive diameter of the vessels. *Significant difference from the curve of apocynin. B: 2-hydroxyethidium was determined in coronary arteries of LS + Losa dogs (n = 2 dogs/5–7 arteries) in the absence and in the presence of PDBu and PDBu plus losartan (Losa) or CLT. *Significant difference from the absence of PDBu. #Significant difference from PDBu. C and D: protein expression of gp91phox in coronary arterioles of NS-, LS-, and LS- plus losartan-treated dogs. *Significant difference from NS. #Significant difference from LS.
DISCUSSION
The major finding of the present study is that endothelial response to shear stress, a primary physiological stimulus for the release of endothelial NO to control arteriolar tone, is impaired in coronary arterioles of dogs fed LS diet. The signaling cascade responsible for the endothelial dysfunction involves the ANG II/AT1R-dependent activation of PKC, which, in turn, upregulates NAD(P)H oxidase to produce superoxide, leading to a reduced NO bioavailability, as manifested by the attenuated flow-induced dilation.
Activity of the RAAS is regulated by sodium intake. Salt depletion is a potent stimulus for the secretion of renin and the generation of angiotensin, as well as aldosterone (8). However, the mechanism contributing to the failure of hyperreninemia to elevate blood pressure has not been convincingly explained. Indeed, in the present study, we observed a significant reduction of MAP in LS compared with NS dogs, which was paradoxically associated with a threefold increase in plasma ANG II. Therefore, our results provide strong evidence in favor of the hypothesis that LS intake initiates blood pressure-independent effects on the cardiovascular system. We demonstrated previously that arterial/arteriolar endothelium contributes to circulatory homeostasis and tissue perfusion by the flow/shear stress-dependent regulation of vascular resistance (21) through release of endothelial NO. Flow-induced dilation of coronary arterioles has not yet been investigated in dogs with sodium restriction, preventing the assessment of the effects of ANG II on shear stress-dependent mechanisms in the coronary circulation. Motivated by this, flow-induced dilation of coronary arterioles was assessed. As demonstrated previously, NO is the primary mediator in coronary arteries of dogs (37). Thus, the significantly attenuated flow-induced dilation in coronary arterioles of LS dogs (Fig. 1) is indicative of an impaired NO-mediated portion of the response. Consistent with our findings, an impaired ACh-initiated NO-mediated vasodilation was also reported in aorta of rats fed LS (6). Because the endothelium-independent dilator responses in vessels of both groups of dogs were similar, we hypothesized that an impaired NO availability was responsible for the attenuated flow-induced dilation of LS vessels. To further characterize this response, the role of superoxide in this endothelial dysfunction was evaluated by inhibition of vascular NAD(P)H oxidase, since, among the many enzymatic sources of superoxide, NAD(P)H oxidase appears to be most prevalent in the vasculature (23, 41). The results indicated that apocynin significantly restored the attenuated dilator responses to shear stress in LS vessels and that the restored portion of the responses was inhibited by l-NAME, indicating that NAD(P)H-derived superoxide impairs flow-induced dilation through scavenging of NO. However, shear stress-induced release of NO (36) and superoxide-induced eNOS nitration (42) were not determined in the present study. Thus, effects of LS on eNOS function (NO production) need to be further investigated. In our recent study, we found that in vivo administration of veratrine initiated NO-mediated increases in coronary blood flow in NS dogs. In LS dogs, however, the veratrine-induced increases in coronary blood flow were reduced by 44% and were completely reversed by ascorbic acid or apocynin (35). In line with these results, Fig. 2 demonstrates that vascular (including the endothelium and smooth muscle layers) generation of superoxide was indeed significantly greater in LS than NS dogs. This was normalized by apocynin, confirming that NAD(P)H oxidase is the major source of superoxide, although we could not, in the present study, exclude the possibility that apocynin has additional antioxidant effects besides the inhibition of NAD(P)H oxidase. NAD(P)H oxidase is a multicomponent enzyme consisting of membrane-bound gp91phox and p22phox, three cytoplasmic subunits including p47phox, p67phox, and p40phox, and the small GTPase Rac 1/2 (4). Vascular endothelial cells express all of these components, as well as gp91phox homologs nox1, nox4, and nox5 (1, 23, 25). Of the numerous vasoactive agents regulating vascular NAD(P)H oxidase, ANG II appears to be one of the most important. ANG II activates NAD(P)H oxidase via translocation of cytosolic p47phox, p67phox, and p40phox to membrane-associated gp91phox and p22phox, leading to assembly and activation of the oxidase to generate superoxide (31, 39). Additionally, we also observed in the present study that ANG II is able to control the expression of NAD(P)H oxidase subunits (23) by increased protein expression of gp91phox and p47phox in LS coronary arterioles (Fig. 3). A specific role of p47phox in functionally active NAD(P)H oxidase was shown by studies using p47phox knockout (KO) mice. These studies indicated that vascular smooth muscle cells isolated from p47phox-KO mice failed to produce superoxide in response to ANG II (24). Moreover, ANG II-stimulated superoxide production was completely absent in coronary microvascular endothelial cells isolated from p47phox-KO mice, which could be restored by transfection of the cells with p47phox cDNA (26). These studies strongly support our conclusions that LS-initiated increases in plasma ANG II stimulate the production of vascular superoxide derived from NAD(P)H oxidase and that the chronic presence of high plasma ANG II upregulates p47phox and gp91phox that, in turn, further increases superoxide synthesis. Moreover, in the presence of high levels of aldosterone, as a consequence of increased ANG II, the increased production of reactive oxygen species (ROS) leads to eNOS uncoupling, which, in turn, further reduces NO synthesis and enhances superoxide production (22), although we indicated recently that, in physiological conditions, aldosterone evokes an endothelium-dependent NO-mediated vasodilation (17).
In the present study, we were particularly interested in the role of AT1R, which participates in ANG II-dependent regulation of superoxide generation (9, 29). AT1R serves as a control point for regulating the ulterior effects of ANG II on its target tissue. This issue was well clarified by a previous study showing that superoxide-induced endothelial dysfunction in aged cerebral arteries, represented by an impaired NO-mediated vasodilation, was absent in AT1R-KO mice of the same age (30). Our results also provide evidence showing that the increased production of superoxide in ANG II-treated vessels was reversed by blockade of AT1R (Fig. 4). The expression/activity of AT1R links, mechanistically, a variety of cardiovascular diseases, such as hypertension and hypercholesterolemia. For instance, low-density lipoprotein upregulates AT1R via posttranscriptional mRNA stabilization (32) that could serve as an explanation for the association between hyperlipidemia and hypertension. In the present study, vascular protein expression of AT1R was unchanged after LS diet for 2 wk, but its essential role in the mediation of ANG II-induced superoxide production was evidenced by the normalization of the responses by in vitro treatment of LS vessels (Fig. 4A) and in vivo treatment of LS dogs with losartan (Fig. 6).
To further clarify whether the ANG II/AT1R accounts directly for the upregulation of vascular NAD(P)H oxidase and superoxide production, and, if so, what mechanism(s) is involved, we tested generation of superoxide in LS vessels that were additionally treated in vitro with ANG II. As expected, the vessels, having been subjected to ANG II, produced significantly more than the originally elevated basal level of superoxide. The additional increase was normalized by CLT (Fig. 4A), suggesting that activation of PKC plays a critical role in the signaling cascade. The specific role of PKC in the responses was then further evaluated by exposure of the vessels to the PKC agonist PDBu. Indeed, PDBu increased superoxide production (Fig. 4B) to the level induced by ANG II (Fig. 4A). PKC is a family of serine-threonine kinases, among which class/conventional PKC isoforms (cPKC-α, -βI, -βII, and -γ) are the main subgroups present in endothelial cells (28). Inhibition of PKC activity is associated with suppression of ROS production in a variety of vascular cell types (18, 40), suggesting that the activation of PKC and generation of intracellular ROS are interdependent. Activated PKC stimulates NAD(P)H oxidase activity through phosphorylation of p47phox (5, 7). In this context, PKC-dependent phosphorylation of p47phox has been reported to be essential for platelet-derived growth factor-stimulated ROS generation in human umbilical venous endothelial cells (34). Consistent with these findings, we demonstrated significantly increased phosphorylation at the βII/Ser660 position, but not unphosphorylated PKC (Fig. 5, C and D), confirming further the essential role of PKC activation in ANG II-dependent upregulation of superoxide synthesis in LS vessels (Fig. 4). Although phosphorylation of p47phox, downstream to PKC activation during the signaling cascade for ANG II-dependent regulation of NAD(P)H oxidase, was not measured in the present study, its role is implied by upregulation of p47phox (Fig. 3), accompanied by an increased PKC activity (Fig. 5). Thus, the ANG II-AT1R-PKC-NAD(P)H-superoxide signal transduction cascade is likely to account for the generation of superoxide in LS vessels. It is noteworthy that PKC-dependent generation of superoxide was also observed in ANG II-treated NS vessels, implying the universal nature of the response. Moreover, the increased activation of PKC (phosphorylated PKC, Fig. 5) contributes to a greater increase in the production of superoxide in LS vessels in response to ANG II. Interestingly, a recently published study revealed a novel mechanism in phagocytic cells by which the metabolite of losartan blocks NADPH oxidase-mediated superoxide production by inhibiting PKC (12), although the significance of these findings in vascular endothelial cells needs to be clarified.
In conclusion, LS for 2 wk increases coronary vascular superoxide production that decreases NO bioactivity to impair the mechanism of endothelium-mediated responses to shear stress. The specific signal transduction pathway involves an ANG II-dependent upregulation of NAD(P)H oxidase, via AT1R-mediated activation of PKC. It is noted that normal dogs were used in the present study, whereas in patients with hypertension or heart failure hormone levels affecting salt retention may already be altered. Nevertheless, in regard to the net effect of LS intake, results of our study suggest that a dietary recommendation of salt restriction should take into account its multiple consequences for cardiovascular function in devising a clinical strategy for the prevention/treatment of cardiovascular diseases.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-68813, HL-43023, HL-083647, HL-50142, and HL-070653.
DISCLOSURES
NO.
REFERENCES
- 1.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109: 227– 233, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Alderman MH. Salt, blood pressure, and human health. Hypertension 36: 890– 893, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Alderman MH, Cohen H, Madhavan S. Dietary sodium intake and mortality: the National Health and Nutrition Examination Survey (NHANES I). Lancet 351: 781– 785, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Babior BM. NADPH oxidase. Curr Opin Immunol 16: 42– 47, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys 397: 342– 344, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Boonstra AH, Gschwend S, Kocks MJ, Buikema H, de Zeeuw D, Navis GJ. Does a low-salt diet exert a protective effect on endothelial function in normal rats? J Lab Clin Med 138: 200– 205, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res 65: 16– 27, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Cowley AW, McCaa RE. Acute and chronic dose-response relationships for angiotensin, aldosterone, and arterial pressure at varying levels of sodium intake. Circ Res 39: 788– 797, 1976 [DOI] [PubMed] [Google Scholar]
- 9.Dechend R, Muller DN, Wallukat G, Homuth V, Krause M, Dudenhausen J, Luft FC. AT1 receptor agonistic antibodies, hypertension, and preeclampsia. Semin Nephrol 24: 571– 579, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Egan BM. Pleiotropic benefits of moderate salt reduction. Hypertension 54: 447– 448, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 287: C895– C902, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Fortuno A, Bidegain J, Robador PA, Hermida J, Lopez-Sagaseta J, Beloqui O, Diez J, Zalba G. Losartan metabolite EXP3179 blocks NADPH oxidase-mediated superoxide production by inhibiting protein kinase C: potential clinical implications in hypertension. Hypertension 54: 744– 750, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Giardina JB, Cockrell KL, Granger JP, Khalil RA. Low-salt diet enhances vascular reactivity and Ca(2+) entry in pregnant rats with normal and reduced uterine perfusion pressure. Hypertension 39: 368– 374, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Graudal NA, Galloe AM, Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: a meta-analysis. J Am Med Assoc 279: 1383– 1391, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res 86: E85– E90, 2000 [DOI] [PubMed] [Google Scholar]
- 16.He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, MacGregor GA. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension 54: 482– 488, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Heylen E, Huang A, Sun D, Kaley G. Nitric oxide-mediated dilation of arterioles to intraluminal administration of aldosterone. J Cardiovasc Pharmacol 54: 535– 542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49: 1939– 1945, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Ivanovski O, Szumilak D, Nguyen-Khoa T, Dechaux M, Massy ZA, Phan O, Mothu N, Lacour B, Drueke TB, Muntzel M. Dietary salt restriction accelerates atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 180: 271– 276, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G, Sun D. Aging enhances pressure-induced arterial superoxide formation. Am J Physiol Heart Circ Physiol 293: H1344– H1350, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koller A, Kaley G. Endothelial regulation of wall shear stress and blood flow in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol 260: H862– H868, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201– 1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277– R297, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation 104: 79– 84, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem 277: 19952– 19960, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem 278: 12094– 12100, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Luft FC. Proinflammatory effects of angiotensin II and endothelin: targets for progression of cardiovascular and renal diseases. Curr Opin Nephrol Hypertens 11: 59– 66, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Mahrouf M, Ouslimani N, Peynet J, Djelidi R, Couturier M, Therond P, Legrand A, Beaudeux JL. Metformin reduces angiotensin-mediated intracellular production of reactive oxygen species in endothelial cells through the inhibition of protein kinase C. Biochem Pharmacol 72: 176– 183, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82– C97, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol Heart Circ Physiol 296: H1914– H1919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol 25: 274– 278, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Nickenig G, Sachinidis A, Michaelsen F, Bohm M, Seewald S, Vetter H. Upregulation of vascular angiotensin II receptor gene expression by low-density lipoprotein in vascular smooth muscle cells. Circulation 95: 473– 478, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Roks AJ, Nijholt J, van Buiten A, van Gilst WH, de Zeeuw D, Henning RH. Low sodium diet inhibits the local counter-regulator effect of angiotensin-(1–7) on angiotensin II. J Hypertens 22: 2355– 2361, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Simon F, Stutzin A. Protein kinase C-mediated phosphorylation of p47 phox modulates platelet-derived growth factor-induced H2O2 generation and cell proliferation in human umbilical vein endothelial cells. Endothelium 15: 175– 188, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Suematsu N, Ojaimi C, Recchia FA, Wang Z, Skayian Y, Xu X, Zhang S, Kaminski P, Sun D, Wolin MS, Kaley G, Hintze TH. Potential mechnisms of low salt-induced cardiac disease: superoxide-NO in the heart. Circ Res 106: 593– 600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol 286: H2249– H2256, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun D, Huang A, Zhao G, Bernstein R, Forfia P, Xu X, Koller A, Kaley G, Hintze TH. Reduced NO-dependent arteriolar dilation during the development of cardiomyopathy. Am J Physiol Heart Circ Physiol 278: H461– H468, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension 42: 1075– 1081, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Touyz RM, Yao G, Quinn MT, Pagano PJ, Schiffrin EL. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol 25: 512– 518, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108: 1253– 1258, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Wolin MS. Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol 296: H539– H549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297: H1829– H1836, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34: 1359– 1368, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Zhou MS, Schulman IH, Raij L. Nitric oxide, angiotensin II, hypertension. Semin Nephrol 24: 366– 378, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc 3: 8– 21, 2008 [DOI] [PubMed] [Google Scholar]