Abstract
The objectives of this study were to determine the role of calcium-activated, small (SK), intermediate (IK), and large (BK) conductance potassium channels in initiating the development of an anti-inflammatory phenotype elicited by preconditioning with an exogenous hydrogen sulfide (H2S) donor, sodium hydrosulfide (NaHS). Intravital microscopy was used to visualize rolling and firmly adherent leukocytes in vessels of the small intestine of mice preconditioned with NaHS (in the absence and presence of SK, IK, and BK channel inhibitors, apamin, TRAM-34, and paxilline, respectively) or SK/IK (NS-309) or BK channel activators (NS-1619) 24 h before ischemia-reperfusion (I/R). I/R induced marked increases in leukocyte rolling and adhesion, effects that were largely abolished by preconditioning with NaHS, NS-309, or NS-1619. The postischemic anti-inflammatory effects of NaHS-induced preconditioning were mitigated by BKB channel inhibitor treatment coincident with NaHS, but not by apamin or TRAM-34, 24 h before I/R. Confocal imaging and immunohistochemistry were used to demonstrate the presence of BKα subunit staining in both endothelial and vascular smooth muscle cells of isolated, pressurized mesenteric venules. Using patch-clamp techniques, we found that BK channels in cultured endothelial cells were activated after exposure to NaHS. Bath application of the same concentration of NaHS used in preconditioning protocols led to a rapid increase in a whole cell K+ current; specifically, the component of K+ current blocked by the selective BK channel antagonist iberiotoxin. The activation of BK current by NaHS could also be demonstrated in single channel recording mode where it was independent of a change in intracellular Ca+ concentration. Our data are consistent with the concept that H2S induces the development of an anti-adhesive state in I/R in part mediated by a BK channel-dependent mechanism.
Keywords: ischemia-reperfusion; leukocyte rolling; leukocyte adhesion; human microvascular endothelial cells; coronary microvascular endothelial cells; calcium activation; small, intermediate, and large conductance potassium channels
although the cytotoxic properties of hydrogen sulfide (H2S) have long been appreciated, it is now recognized that this noxious gas is produced endogenously and plays a prominent role in cellular signaling. H2S is enzymatically generated by the vasculature through the actions of cystathionine γ-lyase (CSE, also referred to as cystathionase). Like the other two endogenous gaseous signaling molecules, nitric oxide (NO) and carbon monoxide (CO), H2S produces vasorelaxation but does so by a mechanism distinct from these gaseous monoxides, activating ATP-sensitive K+ channels (KATP) channels rather than guanylyl cyclase (9, 12, 50, 69, 70). However, H2S can enhance the vasorelaxant effects of NO (23) and, conversely, the production of H2S can be upregulated by NO (68).
We (64) recently demonstrated that preconditioning with an exogenous H2S donor (NaHS-PC) 24 h before ischemia-reperfusion (I/R) causes postcapillary venules to shift to an anti-inflammatory phenotype in C57BL/6J wild-type mice such that these vessels fail to support leukocyte rolling and leukocyte adhesion during reperfusion. Moreover, we showed that the development of NaHS-PC is triggered by an endothelial nitric oxide synthase (eNOS)-dependent mechanism. The latter observation, when coupled with recent work (25, 34, 48, 56) demonstrating that endothelial BK channels play a role in regulating the synthesis of NO, suggests the possibility that these potassium channels may also be involved in the genesis of the preconditioned anti-inflammatory phenotype that develops in response to antecedent NaHS treatment. BK channels are a class of voltage- and calcium-activated K+ channel (also termed Slo or MaxiK) characterized by their large unitary conductance (210–270 pS) and sensitivity to blockade by iberiotoxin (IBTX; Refs. 26, 39). BK channels are found in many excitable cells (51) and have also been reported to be expressed in some types of endothelium (13, 24, 29, 33, 36, 55), although their expression and functional roles in native endothelial cells are controversial (19, 28, 41). BK channel activation would hyperpolarize the endothelial cell membrane, enhance calcium influx through non-voltage-gated calcium channels (10, 22, 34, 42), and thereby promote NO production through calcium-sensitive eNOS (17). A possible role for BK channels in preconditioning is supported by the fact that preconditioning with the selective BK channel opener NS-1619 induces both early and late phase preconditioning in the myocardium, effects that were not blocked by KATP channel antagonists but that were mitigated by the BK channel antagonists IBTX and paxilline (45, 54, 58). In addition, the cardioprotective effects of estradiol in rat ventricular myocytes exposed to simulated ischemia appear to involve BK channel activation (31, 35), while estrogens are also known to enhance BK activity. For these reasons, we hypothesized that H2S donor treatment (NaHS-PC) elicits the development of a preconditioned anti-adhesive phenotype via a BK channel-dependent mechanism. In addition to testing this postulate, we also sought to determine whether direct application of NaHS could activate endothelial cell BK channels via use of patch-clamp techniques to record K+ currents in single, cultured microvascular endothelial cells. Finally, we evaluated the role of small and intermediate conductance, calcium-activated potassium channels (SK and IK, respectively) and determined whether an anti-inflammatory phenotype could be invoked by antecedent treatment with SK, IK, as well as BK channel activators.
MATERIALS AND METHODS
Animals
Wild-type (WT) male C57BL/6J WT mice (6–7 wk of age) were obtained from the Jackson Laboratories (Bar Harbor, ME). All mice were maintained on standard mouse chow and used at 8–12 wk of age. The experimental procedures described herein were performed according to the criteria outlined in the National Institutes of Health Guidelines and were approved by the University of Missouri–Columbia Institutional Animal Care and Use Committee.
Surgical Procedures and Induction of I/R
The mice were anesthetized initially with a mixture of ketamine (150 mg/kg body wt ip) and xylazine (7.5 mg/kg body wt ip). The right carotid artery was cannulated and systemic arterial pressure was measured with a Statham P23A pressure transducer (Gould) connected to the carotid artery catheter. Systemic blood pressure was recorded continuously with a personal computer (Power Macintosh 8600; Apple) equipped with an analog-to-digital converter (MP 100; Biopac Systems). Carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE; Molecular Probes, Eugene, OR) was dissolved in DMSO at a stock concentration of 5 mg/ml, divided into 25 μl aliquots, and stored in light-tight containers at −20°C until further dilution immediately before intravenous injection. The left jugular vein was cannulated for administration of CFDA-SE. After these procedures, a midline abdominal incision was performed, and the superior mesenteric artery was occluded with a microvascular clip for 0 (sham) or 45 min. After the ischemic period, the clip was gently removed and leukocytes were labeled with CFDA-SE by intravenous administration of the fluorochrome solution (250 μg/ml in saline) at 20 μl/min for 5 min. During the preparation, storage, and administration of CFDA-SE, care was taken to minimize light exposure. Leukocyte/endothelial cell adhesive interactions were quantified over 30–40 and 60–70 min of reperfusion, as described below.
Intravital Fluorescence Microscopy
The mice were positioned on a 20 × 30-cm Plexiglas board in a manner that allowed a selected section of small intestine to be exteriorized and placed carefully and gently over a glass slide covering a 4 × 3-cm hole centered in the Plexiglas. The exposed small intestine was superfused with warmed (37°C) bicarbonate-buffered saline (BBS, pH 7.4) at 1.5 ml/min using a peristaltic pump (Model M312; Gilson). The exteriorized region of the small bowel was covered with BBS-soaked gauze to minimize tissue dehydration, temperature changes, and the influence of respiratory movements. The superfusate was maintained at 37 ± 0.5°C by pumping the solution through a heat exchanger warmed by a constant-temperature circulator (model 1130; VWR). Body temperature of the mouse was maintained at 36.5–37.5°C by use of a thermostatically controlled heat lamp. The board was mounted on the stage of an inverted microscope (Diaphot TMD-EF; Nikon), and the intestinal microcirculation was observed through a ×20 objective lens. Intravital fluorescence images of the microcirculation (excitation wavelength, 420–490 nm; emission wavelength, 520 nm) were detected with a charge-coupled device camera (XC-77; Hamamatsu Photonics), a charge-coupled device camera control unit (C2400; Hamamatsu Photonics), and an intensifier head (M4314; Hamamatsu Photonics) attached to the camera. Microfluorographs were projected on a television monitor (PVM-1953MD; Sony) and recorded on digital video using a digital video recorder (DMR-E50; Panasonic) for off-line quantification of measured variables during playback of the recorded image. A video time-date generator (WJ810; Panasonic) displayed a stopwatch function on the monitor.
The intravital microscopic measurements described below were obtained over 30–40 and 60–70 min of reperfusion or at equivalent time points in the control groups. Each intestinal segment was scanned from the oral to aboral section and 10 single, unbranched venules (20- to 50-μm diameter, 100-μm length) were observed, each for 30 s. Leukocyte-endothelial cell interactions (the numbers of rolling and firmly adherent leukocytes) were quantified in each of the 10 venules, followed by calculation of the mean value, which was used in the statistical analysis of the data. Circulating leukocytes were considered to be firmly adherent if they did not move or detach from the venular wall for a period of ≥30 s. Rolling cells were defined as cells crossing an imaginary line in the microvessel at a velocity that was significantly lower than centerline velocity; their numbers were expressed as rolling cells per minute. The numbers of rolling or adherent leukocytes were normalized by expressing each as the number of cells per millimeters squared vessel area.
Experimental Protocols–Intravital Microscopy
The general design of the experimental protocols for each group in the study is shown in Fig. 1 and described below.
Fig. 1.
Illustration of the experimental protocols assigned to each group. Numbers along the top of the diagram in minutes refer to the time line of the protocol on day 1, 24 h before ischemia-reperfusion (I/R), and day 2, the day of I/R. Hatched bars indicate digital video recording (10 min). Solid black bars indicate the 45-min period of ischemia during which the superior mesenteric artery had no blood flow. ▴, Administration of drug. See text for further details.
Group 1: sham.
As a time control for the effects of experimental duration, the mesentery of each mouse in this group (n = 6) was superfused with BBS. The superior mesenteric artery was exposed but not subjected to occlusion, with leukocyte-endothelial cell adhesive interactions quantified at time points comparable to those described for mice subjected to 45 min of intestinal ischemia followed by 70 min of reperfusion (group 2, below).
Group 2: I/R alone.
Mice in this group (n = 6) were treated as described for group 1 above except that I/R was induced by occlusion of the superior mesenteric artery for 45 min followed by reperfusion for 70 min. leukocyte rolling and leukocyte adhesion were quantified during 30–40 and 60–70 min of reperfusion.
Group 3: NaHS + I/R.
To determine whether H2S can act as a preconditioning stimulus and prevent I/R-induced leukocyte rolling and leukocyte adhesion, a solution of NaHS was used as a H2S donor. NaHS (Sigma Chemical, St. Louis, MO) was weighed and diluted in saline at a concentration of 1.4 mM. Mice in this group (n = 6) were treated as described for group 2 except that NaHS (14 μmol/kg ip, 0.3 ml) was administered 24 h before I/R.
Group 4: paxilline + NaHS + I/R.
To explore the role of BK channels as a trigger for the development of NaHS-PC, we investigated the effects of administration of the selective BK channel inhibitor paxilline (25 mg/kg ip) (40) coincident with NaHS 24 h before I/R. Mice in this group (n = 6) were treated as described for group 3 except that paxilline was administered 10 min before NaHS.
Group 5: paxilline + I/R.
To determine whether paxilline treatment 24 h before I/R influenced postischemic leukocyte rolling and adhesion, we repeated the studies outlined above for group 4, except that an equal volume of saline vehicle was administered 10 min after paxilline injection, in lieu of NaHS (n = 6).
Group 6: NS-1619 + I/R.
The aim of this group of experiments was to determine whether preconditioning with the BKB channel opener NS-1619 [1-(2′-hydroxy-5′-trifluoromethylphenyl)-5-trifluoromethyl-2(3H)benzimid-axolone] would mimic the effects of NaHS-PC and prevent postischemic leukocyte rolling and leukocyte adhesion on subsequent exposure of the small intestine to I/R 24 h later. Mice in this group (n = 6) were treated as described in group 3 except that they received NS-1619 (100 μM, 0.5 ml ip) 24 h before I/R in lieu of NaHS.
Group 7: paxilline + NS-1619 + I/R.
To demonstrate that the effect of NS-1619 to invoke preconditioning was due to BK activation, we repeated the studies outlined for group 4 in a separate group of mice, except that paxilline was administered 10 min before NS-1619 (instead of NaHS). Leukocyte rolling and adhesion was assessed during I/R 24 h later.
Groups 8–10.
The following groups were used to assess the role of IKCa and SKCa in NaHS-PC. As part of this study, we repeated groups 1–3 above (sham = group 8, n = 6; I/R alone = group 9, n = 6; and NaHS + I/R = group 10, n = 6) because all studies for this aspect of the work were conducted using a different microscope system and experimentalist. The inverted microscope used in this setting was a Nikon Eclipse TE2000-U, while the camera was a Photometric Coolsnap ES. All other conditions were identical in the setup.
Group 11: TRAM-34 + NaHS + I/R.
To determine whether IKCa played a role in NaHS-PC, mice in this group (n = 7) were treated as described for group 4, except that the IK inhibitor TRAM-34 (20 mg/kg ip) was administered in lieu of paxilline.
Group 12: apamin + NaHS + I/R.
Studies outlined for this group were designed to determine whether apamin (0.4 mg/kg ip), an inhibitor of SK, would prevent the postischemmic anti-inflammatory effects of antecedent NaHS treatment.
Group 13: NS-309 + I/R.
To determine whether activation of IK and SK would mimic the effects of NaHS-PC, mice in this group (n = 6) were treated as described for group 6, except that NS-309 (Sigma Chemical), an IKCa and SKCa agonist, was administered (1 mg/kg in 2% DMSO saline mixture, 100 μM, 0.5 ml ip) in lieu of NaHS.
Group 14: verapamil + I/R.
Since most pharmacological agents that induce preconditioning are also vasodilators that activate either adenylyl cyclase-dependent (e.g., adenosine, CGRP), guanylyl cyclase-dependent (e.g., NO), or BKCa-dependent (e.g., NS-1619) signaling pathways, vasodilation per se could serve as the trigger for entrance into preconditioned states. Thus we sought to determine whether verapamil (25 mg/kg ip), which induces vasodilation secondary to inhibition of L-type calcium channels and not by the mechanisms described above, would mimic the effects of these other vasodilators and produce the development of an anti-inflammatory phenotype. Mice in this group (n = 6) were treated as described for group 6 above, except that verapamil (25 mg/kg ip) was administered in lieu of NS-1619.
Human Microvascular Endothelial Cell Culture
Human microvascular endothelial cells (HMEC-1) were purchased from ATCC and cultured as described previously (1). The HMEC-1 cell line has been previously shown to express functional BK channels (21). The cells were seeded and grown in MCDB-131 (Sigma) medium containing l-glutamine (1 mM) and sodium bicarbonate (11.6g/l), glucose, and 10% (vol/vol) FBS (Invitrogen) and were maintained at 37°C in 5% CO2. The cells were grown to 80–90% confluency and then detached using 0.25% trypsin/0.5 mM EDTA in PBS and reseeded onto sterile glass coverslips in 35-mm culture dishes. The cells were allowed to attach to uncoated glass coverslips and were incubated at 370 C in 5% CO2 for 1 h, and then the dishes were moved to a 28°C incubator just before electrophysiological recordings.
Electrophysiological Recordings
A coverslip with cells was placed in a recording chamber (0.5 ml) with continuous perfusion (∼1 ml/min) on the stage of an inverted microscope (Zeiss IM-405). The patch-clamp technique was employed using an EPC-9 amplifier (HEKA) to measure membrane currents in the whole cell and inside-out configurations, as described previously. The amplifier was controlled through an ITC-16 interface (Instrutech, Port Washington, NY) by a Dell XPS computer running Pulse + Pulsefit software (HEKA). Igor Pro (WaveMetrics, Oswego, OR) and SigmaPlot 9.0 (SPSS, Chicago, IL) were used for data analysis. Currents were sampled at 10 kHz and filtered at 3.3 kHz (whole cell mode) or 1 kHz (single-channel mode). From a typical holding potential of −60 mV, whole cell currents were activated by voltage step pulses (to potentials from −80 to +80 mV in 10-mV increments, duration = 300 ms) or voltage ramps (from −100 mV to +100 mV, duration = 1 s). Micropipettes were pulled from borosilicate glass capillaries (ID, 1.2 mm; OD, 1.5 mm; World Precision Instruments, Sarasota, FL) using a Sutter P-97 electrode puller (Sutter Instruments, Novato, CA). Resistances ranged from 3.0–3.5 MΩ when filled with pipette solution. All electrophysiological protocols were performed at room temperature.
Solutions
For whole cell recordings using HMEC-1 cells, the bath solution contained the following (in mM): 140 NaCl, 5.6 KCl, 2 CaCl2, 1 MgCl2, 15 glucose, 10 HEPES, and 2 Na-pyruvate (pH 7.4). The pipette solution contained the following (in mM): 140 KCl, 8 NaCl, 1 EGTA, 3 Mg-ATP, and 10 HEPES (pH 7.2). The 0.1 M CaCl2 solution was added to give a free [Ca2+] of 600 nM or 1 μM. The level of free Ca2+ in each solution was confirmed using a calcium electrode (Orion model 93–20; World Precision Instruments). ATP (3 mM) was included to inhibit KATP and provide substrate for energy-dependent processes.
Single-channel BK current recordings were made using the inside-out configuration. The bath solution contained 140 KCl, 10 HEPES, 2 EGTA, and 1 MgCl2 (pH 7.2 with KOH), with variable amounts of a 0.1 M CaCl2 solution added to give the desired free [Ca2+]. The pipette solution contained 140 KCl, 2 EGTA, 1.8 CaCl2, 10 HEPES, and 1 MgCl2 (pH 7.4).
Chemicals
IBTX was obtained from Sigma and paxilline was obtained from Transduction Laboratories (Lexington, KY). All stocks were made in bath solution. The final bath concentrations were 100 nM IBTX, 10 μM paxilline, and 100 μM NaHS. Apamin (50 nM) and Tram 34 (1 μM) were used to block SK- and IK-conductance calcium-activated K+ channels, respectively.
Confocal Microscopy and Immunohistochemistry
NaHS (14 μmol/kg ip, 0.3 ml) was administered to mice for 24 h after which thris-order mesenteric veins were isolated, cannulated, and pressurized at ∼10 mmHg. The vessels were then fixed with 2% paraformaldehyde for 20 min and stained with primary BK-α subunit Ab [rabbit anti-mouse Ab (1:50); Alomone Labs, Israel], followed by goat-anti rabbit IgG (1:100; Alexa fluor conjugated; 488 nm excitation wavelength). To assist in identification of the cells, nuclei were stained with propidium iodide (20 μM) for 15 min. Vessel segments were examined while remaining cannulated using a Leica TCS SP5 fluorescence confocal microscopy system, with Argon laser excitation at 488 nm, using a ×60 water objective, Z step at 0.1 μm, with resolution 512 × 512, 700 Hz.
Microvascular Endothelial Cell Isolation
Cell lysates from freshly isolated coronary microvascular endothelial cells were prepared to examine BK channel expression in native endothelial cells. Rat ventricular tissue was minced in HEPES-buffered (25 mM) DMEM and digested with Liberase Blendzyme 3 (Roche Applied Science, Indianapolis, IN) for 60 min at 37°C in a shaking apparatus. The digested tissue was passed through a nylon cell strainer (100 μm opening; Falcon no. 352360) suspended over a 50-ml sterile centrifuge tube. Cells were pelleted by centrifugation, washed with HEPES-buffered DMEM, and pelleted again. Cells were then resuspended in 1 ml of HEPES-buffered DMEM containing biotinylated anti-rat PECAM-1 (CD31) antibody (10 μg/ml; AbD Serotec, Raleigh, NC), placed on a circular rotator, and incubated 60 min at room temperature. Cells were pelleted again and rinsed to remove unbound antibody and then pelleted again and resuspended in 1 ml of HEPES-buffered DMEM containing 10 μl of streptavidin-coated magnetic beads (Dynabeads M-280; Invitrogen, Carlsbad, CA). The cells were placed on a circular rotator and incubated 45 min at room temperature. The tube was placed in a magnetic stand to collect the endothelial cells bound to the beads, with the supernatant containing nonendothelial cells carefully aspirated and discarded. The tubes were then removed from the magnetic stand, and the cells were resuspended in PBS and placed back on the magnetic stand. This procedure was repeated twice, and the final cell suspension was pelleted by centrifugation. Forty microliters of lysis buffer were added to the cell pellet, and lysates were stored at −20°C until utilized for Western blotting.
Western Blots of Freshly Isolated Microvascular Endothelial Cell Lysates
Graded amounts of endothelial cell lysate (0.2–2.0 μl) were subjected to SDS-PAGE on a 7% gel and then electroblotted to nitrocellulose membrane. Blots were probed with a rabbit anti-BKCa α-subunit (APC-107; Alomone Labs) at 1:300 dilution in TBS containing 0.1% Tween-20 + 5% BSA and then with a horseradish peroxidase-coupled anti-rabbit secondary antibody (Cell Signaling Technologies, Danvers, MA) at 1:2,000 dilution in TBS + 0.1% Tween-20 + 5% milk. Bands were detected using the Super Signal West Pico chemiluminescent detection system (Pierce, Rockford, IL).
Statistical Analysis
For intravital studies, the data were analyzed with standard statistical analysis, i.e., ANOVA with Scheffé's (post hoc) tests for multiple comparisons (SigmaStat). All values are expressed as means ± SE. For whole cell current analyses, raw current values were normalized to cell capacitance and expressed as current density (pA/pF). For single channel analysis, amplitude histograms were constructed from continuous recordings at constant holding potential and fitted with Gaussian curves to determine average current amplitude. NPo (number of open channels × open probability) was computed using the method and program previously described (47). Summary data are expressed as means ± SE. Statistical significance was determined using t-tests, paired t-tests, or ANOVA (SigmaStat), as indicated for specific protocols. Statistical significance was defined at P < 0.05.
RESULTS
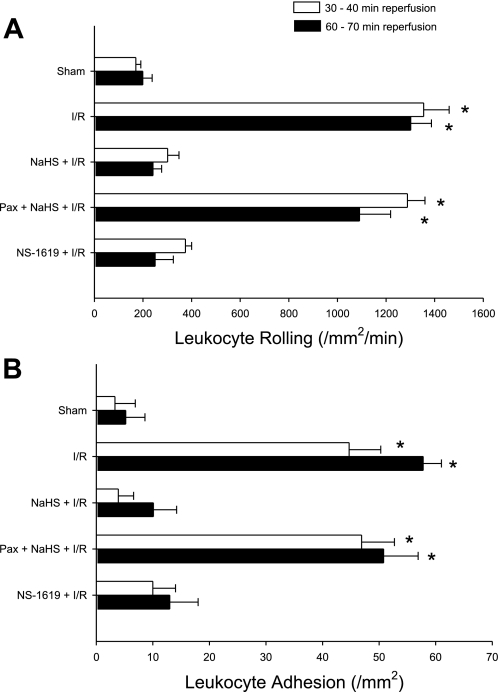
Figure 2 illustrates the average numbers of rolling (A) and adherent (B) leukocytes in postcapillary venules of the murine small intestine exposed to I/R alone (I/R, group 2) or H2S donor 24 h before I/R (NaHS + I/R, group 3) relative to nonischemic controls (sham, group 1). I/R induced marked increases in the numbers of rolling and adherent leukocytes after 30 and 60 min of reperfusion; proadhesive effects that were abolished by preconditioning with the H2S donor NaHS.
Fig. 2.
Effects of large (BK) conductance potassium channel inhibition on the preconditioning effects of NaHS. Effects of I/R, I/R following pretreatment with a hydrogen sulfide (H2S) donor (NaHS + I/R) or paxilline (paxilline + I/R), BK channel inhibition just before treatment with H2S donor (paxilline + NaHS +I/R), or BK channel activation with NS-1619 (in lieu of NaHS) in the absence (NS-1619 + I/R) and presence of paxilline (paxilline + NS-1619 + I/R) on postischemic leukocyte rolling (A) or stationary leukocyte adhesion (B) determined after 30 and 60 min of reperfusion (n = 6 in all groups). *Mean values that were statistically different from the NaHS + IR group.
To investigate the role of BK channels in the triggering mechanism involved in the development of this anti-inflammatory phenotype in response to antecedent H2S, postischemic leukocyte rolling and leukocyte adhesion were quantified in mice treated with the BK channel antagonist paxilline coincident with NaHS-PC 24 h before I/R (Fig. 2). Paxilline effectively abolished the effects elicited by H2S donor treatment to limit postischemic leukocyte rolling and leukocyte adhesion. This result suggests that BK channel activation secondary to NaHS treatment plays a crucial role in preventing I/R-induced leukocyte rolling and leukocyte adhesion. It is important to note that paxilline treatment alone (i.e., no NaHS) 24 h before I/R did not alter postischemic leukocyte rolling (1,527 ± 81 and 1,183±155 leukocytes·mm−2·min−1 at 30 and 60 of min of reperfusion, respectively) or adhesion (105 ± 9 and 109 ± 14 leukocytes/mm2 at 30 and 60 min of reperfusion, respectively; Fig. 2). Interestingly, preconditioning with the selective BK channel activator NS-1619, in lieu of H2S, was as effective as NaHS-PC in attenuating I/R-induced leukocyte rolling and adhesion, further supporting a role for BK channel activation as a trigger for the development of an anti-inflammatory phenotype in I/R. We also demonstrated that paxilline prevented the ability of NS-1619 to induce an anti-inflammatory phenotype (1,320 ± 45 and 1,285 ± 94 rolling leukocytes·mm−2·min−1 at 30 and 60 min of reperfusion, respectively, and 65 ± 13 and 75 ± 16 adherent leukocytes/mm2 at 30 and 60 min of reperfusion, respectively), an observation that supports the conclusion that this compound invokes preconditioning by activating BK.
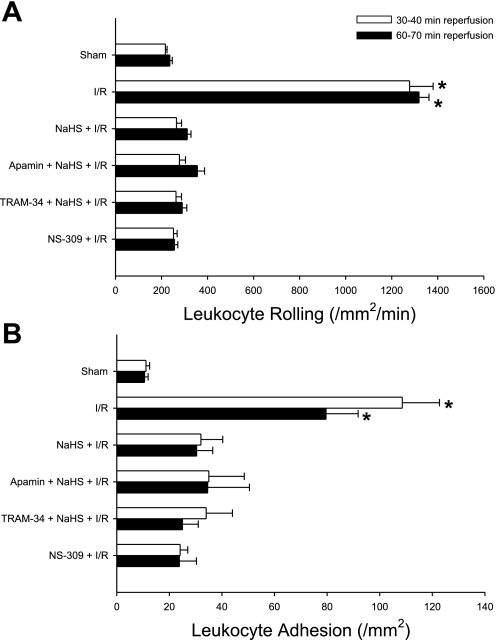
The data illustrated in Fig. 3 demonstrate that treatment with apamin or TRAM-34 failed to affect the ability of antecedent NaHS to prevent postischemic leukocyte rolling and adhesion. Treatment with the vehicle for TRAM-34 (peanut oil) did not affect postischemic leukocyte rolling (255 ± 34 and 234 ± 18 leukocytes·mm−2·min−1 at 30 and 60 min of reperfusion, respectively) or adhesion (12 ± 4 and 12 ± 1 leukocytes/mm2 at 30 and 60 min of reperfusion, respectively). While these results suggest that SK and IK are not involved in NaHS preconditioning, administration of the SK and IK activator NS-309, in lieu of NaHS, was effective in reducing I/R-induced leukocyte rolling and adhesion. Since NS-309 activates both SK and IK, we cannot determine whether activation of either or both channels is required to elicit preconditioning by this agent. However, because treatment with either apamin and TRAM-34 failed to abrogate the effect of antecedent NaHS to abrogate postischemic leukocyte rolling and adhesion, our data are consistent with the concept that SK and IK do not contribute to NaHS-PC.
Fig. 3.
Role of small (SK) and intermediate (IK) conductance potassium channels preconditioning. Effects of I/R alone, I/R following pretreatment with a H2S donor (NaHS + I/R), SK or IK channel inhibition with apamin or TRAM-34, respectively, just before treatment with H2S donor (apamin + NaHS + I/R; or TRAM-34 + NaHS + I/R), or preconditioning with the SK/IK activator NS-309, in lieu of NaHS (NS-309 + I/R), on postischemic leukocyte rolling (A) or stationary leukocyte adhesion (B) determined after 30 and 60 min of reperfusion (n = 6 in all groups). *Mean values that were statistically different from the NaHS + IR group.
Since most pharmacological agents that induce preconditioning are also vasodilators that activate either adenylyl cyclase-dependent (e.g., adenosine, CGRP), guanylyl cyclase-dependent (e.g., NO), or BKCa-dependent (e.g., NS-1619) signaling pathways, vasodilation per se could serve as the trigger for entrance into preconditioned states. However, antecedent verapamil treatment, which induces vasodilation secondary to inhibition of L-type calcium channels and not by the mechanisms described above, did not limit leukocyte rolling (1,349 ± 46 and 1,048 ± 148 rolling leukocytes·mm−2·min−1) or adhesion (45 ± 8 and 42 ± 7 adherent leukocytes/mm2) when assessed at 30 and 60 min of reperfusion, respectively, compared with NaHS preconditioning.
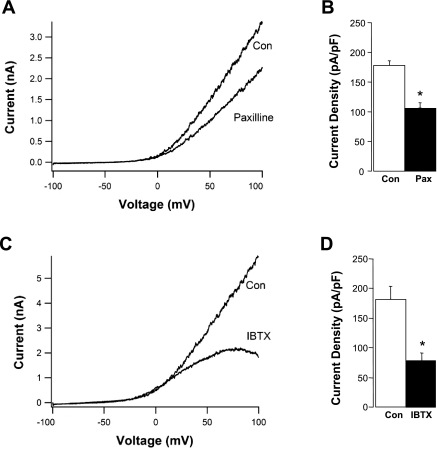
To test if direct application of NaHS could activate endothelial cell BK channels, we used patch-clamp techniques to record K+ currents in single, cultured endothelial cells. HMEC-1 cells have previously been shown to exhibit functional BK channel activity (21), which we verified in our experiments. Figure 4A shows an example of the current-voltage (I-V) relationship for outward, whole cell current in a single HMEC under conditions optimal for recording K+ current. Very little current is evident at potentials between −100 and −50 mV, with an outwardly rectifying current evident at potentials positive to −40 mV. Subsequent application of paxilline (10 μM) inhibited current by 45% in this cell, suggesting that BK channels contributed ∼45% to whole cell K+ current. Figure 4B shows summary data from four HMECs in which paxilline inhibited, on average, to 43% of whole cell K+ current. Figure 4, C and D, shows similar recordings of whole cell current in the absence and presence of the more selective BK channel inhibitor IBTX (100 nM). This concentration of IBTX inhibited, on average, 67% of whole cell K+ current (n = 7). These recordings were performed in the presence of 3 mM ATP in the recording pipette to inhibit KATP channels, so that the remaining K+ current most likely was contributed by IK and SK calcium-activated K+ channels (7, 11, 33). Qualitatively similar findings were obtained using solutions that minimized the possible contribution of Cl− channels (not shown), suggesting that the large outward currents under these conditions were carried by K+ rather than Cl−.
Fig. 4.
A: whole cell K+ current in a single human microvascular endothelial cells (HMEC). Current-voltage (I-V) relationship was obtained using a voltage ramp from −100 to +100 mV from a holding potential of −60 mV. Recording solutions are the same as stated in Materials and Methods, with pipette solution buffered to 1 μM Ca2+. Application of paxilline (10 μM) in the bath solution partially inhibited current at all potentials positive to 0 mV. B: summary of HMEC current density recorded at +80 mV in 4 cells using a voltage step in the absence and presence of paxilline (10 μM). C: whole cell K+ current before (Con) and after bath application of iberiotoxin (IBTX; 100 nM). Pipette solution was buffered to 1 μM Ca2+. D: summary of HMEC current density recorded at +80 mV in 7 cells using a voltage step in the absence and presence of IBTX (100 nM). *Mean values that were statistically different from the control group.
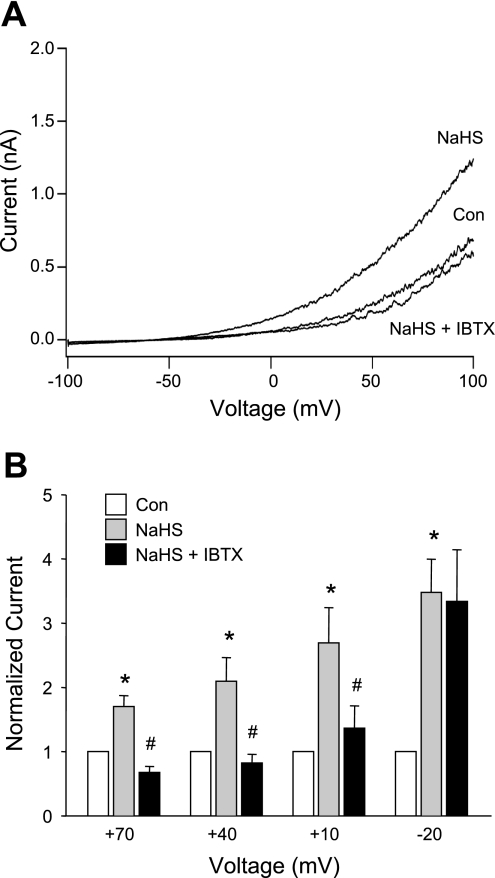
Next, we tested the effect of NaHS on endothelial cell K+ current. NaHS, at the same concentration estimated to be achieved in our in vivo studies, produced a rapid and substantial increase in whole cell K+ current (Fig. 5A). The magnitude of the effect varied with membrane potential, as shown using voltage step protocols in Fig. 5B; NaHS increased K+ current by 80% at a test potential of +70 mV, (n = 6), by 100% at +40 mV, by 150% at +10 mV, and by 250% at −20 mV. The effects of NaHS at more negative (and physiological) potentials were more difficult to quantify because of the much smaller size of the currents. However, the trend suggested a greater effect of NaHS at more negative potentials (up to ∼3.5-fold). The subsequent application of IBTX blocked the increase in current produced by NaHS (Fig. 5A) and reduced current below control levels (to levels comparable to the effect of IBTX alone in Fig. 4B). As shown in Fig. 5B, IBTX blocked almost all of the increase in K+ current elicited by NaHS at membrane potentials between +10 and +70 mV but had little effect on NaHS-activated K+ current at −20 mV. Similar results were obtained in another set of experiments at higher intracellular Ca2+ levels (1 μM, n = 5; not shown). This result suggests that NaHS also activated another K+ current, possibly IK and/or SK, both of which are known to be activated at more negative potentials than BK current (51).
Fig. 5.
Effect of NaHS on whole cell K+ current in HMECs. A: sample I-V plot from a single cell obtained using a voltage ramp. Pipette solution was buffered to 600 nM Ca2+. After the control recording (Con), NaHS (100 μM) was applied via the bath (at time = 1 min) and resulted in a substantial increase in current. Subsequent application of IBTX in the continued presence of NaHS reduced current below the control level (NaHS + IBTX). B: summary of effects of NaHS on HMEC K+ current in 6 cells. Measurements of current were obtained from voltage steps to the indicated step potentials (duration = 300 ms, with current measurements made at the end of the step). For each cell, current was normalized to basal (control) current. *Significant difference from the NaHS group. #Significant difference from the Con group.
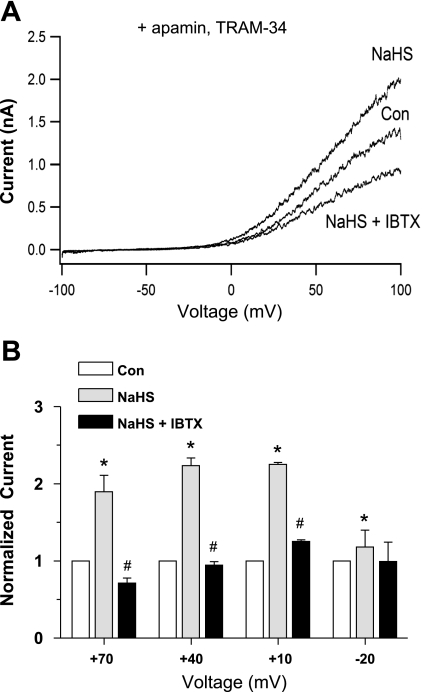
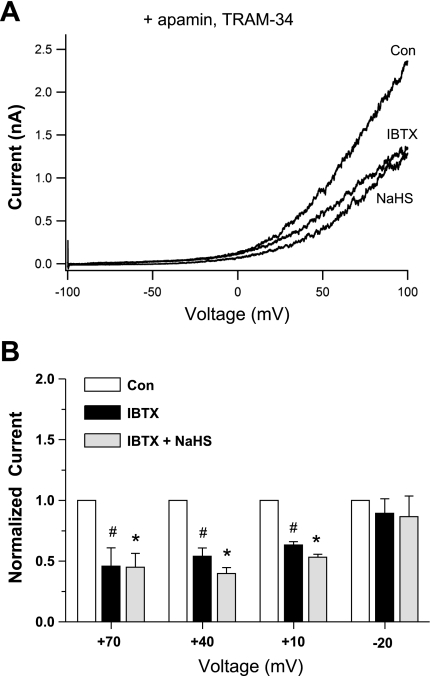
To more specifically test for activation of BK current by NaHS, we repeated the above protocol in the presence of the selective SK channel blocker apamin (50 nM) combined with the selective IK channel blocker TRAM-34 (1 μM). Consistent with the basal inhibition of IK and SK channels, the resulting I-V relationship was right-shifted (Fig. 6A), compared with that in Fig. 5A, with substantial K+ current being activated only at potentials positive to approximately −20 mV. Subsequent application of NaHS produced an approximately twofold increase in current at +70, +40, and +10 mV, almost all of which was blocked by IBTX (Fig. 6B). At −20 mV, there was a slight but significant enhancement of the apparent BK current by NaHS (paired t-test, control vs. post-H2S). Examination of the individual I-V curves (n = 10) revealed an enhancement of current in seven cells, slight inhibition in one cell and no effect in two cells. Similar results were obtained in another set of experiments at higher intracellular Ca2+ levels (1 μM, n = 6; not shown). A similar protocol, also in the presence of apamin and TRAM-34, was performed with IBTX applied first, followed by NaHS. As shown in Fig. 7, IBTX reduced basal K+ current by about half and prevented any increase in K+ current during subsequent application of NaHS. The difference in current between the control and NaHS group in the presence of IBTX was not significant even using a paired t-test.
Fig. 6.
Effect of NaHS on whole cell K+ current in the presence of apamin (50 nM) and TRAM-34 (1 μM). A: I-V relationship from a single cell obtained using a voltage ramp. Pipette solution was buffered to 600 nM Ca2+. After the control recording, NaHS (100 μM) was applied via the bath (at time = 1 min) and resulted in a substantial increase in current. Subsequent application of IBTX in the continued presence of NaHS reduced current below the control level (NaHS + IBTX). B: summary of effects of NaHS on HMEC K+ current in 9 cells. Measurements of current were obtained from voltage steps to the indicated step potentials (duration = 300 ms, with current measurements made at the end of the step). For each cell, current was normalized to basal (control) current. *Significant difference from the NaHS group. #Significant difference from the Con group.
Fig. 7.
Effect of NaHS on whole cell K+ current in the presence of apamin (50 nM) and TRAM-34 (1 μM). A: current-voltage (I-V) relationship from a single cell obtained using a voltage ramp. Pipette solution was buffered to 600 nM Ca2+. After the control recording, IBTX (100 nM) was applied via the bath and resulted in a substantial inhibition in current. Subsequent application of NaHS (100 μM) in the continued presence of IBTX (100 nM) failed to increase current. B: summary of effects of NaHS (100 μM) on HMEC K+ current in 9 cells when IBTX (100 nM) was given first. Measurements of current were obtained from voltage steps to the indicated step potentials (duration = 300 ms, with current measurements made at the end of the step). For each cell, current was normalized to basal (control) current. *Significant difference from the IBTX group. #Significant difference from the Con group.
To test whether BK channels contribute to the resting membrane potential of HMECs, we performed current clamp recordings of resting membrane potential (Em) before and after the application of IBTX. Figure 8A shows a recording from one cell, where Em was −37 mV and IBTX application was associated with a depolarization to −31 mV. The data for 7 cells are shown in Fig. 8B, where, on average, IBTX caused ∼2 mV depolarization, an effect that was just short of being significant (P = 0.053; paired t-test).
Fig. 8.
Effect of IBTX on resting membrane potential (Em) of HMECs. A: sample recording from 1 cell, where IBTX is associated with a depolarization from −37 to −31 mV. B: summary of results from 7 cells using the same protocol. IBTX caused a slight (2 mV) depolarization, but the effect was not statistically significant at the P < 0.05 level (paired t-test).
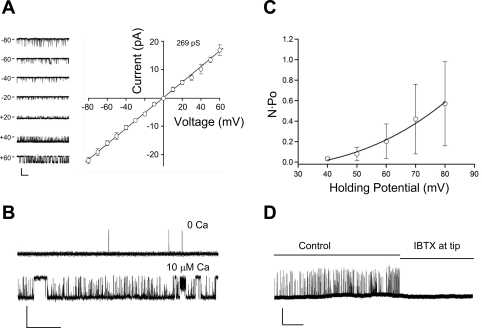
We hypothesized that similar results should be obtained when recording the activity of single BK channels. Figure 9 shows examples of single BK channel activity recorded in inside-out patches from HMECs. After the gigaseal was achieved, the patch was excised into bath solution containing 140 mM K+ to bring the membrane potential close to zero. Figure 9A shows sample traces of the activity of a single, large-amplitude channel at various holding potentials between −80 and +60 mV, along with the I-V relationship for that channel. The reversal potential for current was ∼0 mV in symmetric K+ solutions, consistent with the behavior of a K+ channel. The slope of the I-V curve was 269 pS, which is consistent with a BK channel. For comparison, the unitary slope conductance is known to range from 20 to 80 pS for IK channels and <15 pS for SK channels (33, 51).
Fig. 9.
Single channel BK current recordings in HMECs; all recordings were made in symmetrical 140 mM K+ solutions in excised, inside-out patches. A, left: sample recordings of a K+ channel at 7 different holding potentials with the inside of the patch exposed to 1 μM Ca2+. A, right: I-V relationship of single channel current recorded in the same cell. Each point represents the average current amplitude ± SE over a 3-s period at the indicated holding potential. Slope conductance was 269 pS for this cell. Calibration bar = 15 pA, 0.5 s. B: BK channel activity is increased when the bath solution (the inside surface of the patch) is switched from low (nominally 0 Ca2+) to high (10 μM Ca2+). Holding potential (HP) = +20 mV. Calibration bar = 10 pA, 0.5 s. C: a plot of N·Po (where N is number of channels and Po is open probability) vs. holding potential for 4 cells. Ca2+ was buffered to 300 nM. D: BK channel recording for an ∼8-min period before and after diffusion of IBTX to the outer surface of the patch (see text for backfilling details). The inside of the patch was exposed to 600 nM Ca2+. HP = +60 mV. Calibration bar = 10 pA, 1 min.
In Fig. 9B, the activity of a similar channel in another patch markedly increased when the bath solution was switched from ∼0 to 10 μM Ca2+, consistent with the known Ca2+ sensitivity of BK. Figure 9C is a plot of the calculated N·Po (where N is number of channels and Po is open probability) for four recordings under conditions similar to those in Fig. 9A. N·Po was calculated using previously described methods (47). The increase in N·Po at positive membrane potentials is also consistent with the behavior of a BK channel. Finally, Fig. 9D shows a recording where the pipette tip was loaded with the normal pipette solution for a distance of ∼100 μm after which the rest of the pipette was backfilled with the same solution containing IBTX (100 nM). We (57) have previously shown that this procedure allows the recording of BK channel activity for the 3–5 min required for IBTX diffusion to the outer surface of the patch. The activity of the channel in this patch (∼250 pS) abruptly ceased at ∼5 min, whereas in other patches not exposed to IBTX, stable BK channel activity could be recorded for much longer periods of time (57) (see also Fig. 10C).
Fig. 10.
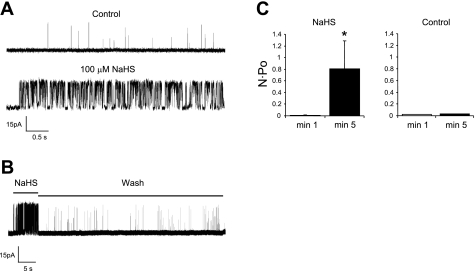
Effect of NaHS on single channel BK current. A: 2 tracings from the same HMEC showing BK channel activity before and after bath application of NaHS (100 μM). The inside of the patch was exposed to 600 nM Ca2+. HP = +60 mV. Calibration bar = 15 pA, 0.5 s. B: recording of a single BK channel in another patch showing that the effect of NaHS was reversible with washout. N·Po for this channel was 0.0014 for control and 0.42 for NaHS. The inside of the patch was exposed to 600 nM Ca2+. HP = +60 mV. Calibration bar = 15 pA, 5 s. C: average N·Po from 4 cells during 1 min (patch exposed to control solution) and 2 min (patch exposed to NaHS); right: time control when no NaHS was present at 5 min (n = 2). *Significant difference from the 1-min group.
Having demonstrated that we could record single BK channels in excised, inside-out patches from HMECs, we tested the effect of NaHS on channel activity. Bath application of NaHS (100 μM) resulted in a rapid and large increase in the open probability of the single BK channel (∼150 pS) shown in Fig. 10A. The effect could also be rapidly washed out (Fig. 10B; recording from a different cell). The increase in N·Po induced by NaHS is summarized in Fig. 10C (4 cells), where, on average, NaHS produced an 83-fold increase in N·Po. Control experiments under similar conditions in the absence of NaHS showed no substantial change in N·Po over the same time course (n = 2).
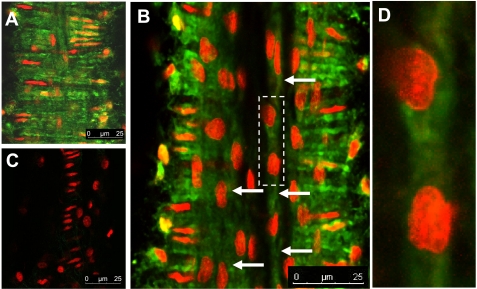
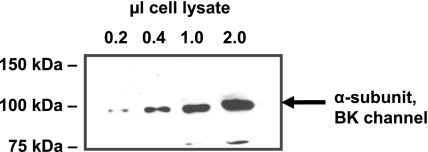
In light of recent controversy regarding the effects of endothelial cell isolation and culture on BK channel expression relative to native vessels (41), we examined BKα subunit expression in isolated mesenteric venules using an immunohistochemical approach and confocal microscopy. Figure 11A illustrates marked BKα staining in vascular smooth muscle cells. Figure 11, B and D, shows comparatively less intense, but positive, staining for BKα in endothelial cells (Fig. 9B, arrows). Nuclear staining confirms cellular orientation consistent with endothelial cells. Figure 11C shows a control with secondary antibody only, plus propidium iodide staining to identify cell nuclei. Previous studies (63) have demonstrated the specifity of the primary antibody directed at BKα. Finally, to confirm the presence of the BKα subunit in microvascular endothelial cells, freshly isolated rat microvascular endothelial cells were lysed, and their proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with BKα subunit antibody (Fig. 12). Our data show that the BKα subunit is expressed in native (noncultured) coronary endothelial cells.
Fig. 11.
Immunohistochemical identification of BK in a cannulated mesenteric venule taken from a control animal. Small mesenteric venules were cannulated and pressurized (∼10 mmHg) as described in the text. BK was identified with an antibody (Alomone APC-107) directed against the α-subunit (green). Nuclei were stained with propidium iodide (red) to assist in identification of cells. Images of cannulated vessels were taken using confocal microscopy. A: marked BK staining in vascular smooth muscle cells. B: comparatively less intense, but positive, staining for BK in endothelial cells (arrows). Nuclear staining confirms cellular orientation consistent with endothelial cells. Image represents a composite 10 Z-stack images taken at 0.1-μm distances. C: secondary antibody only control with propidium iodide staining to identify cell nuclei. Previous studies (63) using Western blotting have confirmed specificity of the primary antibody. D: enlargment of the area indicated by the dashed rectangle overlying B. Endothelial staining for BK was obtained in 6 additional preparations processed in a similar manner.
Fig. 12.
Western blot verifying expression of BKα in noncultured endothelial cells. Increasing amounts of freshly isolated coronary endothelial cell lysates were subjected to SDS-PAGE. Separated proteins were blotted to nitrocellulose, and blots were probed with antibody specific for BKα subunit. A 100-kDa band representing BKα subunit is shown in the blot, indicating the expression of BK channels in coronary endothelial cells.
DISCUSSION
A growing body of evidence indicates that H2S exerts a variety of effects that may limit I/R-induced injury and inflammation. For example, H2S produces vasorelaxation by activating KATP channels (9, 16, 23, 30, 50, 69, 70) and can enhance the vasodilatory effects of NO (23, 68), actions that may improve tissue perfusion in I/R. In addition, this gaseous signaling molecule reduces mitochondrial respiration, which may conserve ATP levels in ischemic tissues (3, 4, 17, 20, 21, 24, 33, 34, 37). Recent evidence (66) has shown that inhibition of endogenous H2S synthesis reduces leukocyte rolling velocity and increases leukocyte adherence to mesenteric postcapillary venules under baseline conditions. The latter results indicate that H2S production serves as an endogenous modulator of leukocyte/endothelial cell adhesive interactions. While the aforementioned results suggest that H2S may be effective in reducing I/R injury when applied during the ischemic insult, the results of the present study (Figs. 2 and 3) and our earlier work (64) provide the first evidence that H2S may also induce protection in I/R by instigating the development of a preconditioned, anti-inflammatory phenotype such that postcapillary venules fail to support leukocyte rolling and adhesion in tissues exposed to I/R 24 h after treatment with this gaseous signaling molecule.
Potassium channels are ubiquitously expressed cell membrane proteins that participate in a wide variety of physiological processes, including regulation of vasomotor tone, heart rate, neurotransmitter release, and muscle contraction. Given the broad range of functional activities, it is not surprising that a diverse spectrum of functionally distinct potassium channels exist. With regard to preconditioning, ATP-sensitive potassium channels have received the most attention. However, the results of several recent studies (5, 20, 51) point to the importance of BK channels, as critical triggers for the development of the protected phenotype in response to preconditioning stimuli. These channels are expressed by parenchymal cells in a variety of organs but also in vascular tissues, including endothelial and vascular smooth muscle cells (20). BK channels can be activated by elevations in intracellular calcium and by membrane depolarization and are characterized by a large single channel conductance and sensitivity to IBTX. Each BK channel is comprised of pore-forming α-subunits and modulatory β-subunits (20). In the heart, BK channels are thought to be present only in the mitochondria, whereas endothelial and other cells express these channels on the plasma membrane (20).
In earlier work, we (18, 27, 43, 60, 61, 65) demonstrated that a variety of preconditioning stimuli, including antecedent ethanol ingestion, short bouts of ischemia, adenosine A2-receptor agonists, exogenous CGRP or bradykinin, and AMP-activated protein kinase activators induce the development of an anti-inflammatory phenotype that is triggered by NO formed by eNOS. We (Zuidema, M., et al., unpublished observations) recently demonstrated that H2S donor treatment 24 h before I/R also induced preconditioning by an eNOS-dependent mechanism that also involves activation of KATP channels. Due to the recently suggested cross-talk between KATP channels and BK channels in sildenafil-induced late preconditioning (53), the present study is of particular interest. As we have already shown that the H2S donor elicits a preconditioned phenotype by activation of KATP channels, we sought to determine whether BK channels also play a role in NaHS-PC.
The present study provides strong support for the notion that BK channels play an important role in the development of an anti-inflammatory phenotype in mice treated with NaHS, such that postcapillary venules fail to support increased leukocyte rolling and adhesion induced by exposing the bowel to I/R 24 h after application of this preconditioning stimulus. Three lines of evidence support this conclusion. First, coincident administration of the BK channel inhibitor paxilline with NaHS 24 h before I/R effectively abolished the effects of NaHS-PC to prevent postischemic leukocyte rolling and adhesion. Second, the anti-inflammatory effects of NaHS-PC were mimicked by preconditioning with the BK channel activator NS-1619. Third, we provided evidence that intact mesenteric venules express the BKα subunit in both endothelial and vascular smooth muscle cells and that exposing cultured endothelial cells to NaHS, at the same concentration achieved in our in vivo studies, activates an IBTX-sensitive current.
A cardioprotective role for BK channels was first suggested by Ghatta et al. (20), who demonstrated that administration of NS-1619 just before I/R reduced myocardial infarct size. The powerful infarct-sparing effects of BKCa channel activation were prevented by paxilline, providing additional support for the notion that these specific channels limit infarct size. Subsequent work has demonstrated NS-1619 induces both early and late phase preconditioning in myocardium, effects that were not blocked by KATP channel antagonists but that were mitigated by the selective BK blockers iberiotoxin or paxilline (20, 51), especially IBTX. In addition, the cardioprotective effects of estradiol appear to involve BK channel activation in rat ventricular myocytes exposed to simulated ischemia (35). There is also evidence that the infarct-sparing effects of ischemic preconditioning may involve BK channel activation (5, 20). Our results demonstrate that in addition to infarct-sparing effects in the myocardium, BK channel activation also promotes the development of an anti-inflammatory phenotype that may reduce leukocyte-dependent reperfusion injury. Our data also extend the notion that antecedent BK channel activation produces protective effects in the small intestine in addition to the heart. Finally, we also demonstrate for the first time the contribution of BK channels in response to a novel preconditioning stimulus, NaHS.
Our conclusions are partly based on the specificity of paxilline and NS-1619 for BK channels in vivo. However, it is known that these agents produce other effects. For example, paxilline has been shown to inhibit sarco(endo)plasmic reticulum Ca2+-ATPase both in vitro and in vivo (52, 54), although at concentrations higher than were likely achieved in our in vivo experiments. IBTX is a more specific BK channel inhibitor, but use of this neurotoxin in vivo is precluded by its expense for in vivo experiments. On the other hand, NS-1619 or H2S can inhibit complex I of the mitochondrial respiratory chain in tumor cells and modify mitochondrial membrane potential (32) . This has potential implications for our studies, since H2S has been reported to inhibit mitochondrial cytochrome oxidase, leading to oxidative stress. Since the generation of reactive oxygen species has been implicated as a trigger for ischemic and ethanol preconditioning (60), it is possible that the anti-inflammatory state induced by NS-1619 preconditioning may be related to oxidant production. Although the actions of NS-1619 are not inhibited by KATP channel antagonists (5), more recent work indicates that NS-1619 stimulates Ca2+-activated chloride channels (38) and there is some evidence that chloride channels participate in ischemic preconditioning in the myocardium (8).
While we have not directly tested these potential complicating side effects, our observation that NaHS activates BK channels in endothelial cells, when coupled with the demonstration that paxilline blocks the protective actions of NS-1619 in our model, argues in favor of the interpretation that NaHS-PC occurs by a BK channel-dependent mechanism. Nevertheless, our electrophysiological studies suggest that NaHS also activated IK and/or SK channels (Figs. 6–7), both of which are known to be activated at more negative potentials than BK channels. However, activation of IK or SK by NaHS does not appear to participate in the development of an anti-inflammatory phenotype invoked by treatment with this H2S donor, because inhibition of these channels failed to abrogate its ability to prevent postischemic leukocyte rolling and adhesion.
Even though our data do not support a role for SK and IK in preconditioning by NaHS, we show for the first time that an anti-inflammatory state can be invoked by activation of one or both of these channels with NS-309. This observation is important because it suggests another avenue for potential therapeutic production of a protected phenotype and may imply that SK and/or IK activation induces preconditioning by downstream signaling mechanisms that are distinct from those provoked by H2S donors and perhaps other interventions that instigate the development of a protected state and limit I/R injury. Clearly, much additional work will be required to investigate these interesting possibilities.
If BK channels play a critical role in the preconditioning effect of H2S, why do our electrophysiological measurements suggest only a small contribution of BK channels at physiological (resting) membrane potentials for endothelial cells? In Fig. 8, we recorded a slight but not significant depolarizing effect of IBTX on Em of HMECs, and in Fig. 6, we detected only a slight enhancement of BK current by NaHS (in the presence of SK/IK blockade) at −20 mV. There are several possible explanations for these results. First, small BK currents at −20 mV do not necessarily mean an insignificant effect of BK channels because their effect on Em would depend on the input resistance of the endothelial cell, which is not known in vivo. These results suggest that membrane potentials in the −20- to −35-mV range appear to be near a threshold for seeing a consistent effect of NaHS, which is not surprising since it is near the threshold for voltage activation of BK (at low Ca2+ levels) in other cell types (26, 39). Second, a small or barely significant change in Em or BK current under whole cell patch-clamp conditions does not preclude a more robust effect under physiological conditions in vivo. Recall that the cells are dialyzed with an EGTA-containing pipette solution during these recordings. Thus any contribution to BK activation by an increase in intracellular [Ca2+] would be missed. The same argument applies to cGMP (26, 32) and other small molecules known to regulate BK channels (e.g., causing a significant leftward shift in the membrane potential-open probability relationship), which would be diluted or completely washed out by pipette dialysis. Additionally, a recent study (62) has identified a family of auxiliary proteins that allow significant activation of the BK channel at resting membrane potentials and low intracellular [Ca2+] levels, and it is possible that such proteins are also affected by whole cell dialysis and/or lose expression with passage in cell lines such as HMECs. Although we can only speculate at this time, it is possible that the effect of H2S on BK channels is mediated by a combination of several intracellular signaling pathways. Alternatively, as mentioned below, it is also possible that the requirement of BK channel activation in the preconditioning effect of H2S in the in vivo mouse intestine occurs in another cell type, not the endothelium.
In our earlier work (18, 27, 43, 60, 61, 65), we demonstrated that preconditioning with short bouts of ischemia (ischemic preconditioning) or agents such as NO donors, adenosine A2 receptor agonists, β2-adrenergic receptor agonists, KATP activators, bradykinin, or CGRP all act to prevent postischemic leukocyte rolling and adhesion. Since ischemic preconditioning and each of these agents also produce vasodilation, as do activators of SK, IK, and BK, it is possible that vasodilation per se plays a role in the development of the anti-inflammatory phenotype that occurs in response to antecedent treatment with these agents before I/R. However, treatment with verapamil, which produces vasodilation via inhibition of L-type calcium channels (and not through activation of adenylyl cyclase-, guanylyl cyclase-, KATP-, or calcium-activated potassium channel-dependent mechanisms), 24 h before I/R did not prevent postischemic leukocyte rolling and adhesion (Fig. 3). While this result suggests that vasodilation per se does not account for preconditioning, our interpretation should be viewed with caution, since our experimental preparation does not allow assessment of vasodilator responses during the period of exposure to the preconditioning stimulus with subsequent measurement of leukocyte rolling and adhesion during I/R 24 h later. This would require at least two aseptic surgical procedures, with experimental observations, in the same animal. Nevertheless, the notion that vasodilation per se is not required for development of an anti-inflammatory phenotype is consistent with the fact that other approaches to instigate preconditioning do not relax vascular smooth muscle (e.g., low dose ethanol ingestion), while others elicit vasoconstriction (e.g., angiotensin II).
It is possible that the effects of NaHS-PC to prevent leukocyte rolling and leukocyte adhesion are likely manifest in the endothelial cells of postcapillary venules and cannot be attributed to a direct action on neutrophils. This notion is based on the recent report that BK channel activity is absent in both human and mouse neutrophils (15) and the fact that H2S is unlikely to persist 24 h after NaHS treatment owing to a short circulating half-life. Our electrophysiologic data also support this concept in that BK channels were activated by NaHS exposure in cultured microvascular endothelial cells. Our immunohistochemistry studies indicate that BKα subunit expression occurs in native mesenteric venules (Fig. 11), in contrast to endothelium in intact large arteries, which do not express this protein (19, 41). This is a controversial area, however, as others (2) have provided functional evidence for endothelial BK by comparing vasodilator responses to nitroglycerin in the presence and absence of iberiotoxin in endothelium-intact vs. -denuded porcine coronary arterial rings. BK channel expression has also been reported in primary cultures of pig coronary artery endothelium (3, 4). Interestingly, we did not detect BKα subunit expression in the endothelium of superior mesenteric artery or vein, although subunit staining was intense in the vascular smooth muscle cells of these large vessels in mice (data not shown). These observations are consistent with earlier reports indicating that BK channel expression was limited to vascular smooth muscle cells of large arteries isolated from bovine hearts discussed above (17). Finally, we confirmed that freshly isolated coronary microvascular endothelial cells show expression of the α-subunit of the BK channel (Fig. 12).
Taken together, our results support the possibility that microvascular endothelial cells express functional BK channels that may be involved in the anti-inflammatory effects of antecedent H2S. However, it is important to emphasize that vascular smooth muscle cells (and perhaps pericytes) express the BKα subunit in mesenteric venules (Fig. 11), suggesting that H2S could activate BK channels in these cells to invoke preconditioning. Recent work (67) indicates that pericytes are closely apposed to mesenteric venular endothelial cells, typically separated by just 100–200 nm. Moreover, 85% of the surface area of mesenteric venules is covered by pericytes. In addition, a smaller number of fibroblasts, which express BK channels (37), were noted just outside the pericyte layer (67). These observations suggest the possibility that pericytes and/or fibroblasts may communicate BK-dependent signals to venules to modulate leukocyte-endothelial cell adhesive interactions. According to this scenario, H2S-induced BK channel activation on vascular smooth muscle cells, pericytes, or fibroblasts may result in communication of signaling events that, either by a separate mechanism or through augmentation of the effects of endothelial BK activation, prevent the ability of venular endothelium to support leukocyte rolling and adhesion after I/R. Clearly, much additional work will have to be conducted to determine the relative contribution of BK channel activation in the different cell types comprising the venular wall to the ability of the endothelium to resist the proinflammatory effects of I/R.
The mechanism whereby NaHS activates BK channels to elicit preconditioning is unclear as are the downstream signaling events that mediate NaHS-PC. However, H2S is known to elicit an oxidative stress secondary to its effect to inhibit cytochrome oxidase, which may activate BK channels (14). Others (46) have recently suggested that H2S directly increases BK channel activity by modulating the redox status of critical sulfhydryl groups located on the cytoplasmic side of the channel protein. Conversely, it has also been proposed that mitochondrial BK channel opening increases matrix K+ concentration, which in turn alters mitochondrial matrix H+ by K+/H+ exchange, thereby stabilizing mitochondrial membrane potential despite increased respiration. This results in enhanced generation of superoxide (49). In light of our earlier work demonstrating a role for eNOS-derived NO in the development of NaHS-PC (64) and the fact that NO can modulate BK channel activity in mesenteric vessels (6), it is tempting to speculate that NaHS-induced eNOS activation may be an early upstream event. With regard to downstream effectors, it is unlikely that NOS activation occurs secondary (i.e., downstream) to BK channel activation because Wang et al. (54) have provided evidence that NS-1619 produced myocardial preconditioning by a NOS-independent mechanism. However, we have obtained strong evidence that heme oxygenase-1 is an end effector of the anti-inflammatory phenotype during I/R 24 h, a conclusion based on the observations that NaHS-PC is ineffective in wild-type mice treated with a heme oxygenase inhibitor during I/R and is absent in HO-1 knockout animals (Zuidemam, M., et al., unpublished observations). Nevertheless, the mechanistic links between BK channel activation induced by NaHS and increased HO-1 activity during I/R 24 h later are unknown. Clearly, much additional work will be required to answer these important questions.
In summary, the results of this study provide evidence for a novel triggering mechanism for the development of the anti-inflammatory effects induced by late phase NaHS-PC. Specifically, the effect of NaHS-PC to prevent the postischemic increases in leukocyte rolling and adherence appears to be initiated by BK channel-dependent mechanisms. Moreover, our data show that an anti-inflammatory phenotype can be elicited by activation of the BK channels with NS-1619 in the murine small intestine. Our studies also demonstrate the presence of BKα in endothelial and vascular smooth muscle cells of intact mesenteric venules as well as freshly isolated coronary endothelial cells and that NaHS elicits an iberiotoxin-sensitive whole cell potassium current in microvascular endothelial cells. These observations provide new insight regarding the potential use of BK channel activators and NaHS as rational therapeutic agents to limit leukocyte-dependent reperfusion injury in I/R and perhaps other inflammatory conditions. Although IK and SK do not appear to play a role in NaHS preconditioning, interventions that promote IK/SK activation may be useful as therapeutic interventions in ischemic states. Finally, our results suggest the possibility that development of interventions directed at enhancing the endogenous production of H2S may be effective in limiting the inflammatory component of I/R injury.
GRANTS
This work was supported by grants from the National Institutes of Health (AA-014945, HL-082816, HL-071796, and HL-092241).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Alaina Boyett and Christine Korthuis contributed to the completion of this work and their help is greatly appreciated. We also thank Srikanth Ella for assistance in the confocal microscopy studies.
REFERENCES
- 1.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 99: 683– 690, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Bang L, Boesgaard S, Nielsen-Kudsk JE, Vejlstrup NG, Aldershvile J. Nitroglycerin-mediated vasorelaxation is modulated by endothelial calcium-activated potassium channels. Cardiovasc Res 43: 772– 778, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Baron A, Frieden M, Beny JL. Epoxyeicosatrienoic acids activate a high-conductance, Ca(2+)-dependent K+ channel on pig coronary artery endothelial cells. J Physiol 504: 537– 543, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron A, Frieden M, Chabaud F, Beny JL. Ca(2+)-dependent non-selective cation and potassium channels activated by bradykinin in pig coronary artery endothelial cells. J Physiol 493: 691– 706, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao CM, Xia Q, Gao Q, Chen M, Wong TM. Calcium activated potassium channel triggers cardioprotection of ischemic preconditioning. J Pharmacol Exp Ther 312: 644– 650, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Carrier GO, Fuchs LC, Winecoff AP, Giulumian AD, White RE. Nitrovasodilators relax mesenteric microvessels by cGMP-induced stimulation of Ca-activated K channels. Am J Physiol Heart Circ Physiol 273: H76– H84, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Ceroni L, Ellis A, Wiehler WB, Jiang YF, Ding H, Triggle CR. Calcium-activated potassium channel and connexin expression in small mesenteric arteries from eNOS-deficient (eNOS−/−) and eNOS-expressing (eNOS+/+) mice. Eur J Pharmacol 560: 193– 200, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Liu LL, Ye LL, McGuckin C, Tamowski S, Scowen P, Tian H, Murray K, Hatton WJ, Duan D. Targeted inactivation of cystic fibrosis transmembrane conductance regulator chloride channel gene prevents ischemic preconditioning in isolated mouse heart. Circulation 110: 700– 704, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316– H2323, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Colden-Stanfield M, Schilling WP, Ritchie AK, Eskin SG, Navarro LT, Kunze DL. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res 61: 632– 640, 1987 [DOI] [PubMed] [Google Scholar]
- 11.Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol 553: 183– 189, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominy JE, Stipanuk MH. New roles for cysteine and transsulfuration enzymes: production of H2S, a neuromodulator and smooth muscle relaxant. Nutr Rev 62: 348– 353, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Dong DL, Zhang Y, Lin DH, Chen J, Patschan S, Goligorsky MS, Nasjletti A, Yang BF, Wang WH. Carbon monoxide stimulates the Ca2(+)-activated big conductance k channels in cultured human endothelial cells. Hypertension 50: 643– 651, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Douglas RM, Lai JC, Bian S, Cummins L, Moczydlowski E, Haddad GG. The calcium-sensitive large-conductance potassium channel (BK/MAXI K) is present in the inner mitochondrial membrane of rat brain. Neuroscience 139: 1249– 1261, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Essin K, Salanova B, Kettritz R, Sausbier M, Luft FC, Kraus D, Bohn E, Autenrieth IB, Peschel A, Ruth P, Gollasch M. Large-conductance calcium-activated potassium channel activity is absent in human and mouse neutrophils and is not required for innate immunity. Am J Physiol Cell Physiol 293: C45– C54, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V, Morelli A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology 42: 539– 548, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J Pharmacol Exp Ther 299: 818– 824, 2001 [PubMed] [Google Scholar]
- 18.Gaskin FS, Kamada K, Yusof M, Korthuis RJ. 5[prime]-AMP-activated protein kinase activation prevents postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol 292: H326– H332, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Gauthier KM, Liu C, Popovic A, Albarwani S, Rusch NJ. Freshly isolated bovine coronary endothelial cells do not express the BK Ca channel gene. J Physiol 545: 829– 836, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghatta S, Nimmagadda D, Xu X, O'Rourke ST. Large-conductance, calcium-activated potassium channels: structural and functional implications. Pharmacol Ther 110: 103– 116, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Grgic I, Eichler I, Heinau P, Si H, Brakemeier S, Hoyer J, Kohler R. Selective blockade of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler Thromb Vasc Biol 25: 704– 709, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Himmel HM, Whorton AR, Strauss HC. Intracellular calcium, currents, and stimulus-response coupling in endothelial cells. Hypertension 21: 112– 127, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527– 531, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Hoyer J, Distler A, Haase W, Gogelein H. Ca2+ influx through stretch-activated cation channels activates maxi K+ channels in porcine endocardial endothelium. Proc Natl Acad Sci USA 91: 2367– 2371, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaggar JH, Wellman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, Kleppisch T, Rubart M, Stevenson AS, Lederer WJ, Knot HJ, Bonev AD, Nelson MT. Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand 164: 577– 587, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Kaczorowski GJ, Knaus HG, Leonard RJ, McManus OB, Garcia ML. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J Bioenerg Biomembr 28: 255– 267, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Kamada K, Gaskin FS, Yamaguchi T, Carter P, Yoshikawa T, Yusof M, Korthuis RJ. Role of calcitonin gene-related peptide in the postischemic anti-inflammatory effects of antecedent ethanol ingestion. Am J Physiol Heart Circ Physiol 290: H531– H537, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kamouchi M, Trouet D, De Greef C, Droogmans G, Eggermont J, Nilius B. Functional effects of expression of hslo Ca2+ activated K+ channels in cultured macrovascular endothelial cells. Cell Calcium 22: 497– 506, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki J, Davis GE, Davis MJ. Regulation of Ca2+-dependent K+ current by alphavbeta3 integrin engagement in vascular endothelium. J Biol Chem 279: 12959– 12966, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol 26: 13– 19, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Muraki K, Imaizumi Y, Ohya S, Sato K, Takii T, Onozaki K, Watanabe M. Apamin-sensitive Ca2+-dependent K+ current and hyperpolarization in human endothelial cells. Biochem Biophys Res Commun 236: 340– 343, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Nardi A, Calderone V, Chericoni S, Morelli I. Natural modulators of large-conductance calcium-activated potassium channels. Planta Med 69: 885– 892, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415– 1459, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Nilius B, Wohlrab W. Potassium channels and regulation of proliferation of human melanoma cells. J Physiol 445: 537– 548, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohya S, Kuwata Y, Sakamoto K, Muraki K, Imaizumi Y. Cardioprotective effects of estradiol include the activation of large-conductance Ca2+-activated K+ channels in cardiac mitochondria. Am J Physiol Heart Circ Physiol 289: H1635– H1642, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Papassotiriou J, Kohler R, Prenen J, Krause H, Akbar M, Eggermont J, Paul M, Distler A, Nilius B, Hoyer J. Endothelial K(+) channel lacks the Ca(2+) sensitivity-regulating beta subunit. FASEB J 14: 885– 894, 2000 [PubMed] [Google Scholar]
- 37.Roh S, Choi S, Lim I. Involvement of protein kinase A in nitric oxide stimulating effect on a BK(Ca) channel of human dermal fibroblasts. J Invest Dermatol 127: 2533– 2538, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Saleh SN, Angermann JE, Sones WR, Leblanc N, Greenwood IA. Stimulation of Ca2+-gated Cl− currents by the calcium-dependent K+ channel modulators NS1619 {1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2 H-benzimidazol-2-one} and isopimaric acid. J Pharmacol Exp Ther 321: 1075– 1084, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7: 921– 931, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Sanchez M, McManus OB. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35: 963– 968, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Sandow SL, Grayson TH. Limits of isolation and culture: intact vascular endothelium and BKCa. Am J Physiol Heart Circ Physiol 297: H1– H7, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Sharma NR, Davis MJ. Mechanism of substance P-induced hyperpolarization of porcine coronary artery endothelial cells. Am J Physiol Heart Circ Physiol 266: H156– H164, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Shigematsu S, Ishida S, Gute DC, Korthuis RJ. Bradykinin-induced proinflammatory signaling mechanisms. Am J Physiol Heart Circ Physiol 283: H2676– H2686, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Shigematsu S, Ishida S, Gute DC, Korthuis RJ. Postischemic anti-inflammatory effects of bradykinin preconditioning. Am J Physiol Heart Circ Physiol 280: H441– H454, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Shintani Y, Node K, Asanuma H, Sanada S, Takashima S, Asano Y, Liao Y, Fujita M, Hirata A, Shinozaki Y, Fukushima T, Nagamachi Y, Okuda H, Kim J, Tomoike H, Hori M, Kitakaze M. Opening of Ca(2+)-activated K(+) channels is involved in ischemic preconditioning in canine hearts. J Mol Cell Cardiol 37: 1213– 1218, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Sitdikova GF, Weiger TM, Hermann A. Hydrogen sulfide increases calcium-activated potassium (BK) channel activity of rat pituitary tumor cells. Pflügers Arch 459: 389– 397 [DOI] [PubMed] [Google Scholar]
- 47.Sohma Y, Harris A, Wardle CJ, Argent BE, Gray MA. Two barium binding sites on a maxi K+ channel from human vas deferens epithelial cells. Biophys J 70: 1316–1325, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoen R, Lossius K, Persson AA, Karlsson JO. Relative significance of the nitric oxide (NO)/cGMP pathway and K+ channel activation in endothelium-dependent vasodilation in the femoral artery of developing piglets. Acta Physiol Scand 171: 29– 35, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Stowe DF, Aldakkak M, Camara AK, Riess ML, Heinen A, Varadarajan SG, Jiang MT. Cardiac mitochondrial preconditioning by big Ca2+-sensitive K+ channel opening requires superoxide radical generation. Am J Physiol Heart Circ Physiol 290: H434– H440, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol 137: 139– 145, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol 8: 321– 329, 1998 [DOI] [PubMed] [Google Scholar]
- 52.WANGR Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792– 1798, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Fisher PW, Xi L, Kukreja RC. Essential role of mitochondrial Ca2+-activated and ATP-sensitive K+ channels in sildenafil-induced late cardioprotection. J Mol Cell Cardiol 44: 105– 113, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Yin C, Xi L, Kukreja RC. Opening of Ca2+-activated K+ channels triggers early and delayed preconditioning against I/R injury independent of NOS in mice. Am J Physiol Heart Circ Physiol 287: H2070– H2077, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Wang XL, Ye D, Peterson TE, Cao S, Shah VH, Katusic ZS, Sieck GC, Lee HC. Caveolae targeting and regulation of large conductance Ca(2+)-activated K+ channels in vascular endothelial cells. J Biol Chem 280: 11656– 11664, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Wiecha J, Munz B, Wu Y, Noll T, Tillmanns H, Waldecker B. Blockade of Ca2+-activated K+ channels inhibits proliferation of human endothelial cells induced by basic fibroblast growth factor. J Vasc Res 35: 363– 371, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Wu X, Yang Y, Gui P, Sohma Y, Meininger GA, Davis GE, Braun AP, Davis MJ. Potentiation of large conductance, Ca2+-activated K+ (BK) channels by alpha5beta1 integrin activation in arteriolar smooth muscle. J Physiol 586: 1699– 1713, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O'Rourke B. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science 298: 1029– 1033, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi T, Dayton C, Shigematsu T, Carter P, Yoshikawa T, Gute DC, Korthuis RJ. Preconditioning with ethanol prevents postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol 283: H1019– H1030, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi T, Dayton CB, Ross CR, Yoshikawa T, Gute DC, Korthuis RJ. Late preconditioning by ethanol is initiated via an oxidant-dependent signaling pathway. Free Radic Biol Med 34: 365– 376, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Yamaguchi T, Kamada K, Dayton C, Gaskin FS, Yusof M, Yoshikawa T, Carter P, Korthuis RJ. Role of eNOS-derived NO in the postischemic anti-inflammatory effects of antecedent ethanol ingestion in murine small intestine. Am J Physiol Heart Circ Physiol 292: H1435– H1442, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Yan J, Aldrich RW. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 466: 513– 516 [DOI] [PubMed] [Google Scholar]
- 63.Yang Y, Murphy TV, Ella SR, Grayson TH, Haddock R, Hwang YT, Braun AP, Peichun G, Korthuis RJ, Davis MJ, Hill MA. Heterogeneity in function of small artery smooth muscle BKCa: involvement of the beta1-subunit. J Physiol 587: 3025–3044, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yusof M, Kamada K, Kalogeris T, Gaskin FS, Korthuis RJ. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO- and p38 MAPK-dependent mechanism. Am J Physiol Heart Circ Physiol 296: H868– H876, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yusof M, Kamada K, Spencer Gaskin F, Korthuis RJ. Angiotensin II mediates postischemic leukocyte-endothelial interactions: role of calcitonin gene-related peptide. Am J Physiol Heart Circ Physiol 292: H3032– H3037, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J 20: 2118– 2120, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Zhang X, Adamson RH, Curry FE, Weinbaum S. Transient regulation of transport by pericytes in venular microvessels via trapped microdomains. Proc Natl Acad Sci USA 105: 1374– 1379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol 81: 848– 853, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol 283: H474– H480, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008– 6016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]