Abstract
Angiotensin II (ANG II) contributes to hypertension, cardiac hypertrophy, fibrosis, and dysfunction; however, it is difficult to separate the cardiac effect of ANG II from its hemodynamic action in vivo. To overcome the limitations, we used transgenic mice with cardiac-specific expression of a transgene fusion protein that releases ANG II from cardiomyocytes (Tg-ANG II) and treated them with deoxycorticosterone acetate (DOCA)-salt to suppress their systemic renin-angiotensin system. Using this unique model, we tested the hypothesis that cardiac ANG II, acting on the angiotensin type 1 receptor (AT1R), increases inflammation, oxidative stress, and apoptosis, accelerating cardiac hypertrophy and fibrosis. Male Tg-ANG II mice and their nontransgenic littermates (n-Tg) were uninephrectomized and divided into the following three groups: 1) vehicle-treated normotensive controls; 2) DOCA-salt; and 3) DOCA-salt + valsartan (AT1R blocker).Under basal conditions, systolic blood pressure (SBP) and cardiac phenotypes were similar between strains. In DOCA-salt hypertension, SBP increased similarly in both n-Tg and Tg-ANG II, and cardiac function did not differ between strains; however, Tg-ANG II had 1) greater ventricular hypertrophy as well as interstitial and perivascular fibrosis; 2) a higher number of deoxynucleotidyl-transferase-mediated dUTP nick end labeling-positive cells and infiltrating macrophages; 3) increased protein expression of NADPH oxidase 2 and transforming growth factor-β1; and 4) downregulation of phosphatidylinositol 3-kinase (PI 3-kinase) and protein kinase B (Akt) phosphorylation. Valsartan partially reversed these effects in Tg-ANG II but not in n-Tg. We conclude that, when hemodynamic loading conditions remain unchanged, cardiac ANG II does not alter heart size or cardiac functions. However, in animals with hypertension, cardiac ANG II, acting via AT1R, enhances inflammation, oxidative stress, and cell death (most likely via downregulation of PI 3-kinase and Akt), contributing to cardiac hypertrophy and fibrosis.
Keywords: inflammation, oxidative stress, cardiac hypertrophy, fibrosis
angiotensin ii (ANG II), the principal effector of renin-angiotensin system (RAS) binds to two types of cell surface receptors, type 1 (AT1R) and type 2 (AT2R). Most well-known biological actions of ANG II are mediated by the AT1R (26). Studies have indicated that ANG II not only functions as a circulating vasoconstrictor, causing hypertension, but also acts locally as a paracrine and autocrine hormone to promote cell growth, apoptosis, inflammation, oxidative stress, and tissue damage, ultimately leading to cardiac hypertrophy, fibrosis, and heart failure (20, 30).
There is evidence that all components of the RAS, including its receptors, are found in the heart and become upregulated in hypertrophy and ischemia (3, 17, 37). ANG II concentration is also reportedly higher in the heart than in plasma (17, 37). Thus locally produced ANG II might act in both a paracrine and autocrine manner to promote inflammation, oxidative stress, and cell growth and/or death and in this way contribute to cardiac hypertrophy, fibrosis, and dysfunction (10, 16, 19, 39). However, in vivo, it is difficult to separate local effects of ANG II from its hemodynamic action. Moreover, although blocking the RAS with angiotensin-converting enzyme inhibitors or ANG II receptor blockers has been shown to reduce morbidity and mortality in patients with cardiovascular disease, it is unclear whether their cardioprotective effects are due to systemic RAS inhibition and/or a direct action on the heart.
To overcome these limitations, van Kats et al. (37) developed transgenic mice with cardiac-specific overexpression of ANG II (Tg-ANG II). These mice have a transgene construct encoding a fusion peptide that releases ANG II directly from cardiomyocytes without involvement of renin. We previously reported that cardiac homogenates from these mice contain 10- to 15-fold higher concentration of ANG II than their nontransgenic littermates (n-Tg), even though systolic blood pressure (SBP) and plasma ANG II are the same in both strains (40). Under basal conditions, Tg-ANG II mice exhibit a slight increase in cardiac collagen but not cardiac hypertrophy (37). When a hemodynamic load such as myocardial infarction (MI) is applied, Tg-ANG II develop more severe myocyte hypertrophy and interstitial fibrosis than n-Tg (37, 40). Unfortunately, these findings are of limited value because MI activates not only the cardiac but also the systemic RAS, which makes it difficult to separate the cardiac effect of ANG II from its hemodynamic action.
To overcome these limitations, we induced deoxycorticosterone acetate (DOCA)-salt hypertension in Tg-ANG II mice to create a model of low-circulating renin-ANG II but high ANG II in the heart. In this model, cardiac ANG II is generated by the protease furin acting on the bioengineered fusion protein encoded by the transgene, and its production is completely independent of blood pressure or systemic renin concentration. Using this approach, we tested the hypothesis that cardiac ANG II acts locally via the AT1R, increasing inflammation, oxidative stress, and apoptosis and accelerating cardiac hypertrophy and fibrosis.
MATERIALS AND METHODS
Animals.
Twelve-week-old male cardiac Tg-ANG II mice were developed by van Kats et al. (37). These mice have a transgene construct encoding a fusion peptide that releases ANG II directly from cardiomyocytes when the ANG II-containing precursor protein is cleaved by natural protease furin. The cleavage site for the protease that releases the ANG II peptide is very specific for furin, which is ubiquitously found only in the secretory pathway. Because of this design, ANG II can only be generated in the secretory pathway and gets released from the cells in conjunction with the remainder of the fusion protein without requirement of the RAS.
Tg-ANG II mice were on mixed FVB/N and C57BL/6 genetic background. They were generated by mating transgene hemizygotes and nontransgenic littermates, which provides hemizygotes (Tg-ANG II) and nontransgenic offspring (n-Tg) in approximately equal numbers. Their genotypes were determined by PCR using genomic DNA isolated from tail biopsies as the template and primers. Four primers are used in a single reaction: Tim210 (5′-TTCTCATGCAACGTGAGAC-3′); Tim547 (5′-TGGTGATACAAGGGACATC-3′); Nos3A (5′-ATGGCGAAGCGTGTGAAGGCAACCAT-3′); and Nos3B (5′-CCATTGCCAAATGTGCTGGTCACCAC-3′).
Tim210 and Tim547 amplify an ≈400-bp fragment in the presence of an ANG II transgene, whereas Nos3A and Nos3B amplify an ≈550 bp fragment from the wild-type eNOS gene and are used as internal amplification control n-Tg (40). Tg-ANG II and n-Tg mice were housed in an air-conditioned room with a 12:12-h light-dark cycle, received standard mouse chow, and drank tap water. This study was approved by the Institutional Animal Care and Use Committee of Henry Ford Health System. All studies in animals were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Induction of DOCA-salt hypertension.
Tg-ANG II and their n-Tg controls were anesthetized with pentobarbital sodium (50 mg/kg ip), uninephrectomized via a retroperitoneal flank incision, and divided into the following three groups: 1) normotensive controls (vehicle): mice were subcutaneously implanted with a silicone rubber sheet without DOCA and received tap water as drinking fluid; 2) DOCA-salt hypertension: mice were implanted with a silicone rubber sheet containing 10 mg/10 g of body wt DOCA and drank 1% saline containing 0.2% KCl; and 3) DOCA-salt + valsartan (50 mg·kg−1·day−1, via gavage initiated 4 wk after DOCA-salt and continued for 4 wk) (31). The total experimental period was 8 wk.
Measurement of blood pressure and cardiac function and remodeling.
SBP and heart rate were measured weekly using a noninvasive computerized tail-cuff system (BP-2000; Visitech, Apex, NC) as described previously (41). Left ventricular (LV) ejection fraction and LV mass were evaluated with a Doppler echocardiographic system equipped with a 15-MHz linear transducer (c256; Acuson, Mountain View, CA) in awake animals as described previously (43, 44).
Urine volume, albuminuria, and urinary 8-isoprostane excretion.
At the end of the 8-wk experiment, mice were placed in metabolic cages. After 2 days acclimatization, 24-h urine was collected. Urine samples were centrifuged, filtered, and stored at −20°C until assay. Urinary albumin was measured with a commercially available ELISA kit (Alpha Diagnostic, San Antonio, TX), and 8-isoprostane was measured with a enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) (27).
Plasma renin concentration.
After urine collection, mice were anesthetized with pentobarbital sodium (50 mg/kg ip) and blood was collected in a microhematocrit tube by puncturing the retroorbital plexus. Plasma renin concentration (PRC) was determined with a commercially available RIA kit (DiaSorin, Stillwater, MN) and expressed as microgram ANG I per milliliter per hour.
Histopathological studies.
After blood was withdrawn, the heart was stopped at diastole by intraventricular injection of 15% KCl (50 μl). The hearts were removed and weighed. Heart weight was corrected by body weight and expressed as milligrams per 10 grams body weight. Left ventricle was then dissected and stored at −80°C for further assessment of myocyte cross-sectional area (MCSA) and interstitial collagen fraction (ICF) and perivascular collagen deposition (PVC). For MCSA and ICF, 6-μm sections were double-stained with 1) fluorescein-labeled peanut agglutinin to delineate the MCSA and interstitial apace and 2) rhodamine-labeled Griffonia simplicifolia lectin I to show the capillaries (12, 42). Images were taken at ×400 magnification using a microscope (IX81; Olympus America, Center Valley, PA) and a digital camera (DP70; Olympus America) and analyzed with a computerized image analysis system (MicroSuite Biological imaging software; Olympus America). ICF was determined by total interstitial space area minus capillaries and expressed as percent of total image area. Perivascular collagen was stained with picrosirius red using a modification of Sweat et al.'s (33) method. Perivascular fibrosis was expressed as the ratio of the fibrotic area immediately surrounding the vessel to cross-sectional area of the vessel (22).
Immunohistochemical staining for macrophages.
Frozen sections (6 μm) were immunostained with rat anti-mouse CD68 antibody (a marker for mouse macrophages, 1:200; AbD Serotec, Raleigh, NC) and then counterstained with hematoxylin to show the nuclei. Images of 12 regions of the section were captured at ×400 magnifications. Positive cells, identified by their reddish-brown staining, were counted in a double-blind fashion by three individuals using MicroSuite Biological Suite software and expressed as cells per square millimeter of myocardium (40).
Terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling staining.
To evaluate myocardial apoptotic activity, the terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling (TUNEL) assay was performed with the ApopTag Plus Peroxidase in Situ Apoptosis Detection Kit (Millipore). This method takes advantage of DNA fragmentation, which is characteristic of apoptosis. Briefly, frozen sections (6 μm) were fixed in freshly prepared 1% paraformaldehyde for 10 min and postfixed in a precooled 2:1 mixture of ethanol and acetic acid for 5 min at −20°C and incubated with 3% hydrogen peroxide to inhibit endogenous peroxidase for 5 min. After being washed with PBS for 15 min, the sections were soaked in the equilibration buffer of the kit for 10 s at room temperature (RT) and then terminal deoxynucleotidyl transferase reaction was carried out for 1 h at 37°C. The reaction was stopped by incubating the stop/wash buffer, and sections were incubated with an anti-digoxigenin peroxidase conjugate at RT for 30 min, and then 3,3′-diaminobenzidine was applied to develop color for 3 min at RT. Harris' hematoxylin was used as a counterstain and mounted in Cytoseal. A negative control was also prepared from additional slides. TUNEL-positive cells were determined by randomly counting 10 fields of the section and counted in a double-blind fashion using MicroAuite Biological Suite software and expressed as cells per square millimeter of myocardium (18, 40).
Western blot for phospho-protein kinase b and NADPH oxidase 2, phosphatidylinositol 3-kinase, transforming growth factor-β1, and AT1R protein expression.
LV tissue was homogenized in lysis buffer, the supernatant was collected, and protein content was detected with a Coomassie protein assay reagent (Thermo Scientific). Protein extracts (60 μg/lane) were separated out in 10% SDS-PAGE under reducing conditions and electrotransferred to a nitrocellulose membrane (Amervehicle Biosciences). Membranes were blocked in buffer (TBS-0.1% Tween 20 containing 5% nonfat milk) and incubated overnight at 4°C with either 1) a rabbit polyclonal antibody against total protein kinase B (Akt) or phospho-Akt (p-Akt) (1:1,000; Cell Signaling Technology), 2) a monoclonal antibody against NADPH oxidase (Nox) 2 (1:1,000; BD Transduction), 3) a rabbit polyclonal antibody against phosphatidylinositol 3-kinase (PI 3-kinase) p85 (1:1,000; Cell Signaling Technology), 4) a monoclonal antibody against transforming growth factor (TGF)-β1 (2 μg/ml; R&D System), 5) a rabbit polyclonal antibody against the NH2-terminal extracellular domain of AT1R (1:500, SC-1173; Santa Cruz Biotechology), or 6) a rabbit polyclonal antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:3,000; Cell Signaling Technology). Membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibodies (1:2,000; Amersham Biosciences) for 1 h at RT. Immunoreactive bands were detected by a chemiluminescent reaction (ECL kit; Amersham Pharmacia) and semiquantified by densitometry. p-Akt was normalized by total Akt, and Nox2, PI 3-kinase, TGF-β1, and AT1R proteins were normalized by GAPDH. Results were expressed as the ratio of the density of specific bands to the corresponding internal controls.
Data analysis.
All data were expressed as means ± SE. Student's two-sample t-test was used to compare differences between strains or between treatments within strains. When multiple comparisons were performed, Hochberg's step-up procedure was used to adjust the P values. The family-wise type I error rate was set at 0.05.
RESULTS
SBP, heart rate, and cardiac function.
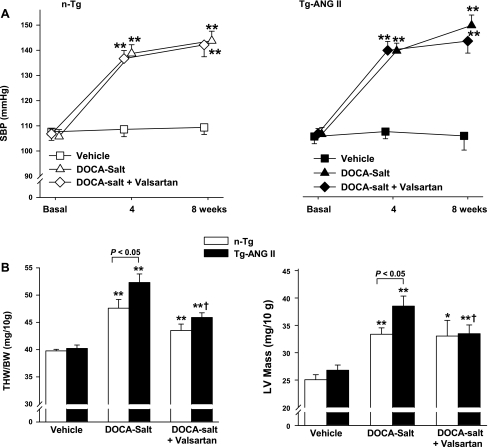
Basal SBP was similar among groups and remained unchanged in vehicle-treated groups. DOCA-salt increased SBP during the 8-wk treatment period, and no strain difference was found (Fig. 1A). Valsartan had no effect on SBP in either strain (Fig. 1A). Total heart weight (THW) and LV mass were similar in control groups of both strains, confirming that, in the absence of hypertension, cardiac ANG II does not cause hypertrophy. DOCA-salt hypertension increased THW and LV mass in both strains, but had a greater effect in Tg-ANG II (Fig. 1B). Valsartan lessened THW and LV mass in the Tg-ANG II but had no effect on n-Tg. Heart rate and ejection fraction were similar in both normotensive controls, and DOCA-salt had no significant effect on either strain despite increased SBP and hypertrophy. Valsartan also had no effect on heart rate and ejection fraction (Table 1).
Fig. 1.
Effect of cardiac overexpression of angiotensin II (ANG II) and angiotensin type 1 (AT1) receptor blocker (valsartan) on systolic blood pressure (SBP) and cardiac hypertrophy in mice with deoxycorticosterone acetate (DOCA)-salt hypertension. A: SBP. B: total heart weight (THW, left) and left ventricular (LV) mass (right). Tg-ANG II, transgenic mice with cardiac overexpression of ANG II; n-Tg, nontransgenic littermates; MI, myocardial infarction; BW, body wt. n = 8–11 mice. *P <0.05 and **P <0.01, DOCA-salt or DOCA-salt + valsartan vs. vehicle within strain. †P < 0.05, DOCA-salt + valsartan vs. DOCA-salt within strain.
Table 1.
Effect of ANG II overexpression in the heart and an angiotensin AT1 receptor blocker (valsartan) on hemodynamics, cardiac function, urine volume, and albuminuria in mice with DOCA-salt hypertension
| Groups |
||||||
|---|---|---|---|---|---|---|
| Vehicle |
DOCA-salt |
DOCA-salt + valsartan |
||||
| Parameters | n-Tg (n = 10) | Tg-ANG II (n = 11) | n-Tg (n = 11) | Tg-ANG II (n = 8) | n-Tg (n = 10) | Tg-ANG II (n = 12) |
| Body wt, g | 33.3 ± 0.67 | 32.1 ± 0.64 | 34.8 ± 0.67 | 32.5 ± 0.77 | 32.6 ± 0.85 | 34.7 ± 1.2 |
| HR, beats/min | 712 ± 20.3 | 756 ± 11.3 | 732 ± 8.3 | 734 ± 19.2 | 710 ± 16.7 | 708 ± 11.6 |
| EF, % | 71.1 ± 0.7 | 71.8 ± 1.3 | 68.7 ± 1.02 | 67.9 ± 1.2 | 67.6 ± 1.2 | 68.7 ± 1.1 |
| U volume, ml/24 h | 3.2 ± 0.3 | 2.7 ± 0.3 | 7.9 ± 1.4‡ | 6.3 ± 0.7‡ | 4.3 ± 0.7 | 4.2 ± 0.6 |
| U albumin, μg/24 h | 41.5 ± 5.3 | 39.7 ± 4.6 | 369 ± 116* | 300 ± 44† | 258 ± 95 | 372 ± 112 |
Values are means ± SE; n, no. of mice. HR, heart rate; EF, ejection fraction; U, urinary; DOCA, deoxycorticosterone acetate; valsartan, the angiotensin type 1 (AT1) receptor blocker; n-Tg, nontransgenic littermates; Tg-ANG II, transgenic mice with cardiac overexpression of angiotensin II.
P < 0.05,
P < 0.01, and
P < 0.001, DOCA-salt vs. vehicle within strains.
Myocyte size and LV interstitial and perivascular collagen.
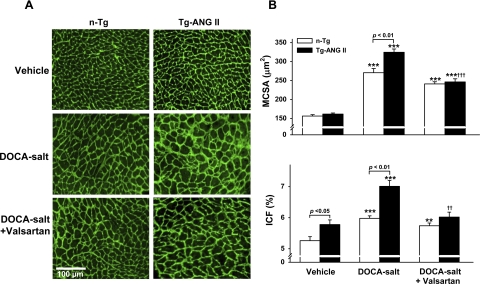
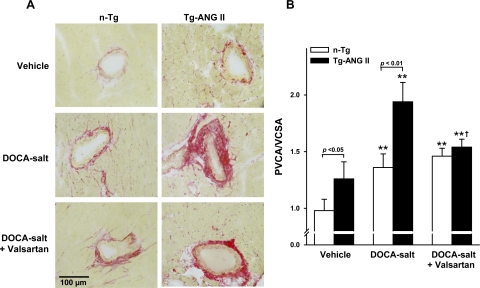
MCSA was similar in normotensive controls of both strains, but ICF and PVC were significantly higher in Tg-ANG II than in n-Tg mice (Figs. 2 and 3). DOCA-salt increased MCSA, ICF, and PVC in both strains; however, they were significantly higher in Tg-ANG II. Valsartan decreased all three parameters in Tg-ANG II but not in n-Tg (Figs. 2 and 3).
Fig. 2.
Effect of cardiac overexpression of ANG II and valsartan on myocyte cross-sectional area (MCSA) and interstitial collagen fraction (ICF). A: representative images showing MCSA (delineated by the interstitial space) and interstitial collagen deposition (green staining) in n-Tg and Tg-ANG II mice given vehicle, DOCA-salt, or DOCA-salt plus valsartan. B: quantitative data on MCSA (top) and ICF (bottom); n = 8–11. **P <0.01 and ***P <0.001, DOCA-salt or DOCA-salt + valsartan vs. vehicle within strain. ††P < 0.01 and †††P < 0.001, DOCA-salt + valsartan vs. DOCA-salt within strain.
Fig. 3.
Effect of cardiac overexpression of ANG II and valsartan on perivascular collagen deposition. A: representative images showing myocardial perivascular collagen deposition (red staining) in n-Tg and Tg-ANG II mice given vehicle, DOCA-salt, or DOCA-salt plus valsartan. B: quantitative analysis of myocardial perivascular fibrosis, expressed as the ratio of perivascular collagen area (PVCA) to vessel cross-sectional area (VCSA) of the coronary arteries; n = 7–9. **P <0.01, DOCA-salt or DOCA-salt + valsartan vs. vehicle within strain. †P < 0.05, DOCA-salt + valsartan vs. DOCA-salt within strain.
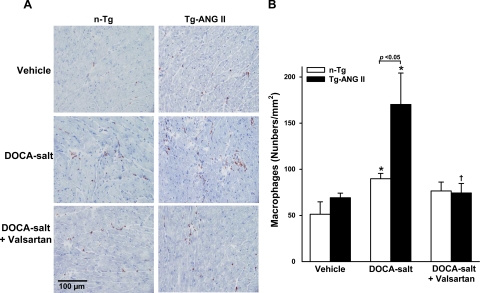
Macrophage infiltration, TGF-β1 protein expression.
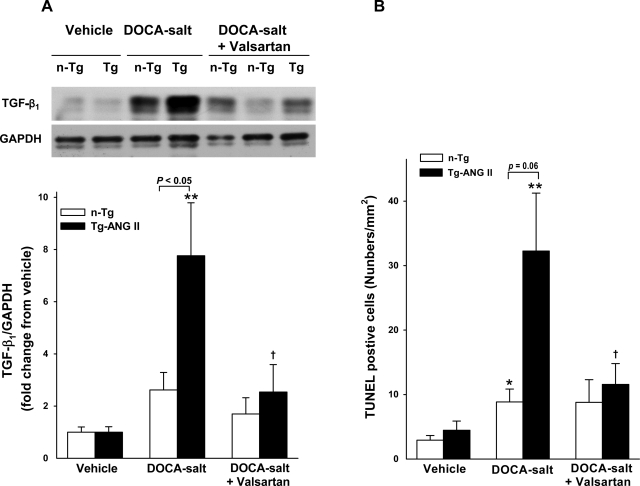
Macrophages were seen infiltrating the myocardial interstitium similarly in vehicle-treated controls. DOCA-salt increased macrophage infiltration in both strains but more so in Tg-ANG II. Valsartan lowered the macrophage count in Tg-ANG II but not in the n-Tg group (Fig. 4, A and B). TGF-β1 protein expression was the same in both control groups and rose in response to DOCA-salt in both strains, although the increase was significantly greater in Tg-ANG II. Valsartan significantly reduced TGF-β1 protein expression in Tg-ANG II but not in n-Tg (Fig. 5A).
Fig. 4.
Effect of cardiac overexpression of ANG II and valsartan on macrophage infiltration. A: representative images showing infiltrated macrophages (reddish-brown stained) in n-Tg and Tg-ANG II mice given vehicle, DOCA-salt, or DOCA-salt plus valsartan. B: quantitative analysis of the no. of macrophages infiltrating the LV myocardium; n = 6–8. *P < 0.05, DOCA-salt vs. vehicle within strain. †P < 0.05, DOCA-salt + valsartan vs. DOCA-salt within strain.
Fig. 5.
Effect of cardiac overexpression of ANG II and valsartan on transforming growth factor (TGF)-β1 protein expression and apoptosis. A: representative Western blots of TGF-β1 and glyceraldehydes-3-phosphate dehydrogenase (GAPDH, top) and semiquantitative analysis of TGF-β1 protein corrected by GAPDH and expressed as the degree of changes relative to their vehicle control (bottom). B: quantitative analysis of the no. of terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling (TUNEL)-positive staining cells in the LV myocardium; n = 6–8. *P < 0.05 and **P < 0.01, DOCA-salt vs. vehicle within strain. †P < 0.05, DOCA-salt + valsartan vs. DOCA-salt within strain.
TUNEL-positive cells, Akt phosphorylation, and PI 3-kinase protein expression.
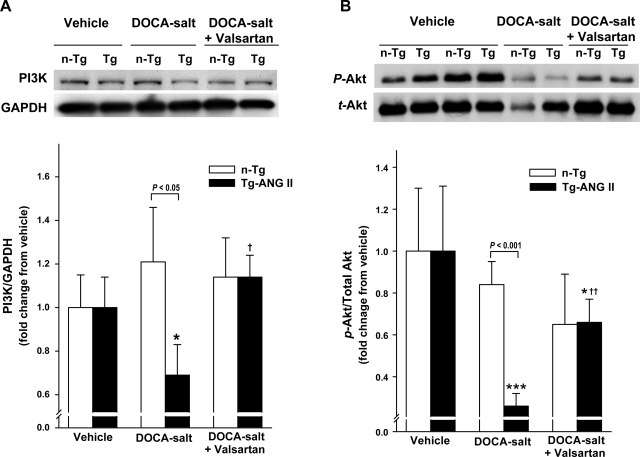
A few TUNEL-positive cells were found in the heart of normotensive controls, and no strain difference was detected. Their number increased with DOCA-salt in both strains, but more so in Tg-ANG II; moreover, valsartan reduced TUNEL-positive cells in Tg-ANG II but not in n-Tg (Fig. 5B). PI 3-kinase protein expression and Akt phosphorylation were similar in both control groups. DOCA-salt reduced PI 3-kinase protein expression and downregulated Akt phosphorylation in Tg-ANG II but not in n-Tg. Valsartan restored PI 3-kinase protein expression and partially Akt phosphorylation in Tg-ANG II and had no effect on these parameters in the n-Tg mice (Fig. 6, A and B).
Fig. 6.
Effect of cardiac overexpression of ANG II and valsartan on phosphatidylinositol 3-kinase (PI3K) protein expression and protein kinase B (Akt) phosphorylation. A: representative Western blots of PI 3-kinase and GAPDH (top) and semiquantitative analysis of PI 3-kinase protein corrected by GAPDH and expressed as the degree of changes relative to their vehicle control. B: representative Western blots of phosphorylated Akt (p-Akt) and total Akt (t-Akt) (top) and semiquantitative analysis of Akt phosphorylation expressed as the degree of changes relative to their vehicle (bottom); n = 6–8. *P < 0.05 and ***P < 0.001, DOCA-salt or DOCA-salt + valsartan vs. vehicle within strain. †P < 0.05 and ††P < 0.01, DOCA-salt + valsartan vs. DOCA-salt within strain.
Nox2 and urinary 8-isoprostane excretion.
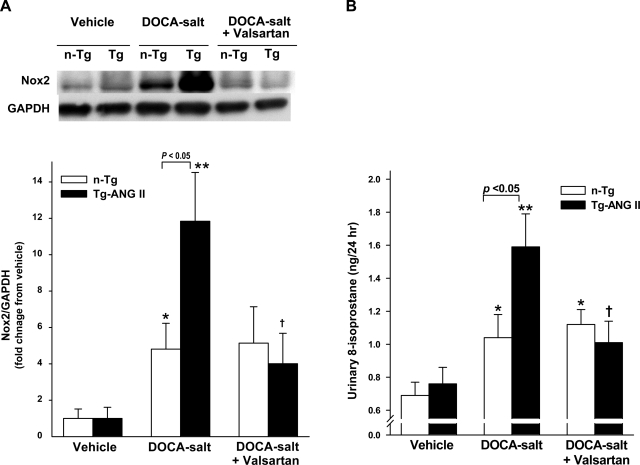
Low levels of Nox2 protein were detected in the LV in vehicle-treated controls of both strains. DOCA-salt increased Nox2 protein significantly more in Tg-ANG II. Valsartan significantly reduced Nox2 protein expression, whereas Nox2 in n-Tg was not affected by valsartan (Fig. 7A). Urinary 8-isoprostane, an index of oxidative stress, was similar in both controls. DOCA-salt increased urinary 8-isoprostane excretion significantly in both strains but more so in Tg-ANG II. Valsartan decreased urinary 8-isoprostane excretion in Tg-ANG II but not in n-Tg (Fig. 7B).
Fig. 7.
Effect of cardiac overexpression of ANG II and valsartan on NADPH oxidase (Nox) 2 protein expression and urinary 8-isoprostane excretion. A: representative Western blots of Nox2 and GAPDH (top) and semiquantitative analysis of Nox2 protein corrected by GAPDH and expressed as the degree of changes relative to their vehicle control (bottom); n = 6–8. B: 24-h urinary 8-isoprostane; n = 8–12. *P < 0.05 and **P < 0.01, DOCA-salt vs. vehicle within strain. †P < 0.05, DOCA-salt + valsartan vs. DOCA-salt within strain.
Urine volume and albuminuria.
Urine volume and urinary albumin excretion were similar in control groups. DOCA-salt increased urine volume and albumin excretion similarly in both n-Tg and Tg-ANG II groups. Valsartan caused an insignificant drop in urine volume in both strains and had no effect on albuminuria (Table 1).
Cardiac AT1R protein expression and PRC.
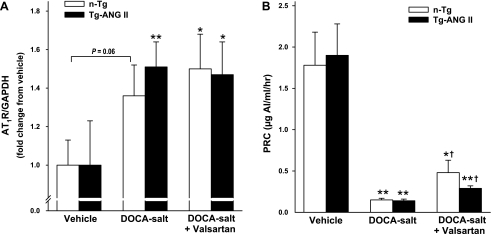
AT1R protein expression was similar in both vehicle-treated controls. DOCA-salt had no significant effect on AT1R expression in either strain with or without valsartan treatment (Fig. 8A). PRC was the same in control groups. DOCA-salt suppressed PRC similarly in both n-Tg and Tg-ANG II. Valsartan increased PRC slightly but significantly in both strains, and no strain difference was detected (Fig. 8B).
Fig. 8.
Effect of cardiac overexpression of ANG II and valsartan on AT1 receptor (AT1R) protein expression and plasma renin concentration (PRC) in mice with DOCA-salt hypertension. A: semiquantitative analysis of AT1R protein expression corrected by GAPDH and expressed as the degree of changes relative to their vehicle; n = 8–10. B: PRC; n = 8–12. *P < 0.05 and **P < 0.01, DOCA-salt or DOCA-salt + valsartan vs. vehicle within strain. †P < 0.05, DOCA-salt + valsartan vs. DOCA-salt within strain.
DISCUSSION
Several clinical trials have documented the beneficial effects of RAS inhibition in preventing secondary cardiovascular events (2, 5, 24, 38). However, because of the pleiotropic effects of ANG II, it is difficult to determine whether this benefit is solely due to a reduction in blood pressure or depends on inhibiting the direct actions of ANG II on the heart as well. Tg-ANG II mice provide a unique model to investigate the contribution of the local RAS to cardiac pathology, since ANG II only increases in the heart. In agreement with previous reports (25), our results confirm that cardiac ANG II does not cause cardiac hypertrophy in the absence of other contributing factors such as hypertension or ischemia. However, when pathological stimuli were applied such as MI as we reported previously, Tg-ANG II mice exhibited a more severe reduction in contractility than their n-Tg littermates (40), suggesting that ANG II could aggravate disease by acting directly on the heart. For this reason, we sought to extend our previous findings by testing whether local ANG II also potentiates cardiac remodeling in animals with hypertension. To test this hypothesis, we gave DOCA-salt to Tg-ANG II mice to suppress their circulating renin and ANG II but at the same time to induce hypertension (1, 21), which allows us to assess the effects of elevated cardiac ANG II in the context of a decreased circulating ANG II and increased systemic blood pressure. We found that DOCA-salt-treated mice had significantly low levels of plasma renin and increased blood pressure that did not respond to valsartan, confirming that the endogenous RAS was suppressed by DOCA-salt. Although blood pressure increased similarly with DOCA-salt in both strains, the Tg-ANG II mice exhibited greater increases in cardiac and perivascular collagen and hypertrophy as well as inflammation and oxidative stress, as evidenced by increased macrophage infiltration, TGF-β1 and Nox2 expression, and urinary 8-isoprostane excretion. They also had increased AT1R expression and decreased PI 3-kinase protein and Akt phosphorylation together with an increased number of TUNEL-positive cells compared with n-Tg hypertensive mice. Moreover, following valsartan administration, only the Tg-ANG II mice had reduced LV hypertrophy, interstitial and perivascular fibrosis, macrophage infiltration, TGF-β1 and Nox2 expression, and urinary 8-isoprostane excretion, which all developed without significant changes in heart function. Taken together, our data may suggest that upregulation of cardiac AT1R induced by mechanical stretch (pressure overload due to hypertension and volume overload due to DOCA-salt) and ANG II acting on its receptor as well as DOCA per se acting on the mineralocorticoid receptor all contribute to the enhanced inflammation, oxidative stress, and apoptosis observed in Tg-ANG II mice, thereby exaggerating cardiac remodeling.
Growing evidence indicates that production of ROS plays a crucial role in ANG II-induced cardiac damage (23, 35). The predominant source of ROS is Nox (34, 45). To date, five Nox isoforms have been identified, with the heart primarily expressing Nox2 (34, 45). In vitro studies have demonstrated that ANG II is one of the most potent stimuli for Nox2 activation and generation of ROS (35). ROS could serve as both intercellular and intracellular second messengers, modulating the signaling molecules involved in myocyte growth, apoptosis, and release of proinflammatory mediators (34, 35). DOCA is a mineralocorticoid receptor agonist (28) and is reportedly involved in myocardial Nox activation, causing interstitial fibrosis (9). In the present study, we found that, under basal conditions, overexpression of cardiac ANG II had no effect on Nox2 expression and 8-isoprostane excretion, a marker of oxidative stress. DOCA-salt increased Nox2 protein expression and 8-isoprostane excretion in both strains; however, the increases were greater in Tg-ANG II even though blood pressure was similar in both strains. These data may indicate that the mechanism(s) behind the increase in Nox2 and 8-isoprostane may be different between the two strains. In n-Tg mice, DOCA-salt suppressed the RAS but increased Nox2 and 8-isoprostane, and this increase was not affected by valsartan, indicating that the increased oxidative stress in n-Tg mice was not related to angiotensin or its receptors but rather was the direct result of the DOCA-salt. On the other hand, in Tg-ANG II mice, the increase in Nox2 and 8-isoprostane stemmed from not only a direct effect of DOCA-salt but also cardiac ANG-induced oxidative stress via the AT1R, which could explain why Nox-2 and 8-isoprostane rose more and was reduced by valsartan in the Tg-ANG II mice.
Increased inflammation also contributes to ANG II-induced cardiovascular injury. ANG II is known to exert a profound proinflammatory action in addition to its vasoconstrictor effect (29, 32). In particular, it increases monocyte chemoattractant protein (MCP-1), a prominent chemokine that regulates monocyte/macrophage infiltration via the AT1R (11, 13, 29) and enhances the release of ROS from these inflammatory cells (4, 7). We found that macrophage infiltration and TGF-β1 expression in Tg-ANG II mouse hearts were markedly increased compared with n-Tg in response to DOCA-salt. The heightened macrophage infiltration is most likely due to the increase in MCP-1 brought about by ANG II. Valsartan significantly reduced both macrophage infiltration and TGF-β1 expression in Tg-ANG II, suggesting that enhanced inflammatory response and oxidative stress induced by cardiac ANG II may be largely responsible for the exaggerated myocyte hypertrophy and fibrosis observed in hypertensive Tg-ANG II mice.
ANG II-induced ROS generation reportedly leads to myocyte apoptosis and necrosis (36). One of the targets of ROS is the serine-threonine kinase Akt, a key anti-apoptotic molecule that regulates cell growth and survival (8, 14). Activation of Akt preserves cardiomyocyte function both in vitro and in vivo, whereas inhibition of Akt greatly accelerates hypoxia-induced cardiomyocyte dysfunction (15). Furthermore, Akt gene transfer to the heart diminishes cardiomyocyte apoptosis and limits infarct size following ischemia-reperfusion injury (6). We found that cardiac overexpression of ANG II reduced PI 3-kinase protein expression in mice treated with DOCA-salt. Phosphorylation of Akt, the downstream target of PI 3-kinase, was also impaired in Tg-ANG II mice with DOCA-salt hypertension, associated with an increased number of TUNEL-positive nuclei in cardiomyocytes. Valsartan restored PI 3-kinase expression and Akt phosphorylation and decreased apoptosis. Taken together, these data suggest that the increased myocyte apoptosis seen in Tg-ANG II mice may be mediated by ANG II-induced downregulation of PI 3-kinase and Akt via the AT1R.
Study limitations are as follows. 1) First is evaluation of LV diastolic function. Although LV systolic function was well preserved in Tg-ANG II mice, we could not exclude the possibility that cardiac ANG II impairs diastolic function, particularly in the presence of DOCA-salt. Although the pressure-volume conductance system offers sensitive measurement of LV diastolic function, we cannot use it with DOCA-salt, since high plasma sodium interferences with the conductance signal, making data unreliable. Doppler mitral velocity (E/A ratio) could be an indication for diastolic properties; however, obtaining E/A is not feasible, since we could not record ECG in awake mice. Without an ECG trace, it is almost impossible to correctly identify E and A waveforms in mice that have a heart rate >600 beats/min. 2) Second is the possible role of the AT2R in the cardioprotective effect of valsartan. We previously showed that the therapeutic effects of AT1R blockers were mediated in part by activation of AT2R. Thus it is possible that, during AT1R blockade in Tg-ANG II mice, cardiac ANG II acts on the AT2R, contributing to the cardioprotective effect of valsartan. This needs to be investigated further. 3) Thirdly, despite their enhanced inflammatory, hypertrophic, and fibrotic responses to DOCA-salt, Tg-ANG II mice have well-preserved LV systolic function, possibly because the experiments are short and heart function is still undergoing compensation. Prolonging the experimental period would be a future consideration. Nevertheless, these limitations do not detract from the importance of the present work demonstrating that ANG II released from the heart acts as a paracrine/autocrine hormone, promoting inflammation and oxidative stress and causing cardiac hypertrophy and fibrosis via the AT1R.
In summary, we believe our data demonstrate for the first time that ANG II released from the heart acts locally to exacerbate hypertrophy and fibrosis in mice with DOCA-salt hypertension, working independently of the systemic RAS. Inflammation, activation of redox-dependent signaling, and downregulation of Akt phosphorylation may be largely responsible for the detrimental action of cardiac ANG II. Blockade of AT1R partially reversed all of these harmful effects of cardiac ANG II without altering blood pressure. Taken together, our findings suggest that cardiac ANG II, acting on the AT1R, promotes inflammation and oxidative stress and aggravates cardiac remodeling in hypertensive heart.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-28982 (Project I to O. A. Carretero and II to X.-P. Yang) and HL-078951 (X.-P. Yang).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Barrett G, Morgan T, Smith M, Aldred P. Effect of mineralocorticoids and salt loading on renin release, renal renin content and renal renin mRNA in mice. Clin Exp Pharmacol Physiol 16: 631–639, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Basile J, Toth PP. Angiotensin receptor blockers: role in hypertension management, cardiovascular risk reduction, and nephropathy. South Med J 102: S1–S12, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Danser AH. Local renin-angiotensin systems: the unanswered questions. Int J Biochem Cell Biol 35: 759–768, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Ferder L, Inserra F, Martinez-Maldonado M. Inflammation and the metabolic syndrome: role of angiotensin II and oxidative stress. Curr Hypertens Rep 8: 191–198, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Fintel DJ. The role of RAS modification for primary and secondary stroke prevention. Postgrad Med 121: 115–122, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101: 660–667, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernández-Presa M, Bustos C, Ortego M, Tuñon J, Renedo G, Ruiz-Ortega M, Egido J. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-κB activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation 95: 1532–1541, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, Sharma RV, Engelhardt JF, Davisson RL. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics 26: 180–191, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J 20: 1546–1548, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Liao TD, Yang XP, Liu YH, Shesely EG, Cavasin MA, Kuziel WA, Pagano PJ, Carretero OA. Role of inflammation in the development of renal damage and dysfunction in angiotensin-II-induced hypertension. Hypertension 52: 256–263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu YH, Yang XP, Sharov VG, Nass O, Sabbah HN, Peterson E, Carretero OA. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest 99: 1926–1935, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mateo T, bu Nabah YN, Abu TM, Mata M, Cerda-Nicolas M, Proudfoot AE, Stahl RA, Issekutz AC, Cortijo J, Morcillo EJ, Jose PJ, Sanz MJ. Angiotensin II-induced mononuclear leukocyte interactions with arteriolar and venular endothelium are mediated by the release of different CC chemokines. J Immunol 176: 5577–5586, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Matsui T, Nagoshi T, Rosenzweig A. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle 2: 220–223, 2003 [PubMed] [Google Scholar]
- 15.Matsui T, Tao J, del MF, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104: 330–335, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Matsusaka T, Katori H, Inagami T, Fogo A, Ichikawa I. Communication between myocytes and fibroblasts in cardiac remodeling in angiotensin chimeric mice. J Clin Invest 103: 1451–1458, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neri Serneri GG, Boddi M, Cecioni I, Vanni S, Coppo M, Papa ML, Bandinelli B, Bertolozzi I, Polidori G, Toscano T, Maccherini M, Modesti PA. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circ Res 88: 961–968, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Ohno M, Takemura G, Ohno A, Misao J, Hayakawa Y, Minatoguchi S, Fujiwara T, Fujiwara H. “Apoptotic” myocytes in infarct area in rabbit hearts may be oncotic myocytes with DNA fragmentation: analysis by immunogold electron microscopy combined with In situ nick end-labeling. Circulation 98: 1422–1430, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Paradis P, Dali-Youcef N, Paradis FW, Thibault G, Nemer M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc Natl Acad Sci USA 97: 931–936, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Peng H, Carretero OA, Alfie ME, Masura JA, Rhaleb NE. Effects of angiotensin-converting enzyme inhibitor and angiotensin type 1 receptor antagonist in deoxycorticosterone acetate-salt hypertensive mice lacking Ren-2 gene. Hypertension 37: 974–980, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Peng H, Carretero OA, Liao TD, Peterson EL, Rhaleb NE. Role of Ac-SDKP in the antifibrotic effect of angiotensin-converting enzyme inhibitors in hypertension-induced target organ damage. Hypertension 49: 1–9, 2007. 17145983 [Google Scholar]
- 23.Polizio AH, Balestrasse KB, Yannarelli GG, Noriega GO, Gorzalczany S, Taira C, Tomaro ML. Angiotensin II regulates cardiac hypertrophy via oxidative stress but not antioxidant enzyme activities in experimental renovascular hypertension. Hypertens Res 31: 325–334, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Ravandi A, Teo KK. Blocking the renin-angiotensin system: dual- versus mono-therapy. Expert Rev Cardiovasc Ther 7: 667–674, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Reudelhuber TL, Bernstein KE, Delafontaine P. Is angiotensin II a direct mediator of left ventricular hypertrophy? Time for another look. Hypertension 49: 1196–1201, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadoshima J, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res 73: 413–423, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-iso prostaglandin F2α. Hypertension 33: 424–428, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Shapiro BP, Owan TE, Mohammed S, Kruger M, Linke WA, Burnett JC, Jr, Redfield MM. Mineralocorticoid signaling in transition to heart failure with normal ejection fraction. Hypertension 51: 289–295, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Skultetyova D, Filipova S, Riecansky I, Skultety J. The role of angiotensin type 1 receptor in inflammation and endothelial dysfunction. Recent Pat Cardiovasc Drug Discov 2: 23–27, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res 81: 482–490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Carretero OA, Xu J, Rhaleb NE, Wang F, Lin C, Yang JJ, Pagano PJ, Yang XP. Lack of inducible NO synthase reduces oxidative stress and enhances cardiac response to isoproterenol in mice with deoxycorticosterone acetate-salt hypertension. Hypertension 46: 1355–1361, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol 35: 881–900, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Sweat F, Puchtler H, Rosenthal SI. Sirius Red F3BA as a stain for connective tissue. Arch Pathol 78: 69–72, 1964 [PubMed] [Google Scholar]
- 34.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49: 241–248, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells-implications in cardiovascular disease. Braz J Med Biol Res 37: 1263–1273, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem 274: 22699–22704, 1999 [DOI] [PubMed] [Google Scholar]
- 37.vanKats JP, Methot D, Paradis P, Silversides DW, Reudelhuber TL. Use of a biological peptide pump to study chronic peptide hormone action in transgenic mice. Direct and indirect effects of angiotensin II on the heart. J Biol Chem 276: 44012–44017, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Werner CM, Bohm M. The therapeutic role of RAS blockade in chronic heart failure. Ther Adv Cardiovasc Dis 2: 167–177, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Whaley-Connell A, Govindarajan G, Habibi J, Hayden MR, Cooper SA, Wei Y, Ma L, Qazi M, Link D, Karuparthi PR, Stump C, Ferrario C, Sowers JR. Angiotensin II-mediated oxidative stress promotes myocardial tissue remodeling in the transgenic (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab 293: E355–E363, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Carretero OA, Cavasin MA, Lin C, Shesely EG, Yang JJ, Reudelhuber TL, Yang XP. Role of cardiac overexpression of angiotensin ii in the regulation of cardiac function and remodeling post-myocardial infarction. Am J Physiol Heart Circ Physiol 293: H1900–H1907, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Carretero OA, Liu YH, Shesely EG, Yang F, Kapke A, Yang XP. Role of AT2 receptors in the cardioprotective effect of AT1 antagonists in mice. Hypertension 40: 244–250, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Xu J, Carretero OA, Liu YH, Yang F, Shesely EG, Oja-Tebbe N, Yang XP. Dual inhibition of ACE and NEP provides greater cardioprotection in mice with heart failure. J Card Fail 10: 83–89, 2004 [PubMed] [Google Scholar]
- 43.Xu J, Carretero OA, Sun Y, Shesely EG, Rhaleb NE, Liu YH, Liao TD, Yang JJ, Bader M, Yang XP. Role of the B1 kinin receptor in the regulation of cardiac function and remodeling after myocardial infarction. Hypertension 45: 747–753, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol Heart Circ Physiol 277: H1967–H1974, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Zhang M, Kho AL, Anilkumar N, Chibber R, Pagano PJ, Shah AM, Cave AC. Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation 113: 1235–1243, 2006 [DOI] [PubMed] [Google Scholar]