Abstract
A successful pregnancy outcome relies on extensive maternal cardiovascular adaptation, including enhanced uteroplacental vasodilator mechanisms. The objective of the present study was to determine the contribution of the endothelium-derived hyperpolarizing factor (EDHF) signaling in pregnancy-enhanced uterine vasodilation, to define the role of Ca2+-activated K+ channels in mediating EDHF effects, and to explore the impact of endothelial Ca2+ signaling in pregnancy-specific upregulation of EDHF. Fura 2-based measurements of smooth muscle cell (SMC) and endothelial cell cytosolic Ca2+ concentration ([Ca2+]i) were performed simultaneously with measurements of the diameter of uterine radial arteries from nonpregnant (NP) and late pregnant (LP) rats. Changes in SMC membrane potential of pressurized arteries from LP rats were assessed using glass microelectrodes. After blockade of nitric oxide and prostacyclin production, a cumulative application of ACh induced rapid and effective dilatation of uterine vessels from both NP and LP rats. This vasodilation was associated with SMC hyperpolarization and SMC [Ca2+]i reduction and was abolished by a high-K+ solution, demonstrating that NG-nitro-l-arginine (l-NNA)- and indomethacin-resistant responses are attributable to EDHF. Pregnancy significantly potentiates EDHF-mediated vasodilation in part due to enhanced endothelial Ca2+ signaling. l-NNA- and indomethacin-resistant responses were insensitive to iberiotoxin but abolished by a combined treatment with apamin and charybdotoxin, supporting the key role of small- and intermediate-conductance K+ channels in mediating EDHF signaling in the maternal uterine resistance vasculature.
Keywords: acetylcholine, smooth muscle cell hyperpolarization, endothelial calcium signaling, calcium-activated potassium channels
successful pregnancy outcome relies on extensive maternal cardiovascular adaptation, including dramatic increase in uteroplacental blood flow. Current studies implicate growth and remodeling of the maternal uterine vasculature and enhanced uterine vasodilation as major underlying mechanisms (1, 3, 36, 37, 43, 44). Pregnancy is associated with a marked change in uterine endothelial function, resulting in increased basal and stimulated release of nitric oxide (NO) and prostacyclin (PGI2). In large conductive uterine arteries of animals and humans, these two autocoids mostly mediate endothelium-dependent dilatation (3, 50). Recent studies indicate that a significant part of agonist-induced vasodilation of more distal human myometrial arteries or smaller rat uterine radial arteries is resistant to inhibitors of nitric oxide synthase (NOS) and cyclooxygenase (COX), implicating the role of endothelium-derived hyperpolarizing factor (EDHF) (6, 28).
Extensive in vivo and in vitro studies during the last decade convincingly demonstrate an essential role of EDHF in regulating the tone of small-resistance arteries and arterioles in a variety of vascular beds (5, 8, 13, 16, 22, 27, 30, 48). The nature of EDHF remains the matter of substantial controversy, and EDHF is no longer considered as a single endothelium-derived factor. Several diffusible substances or even the mechanism of electrical coupling between endothelial cells (ECs) and smooth muscle cells (SMCs) were proposed for the role of EDHF in different vascular beds. These multiple mechanisms can work separately or in combination depending on the type of EC stimulation as well as the origin of blood vessels (5, 8, 9, 16, 22). An elevation of endothelial cytosolic Ca2+ concentration ([Ca2+]i), a key event in response to stimulation of ECs by numerous neurohumoral mediators or mechanical forces, is critically involved in EDHF-evoked vasodilation (5, 8, 22, 34, 41). This [Ca2+]i rise in endothelial cells results in activation of multiple Ca2+-dependent pathways mediating EDHF. For example, Ca2+-induced activation of arachidonic acid can cause formation of epoxyeicosatrienoic acids (EETs), a diffusible mediator of EDHF-induced vasodilation in coronary, renal, and skeletal circulations. One of the established mechanisms of EET-induced vasodilation is activation of large-conductance Ca2+-activated K+ channels (BKCa) of vascular SMCs, resulting in their hyperpolarization and relaxation (5, 8, 9, 16). In the majority of resistance arteries and arterioles, endothelial [Ca2+]i rise activates Ca2+-dependent small (SKCa)- and intermediate (IKCa)-conductance K+ channels (5, 8, 16, 22, 27, 34, 41). Subsequent efflux of K+ from ECs through SKCa and IKCa channels can hyperpolarize and relax underlying vascular SMCs through activation of inward-rectifier K+ channels, Na+-K+ pump, or both (15). Recently demonstrated colocalization of IKCa channels and myoendothelial gap junctions provides a structural basis for such mechanism (33, 46). Hyperpolarization of ECs due to activation of SKCa and IKCa channels can also electrotonically spread to neighboring SMCs through myoendothelial gap junctions, resulting in SMC hyperpolarization and relaxation. Contribution of other proposed mediators, such as hydrogen peroxide and C-type natriuretic peptide, in EDHF-related mechanisms is currently under investigation (5, 8, 16).
In the course of rodent and human pregnancy, maternal preplacental spiral arteries are transformed into large dilated vessels with a loss of their constrictor function. More proximal radial uterine arteries become a major site regulating uteroplacental vascular resistance and, as a consequence, fetal growth and development (4, 21, 37, 43, 44). In spite of the acknowledged importance of these vessels in the control of uteroplacental blood flow, the nature of EDHF and its role in the regulation of uteroplacental vascular tone remains unknown. In the present study, we hypothesized that EDHF importantly contributes to pregnancy-specific enhancement of vasodilation in the maternal uterine circulation because of augmented EC Ca2+ signaling and activation of SKCa and IKCa channels. Therefore, the purpose of this study was to: 1) characterize the contribution of EDHF to ACh-induced uterine vasodilation; 2) define the effect of pregnancy on EDHF-mediated responses; 3) explore the role of EC [Ca2+]i in pregnancy-induced modulation of EDHF; and 4) study the role of BKCa, SKCa, and IKCa channels in EDHF-induced responses of uterine vessels.
METHODS
Animals and preparation of arteries.
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 85–23, Revised 1996), and the experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Vermont.
Virgin cycling nonpregnant (NP, n = 50) or late pregnant (19–20 day, LP, n = 66) female Sprague-Dawley rats of 14–16 wk old were used for this study. The estrous cycle of NP rats was determined by examination of vaginal smears on the day of experimentation. In the current study, we used 11 rats in proestrous, 23 rats in metestrous, and 16 rats in diestrous stage of the estrous cycle. Animals were anesthetized by an intraperitoneal injection of Nembutal (50 mg/kg) and killed by decapitation. The abdominal wall was transected, and the entire uterus and uterine vasculature was rapidly removed and pinned in a dissecting dish filled with aerated cold physiological salt solution (PSS; see Solutions and drugs for composition). Second-order uterine radial arteries were identified within the mesometrial arcade and dissected free of connective tissue. Only radial arteries feeding the placenta (uteroplacental arteries) were dissected from LP rats. Arterial segments were cannulated from both ends in the arteriograph and continuously superfused at 3 ml/min with aerated (10% O2-5% CO2-85% N2) PSS at 37°C. To minimize mechanical stimulation of ECs and SMCs within the arterial wall during the equilibration period, cannulated arteries were initially pressurized to 10 mmHg using the servo pressure system (Living System Instrumentation, Burlington, VT). All experiments were performed at 50 mmHg and under no intraluminal flow conditions. In contrast to uterine radial arteries from NP rats, uteroplacental arteries from LP animals can develop vasoconstriction (myogenic tone) in response to elevations of pressure exceeding 50 mmHg (52). Therefore, to avoid development of myogenic tone and to equalize experimental conditions for arteries of NP and LP rats, they were pressurized to a similar level: 50 mmHg. Blood pressure measured in vivo in rat distal uteroplacental arteries just before entering the placenta was ∼14 mmHg (37). Uteroplacental arteries are located in the middle section of the mesometrial vasculature between the main uterine artery and the placenta. Therefore, physiological levels of pressure experienced by these vessels in vivo should approximate 50–70 mmHg.
Selective loading of endothelial or SMCs with fura 2 and measurement of intracellular [Ca2+]i.
Detailed description of the procedure for selective loading of ECs or SMCs of uterine arteries with the Ca2+-sensitive dye fura 2 was previously published (20, 52). Briefly, heat-polished glass cannulas were used in all experiments to prevent accidental damage of the endothelial layer during the cannulation procedure and to avoid diffusion of fura 2 to the SMC layer. ECs were loaded with fura 2 at room temperature by intraluminal perfusion of pressurized arteries with fura 2-AM-containing solution (5 μM) for 5 min followed by 10 min of washout with regular PSS. A similar protocol was used in our previous study where preferential loading of ECs with fura 2 was confirmed by nearly complete disappearance of fluorescent signal after arterial denudation (20). SMC loading with fura 2 was performed by extraluminal incubation of arteries in fura 2-AM (5 μM) solution at room temperature in the dark for 60 min. Fura 2-loaded arteries were washed two to three times and then continuously superfused with aerated PSS at 37°C. Preferential loading of SMCs over ECs with this protocol can be confirmed by lack of [Ca2+]i responses to 3 μM ACh in uterine arteries depolarized with high (35–45 mM)-K+ solution (see Fig. 4B). It has been shown that, in pressurized arterioles, ACh-induced increase in EC [Ca2+]i was not modulated by high-K+ depolarization (10). Similar experimental protocols were previously used for selective loading of ECs or SMCs in microvessels (7, 35, 53).
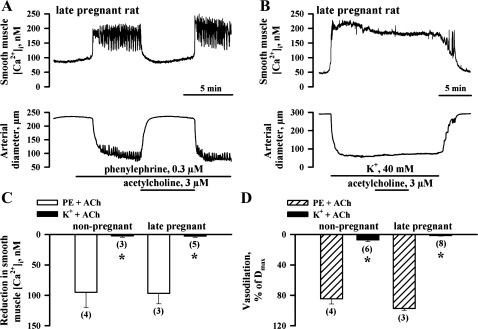
Fig. 4.
Inhibition of EDHF-mediated responses of uterine arteries by high-K+ solution. A: representative tracings showing changes in SMC [Ca2+]i and lumen diameter of the uteroplacental artery from a LP rat in response to application of 3 μM ACh. B: ACh at the same concentration failed to induce changes in SMC [Ca2+]i and the diameter of the artery preconstricted with 40 mM of K+. l-NNA (200 μM) and indomethacin (10 μM) were present throughout all experiments. C and D: bar graphs summarizing ACh-induced SMC [Ca2+]i and dilator responses of uterine arteries from NP and LP rats preconstricted with PE or high-K+ solution. Vasodilation is expressed as Dmax. Nos. in parentheses indicate the no. of tested arteries. *Significantly different at P < 0.05 (unpaired Student's t-test).
Ratiometric measurements of fura 2 fluorescence from ECs or SMCs were performed using a photomultiplier system (IonOptix, Milton, MA). Experimental ratios were corrected for background fluorescence taken from each artery before loading with fura 2. Background-corrected ratios of 510 nm emission were obtained at a sampling rate of 5 Hz from arteries alternately excited at 340 and 380 nm. The arterial lumen diameter was simultaneously monitored using the SoftEdge Acquisition Subsystem (IonOptix). All experimental protocols were started following an additional 15-min equilibration period at 10 mmHg to allow intracellular deesterification of fura 2-AM.
Measurements of membrane potential from SMCs of pressurized uterine arteries.
For intracellular measurement of SMC membrane potential (MP) from pressurized arteries, we used short arterial segments (400–500 μm) that were carefully cleaned of any residual connective tissue. Lumen diameter and MP changes were simultaneously recorded from arteries pressurized at 50 mmHg. Each glass microelectrode was positioned on the top of the arterial segment, and impalement was made from the adventitial surface of the artery by advancing the electrode down the long axis of SMCs. Such position of the glass microelectrode and some flexibility of the microelectrode tip allowed for better maintenance of the impalement during movements of the vessel wall. All MP measurements were performed on uteroplacental arteries of LP rats. Small diameters and stiff vessel walls of radial uterine arteries of NP rats did not allow continuous recordings of SMC MP during ACh application. For measurement of MP, we used microelectrodes filled with 0.5 M KCl having tip resistances of 110–150 MΩ; an Ag-AgCl pellet was used as an indifferent electrode. A microelectrode was connected to a motorized micromanipulator (World Precision Instruments), and MP was recorded using a high-input impedance amplifier Electro 705 (World Precision Instruments). Changes in MP and arterial diameter were simultaneously displayed and recorded on a desktop computer using a data acquisition program (IonOptix). The following criteria were used for acceptance of MP recordings: 1) abrupt negative change in voltage upon impalement of the cells; 2) a sharp return to zero voltage following withdrawal of a microelectrode tip; 3) tip potential of <7 mV; and 4) unchanged resistance of microelectrodes after impalement. A stable MP recording for at least 1 min was accepted for data collection.
Protocols for studying EDHF-mediated responses of uterine arteries.
After equilibration and loading of cells with fura 2, arteries were incubated with 200 μM NG-nitro-l-arginine (l-NNA, NOS inhibitor) and 10 μM indomethacin (COX inhibitor) to abolish the production of NO and PGI2, respectively. A number of published observations demonstrated a nearly complete inhibition of agonist-induced endothelial NO production in the presence of 100–200 μM NOS inhibitors [l-NNA or NG-monomethyl-l-arginine (l-NMMA)] measured with the NO-sensitive dye DAF-2 (14, 31, 57). The arterial diameters and levels of EC or SMC [Ca2+]i were recorded during 5 min at 10 mmHg followed by an elevation of intraluminal pressure to 50 mmHg.
After 20 min of treatment of vessels with l-NNA and indomethacin, phenylephrine (PE) was added in increasing concentrations (1–3 doses) to produce a constriction of 50–70% of the initial diameter. Following stabilization of vasoconstriction, ACh was applied in increasing concentrations. For each artery, dose-dependent effects of ACh were studied only one time. A combination of papaverine (100 μM, a phosphodiesterase inhibitor) and diltiazem (10 μM, a Ca2+ channel blocker) was added at the end of each experiment to obtain the diameter under maximally dilated conditions. ACh-induced vasodilation was expressed as the percentage of maximal vasodilation in response to papaverine and diltiazem (Dmax). Additional experiments were performed to determine the effects of 3 μM ACh in arteries treated with l-NNA and indomethacin and preconstricted by 50–70% of the initial diameters with moderate (35–45 mM) concentrations of K+.
To test the role of SMC BKCa channels in EDHF-mediated responses, l-NNA- and indomethacin-treated uterine arteries were preconstricted with PE by ∼20–30% of their initial diameters. Subsequent extraluminal application of iberiotoxin (IBTX), a specific inhibitor of BKCa channels, resulted in an additional constriction that stabilized within 5 min. Final levels of preconstriction by combined treatment with PE and IBTX were 60–70% of the initial diameters. ACh was then tested in concentrations of 0.03, 0.1, and 10 μM. In a separate set of experiments, we studied ACh-induced responses after treatment of the vessels with a combination of 100 nM apamin and 50 nM charybdotoxin (CTX) or 100 nM apamin and 100 nM IBTX. All toxins were delivered intraluminally by producing a transient flow of 30–50 μl/min for 5–8 min. PE was then applied in increasing concentrations to preconstrict arteries, and a combination of apamin and CTX (or IBTX) was added extraluminally with a superfusion solution for an additional 5–10 min. ACh was applied to PE-preconstricted vessels in the presence of apamin and CTX (or IBTX) in 30–40 min after cessation of intraluminal flow.
In our electrophysiological experiments, each artery was pressurized to 50 mmHg in the presence of l-NNA and indomethacin, and two to three concentrations of PE were added to preconstrict arteries. After stabilization of the PE-induced constriction, microelectrode impalement of SMC was made, and MP was recorded for 2–3 min. Simultaneous changes in SMC MP and the arterial diameter in response to application of 0.1 ACh were then recorded during the next 3–5 min. A combination of papaverine and diltiazem was added at the end of each experiment to maximally dilate the artery.
Solutions and drugs.
The PSS contained (in mM): 119 NaCl, 4.7 KCl, 24.0 NaHCO3, 1.2 KH2PO4, 1.6 CaCl2, 1.2 MgSO4, 0.023 EDTA, and 11.0 glucose, pH = 7.4. For the fura 2 calibration procedure, we used a solution of the following composition: 140 mM KCl, 20 mM NaCl, 5 mM HEPES, 5 mM EGTA, 1 mM MgCl2, 5 μM nigericin, and 10 μM ionomycin, pH = 7.1.
The majority of chemicals was purchased from Sigma Chemical (St. Louis, MO) with the exception of ionomycin and nigericin, which were obtained from Calbiochem (La Jolla, CA). Fura 2-AM and pluronic acid were purchased from Invitrogen (Carlsbad, CA). Fura 2-AM was dissolved in dehydrated DMSO as a 1 mM stock solution, frozen in small aliquots, and used within 1 wk of preparation. l-NNA, PE, ACh, and papaverine were dissolved in deionized water on the experimental day. Diltiazem and indomethacin were prepared as 10 mM stock solutions in deionized water and alcohol, respectively, and kept refrigerated until use. Ionomycin and nigericin were dissolved in methanol (10 mM) and kept at −20°C. Stock solutions of IBTX, apamin, and CTX were prepared in deionized water and stored at −20°C until use.
Calculations and statistical analysis.
EC or SMC [Ca2+]i was calculated using the following equation (23): [Ca2+]i = Kdβ(R − Rmin)/(Rmax − R), where Kd is the dissociation constant, R is an experimentally measured ratio (340/380 nm) of fluorescence intensities, Rmin is a ratio in the absence of [Ca2+]i, Rmax is a ratio at Ca2+-saturated fura 2 conditions, and β is a ratio of the fluorescence intensities at 380 nm excitation wavelength at Rmin and Rmax. Rmin, Rmax, and β were determined by an in situ calibration procedure from the arteries treated with the ionophores ionomycin (10 μM) and nigericin (5 μM) to increase cell membrane permeability to Ca2+ and minimize Ca2+ extrusion mechanisms through Na+/Ca2+ exchange, respectively (56). Calibration was performed for two separate sets of vessels loaded intraluminally (endothelial cell loading, n = 9) or extraluminally (SMC loading, n = 8) with fura 2. These values were then pooled and used to convert the ratio values into a [Ca2+]i. The Kd (the dissociation constant for fura 2) was 282 nM, as determined by in situ titration of Ca2+ in fura 2-loaded small arteries (29). Arterial diameter and pressure and ratio values were simultaneously recorded using an IonOptix data acquisition program and imported into SigmaPlot and SigmaStat programs for graphical representation, calculations, and statistical analysis. In view of significant oscillatory activity of uterine arteries, all measurements were made by averaging records of arterial diameters or SMC [Ca2+]i during 15–20 s. Data are expressed as means ± SE, where each n is the number of arterial segments studied. One or two arteries from the same animal were used on each experimental day with one vessel per animal used for a particular protocol. A paired or unpaired Student's t-test or two-way repeated-measures ANOVA was used to determine the significance of differences between sets of data, with P < 0.05 considered significant. The concentration of ACh required to produce half-maximal vasodilation, EC50, was determined for each tested artery using standard curve analysis from data imported into a SigmaPlot program.
RESULTS
Temporal characterization of uterine vasodilation before and after inhibition of NOS and COX.
Late pregnancy was associated with a significant increase in passive lumen diameters of radial uterine arteries at 50 mmHg that averaged 195.8 ± 5.7 μm (n = 63) vs. 120.3 ± 4.0 μm (n = 53) in the nonpregnant state.
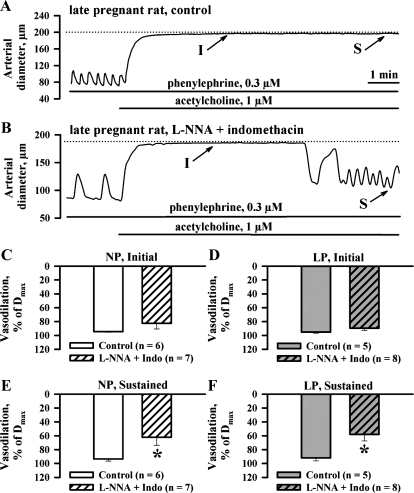
Under control conditions, an application of 1 μM ACh resulted in a rapid and complete vasodilation of arteries preconstricted with PE (Fig. 1A). This response remained stable until washout of ACh. Similar experiments were performed after blockade of NO and PGI2 production with l-NNA and indomethacin. Initial lumen diameters of arteries in the presence of these inhibitors were comparable to passive lumen diameters of the same arteries treated with papaverine and diltiazem (NP rats: 119.9 ± 4.2 vs. 120.3 ± 4.0 μm, n = 53; LP rats: 193.3 ± 5.8 vs. 195.8 ± 5.7 μm, n = 63). These data indicate that arteries of both NP and LP rats pressurized at 50 mmHg develop no significant spontaneous tone in the presence of l-NNA and indomethacin. To test vasodilatory effects of ACh in these and other experiments, arteries were preconstricted with PE by 58.4 ± 1.8% (NP, n = 38) and 58.7 ± 2.0% (LP, n = 49) of their initial diameters. The concentrations of PE used to preconstrict vessels from NP rats (0.70 ± 0.10 μM) were significantly higher than those used for arteries of LP rats (0.26 ± 0.04 μM). Application of 1 μM ACh induced vasodilation that reached the maximal value within 3 min and was followed by a partial restoration of the constriction (Fig. 1B). Initial maximal responses were similar in control and l-NNA- and indomethacin-treated arteries (Fig. 1, C and D). Sustained vasodilation at the end of a 10-min period of ACh administration was significantly diminished from 93.5 ± 3.2 and 92.0 ± 4.2% to 62.2 ± 11.8 and 58.0 ± 9.4% in arteries of NP and LP rats, respectively (Fig. 1, F and E). Time for reaching two-thirds of maximal vasodilation in treated arteries from NP (23.1 ± 5.3 s, n = 7) and LP (19.7 ± 3.4 s, n = 8) rats was not significantly different from NP (20.8 ± 3.7 s, n = 6) and LP (15.8 ± 2.2 s, n = 5) controls.
Fig. 1.
Relative contribution of endothelium-derived hyperpolarizing factor (EDHF) to initial (I) and sustained (S) components of ACh-induced uterine vasodilation. A and B: representative tracings showing changes in lumen diameter of the control uteroplacental artery (A) and the artery treated with NG-nitro-l-arginine (l-NNA) and indomethacin (B) in response to application of 1 μM ACh. Solid lines indicate the exposure of arteries to tested compounds. Dotted lines show maximally dilated diameters of the arteries in the presence of 100 μM papaverine and 10 μM diltiazem. C and D: summary graphs showing no difference in initial dilatation to 1 μM ACh in control arteries of nonpregnant (NP) and late pregnant (LP) rats and arteries treated with l-NNA and indomethacin. Dmax, maximal vasodilation in response to papaverine and diltiazem. E and F: summary graphs demonstrating the effects of l-NNA and indomethacin on sustained vasodilation induced by ACh in arteries from NP and LP rats. Initial and sustained components were calculated at ∼3 and 10 min of ACh application and are expressed as Dmax. *Significantly different at P < 0.05 (unpaired Student's t-test); n, no. of arteries tested.
Pregnancy enhances l-NNA- and indomethacin-resistant vasodilation of uteroplacental arteries.
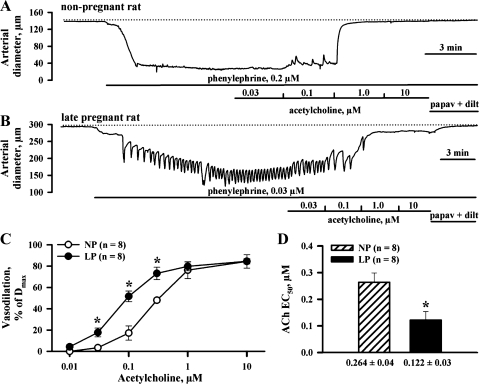
The effect of pregnancy on EDHF-mediated responses of uterine arteries was characterized next. Figure 2 shows representative changes in the diameters of arteries from NP (A) and LP (B) rats in response to cumulative application of ACh. Vessels were pretreated with l-NNA and indomethacin and preconstricted with PE. The threshold concentration of ACh producing a minimal response was lower for LP arteries (0.03 μM) compared with NP controls (0.1 μM). In both types of vessels, maximal vasodilation was induced by ACh at 10 μM (Fig. 2C). The EC50 was significantly decreased at near-term pregnancy from 0.264 ± 0.04 μM (n = 8, NP rats) to 0.122 ± 0.03 μM (n = 8, LP rats; Fig. 2D).
Fig. 2.
Pregnancy enhances l-NNA- and indomethacin-resistant vasodilation of uteroplacental arteries. A and B: representative changes in lumen diameters of pressurized uterine arteries from NP and LP rats in response to cumulative application of ACh in increasing concentrations. Arteries were treated with l-NNA and indomethacin and preconstricted with phenylephrine (PE) before testing ACh. Dotted lines indicate the diameters of maximally dilated arteries obtained at the end of each experiment by treating vessels with a combination of papaverine and diltiazem. Solid horizontal lines depict the time of exposure of arteries to tested compounds. C: summary graphs demonstrating the degree of initial vasodilation as a function of ACh concentrations in arteries from NP and LP rats. ACh-induced vasodilation is expressed as Dmax. *Significantly different at P < 0.05 (2-way repeated-measures ANOVA). D: bar graph showing significant decrease in the concentration of ACh required for half-maximal dilatation (EC50) of uterine arteries in late gestation. *Significantly different at P < 0.05 (unpaired Student's t-test); n, no. of arteries tested.
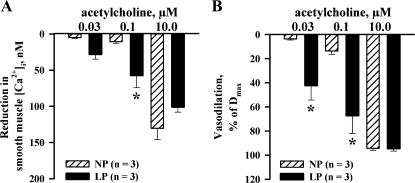
EDHF-mediated uterine vasodilation is associated with a marked reduction in SMC [Ca2+]i.
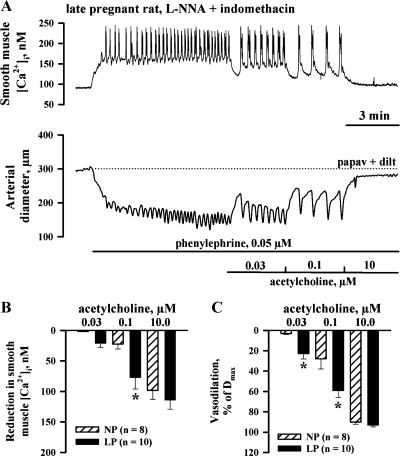
We next determined whether EDHF-mediated reduction in SMC [Ca2+]i was different in arteries of NP and LP rats. Changes in SMC [Ca2+]i and the diameter of uteroplacental artery from a LP rat in response to threshold, intermediate, and maximally effective concentrations of ACh are shown in Fig. 3A. An application of PE resulted in a marked elevation of SMC [Ca2+]i with superimposed [Ca2+]i oscillations that were associated with synchronous oscillations in the arterial diameter. PE-induced oscillations in SMC [Ca2+]i and the diameter were smaller and less frequent in arteries of NP rats. The averaged levels of PE-induced SMC [Ca2+]i and the frequency of oscillations were significantly reduced or abolished by ACh with a resultant concentration-dependent arterial dilatation. As evident from summary graphs, both EDHF-mediated [Ca2+]i responses and vasodilatation to ACh were significantly enhanced by pregnancy (Fig. 3, B and C).
Fig. 3.
Increased EDHF-mediated uterine vasodilation in late gestation is associated with enhanced smooth muscle cell (SMC) cytosolic Ca2+ concentration ([Ca2+]i) response. A: representative changes in SMC [Ca2+]i and the lumen diameter of the artery from a LP rat to cumulative application of ACh. The artery was pretreated with l-NNA and indomethacin and preconstricted with PE before testing ACh. B and C: bar graphs showing the reduction in SMC [Ca2+]i and vasodilation induced by ACh in arteries of NP and LP rats. Vasodilation is expressed as Dmax. *Significantly different at P < 0.05 (2-way repeated-measures ANOVA); n, no. of arteries tested.
Abolition of EDHF-mediated responses of uterine arteries depolarized with high-K+ solution.
We next studied the effect of high-K+ depolarization on EDHF-mediated responses of uterine arteries to 3 μM ACh, the concentration that produced near-maximal dilatation of arteries from both NP and LP rats. As shown in Fig. 4A, in the artery from a LP rat preconstricted with PE in the presence of l-NNA and indomethacin, 3 μM ACh induced a marked reduction in SMC [Ca2+]i associated with vasodilation. In contrast, in arteries treated with 35–45 mM K+, application of ACh resulted in no significant reduction in [Ca2+]i or vasodilation (Fig. 4B). Similar data were obtained in our experiments using vessels from NP rats (Fig. 4, C and D). These findings demonstrate that depolarization of SMCs with high-K+ prevents ACh-induced reduction in [Ca2+]i and vasodilation, suggesting that these responses are mediated by hyperpolarization of SMCs.
l-NNA- and indomethacin-resistant uteroplacental vasodilation is associated with SMC hyperpolarization.
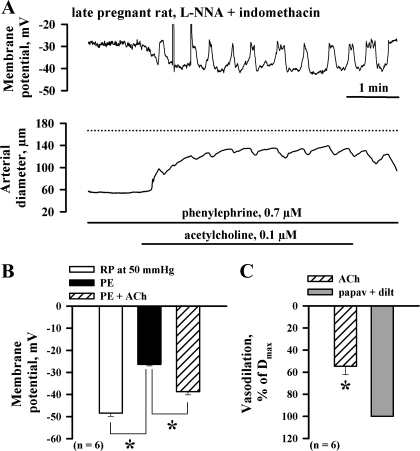
Direct evidence that ACh can hyperpolarize SMCs after blockade of NO and PGI2 production was obtained by microelectrode measurements of MP from SMCs in the wall of uteroplacental arteries from LP rats pressurized at 50 mmHg. Resting MP of SMCs was −48.4 ± 1.5 mV (n = 6). Application of PE resulted in marked SMC depolarization to −26.3 ± 0.7 mV that was associated with vasoconstriction (data not shown). Administration of ACh in the concentration 0.1 μM hyperpolarized SMCs to 38.7 ± 1.5 mV and resulted in a considerable vasodilation. Representative recordings of simultaneous changes in MP and in the arterial diameter in response to ACh are shown in Fig. 5A. ACh-induced partial hyperpolarization of PE-depolarized SMCs resulted in the appearance of oscillations in MP that were followed by transient arterial constrictions. These findings are summarized in Fig. 5, B and C.
Fig. 5.
l-NNA- and indomethacin-resistant uteroplacental vasodilation is caused by SMC hyperpolarization. A: representative tracings showing simultaneous changes in SMC membrane potential and lumen diameter of the artery from a LP rat in response to application of 0.1 μM ACh. The artery was pressurized to 50 mmHg and depolarized with PE in the presence of l-NNA and indomethacin before testing ACh. Note a close association between membrane hyperpolarization and vasodilation in response to ACh. Depolarizing oscillations in membrane potential were followed by transient arterial constrictions. B: bar graphs summarizing the effect of PE and ACh on SMC membrane potential. RP, resting membrane potential measured from SMCs of pressurized (50 mmHg) arteries before application of PE. C: bar graph showing dilatation of the same vessels in response to 0.1 ACh, which is expressed as the percentage of maximal responses induced by papaverine and diltiazem. *Significantly different at P < 0.05 (paired Student's t-test); n, no. of arteries tested.
Pregnancy-increased EDHF-mediated vasodilation is associated with enhanced endothelial [Ca2+]i responses.
In our previous study, we demonstrated that ACh-induced uterine vasodilation is preceded by a significant rise in EC [Ca2+]i (20). Similar responses were obtained in uterine arteries after blockade of NO and PGI2 production (data not shown). The onset of vasodilation was delayed from the onset of [Ca2+]i elevation in response to 10 μM ACh by 4.8 ± 0.4 and 5.7 ± 0.8 s in arteries of NP (n = 5) and LP (n = 6) rats, respectively.
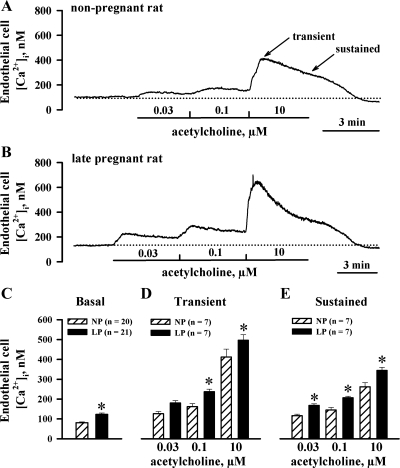
Recent studies indicate that Ca2+ and/or inositol trisphosphate (IP3), elevated in response to agonist stimulation of SMCs, can diffuse to ECs through myoendothelial gap junctions and modulate Ca2+ signaling in endothelial cells (26, 32). To minimize a possible contribution of this mechanism to endothelial Ca2+ signaling, we studied ACh-induced endothelial [Ca2+]i responses in vessels that were not preconstricted with PE. In the presence of l-NNA and indomethacin, basal levels of EC [Ca2+]i measured at 50 mmHg were significantly higher in uterine arteries of LP animals (123 ± 7 nM, n = 21) compared with NP controls (81 ± 5 nM, n = 20; Fig. 6, A, B, and C). Results of these studies, shown in Fig. 6, D and E, indicate that, in the absence of SMC stimulation with PE, ACh-induced EC [Ca2+]i responses were significantly enhanced in late gestation.
Fig. 6.
Late gestation results in increased basal levels of cytoplasmic concentration of Ca2+ in endothelial cells (EC [Ca2+]i) and in augmented EC [Ca2+]i responses to ACh. A and B: representative changes in EC [Ca2+]i in response to increasing concentrations of ACh in pressurized uterine arteries from a NP rat (A) and a LP rat (B) that were not preconstricted with PE. The arteries were treated with 200 μM l-NNA and 10 μM indomethacin. Solid lines indicate time of exposure of arteries to ACh, while dotted lines show basal levels of EC [Ca2+]i. Transient (maximal) and sustained (3 min after ACh application) components of the response are shown in A. C–E: summary graphs showing a significant increase in basal levels of EC [Ca2+]i (C) and in transient (D) and sustained (E) EC [Ca2+]i responses to ACh of uterine arteries from LP rats compared with NP controls. *Significantly different at P < 0.05 (2-way repeated-measures ANOVA); n, no. of arteries tested.
Blockade of SKCa and IKCa channels abolishes EDHF-mediated uterine vasodilation.
Ca2+-activated K+ channels are commonly implicated in EDHF-mediated vasodilation. We first tested the contribution of SMC BKCa channels to EDHF in uterine arteries by studying the effects of ACh in the presence of extraluminal IBTX. As evident from the graphs in Fig. 7, EDHF-mediated SMC [Ca2+]i (Fig. 7A) and dilator responses (Fig. 7B) of uterine arteries in the presence of IBTX were not different from the control responses shown in Fig. 3. Responses of arteries from LP rats were significantly enhanced compared with those of vessels from NP controls.
Fig. 7.
Iberiotoxin failed to inhibit EDHF-mediated changes in SMC [Ca2+]i and dilatation induced by ACh. A and B: graphs showing reduction in SMC [Ca2+]i and vasodilation induced by ACh in arteries treated with l-NNA and indomethacin and preconstricted with 100 nM iberiotoxin and PE. *Significantly different at P < 0.05 (2-way repeated-measures ANOVA); n, no. of arteries tested.
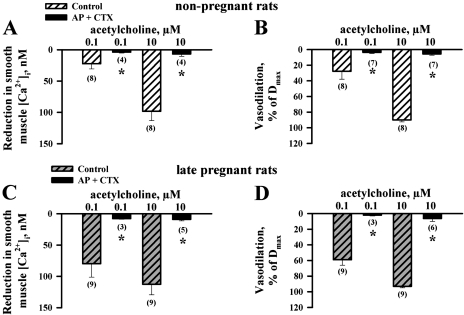
To evaluate the role of endothelial BKCa, SKCa, and IKCa channels in EDHF-mediated uterine vasodilation, we tested ACh-induced changes in SMC [Ca2+]i and arterial diameter after blockade of these channels with a combination of apamin and CTX (or apamin and IBTX). To ensure effective inhibition of endothelial K+ channels, all toxins were delivered to the arteries intra- and extraluminally. Summary graphs in Fig. 8 demonstrate an abolition of both SMC [Ca2+]i (Fig. 8, A and C) and dilator (Fig. 8, B and D) responses to ACh of uterine arteries treated with apamin and CTX. At the same time, the vasodilation induced by 10 μM ACh was preserved after combined treatment of arteries with apamin and IBTX (NP rats: 84 ± 5%, n = 5; LP rats: 81 ± 4%, n = 5).
Fig. 8.
Abolition of EDHF-mediated responses of uterine arteries by charybdotoxin (CTX) and apamin (AP). A–D: bar graphs demonstrating inhibition of ACh-induced SMC [Ca2+]i and dilator responses by a combined treatment of arteries with 50 nM CTX and 100 nM AP. Both toxins were applied intra- and extraluminally. Arteries were preconstricted with PE before testing ACh. l-NNA (200 μM) and indomethacin (10 μM) were present throughout the whole experiment. ACh-induced vasodilation is expressed as Dmax. Nos. in parentheses indicate the no. of tested arteries. *Significantly different at P < 0.05 (2-way repeated-measures ANOVA).
DISCUSSION
This study aimed to explore the role of EDHF in endothelium-dependent vasodilation of maternal uterine resistance arteries and to define the effect of pregnancy on EDHF-mediated vascular responses with a specific focus on endothelial cell Ca2+ signaling. The main findings of this study are: 1) ACh can effectively dilate uterine resistance arteries after blockade of NO and PGI2 production; 2) l-NNA- and indomethacin-resistant vasodilation to ACh is abolished by high-K+ solution and is associated with SMC hyperpolarization and a reduction in SMC [Ca2+]i; 3) late pregnancy increases EDHF-mediated vasodilation in part due to enhanced endothelial Ca2+ signaling; 4) EDHF-dependent responses are insensitive to IBTX; and 5) combined treatment of uterine arteries with apamin and CTX abolishes NO- and PGI2-resistant responses to ACh, supporting the key role of SKCa and IKCa channels in mediating EDHF effects.
The importance of EDHF in the control of regional vascular resistance is well established (16); however, the exact nature and role of EDHF in regulating maternal uterine blood flow remains unclear. The present study utilizing small-resistance-size radial uterine arteries from both NP and LP rats demonstrates that ACh can induce rapid and effective dilatation of these vessels after blockade of NO and PGI2 production. This vasodilation was associated with SMC [Ca2+]i reduction, SMC hyperpolarization, and was abolished by a high-K+ solution, suggesting that l-NNA- and indomethacin-resistant responses are attributable to EDHF.
Prevalence of EDHF in the endothelium-dependent control of small uterine artery tone.
Previous studies performed on large (main or arcuate) uterine arteries of rodents demonstrated only small and transient endothelium-dependent uterine vasodilation to ACh after inhibition of the production of NO and PGI2 (12, 40, 51). In experiments using uterine arteries from both nonpregnant and pregnant women, NOS inhibition resulted in a complete abolition of ACh-induced dilatation (39). These data are consistent with a relatively minor contribution of EDHF to endothelium-dependent dilatation of large uterine arteries. However, smaller myometrial arteries from nonpregnant or late pregnant women can be markedly dilated with bradykinin or placental growth factor in the presence of NOS and COX inhibitors (28, 42). EDHF also importantly contributes to ACh-induced dilatation of radial uterine arteries from ovariectomized NP rats (6). Collectively, previous observations and our current findings indicate that contribution of EDHF to the control of maternal uterine vascular tone increases in more distal uterine vasculature. The inverse relationship between arterial size and magnitude of EDHF-mediated dilatation was previously established in mesenteric, cerebral, and rabbit ear circulations (2, 5, 24, 49).
The exact mechanism(s) underlying an increased role of EDHF in endothelium-dependent vasodilation of smaller uterine arteries remains unknown. It has been demonstrated that the augmented contribution of EDHF to the control of vascular tone in more distally located mesenteric arteries correlates well with a higher incidence of myoendothelial gap junctions (47). An increased density of myoendothelial gap junctions may result in more effective electrotonic spreading of agonist-induced hyperpolarization from ECs to neighboring SMCs. It is also conceivable that electrical spreading of hyperpolarization from ECs to SMCs might be more effective in the wall of smaller arteries and arterioles with one to two layers of SMCs compared with large conductive vessels with five to six layers of SMCs.
It is generally accepted that endothelial SKCa and IKCa channels play a pivotal role in EDHF effects in the majority of microcirculatory vascular beds (5, 8, 16, 22, 27, 34). In this regard, relative expression of SKCa and IKCa channels was significantly higher in fourth- compared with first-order mesenteric arteries (24). Therefore, both increased density of myoendothelial gap junctions and/or upregulation in SKCa and IKCa channel gene expression may be responsible for augmented EDHF signaling in more distal resistance uterine vasculature.
Late gestation enhances the contribution of EDHF to the control of uteroplacental vascular tone.
In this study, we demonstrated for the first time that both EDHF-mediated uterine vasodilation and associated reduction in SMC [Ca2+]i were significantly enhanced in late pregnancy. These findings convincingly establish the importance of EDHF in pregnancy-specific upregulation of vasodilatory mechanisms in the maternal uteroplacental circulation. Late gestation was also associated with an increased functional role of EDHF in the mesenteric arteries (17). The previous observation and our current findings suggest that a common mechanism might be responsible for pregnancy-induced upregulation of EDHF in different vascular beds. The role of estrogen in enhancement of endothelium-dependent vasodilation is well documented in human and animal studies (1, 25, 48). Estrogen effects are especially prominent in the reproductive vasculature, and marked estrogen-induced sensitization of the uterine artery to ACh was first demonstrated by Bell (1). The high estrogen state of pregnancy increases endothelial NOS activity in the main uterine artery of the guinea pig (54). Several recent studies suggest that estrogen can also regulate vascular tone through modulation of EDHF-mediated vascular responses (25, 45, 48). We recently demonstrated that EDHF-mediated responses of uterine arteries were significantly potentiated by supplementation of ovariectomized rats with estrogen (6). Taken together, these data strongly suggest an important role of estrogen in pregnancy-induced upregulation of EDHF in uterine and systemic circulations.
Increased endothelial Ca2+ signaling contributed to pregnancy-enhanced EDHF.
Although the exact nature of EDHF remains the matter of controversy, it is generally accepted that elevation of intracellular [Ca2+]i in response to chemical or mechanical stimulation of endothelial cells is an initial critical step in EDHF-induced vasodilation (5, 8, 16, 22, 34, 41, 46). In our previous study, we found that chelation of endothelial [Ca2+]i with BAPTA abolished ACh-induced [Ca2+]i rise and associated vasodilation of rat radial uterine arteries, indicating that Ca2+ is a key mediator for production of endothelium-derived factors, including EDHF (20). In the current study, ACh-induced EC [Ca2+]i elevation was followed by EDHF-mediated vasodilation with a time delay of only ∼5 s. In this regard, it has been reported that onset of endothelial NO production determined with the NO-sensitive dye DAF-2 significantly delayed by 20–60 s from the onset of agonist-induced elevation in EC [Ca2+]i (14, 31, 57). These data suggest that initial uterine vasodilation in response to the Ca2+-mobilizing agonist ACh is mostly mediated by EDHF. This conclusion is also supported by the fact that the magnitude and time for reaching two-thirds of initial ACh-induced dilatation in the presence of l-NNA and indomethacin was not different from those of control untreated vessels. Sustained component, however, was significantly diminished, demonstrating that NO and PGI2, in addition to EDHF, contribute to sustained uterine vasodilation in the nonpregnant and pregnant states.
In uterine vessels treated with l-NNA and indomethacin, both basal levels of EC [Ca2+]i and ACh-stimulated [Ca2+]i responses were significantly enhanced in late pregnancy through as-yet-unidentified mechanisms. The levels of cytosolic [Ca2+]i in ECs are finely regulated through Ca2+ release from internal stores and Ca2+ influx into cells as well as by cellular Ca2+ extrusion and sequestration mechanisms. Binding of ACh to endothelial muscarinic receptors results in activation of phospholipase C with subsequent Ca2+ release from internal stores and Ca2+ influx through Ca2+ permeable channels (41); both of these processes may be modulated in late pregnancy. In view of the absence of voltage-gated Ca2+ channels in endothelium of the majority of blood vessels, Ca2+ entry in cells is determined by Ca2+ electrochemical potential. In addition to a strong Ca2+ concentration gradient, membrane hyperpolarization is another driving force for endothelial Ca2+ influx (41). Therefore, pregnancy may affect endothelial Ca2+ signaling through modulation of the expression and/or function of ion channels that regulate MP.
Finally, close anatomical and functional communications between ECs and SMCs through myoendothelial gap junctions are convincingly demonstrated in recent publications (8, 13, 16, 26, 32, 33, 46). Previous studies indicate that Ca2+ and/or IP3 can diffuse from SMCs through gap junctions and subsequently modulate Ca2+ signaling and Ca2+-dependent mechanisms in endothelial cells (26, 32, 33). We found that pressure-induced elevation of SMC [Ca2+]i in small uterine arteries is significantly enhanced in late pregnancy (52). This observation raises the possibility that IP3/Ca2+ diffusion from SMCs may contribute to a pregnancy-induced increase in basal levels of EC [Ca2+]i and also modulate Ca2+ responses to ACh in pressurized uterine vessels. Earlier, we did not find significant changes in basal EC [Ca2+]i during development of myogenic tone in uteroplacental arteries (20). Although our data do not exclude the role of SMCs in regulation of Ca2+ signaling in adjacent endothelial cells, this mechanism is unlikely responsible for the higher basal levels of [Ca2+]i in endothelial cells of uterine vessels in LP compared with NP rats.
In our experiments, PE application was associated with SMC [Ca2+]i elevation and vasoconstriction of uterine radial arteries from LP and NP rats. We also noted a remarkable [Ca2+]i oscillatory activity that was more frequent in vessels of LP rats compared with NP controls. Previously, we observed similar [Ca2+]i oscillations and associated uteroplacental vasomotions in response to elevation of intracellular pressure. Pressure-induced vasomotions were preceded by oscillations in SMC MP that were most likely responsible for [Ca2+]i transients (52). The origin of [Ca2+]i and vessel wall rhythmic activity in response to PE in the current study, as well as mechanisms underlying its augmentation in late pregnancy, still remain unknown and deserves further investigation.
A key role of endothelial SKCa-IKCa channels in EDHF-mediated uterine vasodilation.
In the current experiments, we observed a close relationship between EC [Ca2+]i rise and EDHF-mediated vasodilation. Elevation of EC [Ca2+]i results in activation of several Ca2+-dependent intracellular mechanisms accompanied by activation of the EDHF system. It is generally accepted that endothelial SKCa and IKCa channels play a pivotal role in EDHF effects in the majority of microcirculatory vascular beds. In the present study, ACh-induced responses were abolished by a combined treatment with apamin and CTX (but not with apamin and IBTX), demonstrating a critical involvement of SKCa and IKCa channels in EDHF-mediated vasodilation of rat uterine arteries as well. Similar data were published for small human myometrial arteries (18). From our study, we concluded that increased EC [Ca2+]i responses followed by activation of SKCa and IKCa channels is an important mechanism of enhanced EDHF-mediated uterine vasodilation during pregnancy. This, however, does not rule out a contribution of other mechanisms. [Ca2+]i elevation in ECs resulted in generation of arachidonic acid and formation of a number of diffusible molecules proposed for the role of EDHF (8, 16). So far, the most convincing evidence was obtained on the role of EETs as EDHF in coronary and renal arteries. In these vessels, EETs can hyperpolarize SMCs through activation of SMC BKCa channels (9). In our experiments, IBTX, a highly potent and selective inhibitor of these channels, did not modify l-NNA- and indomethacin-resistant uterine vasodilation, indicating no role for BKCa channels in EDHF. We cannot, however, exclude an involvement of EETs in paracrine modulation of endothelial SKCa and IKCa channel function of uterine vessels. As was reported recently, EETs can promote Ca2+ influx in endothelial cells through Ca2+-permeable channels or directly activate endothelial K+ channels (38, 55). Finally, expression of SKCa and IKCa channels and/or connexins may also be increased during late gestation, adding more complexity to the potential mechanisms contributing to augmented EDHF signaling in the maternal uterine circulation.
The role of EDHF in the control of regional blood flow as well as in systemic blood pressure regulation is confirmed by numerous current studies. A number of cardiovascular diseases, including hypertension and diabetes, are accompanied by endothelial dysfunction, which in part may be due to defects in the EDHF system (5, 11, 22, 30). Recently, it has been shown that the development of preeclampsia in pregnant women is associated with compromised EDHF function of small myometrial arteries (28). We have found that EDHF-mediated uterine vasodilation is impaired in the rat model of diabetic pregnancy (19). Further studies on the mechanisms contributing to an impaired EDHF system are essential in the search for pharmacological strategies to improve maternal uteroplacental circulation in pregnancies complicated by hypertension, preeclampsia, or diabetes.
In conclusion, we demonstrated a prominent role of EDHF in ACh-induced vasodilation of small-resistance uterine arteries and established the importance of EDHF in pregnancy-specific upregulation of vasodilatory mechanisms in the maternal uteroplacental circulation. Increased EC [Ca2+]i signaling and concomitant activation of SKCa and IKCa channels importantly contribute to the augmented role of EDHF in the regulation of maternal uteroplacental blood flow in late gestation.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-067250 and HL-088245.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Tara Goecks and Ashley Zucker for excellent technical assistance and Rita Lemire for help with editing of the manuscript.
REFERENCES
- 1.Bell C. Control of uterine blood flow in pregnancy. Med Biol 52: 219–228, 1974 [PubMed] [Google Scholar]
- 2.Berman RS, Martin PE, Evans WH, Griffith TM. Relative contributions of NO and gap junctional communication to endothelium-dependent relaxations of rabbit resistance arteries vary with vessel size. Microvasc Res 63: 115–128, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am J Physiol Regul Integr Comp Physiol 284: R245–R258, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol 187: 1416–1423, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Bryan RM, Jr, You J, Golding EM, Marrelli SP. Endothelium-derived hyperpolarizing factor: a cousin to nitric oxide and prostacyclin. Anesthesiology 102: 1261–1277, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Burger NZ, Kuzina OY, Osol G, Gokina NI. Estrogen replacement enhances EDHF-mediated vasodilation of mesenteric and uterine resistance arteries: role of endothelial cell Ca2+. Am J Physiol Endocrinol Metab 296: E503–E512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation 11: 279–293, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci 23: 374–380, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49: 590–596, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Cohen KD, Jackson WF. Membrane hyperpolarization is not required for sustained muscarinic agonist-induced increases in intracellular Ca2+ in arteriolar endothelial cells. Microcirculation 12: 169–182, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin Exp Pharmacol Physiol 31: 641–649, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Cooke CL, Davidge ST. Pregnancy-induced alterations of vascular function in mouse mesenteric and uterine arteries. Biol Reprod 68: 1072–1077, 2003 [DOI] [PubMed] [Google Scholar]
- 13.de Wit C, Wolfle SE. EDHF and gap junctions: important regulators of vascular tone within the microcirculation. Curr Pharm Biotechnol 8: 11–25, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Dedkova EN, Blatter LA. Nitric oxide inhibits capacitative Ca2+ entry and enhances endoplasmic reticulum Ca2+ uptake in bovine vascular endothelial cells. J Physiol (Lond) 539: 77–91, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 117: 139–155, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Gerber RT, Anwar MA, Poston L. Enhanced acetylcholine induced relaxation in small mesenteric arteries from pregnant rats: an important role for endothelium-derived hyperpolarizing factor (EDHF). Br J Pharmacol 125: 455–460, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillham JC, Myers JE, Baker PN, Taggart MJ. Regulation of endothelial-dependent relaxation in human systemic arteries by SKCa and IKCa channels. Reprod Sci 14: 43–50, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Gokina N, Kuzina O, Pryor L. Induction of endothelial dysfunction of rat uteroplacental arteries during experimental diabetes (Abstract). Reprod Sci 15: 267A, 2008 [Google Scholar]
- 20.Gokina NI, Goecks T. Upregulation of endothelial cell Ca2+ signaling contributes to pregnancy-enhanced vasodilation of rat uteroplacental arteries. Am J Physiol Heart Circ Physiol 290: H2124–H2135, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Gokina NI, Mandala M, Osol G. Induction of localized differences in rat uterine radial artery behavior and structure during gestation. Am J Obstet Gynecol 189: 1489–1493, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Grgic I, Kaistha BP, Hoyer J, Kohler R. Endothelial Ca2+-activated K+ channels in normal and impaired EDHF-dilator responses–relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol 157: 509–526, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 24.Hilgers RH, Todd J, Jr, Webb RC. Regional heterogeneity in acetylcholine-induced relaxation in rat vascular bed: role of calcium-activated K+ channels. Am J Physiol Heart Circ Physiol 291: H216–H222, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation 11: 9–38, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res 100: 246–254, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation 12: 113–127, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenny LC, Baker PN, Kendall DA, Randall MD, Dunn WR. Differential mechanisms of endothelium-dependent vasodilator responses in human myometrial small arteries in normal pregnancy and pre-eclampsia. Clin Sci (Lond) 103: 67–73, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol (Lond) 508: 199–209, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohler R, Hoyer J. The endothelium-derived hyperpolarizing factor: insights from genetic animal models. Kidney Int 72: 145–150, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Koyama T, Kimura C, Park SJ, Oike M, Ito Y. Functional implications of Ca2+ mobilizing properties for nitric oxide production in aortic endothelium. Life Sci 72: 511–520, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Lamboley M, Pittet P, Koenigsberger M, Sauser R, Beny JL, Meister JJ. Evidence for signaling via gap junctions from smooth muscle to endothelial cells in rat mesenteric arteries: possible implication of a second messenger. Cell Cal 37: 311–320, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA 105: 9627–9632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21: 69–78, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Marrelli SP. Selective measurement of endothelial or smooth muscle [Ca2+]i in pressurized/perfused cerebral arteries with fura-2. J Neurosci Methods 97: 145–155, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Meschia G. Circulation to female reproductive organs. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow. Bethesda, MD: Am Physiol Soc, 1983, sect. 2, vol. III, pt. 1, p. 241–269 [Google Scholar]
- 37.Moll W. Structure adaptation and blood flow control in the uterine arterial system after hemochorial placentation. Eur J Obstet Gynecol Reprod Biol 110, Suppl 1: S19–S27, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Mombouli JV, Holzmann S, Kostner GM, Graier WF. Potentiation of Ca2+ signaling in endothelial cells by 11,12-epoxyeicosatrienoic acid. J Cardiovasc Pharmacol 33: 779–784, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Nelson SH, Steinsland OS, Suresh MS, Lee NM. Pregnancy augments nitric oxide-dependent dilator response to acetylcholine in the human uterine artery. Hum Reprod 13: 1361–1367, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Ni Y, Meyer M, Osol G. Gestation increases nitric oxide-mediated vasodilation in rat uterine arteries. Am J Obstet Gynecol 176: 856–864, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415–1459, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Osol G, Celia G, Gokina N, Barron C, Chien E, Mandala M, Luksha L, Kublickiene K. Placental growth factor is a potent vasodilator of rat and human resistance arteries. Am J Physiol Heart Circ Physiol 294: H1381–H1387, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58–71, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramsey E. Placental vasculature and circulation. In: Handbook of Physiology. Endocrinology. Female Reproductive System. Bethesda, MD: Am Physiol Soc, 1973, vol. II, pt. 2, p. 323–337 [Google Scholar]
- 45.Sakuma I, Liu MY, Sato A, Hayashi T, Iguchi A, Kitabatake A, Hattori Y. Endothelium-dependent hyperpolarization and relaxation in mesenteric arteries of middle-aged rats: influence of oestrogen. Br J Pharmacol 135: 48–54, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, Plane F. What's where and why at a vascular myoendothelial microdomain signalling complex. Clin Exp Pharmacol Physiol 36: 67–76, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res 86: 341–346, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, Hobbs AJ, Ahluwalia A. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation 111: 796–803, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28: 703–711, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol Regul Integr Comp Physiol 272: R441–R463, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Tare M, Parkington HC, Coleman HA, Neild TO, Dusting GJ. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature 346: 69–71, 1990 [DOI] [PubMed] [Google Scholar]
- 52.Telezhkin V, Goecks T, Bonev AD, Osol G, Gokina NI. Decreased function of voltage-gated potassium channels contributes to augmented myogenic tone of uterine arteries in late pregnancy. Am J Physiol Heart Circ Physiol 294: H272–H284, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Ungvari Z, Csiszar A, Koller A. Increases in endothelial Ca2+ activate KCa channels and elicit EDHF-type arteriolar dilation via gap junctions. Am J Physiol Heart Circ Physiol 282: H1760–H1767, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci USA 91: 5212–5216, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weston AH, Feletou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids. Br J Pharmacol 145: 775–784, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams DA, Fay FS. Intracellular calibration of the fluorescent calcium indicator Fura-2. Cell Cal 11: 75–83, 1990 [DOI] [PubMed] [Google Scholar]
- 57.Zhu L, He P. Platelet-activating factor increases endothelial [Ca2+]i and NO production in individually perfused intact microvessels. Am J Physiol Heart Circ Physiol 288: H2869–H2877, 2005 [DOI] [PubMed] [Google Scholar]