Abstract
Atrial and brain natriuretic peptides (ANP and BNP) regulate blood pressure and cardiac function. In patients with heart failure (HF), plasma levels of pro-ANP and pro-BNP, the precursor forms of ANP and BNP, are highly elevated, but the mechanism underlying the apparent deficiency in natriuretic peptide processing is unclear. Corin is a cardiac protease that activates natriuretic peptides. In this study, we examined corin protein expression and activity in mouse and human failing hearts. Tissue samples were obtained from a mouse model of HF induced by myotrophin overexpression and from human nonfailing, hypertrophic, and failing hearts. Corin protein levels in the membrane fraction and tissue lysate were measured by Western blotting and ELISA. Corin catalytic and biological activities were measured by fluorescent substrate and pro-ANP processing assays. In mice, corin protein levels did not change with age in normal hearts but increased significantly in failing hearts. In humans, corin protein levels were similar in the atrium from nonfailing and failing hearts but were increased in the ventricle in failing hearts compared with those in nonfailing or hypertrophic hearts. Unlike the protein level, however, corin activity did not increase in failing hearts, as measured by fluorogenic substrate and pro-ANP processing assays. Our results indicate that corin activation is a rate-limiting step in failing hearts. Insufficient corin activation is expected to prevent natriuretic peptide processing and may contribute to body fluid retention and impaired cardiac function in patients with HF.
Keywords: natriuretic peptides, protease, myotropin
atrial and brain natriuretic peptides (ANP and BNP) are cardiac hormones that regulate blood pressure by promoting natriuresis, diuresis, and vasodilation (6, 19, 28). As a compensatory mechanism in heart failure (HF), natriuretic peptide expression is upregulated to lower blood pressure and improve cardiac function. Like many peptide hormones, the natriuretic peptides are made as precursors that are converted to active forms by proteolysis. In patients with HF, plasma pro-ANP and pro-BNP levels are highly increased (1, 2, 5, 11, 20, 33, 42), suggesting that the processing of these peptides may be compromised under pathological conditions.
Corin is a cardiac transmembrane protease that processes natriuretic peptides (38, 39). In mice, lack of corin prevented natriuretic peptide processing and caused hypertension (4). Corin knockout mice also developed cardiac hypertrophy (4, 25), indicating the importance of corin in regulating blood pressure and cardiac function in vivo. In humans, corin gene variants are associated with hypertension and cardiac hypertrophy (9, 29, 37). HF patients with the corin variants had impaired pro-BNP processing and poor clinical outcomes (30), suggesting that corin deficiency may contribute to hypertensive disease.
Corin is made primarily in cardiomyocytes (13, 40). This cell-specific expression was mediated by a GATA-4-dependent transcriptional mechanism (26). In cultured cardiomyocytes, corin mRNA and activity were upregulated upon hypertrophic stimuli (36). In vivo, however, corin gene regulation is less clear. In a rat model of HF induced by left coronary artery ligation, corin mRNA levels in the noninfarcted left ventricular myocardium were increased at 8 wk after the surgery (36). Similar results were reported in a rat model of chemically induced myocardial necrosis (15). In contrast, upregulation of corin mRNA expression was not detected in the atrium in another rat HF model induced by aortacaval shunt (18). The reason for the apparent difference was not clear and may be due to different HF models and/or experimental methods for tissue sampling and mRNA analysis.
To understand the regulation of corin function in HF, here we examined corin protein and activity in a transgenic mouse model of HF and in human nonfailing and failing hearts. Our results showed that corin protein levels, but not its activity, were increased in both mouse and human failing hearts. Our finding suggests that insufficient corin activity may limit natriuretic peptide processing and contribute to the pathogenesis of HF.
METHODS
Mouse model of HF.
A transgenic (Tg) mouse model of HF was used in this study (31), in which overexpression of cardiospecific myotrophin leads to cardiac hypertrophy and subsequently HF. The mice had cardiac morphological, functional, and gene profiling changes that resembled those in human HF (31). Hearts were collected from the Tg and wild-type (WT) mice at 4, 16, and 36 wk, when Tg mice had normal, hypertrophic [ejection fraction (EF), >80%], and failing (EF < 15%) hearts, respectively. This well characterized mouse HF model offered an opportunity to analyze corin gene and protein expression during transitional stages from normal to hypertrophic and to failing hearts. The animal care and experimental procedures were approved by and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Cleveland Clinic.
Human hearts.
Human failing hearts were collected from transplant recipients with dilated cardiomyopathy. Nonfailing hearts were from unmatched donors whose hearts were no longer suitable for transplantation. Heart samples were kept in an ice-cold cardioplegic solution (34) and tissues from each heart chamber were separated, frozen in liquid nitrogen, and stored at −80°C. Protocols for tissue procurement were in compliance with institutional guidelines for human research and approved by the local Institutional Review Board. The cardiac function and hypertrophy were assessed by echocardiography. HF stages were based on the New York Heart Association functional classification. Clinical characteristics of the patients whose hearts were used are shown in Table 1. More detailed information for individual donors is provided in the supplemental material (Supplemental Table S1; Supplemental Material for this article is available online at the Journal website).
Table 1.
Clinical characteristics of the patients whose heart samples were used
| Nonfailing (n = 8) | Hypertrophic (n = 5) | HF (n = 12) |
|
|---|---|---|---|
| Age, yr | 48 ± 8 | 52 ± 3 | 51 ± 10 |
| Male, % | 80 | 88 | 100 |
| Diagnosis | Head trauma, MVA, CVA | CVA | DCM |
| Hypertensive, % | 38 | 100 | 17 |
| HW/BW, g/kg | 4.8 ± 1.6 | 5.4 ± 1.5 | 6.3 ± 1.6 |
| %EF | 64 ± 8 | 68 ± 10 | 16 ± 4* |
Values are means ± SD. HF, heart failure; MVA, motor vehicle accident; CVA, cerebrovascular accident; DCM, dilated cardiomyopathy; HW/BW, heart weight/body weight; EF, ejection fraction.
P < 0.001 vs. nonfailing or hypertrophic.
Tissue sample preparation.
Heart tissues were homogenized in a buffer with 20 mmol/l Tris·HCl, pH 8.0, 100 mmol/l NaCl, 1 mmol/l EDTA, 0.5% Nonidet P-40 (NP-40), and 1% (vol/vol) protease inhibitor cocktail. After centrifugation to remove cellular debris, protein concentrations in the homogenate were measured by a Bradford assay. Membrane fractions from heart tissues were prepared by homogenizing tissues in 25 mmol/l Tris·HCl, pH 7.4, 5 mmol/l EDTA, pH 8.0, with or without aprotinin (200 ng/ml) and leupeptin (12.5 μg/ml). The homogenate was centrifuged at 200,000 g for 1 h. The pellet was washed and resuspended in an NP-40 buffer.
To extract total RNAs from heart samples, tissues were homogenized in the TRIzol reagent (Invitrogen) and centrifuged at 12,000 g at 4°C for 10 min. The supernatant was extracted with chloroform. RNAs in the aqueous phase were precipitated and dissolved in distilled water.
ELISA and Western blotting.
Human corin protein was measured by ELISA (7). Briefly, 96-well plates were coated with an anti-human corin antibody (R&D Systems). Tissue lysate or recombinant human corin standard was added and incubated at room temperature for 2 h. A biotinylated anti-human corin antibody was added and incubated for 2 h. A streptavidin-horseradish peroxidase (HRP) solution was added and incubated at room temperature for 20 min followed by adding a substrate solution (1:1 mixture of H2O2 and 3,3′,5,5′-tetramethylbenzidine). The optical density in wells was monitored by a plate reader (Molecular Devices) at 450-nm wavelength.
In Western blot analysis, protein samples in a Laemmli buffer with (reducing) or without (nonreducing) 5% β-mercaptoethanol were separated in 4–20% Tris-glycine gels and transferred onto polyvinylidene difluoride membranes. An anti-corin polyclonal antibody and an HRP-conjugated secondary antibody were used to detect corin fragments. Corin levels, normalized with a GAPDH internal control, were quantified using a densitometer (Bio-Rad), as described previously (21).
Quantitative RT-PCR.
To quantify corin mRNA expression in heart tissues, total RNAs were isolated and analyzed by real-time RT-PCR. Reactions were done using TaqMan Gene Expression Master Mix according to the manufacturer's protocol. Amplification was done by the ABI PRISM 7900HT sequence detection system (Applied Biosystems). The thermal cycling conditions were 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. β-Actin was used as an internal control.
Corin catalytic activity assay.
Corin catalytic activity was measured by using the fluorogenic substrate (p-tosyl-Gly-Pro-Arg)2-rhodamine 110 (Molecular Probes). The assay was done in 96-well plates with 20 μmol/l of the substrate and 5 μg of heart membrane proteins. To determine the specificity of corin activity, benzamidine (a nonspecific serine protease inhibitor) (Amresco) or hirudin (a specific thrombin inhibitor) (Sigma-Aldrich) was mixed with the heart samples and incubated at 37°C for 5 min before addition to the substrate. Another control in these experiments included assay buffer alone (10 mmol/l Tris·HCl, pH 7.5, and 100 μmol/l CaCl2). The fluorescence was monitored at wavelengths of 485 nm (excitation) and 538 nm (emission) at room temperature at 2-min intervals for 1 h in a plate reader. Corin catalytic activity was presented as Vmax for the maximal rate of reaction.
Pro-ANP processing assay.
To examine the pro-ANP processing activity of corin, the conditioned medium from transfected HEK 293 cells expressing human pro-ANP was prepared and incubated with heart membrane fractions. The corin concentration in the membrane fractions was determined by ELISA. Pro-ANP processing was performed at 37°C for 1 h in the presence of increasing concentrations of corin. Pro-ANP and ANP in the conditioned medium were analyzed by immunoprecipitation and Western blotting, as described previously (16, 17).
Statistical analysis.
Data are presented as means ± SE. Statistical analysis was done by use of the GraphPad Prism software. Comparisons were made by Student's t-test for two groups or one-way ANOVA followed by Tukey's posttest for three or more groups. A P value of <0.05 was considered statistically significant.
RESULTS
Corin protein expression in mouse failing hearts.
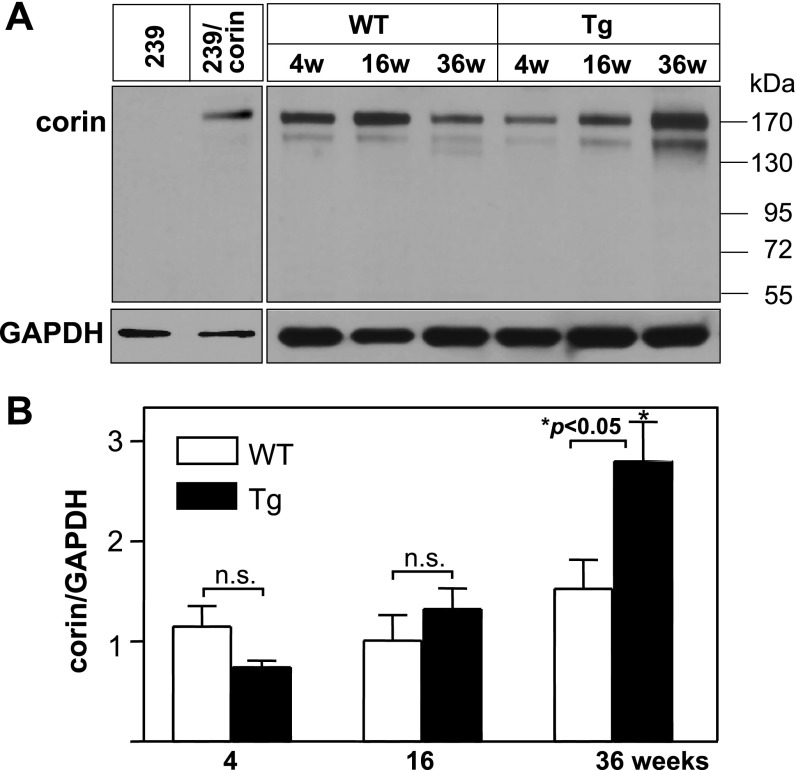
By Western blot analysis, corin levels in heart samples did not increase in WT mice at 4, 16, and 36 wk of age (Fig. 1). In Tg mice overexpressing myotrophin, corin levels were similar to that in age-matched WT mice at 4 and 16 wk but were significantly increased at 36 wk (Fig. 1). In this Tg mouse model, hearts appeared normal at 4 wk, became hypertrophic at 16 wk, and were failing at 36 wk (31), indicating increased corin protein expression in failing hearts.
Fig. 1.
Corin protein expression in mouse hearts. A: heart membranes were from wild-type (WT) and transgenic (Tg) mice of the indicated ages (w, weeks). Corin protein was analyzed by Western blotting. Samples from parental HEK 293 cells (negative) or HEK 293 cells expressing human corin (positive) were used as controls. Blots were reprobed with an anti-GAPDH antibody, as an internal control. B: corin protein levels were quantified by densitometric analysis of Western blots. The data were from 3 independent experiments. *P < 0.05 vs. WT of the same age group; n.s., not statistically significant.
Corin mRNA and protein expression in human normal hearts.
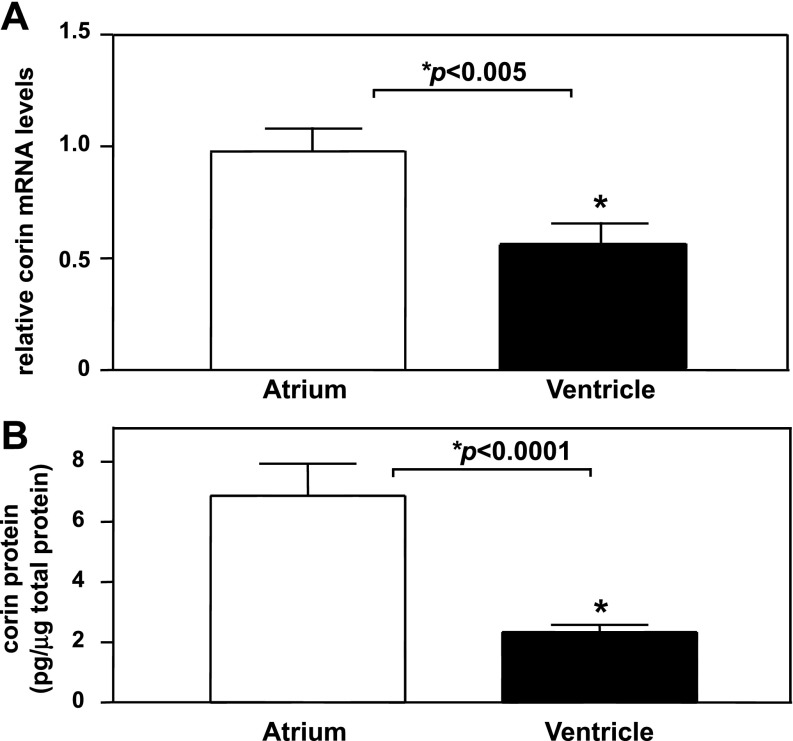
Next, we examined corin mRNA expression in human normal hearts by quantitative RT-PCR. Corin mRNA levels were significantly higher in the atrium than that in the ventricle (P < 0.005) (Fig. 2A). By ELISA, corin protein levels were also higher in the atrium than that in the ventricle (6.9 ± 1.1 vs. 2.3 ± 0.2 pg/μg total protein, P < 0.0001) (Fig. 2B). The results were consistent with a previous study showing that corin mRNA expression was higher in the atrium than the ventricle in mouse developing and adult hearts (40).
Fig. 2.
Corin mRNA and protein expression in human nonfailing hearts. A: total RNAs from atria and ventricles were analyzed by quantitative RT-PCR. Corin mRNA levels were higher in the atrium than that in the ventricle. *P < 0.005; n = 8. B: tissue lysates from atria and ventricles were analyzed by ELISA. Corin protein levels were higher in the atrium than in the ventricle. *P < 0.0001; n = 10.
Corin protein expression in human failing hearts.
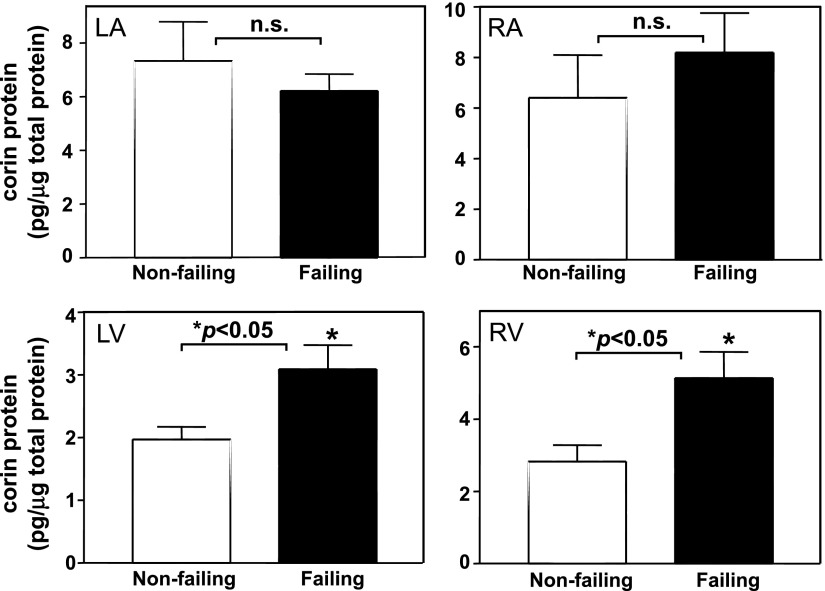
We measured corin protein levels by ELISA in human failing hearts. In either left or right atria, no significant differences in corin protein expression were found between nonfailing and failing hearts (P > 0.05) (Fig. 3, top). In both left and right ventricles, however, corin protein levels were considerably higher in failing hearts than that in nonfailing hearts (P < 0.05) (Fig. 3, bottom). Western blot analysis and quantitative RT-PCR confirmed increased corin protein and mRNA levels in ventricular samples from failing hearts compared with that from nonfailing controls (P < 0.05) (data not shown).
Fig. 3.
Corin protein levels in human failing hearts. Tissue lysate was from left and right atria (LA and RA, top) and ventricles (LV and RV, bottom) from nonfailing and failing hearts. Corin protein levels were measured by ELISA. Samples numbers in each group were ≥5. *P < 0.05 vs. nonfailing hearts of the same group.
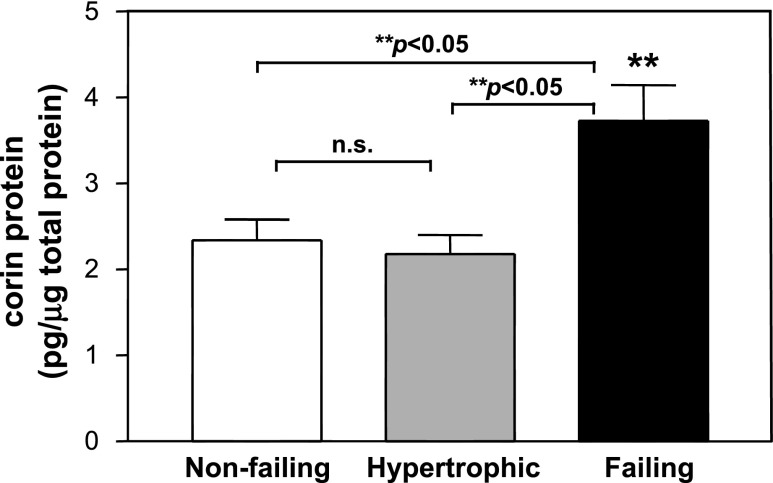
During the course of HF progression, hearts often become hypertrophic before failing (14, 22, 24). By ELISA, we examined corin protein levels in ventricular tissues from human nonfailing, hypertrophic, and failing hearts. The results showed that corin levels were increased only in failing hearts but not hypertrophic hearts (Fig. 4), indicating that human corin protein expression was upregulated only in late stages of HF.
Fig. 4.
Corin protein levels in human nonfailing, hypertrophic, and failing hearts. Tissue lysate was prepared with ventricular samples from nonfailing, hypertrophic, and failing hearts. Corin expression levels were measured by ELISA. At least 9 samples were included in each group. **P < 0.05 vs. nonfailing or hypertrophic.
Corin activity in human failing hearts.
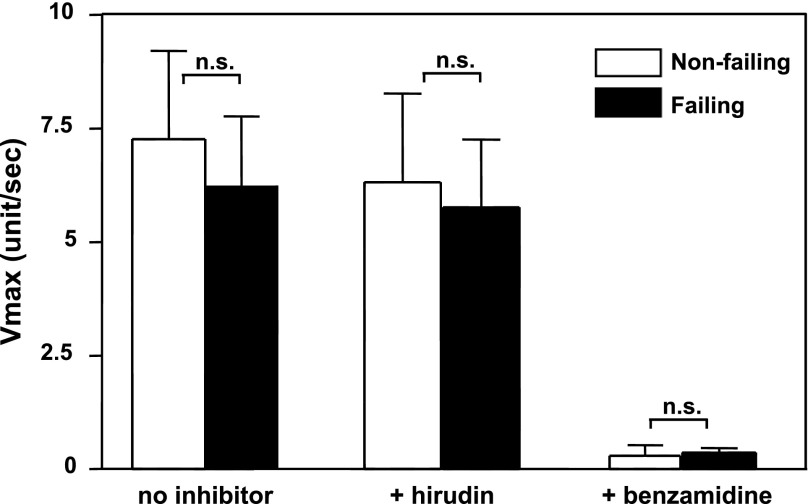
To determine corin enzyme activity in human hearts, we developed a fluorogenic substrate assay using a peptide (Gly-Pro-Arg) that was similar to the corin cleavage sequence in pro-ANP (41). Because corin is a transmembrane protein, we prepared membrane fractions from hearts to measure corin activity. Heart membrane fractions from corin knockout mice were used as a background control for other potential protease activities that might be associated with heart membranes.
In the fluorogenic assay, protease enzymatic activities were similar in membrane fractions from left ventricles of human nonfailing and failing hearts (P > 0.05) (Fig. 5). We verified the assay specificity with two protease inhibitors. Hirudin, a highly specific thrombin inhibitor, was used to exclude possible blood clotting enzymes in the membrane fractions, and benzamidine, a nonspecific serine protease inhibitor, was used to confirm fluorogenic signals were from serine protease activity. As shown in Fig. 5, hirudin had little inhibitory effect whereas benzamidine inhibited virtually all the activity in this assay. The results suggested that corin enzyme activity, as measured by this substrate assay, was not increased in human failing hearts compared with that in nonfailing hearts.
Fig. 5.
Serine protease activity in human nonfailing and failing hearts. Serine protease activity in membrane fractions from left ventricles of nonfailing and failing hearts was measured by a fluorescent substrate assay. The reaction was monitored by a plate reader using a kinetic mode. Hirudin and benzamidine were used to determine assay specificity. The data were from 3 independent experiments, each of which included 4 samples. n.s., Not statistically significant vs. nonfailing hearts.
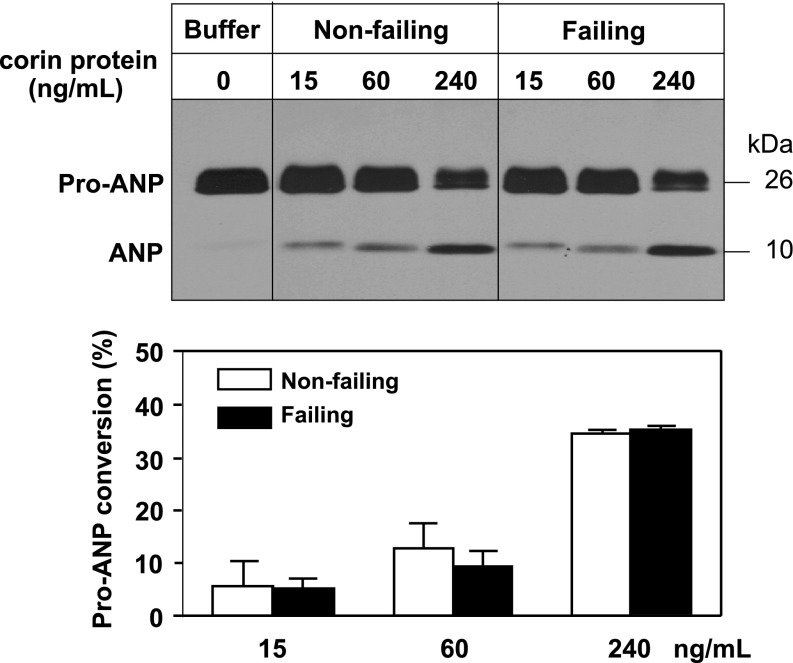
To confirm this result, we performed a pro-ANP processing assay. Recombinant pro-ANP was incubated with membrane fractions from left ventricles of human nonfailing and failing hearts. Corin concentration in both preparations was normalized to 3.1 ng/ml. Pro-ANP to ANP conversion was examined by Western blot analysis (16). The result showed that conversion of pro-ANP to ANP was similar in samples from nonfailing and failing hearts (Fig. 6), which were consistent with the data from the fluorogenic substrate assay (Fig. 5) and indicated that corin activities did not increase in human failing hearts.
Fig. 6.
Pro-atrial natriuretic peptide (ANP) processing activity in human nonfailing and failing hearts. Membrane fractions from left ventricles of nonfailing and failing hearts with indicated corin concentrations were incubated with human pro-ANP. Conversion of pro-ANP to ANP was analyzed by immunoprecipitation and Western blotting (top). Data from 3 independent experiments were quantified by densitometry (bottom).
DISCUSSION
Natriuretic peptides are essential for regulating blood pressure and cardiac function. In cells, these peptides are made as pro-ANP and pro-BNP, which are processed to mature ANP and BNP upon secretion (3, 28, 32). In patients with end-stage HF, plasma pro-ANP and pro-BNP levels are elevated (1, 2, 5, 11, 20, 33, 42), suggesting that processing of these peptides is compromised as the disease progresses. Since ANP and BNP, but not pro-ANP and pro-BNP, are biologically active (8, 12, 28), the impaired processing of these peptides is expected to reduce their activities, which may contribute to water retention and worsening cardiac function in patients with HF.
Corin processes natriuretic peptides in the heart. Corin gene variants are associated with the risk of hypertension and cardiac hypertrophy and poor clinical outcomes (9, 29, 30, 37). To date, however, corin expression and activity in HF remains unclear. In this study, we examined corin mRNA and protein expression in falling hearts. We used a Tg mouse HF model induced by myotrophin overexpression (31). Hearts from these Tg mice undergo phenotypic and functional changes that are similar to those in human HF (31). In these mice, corin protein levels did not change up to 16 wk of age even though hearts became hypertrophic. By 36 wk, however, corin protein levels were increased when their hearts were in failure. The results were consistent with our previous findings in a rat model, in which upregulation of corin mRNA occurred only at a late stage in HF (36).
We extended our studies and examined corin expression in humans. In human normal hearts, both corin mRNA and protein levels were higher in the atrium than the ventricle. As natriuretic peptides, especially ANP, are abundant in the atrium, the higher atrial corin expression may reflect its physiological function in processing natriuretic peptides in the heart.
In patients with HF, corin levels were similar in the atrium in nonfailing and failing hearts but were increased in the ventricle in failing hearts. Like the findings in the mouse HF model (Fig. 1), the increase in corin protein levels was observed only in failing, but not hypertrophic, hearts. In contrast, natriuretic peptide expression is elevated in both hypertrophic and failing hearts (23, 35). In our study, the hypertrophic hearts were from patients with hypertension (Table 1). It appeared, therefore, that corin expression did not directly correlate with high blood pressure but was upregulated only in failing hearts where its activity might be no longer sufficient for processing increasing amounts of natriuretic peptides. Previous studies showed that the transcription factor GATA-4 played a major role in the cardiac specific expression of the corin gene (26). It remains to be determined whether a similar GATA-4-dependent mechanism is involved in upregulating corin expression in failing hearts.
High corin levels in human failing hearts seemed to be paradoxical to elevated levels of circulating pro-ANP and pro-BNP in patients with HF. As a trypsinlike protease, corin is synthesized as an inactive zymogen, which is activated by cleavage at a conserved site (40). For many serine proteases, zymogen activation is critical in controlling their activities. To date, however, the corin activator has not been identified. Studies have shown that in normal heart cells only a small fraction of total corin molecules was active (10, 21). It is possible that the activity from such a fraction of corin molecules is sufficient under normal conditions and that corin zymogen activation might increase when more of its activity is needed under pathological conditions. Because of the lack of a suitable antibody that recognizes the activated corin protease fragment, we were unable to directly assess corin zymogen activation in heart samples by Western blotting. In two functional assays, however, we did not detect any increase in corin activity in failing hearts despite apparent higher levels of corin protein. It appears, therefore, that corin activation is a critical step that limits its activity in failing hearts. Further identification and characterization of the corin activator shall help to understand the regulation of corin expression and activity.
Recently, we and others detected soluble corin in human plasma (7, 27), suggesting that corin is shed from the cell surface and that the cleaved corin fragment(s) enter the circulation. Interestingly, plasma-soluble corin levels were lower in patients with HF and the reduction correlated with disease severity (7). If corin protein levels were higher in failing hearts, why were plasma-soluble corin levels low in HF patients? The exact reason is unclear. It is possible that the expression/activity of corin sheddase, which remains unknown, are reduced in failing hearts, leading to low levels of plasma-soluble corin. Alternatively, corin cleaves itself on the cell surface and in failing hearts such a process is inhibited or impaired, resulting in higher corin levels in the heart but lower levels in plasma. Other possibilities may also include accelerated plasma corin degradation or clearance in patients with HF.
In summary, we found that corin mRNA and protein expression was upregulated in mouse and human failing hearts. In contrast, corin activity did not increase proportionally. Natriuretic peptide levels are increased in HF as a compensatory mechanism to counter high blood pressure and excessive body fluid retention. When the production of these peptides reaches a certain level, apparently, the heart also starts to produce more corin protein to increase natriuretic peptide processing, thereby generating more natriuretic and diuretic activities. In failing hearts, however, corin activation does not appear to increase proportionally to meet the demand. As a result, when the heart produces more natriuretic peptides, corin enzyme activity becomes rate limiting and significant portions of these peptides remain unprocessed. Our results may explain high plasma levels of pro-ANP and pro-BNP found in HF patients and also suggest that strategies to enhance corin activation may be used as a therapy to treat patients with HF.
GRANTS
This work was supported in part by the Ralph Wilson Medical Research Foundation, Bakken Heart-Brain Institute, and the National Heart, Lung, and Blood Institute (R01HL089298, HL089298-S1 to Q. Wu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Sathyamangla Naga Prasad, Neelakantan Vasudevan, Jingjing Jiang, and Xiaofei Qi for helpful suggestions; Life Banc of Northeastern Ohio for human heart tissues; and the Kaufman Center for Heart Failure at the Cleveland Clinic for supporting the human heart tissue bank.
REFERENCES
- 1. Ando K, Hirata Y, Emori T, Shichiri M, Kurosawa T, Sato K, Marumo F. Circulating forms of human atrial natriuretic peptide in patients with congestive heart failure. J Clin Endocrinol Metab 70: 1603–1607, 1990. [DOI] [PubMed] [Google Scholar]
- 2. Azizi C, Maistre G, Kalotka H, Isnard R, Barthelemy C, Masson F, Pham P, Pousset F, Eurin J, Lechat P, Komajda M, Carayon A. Plasma levels and molecular forms of proatrial natriuretic peptides in healthy subjects and in patients with congestive heart failure. J Endocrinol 148: 51–57, 1996. [DOI] [PubMed] [Google Scholar]
- 3. Bloch KD, Scott JA, Zisfein JB, Fallon JT, Margolies MN, Seidman CE, Matsueda GR, Homcy CJ, Graham RM, Seidman JG. Biosynthesis and secretion of proatrial natriuretic factor by cultured rat cardiocytes. Science 230: 1168–1171, 1985. [DOI] [PubMed] [Google Scholar]
- 4. Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci USA 102: 785–790, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen HH. Heart failure: a state of brain natriuretic peptide deficiency or resistance or both! J Am Coll Cardiol 49: 1089–1091, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 50: 2357–2368, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Dong N, Chen S, Yang J, He L, Liu P, Zhen D, Li L, Zhou Y, Ruan C, Plow E, Wu Q. Plasma soluble corin in patients with heart failure. Circ Heart Fail 3: 207–211, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dries DL. Relevance of molecular forms of brain natriuretic peptide for natriuretic peptide research. Hypertension 49: 971–973, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W, Drazner MH. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation 112: 2403–2410, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Gladysheva IP, Robinson BR, Houng AK, Kovats T, King SM. Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both zymogen and catalytically active forms. J Mol Cell Cardiol 44: 131–142, 2008. [DOI] [PubMed] [Google Scholar]
- 11. Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC, Jr, Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci USA 102: 17442–17447, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heublein DM, Huntley BK, Boerrigter G, Cataliotti A, Sandberg SM, Redfield MM, Burnett JC., Jr Immunoreactivity and guanosine 3′,5′-cyclic monophosphate activating actions of various molecular forms of human B-type natriuretic peptide. Hypertension 49: 1114–1119, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Hooper JD, Scarman AL, Clarke BE, Normyle JF, Antalis TM. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur J Biochem 267: 6931–6937, 2000. [DOI] [PubMed] [Google Scholar]
- 14. Jessup M, Brozena S. Heart failure. N Engl J Med 348: 2007–2018, 2003. [DOI] [PubMed] [Google Scholar]
- 15. Jiang W, Cai DY, Pan CS, Qi YF, Jiang HF, Geng B, Tang CS. Changes in production and metabolism of brain natriuretic peptide in rats with myocardial necrosis. Eur J Pharmacol 507: 153–162, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Knappe S, Wu F, Madlansacay MR, Wu Q. Identification of domain structures in the propeptide of corin essential for the processing of proatrial natriuretic peptide. J Biol Chem 279: 34464–34471, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Knappe S, Wu F, Masikat MR, Morser J, Wu Q. Functional analysis of the transmembrane domain and activation cleavage of human corin. J Biol Chem 278: 52363–52370, 2003. [DOI] [PubMed] [Google Scholar]
- 18. Langenickel TH, Pagel I, Buttgereit J, Tenner K, Lindner M, Dietz R, Willenbrock R, Bader M. Rat corin gene: molecular cloning and reduced expression in experimental heart failure. Am J Physiol Heart Circ Physiol 287: H1516–H1521, 2004. [DOI] [PubMed] [Google Scholar]
- 19. Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 339: 321–328, 1998. [DOI] [PubMed] [Google Scholar]
- 20. Liang F, O'Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, Apple FS, Maisel AS, Pollitt NS, Protter AA. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol 49: 1071–1078, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Liao X, Wang W, Chen S, Wu Q. Role of glycosylation in corin zymogen activation. J Biol Chem 282: 27728–27735, 2007. [DOI] [PubMed] [Google Scholar]
- 22. McMurray JJ, Pfeffer MA. Heart failure. Lancet 365: 1877–1889, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Molkentin JD, Dorn GW., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol 63: 391–426, 2001. [DOI] [PubMed] [Google Scholar]
- 24. Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 356: 1140–1151, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, Gurish M, Mathis D, Benoist C, Lee DM. Genetic inversion in mast cell-deficient (W(sh)) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol 173: 1693–1701, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan J, Hinzmann B, Yan W, Wu F, Morser J, Wu Q. Genomic structures of the human and murine corin genes and functional GATA elements in their promoters. J Biol Chem 277: 38390–38398, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Peleg A, Jaffe AS, Hasin Y. Enzyme-linked immunoabsorbent assay for detection of human serine protease corin in blood. Clin Chim Acta 409: 85–89, 2009. [DOI] [PubMed] [Google Scholar]
- 28. Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev 27: 47–72, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Rame JE, Drazner MH, Post W, Peshock R, Lima J, Cooper RS, Dries DL. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension 49: 857–864, 2007. [DOI] [PubMed] [Google Scholar]
- 30. Rame JE, Tam SW, McNamara D, Worcel M, Sabolinski ML, Wu AH, Dries DL. Dysfunctional corin I555(P568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: results from the Genetic Risk Assessment in Heart Failure substudy. Circ Heart Fail 2: 541–548, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sarkar S, Leaman DW, Gupta S, Sil P, Young D, Morehead A, Mukherjee D, Ratliff N, Sun Y, Rayborn M, Hollyfield J, Sen S. Cardiac overexpression of myotrophin triggers myocardial hypertrophy and heart failure in transgenic mice. J Biol Chem 279: 20422–20434, 2004. [DOI] [PubMed] [Google Scholar]
- 32. Shields PP, Glembotski CC. The post-translational processing of rat pro-atrial natriuretic factor by primary atrial myocyte cultures. J Biol Chem 263: 8091–8098, 1988. [PubMed] [Google Scholar]
- 33. Shimizu H, Masuta K, Aono K, Asada H, Sasakura K, Tamaki M, Sugita K, Yamada K. Molecular forms of human brain natriuretic peptide in plasma. Clin Chim Acta 316: 129–135, 2002. [DOI] [PubMed] [Google Scholar]
- 34. Sivakumar P, Gupta S, Sarkar S, Sen S. Upregulation of lysyl oxidase and MMPs during cardiac remodeling in human dilated cardiomyopathy. Mol Cell Biochem 307: 159–167, 2008. [DOI] [PubMed] [Google Scholar]
- 35. Stein BC, Levin RI. Natriuretic peptides: physiology, therapeutic potential, and risk stratification in ischemic heart disease. Am Heart J 135: 914–923, 1998. [DOI] [PubMed] [Google Scholar]
- 36. Tran KL, Lu X, Lei M, Feng Q, Wu Q. Upregulation of corin gene expression in hypertrophic cardiomyocytes and failing myocardium. Am J Physiol Heart Circ Physiol 287: H1625–H1631, 2004. [DOI] [PubMed] [Google Scholar]
- 37. Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, Qin J, Wu Q. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res 103: 502–508, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu Q. The serine protease corin in cardiovascular biology and disease. Front Biosci 12: 4179–4190, 2007. [DOI] [PubMed] [Google Scholar]
- 39. Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int 75: 142–146, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem 274: 14926–14935, 1999. [DOI] [PubMed] [Google Scholar]
- 41. Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA 97: 8525–8529, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yandle TG, Richards AM, Gilbert A, Fisher S, Holmes S, Espiner EA. Assay of brain natriuretic peptide (BNP) in human plasma: evidence for high molecular weight BNP as a major plasma component in heart failure. J Clin Endocrinol Metab 76: 832–838, 1993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.