Abstract
We recently demonstrated a cardiac therapeutic regimen based on injection of bone marrow mesenchymal stem cells (MSCs) into the skeletal muscle. Although the injected MSCs were trapped in the local musculature, the extracardiac cell delivery approach repaired the failing hamster heart. This finding uncovers a tissue repair mechanism mediated by trophic factors derived from the injected MSCs and local musculature that can be explored for minimally invasive stem cell therapy. However, the trophic factors involved in cardiac repair and their actions remain largely undefined. We demonstrate here a role of MSC-derived IL-6-type cytokines in cardiac repair through engagement of the skeletal muscle JAK-STAT3 axis. The MSC IL-6-type cytokines activated JAK-STAT3 signaling in cultured C2C12 skeletal myocytes and caused increased expression of the STAT3 target genes hepatocyte growth factor (HGF) and VEGF, which was inhibited by glycoprotein 130 (gp130) blockade. These in vitro findings were corroborated by in vivo studies, showing that the MSC-injected hamstrings exhibited activated JAK-STAT3 signaling and increased growth factor/cytokine production. Elevated host tissue growth factor levels were also detected in quadriceps, liver, and brain, suggesting a possible global trophic effect. Paracrine actions of these host tissue-derived factors activated the endogenous cardiac repair mechanisms in the diseased heart mediated by Akt, ERK, and JAK-STAT3. Administration of the cell-permeable JAK-STAT inhibitor WP1066 abrogated MSC-mediated host tissue growth factor expression and functional improvement. The study illustrates that the host tissue trophic factor network can be activated by MSC-mediated JAK-STAT3 signaling for tissue repair.
Keywords: IL-6-type cytokine, signal transducer and activator of transcription 3, skeletal muscle
mesenchymal stem cells (MSCs) are currently being used in clinical trials for treating a wide range of diseases (http://www.clinicaltrials.gov). Although MSCs exhibit prominent multilineage potential (or stemness), recent studies have suggested that this cellular feature contributes little to their therapeutic benefits (4, 12, 47). Instead, the secretion of multiple growth factors and cytokines (trophic action) by MSCs appears to provide the underlying tissue-healing mechanism (3, 23, 44). We have taken advantage of this trophic action of MSCs in formulating a minimally invasive therapeutic approach for heart failure treatment, which is based on injection of MSCs or MSC-derived factors into the limb muscle away from the diseased heart (54). Cardiac repair is evidenced by increased cardiomyogenesis and angiogenesis, attenuated myocardial apoptosis and fibrosis, and improved ventricular function (54). This extracardiac cell administration strategy targeting the skeletal muscle contrasts with the traditional intramyocardial or intracoronary delivery approaches and provides the proof that cardiac repair can be achieved through stem cell trophic actions independent of stemness.
Skeletal muscle is a dynamic tissue capable of responding to environmental stimuli and functioning as a trophic factor-producing organ (45, 69). Its impressive ability to regenerate after injury or ischemic insult is coupled with the ability to produce many trophic factors, some of which possess cardioprotective effects. These trophic factors can potentially exert a wide range of physiological effects as illustrated for those secreted by the adipose tissue (32, 68, 69). For instance, skeletal muscle produces several well-known cardioprotective growth factors such as FGF, hepatocyte growth factor (HGF), and VEGF (17, 48, 60), which have been used in preclinical or clinical studies for cardiovascular therapy (38, 59, 61). Exercise training can further promote muscle production of HGF, IGF, IL-6, VEGF, and NGF (5, 41, 58, 63, 74). Indeed, physical activity is known to prevent coronary artery disease and cognitive decline (31, 62), highlighting the critical roles of skeletal muscle-derived growth factors and cytokines in maintaining the cardiovascular system.
Among the many soluble mediators released by skeletal muscle in response to contraction is the cytokine IL-6, which can increase up to 100-fold in the circulation during intense exertion (45). IL-6 along with IL-11, leukemia inhibitory factor (LIF), cardiotrophin-1 (CT-1), cardiotrophin-like cytokine (CLC), oncostatin M (OSM), and ciliary neurotrophic factor (CNTF) forms the IL-6 family of cytokines, which signal through the common gp130 receptor (10) and activate the STATs via the JAKs (7). Initially identified as an IL-6-dependent transcription factor, STAT3 is now known to play a dominant role in transducing signals for the IL-6-type cytokines. Among the many downstream gene targets activated by STAT3 are HGF and VEGF (11, 70, 72), suggesting that the JAK-STAT3 axis may be harnessed for therapeutic regeneration.
We (54, 76, 77) recently demonstrated a cardiac repair regimen based on injections of MSCs, MSC-conditioned medium (MSC-CM), or VEGF into the hind limb muscle of TO2 cardiomyopathic hamsters. Although the intramuscularly injected MSCs were found to be trapped in the local musculature (53), the cell injection significantly improved ventricular function, which was associated with mobilization of bone marrow progenitor cells, myocardial regeneration, and attenuation of apoptosis and fibrosis (54). Elevated circulating levels of LIF (an IL-6-type cytokine) and HGF (a STAT3 target gene) after MSC administration were noted in the study (54), suggesting an involvement of the JAK-STAT3 pathway. In support of this notion, intramuscular administration of VEGF, also a STAT3 target gene, was found to improve ventricular function through similar mechanisms (76, 77). We hypothesize that MSC-derived IL-6-type cytokines activate the skeletal muscle JAK-STAT3 axis, and this upstream signaling event leads to amplification of the host trophic factor network and cardiac repair. The work presented here was initiated to test this hypothesis.
MATERIALS AND METHODS
Animals.
F1B (normal) and TO2 (cardiomyopathic) male hamsters (4–5 mo of age) were obtained from Bio Breeders (Watertown, MA). All procedures and protocols conformed to institutional guidelines for the care and use of animals in research, and the study was approved by the University at Buffalo institutional animal care and use committee (IACUC).
Echocardiography.
Animals were anesthetized by intraperitoneal injection of xylazine (2 mg/kg) and ketamine (25 mg/kg) and remained semiconscious during the measurement procedure. Multiple M-mode images were obtained from the short-axis view of the left ventricle at the level of the papillary muscles with a GE Vingmed echo machine using a 10-MHz transducer. From this image, left ventricular end-systolic dimension and left ventricular end-diastolic dimension were measured in a blindfolded manner. These dimensions were measured and averaged from at least two consecutive cardiac cycles.
MSC implantation and drug administration.
Porcine MSCs and intramuscular MSC injections were described previously (29, 54, 65). In brief, each TO2 hamster received injections of 4 million MSCs resuspended in 0.6-ml HBSS. Cells were equally divided in the left and right hamstrings. Control TO2 hamsters received the same volume of HBSS. Although the animals received a 2nd intramuscular MSC implantation after 2 wk, the 2nd injection was later found to be unnecessary. The JAK-STAT3 inhibitor WP1066 (Calbiochem) was dissolved in polyethylene glycol (PEG)-300/dimethyl sulfoxide (4:1). The drug was administered by intraperitoneal injection twice a week at a dose of 40 mg/kg as documented (18). The IC50 of WP1066 against the malignant glioma U87-MG and U373-MG cells were 5.6 and 3.7 μM, respectively (18). The mean achieved peak plasma concentration following a 40 mg/kg intravenous dose in mice was 4.13 μM, and mean drug half-life was 4.5 h (33).
Cell culture.
Porcine MSCs, human MSCs, C2C12 skeletal myocytes, and human embryonic kidney (HEK-293) cells were maintained in DMEM/F-12 supplemented with 10% FBS, 2 mM glutamine, 50 μg/ml gentamicin, and 0.125 μg/ml Fungizone. MSC-CM was prepared by exposing an ∼80% confluent MSC culture to a 5% FBS-containing DMEM/F-12 for 2 days. Recombinant mouse IL-6 was from R&D Systems (Minneapolis, MN). Recombinant mouse LIF and human IL-11 were from Millipore (Temecula, CA). For measurement of JAK-STAT3 signaling, C2C12 cells seeded in 6-well plates at a density of 100,000 cells per well were treated with MSC-CM, HEK-293-CM, IL-6, LIF, or IL-11 for 30 min. Cells were then washed twice and lysed in 1% SDS. Whole cell lysates were clarified briefly by centrifugation, and protein concentrations were determined using the Pierce BCA protein assay kit (Pierce Biotechnology, Rockford, IL).
Western blotting.
Proteins were resolved by SDS-PAGE and electrotransferred to Immobilon-P membrane, which was incubated with a 1,000-fold diluted primary antibody solution overnight at 4°C. Washed membrane was probed with a horseradish peroxidase (HRP)-conjugated secondary antibody at ∼10 ng/ml. Signals were developed using the SuperSignal chemiluminescent substrate from Pierce Biotechnology and imaged by Fuji imager. The following primary antibodies were purchased from Cell Signaling Technology (Danvers, MA): anti-phosphorylated Tyr705 (anti-p-Tyr705) of STAT3 (cat. no. 9145), anti-total STAT3 (cat. no. 9143), anti-p-Tyr1022/1023 of JAK1 (cat. no. 3133), anti-total JAK1 (cat. no. 3332), anti-p-Tyr1007/1008 of JAK2 (cat. no. 3766), anti-total JAK2 (cat. no. 3230), anti-p-Ser473 of Akt (cat. no. 4060), anti-total Akt (cat. no. 4691), anti-p-Thr202/204 of ERK1/2 (cat. no. 4370), and anti-total ERK1/2 (cat. no. 4695).
ELISA analysis.
Trophic factor concentrations of cell culture media and whole tissue/cell extracts (soluble fraction) were measured using ELISA kits from R&D Systems: IL-6 DuoSet (cat. no. DY686), IL-11 DuoSet (cat. no. DY218), HGF DuoSet (cat. no. DY2207), IGF-II DuoSet (cat. no. DY792), and VEGF DuoSet (cat. nos. DY564 and DY293B). Snap-frozen tissues or cells were homogenized in an ice-cold lysis solution (normal saline supplemented with 0.1% Triton X-100 and 2 mM EDTA). Tissue homogenates were clarified, diluted to 1 mg proteins/ml, and stored at −80°C.
qRT-PCR.
RNA extraction from C2C12 skeletal myocytes, MSCs, and hamster tissues were performed using Qiagen RNA isolation kits, and quantitative RT-PCR (qRT-PCR) protocols were as previously described (29, 76). In brief, PCR was performed using the MyiQ machine with the SYBR Green kit (Bio-Rad, Hercules, CA). Amplification conditions after an initial denaturation step for 3 min at 95°C were 45 cycles of 95°C, 10 s, for denaturation, and 55°C, 30 s, for annealing and elongation. Melting curve analysis was performed to check for a single amplicon. MyiQ analysis software was used for determining crossing points. β2-Microglobulin was used as the reference gene for calculations. Data were analyzed by the 2ΔΔCT threshold cycle method. Oligonucleotides were synthesized by Midland Certified Reagent (Midland, TX). Primer sequences are listed in Table 1.
Table 1.
Primer sequences for qRT-PCR
| Gene | 5′ Primer | 3′ Primer |
|---|---|---|
| Rodent | ||
| Beta-2-microglobulin (B2M) | TCTCTTGGCTCACAGGGAGT | ATGTCTCGTTCCCAGGTGAC |
| ANP | ATATTGGAGCGAACCCTGTG | GGGCACGACCTCATCTTCTA |
| BNP | GTCTCCGGAACAATCCAAGA | CTATCCTCTGCCCAAAGCAG |
| c-myc | CTGCTCCACCTCTAGCCTGT | GAGAGAAGGCTGTGGAGTCG |
| CYCLIN A2 | CCTGCAAACTGCAAAGTTGA | AAAGGCAGCTCCAGCAATAA |
| CYCLIN D2 | AGAACCTGCTGACCATCGAG | CCTCGCAGACCTCTAGCATC |
| HGF | AGAGGTCCCATGGATCACAC | AGCCCTTGTCGGGATATCTT |
| IGF-1 | GGACGCTCTTCAATTCGTGT | GATAGAGCGGGCTGATTTTG |
| IGF-2 | CAAGTCCGAGAGGGATGTGT | GGACTGTCTCCAGGTGTCGT |
| IL-1ra | TTGTGCCAAGTCTGGAGATG | TTCTCAGAGCGGATGAAGGT |
| IL-6 | CTCCGCAAGAGACTTCCATC | ACCAAACCTCCGACTTGTTG |
| LIF | ATTGTGCCCTTACTGCTGCT | TGAGCTGTGCCAGTTGATTC |
| NGF | GGACGCAGCTTTCTATCCTG | TGGGACAATGCTGTCTGTGT |
| SDF-1 | GCTCTGCATCAGTGACGGTA | CAGCCTTGCAACAATCTGAA |
| SOCS3 | GACCTCTCTCCTCCAACGTG | TCCAGGAACTCCCGAATGG |
| VEGF | TCACCAAAGCCAGCACATAG | AAATGCTTTCTCCGCTCTGA |
| vWF | GTGTTTGTGCAGCAGAGGAA | AATGATGGTGCCATTGAGC |
| Human | ||
| Beta-2-Microglobulin (B2M) | TGCTGTCTCCATGTTTGATGTATCT | TCTCTGCTCCCCACCTCTAAGT |
| CLC | GACTCGTGGGGGATGTTAGC | AGGTCATAGGTTTTCTGGATGGA |
| CNTF | ATGGCTTTCACAGAGCATTCA | GGATTCCGTAAGAGCAGTCAGG |
| CT-1 | AGACCCCCAGACTGATTCCTC | AGCTGCACATATTCCTGGAGC |
| GAPDH | CTCTCTGCTCCTCCTGTTCGAC | TGAGCGATGTGGCTCGGCT |
| IL-11 | CGAGCGGACCTACTGTCCTA | GCCCAGTCAAGTGTCAGGTG |
| IL-6 | AAATTCGGTACATCCTCGACGG | GGAAGGTTCAGGTTGTTTTCTGC |
| LIF | CCCATCACCCCTGTCAACG | GGGCCACATAGCTTGTCCA |
| Oncostatin M (OSM) | GCTCACACAGAGGACGCTG | GGAGCACGCGGTACTCTTTC |
qRT-PCR, quantitative RT-PCR.
In situ immunostaining.
C2C12 myocytes were treated with MSC-CM for 30 min and fixed with 4% paraformaldehyde and permeabilized with 100% methanol. Stained sections were imaged with the Zeiss Axio Imager Z1 epifluorescence microscope. Hamstring cryosections were stained with diluted primary antibodies overnight at 4°C. Sections were further incubated with Alexa 647- and/or Alexa 488-conjugated secondary antibody for 30 min and then mounted in Vectashield Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA). Stained sections were imaged with an epifluorescence microscope (Zeiss Axio Imager Z1). The myosin heavy chain (MHC) antibody was described previously (25). The β-actin antibody was purchased from Sigma (cat. no. B5186).
Statistical analysis.
Data are expressed as means ± SE. Comparisons of parameters between groups were made with unpaired Student's t-test or ANOVA where applicable. A value of P < 0.05 was considered significant.
RESULTS
MSCs activate JAK-STAT3 signaling in cultured skeletal myocytes.
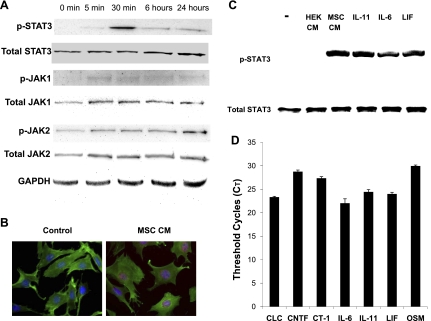
Several IL-6-type cytokines are known to be expressed by MSCs (29, 40, 77). To test the hypothesis that MSC-derived IL-6-type cytokines activate the skeletal muscle JAK-STAT3 axis, we first studied JAK-STAT3 signaling in cultured C2C12 skeletal myocytes in response to MSC-derived factors. C2C12 cells were incubated with MSC-CM, and JAK-STAT3 signaling was monitored by Western blot assays of protein phosphorylation. Figure 1A shows that MSC-CM induced phosphorylation of Tyr1022/1023 of JAK1 and Tyr1007/1008 of JAK2. Prominent phosphorylation Tyr705 of STAT3 appeared at 30 min (Fig. 1A), which was further validated by in situ immunostaining of nuclear p-STAT3 (Fig. 1B). Recombinant IL-6, IL-11, and LIF each activated STAT3 phosphorylation as expected (Fig. 1C). In contrast, the conditioned medium prepared from HEK-293 had no detectable effect on STAT3 activation (Fig. 1C). qRT-PCR shows that MSCs express multiple members of the IL-6-type cytokine family including CLC, CNTF, CT-1, IL-6, IL-11, LIF, and OSM. Analysis of threshold cycles (CT) shows that IL-6, IL-11, CLC, and LIF of the cytokine family are the more abundantly expressed cytokines with a CT range of 22–24.
Fig. 1.
Trophic actions of mesenchymal stem cells (MSCs) stimulate JAK-STAT3 signaling in cultured skeletal myocytes. C2C12 myocytes were plated in 6-well plates (105 cells per well). After overnight plating, cells were exposed to the indicated conditioned medium (CM). A: Western blot assays of C2C12 cells exposed to MSC-CM for 5 min, 30 min, 6 h, and 24 h. B: in situ immunostaining of control medium- and MSC-CM-treated C2C12 cells (30-min incubation) using a phosphorylated STAT3 (p-STAT3) antibody (red) and β-actin antibody (green). Alexa 647- and FITC-conjugated secondary antibodies were used. Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI). C: Western blot assays comparing the effects of porcine MSC-CM, human embryonic kidney (HEK)-293-CM, IL-6, IL-11, and leukemia inhibitory factor (LIF) on STAT3 phosphorylation in C2C12 myocytes 30 min after treatment. Fifty nanograms per milligram recombinant proteins were used. −, No addition. D: threshold cycles (CT) for human MSC IL-6-type cytokines. The CT for the housekeeping gene β2-microglobulin and GAPDH are ∼17 and ∼15, respectively. CLC, cardiotrophin-like cytokine; CNTF, ciliary neurotrophic factor; CT-1, cardiotrophin-1; OSM, oncostatin M.
STAT3 signaling in cultured skeletal myocytes mediates growth factor production.
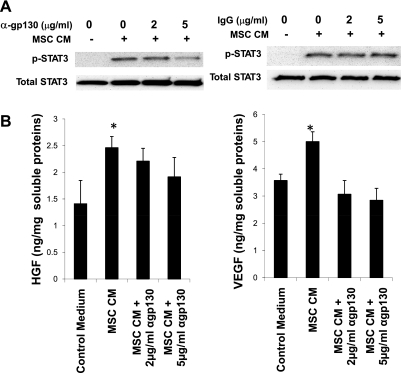
To demonstrate the specificity and function of STAT3 signaling, we determined whether blocking the gp130 receptor might inhibit STAT3 phosphorylation in C2C12 skeletal myocytes exposed to MSC-CM. Indeed, a gp130 receptor blocking antibody at 5 μg/ml was able to attenuate STAT3 phosphorylation (Fig. 2A). Downstream gene targets of STAT3 signaling include the two therapeutically important factors HGF and VEGF (11, 70, 72). ELISA assays (Fig. 2B) confirmed that C2C12 myocytes produced elevated levels of HGF and VEGF in response to MSC-CM, and the increase in growth factor production was abrogated by the gp130 receptor blocking antibody. Collectively, these data indicate that MSC-derived IL-6-type cytokines activate JAK-STAT3 signaling in cultured skeletal myocytes, and this upstream signaling event led to amplified expression of HGF and VEGF.
Fig. 2.
Glycoprotein 130 (gp130)-STAT3 signaling stimulates growth factor expression in cultured skeletal myocytes. C2C12 myocytes were treated with 2 and 5 μg/ml gp130 antibody (cat. no. AF468; R&D Systems) or control IgG for 1 h at 37°C. The IgG isotype antibody was used as a negative control. Cells were then briefly rinsed and treated with MSC-CM for 30 min to assess STAT3 activation or 3 days for growth factor production. A: Western blot assays of C2C12 whole cell lysates using STAT3 antibodies, showing an inhibitory effect of the gp130 antibody on STAT3 phosphorylation. B: analysis of C2C12 whole cell lysates using rodent-specific VEGF and hepatocyte growth factor (HGF) ELISA kits. Rat VEGF and mouse HGF were used as standards. *P < 0.05 vs. control medium.
MSC-mediated STAT3 signaling stimulates growth factor/cytokine production in vivo.
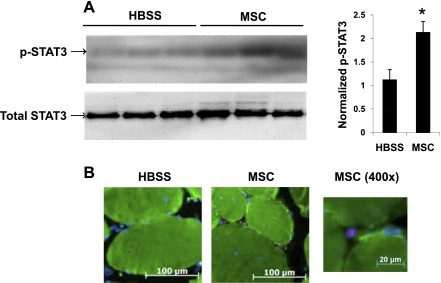
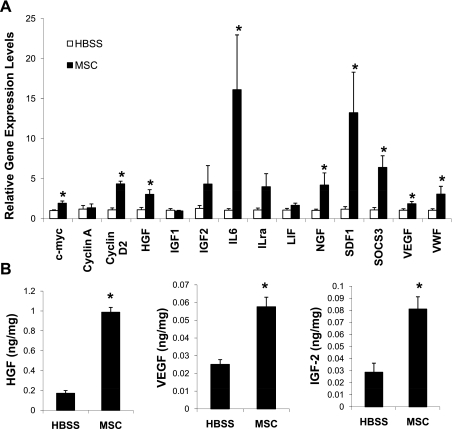
Extracardiac MSC injection experiments as described previously (54) were performed to confirm the in vitro data. The MSC-injected hamstrings exhibited a significant increase in STAT3 phosphorylation compared with the saline (HBSS)-injected muscles as shown by Western blotting (Fig. 3A) and in situ immunostaining (Fig. 3B). The p-STAT3-positive nuclei were not detected in the control hamstrings. STAT3 signaling controls a wide array of cellular processes by targeting the expression of many genes such as those involved in cell cycle control (c-myc and cyclin) and encoding trophic factors (HGF, VEGF, and IGF-II). qRT-PCR assays of the injected hamstrings using rodent-specific primers reveal that there was significant induction of the STAT3-regulated genes HGF, VEGF, cyclin D, IL-6, c-myc, and suppressor of cytokine signaling 3 (SOCS3; Fig. 4A). Assays of hamstring homogenates using rodent-specific ELISA kits showed increased amounts of host muscle-derived HGF, VEGF, and IGF-II (Fig. 4B). HGF was ∼10 times more abundant than VEGF in the control hamstrings (∼0.2 ng HGF/mg vs. ∼0.02 ng VEGF/mg soluble proteins). After MSC stimulation, the hamstring HGF concentration increased to ∼1 ng/mg, whereas that of VEGF was doubled (Fig. 4B). As illustrated for contracting skeletal muscle (45), some of the muscle-derived growth factors were likely released to the systemic circulation, causing increased circulating levels of HGF and VEGF (see below).
Fig. 3.
Activation of skeletal muscle STAT3 signaling by MSCs. MSCs were injected into the hamstring muscles of 4-mo-old TO2 cardiomyopathic hamsters as described (54). The injected muscles were harvested 3 days postinjection (n = 3) for Western blotting and immunohistochemical analyses. A: Western blot assays were performed to assess STAT3 phosphorylation. Densitometric analysis revealed statistical significance (*P < 0.05 vs. HBSS). B: in situ immunostaining of cryostat sections demonstrated nuclear p-STAT3 signals (pink) in the MSC-injected hamstrings. Myofibers were stained by a myosin heavy chain antibody (green). Nuclei were stained with DAPI (blue). A higher magnification (×400) of a p-STAT3-positive nucleus is also shown.
Fig. 4.
Activation of the skeletal muscle growth factor network. The injected hamstrings were collected 3 days after injections, and tissue samples (n = 4) were processed for quantitative RT-PCR (qRT-PCR) and ELISA assays. A: gene expression analysis by qRT-PCR using β2-microglobulin as the reference gene. Rodent-specific PCR primers were designed to amplify the hamster sequences. *P < 0.05 vs. HBSS control. ILra, IL receptor antagonist; SDF-1, stromal cell-derived factor-1; SOCS3, suppressor of cytokine signaling 3; VWF, von Willebrand factor. B: immunoassays of hamstring tissue homogenates for HGF, IGF-II, and VEGF using rodent-specific HGF, IGF-II, and VEGF ELISA kits. Mouse IGF-II was used as standard. Growth factor concentrations were expressed as nanograms per milligram soluble proteins. *P < 0.05 vs. HBSS control.
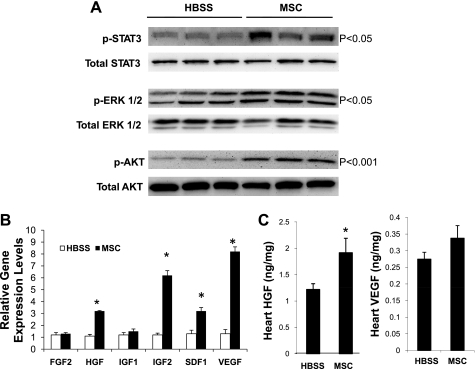
Paracrine activation of growth factor signaling and expression in the myocardium.
Since the hamstring-injected MSCs were trapped in the local musculature (53), growth factors derived from the MSC-activated hamstrings likely exert their trophic or paracrine actions on the myocardium through multiple signaling pathways, resulting in attenuation of myocardial apoptosis and promotion of cardiac regeneration (54). Figure 5A shows significant activation of Akt (phosphorylation of Ser473) and ERK1/2 (phosphorylation of Thr202/204) along with enhanced STAT3 signaling in the TO2 hamster heart after MSC therapy. These prosurvival signaling events further caused increased expression of several myocardial growth factors including HGF and VEGF (Fig. 5B), which was also confirmed by ELISA assays of tissue homogenates (Fig. 5C). Taken together, these results illustrate a therapeutically relevant trophic factor signaling cascade initiated by MSC IL-6-type cytokines, which stimulate the skeletal muscle trophic factor network through JAK-STAT3 signaling. Paracrine actions of these host muscle-derived growth factors serve to activate growth factor expression in the diseased heart and mediate myocardial regeneration (54).
Fig. 5.
Activation of myocardial growth factor signaling and expression. Cardiac ventricular tissues were harvested as described in Fig. 3 (n = 3 per group). A: signaling pathways were examined by Western blot assays. Statistical significance for each pathway was indicated. B: qRT-PCR assays of hamster growth factor expression 1 mo post-MSC treatment. *P < 0.05 compared with HBSS control. C: assays of myocardial tissue levels of HGF and VEGF using rodent-specific ELISA kits 1 mo post-MSC treatment. Growth factor concentrations were expressed as nanograms per milligram soluble proteins. *P < 0.05 vs. HBSS control.
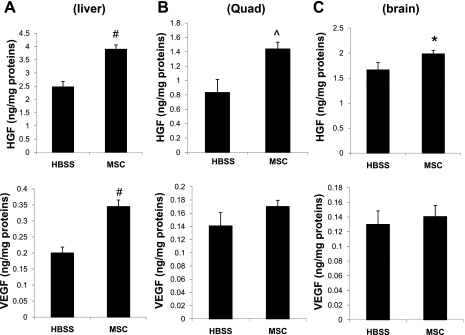
Increased growth factor levels in multiple host tissues.
We further determined whether the hamstring-injected MSCs, which were trapped in the local musculature (53), might affect the growth factor levels in other noninjected tissues. Rodent-specific ELISA revealed that liver homogenates contained significantly elevated levels of HGF and VEGF after MSC therapy (Fig. 6A) despite the finding from PCR assays that the intramuscularly injected MSCs could not be detected in the liver (53). A similar paracrine effect was observed for the noninjected skeletal muscle quadriceps (Fig. 6B). Interestingly, a slight but significant increase in HGF content was also detected in the brain after MSC therapy (Fig. 6C). Thus the growth factor trophic mechanism mediated by the MSC-skeletal muscle circuit might have a global physiological effect not restricted to the diseased heart.
Fig. 6.
Extracardiac MSCs caused increased growth factor levels in multiple tissues. Liver (A), quadriceps (Quad; B), and brain (C) were harvested 1 mo after the extracardiac MSC therapy (n = 3). Soluble fractions of total tissue extracts were prepared and assayed by rodent-specific ELISA kits. Growth factor concentrations were expressed as nanograms per milligram soluble proteins. *P < 0.05, #P < 0.001, and ^P < 0.01 compared with HBSS.
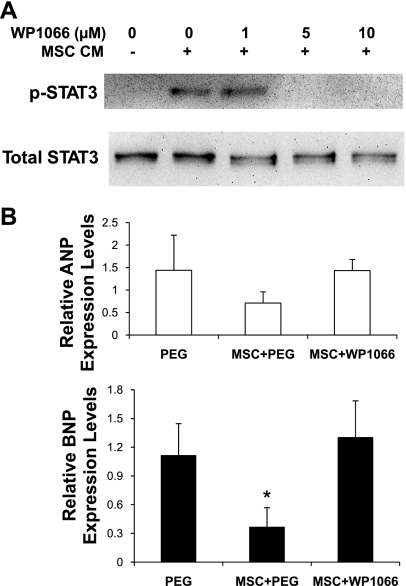
Inhibition of JAK-STAT3 signaling abrogates the therapeutic effect of MSCs.
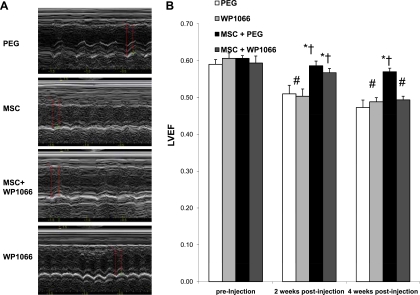
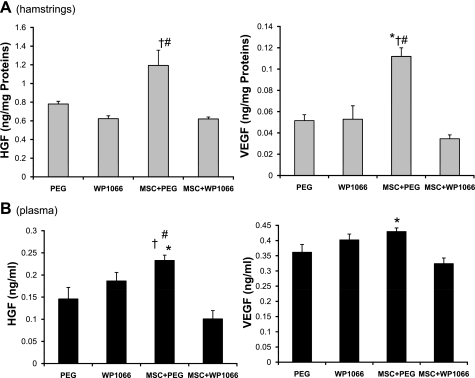
To further demonstrate the functional relevance of the proposed signaling cascade, we assessed whether the JAK-STAT3 inhibitor WP1066, which is a cell-permeable analog of AG490 (18), might block the cardiac therapeutic effects of MSCs. Incubation of C2C12 myocytes with WP1066 in culture confirmed the STAT3-inhibitory effect of the drug (Fig. 7A). Cardiomyopathic hamsters were divided into 4 groups: WP1066; WP1066 vehicle (PEG); MSC and WP1066; and MSC and WP1066 vehicle (MSC+PEG). The TO2 hamster heart exhibits progressive decline in left ventricular function (35). Myocardial expression of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), which are typically increased in congestive heart failure (37), were increased by ∼10- and ∼3-fold, respectively, in the 5-mo-old TO2 hamster heart. These changes are associated with dilation of the left ventricular chamber and decreased capillary density, which can be reversed by MSC administration (54). As expected, qRT-PCR assays show that although MSCs attenuated the expression of ANP and BNP, administration of WP1066 blocked this effect of MSCs (Fig. 7B). Furthermore, echocardiography shows that whereas MSCs prevented the progressive decline in left ventricular ejection fraction, WP1066 again abolished this effect of MSCs (Fig. 8). Significantly, these inhibitory effects of WP1066 on heart function were associated with abrogation of MSC-induced HGF and VEGF in the injected hamstrings (Fig. 9A) and in the circulation (Fig. 9B). These studies thus confirm the role of MSC-mediated JAK-STAT3 signaling in amplifying the host tissue trophic factor network.
Fig. 7.
WP1066 inhibits JAK-STAT3 signaling and abrogates MSC-mediated cardiac repair. A: inhibitory effect of WP1066 on STAT3 phosphorylation in C2C12 myocytes. Cells were pretreated with WP1066 at the indicated dosages for 2 h, following which, cells were stimulated with MSC-CM for 30 min. Western blot assays were performed to determine STAT3 activation. B: the extracardiac MSC therapy was carried out as described previously (54). The 4 treatment groups (n = 5 per group) were: WP1066; drug vehicle [polyethylene glycol (PEG)]; MSC and WP1066; and MSC and drug vehicle (MSC+PEG). qRT-PCR assays of myocardial atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) expression (n = 4 per group) after 1 mo are presented. ANOVA was performed to assess statistical significance. *P < 0.05 vs. PEG.
Fig. 8.
WP1066 blocks MSC-mediated improvement in cardiac function. Cardiac function was determined by blindfolded echocardiography after MSC administration. A: representative M-mode recording for the 4 animal groups at 4 wk. Red arrows were placed to measure left ventricular end-diastolic and -systolic diameters and wall thickness. B: left ventricular ejection fraction (LVEF) at 2 and 4 wk after MSCs. ANOVA was performed to assess statistical significance. *P < 0.05 vs. PEG; †P < 0.05 vs. WP1066; #P < 0.05 vs. preinjection.
Fig. 9.
Inhibition of JAK-STAT3 signaling abolishes MSC-induced tissue growth factor levels. Soluble fractions of total hamstring extracts (A) and plasma samples (B) were assayed using rodent-specific HGF and VEGF ELISA kits (n = 4 per group). Tissue growth factor concentrations were expressed as nanograms per milligram soluble proteins. ANOVA was performed to assess statistical significance. *P < 0.05 vs. PEG; †P < 0.05 vs. WP1066; #P < 0.05 vs. MSC+WP1066.
DISCUSSION
Although it has increasingly been recognized that the trophic actions of MSCs underlie their therapeutic effects in various preclinical and clinical studies (4, 12, 47), the trophic factors responsible for tissue functional improvement remain largely undefined. We demonstrate that MSC IL-6-type cytokines through JAK-STAT3 signaling are involved in the tissue-healing mechanisms. The hallmark of the MSC cardiac repair mechanism lies in the synergistic trophic actions mediated initially by MSC IL-6-type cytokines and subsequently by the myriad of host tissue-derived growth factors, activating the myocardial prosurvival and myogenic pathways orchestrated by Akt, ERK, and JAK-STAT3.
The hamster heart failure model.
The δ-sarcoglycan-null strain of TO2 hamster exhibits cardiac dysfunction between 1 and 2 mo of age and develops dilated cardiomyopathy (DCM), which leads to congestive heart failure resembling that seen in many patients (13, 34, 50). The δ-sarcoglycan protein deficiency causes cell membrane fragility and permeability to Ca2+, inducing damages to myocytes, smooth muscle cells, and endothelial cells (20, 30, 49, 55). We (35) previously showed that cardiac dysfunction in TO2 hamsters exhibit presymptomatic and symptomatic stages associated with unique biochemical events, and a major pathological hallmark is oxidative stress injury caused by inflammation. Since the hamster DCM model displays successive and uniform phases of pathophysiological changes, it has been used in growth factor and pharmacological therapeutic studies (8, 22, 36, 51). Using this heart failure model, we recently demonstrated cardiac therapeutic effects of extracardiac MSCs, which are mediated by increased circulating growth factors, mobilization of bone marrow progenitor cells, attenuation of myocardial cell death and fibrosis, amplification of cardiac progenitor cells, and accelerated cardiomyogenesis and angiogenesis (54, 77).
MSC-mediated JAK-STAT3 signaling in the maintenance of muscle function.
JAK-STAT3 signaling is known to promote normal functioning of the skeletal muscle and cardiac muscle systems (15, 26, 27, 63). In particular, altered JAK-STAT3 signaling has been associated with end-stage DCM and myocardial aging (2, 28, 46). We demonstrate a role of MSC IL-6-type cytokines in cardiac repair through engagement of the skeletal muscle JAK-STAT3 axis. The cytokines activate skeletal muscle JAK-STAT3 signaling both in vitro and in vivo with induction of many STAT3 target genes, most notably HGF and VEGF. Functional relevance of this signaling cascade is revealed by attenuated growth factor expression and loss of cardiac therapeutic effect after gp130-JAK-STAT3 blockade. In agreement with our findings here, activation of JAK-STAT3 in skeletal myocytes was found to stimulate HGF and IGF-II expression (70). IL-6 produced by growing myofibers stimulates satellite cell proliferation and skeletal muscle hypertrophy in a STAT3-dependent manner (52), and similar findings have also been presented for LIF (57). Notably, although MSCs were delivered to the hamstrings in our therapeutic trials, the MSC trophic actions further activated STAT3 signaling in the myocardium, which likely plays a significant role in rescuing the failing hamster heart because mice harboring a heart-restricted knockout of gp130 or STAT3 develop heart failure (15, 16). At the cellular level, cardiomyocytes with STAT3 deletion produce significantly more TNF-α in response to lipopolysaccharide than normal cardiomyocytes, indicating crucial functions of STAT3 in cardiac resistance to inflammation and acute injury (19). Conversely, cardiac-specific overexpression of STAT3 protects the heart from hypoxia/reoxygenation-induced oxidative stress (39) and promotes myocardial vascular growth (43). Since MSCs abundantly produce IL-6-type cytokines, synergistic actions of these cytokines can efficiently activate the host JAK-STAT3 machinery for tissue repair.
Amplification of the host trophic factor network for tissue repair.
Among the many STAT3-regulated genes are the growth factors HGF, VEGF, and IGF-II (11, 70, 72), and these trophic factors were induced by MSCs in cultured C2C12 myocytes and after extracardiac MSC delivery. A feature of the growth factor network are cross talk mechanisms that enable induction and amplification of more than one growth factor by another (56, 66, 76). Coordinated induction of muscle HGF and VEGF as observed in our study is noteworthy because combined administrations of HGF and VEGF have been found to result in a much more robust angiogenic response than either growth factor alone (66, 75). Induced HGF and VEGF can further amplify each other through mutual activation (66, 73). This cross-activation mechanism may explain why intramuscular injection of VEGF or HGF alone also repairs the failing hamster heart (24, 76). Similarly, it has been demonstrated that myocardial expression of HGF, IGF, and VEGF were upregulated after intramyocardial stem cell implantation (6). These findings together illustrate the central roles of host tissue trophic factors in regenerative medicine.
Active skeletal muscle can improve mortality in heart patients (62) and prevent cognitive decline in the elderly (31). The extracardiac MSC injection regimen may be compared with exercise therapy, which is known to increase the release of beneficial factors (HGF, IGF, IL-6, VEGF, and NGF) from skeletal muscle and reduce circulating adiponectin levels in heart failure patients (5, 45, 63, 67, 74). The current work demonstrates that increased host tissue trophic factors are also evident in quadriceps, liver, and brain after the MSC therapy, suggesting a possible global physiological effect of this cell administration approach. Since the production of beneficial trophic factors can be aberrantly downregulated in human heart disease (1, 21), the extracardiac MSC delivery regimen through its engagement and activation of multiple host tissue trophic factors represents a clinically attractive and testable therapeutic strategy.
MSC-mediated JAK-STAT3 signaling in immunomodulation.
MSCs possess prominent immunomodulatory properties that can be explored for nonautologous or xenogeneic stem cell therapy (64). We have shown that human and porcine MSCs can be used for tissue repair in immunocompetent rodents without triggering adverse host tissue inflammation (53, 54, 77). The potent immunomodulatory effects of MSCs appear to nonspecifically target cells of the immune system, and some of the actions appear to be mediated through STAT3 signaling. For instance, MSC-derived IL-6 was found to inhibit the differentiation of bone marrow progenitors into mature dendritic cells (9), thus impairing their stimulatory effect on resting natural killer cells and compromising antigen presentation to T cells. MSC-derived IL-11 and LIF can exert anti-inflammatory actions because these cytokines through STAT3 signaling can inhibit inflammatory cytokine production (14). Indeed, we (53) and others (42, 71) have shown that the trophic actions of MSCs can decrease host production of inflammatory cytokines while inducing the release of anti-inflammatory cytokines. Along this line, we have found that cultured C2C12 myocytes express increased levels of IL-10 and IL-13 (anti-inflammatory cytokines) and decreased levels of TNF-α (inflammatory cytokine) in response to IL-6 (data not shown), suggesting that the MSC/IL-6-type cytokine circuit can potentially modulate the immune system through the paracrine action of muscle cells. Thus it is possible that other cells capable of actively producing multiple IL-6-type cytokines may similarly improve cardiac function of the TO2 hamster heart through host muscle JAK-STAT3 signaling on intramuscular injection.
In summary, we presented a therapeutic mechanism of extracardiac MSC-mediated cardiac repair through engagement of the skeletal muscle JAK-STAT3 axis. The trophic cascade initiated by skeletal muscle JAK-STAT3 signaling increased growth factor levels in multiple tissues, leading to elevated circulating growth factors such as HGF and VEGF. Synergistic actions of these trophic factors further activate the myocardial repair mechanisms orchestrated by the Akt, ERK, and JAK-STAT3 signaling pathways. The therapeutic regimen is minimally invasive, taking advantage of the versatile ability of skeletal muscle to produce and amplify beneficial growth factors and cytokines, most notably HGF and VEGF, in response to multiple MSC IL-6-type cytokines.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-84590.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Abraham D, Hofbauer R, Schafer R, Blumer R, Paulus P, Miksovsky A, Traxler H, Kocher A, Aharinejad S. Selective downregulation of VEGF-A(165), VEGF-R(1), and decreased capillary density in patients with dilative but not ischemic cardiomyopathy. Circ Res 87: 644–647, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, Schulz R. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res 102: 131–135, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Brunswig-Spickenheier B, Boche J, Westenfelder C, Peimann F, Gruber AD, Jaquet K, Krause K, Zustin J, Zander AR, Lange C. Limited immune-modulating activity of porcine mesenchymal stromal cells abolishes their protective efficacy in acute kidney injury. Stem Cells Dev 19: 719–729, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Caplan A. Why are MSCs therapeutic? New data: new insight. J Pathol 217: 318–324, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chae CH, Jung SL, An SH, Park BY, Wang SW, Cho IH, Cho JY, Kim HT. Treadmill exercise improves cognitive function and facilitates nerve growth factor signaling by activating mitogen-activated protein kinase/extracellular signal-regulated kinase1/2 in the streptozotocin-induced diabetic rat hippocampus. Neuroscience 164: 1665–1673, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med 204: 3257–3269, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darnell JE., Jr STATs and gene regulation. Science 277: 1630–1635, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Dixon IM, Ju H, Reid NL, Scammell-La Fleur T, Werner JP, Jasmin G. Cardiac collagen remodeling in the cardiomyopathic Syrian hamster and the effect of losartan. J Mol Cell Cardiol 29: 1837–1850, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noel D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 25: 2025–2032, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Ernst M, Jenkins BJ. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet 20: 23–32, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Funamoto M, Fujio Y, Kunisada K, Negoro S, Tone E, Osugi T, Hirota H, Izumi M, Yoshizaki K, Walsh K, Kishimoto T, Yamauchi-Takihara K. Signal transducer and activator of transcription 3 is required for glycoprotein 130-mediated induction of vascular endothelial growth factor in cardiac myocytes. J Biol Chem 275: 10561–10566, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103: 1204–1219, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goineau S, Pape D, Guillo P, Ramee MP, Bellissant E. Hemodynamic and histomorphometric characteristics of dilated cardiomyopathy of Syrian hamsters (Bio TO-2 strain). Can J Physiol Pharmacol 79: 329–337, 2001 [PubMed] [Google Scholar]
- 14.Hendriks JJ, Slaets H, Carmans S, de Vries HE, Dijkstra CD, Stinissen P, Hellings N. Leukemia inhibitory factor modulates production of inflammatory mediators and myelin phagocytosis by macrophages. J Neuroimmunol 204: 52–57, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Hilfiker-Kleiner D, Hilfiker A, Drexler H. Many good reasons to have STAT3 in the heart. Pharmacol Ther 107: 131–137, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J, Jr, Muller W, Chien KR. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell 97: 189–198, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Hughes RA, Sendtner M, Goldfarb M, Lindholm D, Thoenen H. Evidence that fibroblast growth factor 5 is a major muscle-derived survival factor for cultured spinal motoneurons. Neuron 10: 369–377, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, Kondo S, Priebe W, Kondo Y. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene 26: 2435–2444, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Jacoby JJ, Kalinowski A, Liu MG, Zhang SS, Gao Q, Chai GX, Ji L, Iwamoto Y, Li E, Schneider M, Russell KS, Fu XY. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci USA 100: 12929–12934, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato Y, Iwase M, Takagi K, Nishizawa T, Kanazawa H, Matsushita A, Umeda H, Izawa H, Noda A, Koike Y, Nagata K, Yokota M. Differential myolysis of myocardium and skeletal muscle in hamsters with dilated cardiomyopathy. Circ J 70: 1497–1502, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Kaye DM, Vaddadi G, Gruskin SL, Du XJ, Esler MD. Reduced myocardial nerve growth factor expression in human and experimental heart failure. Circ Res 86: E80–E84, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Khalife WI, Tang YD, Kuzman JA, Thomas TA, Anderson BE, Said S, Tille P, Schlenker EH, Gerdes AM. Treatment of subclinical hypothyroidism reverses ischemia and prevents myocyte loss and progressive LV dysfunction in hamsters with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 289: H2409–H2415, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, Halvorsen YD, Cheatham B, Storms RW, Gimble JM. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol 212: 702–709, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Komamura K, Tatsumi R, Miyazaki J, Matsumoto K, Yamato E, Nakamura T, Shimizu Y, Nakatani T, Kitamura S, Tomoike H, Kitakaze M, Kangawa K, Miyatake K. Treatment of dilated cardiomyopathy with electroporation of hepatocyte growth factor gene into skeletal muscle. Hypertension 44: 365–371, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Kositprapa C, Zhang B, Berger S, Canty JM, Lee TC. Calpain-mediated proteolytic cleavage of troponin I induced by hypoxia or metabolic inhibition in cultured neonatal cardiomyocytes. Mol Cell Biochem 214: 47–55, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Kunisada K, Negoro S, Tone E, Funamoto M, Osugi T, Yamada S, Okabe M, Kishimoto T, Yamauchi-Takihara K. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci USA 97: 315–319, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurdi M, Booz GW. Can the protective actions of JAK-STAT in the heart be exploited therapeutically? Parsing the regulation of interleukin-6-type cytokine signaling. J Cardiovasc Pharmacol 50: 126–141, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Lehrke S, Mazhari R, Durand DJ, Zheng M, Bedja D, Zimmet JM, Schuleri KH, Chi AS, Gabrielson KL, Hare JM. Aging impairs the beneficial effect of granulocyte colony-stimulating factor and stem cell factor on post-myocardial infarction remodeling. Circ Res 99: 553–560, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Lin H, Shabbir A, Molnar M, Yang J, Marion S, Canty JM, Lee T. Adenoviral expression of vascular endothelial growth factor splice variants differentially regulate bone marrow-derived mesenchymal stem cells. J Cell Physiol 216: 458–468, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Lipskaia L, Pinet C, Fromes Y, Hatem S, Cantaloube I, Coulombe A, Lompre AM. Mutation of delta-sarcoglycan is associated with Ca(2+) -dependent vascular remodeling in the Syrian hamster. Am J Pathol 171: 162–171, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol Neurobiol 30: 493–503, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDougald OA, Burant CF. The rapidly expanding family of adipokines. Cell Metab 6: 159–161, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Madden T, Kazerooni R, Myer J, Culotta K, Donato N, Johansen MJ, Kondo Y, Mack D, Priebe W. The preclinical pharmacology of WP 1066, a potent small molecule inhibitor of the JAK2/STAT3 pathway. In: AACR Meeting, Experimental and Molecular Therapeutics 40, Abstract #4853, 2006 [Google Scholar]
- 34.Minieri M, Fiaccavento R, Carosella L, Peruzzi G, Di Nardo P. The cardiomyopathic hamster as model of early myocardial aging. Mol Cell Biochem 198: 1–6, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Missihoun C, Zisa D, Shabbir A, Lin H, Lee T. Myocardial oxidative stress, osteogenic phenotype, and energy metabolism are differentially involved in the initiation and early progression of delta-sarcoglycan-null cardiomyopathy. Mol Cell Biochem 321: 45–52, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyata S, Takemura G, Kawase Y, Li Y, Okada H, Maruyama R, Ushikoshi H, Esaki M, Kanamori H, Li L, Misao Y, Tezuka A, Toyo-Oka T, Minatoguchi S, Fujiwara T, Fujiwara H. Autophagic cardiomyocyte death in cardiomyopathic hamsters and its prevention by granulocyte colony-stimulating factor. Am J Pathol 168: 386–397, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature 451: 919–928, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Nakamura T, Matsumoto K, Mizuno S, Sawa Y, Matsuda H, Nakamura T. Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. Am J Physiol Heart Circ Physiol 288: H2131–H2139, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, Osugi T, Izumi M, Oshima Y, Nakaoka Y, Hirota H, Kishimoto T, Yamauchi-Takihara K. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation 104: 979–981, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Novotny NM, Markel TA, Crisostomo PR, Meldrum DR. Differential IL-6 and VEGF secretion in adult and neonatal mesenchymal stem cells: role of NFkB. Cytokine 43: 215–219, 2008 [DOI] [PubMed] [Google Scholar]
- 41.O'Reilly C, McKay B, Phillips S, Tarnopolsky M, Parise G. Hepatocyte growth factor (HGF) and the satellite cell response following muscle lengthening contractions in humans. Muscle Nerve 38: 1434–1442, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 104: 11002–11007, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osugi T, Oshima Y, Fujio Y, Funamoto M, Yamashita A, Negoro S, Kunisada K, Izumi M, Nakaoka Y, Hirota H, Okabe M, Yamauchi-Takihara K, Kawase I, Kishimoto T. Cardiac-specific activation of signal transducer and activator of transcription 3 promotes vascular formation in the heart. J Biol Chem 277: 6676–6681, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Park KS, Kim YS, Kim JH, Choi B, Kim SH, Tan AH, Lee MS, Lee MK, Kwon CH, Joh JW, Kim SJ, Kim KW. Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation 89: 509–517, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88: 1379–1406, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Podewski EK, Hilfiker-Kleiner D, Hilfiker A, Morawietz H, Lichtenberg A, Wollert KC, Drexler H. Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation 107: 798–802, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin Pharmacol Ther 82: 241–243, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Rissanen TT, Vajanto I, Hiltunen MO, Rutanen J, Kettunen MI, Niemi M, Leppanen P, Turunen MP, Markkanen JE, Arve K, Alhava E, Kauppinen RA, Yla-Herttuala S. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol 160: 1393–1403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryoke T, Gu Y, Ikeda Y, Martone ME, Oh SS, Jeon ES, Knowlton KU, Ross J., Jr Apoptosis and oncosis in the early progression of left ventricular dysfunction in the cardiomyopathic hamster. Basic Res Cardiol 97: 65–75, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Sakamoto A, Ono K, Abe M, Jasmin G, Eki T, Murakami Y, Masaki T, Toyo-Oka T, Hanaoka F. Both hypertrophic and dilated cardiomyopathies are caused by mutation of the same gene, delta-sarcoglycan, in hamster: an animal model of disrupted dystrophin-associated glycoprotein complex. Proc Natl Acad Sci USA 94: 13873–13878, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serose A, Prudhon B, Salmon A, Doyennette MA, Fiszman MY, Fromes Y. Administration of insulin-like growth factor-1 (IGF-1) improves both structure and function of delta-sarcoglycan deficient cardiac muscle in the hamster. Basic Res Cardiol 100: 161–170, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7: 33–44, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Shabbir A, Zisa D, Leiker M, Johnston C, Lin H, Lee T. Muscular dystrophy therapy by non-autologous mesenchymal stem cells: muscle regeneration without immunosuppression and inflammation. Transplantation 87: 1275–1282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a non-invasive therapeutic regimen. Am J Physiol Heart Circ Physiol 296: H1888–H1897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimizu T, Okamoto H, Watanabe M, Kumamoto H, Chiba S, Matsui Y, Sugawara T, Onozuka H, Mikami T, Kitabatake A. Altered microvasculature is involved in remodeling processes in cardiomyopathic hamsters. Jpn Heart J 44: 111–126, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Slomiany MG, Black LA, Kibbey MM, Day TA, Rosenzweig SA. IGF-1 induced vascular endothelial growth factor secretion in head and neck squamous cell carcinoma. Biochem Biophys Res Commun 342: 851–858, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Spangenburg EE, Booth FW. Multiple signaling pathways mediate LIF-induced skeletal muscle satellite cell proliferation. Am J Physiol Cell Physiol 283: C204–C211, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Suetta C, Clemmensen C, Andersen JL, Magnusson SP, Schjerling P, Kjaer M. Coordinated increase in skeletal muscle fiber area and expression of IGF-I with resistance exercise in elderly post-operative patients. Growth Horm IGF Res 20: 134–140, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Suzuki G, Lee T, Fallavollita JA, Canty JM., Jr Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circ Res 96: 767–775, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol 194: 114–128, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Testa U, Pannitteri G, Condorelli GL. Vascular endothelial growth factors in cardiovascular medicine. J Cardiovasc Med (Hagerstown) 9: 1190–1221, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. American Heart Association Council on Clinical Cardiology Subcommittee on Exercise, Rehabilitation, and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism Subcommittee on Physical Activity. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 107: 3109–3116, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Trenerry MK, Carey KA, Ward AC, Cameron-Smith D. STAT3 signaling is activated in human skeletal muscle following acute resistance exercise. J Appl Physiol 102: 1483–1489, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Tyndall A, Walker UA, Cope A, Dazzi F, De Bari C, Fibbe W, Guiducci S, Jones S, Jorgensen C, Le Blanc K, Luyten F, McGonagle D, Martin I, Bocelli-Tyndall C, Pennesi G, Pistoia V, Pitzalis C, Uccelli A, Wulffraat N, Feldmann M. Immunomodulatory properties of mesenchymal stem cells: a review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK, 31 October 2005. Arthritis Res Ther 9: 301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Jr, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol 205: 194–201, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Van Belle E, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R, Isner JM. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation 97: 381–390, 1998 [DOI] [PubMed] [Google Scholar]
- 67.Van Berendoncks AM, Beckers P, Hoymans VY, Possemiers N, Wuytss FL, Vrints CJ, Conraads VM. Exercise training reduces circulating adiponectin levels in patients with chronic heart failure. Clin Sci (Lond) 118: 281–289, 2010 [DOI] [PubMed] [Google Scholar]
- 68.Van Berendoncks AM, Garnier A, Beckers P, Hoymans VY, Possemiers N, Fortin D, Martinet W, Van Hoof V, Vrints CJ, Ventura-Clapier R, Conraads VM. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail 3: 185–194, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Walsh K. Adipokines, myokines and cardiovascular disease. Circ J 73: 13–18, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Wang K, Wang C, Xiao F, Wang H, Wu Z. JAK2/STAT2/STAT3 are required for myogenic differentiation. J Biol Chem 283: 34029–34036, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wehner R, Wehrum D, Bornhauser M, Zhao S, Schakel K, Bachmann MP, Platzbecker U, Ehninger G, Rieber EP, Schmitz M. Mesenchymal stem cells efficiently inhibit the proinflammatory properties of 6-sulfo LacNAc dendritic cells. Haematologica 94: 1151–1156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wojcik EJ, Sharifpoor S, Miller NA, Wright TG, Watering R, Tremblay EA, Swan K, Mueller CR, Elliott BE. A novel activating function of c-Src and Stat3 on HGF transcription in mammary carcinoma cells. Oncogene 25: 2773–2784, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Wojta J, Kaun C, Breuss JM, Koshelnick Y, Beckmann R, Hattey E, Mildner M, Weninger W, Nakamura T, Tschachler E, Binder BR. Hepatocyte growth factor increases expression of vascular endothelial growth factor and plasminogen activator inhibitor-1 in human keratinocytes and the vascular endothelial growth factor receptor flk-1 in human endothelial cells. Lab Invest 79: 427–438, 1999 [PubMed] [Google Scholar]
- 74.Wu G, Rana JS, Wykrzykowska J, Du Z, Ke Q, Kang P, Li J, Laham RJ. Exercise-induced expression of VEGF and salvation of myocardium in the early stage of myocardial infarction. Am J Physiol Heart Circ Physiol 296: H389–H395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, Schwall R, Ferrara N, Gerritsen ME. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol 158: 1111–1120, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zisa D, Shabbir A, Mastri M, Suzuki G, Lee T. Intramuscular VEGF repairs the failing heart: role of host-derived growth factors and mobilization of progenitor cells. Am J Physiol Regul Integr Comp Physiol 297: R1503–R1515, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zisa D, Shabbir A, Suzuki G, Lee T. Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair. Biochem Biophys Res Commun 390: 834–838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]