Abstract
The Ca2+-sensitive nuclear factor of activated T cell (NFAT) transcription factors are implicated in cardiac development and cellular remodeling associated with cardiac disease. In adult myocytes it is not resolved what specific Ca2+ signals control the activity of different NFAT isoforms in an environment that undergoes large changes of intracellular Ca2+ concentration with every heart beat. Cardiac myocytes possess the complete inositol 1,4,5-trisphosphate (IP3)/Ca2+-signaling cassette; however, its physiological and pathological significance has been a matter of ongoing debate. Therefore, we tested the hypothesis whether IP3 receptor activation regulates NFAT activity in cardiac myocytes. We used confocal microscopy to quantify the nuclear localization of NFATc1-green fluorescent protein (GFP) and NFATc3-GFP fusion proteins (quantified as the ratio of nuclear NFAT to cytoplasmic NFAT) in response to stimulation with neurohumoral agonists. In rabbit atrial myocytes, an overnight stimulation with endothelin-1, angiotensin II, and phenylephrine induced nuclear accumulation of NFATc1 that was sensitive to calcineurin inhibitors (cyclosporin A or inhibitor of NFAT-calcineurin association-6) and prevented by the IP3 receptor inhibitor 2-aminoethoxydiphenyl borate. Furthermore, a direct elevation of intracellular IP3 with a cell-permeable IP3 acetoxymethyl ester (10 μM) induced nuclear localization of NFATc1. With a fluorescence-based in vivo assay, we showed that endothelin-1 also enhanced the transcriptional activity of NFATc1 in atrial cells. The agonists failed to activate NFATc1 in rabbit ventricular cells, which express IP3 receptors at a lower density than atrial cells. They also did not activate NFATc3, an isoform that is highly influenced by nuclear export processes, in both cell types. Our data show that the second messenger IP3 is directly involved in the activation of NFATc1 in adult atrial cardiomyocytes.
Keywords: nuclear factor of activated T cells; adult myocytes; inositol 1,4,5-trisphosphate; endothelin-1
nuclear factor of activated T cell (NFAT) transcription factors play a key role in the cardiac development and cellular remodeling associated with cardiac disease. Four Ca2+-sensitive NFAT isoforms are expressed in mammalian hearts (NFATc1 to NFATc4) that are activated by the Ca2+/calmodulin-dependent phosphatase calcineurin (CaN). The dephosphorylation of NFAT induces an accumulation of active NFAT in the nucleus and transcriptional activity. The rephosphorylation by cellular kinases (e.g., p38, JNK2, and GSK3) inactivates NFAT and initiates export back to the cytoplasm (25, 33). In addition to these enzymatic activities, the precise regulation of NFAT in muscle cells also depends on NFAT isoform, cell and tissue type (28, 29).
Several Ca2+-dependent mechanisms have been hypothesized to selectively activate CaN and dephosphorylate NFAT in the heart; however, the exact Ca2+ source, the spatiotemporal organization of the relevant Ca2+ signal, and the signaling domain are unknown. Because cardiac myocytes physiologically display large rhythmic changes in intracellular Ca2+ concentration ([Ca2+]i) with every heart beat, this raises the intriguing question of how NFAT can be activated in a Ca2+-dependent fashion (or why NFAT can remain inactive), given the continuous physiological Ca2+ oscillations in normal and diseased hearts. Candidates for NFAT activation are Ca2+ fluxes through voltage-gated L-type Ca2+ channels and Ca2+-induced Ca2+ release from ryanodine receptor (RyR) Ca2+ release channels, Ca2+ entry via T-type Ca2+ channels, store-operated Ca2+ entry, Ca2+-permeable transient receptor potential channels, and inositol 1,4,5-trisphosphate (IP3)-dependent Ca2+ release (32, 34).
IP3 is a ubiquitous intracellular messenger that induces Ca2+ release from endogenous stores through IP3 receptors (IP3Rs). Neurohumoral agonists [e.g., angiotensin II (ANG II), endothelin-1 (ET-1), and phenylephrine (Phe)] stimulate Gq protein-coupled receptors, a pathway that has been suggested to activate NFAT in cardiac myocytes (19, 21). Gq proteins stimulate the enzyme phospholipase C, which generates IP3 and diacylglycerol. Cardiac excitation-contraction coupling relies largely on RyR-induced Ca2+-induced Ca2+ release from the sarcoplasmic reticulum (SR), and myocytes express a significantly larger number of RyRs compared with IP3Rs. Furthermore, IP3Rs are expressed at a higher density in atrial than in ventricular tissue but are upregulated in heart failure. The role of IP3 and IP3-mediated Ca2+ signaling in the heart has long been enigmatic (cf. 37). Nonetheless, the strategic localization of IP3Rs in cytoplasmic compartments and the nucleus enables them to participate in subsarcolemmal, bulk cytoplasmic, and nuclear Ca2+ signaling. IP3R activation leads to changes in basal (diastolic) [Ca2+]i, positive inotropic effects, but also in proarrhythmic Ca2+ release (17, 38). The nuclear envelope, a Ca2+-storing membrane system that is contiguous with the SR, contains significant densities of IP3R that allow Ca2+ release into the nucleoplasm (37), suggesting control of nuclear functions. Prime candidates for targets of nuclear IP3R-dependent Ca2+ signals are Ca2+-dependent transcription factors (e.g., NFAT and histone deacetylase), thus suggesting a key role in excitation-transcription coupling. For example, there is evidence that ET-1 affects cytoplasmic and nuclear Ca2+, activates the CaN/NFAT pathway, and induces hypertrophy in ventricular myocytes (13), and we have recently shown that an overexpression of IP3Rs enhanced cardiac hypertrophy downstream of G protein-coupled receptor signaling, partially through a CaN-dependent mechanism (22).
Here we tested the hypothesis whether IP3 itself is involved in the activation of NFAT in atrial and ventricular myocytes from rabbits. We analyzed the subcellular localization of NFATc1-green fluorescent protein (GFP) and NFATc3-GFP fusion proteins in response to neurohumoral agonists and direct administration of IP3 and quantified it as the ratio of nuclear NFAT to cytoplasmic NFAT (NFATNuc/NFATCyt). We present direct evidence that IP3 induced nuclear accumulation of NFATc1 followed by increased NFAT-dependent transcriptional activity in atrial myocytes but not in ventricular cells. In contrast, the same agonists failed to significantly activate NFATc3 in rabbit atrial and ventricular myocytes. Taken together, we demonstrate that IP3-dependent Ca2+ release directly activates NFATc1 in adult atrial myocytes.
MATERIALS AND METHODS
Isolation, cell culture, and viral transduction of cardiac myocytes.
Atrial and ventricular myocytes from New Zealand white rabbits were isolated as previously described (6). The procedure was approved by the Institutional Animal Care and Use Committee. Briefly, animals of either sex were anesthetized and hearts were removed quickly and mounted on a Langendorff perfusion apparatus. The heart was then retrogradely perfused via the aorta, and single myocytes were obtained after perfusion with a Liberase Blendzyme 4 (Roche Applied Science, Indianapolis, IN) containing solution. Ca2+-tolerant myocytes were obtained by slow adaptation to the final Ca2+ concentration of the culture medium, and the cells were cultured on sterile, laminin-coated glass coverlips in medium 199, supplemented with 25 μg/ml gentamycin and 25 μg/ml kanamycin (all Mediatech, Hernon, VA). The cells were infected with adenoviruses (Ad) for NFATc1-GFP or NFATc3-GFP (28) and were imaged 48 h after infection. NFAT-GFP fusion proteins are widely used to study NFAT in living cells because they behave similarly to endogenous proteins (4, 9, 14, 18, 26, 28, 29).
Solutions and chemicals.
During the experiments, the cells were bathed in a HBSS containing (in mM) 135 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES (pH = 7.3 with NaOH). Agonists or antagonists were prepared in HBSS for acute experiments or added to the cell culture medium for overnight incubations. All chemicals, agonists, or inhibitors were purchased from Sigma (St. Louis, MO) or Tocris (Ellisville, MO). The cell-permeable IP3 acetoxymethyl ester (IP3-AM) was from SiChem (Bremen, Germany).
Fluorescence measurements of NFAT-GFP.
The subcellular localization of NFAT-GFP was analyzed with confocal microscopy (Bio-Rad Radiance 2000/MP). GFP was excited with an argon ion laser line at 488 nm, and emitted fluorescence was collected at 500–520 nm. The subcellular distribution of NFAT-GFP was quantified as NFATNuc/NFATCyt (26, 28) using a region of interest (ROI) that covered the area of the nucleus (NFATNuc) and a cytoplasmic ROI (NFATCyt) of the same size (number of pixels). Images were background subtracted, and the mean fluorescence of a particular ROI was analyzed using ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, MD).
Staining of cell nuclei with SYTO-59.
To identify nuclei, cardiomyocytes were stained for 30 min with 5 μM of the membrane-permeable DNA dye SYTO-59 (Invitrogen/Molecular Probes, Carlsbad, CA). For confocal imaging, the cells were excited at 637 nm (red diode laser) and emitted fluorescence was collected at λ > 660 nm. The colocalization of SYTO-59 and NFAT-GFP was used to confirm the nuclear localization of NFAT (26).
In vivo assay for transcriptional activity of NFAT.
To monitor the transcriptional activity of NFAT in living cells, atrial myocytes were double infected with Ad-NFAT-red fluorescent protein (RFP) (27), which expresses RFP under control of a NFAT-sensitive interleukin-2 promoter, and with Ad-NFATc1-GFP. After infections (24 h), a subset of cells was stimulated with 100 nM ET-1 overnight and red fluorescence was measured on the next day. RFP was excited at 535 nm (green He-Ne laser line), and emitted fluorescence was collected at λ > 570 nm. NFAT-GFP was imaged as described in Fluorescence measurements of NFAT-GFP. To avoid the detection of GFP emission in the RFP channel, GFP and RFP images were taken sequentially, avoiding simultaneous excitation. The mean fluorescence of RFP was averaged over the entire surface area of the cell.
Data analysis and presentation.
Data are presented as individual observations or as means ± SE and were analyzed using Student's t-test; n represents the number of individual cells, and differences were considered significant at P < 0.05.
RESULTS
Basal localization of NFATc1 and NFATc3 in resting myocytes.
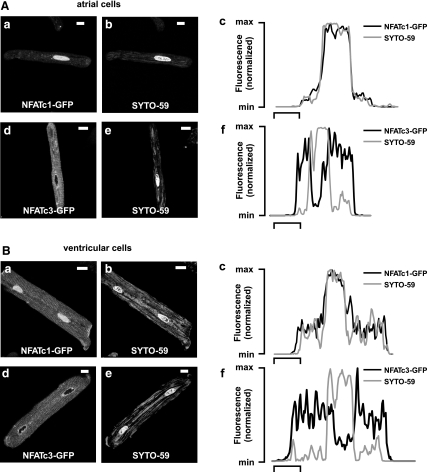
Subcellular localizations of NFATc1- and NFATc3-GFP fusion proteins were analyzed with confocal microscopy 48 h after infections. The isoform NFATc1 was localized to the nucleus in resting atrial (Fig. 1A,a) and ventricular (Fig. 1B,a) myocytes. The corresponding nuclei were stained with SYTO-59 in atrial (Fig. 1A,b) and ventricular (Fig. 1B,b) myocytes. The average NFATNuc/NFATCyt ratios were 9.82 ± 0.36 (n = 234) for atrial myocytes and 9.08 ± 0.33 (n = 126) for ventricular myocytes. The line profiles of the raw fluorescence intensities of NFATc1-GFP and SYTO-59 across the nucleus overlap, confirming localization to the same cellular compartment in atrial (Fig. 1A,c) and ventricular (Fig. 1B,c) myocytes. In contrast, the isoform NFATc3-GFP was distributed to the cytoplasm of atrial (Fig. 1A,d) and ventricular (Fig. 1B,d) myocytes, indicated by different staining of the corresponding nuclei (atria, Fig. 1A,e; and ventricle, Fig. 1B,e) and not overlapping fluorescence intensity profiles (atrial, Fig. 1A,f; and ventricle, Fig. 1B,f). The average NFATNuc/NFATCyt ratios were 0.34 ± 0.03 (n = 66) for atrial myocytes and 0.59 ± 0.05 (n = 23) for ventricular myocytes. The basal nuclear localization of NFATc1 is consistent with our recent data from adult cat myocytes (28). The cytoplasmic distribution of NFATc3 is due to the enhanced regulation of this isoform by nuclear export processes (26, 29).
Fig. 1.
Subcellular distribution of Ca2+-sensitive nuclear factor of activated T cell isoforms c1 and c3 (NFATc1 and NFATc3) in adult myocytes from rabbit. The isoform NFATc1 displayed nuclear localization in resting atrial (A,a) and ventricular (B,a) myocytes. A,b and B,b: corresponding cell nuclei were identified by staining with the cell-permeable DNA dye SYTO-59. A,c and B,c: fluorescence intensity profiles of NFATc1-green fluorescent protein (GFP) and SYTO-59 fluorescence across the nucleus and adjacent cytoplasmic regions, normalized to maximum fluorescence. In contrast to NFATc1, the isoform NFATc3 was distributed to the cytoplasm of atrial (A,d) and ventricular (B,d) myocytes. A,e and B,e: SYTO-59 staining of the cell nuclei. Corresponding NFATc3-GFP and SYTO-59 fluorescence intensity profiles for atrial (A,f) and ventricular (B,f) myocytes are shown. For images, scale bar = 10 μm. For line profiles, scale bar = 5 μm.
We then investigated how the stimulation of the Gq protein/IP3 pathway influences the nuclear accumulation of NFAT in adult myocytes.
Gq protein-coupled agonists induced nuclear accumulation and transcriptional activity of NFATc1.
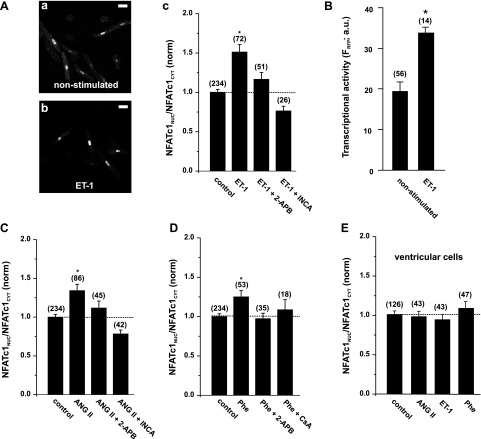
Neurohumoral agonists (e.g., ET-1) have been linked to the activation of the CaN/NFAT pathway and to inducing hypertrophy (13, 35). To test whether NFATc1 is regulated by the phospholipase C/IP3 pathway, we stimulated atrial myocytes expressing NFATc1-GFP with the agonists ET-1 (100 nM, Fig. 2A), ANG II (2 μM, Fig. 2C), or Phe (10 μM, Fig. 2D) overnight and analyzed the nuclear localization of NFATc1 with confocal microscopy. All agonists induced the enhanced nuclear accumulation of NFATc1, indicated by increases of NFATNuc/NFATCyt ratios between 25 and 50%, depending on the agonist used (Fig. 2, A–D). This effect was prevented by 2 μM 2-aminoethoxydiphenyl borate (2-APB), which inhibits cardiac SR IP3R Ca2+ release channels at this concentration (7). The activation of NFATc1 was also sensitive to the inhibition of CaN with 1 μM inhibitor of NFAT-CaN association-6 or 1 μM cyclosporine A (Fig. 2, A–D).
Fig. 2.
Neurohumoral agonists induce nuclear accumulation and transcriptional activity of NFATc1 in atrial (A–D) and ventricular (E) cells. Overnight stimulation with 100 nM endothelin-1 (ET-1) resulted in an increase in nuclear-localized NFATc1 (A,b compared with nonstimulated cells in A,a) by 50% [ratio of nuclear NFAT to cytoplasmic NFAT (NFATNuc/NFATCyt); A,c]. This effect was prevented by the inositol 1,4,5-trisphosphate (IP3) receptor blocker 2-aminoethoxydiphenyl borate (2-APB; 2 μM) and was sensitive to the calcineurin (CaN) inhibitor of NFAT-CaN association-6 (INCA-6; 1 μM). B: In atrial myocytes expressing NFATc1-GFP, stimulation with ET-1 resulted in higher transcriptional activity, measured with NFAT-sensitive expression of the red fluorescent protein (RFP) (NFAT-RFP reporter; see text for details). FRFP, fluorescence of RFP [in arbitrary units (AU)]. C and D: increases in nuclear localization of NFATc1 were also induced by ANG II (2 μM) and phenylephrine (Phe; 10 μM) and were prevented by 2-APB and CaN inhibition. CsA, cyclosporin A. E: all agonists failed to induce activation of NFATc1 in ventricular cells. Numbers in parentheses indicate the number of individual cells tested. NFATNuc/NFATCyt ratios are normalized (norm) to control. *P < 0.05, significantly different from control. Scale bar = 30 μm.
We further tested the hypothesis whether ET-1 stimulation induced not only the nuclear accumulation of NFATc1 but also NFAT-regulated transcriptional activity (Fig. 2B). We measured transcriptional activity of NFATc1-GFP with NFAT-RFP, a tool that allows analyzing transcriptional activity of NFAT dynamically in individual living cells (27). Atrial myocytes were coinfected with NFATc1-GFP and NFAT-RFP. Indeed, overnight stimulation with ET-1 induced a 1.5-fold increase in RFP fluorescence (Fig. 2B). The expression of NFAT-RFP alone did not result in a comparable RFP fluorescence intensity (data not shown).
In ventricular myocytes, where SR IP3Rs are expressed at a lower density compared with atrial tissue, all agonists tested did not induce the nuclear accumulation of NFATc1 (Fig. 2E). These data suggest that IP3 is directly involved in the activation of NFATc1 only in atrial tissue.
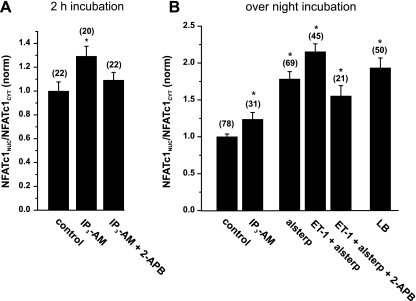
To test this hypothesis, we incubated atrial cells with 10 μM of a membrane-permeable IP3-AM for 2 h and analyzed the nuclear localization of NFATc1-GFP. Indeed, the direct administration of IP3 resulted in an enhanced nuclear localization of NFATc1, which was sensitive to 2-APB (Fig. 3A). Overnight incubations with IP3-AM induced a nuclear accumulation that was comparable to agonist stimulations (∼25% increase in NFATNuc/NFATCyt ratio; Fig. 3B, left). We have previously demonstrated that nuclear localization of NFAT in cardiac myocytes is negatively regulated by cellular kinases and nuclear export processes (28). To test whether this pathway also influences NFATc1, we incubated atrial cells with 1 μM alsterpaullone (inhibitor of glycogen synthase kinase 3), which resulted in a 75% increase in the nuclear localization of NFATc1. This effect was further facilitated by ET-1 in a 2-APB-sensitive manner (Fig. 3B, middle). This additive effect was in the range of nuclear accumulation observed under the conditions of a full inhibition of nuclear export with 40 nM leptomycin B (Fig. 3B, right). These data suggest that the nuclear localization of NFATc1 is influenced by export pathways. IP3 activates the nuclear import machinery for NFATc1 to overcome these export rates.
Fig. 3.
Activation of NFATc1 is directly mediated by IP3 in atrial myocytes. A: short-term incubation with the membrane-permeable IP3 acetoxymethyl ester (IP3-AM; 10 μM, 2 h) resulted in nuclear accumulation of NFATc1, which was sensitive to 2-APB (2 μM). B: IP3 induced activation of NFAT in overnight incubations. Inhibition of GSK3 with 1 μM alsterpaullone (Alsterp) resulted in robust nuclear accumulation of NFATc1, which was further enhanced by ET-1 in a 2-APB-sensitive manner (middle). B, right: nuclear accumulation of NFATc1 after full inhibition of nuclear export with 40 nM leptomycin B (LB). Numbers in parentheses indicate the number of individual cells tested. NFATNuc/NFATCyt ratios are normalized to control. *P < 0.05, significantly different from control.
NFATc3-GFP was not activated by the Gq/IP3 pathway.
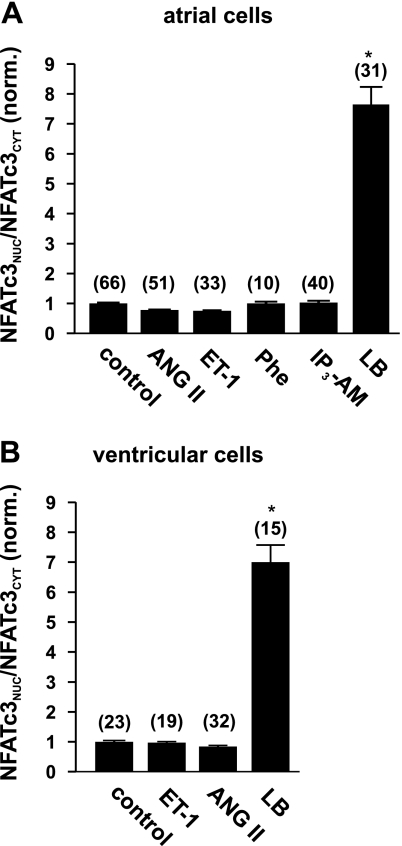
In contrast to NFATc1, the isoform NFATc3 was not activated by the Gq protein-coupled agonists ET-1, ANG II, and Phe or by IP3-AM, neither in atrial (Fig. 4A) nor in ventricular (Fig. 4B) cells. However, the inhibition of nuclear export with 40 nM leptomycin B resulted in a strong nuclear localization of NFATc3 in both types of myocytes. This result indicates that NFATc3 is accessible for nuclear import in rabbit myocytes but also confirms the observation that nuclear localization of NFATc3 is prevented and highly controlled by export mechanisms (10, 26).
Fig. 4.
NFATc3-GFP is not activated by neurohumoral agonists in rabbit myocytes. Incubation with the agonists ET-1 (100 nM), ANG II (2 μM), Phe (10 μM), or with IP3-AM (10 μM) overnight did not activate NFATc3 in rabbit atrial (A) or ventricular (B) myocytes. Full inhibition of nuclear export with LB (40 nM) did result in nuclear accumulation of NFATc3. Numbers in parentheses indicate the number of individual cells tested. NFATNuc/NFATCyt ratios are normalized to control. *P < 0.05, significantly different from control.
DISCUSSION
Transcription factors of the NFAT family are activated in cardiac myocytes during cardiac development and pathological cellular remodeling (24, 36). Although the CaN-dependent activation of NFAT and the underlying Ca2+ signals are well characterized in several excitable and nonexcitable cells (2, 11, 29, 33), it is not fully understood how a Ca2+ signal can activate NFAT in adult cardiac myocytes in the surroundings of the large and normal beat-to-beat Ca2+ fluctuations (1, 20). Here we demonstrate that IP3 is directly involved in the activation of NFATc1 in atrial myocytes. Three independent agonists (ET-1, ANG II, and Phe) enhanced the accumulation of NFATc1 in the nucleus (Fig. 2). Several lines of evidence support a direct involvement of IP3 in this process. 2-APB, an inhibitor of the SR IP3-dependent Ca2+ release channels (IP3Rs), prevented agonist-induced nuclear translocation of NFATc1. Furthermore, the direct application of IP3 in form of a cell-permeable IP3-AM induced the activation of NFAT c1 in atrial myocytes (Fig. 3). The same agonists did not activate NFATc1 in ventricular cells (Fig. 2E), an effect that may be explained by a lower density of IP3Rs in the ventricular SR membrane (7). In atrial cells, ET-1 not only induced a stronger nuclear localization of NFATc1-GFP but also enhanced the transcriptional activity of NFATc1. By measuring nuclear NFATc1-GFP and NFAT-sensitive expression of RFP simultaneously in living cells, we observed a 1.5-fold increase in RFP expression after overnight stimulation with ET-1 (Fig. 2B).
The precise Ca2+ signal by which IP3 activates NFAT remains to be clarified. Our previous work together with studies by others implicate at least three distinct mechanisms by which IP3 may influence intracellular Ca2+ signaling in cardiac myocytes. First, we showed that IP3-mediated Ca2+ release can act as a locally restricted Ca2+ source, e.g., around the nucleus. The nuclear envelope contains a significant amount of IP3Rs. IP3-mediated Ca2+ release from the nuclear envelope dominates over RyR-mediated Ca2+ release and controls nuclear Ca2+ concentration (15, 37). This is consistent with the demonstration that ET-1 induces intracellular Ca2+ elevations that affect nuclear Ca2+ concentration and activate NFAT in neonatal cardiac myocytes (13). Second, we demonstrated cytosolic IP3R-mediated Ca2+ release in the form of locally restricted Ca2+ puffs (38). Furthermore, IP3-mediated Ca2+ release modulates Ca2+ release through RyRs and modifies normal beat-to-beat Ca2+ by increasing diastolic [Ca2+]i and enhancing action potential-dependent Ca2+ transients but also by inducing arrythmogenic Ca2+ release (38). Importantly, CaN has been shown to be sensitive to sustained elevations of resting [Ca2+]i (5), and arrhythmogenic Ca2+ signals, experimentally induced by tachycardic pacing of myocytes or Ca2+ overload protocols (28, 30), are indeed capable of activating NFAT in adult myocytes. Third, as a canonical pathway for nonexcitable cells, IP3-mediated Ca2+ release triggers an influx of Ca2+ from the extracellular space (store-operated Ca2+ entry), which activates CaN and NFAT (8, 12, 26). A recent study elegantly demonstrated that this pathway is also implicated in maintaining hypertrophy in adult cardiac myocytes (34); channel members of the transient receptor potential channel family are upregulated, resulting in enhanced transcriptional activity of NFAT (16, 23). However, the isoform NFATc1 has not been implicated in cardiac hypertrophy [where NFATc3 plays a dominant role; (28, 31)], and evidence is lacking that store-operated Ca2+ entry is involved in the regulation of NFATc1 in the heart.
Aside from the differences in the IP3/Ca2+ dependence of the regulation of NFAT isoforms (c1 vs. c3) in different tissues (atrium vs. ventricle), a striking difference between isoforms c1 and c3 is their basal subcellular distribution. Whereas under basal resting conditions NFATc3 is predominantly localized to the cytosol, NFATc1 resides in the nucleus. Furthermore, as shown here, in atrial myocytes the extent of nuclear localization is modulated by IP3/Ca2+ signaling. While NFATc3 has been undoubtedly linked to cardiac hypertrophy [e.g., NFATc3-deficient mice have a reduced ability to develop CaN-dependent hypertrophy (31, 32)] and heart failure, evidence is lacking that NFATc1 plays a leading role in this disease process. In contrast to the c3 isoform, NFATc1 plays a dominant role in cardiac development. Cardiac NFATc1 controls valve formation and morphogenesis of the heart, and a disruption of this isoform results in lethal defects (3). Thus the basal activity of NFATc1 in cardiac myocytes is likely to be required to maintain the differentiated phenotype of adult myocytes. Along this line, if the maintenance of a differentiated phenotype is controlled by NFATc1 activity, a basal nuclear localization seems prerequisite for a continuous transcriptional activity. It may be further speculated that circulating neurohumoral agonists exert a modulatory control function of NFATc1 transcriptional activity via the IP3/Ca2+ signaling cascade. In contrast, robust and efficient nuclear extrusion mechanisms are required for the regulation of transcription factors (such as NFATc3 in this study; cf. Fig. 4) that remain quiescent in normal cardiac tissue and only become active in a disease state such as pathological cardiac hypertrophy. As we have shown previously (28), NFATc3 reveals an enhanced nuclear localization in myocytes from failing hearts, supporting the notion that NFATc3 (but not c1) is implicated in hypertrophic remodeling.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-62231 and HL-80101 (to L. A. Blatter) and the American Heart Association Grant 0820080Z (to A. Rinne).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell 109, Suppl: S67–S79, 2002 [DOI] [PubMed] [Google Scholar]
- 3.de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E, Potter J, Wakeham A, Marengere L, Langille BL, Crabtree GR, Mak TW. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 392: 182–186, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Dean DA, Urban G, Aragon IV, Swingle M, Miller B, Rusconi S, Bueno M, Dean NM, Honkanen RE. Serine/threonine protein phosphatase 5 (PP5) participates in the regulation of glucocorticoid receptor nucleocytoplasmic shuttling. BMC Cell Biol 2: 6, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386: 855–858, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Domeier TL, Blatter LA, Zima AV. Alteration of sarcoplasmic reticulum Ca2+ release termination by ryanodine receptor sensitization and in heart failure. J Physiol 587: 5197–5209, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA. IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol 294: H596–H604, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald DJ, Burgoyne RD, Haynes LP. Neuronal calcium sensor proteins are unable to modulate NFAT activation in mammalian cells. Biochim Biophys Acta 1780: 240–248, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez MF, Bosc LV, Stevenson AS, Wilkerson MK, Hill-Eubanks DC, Nelson MT. Constitutively elevated nuclear export activity opposes Ca2+-dependent NFATc3 nuclear accumulation in vascular smooth muscle: role of JNK2 and Crm-1. J Biol Chem 278: 46847–46853, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Graef IA, Chen F, Crabtree GR. NFAT signaling in vertebrate development. Curr Opin Genet Dev 11: 505–512, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium 42: 145–156, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Higazi DR, Fearnley CJ, Drawnel FM, Talasila A, Corps EM, Ritter O, McDonald F, Mikoshiba K, Bootman MD, Roderick HL. Endothelin-1-stimulated InsP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol Cell 33: 472–482, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Kehlenbach RH, Dickmanns A, Gerace L. Nucleocytoplasmic shuttling factors including Ran and CRM1 mediate nuclear export of NFAT In vitro. J Cell Biol 141: 863–874, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kockskamper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol 45: 128–147, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 116: 3114–3126, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate (IP3)-receptor type 2-deficient mice. Circ Res 96: 1274–1281, 2005 [DOI] [PubMed] [Google Scholar]
- 18.MacDonnell SM, Weisser-Thomas J, Kubo H, Hanscome M, Liu Q, Jaleel N, Berretta R, Chen X, Brown JH, Sabri AK, Molkentin JD, Houser SR. CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes. Circ Res 105: 316–325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mende U, Kagen A, Cohen A, Aramburu J, Schoen FJ, Neer EJ. Transient cardiac expression of constitutively active Galphaq leads to hypertrophy and dilated cardiomyopathy by calcineurin-dependent and independent pathways. Proc Natl Acad Sci USA 95: 13893–13898, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molkentin JD. Dichotomy of Ca2+ in the heart: contraction versus intracellular signaling. J Clin Invest 116: 623–626, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama H, Bodi I, Maillet M, DeSantiago J, Domeier TL, Mikoshiba K, Lorenz JN, Blatter LA, Bers DM, Molkentin JD. The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circ Res 107: 659–666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, Mori Y, Ono K, Iijima T, Ito H. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol 42: 498–507, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Qi XY, Yeh YH, Xiao L, Burstein B, Maguy A, Chartier D, Villeneuve LR, Brundel BJ, Dobrev D, Nattel S. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ Res 103: 845–854, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Rao A. Signaling to gene expression: calcium, calcineurin and NFAT. Nat Immunol 10: 3–5, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Rinne A, Banach K, Blatter LA. Regulation of nuclear factor of activated T cells (NFAT) in vascular endothelial cells. J Mol Cell Cardiol 47: 400–410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rinne A, Blatter LA. A fluorescence-based assay to monitor transcriptional activity of NFAT in living cells. J Physiol 588: 3211–3216, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinne A, Kapur N, Molkentin JD, Pogwizd SM, Bers DM, Banach K, Blatter LA. Isoform- and tissue-specific regulation of the Ca2+-sensitive transcription factor NFAT in cardiac myocytes and in heart failure. Am J Physiol Heart Circ Physiol 298: H2001–H2009, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen T, Liu Y, Cseresnyes Z, Hawkins A, Randall WR, Schneider MF. Activity- and calcineurin-independent nuclear shuttling of NFATc1, but not NFATc3, in adult skeletal muscle fibers. Mol Biol Cell 17: 1570–1582, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voigt N, Maguy A, Yeh YH, Qi X, Ravens U, Dobrev D, Nattel S. Changes in IK,ACh single-channel activity with atrial tachycardia remodelling in canine atrial cardiomyocytes. Cardiovasc Res 77: 35–43, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Wilkins BJ, Molkentin JD. Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J Physiol 541: 1–8, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun 322: 1178–1191, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Wu H, Peisley A, Graef IA, Crabtree GR. NFAT signaling and the invention of vertebrates. Trends Cell Biol 17: 251–260, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci USA 107: 7000–7005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest 116: 675–682, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao L, Coutu P, Villeneuve LR, Tadevosyan A, Maguy A, Le Bouter S, Allen BG, Nattel S. Mechanisms underlying rate-dependent remodeling of transient outward potassium current in canine ventricular myocytes. Circ Res 103: 733–742, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Zima AV, Bare DJ, Mignery GA, Blatter LA. IP3-dependent nuclear Ca2+ signalling in the mammalian heart. J Physiol 584: 601–611, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol 555: 607–615, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]