Abstract
Kallistatin is a regulator of vascular homeostasis capable of controlling a wide spectrum of biological actions in the cardiovascular and renal systems. We previously reported that kallistatin inhibited intracellular reactive oxygen species formation in cultured cardiac and renal cells. The present study was aimed to investigate the role and mechanisms of kallistatin in protection against oxidative stress-induced vascular injury and endothelial cell apoptosis. We found that kallistatin gene delivery significantly attenuated aortic superoxide formation and glomerular capillary loss in hypertensive DOCA-salt rats. In cultured endothelial cells, kallistatin suppressed TNF-α-induced cellular apoptosis, and the effect was blocked by the pharmacological inhibition of phosphatidylinositol 3-kinase and nitric oxide synthase (NOS) and by the knockdown of endothelial NOS (eNOS) expression. The transduction of endothelial cells with adenovirus expressing dominant-negative Akt abolished the protective effect of kallistatin on endothelial apoptosis and caspase activity. In addition, kallistatin inhibited TNF-α-induced reactive oxygen species formation and NADPH oxidase activity, and these effects were attenuated by phosphatidylinositol 3-kinase and NOS inhibition. Kallistatin also prevented the induction of Bim protein and mRNA expression by oxidative stress. Moreover, the downregulation of forkhead box O 1 (FOXO1) and Bim expression suppressed TNF-α-mediated endothelial cell death. Furthermore, the antiapoptotic actions of kallistatin were accompanied by Akt-mediated FOXO1 and eNOS phosphorylation, as well as increased NOS activity. These findings indicate a novel role of kallistatin in the protection against vascular injury and oxidative stress-induced endothelial apoptosis via the activation of Akt-dependent eNOS signaling.
Keywords: endothelium, endothelial nitric oxide synthase superoxide, tumor necrosis factor-α, Bim, forkhead box O 1
because of its unique position, the vascular endothelium acts as a barrier against various injuries. Oxidative stress plays an important role in the development of endothelial dysfunction, apoptosis, hypertension, and organ injury (23, 25). Apoptosis of endothelial cells critically disturbs the integrity of the endothelial monolayer and contributes to the initiation of proinflammatory events. The continuous production of nitric oxide (NO) by endothelial cells is essential to the normal function of the endothelium, as evidenced by in vitro and in vivo studies (1, 18). Endothelial-derived NO also prevents the adhesion of neutrophils and the expression of macrophage chemotactic proteins (9, 17, 20). NO donors, in physiologically relevant concentrations, block endothelial cell apoptosis induced by various stimuli, including the proinflammatory effectors tumor necrosis factor-α (TNF-α) and oxidative stress (19, 26). Therefore, endothelial NO synthase (eNOS) synthesis and NO production from endothelial cells may have an important role in the inhibition of oxidative stress and subsequent endothelial apoptosis.
Kallistatin is a plasma protein that exhibits pleiotropic effects in hypertension, inflammation, apoptosis, angiogenesis, hypertrophy, and fibrosis (5, 7, 13, 21, 27, 33). Transgenic mice overexpressing kallistatin are more resistant to lipopolysaccharide-induced inflammation and lethality compared with wild-type mice (7). Kallistatin was also observed to reduce ankle swelling, inflammatory cell accumulation, and TNF-α levels in an inflammatory arthritic rat model (33). Moreover, kallistatin gene delivery in rats improved cardiac function and decreased oxidative stress, cardiomyocyte apoptosis, inflammation, and cardiac remodeling after acute myocardial ischemia-reperfusion and chronic myocardial infarction (5, 13). Furthermore, kallistatin administration attenuated salt-induced renal damage, inflammation, and fibrosis in association with increased NO levels and reduced oxidative stress (27). Diminished NO and enhanced oxidative stress play a central role in pathophysiological pathways leading to vascular damage, such as endothelial dysfunction and vascular inflammation. We previously demonstrated that kallistatin inhibited intracellular reactive oxygen species (ROS) formation through NO production in cultured cardiac and renal cells (5, 27). Moreover, our recent study showed that kallistatin inhibited endothelial inflammation by stimulating eNOS expression and NO formation through the interaction with Kruppel-like factor-4 (KLF4) in endothelial cells (28). Therefore, kallistatin may protect against vascular injury by preventing oxidative stress-induced endothelial cell apoptosis via increasing eNOS levels and NO production. This study was designed to investigate the role and signaling mechanisms of kallistatin in endothelial cell apoptosis via Akt-dependent eNOS activation.
MATERIALS AND METHODS
Molecular biological and biochemical reagents.
Adenovirus harboring dominant-negative mutant Akt (Ad.DN-Akt) was kindly provided by Dr. Kenneth Walsh (St. Elizabeth's Medical Center in Boston, MA). Adenoviral vector carrying the human kallistatin cDNA (Ad.KS) or the adenoviral vector alone (Ad.Null) were constructed and prepared as previously described (21). The expression and purification of recombinant human kallistatin were performed as previously described (8). Scrambled eNOS, FOXO1, and Bim small interfering RNA (siRNA) oligonucleotides were purchased from Dharmacon (Lafayette, CO). TNF-α and Nω-nitro-l-arginine methyl ester (l-NAME, NOS inhibitor) were purchased from Sigma (St. Louis, MO); LY-294002 [a phosphatidylinositol 3-kinase (PI3K) inhibitor] and caspase-3 activity kit were obtained from Calbiochem (La Jolla, CA); and antibodies against total and phosphorylated Akt, eNOS, and Bim were purchased from Cell Signaling (Beverly, MA). Human umbilical vein endothelial cells (HUVECs; Lonza, Allendale, NJ) were maintained in endothelial basal medium 2 supplemented with endothelial growth medium-2 kit.
Animal treatments.
All procedures complied with the standard for care and use of animal subjects as stated in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Resources, National Academy of Sciences, Bethesda, MD). The protocols for animal studies were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina. Male Wistar rats (Sprague-Dawley, Harlan, Indianapolis, IN), weighing 200–220 g, were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg) before undergoing left unilateral nephrectomy. One week after surgery, the rats in the sham-operation group (n = 6) received weekly subcutaneous injections of sesame oil and were provided with tap water. Experimental animals received weekly subcutaneous injections of deoxycorticosterone acetate (DOCA; 25 mg/kg body wt; Sigma) suspended in sesame oil and were provided with 1% NaCl drinking water. Two weeks after the initiation of DOCA-salt treatment, each rat was injected with 1 × 1010 plaque-forming units of either Ad.Null or Ad.KS intravenously via the tail vein. Two weeks after gene delivery, the kidneys and aortas were collected for histological analyses.
Measurement of superoxide levels in aorta.
Superoxide levels in aortas were determined by in situ and chemiluminescent methods (22, 30). For the in situ method, aortic sections were immediately placed in optimal cutting temperature-embedding medium and frozen. Aortic ring segments (30 μm thick) received a topical application of the fluorescent dye hydroethidine (2 μM; Molecular Probes, Eugene, OR) and were then placed in a light-protected humidified chamber at 37°C for 30 min. Images were obtained with a laser-scanning confocal microscope. For the measurement of superoxide production by lucigenin-enhanced chemiluminescence (ECL), aortic ring segments were incubated with 1 ml of PBS and lucigenin (5 μM) and immediately placed in a TD20/20 luminometer (Turner Designs, Sunnyvale, CA). The relative light units emitted were integrated for 8 min. Dark current readings (photomultiplier background signal) were automatically subtracted. Background counts were determined from identically treated vessel-free preparations and subtracted from the readings obtained with vessels. Dry weights were obtained for each vascular segment to allow the normalization of activity.
Evaluation of capillary density in renal glomeruli.
Kidneys were fixed in a formaldehyde solution, dehydrated, and embedded. Immunohistochemistry was performed by incubating renal tissue sections (4 μm thick) with a primary antibody against the endothelial cell marker JG-12 at 4°C overnight. Glomerular capillary density was calculated as the number of capillaries per square millimeters using 10 glomeruli from each rat (16).
Transfection of siRNA oligonucleotides.
RNA interference was used to knockdown the expression of eNOS, forkhead box O 1 (FOXO1), and Bim in HUVECs. The siRNA oligonucleotides were transfected into cells using DharmaFECT1 transfection reagent according to the manufacturer's instructions. As a control, the cells were also transfected with scrambled siRNA oligonucleotides. A successful knockdown of target genes was verified by real-time polymerase chain reaction (PCR) and Western blot analysis.
Detection of endothelial cell apoptosis.
Growth-arrested HUVECs were preincubated with kallistatin (0.2 μM) for 30 min. HUVECs were then treated with TNF-α (10 ng/ml) for an additional 12 h. To determine the role of the PI3K-Akt-eNOS prosurvival pathway, inhibitors against PI3K (LY-294002, 5 μM) and NOS (l-NAME, 100 μM) were added 30 min before kallistatin treatment. Moreover, the cells were transfected with scrambled siRNA, eNOS-siRNA, FOXO1-siRNA, or Bim-siRNA for 24 h or transduced with Ad.Null or Ad.DN-Akt for 16 h. The cells were then incubated in the presence or absence of kallistatin (0.2 μM) for 30 min, followed by the addition of TNF-α (10 ng/ml) for 16 h. The cells were incubated with Hoechst 33342 (0.4 mg/ml) for 5 min and examined under a fluorescence microscope to detect apoptotic nuclei. Caspase-3 activity was measured by incubating cell lysates with caspase-3 substrate (10 μM) as described (6). The fluorescence of the cleaved substrate was measured by a spectrofluorometer with excitation and emission wavelengths of 380 and 460 nm, respectively.
Detection of ROS formation in cultured cells.
Intracellular ROS generation was detected using the peroxide-sensitive fluorescent probe 2′,7′-dichlorofluorescin diacetate (DCFH-DA; Molecular Probes) as described previously (27). HUVECs were grown in six-well plates and, upon reaching 80% confluency, were incubated for 30 min with 50 pM DCF-DA diluted in DMEM. The cells were then pretreated with human kallistatin (0.2 μM) for 30 min before the addition of TNF-α (10 ng/ml) for 30 min. After incubation, the cells were washed twice with PBS and imaged using a fluorescence microscope. To measure ROS levels, the cells were seeded onto a 96-well plate and treated. Relative fluorescence was measured using the fluorescence plate reader Victor3TM (Perkin Elmer Life Science) at excitation and emission wavelengths of 485 and 528 nm, respectively.
Measurement of NADPH oxidase activity.
The enzymatic activity of NADPH oxidase in cell homogenates was assessed by lucigenin-ECL as previously described (15). The assay solution contained 50 mM HEPES (pH 7.4), 1 mM EDTA, 6.5 mM MgCl2, 5 μM lucigenin, and 1 mM NADPH. After preincubation at 37°C for 10 min, the reaction was started by adding 50 μg of the homogenate. Fluorescence intensity was continuously monitored for 15 min with a fluorescence reader. The chemiluminescent signals observed in the absence of the homogenate were subtracted from the chemiluminescent signals of the samples. The chemiluminescence signal was corrected for by the protein concentration of each cell homogenate.
Cellular treatment with H2O2.
Growth-arrested HUVECs were preincubated with kallistatin (0.2 μM) for 30 min. The cells were then treated with H2O2 (200 μM) for an additional 16 h. The expression of Bim protein and mRNA was determined by Western blot analysis and real-time PCR.
Real-time PCR.
Total RNA was isolated from cultured cells with TRIzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Total RNA was reverse-transcribed using the High-Capacity cDNA Reverse Transcription Kit. Real-time PCR was performed with the TaqMan Gene Expression Assay and was normalized against 18S RNA using an ABI 7300 real-time PCR System (Applied Biosystems, Foster City, CA). The primers Hs00375807_ml, Hs00167166_ml, and Hs0037580_ml were used for the detection of eNOS, FOXO1, and Bim expression, respectively.
Western blot analysis and NOS activity.
Cells were lysed in radioimmunoprecipitation assay buffer, and proteins were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA). After being blocked for 1 h in a buffer containing 5% nonfat milk, polyvinylidene difluoride membranes were then probed with primary antibodies to total and/or phosphorylated Akt (Ser473), eNOS (Ser1177), FOXO1 (Ser256), and Bim (1:1,000, Cell Signaling) overnight at 4°C. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Chemiluminescence was detected by an ECL-Plus kit (Perkin-Elmer, Foster City, CA). NOS activity was determined by an NOS assay kit (Calbiochem).
Statistical analysis.
All data are presented as means ± SE. Comparison between groups was made using one-way ANOVA with the Fisher multiple comparison test. A probability value of P < 0.05 was considered statistically significant. All cell culture experiments were performed in triplicates on at least three separate occasions.
RESULTS
Kallistatin gene delivery attenuates salt-induced aortic ROS formation and glomerular capillary loss.
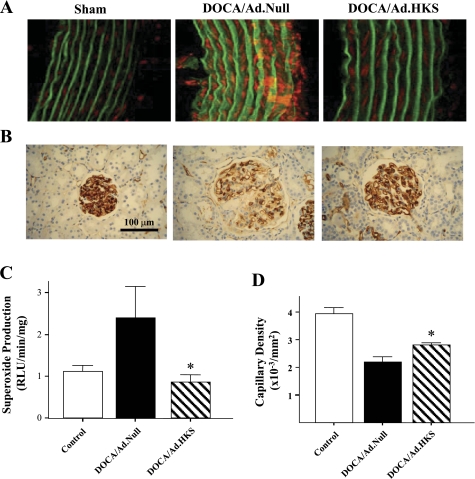
The expression of human kallistatin was identified by immunohistochemistry in the glomerular and tubular cells in DOCA-salt rats after kallistatin gene transfer (data not shown). Kallistatin administration significantly decreased systolic blood pressure and improved renal function compared with DOCA-salt rats receiving Ad.Null injection (data not shown). Representative images showed an elevation of aortic intracellular superoxide levels in rats in the DOCA/Ad.Null group compared with the sham-operated group. Kallistatin gene transfer, however, abolished the rise in salt-induced oxidative stress in the aorta (Fig. 1A). Quantitative analysis confirmed this finding (Fig. 1C). Moreover, immunohistochemical staining against JG-12 (an endothelial cell marker) was performed to examine the effect of high-salt treatment on endothelial cell loss in the kidney. Representative immunostaining and quantitative analysis showed that a significantly lower number of capillaries was detected in rat glomeruli of the DOCA-salt group receiving Ad.Null compared with sham-operated group. However, kallistatin gene transfer caused a 27% increase in glomerular capillary density over DOCA-salt rats given Ad.Null (Fig. 1, B and D). Furthermore, kallistatin gene delivery decreased renal NADH oxidase activity and superoxide formation and increased renal NO production and urinary NO excretion (data not shown). These results indicate that kallistatin gene delivery attenuates oxidative stress in blood vessels and prevents endothelial cell loss in the glomeruli of DOCA-salt rats.
Fig. 1.
Kallistatin (KS) gene delivery prevents aortic superoxide formation and glomerular capillary loss in DOCA-salt rats. Representative images of in situ superoxide production in aorta (A) and JG-12 immunostaining in kidney (B) are shown. Quantitative analysis of aortic superoxide formation (C) and glomerular capillary density (D) are shown. *P < 0.05 vs. other groups. Ad.Null, adenoviral vector alone; Ad.HKS, adenoviral human KS cDNA; RLU, relative light units. Values are expressed as means ± SE; n = 5 animals.
Kallistatin inhibits TNF-α-induced endothelial cell apoptosis via PI3K-Akt-eNOS activation.
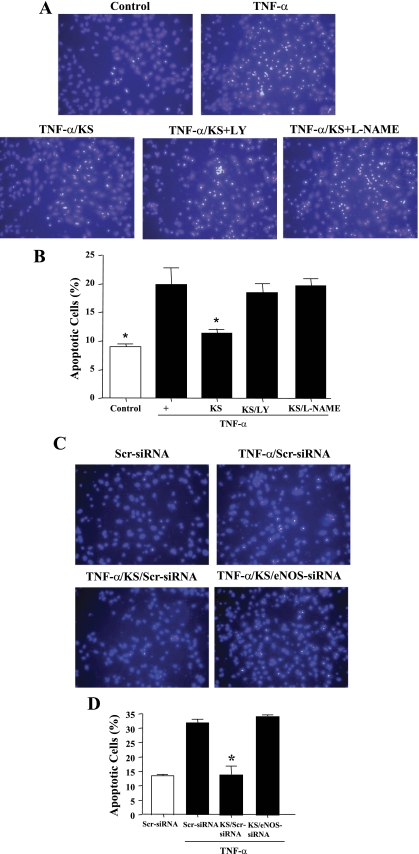
TNF-α treatment induced apoptosis in cultured endothelial cells as shown by Hoechst staining, but the effect was blocked by a pretreatment with kallistatin (Fig. 2, A and B). The protective action of kallistatin on endothelial apoptosis was abolished by the addition of pharmacological inhibitors of PI3K (LY-294002) and NOS (l-NAME). Kallistatin also diminished endothelial cell apoptosis induced by hypoxia/reoxygenation (data not shown). The role of eNOS in preventing endothelial cell apoptosis was verified by the suppression of eNOS expression using siRNA. Cell transfection with eNOS-siRNA oligonucleotides, but not with the scrambled oligonucleotides, significantly blocked the effect of kallistatin on endothelial apoptosis (Fig. 2, C and D). These findings indicate that the protective effect of kallistatin is mediated through the PI3K-Akt-eNOS signaling pathway.
Fig. 2.
KS inhibits TNF-α-induced apoptosis in endothelial cells via phosphatidylinositol 3-kinase (PI3K)-Akt-endothelial nitric oxide synthase (eNOS) signaling. Reduction of TNF-α-induced apoptosis by KS is blocked by LY-294002 (LY; PI3K inhibitor) and Nω-nitro-l-arginine methyl ester (l-NAME; NOS inhibitor), as indicated by representative images of apoptotic nuclei by Hoechst staining (A) and quantitative analysis (B). Knockdown of eNOS expression by small interfering RNA (siRNA) confirmed the role of eNOS in mediating the actions of KS, as indicated by representative images of apoptotic cells by Hoechst staining (C) and quantitative analysis (D). Scr, scrambled. *P < 0.05 vs. other groups. Values are expressed as mean ± SE; n = 3 experiments.
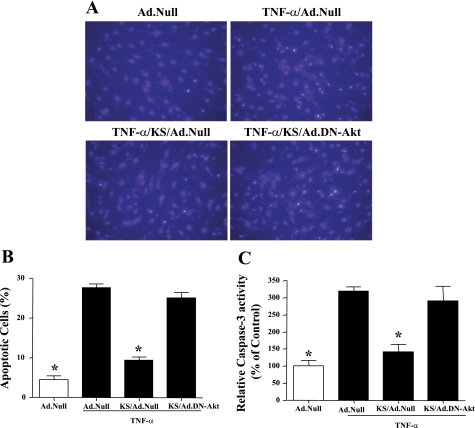
Dominant-negative Akt abolishes the protective effect of kallistatin on endothelial cell apoptosis.
To further confirm the role of Akt in mediating the effect of kallistatin on endothelial apoptosis, adenovirus harboring dominant-negative Akt (DN-Akt) was used to block Akt activity in cultured endothelial cells. Representative images and quantitative analysis showed that the transduction of Ad.DN-Akt blocked the protective effect of kallistatin on TNF-α-induced endothelial cell apoptosis (Fig. 3, A and B). Moreover, kallistatin inhibited TNF-α-induced caspase-3 activity, whereas DN-Akt abolished the actions of kallistatin (Fig. 3C). These results indicate that the effect of kallistatin on endothelial cell apoptosis is dependent on Akt activation.
Fig. 3.
KS inhibits TNF-α-induced apoptosis in endothelial cells, and the effect is blocked by adenovirus harboring dominant-negative mutant Akt (Ad.DN-Akt), as indicated by representative images of apoptotic cells by Hoechst staining (A), quantitative analysis (B), and caspase-3 activity (C). *P < 0.05 vs. other groups. Values are expressed as means ± SE; n = 3 experiments.
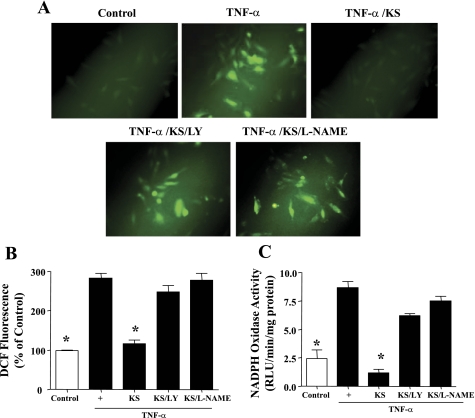
Kallistatin inhibits TNF-α-mediated oxidative stress via Akt-NOS signaling.
TNF-α treatment significantly increased intracellular ROS formation and NADPH oxidase activity, whereas kallistatin significantly blocked these effects (Fig. 4, A–C). The effects of kallistatin on TNF-α-induced ROS formation and NADPH oxidase activity were prevented by LY-294002 and l-NAME, indicating an Akt-NOS-mediated event. Similarly, kallistatin inhibited angiotensin II (100 ng/ml)-induced ROS formation and NADPH oxidase activity via the Akt-NOS signaling pathway (data not shown).
Fig. 4.
KS inhibits TNF-α-induced reactive oxygen species (ROS) formation and NADPH oxidase activity in endothelial cells, as indicated by representative images of ROS-induced dichlorofluorescin (DCF) fluorescence (A), quantification of ROS production (B), and NADPH oxidase activity (C). *P < 0.05 vs. other groups. Values are expressed as means ± SE; n = 3 experiments.
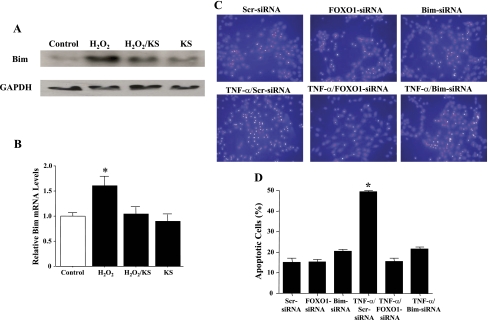
Knockdown of FOXO1 and Bim attenuates TNF-α-induced endothelial cell death.
Previous studies showed that oxidative stress induces endothelial cell apoptosis by promoting the expression of the proapoptotic protein Bim (32). Kallistatin inhibited Bim protein and mRNA expression induced by H2O2 (Fig. 5, A and B). To determine whether TNF-α-induced endothelial cell death involves FOXO1 and Bim, HUVECs were transfected with scrambled FOXO1 or Bim siRNA oligonucleotides. The knockdown efficiency of FOXO1 and Bim by siRNA was 75% and 65%, respectively, as verified by real-time PCR. FOXO1 and Bim knockdown significantly reduced cellular apoptosis (Fig. 5, C and D). The result suggests the involvement of FOXO1 and Bim in endothelial cell apoptosis induced by TNF-α or oxidative stress. These findings indicate that kallistatin may attenuate endothelial cell apoptosis by the inhibition of FOXO1 activity and Bim expression.
Fig. 5.
KS inhibits H2O2-induced Bim expression in endothelial cells, as determined by Western blot analysis (A) and real-time PCR (B). Knockdown of forkhead box O 1 (FOXO1) and Bim by siRNA implicates their role in mediating TNF-α-induced apoptosis, as shown by representative images of apoptotic nuclei by Hoechst staining (C) and quantitative analysis (D). *P < 0.05 vs. other groups. Values are expressed as means ± SE; n = 3 experiments.
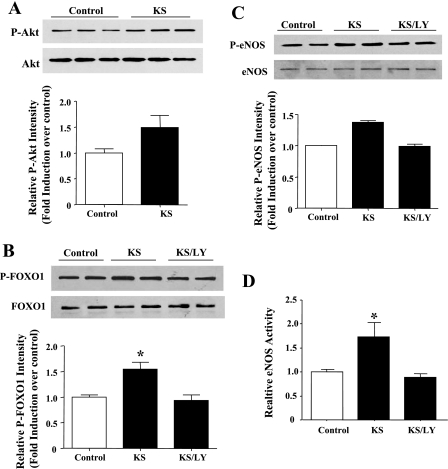
Kallistatin stimulates FOXO1 and eNOS phosphorylation and NOS activity via Akt activation.
In our study, we found that an incubation of endothelial cells with kallistatin for a short period of time (15–30 min) induced a noticeable increase in Akt, FOXO1, and eNOS phosphorylation (Fig. 6, A–C). However, the stimulatory effects of kallistatin on FOXO1 and eNOS phosphorylation were blocked by PI3K inhibition. Kallistatin also increased NOS activity, and the effect was blocked by LY-294002 (Fig. 6D). These results indicate that kallistatin may protect against endothelial apoptosis by a combination of Akt-eNOS activation and Akt-mediated FOXO1 phosphorylation/inactivation.
Fig. 6.
KS stimulates Akt (A), FOXO1 (B), and eNOS phosphorylation (C) in human endothelial cells as determined by Western blot analysis. Cells were stimulated with KS for 15 min to detect Akt phosphorylation or 30 min to detect eNOS and FOXO1 phosphorylation. D: NOS activity. *P < 0.05 vs. other groups. p, Phosphorylated. Values are expressed as means ± SE; n = 3 experiments.
DISCUSSION
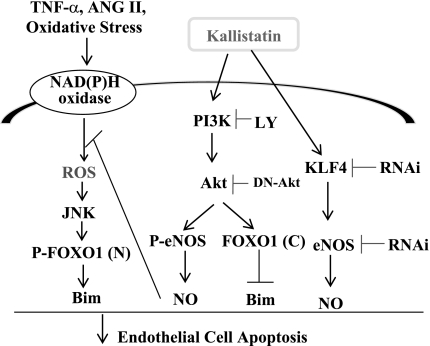
This study identifies a novel role of kallistatin in the protection against oxidative stress and endothelial injury in hypertensive DOCA-salt rats and in cultured cells. We demonstrated that kallistatin protected against endothelial cell apoptosis via an activation of PI3K-Akt-dependent signaling pathways. Using both pharmacological inhibitors and molecular antagonists, we showed that kallistatin increases eNOS and FOXO1 phosphorylation and NOS activity via Akt. Our previous study indicated that kallistatin upregulates eNOS expression through the interaction with KLF4 (28). Therefore, kallistatin appears to increase NO bioavailability by enhancing both eNOS expression and activation. Moreover, kallistatin prevented the c-Jun NH2-terminal kinase (JNK)-mediated nuclear translocation of Bim after an induction by oxidative stress (29). Taken together, these findings indicate that kallistatin contributes to the survival of endothelial cells after oxidative stress-induced arterial injury via the activation of PI3K-Akt-eNOS, Akt-FOXO1-Bim, and KLF4-eNOS signaling pathways, as well as the inhibition of the JNK-Bim cascade (Fig. 7).
Fig. 7.
Signaling pathways mediated by KS in protection against endothelial cell apoptosis. KS attenuates apoptosis by activating the Akt-eNOS, Akt-FOXO1, and Kruppel-like factor-4 (KLF4)-eNOS pathways. FOXO1 (N), nuclear; FOXO1(C), cytosolic; NO, nitric oxide.
Increased oxidative stress and reduced NO bioavailability are important contributing factors in the pathogenesis of endothelial dysfunction, hypertension, and cardiovascular and renal diseases (23, 25). Our previous reports showed that kallistatin gene transfer improved cardiac function and decreased cardiomyocyte apoptosis in association with increased NO levels and reduced oxidative stress in rats after acute myocardial ischemia-reperfusion (5). Moreover, suppressed kallistatin levels were associated with increased circulating thiobarbituric acid reactive substances levels (an indicator of lipid peroxidation) in hypertensive Dahl salt-sensitive rats (27). In addition, our recent study showed that oxidative stress diminished the expression of kallistatin in endothelial cells (29). Conversely, kallistatin gene delivery significantly reduced ROS levels in the circulation and kidney and attenuated renal damage (27). Likewise, the present study showed that kallistatin gene transfer reduced superoxide levels in the aorta and prevented glomerular endothelial loss in DOCA-salt rats. These findings highly suggest that kallistatin may exhibit antioxidant effects in the protection against endothelial dysfunction and vascular damage.
Endothelial dysfunction is often correlated with an increased production of ROS in vascular endothelial cells (23, 25). Elevated ROS levels are primarily derived from the action of NAD(P)H oxidase. Increased vascular NADPH oxidase expression and activity enhance vascular ROS formation and contribute to endothelial dysfunction (24). The vasoconstrictor agent angiotensin II and the cytokine TNF-α both have the ability to stimulate intracellular superoxide generation by activating the NAD(P)H oxidase enzyme on the endothelial cell surface (2, 10, 36). The ensuing rise in ROS levels contributes to the development of endothelial dysfunction by promoting vascular inflammation and cellular apoptosis (23, 25, 36). Conversely, increased NO formation and reduced oxidative stress can protect against vascular injury and oxidative organ damage. We previously showed that kallistatin protein inhibited intracellular ROS production in cultured cardiac and renal cells through NO formation (5, 13, 27). Our present study demonstrated that kallistatin inhibited TNF-α-induced intracellular ROS production via Akt-NOS signaling. Additionally, kallistatin inhibited H2O2 or angiotensin II-induced ROS formation in cultured HUVECs (data not shown). Taken together, these studies indicate that kallistatin suppresses oxidative organ damage by Akt-mediated NO production.
FOXO transcription factors promote a variety of cellular responses by modulating the expression of specific target genes (4, 11). The survival and stress pathways appear to be in a tight balance to regulate FOXO activation. Previous studies have shown that growth factor-activated Akt and stress-activated JNK have opposing effects on FOXO (4, 34). JNK increases FOXO1 activity by stimulating its import into the nucleus. FOXO nuclear translocation can then result in the expression of the proapoptotic protein Bim and endothelial cell apoptosis (3, 32, 34). In contrast, Akt-mediated FOXO1 phosphorylation prevents FOXO nuclear localization and Bim synthesis (4). We recently showed that oxidative stress promoted JNK-induced FOXO1 nuclear translocation and activation (29). In the present study, we found that a knockdown of FOXO1 and Bim expression by siRNA blocked TNF-α-induced endothelial cell apoptosis and that kallistatin inhibited oxidative stress-induced Bim expression in endothelial cells. Moreover, kallistatin increased Akt-mediated FOXO1 phosphorylation, thus attenuating FOXO1 nuclear translocation and Bim expression. These combined findings indicate that kallistatin promotes endothelial cell survival by an inhibition of oxidative stress-induced FOXO1 activation and by a stimulation of Akt-dependent FOXO1 phosphorylation.
Reduced NO bioavailability can occur by decreased activation or expression of eNOS (14, 31). We recently reported that kallistatin has a novel role in inhibiting endothelial inflammation via increasing eNOS expression through KLF4 (28). Taken together with our present observations, our results demonstrate that kallistatin increases NO production via two mechanisms: 1) increasing eNOS protein and mRNA levels (28) and 2) stimulating eNOS activation. The effect of kallistatin on eNOS activation was blocked by a PI3K inhibitor, indicating an Akt-eNOS signaling event. Furthermore, endothelium-derived NO production was previously shown to be mediated by the protein kinase Akt (12). It is therefore likely that kallistatin directly activates an endothelial cell surface receptor or binding protein, thus leading to a rapid activation of PI3K-Akt and eNOS. However, the identity of a kallistatin receptor on endothelial cell surface in mediating kallistatin's activation of the Akt-eNOS pathway is a subject of further investigation.
GRANTS
This work was supported by the National Institutes of Health Grants HL-44083 and HL-29397 and C06-RR-015455.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Albrecht EW, Stegeman CA, Heeringa P, Henning RH, van Goor H. Protective role of endothelial nitric oxide synthase. J Pathol 199: 8–17, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Alvarez E, Rodiño-Janeiro BK, Ucieda-Somoza R, González-Juanatey JR. Pravastatin counteracts angiotensin II-induced upregulation and activation of NADPH oxidase at plasma membrane of human endothelial cells. J Cardiovasc Pharmacol 55: 203–212, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Biswas SC, Shi Y, Sproul A, Greene LA. Pro-apoptotic Bim induction in response to nerve growth factor deprivation requires simultaneous activation of three different death signaling pathways. J Biol Chem 282: 29368–29374, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Chao J, Yin H, Yao YY, Shen B, Smith RS, Jr, Chao L. Novel role of kallistatin in protection against myocardial ischemia-reperfusion injury by preventing apoptosis and inflammation. Hum Gene Ther 17: 1201–1213, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chao J, Yin H, Gao L, Hagiwara M, Shen B, Yang ZR, Chao L. Tissue kallikrein elicits cardioprotection by direct kinin b2 receptor activation independent of kinin formation. Hypertension 52: 715–720, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen LM, Chao L, Chao J. Beneficial effects of kallikrein-binding protein in transgenic mice during endotoxic shock. Life Sci 60: 1431–1435, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Chen VC, Chao L, Pimenta DC, Bledsoe G, Juliano L, Chao J. Identification of a major heparin-binding site in kallistatin. J Biol Chem 276: 1276–1284, 2001 [DOI] [PubMed] [Google Scholar]
- 9.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 96: 60–68, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J 23: 4802–4812, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao L, Yin H, Smith R, Jr, S Chao L, Chao J. Role of kallistatin in prevention of cardiac remodeling after chronic myocardial infarction. Lab Invest 88: 1157–1166, 2008 [DOI] [PubMed] [Google Scholar]
- 14.García-Cardeña G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biol Chem 271: 27237–27240, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Hagiwara M, Bledsoe G, Yang ZR, Smith RS, Jr, Chao L, Chao J. Intermedin ameliorates vascular and renal injury by inhibition of oxidative stress. Am J Physiol Renal Physiol 295: F1735–F1743, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartge MM, Unger T, Kintscher U. The endothelium and vascular inflammation in diabetes. Diab Vasc Dis Res 4: 84–88, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Lee TC, Zhao YD, Courtman DW, Stewart DJ. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation 101: 2345–2348, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Luo T, Xia Z, Ansley DM, Ouyang J, Granville DJ, Li Y, Xia ZY, Zhou QS, Liu XY. Propofol dose-dependently reduces tumor necrosis factor-alpha-Induced human umbilical vein endothelial cell apoptosis: effects on Bcl-2 and Bax expression and nitric oxide generation. Anesth Analg 100: 1653–1659, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, Lowenstein JM, Pevsner J, Wagner DD, Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 115: 139–150, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao RQ, Agata J, Chao L, Chao J. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood 100: 3245–3252, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res 82: 1298–1305, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Papaharalambus CA, Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med 17: 48–54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rush JW, Ford RJ. Nitric oxide, oxidative stress and vascular endothelium in health and hypertension. Clin Hemorheol Microcirc 37: 185–192, 2007 [PubMed] [Google Scholar]
- 25.Schiffrin EL. Oxidative stress, nitric oxide synthase, and superoxide dismutase: a matter of imbalance underlies endothelial dysfunction in the human coronary circulation. Hypertension 51: 31–32, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Sedoris KC, Ovechkin AV, Gozal E, Roberts AM. Differential effects of nitric oxide synthesis on pulmonary vascular function during lung ischemia-reperfusion injury. Arch Physiol Biochem 115: 34–46, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Shen B, Hagiwara M, Yao YY, Chao L, Chao J. Salutary effect of kallistatin in salt-induced renal injury, inflammation and fibrosis via anti-oxidative stress. Hypertension 51: 1358–1365, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Shen B, Smith RS, Jr, Hsu YT, Chao L, Chao J. Kruppel-like factor 4 is a novel mediator of Kallistatin in inhibiting endothelial inflammation via increased endothelial nitric-oxide synthase expression. J Biol Chem 284: 35471–35478, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen B, Chao L, Chao J. Pivotal role of JNK-dependent FOXO1 activation in downregulation of kallistatin expression by oxidative stress. Am J Physiol Heart Circ Physiol 298: H1048–H1054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skatchkov MP, Sperling D, Hink U, Mulsch A, Harrison DG, Sindermann I, Meinertz T, Munzel T. Validation of lucigenin as a chemiluminescent probe to monitor vascular superoxide as well as basal vascular nitric oxide production. Biochem Biophys Res Commun 254: 319–324, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Stuehr DJ, Griffith OW. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol 65: 287–346, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Urbich C, Knau A, Fichtlscherer S, Walter DH, Brühl T, Potente M, Hofmann WK, de Vos S, Zeiher AM, Dimmeler S. FOXO-dependent expression of the proapoptotic protein Bim: pivotal role for apoptosis signaling in endothelial progenitor cells. FASEB J 19: 974–976, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Wang CR, Chen SY, Wu CL, Liu MF, Jin YT, Chao L, Chao J. Prophylactic adenovirus-mediated human kallistatin gene therapy suppresses rat arthritis by inhibiting angiogenesis and inflammation. Arthritis Rheum 52: 1319–1324, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121: 115–125, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Yasuda Y, Yoshinaga N, Murayama T, Nomura Y. Inhibition of hydrogen peroxide-induced apoptosis but not arachidonic acid release in GH3 cell by EGF. Brain Res 850: 197–206, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Yoshida LS, Tsunawaki S. Expression of NADPH oxidases and enhanced H2O2-generating activity in human coronary artery endothelial cells upon induction with tumor necrosis factor-alpha. Int Immunopharmacol 8: 1377–1385, 2008 [DOI] [PubMed] [Google Scholar]