Abstract
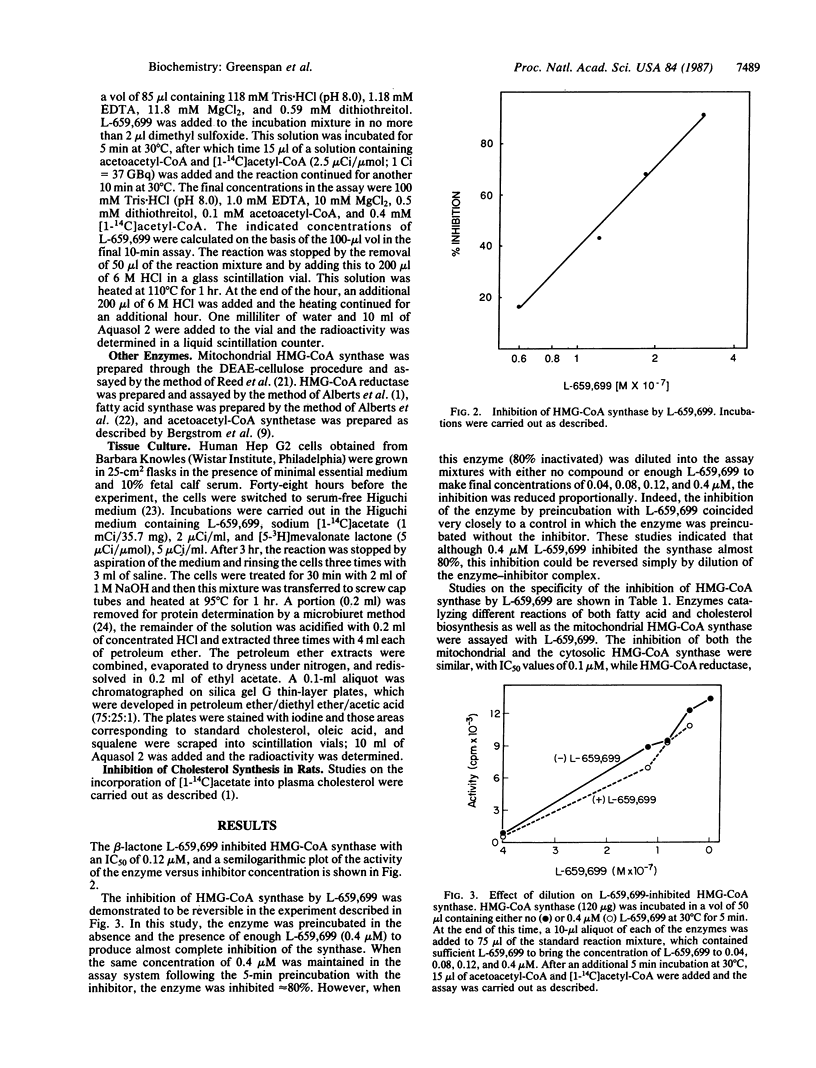
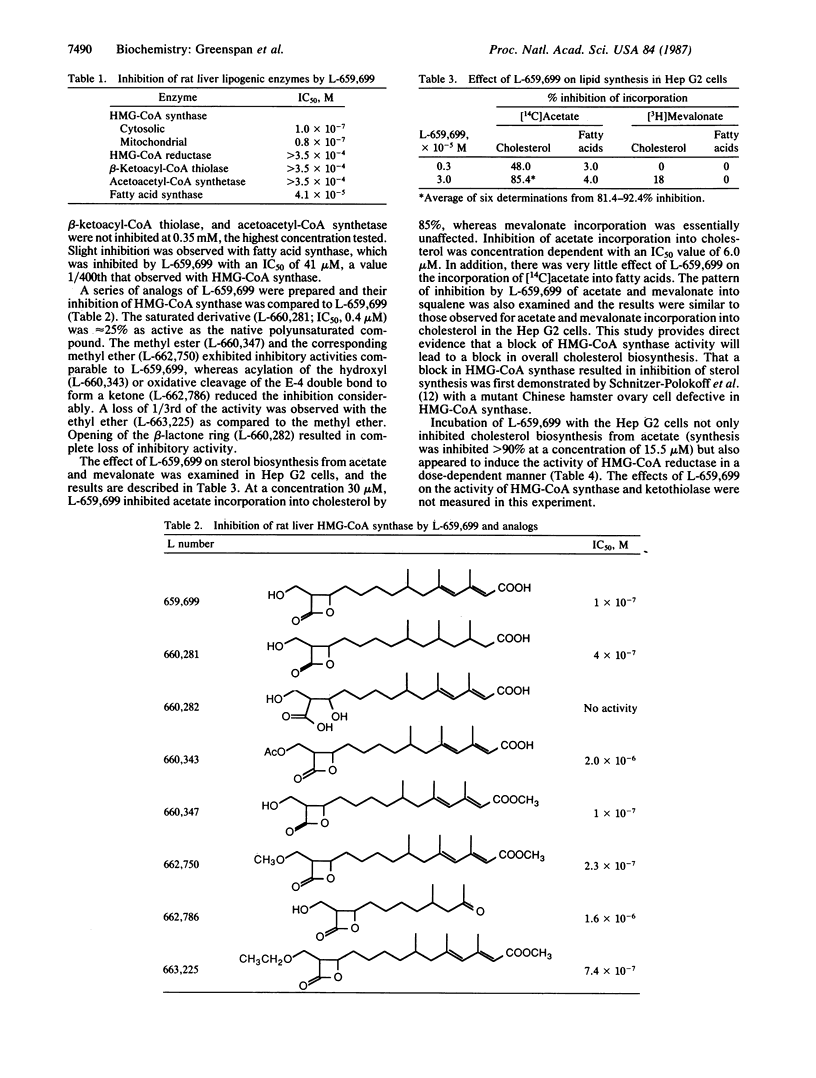
A beta-lactone isolated from Fusarium sp. has been shown to be a potent specific inhibitor of the enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) synthase [(S)-3-hydroxy-3-methylglutaryl-CoA acetoacetyl-CoA-lyase (CoA-acetylating), EC, 4.1.3.5] from rat liver. The structure of this beta-lactone, termed L-659,699, is (E,E)-11-[3-(hydroxy-methyl)-4-oxo-2-oxytanyl]-3,5,7-trimethyl-2,4 - undecadienenoic acid. A partially purified preparation of cytoplasmic HMG-CoA synthase from rat liver was inhibited by L-659,699 with an IC50 of 0.12 microM. The enzyme HMG-CoA reductase, beta-ketoacyl-CoA thiolase, acetoacetyl-CoA synthetase, and fatty acid synthase were not inhibited to any extent by this compound. In cultured Hep G2 cells, the compound inhibited the incorporation of [14C]acetate into sterols with an IC50 of 6 microM, while incorporation of [3H]mevalonate into sterols in these cells was not affected. The activity of HMG-CoA reductase in the cultured Hep G2 cells was induced in a dose-dependent manner by incubation with L-659,699. A 37-fold increase in reductase was observed after a 24-hr incubation with 62 microM L-659,699. The effect of a number of analogs of L-659,699 on HMG-CoA synthase is also discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts A. W., Ferguson K., Hennessy S., Vagelos P. R. Regulation of lipid synthesis in cultured animal cells. J Biol Chem. 1974 Aug 25;249(16):5241–5249. [PubMed] [Google Scholar]
- Aldridge D. C., Giles D., Turner W. B. Antibiotic 1233A: a fungal -lactone. J Chem Soc Perkin 1. 1971;23:3888–3891. doi: 10.1039/j39710003888. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. D., Wong G. A., Edwards P. A., Edmond J. The regulation of acetoacetyl-CoA synthetase activity by modulators of cholesterol synthesis in vivo and the utilization of acetoacetate for cholesterogenesis. J Biol Chem. 1984 Dec 10;259(23):14548–14553. [PubMed] [Google Scholar]
- Bilheimer D. W., Grundy S. M., Brown M. S., Goldstein J. L. Mevinolin and colestipol stimulate receptor-mediated clearance of low density lipoprotein from plasma in familial hypercholesterolemia heterozygotes. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4124–4128. doi: 10.1073/pnas.80.13.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloxham D. P., Chalkley R. A., Coghlin S. J., Salam W. Synthesis of chloromethyl ketone derivatives of fatty acids. Their use as specific inhibitors of acetoacetyl-coenzyme A thiolase, cholesterol biosynthesis and fatty acid synthesis. Biochem J. 1978 Dec 1;175(3):999–1011. doi: 10.1042/bj1750999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L., Kaneko I., Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J Biol Chem. 1978 Feb 25;253(4):1121–1128. [PubMed] [Google Scholar]
- Clinkenbeard K. D., Reed W. D., Mooney R. A., Lane M. D. Intracellular localization of the 3-hydroxy-3-methylglutaryl coenzme A cycle enzymes in liver. Separate cytoplasmic and mitochondrial 3-hydroxy-3-methylglutaryl coenzyme A generating systems for cholesterogenesis and ketogenesis. J Biol Chem. 1975 Apr 25;250(8):3108–3116. [PubMed] [Google Scholar]
- Endo A., Kuroda M., Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot (Tokyo) 1976 Dec;29(12):1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- Greenspan M. D., Alberts A. W., Vagelos P. R. Acyl carrier protein. 13. Beta-ketoacyl acyl carrier protein synthetase from Escherichia coli. J Biol Chem. 1969 Dec 10;244(23):6477–6485. [PubMed] [Google Scholar]
- Higuchi K. An improved chemically defined culture medium for strain L mouse cells based on growth responses to graded levels of nutrients including iron and zinc ions. J Cell Physiol. 1970 Feb;75(1):65–72. doi: 10.1002/jcp.1040750108. [DOI] [PubMed] [Google Scholar]
- Imanaka T., Moriyama Y., Ecsedi G. G., Aoyagi T., Amanuma-Muto K., Ohkuma S., Takano T. Esterastin: a potent inhibitor of lysosomal acid lipase. J Biochem. 1983 Sep;94(3):1017–1020. doi: 10.1093/oxfordjournals.jbchem.a134399. [DOI] [PubMed] [Google Scholar]
- Ishitani K., Niitsu Y., Listowsky I. Characterization of the different polypeptide components and analysis of subunit assembly in ferritin. J Biol Chem. 1975 Apr 25;250(8):3124–3128. [PubMed] [Google Scholar]
- Kovanen P. T., Bilheimer D. W., Goldstein J. L., Jaramillo J. J., Brown M. S. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1194–1198. doi: 10.1073/pnas.78.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. M., Tubbs P. K. 3-Hydroxy-3-methylglutaryl-coenzyme A synthase from ox liver. Purification, molecular and catalytic properties. Biochem J. 1985 Apr 15;227(2):591–599. doi: 10.1042/bj2270591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese W. A., Reitz B. A., Volpe J. J. Changes in sterol biosynthesis accompanying cessation of glial cell growth in serum-free medium. Biochem J. 1980 Nov 15;192(2):709–717. doi: 10.1042/bj1920709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese W. A., Reitz B. A., Volpe J. J. Effects of isoleucine deprivation on synthesis of sterols and fatty acids in LM-cells. J Biol Chem. 1981 Mar 10;256(5):2185–2193. [PubMed] [Google Scholar]
- Mehrabian M., Callaway K. A., Clarke C. F., Tanaka R. D., Greenspan M., Lusis A. J., Sparkes R. S., Mohandas T., Edmond J., Fogelman A. M. Regulation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A synthase and the chromosomal localization of the human gene. J Biol Chem. 1986 Dec 5;261(34):16249–16255. [PubMed] [Google Scholar]
- Middleton B., Tubbs P. K. An enzyme-bound intermediate in the biosynthesis of 3-hydroxy-3-methylglutaryl-coenzyme A. Biochem J. 1974 Jan;137(1):15–23. doi: 10.1042/bj1370015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S., Tomoda H., Kumagai H., Greenspan M. D., Yodkovitz J. B., Chen J. S., Alberts A. W., Martin I., Mochales S., Monaghan R. L. Potent inhibitory effect of antibiotic 1233A on cholesterol biosynthesis which specifically blocks 3-hydroxy-3-methylglutaryl coenzyme A synthase. J Antibiot (Tokyo) 1987 Sep;40(9):1356–1357. doi: 10.7164/antibiotics.40.1356. [DOI] [PubMed] [Google Scholar]
- Ramachandran C. K., Gray S. L., Melnykovych G. Coordinate repression of cholesterol biosynthesis and cytoplasmic 3-hydroxy-3-methylglutaryl coenzyme A synthase by glucocorticoids in HeLa cells. Arch Biochem Biophys. 1978 Jul;189(1):205–211. doi: 10.1016/0003-9861(78)90133-9. [DOI] [PubMed] [Google Scholar]
- Reed W. D., Clinkenbeard D., Lane M. D. Molecular and catalytic properties of mitochondrial (ketogenic) 3-hydroxy-3-methylglutaryl coenzyme A synthase of liver. J Biol Chem. 1975 Apr 25;250(8):3117–3123. [PubMed] [Google Scholar]
- Schnitzer-Polokoff R., Torget R., Logel J., Sinensky M. Analysis of the coordinate expression of 3-hydroxy-3-methylglutaryl coenzyme A synthase and reductase activities in Chinese hamster ovary fibroblasts. Arch Biochem Biophys. 1983 Nov;227(1):71–80. doi: 10.1016/0003-9861(83)90348-x. [DOI] [PubMed] [Google Scholar]
- Stewart P. R., Rudney H. The biosynthesis of beta-hydroxy-beta-methylglutaryl coenzyme A in yeast. IV. The origin of the thioester bond of beta-hydroxy-beta-methylglutaryl coenzyme A. J Biol Chem. 1966 Mar 10;241(5):1222–1225. [PubMed] [Google Scholar]
- Tanaka R. D., Edwards P. A., Lan S. F., Knöppel E. M., Fogelman A. M. The effect of cholestyramine and Mevinolin on the diurnal cycle of rat hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Lipid Res. 1982 Sep;23(7):1026–1031. [PubMed] [Google Scholar]
- Tanzawa K., Endo A. Kinetic analysis of the reaction catalyzed by rat-liver 3-hydroxy-3-methylglutaryl-coenzyme-A reductase using two specific inhibitors. Eur J Biochem. 1979 Jul;98(1):195–201. doi: 10.1111/j.1432-1033.1979.tb13177.x. [DOI] [PubMed] [Google Scholar]
- Tobert J. A., Bell G. D., Birtwell J., James I., Kukovetz W. R., Pryor J. S., Buntinx A., Holmes I. B., Chao Y. S., Bolognese J. A. Cholesterol-lowering effect of mevinolin, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme a reductase, in healthy volunteers. J Clin Invest. 1982 Apr;69(4):913–919. doi: 10.1172/JCI110530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Uotani K., Hamada M., Takeuchi T., Takahashi S. Ebelactone, an inhibitor of esterase, produced by actinomycetes. J Antibiot (Tokyo) 1980 Dec;33(12):1594–1596. doi: 10.7164/antibiotics.33.1594. [DOI] [PubMed] [Google Scholar]
- Volpe J. J., Obert K. A. Coordinate regulation of cholesterol synthesis and 3-hydroxy-3-methylglutaryl coenzyme A synthase but not 3-hydroxy-3-methylglutaryl coenzyme A reductase in C-6 glia. Arch Biochem Biophys. 1981 Nov;212(1):88–97. doi: 10.1016/0003-9861(81)90346-5. [DOI] [PubMed] [Google Scholar]


