Abstract
Sphingosine-1-phosphate (S1P) has been demonstrated to enhance endothelial barrier function in vivo and in vitro. However, different S1P receptor subtypes have been indicated to play different or even opposing roles in the regulation of vascular barrier function. This study aims to differentiate the roles of endogenous endothelial S1P subtype receptors in the regulation of permeability in intact microvessels using specific receptor agonist and antagonists. Microvessel permeability was measured with hydraulic conductivity (Lp) in individually perfused rat mesenteric venules. S1P-mediated changes in endothelial intracellular Ca2+ concentration ([Ca2+]i) was measured in fura-2-loaded venules. Confocal images of fluorescent immunostaining illustrated the spatial expressions of three S1P subtype receptors (S1PR1–3) in rat venules. The application of S1P (1 μM) in the presence of S1PR1–3 inhibited platelet-activating factor- or bradykinin-induced permeability increase. This S1P effect was reversed only with a selective S1PR1 antagonist, W-146, and was not affected by S1PR2 or S1PR3 antagonists JTE-013 and CAY-10444, respectively. S1PR1 was also identified as the sole receptor responsible for S1P-mediated increases in endothelial [Ca2+]i. S1PR2 or S1PR3 antagonist alone affected neither basal Lp nor platelet-activating factor-induced permeability increase. The selective S1PR1 agonist, SEW-2871, showed similar [Ca2+]i and permeability effect to that of S1P. These results indicate that, despite the presence of S1PR1–3 in the intact venules, only the activation of endothelial S1PR1 is responsible for the protective action of S1P on microvessel permeability and that endogenous S1PR2 or S1PR3 did not exhibit functional roles in the regulation of permeability under basal or acutely stimulated conditions.
Keywords: sphingosine-1-phosphate receptors, microvessel permeability, sphingosine-1-phosphate subtype receptor expression, endothelial calcium concentration
an inflammatory mediator-induced increase in microvascular permeability, mainly occurring at postcapillary venules, is a critical event, resulting in edema formation, organ dysfunction, and the pathogenesis of many cardiovascular diseases. The increased microvessel permeability-associated endothelial gap formation also serves as the initiating step for the activation of platelets and leukocytes, resulting in platelet/leukocyte aggregate formation and augmented increases in microvessel permeability (12). Identifying an effective strategy to enhance endothelial barrier function and prevent permeability increases is critical for combating a variety of cardiovascular diseases.
Sphingosine-1-phosphate (S1P), a biologically active lipid mediator, has been identified as an important regulator of a variety of endothelial and vascular functions. Its actions are mediated by a family of G protein-coupled receptors. The differences in subtype receptor distribution and in downstream signaling provide a wide range of actions of S1P in the regulation of vascular functions. One of the important actions of S1P, reported both in vitro and in whole animal studies, is to enhance the barrier function of vascular endothelium (16, 20, 24, 30, 33). Our previous study using individually perfused intact microvessels demonstrated that the application of S1P to the vessel lumen prevents platelet-activating factor (PAF)-induced permeability increases by a Gi protein-dependent signaling (25). Most commonly identified subtype receptors of S1P in cardiovascular system are S1PR1-R3 (31). With the use of genetic approaches, S1PR1 and S1PR2 have been reported to play counteractive roles in the regulation of endothelial function (38). A recent whole animal study indicated that S1PR2 may compromise the S1P protective role in endothelial barrier function (16). Our present study aims to quantitatively evaluate the contributions of S1PR1–3 to S1P-mediated protection in microvessel permeability and changes in endothelial intracellular Ca2+ concentration ([Ca2+]i) in individually perfused intact microvessels, using subtype receptor-specific agonist and antagonists. Microvessel permeability is determined by measuring hydraulic conductivity (Lp) in individually perfused rat mesenteric venules. Permeability increases were induced by two representative inflammatory mediators, PAF and bradykinin (BK). S1P-mediated changes in endothelial [Ca2+]i are examined in fura-2-loaded microvessels before and after exposure to each receptor antagonist. The spatial distributions of the S1P subtype receptors in the same type of venules where the permeability coefficient was measured are illustrated with fluorescent immunostaining using confocal images.
MATERIALS AND METHODS
Animal preparation.
Experiments were carried out on female Sprague-Dawley rats (2 to 3 mo old, 220 to 250 g, Hilltop Laboratory Animal, Scottdale, PA). All procedures and animal use were approved by the Animal Care and Use Committee at West Virginia University. Surgical procedures and tissue preparation have been described (47). For details, see the supplement posted with the online version of this article.
Fluorescent immunostaining and confocal imaging.
The rat mesentery bearing selected venules was fixed with paraformaldehyde (1%) and treated with Triton X-100 before exposure to a specific S1P receptor antibody and the secondary antibody conjugated with Alexa Fluor 488 (Invitrogen). DRAQ5 (Biostatus) was used for nuclei staining. A Leica TCS SL confocal microscope was used for collecting images. A stack of confocal images was obtained from each vessel by optical sectioning at successive X-Y focal planes with vertical step (z-axis) at 0.5 or 0.3 μm using a Leica objective ×20 (HC PL APO, numerical aperature, 0.7) with ×3 electronic zoom or ×63 (HCX PL APO, numerical aperature, 1.2) with ×2 electronic zoom, respectively, and 1,024 × 1,024 scanning format. The selected pinhole diameter (1 airy disk) and the vertical step at z-axis were within the effective resolution limitations calibrated for the objective, the wavelength, and the electronic scanning format used in the Leica confocal system and also comply with the Nyquist criterion. Comparable image acquisition parameters were applied to all groups of studies. Stacks of images were processed and projected using Laica confocal software. The lumen of the fixed vessel was slightly flattened but did not collapse and interfere with the spatial illustration of the receptor distribution.
Measurement of hydraulic conductivity in single perfused rat mesenteric microvessels.
A modified Landis technique was used to measure hydraulic conductivity (Lp) in individually perfused microvessels. The methods have been evaluated in detail (5, 15). Briefly, a single microvessel was cannulated with a micropipette and perfused with albumin-Ringer solution (control) containing red blood cells (1% vol/vol) as markers under certain hydrostatic pressure (40–60 cmH2O). For each measurement, the perfused vessel was briefly occluded downstream with a glass rod for 5–7 s. The initial water flux per unit area of microvessel wall was calculated from the velocity of the marker cell after vessel occlusion, the vessel radius, and the distance between the marker cell and the occlusion site. Lp was calculated as the slope of the relationship between the initial water flow per unit area of vessel wall and the pressure difference across the vessel wall. In each experiment, the baseline Lp and the Lp after the application of testing solutions were measured in the same vessel. The changes in Lp were measured over the entire perfusion period and expressed as the ratio of Lp test to Lp control. See online supplement for details.
Measurements of endothelial [Ca2+]i.
Endothelial [Ca2+]i was measured in individually perfused microvessels using the fluorescent Ca2+ indicator fura-2 AM. A Nikon Diaphod 300 microscope equipped with a Nikon photometer was used for the measurements. In each experiment, the vessel was perfused with albumin-Ringer solution containing 10 μM fura-2 AM for 45 min, followed by albumin-Ringer perfusion for 10 min before collecting the fluorescence intensity (FI) from a segment of the vessel. At the end of each experiment, the microvessel was superfused with a modified Ringer solution (5 mM Mn2+ without Ca2+) while perfused with the same solution containing ionomycin (10 μM) to bleach the Ca2+-sensitive form of fura-2. The remaining background FI was subtracted from the signals. The ratios of the two FI values at 340 and 380 nm, respectively, were converted to Ca2+ concentrations using an in vitro calibration curve (13). See online supplement for details.
Solutions and reagents.
Mammalian Ringer solution (13) was used for the experiments. All the perfusates contained BSA (10 mg/ml). PAF (1-O-alky1–2-acetyl-sn-glycero-3-phosphocholine) and BK were from Sigma (St. Louis, MO). PAF was initially dissolved in 95% ethyl alcohol (5 mM), and BK was in Ringer solution (1 mM). They were further diluted to final concentrations of 10 and 1 nM with albumin-Ringer solution, respectively. (R)-phosphoric acid mono-[2-amino-2-(3-octyl-phenylcarbamoyl)-ethyl]ester (VPC-23019, Avanti Polar Lipid), a S1PR1 and S1PR3 antagonist, and CAY-10444 (CAY, Cayman Chemical), a S1PR3 antagonist, were dissolved in DMSO (10 mM stock). 1-{1,3-Dimethyl-4-(2-methylethyl)-1H-pyrazolo[3,4-b]pyridin-6-yl}-4-(3,5-dichloro-4-pyridinyl)-semicarbazide (JTE-013, Tocris Bioscience), an antagonist of S1PR2, and S1PR1 agonist SEW-2871 (Tocris Bioscience) were dissolved in ethanol (10 and 50 mM stock). W-146 (Avanti Polar Lipid), an S1PR1 antagonist, was prepared following the manufacturer's instructions. Each final concentration was achieved by >1:1,000 dilution with albumin-Ringer solution. All perfusates containing test agents were freshly prepared before each cannulation. S1P receptor antibodies were provided by Dr. Mandala and Jim Milligan (Merck Research Laboratories), which have been characterized by other investigators and demonstrated specific immunoreactivity for S1P subtype receptors (4, 16, 38).
Vessel treatment with pharmacological reagents.
Based on our previous study with Lp measurements during the S1P perfusion period, the S1P effect was initiated fast and remained effective with its presence in the perfusate over the entire experimental period (>1 h) (25). If the baseline Lp was high, a reduction occurred immediately after the start of S1P perfusion but took about 20 min to reach a stable lower Lp level. In this study, the S1P and S1P agonist pretreatments in the presence and absence of the receptor antagonist was 30 min, which appears sufficient to reach a full action of S1P on endothelial cells under our experimental conditions and also consistent with a S1P study by others using the same experimental approach (1). The S1P receptor antagonist was applied to each vessel for 30 min before an S1P addition to the perfusate. So the S1P effect in the presence of receptor antagonist on PAF- or BK-induced Lp increase was measured after 60 min of the initial antagonist perfusion. Antagonist pretreatment for 30–60 min before S1P addition was commonly used by others (16, 34, 41, 45). Based on the receptor antagonist assays, the antagonist activity of JTE-013 and CAY, the S1PR2 and S1PR3 antagonist, respectively, remained effective in cell lines exposed to antagonist for 5 h (41), and no fast but limited duration effect has been identified.
Data analysis and statistics.
All values are means ± SE. Paired t-test was used for paired data analysis. ANOVA was used to compare data between groups. A probability value of P < 0.05 was considered statistically significant. In the summary figures, the asterisk indicates a significant increase from the control.
RESULTS
The spatial distribution of S1P receptors in rat mesenteric venules.
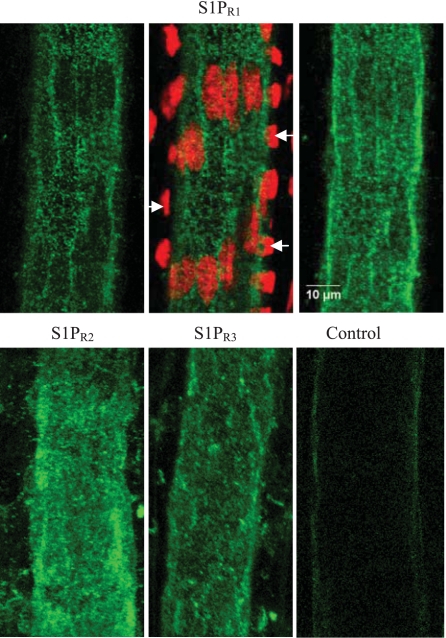
To investigate the specific roles of S1P subtype receptors in the regulation of microvessel permeability, we first examined whether S1PR1–3, the common vascular S1P receptor subtypes, are expressed in the rat mesenteric venules. Specific S1PR1–3 antibodies that have been characterized by other investigators (4, 16, 38) were used for the immunostaining. Confocal images shown in Fig. 1 demonstrated that all three S1P receptors (S1PR1–3) are uniformly expressed in endothelial cells with comparable fluorescence staining and distribution patterns. The fluorescence staining was also detectable in pericytes forming venular walls.
Fig. 1.
Confocal images of fluorescent immunostaining of 3 sphingosine-1-phosphate (S1P) subtype receptors (S1PR1, S1PR2, and S1PR3) in rat mesenteric venules. Top: S1PR1 staining. Top, left: single image section with focus on the center of endothelium at lower half of the vessel wall. Top, middle: low fluorescence areas are the locations of endothelial nuclei that are colocalized with the nuclear dye DRAQ5 staining. The nuclear staining shown on both sides of the vessel wall (indicated by arrows) are the pericyte nuclei. Top, right: projection of the S1PR1 staining from the lower half image stack of the vessel wall. The horizontally oriented fluorescence pattern represents the staining of pericytes. Bottom: projection of lower half image stack of the vessel wall with S1PR2, S1PR3, and the secondary antibody alone (control) staining, respectively. All the images are collected with ×63 objective with ×2 electronic zoom. Each image represents the staining pattern of multiple vessel segments from 3 rats.
VPC-23019, a S1PR1 and S1PR3 antagonist, abolished the inhibitory effect of S1P on PAF-induced increases in Lp.
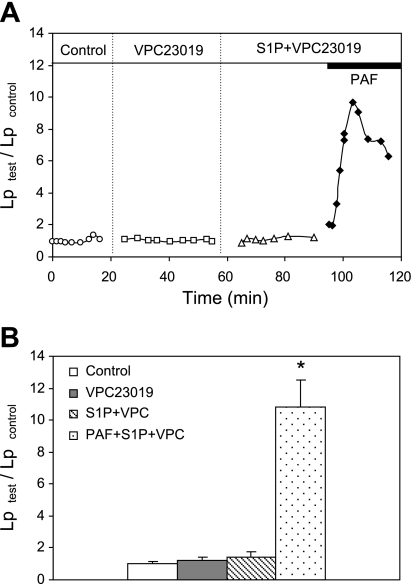
Our previous study showed that purfusing vessels with S1P (1 μM) prevented PAF (10 nM)-induced increases in microvessel permeability (25). To identify the S1P subtype receptor(s) that are responsible for the protective role of S1P in microvessel permeability, VPC-23019, a selective antagonist at both S1PR1 and S1PR3 (6), was used to preperfuse each vessel before examining the effect of S1P on PAF-induced Lp increases. The antagonist activity of VPC-23019 at S1PR1 is about 30 times higher than that at S1PR3 with no binding activity at S1PR2 at concentrations up to 10 μM (6). The binding affinity of VPC-23019 at S1PR1 is about 10 times less than that of S1P (6). In this study we used 10 μM of VPC-23019 to evaluate the effect of S1P (1 μM) in PAF (10 nM)-stimulated vessels. This concentration is similar to others used in ex vivo vascular function studies (32, 42). Experiments were conducted in five microvessels. The mean baseline Lp measured with albumin-Ringer perfusate (control) was 1.5 ± 0.2 × 10−7 cm·s−1·cmH2O−1. Each vessel was then perfused with VPC-23019 for 30 min, followed by S1P plus VPC-23019 for another 30 min. The mean Lp during each perfusion was 1.7 ± 0.2 and 2.0 ± 0.4 × 10−7 cm·s−1·cmH2O−1, respectively, which was not significantly different from that of the control. When PAF was applied to the perfusate in the presence of VPC-23019 and S1P, the inhibitory effect of S1P on the PAF-induced Lp increase was abolished. Lp increased to a mean peak value of 10.8 ± 1.7 times that of the control. The magnitude and the time course of the Lp increase was not significantly different from that with exposure to PAF alone (data in Fig. 4). These results indicate either S1PR1 or S1PR3 alone or both are responsible for the S1P effect. Figure 2A shows the time course of the Lp changes from one of the experiments, and Fig. 2B summarizes the results.
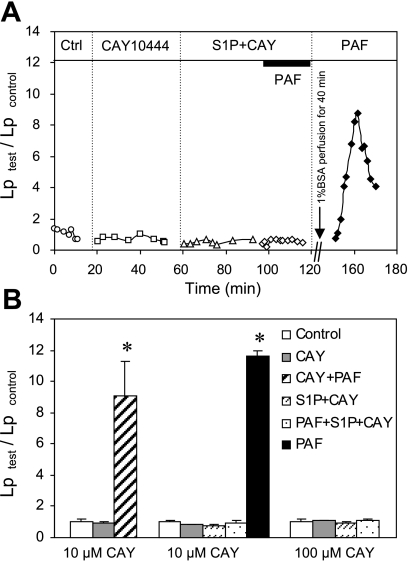
Fig. 4.
Selective inhibition of S1PR3 with CAY-10444 (CAY) did not affect the protective role of S1P in PAF-induced Lp increase. A: an individual experiment shows the time course of the Lp changes. After inhibition of S1PR3 by perfusion of the vessel with CAY (10 μM), S1P still effectively inhibited PAF-induced Lp increase. Removal of S1P and CAY from the vessel lumen with Ringer-albumin perfusion for 40 min recovered the PAF response in the same vessel. Ctrl, control. B: summary figure shows that S1P remains effective in inhibiting PAF-induced Lp increase after selective inhibition of S1PR3 with CAY at 10 (n = 5) and 100 (n = 4) μM. Perfusion of vessels with CAY (10 μM) alone showed no effect on baseline Lp and PAF-induced Lp increases (n = 4). *Significant increase from the control.
Fig. 2.
VPC-23019, a S1PR1 and S1PR3 antagonist, abolished the protective role of S1P in platelet-activating factor (PAF)-induced increases in hydraulic conductivity (Lp). Perfusion of VPC-23019 (10 μM) alone and VPC-23019 plus S1P (1 μM) did not affect the baseline Lp but abolished the S1P effect on PAF-stimulated permeability increase. A: individual experiment shows time course of Lp changes with sequential perfusions of different reagents in the same vessel. B: summary results of 5 microvessels. *Significant increase from the control.
Selective inhibition of S1PR1 abolished the S1P effect on PAF-induced increases in Lp.
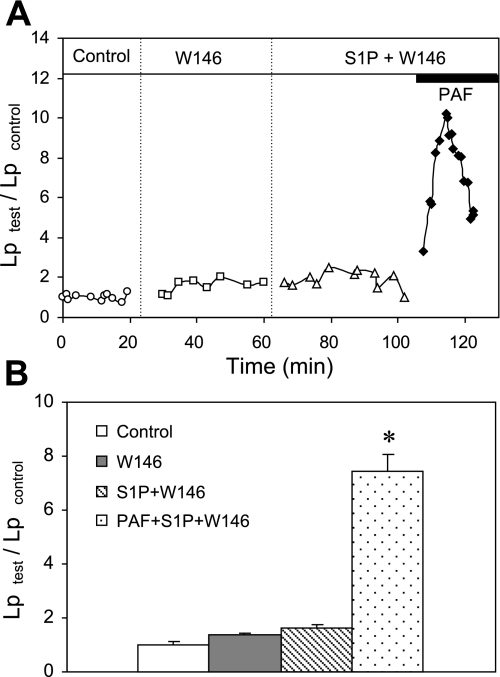
To further differentiate the role of S1PR1 from that of S1PR3 in S1P-mediated inhibition of Lp increases, W-146, a well-characterized selective antagonist of S1PR1 in vivo and in vitro (30, 36), was applied to five microvessels. It was shown that W-146 at a concentration of 10 μM increased the EC50 of S1P by 500-fold at cell membranes expressing human S1PR1 (36). The mean control Lp of five vessels was 1.4 ± 0.2 × 10−7 cm·s−1·cmH2O−1. After perfusion of W-146 (10 μM) and W-146 plus S1P (1 μM) each for 30 min, the mean Lp was 1.8 ± 0.2 and 2.2 ± 0.3 × 10−7 cm·s−1·cmH2O−1, respectively, which were slightly higher than the basal Lp but not statistically significant. When PAF was added to the perfusate in the presence of S1P and W-146, the S1P effect was abolished. Lp increased to a mean peak value of 7.4 ± 0.6 times that of the control within 10 min and then fell toward the control level. These results indicate that the activation of S1PR1 is required for S1P to inhibit the PAF-induced increase in microvessel permeability. Figure 3A shows the time course of the Lp changes from an individual experiment, and Fig. 3B summarizes the results.
Fig. 3.
Inhibition of S1PR1 abolished the protective role of S1P in PAF-induced Lp increases. When S1PR1 was inhibited by the selective antagonist W-146 (10 μM), S1P (1 μM) showed no effect on baseline Lp and PAF-induced Lp increases. A: representative experiment shows the time course of the Lp changes. B: summary results of 5 experiments. *Significant increase from the control.
Selective inhibition of S1PR3 did not affect the protective role of S1P in PAF-induced increases in Lp.
The specific role of S1PR3 in the S1P effect was further examined using a selective S1PR3 antagonist, CAY, with an IC50 of 4.6 μM (41). CAY at concentrations of 5 and 100 μM showed a 70–82% inhibition of S1P (1–5 μM)/S1PR3-mediated cell functions (41, 45). We conducted 13 experiments to examine the effect of CAY at concentrations of 10 and 100 μM on S1P (1 μM)-mediated inhibition of Lp increase. The mean baseline Lp of 13 vessels was 1.5 ± 0.1 × 10−7 cm·s−1·cmH2O−1. CAY at 10 μM was applied to five of the microvessels. Perfusing vessels with CAY (10 μM) for 30 min followed by CAY plus S1P for 30 min did not affect the basal Lp or the inhibitory effect of S1P on PAF-induced increases in Lp. After PAF was added to the perfusate containing CAY and S1P, the PAF response was still completely inhibited by S1P, with a mean value 1.0 ± 0.1 times that of the control (P > 0.05). The vessel responsiveness to PAF was tested in two of the five vessels, in which PAF was applied for the second time after washing out CAY and S1P with albumin-Ringer perfusate for 40 min. The PAF response was restored in both vessels with a mean peak Lp at 11.6 ± 0.3 times the control value. Figure 4A shows the Lp changes in one of the experiments. To ensure a complete inhibition of S1PR3, the effect of CAY at 100 μM was examined in four microvessels. The results were not significantly different from that observed with 10 μM of CAY. The effect of CAY alone on PAF-induced Lp increase was examined in another four vessels, which showed no alterations of PAF-induced increases in Lp. Figure 4B summarizes the results of 13 experiments, indicating that S1PR3 involves neither S1P-mediated permeability effect nor inflammatory mediator-induced permeability increases.
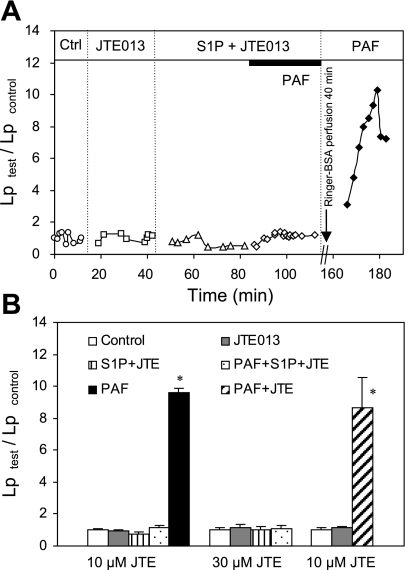
Neither PAF-induced Lp increases nor the protective role of S1P in microvessel permeability was affected by selective inhibition of S1PR2.
S1PR2 has been reported to play opposite roles from that of S1PR1, and the S1PR2 antagonist, JTE-013, showed an inhibition of H2O2-induced lung edema even in the absence of applied S1P (34). In this study, we examined the effects of JTE-013 on the Lp responses to PAF alone and the S1P-mediated protection of microvessel permeability. JTE-013 was characterized as a specific inhibitor of human and rat S1PR2 with IC50 values of 17 ± 6 and 22 ± 9 nM, respectively (27). No binding activity was found at S1PR1 at concentrations up to 10 μM. JTE-013 at concentrations of 1 and 10 μM showed a complete inhibition of S1PR2-mediated S1P (0.1 μM) effect on smooth muscle contraction (27). We consider that 10 μM of JTE-013 should be sufficient to inhibit the effect of 1 μM S1P at S1PR2, based on the functional study and the relative binding affinities reported for S1P and JTE-013 at S1PR2. Experiments were conducted in 15 microvessels. The mean baseline Lp of 15 microvessels was 1.6 ± 0.1 × 10−7 cm·s−1·cmH2O−1. Perfusion of JTE-013 (10 μM) for 30 min showed no effect on basal Lp and PAF-induced Lp increases (n = 3). The mean peak Lp upon the PAF addition was 8.6 ± 1.9 times that of the control, which was not significantly different from that of exposure to PAF alone (Fig. 5B). The S1P effect on microvessel permeability after the inhibition of S1PR2 was examined in 12 microvessels. Perfusing vessels with JTE-013 at 10 μM (n = 6) for 30 min followed by JTE-013 plus S1P perfusion did not affect the basal Lp or the inhibitory role of S1P in microvessel permeability. After the exposure to PAF in the presence of S1P plus JTE-013, the mean Lp was 1.1 ± 0.2 times that of the control. In two of the vessels, a second PAF exposure was given after 40 min albumin-Ringer perfusion, and the mean peak Lp was increased to 9.6 ± 0.2 times that of the control. Figure 5A shows one of the experiments. Because of the negative results, we conducted another six experiments using 30 μM of JTE-013. The results were not different from that of using 10 μM JTE-013, supporting that S1PR2 activation by S1P does not play a role in permeability regulation. Figure 5B summarizes all of the results.
Fig. 5.
Selective inhibition of S1PR2 with JTE-013 (JTE) did not affect the protective role of S1P in PAF-perfused microvessels. A: representative experiment shows the time course of the Lp changes. S1P effectively inhibited PAF-induced Lp increase after preperfusion of the vessel with JTE (10 μM). The PAF response was recovered in the same vessel after Ringer-albumin perfusion for 40 min, indicating the vessel responsiveness to PAF. B: summary that shows that the effect of S1P on inhibition of PAF-induced Lp increases was not altered after inhibition of S1PR2 with JTE at 10 (n = 6) and 30 (n = 6) μM. JTE (10 μM) alone also showed no effect on baseline Lp and PAF-induced Lp increases (n = 3). *Significant increase from the control.
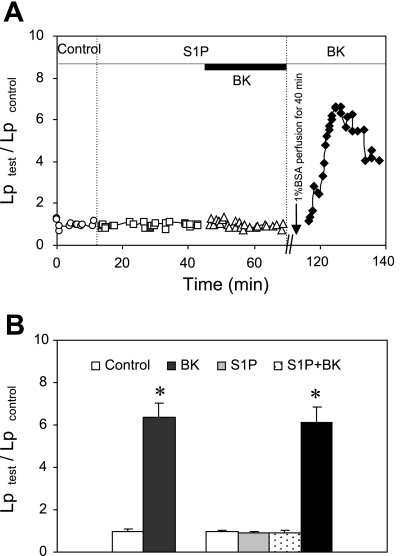
S1P inhibits BK-induced permeability increase via activation of S1PR1.
To investigate whether S1P is effective to prevent permeability increases induced by inflammatory mediators other than PAF, we examined the effect of S1P on BK-induced Lp increase. The mean baseline Lp of nine microvessels was 1.4 ± 0.1 × 10−7 cm·s−1·cmH2O−1. When BK (1 nM) was applied to six of the microvessels, Lp transiently increased to a mean peak value of 6.3 ± 0.7 times that of the control. In another three microvessels, S1P was applied for 30 min before BK administration, which completely inhibited BK-induced Lp increase. A second application of BK was then given to each of the three vessels after 40 min albumin-Ringer perfusion. All three vessels responded well to BK after the removal of S1P, and the mean peak Lp was 6.1 ± 0.7 times that of the control. Figure 6A shows the Lp changes during each perfusion period in a single experiment, and Fig. 6B summarizes the results.
Fig. 6.
S1P inhibited bradykinin (BK)-induced Lp increases. A: paired measurements of Lp in response to BK (1 nM) in the presence and absence of S1P (1 μM) in a single venule. In the presence of S1P, BK-induced Lp increase was completely abolished. After S1P and BK were washed from the vessel lumen with BSA-Ringer perfusion, the Lp response to BK was recovered with a peak value of 6.6 times that of the control. B: summary results compare BK-induced Lp increases in the absence (n = 6) and presence (n = 3) of S1P. *Significant increase from the control.
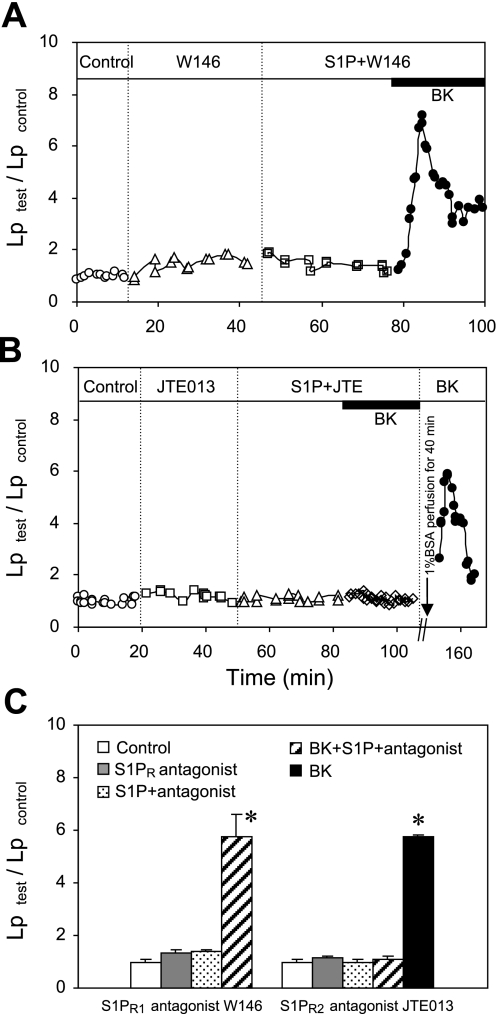
In another six microvessels, specific inhibitors for S1PR1 (W-146) and S1PR2 (JTE-013) were used to examine the subtype receptor(s) responsible for the protective role of S1P in BK-induced Lp increase. The mean baseline Lp was 1.3 ± 0.1 × 10−7 cm·s−1·cmH2O−1. Consistent with the results shown in Figs. 3 and 5, the perfusion of each antagonist alone or antagonist plus S1P did not significantly change the basal Lp. When BK was added to W-146-perfused vessels in the presence of S1P (n = 3), the S1P effect was abolished. Lp increased to a mean peak value of 5.8 ± 0.9 times that of the control, which was not significantly different from BK response in the absence of S1P. In contrast, the protective role of S1P remained effective in JTE-013-perfused vessels (n = 3). In the presence of S1P and JTE-013, the mean Lp upon BK addition was 1.1 ± 0.1 times the control value. These results indicate that the activation of S1PR1 may provide a general protection of the endothelial barrier and not be specific for PAF-induced permeability increases. Figure 7, A and B, shows the Lp changes in two individual vessels treated with W-146 and JTE-013, respectively. Figure 7C summarizes the results.
Fig. 7.
The activation of S1PR1, not S1PR2, is required for the inhibitory effect of S1P on BK-induced Lp increases. A: single experiment shows that addition of BK to S1PR1 antagonist W-146 (10 μM)- and W-146 plus S1P-perfused vessel abolished the S1P effect and transiently increased Lp in a pattern similar to that of exposure to BK alone (Fig. 6). B: another experiment shows that blocking S1PR2 by selective antagonist JTE did not affect the inhibitory effect of S1P on BK-induced Lp increase, and BK response was recovered in the same vessel when the preperfused agents were removed from the microvessel lumen with albumin-Ringer perfusion for 40 min. C: summary results show the effects of blocking S1PR1 (n = 3) and S1PR2 (n = 3) on S1P-mediated inhibition of BK-induced Lp increases. *Significant increase from the control.
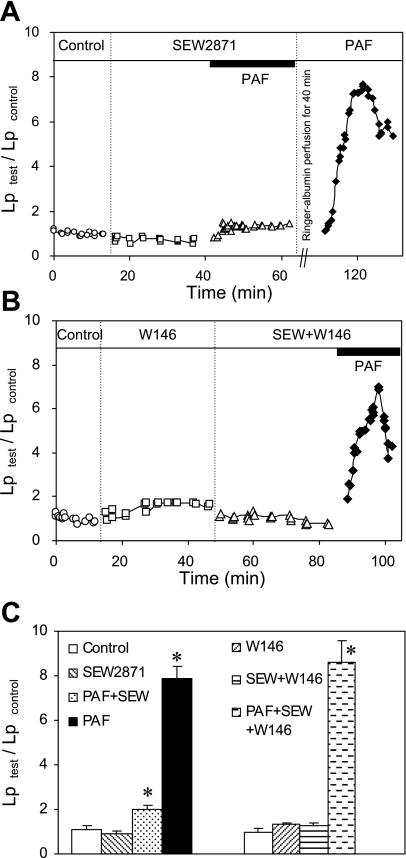
S1PR1 agonist attenuates PAF-induced increases in Lp.
We further examined the role of S1PR1 in PAF-induced permeability increases using a well-characterized S1PR1-selective agonist SEW-2871 (35). When the EC50 values were compared, SEW-2871 was about 30-fold less potent than S1P binding to human S1PR1 and 15-fold less potent than S1P binding to murine S1PR1. No agonist activity of SEW-2871 was identified at S1PR2–5 at concentrations up to 10 μM in both human and murine S1P receptor assays (35). We applied 10 μM of SEW-2871 to five microvessels. The mean baseline Lp of five microvessels was 1.2 ± 0.2 × 10−7 cm·s−1·cmH2O−1. Perfusion of SEW-2871 for 30 min did not change the basal Lp, with a mean of 1.0 ± 0.1 × 10−7 cm·s−1·cmH2O−1. When each vessel was exposed to PAF in the presence of SEW-2871, a PAF-induced Lp increase was significantly attenuated (P < 0.01) and the mean peak Lp was only 1.8 ± 0.2 times that of the control. The reversibility of the agonist and the vessel responsiveness to PAF were examined in the same five vessels. After albumin-Ringer perfusion for 40 min to remove PAF and SEW-2871 from the vessel lumen, the PAF increased Lp to a mean peak value of 7.0 ± 0.5 times that of the control. Although SEW-2871 was categorized as a full agonist, it showed lower potency in receptor binding and downstream signaling than S1P in both in vitro and in vivo studies (14, 30, 35). The incomplete inhibition of PAF-induced Lp increases with 10 μM SEW-2871 may reflect the potency difference between S1P and SEW-2871. We further examined the specificity of SEW-2871 in intact venules using S1PR1 antagonist W-146. Preperfusing vessels with W-146 (10 μM) for 30 min completely abolished the SEW-2871 effect (n = 3). The mean peak Lp increased to 8.6 ± 0.2 times that of the control upon PAF addition in the presence of SEW-2871 and W-146, indicating that the effect of SEW-2871 on permeability was mediated by S1PR1. Figure 8, A and B, shows the Lp changes from individual experiments, and Fig. 8C summarizes the results.
Fig. 8.
SEW-2871 (SEW), a selective S1PR1 agonist, attenuated the PAF-induced increases in Lp. A: single experiment shows that S1PR1 agonist significantly attenuated PAF-induced Lp increases, an effect similar to that of S1P. The second application of PAF in the same vessel after perfusion of albumin-Ringer solution restored the PAF response. B: blocking S1PR1 with selective S1PR1 antagonist W-146 abolished the inhibitory effect of SEW on PAF-induced Lp increases, indicating that the effect of SEW is S1PR1 specific. C: summary results show the effect of SEW on PAF-induced Lp increases with (n = 3, right) and without (n = 5, left) blocking S1PR1 by antagonist W-146. *Significant increase from the control.
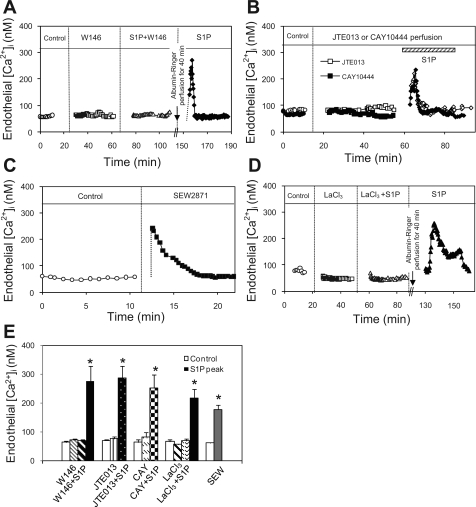
S1PR1 is responsible for S1P-induced increases in endothelial [Ca2+]i.
We previously demonstrated that S1P induces transient increases in endothelial [Ca2+]i without accompanied increases in microvessel permeability (25). To identify the S1P receptors responsible for this Ca2+ effect, endothelial [Ca2+]i was measured in vessels pretreated with each specific receptor antagonist before S1P application. Mean basal endothelial [Ca2+]i of 15 vessels was 68 ± 4 nM. The perfusion of each receptor antagonist did not significantly change the baseline endothelial [Ca2+]i. However, the S1P-induced transient increase in endothelial [Ca2+]i was only abolished by W-146 (10 μM), an antagonist of S1PR1 (Fig. 9A) and not affected by S1PR2 or S1PR3 antagonist (Fig. 9B). The mean endothelial [Ca2+]i upon S1P addition to W-146-perfused vessels (n = 5) was 68 ± 4 nM, which was not significantly different from that of the control. A second exposure to S1P after the removal of W-146 by perfusion of albumin-Ringer solution for 40 min increased endothelial [Ca2+]i to a mean peak of 274 ± 53 nM. In contrast, neither JTE-013 (10 μM, n = 5) nor CAY (10 μM, n = 5) significantly affected the S1P effect on endothelial [Ca2+]i. The mean peak endothelial [Ca2+]i was 285 ± 43 and 253 ± 46 nM, respectively, upon S1P addition. These mean peak Ca2+ values measured during or after the modulation of a specific receptor were higher than the previously reported 193 ± 22 nM when the vessels were directly exposed to S1P but the differences were not statistically significant. About two vessels in each group have higher responses with peaks at 300–400 nM, but the median of the S1P-induced peak Ca2+ of 15 vessels was 233 nM. The increased endothelial [Ca2+]i all returned to the baseline level within 5 to 20 min. Figure 9E summarizes the results.
Fig. 9.
S1P-induced increase in endothelial intracellular Ca2+ concentration ([Ca2+]i) is subtype receptor specific. A: time course of changes in endothelial [Ca2+]i in an individual experiment. W-146 (10 μM), a selective S1PR1 antagonist, had no effect on baseline endothelial [Ca2+]i but abolished S1P-induced endothelial [Ca2+]i increase. The S1P effect was recovered after 40 min perfusion of albumin-Ringer solution. B: superimposed time courses of 2 individual experiments showing that selective inhibition of S1PR2 or S1PR3 by antagonist JTE (10 μM) or CAY (10 μM), respectively, showed no effect on S1P-induced endothelial [Ca2+]i increase. C: SEW, a selective S1PR1 agonist, induced similar transient increases in endothelial [Ca2+]i to that of S1P, further supporting the S1PR1-mediated Ca2+ response. D: LaCl3 (50 μM), a Ca2+ channel blocker, completely abolished S1P-induced endothelial [Ca2+]i increase, and removal of LaCl3 restored the S1P effect in the same vessel, indicating Ca2+ influx is the main source contributing to the S1P-induced increase in endothelial [Ca2+]i. E: summary results show the changes in endothelial [Ca2+]i in each group of 5 experiments. *Significant increase from the control.
The specific role of S1PR1 in S1P-mediated increases in endothelial [Ca2+]i was further examined using a specific S1PR1 agonist, SEW-2871. The mean basal endothelial [Ca2+]i of five microvessels was 60 ± 4 nM. The perfusion of SEW-2871 (10 μM) induced an immediate and transient increase in endothelial [Ca2+]i (Fig. 9C) with a mean peak value of 178 ± 16 nM. The time course was similar to that observed with S1P. The magnitude of SEW-2817-induced peak [Ca2+]i was slightly lower than that of the [Ca2+]i response to S1P alone, but the difference was not statistically significant. This slightly lower peak response may also be attributed to its lower potency than S1P. Figure 9E summarizes the results.
The main source contributing to S1P-induced increases in endothelial [Ca2+]i was investigated in another five microvessels using LaCl3 (50 μM), an inhibitor of the capacitive cationic channels in endothelial cells. The perfusion of LaCl3 for 10–20 min did not significantly change the basal endothelial [Ca2+]i (66 ± 7 vs. 57 ± 2 nM, P > 0.05). When S1P was added to LaCl3-perfused vessels, no increase in endothelial [Ca2+]i was observed, and the mean was 69 ± 7 nM, indicating that S1P-induced increases in endothelial [Ca2+]i were mainly due to Ca2+ influx. When LaCl3 was washed out from the vessel lumen for 30 min, a transient increase in [Ca2+]i was resumed upon S1P addition, and the mean peak [Ca2+]i was 216 ± 33 nM. Figure 9D shows an individual experiment, and 9E summarizes the results.
DISCUSSION
The main new findings of this study are that, in the presence of all three S1P subtype receptors (S1PR1–3) in intact venules of rat mesentery, endothelial S1PR1 was the only subtype receptor involved in the protective effect of S1P on inflammatory mediators, PAF- or BK-induced increases in microvessel permeability. Although the activation of S1PR2 has been reported to disrupt the endothelial barrier, counteracting the effect of S1PR1 (16, 34, 37), our results showed that neither endothelial S1PR2 nor S1PR3 was involved in regulating permeability in intact microvessels. This conclusion is supported by quantitative measurements of the permeability coefficient in intact microvessels using specific S1P receptor antagonists (27, 30, 36, 41), a selective S1PR1 agonist (35), and confocal imaging of fluorescent immunostained S1PR1–3 in microvessel walls.
S1P was reported as a high-affinity ligand for five G protein-coupled S1P receptors (18, 19, 31) that are widely expressed in various mammal tissues. Among the five receptor subtypes, S1PR1–3 have been commonly identified in the vascular system. With an increased recognition of the diverse functions of S1P under physiological and pathological conditions (3, 20, 30, 33, 34, 37, 38, 40), further characterization of the specific roles of S1P subtype receptors in the regulation of a wide range of cellular functions is necessary and important.
The S1P action on enhancing endothelial barrier function has been reported in both in vivo and in vitro studies, which was thought to be via S1PR1 ligation (7, 22, 25, 29, 33, 40). Receptor protein and RNA analysis reported that certain cultured endothelial cells have abundant S1PR1, but the expression of S1PR2 was either undetectable or at very low level (17, 28). The expression of S1P receptor subtypes may also vary in tissues. Our previous study conducted in intact rat mesenteric venules demonstrated that the perfusion of vessels with S1P prevented PAF-induced increases in microvessel permeability and that the inhibitory action of S1P involves the activation of pertussis toxin-sensitive Gi protein-coupled receptors (25). Because Gi protein not only couples to S1PR1 but also couples to other S1P subtype receptors (43), our present study first investigated whether multiple S1P receptor subtypes are expressed in intact rat mesenteric venules. With the use of specific antibody staining in the venules where the S1P effect on permeability has been studied, confocal images illustrated that S1PR1–3 are all expressed in the venular endothelium with comparable fluorescent staining and distribution patterns. Currently, most of the tissue or vessel staining of receptors were demonstrated from immunostaining of cross sections (4, 16, 38). Our confocal fluorescence images provided the first spatial distribution patterns of each receptor subtype at vessel walls. In rat mesenteric venules, the staining was mainly at endothelial cells with uniform distribution over cell membrane and cytosol. Staining is also detectable at pericytes. The existence of these three receptor subtypes in the intact venules provided the fundamental validation for the investigation of the specific action of each receptor subtype in the regulation of microvessel permeability in their native states. We consider that the main S1P effect on microvessel permeability is the S1P action on endothelial cells but could not completely exclude the potential effect of pericytes. Currently, the roles of S1P in venular pericytes and the pericyte roles in the regulation of microvessel permeability remain to be uncovered.
The functions of S1P subtype receptors have been investigated using genetic and pharmacological tools. The recently developed S1P subtype receptor antagonists and agonist showed relatively high selectivity and binding affinity and have been well characterized in vitro and ex vivo (27, 30, 35, 36, 41). These specific pharmacological agents enabled us to investigate the involved subtype receptor(s) of S1P in the regulation of permeability in intact microvessels. The effect of S1P has been reported as concentration dependent (1, 21). The S1P concentration in human plasma is about 200–500 nM (44). The concentrations of S1P at 10 nM-2 μM in cultured cells (21) and 1–5 μM in perfused vessels (2, 25) demonstrated endothelial barrier enhancement. An S1P concentration of 1 μM that has been shown to sufficiently prevent PAF- and BK-induced Lp increases in intact microvessels (1, 25) was used in the present study.
Our results demonstrated that in the presence of three S1P receptor subtypes (S1PR1, S1PR2, and S1PR3) in rat mesenteric venules, the application of S1P inhibited both PAF- and BK-induced permeability increases, and the S1P effect was reversed only with a selective S1PR1 antagonist. Both S1PR2 and S1PR3 antagonists failed to alter either the S1P effect or the inflammatory mediator-induced permeability increases in the absence of S1P application. In addition, the inhibition of S1PR1 showed no effects on basal permeability in the absence and presence of S1P administration, indicating that in the absence of S1PR1-mediated function, the presence or activations of S1PR2 and S1PR3 play no roles in the regulation of basal microvessel permeability. If we assume S1P has a high-binding affinity for all three S1P receptor subtypes in intact venules as that reported in the binding affinity assays on Chinese hamster ovary cell-expressed S1P receptors (6, 19, 35), our results indicate that the activation of endogenous endothelial S1PR2 and S1PR3 by near physiological concentration of S1P (1 μM) is not involved in the regulation of microvessel permeability under basal or acute inflammatory conditions. These results are further supported by studies using a selective S1PR1 agonist, SEW-2871 (14, 35). SEW-2871 has been characterized as a full agonist of S1PR1 without a downregulation of the receptor based on GTPγS35 binding, Ca2+ flux, and cell migration assays (8, 35). Although the structure of SEW-2871 is different from natural ligand S1P, it demonstrated S1P-like functions, downstream signaling, and high selectivity at S1PR1 (3, 14). However, its potency in receptor binding and functional activity was lower than S1P in both in vitro and in vivo studies (14, 35). The potential mechanisms have been evaluated in detail (14). Our results of SEW-2871 are in agreement with others' studies. SEW-2871 at a concentration of 10 μM (slightly less than the binding efficacy of 1 μM of S1P at murine S1PR1) showed a similar effect to that of S1P, but the magnitude of inhibition of PAF-induced Lp increase was not as complete as that of S1P at 1 μM. However, the effect of SEW-2871 was completely reversed by W-146, a selective antagonist of S1PR1, indicating that its action is S1PR1 specific. With the finding that only S1PR1 is involved in the protective role of S1P in microvessel permeability, the high selectivity of SEW-2871 at S1PR1 makes it a valuable therapeutic candidate for enhancing endothelial barrier function during inflammation, even with less potency. The fact that the S1P/S1PR1 interaction is responsible for S1P-mediated inhibitions of both PAF- and BK-induced permeability increases indicates that the S1PR1-mediated S1P effect on microvessel permeability is not only applicable for one specific inflammatory mediator and may represent a universal mechanism of S1P-mediated protection against increases in microvessel permeability during inflammation.
Our results are consistent with studies showing that enhancing endothelial barrier function and the inhibition of permeability increases during inflammation are the dominant effects of S1P in organs expressing S1PR1–3 (20, 22, 29, 33). However, the expression of S1PR2 has been shown to induce endothelial barrier dysfunction that counteracts the actions of S1PR1 activation by S1P (16, 33, 34). When S1PR2 was expressed in human umbilical vein endothelial cells by adenoviral transduction, not only was the effect of S1P on enhancing endothelial barrier function completely abolished, the expression of S1PR2 in the absence of applied S1P showed a disruption of adherens junction (AJ) and increased endothelial cell permeability (34). Additionally, the application of S1PR2 antagonist, JTE-013, reduced H2O2-induced lung edema (34) and enhanced the action of S1P on protecting microvessel permeability (16). S1PR2−/− knockout and silenced S1PR3 mice also showed a reduction of LPS-induced barrier disruption in the absence of applied S1P (33). These results indicated that the expression of S1PR2 causes endothelial barrier dysfunction. In contrast to studies with genetically modified subtype receptors, our results showed that the acute pharmacological inhibition of S1PR2 or S1PR3 in intact rat venules has no effect on basal permeability or inflammatory mediator-induced permeability increases. Specifically, unlike the effect of JTE-013 reported in perfused lungs that reduces H2O2-induced lung edema (34), our study showed that directly perfusing the vessels with JTE-013 did not affect the basal Lp or PAF-induced Lp increase. Additionally, when S1PR1 was blocked by a specific antagonist, S1P ligation with S1PR2 and S1PR3 did not alter the basal Lp. These discrepancies could be attributed to tissue differences in S1P subtype receptor expression or the potential nonspecific effects associated with the genetic manipulations of specific receptors. It should also be noted that our results represent the functions of the native subtype receptors expressed in normal vessels with or without an acute exposure to inflammatory mediators. A retina angiogenesis study reported that an mRNA expression of S1PR2 was increased during hypoxia-induced angiogenesis (38). Whether chronic inflammation changes the relative distributions of S1P subtype receptors and/or the coupling signals, enabling different S1P effects from that of S1PR1 ligation on microvessel permeability, remains to be investigated.
Because S1P receptors are widely expressed on different cell types exhibiting different functions, our study using individually perfused microvessels in the absence of blood cells made a distinction from the whole animal or vascular bed studies (3, 16, 19). While the whole animal or organ studies retained the interactions of circulating blood with endothelium, those studies usually have a limited capacity to differentiate the roles of one cell type from another. For example, a whole animal study showed that S1PR1 activation reduces ischemia-reperfusion injury in mouse kidney, but it was difficult to define the mechanism of the protection, since S1PR1 is expressed in many tissues including lymphocytes and endothelial cells (3). Additionally, permeability changes in whole organ studies were mainly based on the measurement of permeability index, which was determined as a ratio of vascular versus interstitial FI after an intravenous administration of fluorescent dye-labeled albumin. Any changes in hemodynamic parameters, such as pressure and surface area for exchange, may confound the results, especially as S1P has been shown as a vasoconstrictor through S1PR2 or S1PR3 interaction in smooth muscle cells (31). In contrast, our approach enabled us to quantitatively measure the permeability coefficient before and after the treatments in the same vessel with known perfusion pressure and surface area for exchange. The absence of blood cells and single vessel type under well-controlled perfusion conditions provide more exclusive evidence that the protecting role of S1P in vascular barrier functions is mediated by S1PR1 expressed in intact venular microvessels.
Ca2+ mobilization by S1P ligation of G protein-coupled receptors has been reported in many cell types, mainly cultured or genetically modified cell lines (23, 26, 28). Our previous study showed that in intact venules, S1P also induces transient increases in endothelial [Ca2+]i (25). Previously, we showed that increases in endothelial [Ca2+]i correlated with increases in microvessel permeability in agonist-stimulated venules (9, 13). S1P is the first bioactive molecule we have found that increases endothelial [Ca2+]i without an accompanied increase in microvessel permeability. It even inhibits agonist-induced permeability increases without altering an agonist-induced increase in endothelial [Ca2+]i. While agents like cAMP, cGMP agonist, and eNOS inhibitor regulate permeability downstream from the initial Ca2+ signals (10, 11), these modulators do not affect the agonist-induced increases in endothelial [Ca2+]i, similar to S1P. However, unlike S1P, these agents alone have no effect on endothelial [Ca2+]i. Currently, the context of the S1P-induced transient Ca2+ signal in the regulation of microvessel permeability remains unknown. One cultured cell study reported that S1P induces endoplasmic reticulum Ca2+ release and subsequent Ca2+ entry and that the Ca2+ release from intracellular stores was required for S1P-induced Rac activation and the enhancement of endothelial barrier function (23). This conclusion was based on the blockage of S1P-induced AJ assembly when BAPTA was used to chelate intracellular Ca2+ and the failure to prevent S1P-induced AJ assembly when La3+ was used to inhibit Ca2+ entry. However, BAPTA treatment itself interfered with Rac activity and resulted in the disassembly of AJs (23), and the inhibition of Ca2+ entry by La3+, even in the absence of S1P, may tighten endothelial junctions in cultured endothelial cells. Therefore, neither approach is ideal for the evaluation of the causal relationship between Ca2+ signaling and S1P-mediated endothelial barrier function. This is also the reason we did not further investigate their relationship in intact microvessels, since all inflammatory mediator-induced permeability increases are Ca2+ influx dependent. Blocking Ca2+ entry abolishes all inflammatory mediator-induced permeability increases, even in the absence of S1P. Based on in vitro studies, the activation of Rac-1 is considered to be the main signaling mechanism for the S1P effect on endothelial barrier function (1, 7, 17, 23, 31, 40). Whether the activation of Rac-1 by S1P-mediated G protein-coupled receptors requires the Ca2+ signaling remains to be determined.
Although the relationship between S1P-induced increases in endothelial [Ca2+]i and its permeability effect remains to be defined, our present study provided two new findings about S1P-induced Ca2+ signaling. First, our results demonstrated that S1P-mediated transient increases in endothelial [Ca2+]i are only abolished by the inhibition of S1PR1 and not affected by S1PR2 or S1PR3 inhibition, indicating that neither S1PR2 nor S1PR3 is involved in S1P-mediated Ca2+ effect in intact venules. This receptor subtype-specific Ca2+ response can be cell type dependent. For example, JTE-013, a selective S1PR2 antagonist, inhibited the S1P-induced Ca2+ response in smooth muscle cells but failed to affect S1P-induced Ca2+ mobilization in human umbilical vein endothelial cells (28). Our second new finding is that blocking Ca2+ influx with LaCl3, a Ca2+ channel blocker, completely abolished the S1P-mediated increases in endothelial [Ca2+]i, suggesting that S1P/S1PR1 ligation directly triggers Ca2+ influx without the involvement of internal Ca2+ release. This observation also indicates that S1P-induced Ca2+ mobilization is different from the inflammatory mediator or the superoxide-induced increases in endothelial [Ca2+]i in which both Ca2+ influx and Ca2+ release from internal stores are involved and that the application of LaCl3 only affects Ca2+ influx without affecting internal Ca2+ release (13, 46). Currently, the reported sources of S1P-induced Ca2+ mobilization in cultured cells are not consistent (23, 28). While our findings are in agreement with some of the in vitro studies (26), the exact significance of the S1P-induced Ca2+ response remains to be uncovered.
Summary.
Our study demonstrated that in intact rat venules, despite the presence of S1PR1–3, S1PR1 is the only functional S1P receptor responsible for S1P-mediated protection against PAF- or BK-induced increases in microvessel permeability, suggesting that the activation of S1PR1 may be the universal mechanism of S1P that prevents permeability increases in intact microvessels and that the specific S1PR1 agonist may have a great therapeutic potential against permeability increases during inflammation. S1PR1 is also identified as the sole receptor responsible for S1P-induced transient increases in endothelial [Ca2+]i, which only involves Ca2+ influx without an initial internal Ca2+ release. The inhibition of S1PR2 or S1PR3 affected neither baseline permeability nor inflammatory mediator-induced increases in Lp, suggesting that endogenous S1PR2 and S1PR3 in intact rat venules may not directly involve the regulation of microvessel permeability in response to near physiological concentration of S1P. The functional roles of S1PR2 and S1PR3 under physiological and pathological or chronic disease conditions remain to be revealed. This phenomenon does not appear unique for S1P. Similar observations have also been reported in adenosine receptor-mediated permeability responses (39).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-56237 and HL-084338.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Mandala and Jim Milligan (Merck Research Laboratories) for providing us the primary S1P subtype receptor antibodies, Prof. Mary Davis for consulting pharmacological issues, and Prof. Michael Miller for proofreading the manuscript.
REFERENCES
- 1.Adamson RH, Sarai RK, Altangerel A, Thirkill TL, Clark JF, Curry FR. Sphingosine-1-phosphate modulation of basal permeability and acute inflammatory responses in rat venular microvessels. Cardiovasc Res. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson RH, Zeng M, Adamson GN, Lenz JF, Curry FE. PAF- and bradykinin-induced hyperpermeability of rat venules is independent of actin-myosin contraction. Am J Physiol Heart Circ Physiol 285: H406– H417, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290: F1516– F1524, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Chae SS, Paik JH, Furneaux H, Hla T. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest 114: 1082– 1089, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curry PE, Huxley VH, Sarelius IH. Techniques in microcirculation: measurement of permeability, pressure and flow. In: Cardiovascular Physiology. Techniques in the Life Sciences. New York: Elsevier, 1983, p. 1–34 [Google Scholar]
- 6.Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem 280: 9833– 9841, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689– 701, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Cabrera PJ, Hla T, Rosen H. Mapping pathways downstream of sphingosine 1-phosphate subtype 1 by differential chemical perturbation and proteomics. J Biol Chem 282: 7254– 7264, 2007 [DOI] [PubMed] [Google Scholar]
- 9.He P, Curry FE. Endothelial cell hyperpolarization increases [Ca2+]i and venular microvessel permeability. J Appl Physiol 76: 2288– 2297, 1994 [DOI] [PubMed] [Google Scholar]
- 10.He P, Liu B, Curry FE. Effect of nitric oxide synthase inhibitors on endothelial [Ca2+]i and microvessel permeability. Am J Physiol Heart Circ Physiol 272: H176– H185, 1997 [DOI] [PubMed] [Google Scholar]
- 11.He P, Zeng M, Curry FE. Dominant role of cAMP in regulation of microvessel permeability. Am J Physiol Heart Circ Physiol 278: H1124– H1133, 2000 [DOI] [PubMed] [Google Scholar]
- 12.He P, Zhang H, Zhu L, Jiang Y, Zhou X. Leukocyte-platelet aggregate adhesion and vascular permeability in intact microvessels: role of activated endothelial cells. Am J Physiol Heart Circ Physiol 291: H591– H599, 2006 [DOI] [PubMed] [Google Scholar]
- 13.He P, Zhang X, Curry FE. Ca2+ entry through conductive pathway modulates receptor-mediated increase in microvessel permeability. Am J Physiol Heart Circ Physiol 271: H2377– H2387, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, Hla T, Parrill AL, Rosen H. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol 12: 703– 715, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Kendall S, Michel CC. The measurement of permeability in single rat venules using the red cell microperfusion technique. Exp Physiol 80: 359– 372, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Lee JF, Gordon S, Estrada R, Wang L, Siow DL, Wattenberg BW, Lominadze D, Lee MJ. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am J Physiol Heart Circ Physiol 296: H33– H42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99: 301– 312, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279: 1552– 1555, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296: 346– 349, 2002 [DOI] [PubMed] [Google Scholar]
- 20.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem 92: 1075– 1085, 2004 [DOI] [PubMed] [Google Scholar]
- 21.McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal 17: 131– 139, 2005 [DOI] [PubMed] [Google Scholar]
- 22.McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med 170: 987– 993, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Mehta D, Konstantoulaki M, Ahmmed GU, Malik AB. Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J Biol Chem 280: 17320– 17328, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Minnear FL, Patil S, Bell D, Gainor JP, Morton CA. Platelet lipid(s) bound to albumin increases endothelial electrical resistance: mimicked by LPA. Am J Physiol Lung Cell Mol Physiol 281: L1337– L1344, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Minnear FL, Zhu L, He P. Sphingosine 1-phosphate prevents platelet-activating factor-induced increase in hydraulic conductivity in rat mesenteric venules: pertussis toxin sensitive. Am J Physiol Heart Circ Physiol 289: H840– H844, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Muraki K, Imaizumi Y. A novel function of sphingosine-1-phosphate to activate a non-selective cation channel in human endothelial cells. J Physiol 537: 431– 441, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohmori T, Yatomi Y, Osada M, Kazama F, Takafuta T, Ikeda H, Ozaki Y. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc Res 58: 170– 177, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Osada M, Yatomi Y, Ohmori T, Ikeda H, Ozaki Y. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Commun 299: 483– 487, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 169: 1245– 1251, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol 28: 102– 107, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res 94: 724– 734, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Salomone S, Potts EM, Tyndall S, Ip PC, Chun J, Brinkmann V, Waeber C. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br J Pharmacol 153: 140– 147, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sammani S, Moreno-Vinasco L, Mirzapoiazova T, Singleton PA, Chiang ET, Evenoski CL, Wang T, Mathew B, Husain A, Moitra J, Sun X, Nunez L, Jacobson JR, Dudek SM, Natarajan V, Garcia JG. Differential effects of S1P receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol 27: 1312– 1318, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 279: 13839– 13848, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol 2: 434– 441, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Skoura A, Hla T. Regulation of vascular physiology and pathology by the S1P2 receptor subtype. Cardiovasc Res 82: 221– 228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest 117: 2506– 2516, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Huxley VH. Adenosine A2A receptor modulation of juvenile female rat skeletal muscle microvessel permeability. Am J Physiol Heart Circ Physiol 291: H3094– H3105, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res 77: 39– 45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wetter JA, Revankar C, Hanson BJ. Utilization of the Tango beta-arrestin recruitment technology for cell-based EDG receptor assay development and interrogation. J Biomol Screen 14: 1134– 1141, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Whetzel AM, Bolick DT, Hedrick CC. Sphingosine-1-phosphate inhibits high glucose-mediated ERK1/2 action in endothelium through induction of MAP kinase phosphatase-3. Am J Physiol Cell Physiol 296: C339– C345, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. J Biol Chem 274: 27351– 27358, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Yatomi Y. Plasma sphingosine 1-phosphate metabolism and analysis. Biochim Biophys Acta 1780: 606– 611, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Zhao C, Fernandes MJ, Turgeon M, Tancrede S, Di Battista J, Poubelle PE, Bourgoin SG. Specific and overlapping sphingosine-1-phosphate receptor functions in human synoviocytes: impact of TNF-alpha. J Lipid Res 49: 2323– 2337, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Zhou X, Wen K, Yuan D, Ai L, He P. Calcium influx-dependent differential actions of superoxide and hydrogen peroxide on microvessel permeability. Am J Physiol Heart Circ Physiol 296: H1096– H1107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu L, He P. Platelet-activating factor increases endothelial [Ca2+]i and NO production in individually perfused intact microvessels. Am J Physiol Heart Circ Physiol 288: H2869– H2877, 2005 [DOI] [PubMed] [Google Scholar]