Abstract
Normal cardiac excitability depends on the coordinated activity of specific ion channels and transporters within specialized domains at the plasma membrane and sarcoplasmic reticulum. Ion channel dysfunction due to congenital or acquired defects has been linked to human cardiac arrhythmia. More recently, defects in ion channel-associated proteins have been associated with arrhythmia. Ankyrin-B is a multifunctional adapter protein responsible for targeting select ion channels, transporters, cytoskeletal proteins, and signaling molecules in excitable cells, including neurons, pancreatic β-cells, and cardiomyocytes. Ankyrin-B dysfunction has been linked to cardiac arrhythmia in human patients and ankyrin-B heterozygous (ankyrin-B+/−) mice with a phenotype characterized by sinus node dysfunction, susceptibility to ventricular arrhythmias, and sudden death (“ankyrin-B syndrome”). At the cellular level, ankyrin-B+/− cells have defects in the expression and membrane localization of the Na+/Ca2+ exchanger and Na+-K+-ATPase, Ca2+ overload, and frequent afterdepolarizations, which likely serve as triggers for lethal cardiac arrhythmias. Despite knowledge gathered from mouse models and human patients, the molecular mechanism responsible for cardiac arrhythmias in the setting of ankyrin-B dysfunction remains unclear. Here, we use mathematical modeling to provide new insights into the cellular pathways responsible for Ca2+ overload and afterdepolarizations in ankyrin-B+/− cells. We show that the Na+/Ca2+ exchanger and Na+-K+-ATPase play related, yet distinct, roles in intracellular Ca2+ accumulation, sarcoplasmic reticulum Ca2+ overload, and afterdepolarization generation in ankyrin-B+/− cells. These findings provide important insights into the molecular mechanisms underlying a human disease and are relevant for acquired human arrhythmia, where ankyrin-B dysfunction has recently been identified.
Keywords: Na+/Ca2+ exchanger, Na+-K+-ATPase, mathematical model, trafficking, calcium
normal cardiac excitability depends on the coordinated biophysical activity of specific ion channels and transporters on the plasma membrane. Over the past decade, gene mutations that affect ion channel biophysical activity have been linked with fatal human arrhythmias (19). Recently, a new class of gene mutations has been identified that alters the local coupling of ion channels with cytoskeletal and regulatory proteins (5, 20, 22, 27, 28, 36, 41, 42). Based in part on these important findings, it is now clear that ion channel and transporter function depend on normal biophysical properties and proper local membrane localization/organization.
Ankyrin polypeptides are responsible for the targeting and stabilization of ion channels, transporters, and signaling molecules at cell membranes of diverse cell types, including cardiomyocytes, neurons, pancreatic β-cells, and erythrocytes (12). Over the past decade, members of the ankyrin family (ankyrin-R, ankyrin-B, and ankyrin-G) have been linked to a number of human diseases, including hereditary spherocytosis, cardiac arrhythmia, diabetes, and muscular dystrophy (1, 8, 16, 27, 28). Ankyrin-B dysfunction has been linked to both congenital and acquired arrhythmias (15, 17, 23, 26, 28, 35). In fact, nine loss-of-function variants in ANK2 (the gene encoding ankyrin-B) have been identified in human patients with a complex cardiac arrhythmia syndrome (“long QT 4” or “ankyrin-B syndrome”) characterized by sinus node dysfunction, increased susceptibility to ventricular arrhythmias, and sudden death under stress (17, 26, 28, 29, 34). Ankyrin-B-deficient (ankyrin-B+/−) mice display an increased susceptibility to stress-induced arrhythmias and sudden death, similar to human patients (23, 26, 28). Importantly, ankyrin-B+/− mice show defects in ion channel and transporter targeting, abnormal Ca2+ homeostasis, and an increased likelihood of potentially life-threatening afterdepolarizations (28). In ventricular cardiomyocytes, ankyrin-B is responsible for the proper membrane localization and function of Na+-K+-ATPase (NKA), Na+/Ca2+ exchanger (NCX), inositol 1,4,5-trisphosphate (InsP3) receptor, and protein phosphatase 2A (PP2A) (2, 7, 29). Despite the wealth of information gathered from human patients and the mouse model of ankyrin-B syndrome, the direct link between specific membrane protein changes and cardiac arrhythmia has yet to be established.
Mathematical modeling of excitable cells has been used to generate important insights into the cellular mechanisms underlying a wide variety of human diseases, including cardiac arrhythmia, epilepsy, and diabetes (6, 14, 16, 32). In this study, we used mathematical modeling to identify the cellular pathway responsible for abnormal Ca2+ handling and cardiac arrhythmia in ankyrin-B+/− cells. Our computer simulations showed that loss of NCX and NKA membrane targeting in ankyrin-B+/− cells resulted in accumulation of intracellular Na+ and Ca2+ under basal conditions. Furthermore, ankyrin-B reduction predisposed the cell to Ca2+ overload, frequent spontaneous Ca2+ release, and action potential (AP) afterdepolarizations during rapid pacing in the presence of isoproterenol. While loss of NKA function contributed to the increased Ca2+ transients in ankyrin-B+/− cardiomyocytes, abnormal NCX targeting was the dominant mechanism for Ca2+ overload in the sarcoplasmic reticulum (SR), spontaneous Ca2+ release, and afterdepolarizations. These findings provide important insights into the molecular mechanism underlying a human disease and highlight the related, yet distinct, roles of NCX and NKA in ventricular cardiomyocytes. Considering the association between ankyrin-B dysfunction and acquired arrhythmia as well as arrhythmia susceptibility in the general population (15, 35), we expect the insights generated by this study to have broader relevance for human disease.
METHODS
Mathematical model of the ankyrin-B+/− cardiomyocyte.
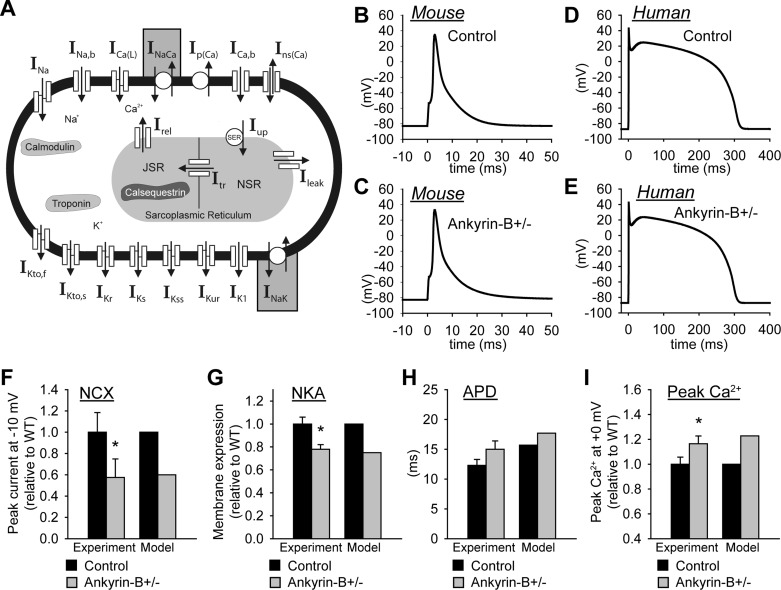
The mathematical model used in this study was based on a model of the murine ventricular AP (3, 4) since much of the experimental data came from the mouse. Importantly, the formulations for NKA and NCX current have been well validated against experimental data from mammalian ventricular myocytes (21) (Supplemental Material, Supplemental Fig. S1).1 Simulations were also performed using a well-validated model of the human ventricular myocyte (38). Modifications to the equations were made to account for experimentally measured changes in NCX and NKA membrane expression in ankyrin-B+/− cardiomyocytes (Fig. 1, A–E) (23, 25, 28, 29). Specifically, NCX was scaled to produce a 40% reduction in current at a test potential of −10 mV compared with control (wild type), consistent with experimental measurements from adult ankyrin-B+/− myocytes (17) (Fig. 1F). Also, the maximum NKA current was decreased 25% based on experimental measurements showing reduced NKA surface expression in ankyrin-B+/− myocytes (as assessed by [3H]ouabain binding) (25) (Fig. 1G). The peak L-type Ca2+ current (ICaL)-voltage relationship was no different between the control and ankyrin-B+/− cell models (not shown), consistent with experimental measurements in ventricular myocytes (28). Importantly, simulated APs and Ca2+ transients showed good agreement with experimental measurements (28) (Fig. 1, H and I). The complete equations and parameters for the models used in this study may be found in the Supplemental Material.
Fig. 1.
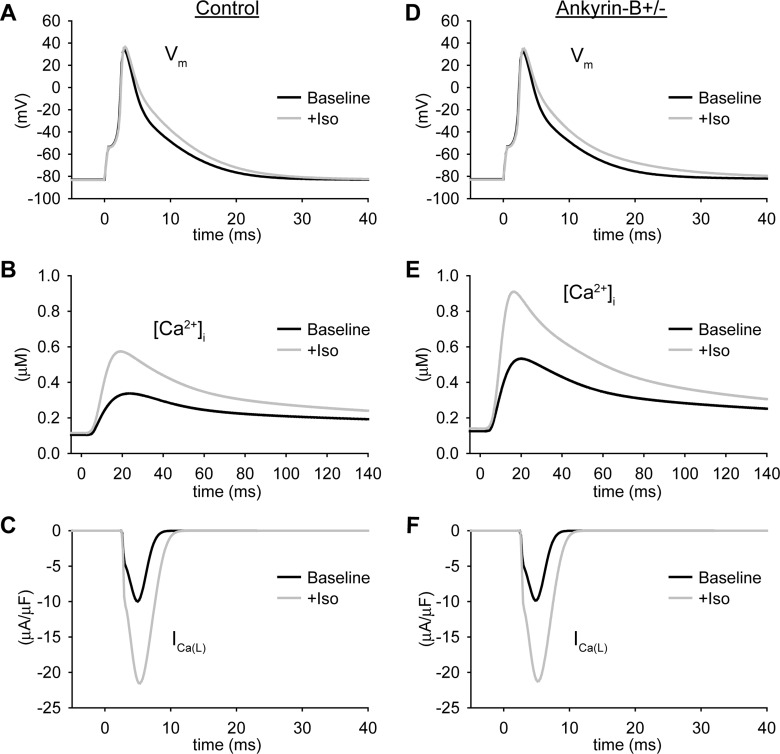
Mathematical model of the ankyrin-B-deficient (ankyrin-B+/−) cell. A: schematic of the mouse ventricular cell model. Na+/Ca2+ exchanger (NCX) current (INaCa) and Na+-K+-ATPase (NKA) current (INaK) were altered in the model of the ankyrin-B+/− cell (shaded boxes). INa, Na+ current; INa,b, background Na+ current; ICaL, L-type Ca2+ current; Ip(Ca), sarcolemmal Ca2+ pump; ICa,b, background Ca2+ current; Ins(Ca), nonspecific Ca2+ current; IKto,f, fast component of the transient outward K+ current; IKto,s, slow component of the transient outward K+ current; IKr, fast component of the delayed recifier K+ current; IKs, slow component of the delayed recifier K+ current; IKss, steady-state K+ current; IKur, ultrarapid K+ current; IK1, inward rectifier K+ current; Irel, Ca2+ release flux; Iup, Ca2+ uptake flux; SER, sarco(endo)plasmic reticulum Ca2+-ATPase; JSR, junctional sarcoplasmic reticulum (SR); NSR, network SR; Itr, Ca2+ transfer flux; Ileak, Ca2+ leak flux. B–E: simulated action potentials (APs) in control (B and D) and ankyrin-B+/− (C and E) cardiomyocytes from the mouse (B and C) and human (D and E) ventricular cell models [10th action potential (AP) shown at a cycle length (CL) of 1,000 ms]. F: simulated INaCa at a test potential of −10 mV from wild-type cell and an ankyrin-B+/− cell compared with experimental measurements (n = 12, *P < 0.05) (17). In both the simulation and experiment, intracellular Na+ concentration = 20 mM, extracellular Na+ concentration = 145 mM, intracellular Ca2+ concentration ([Ca2+]i) = 1 μM, and extracellular Ca2+ concentration = 2 mM. G: simulated and experimentally measured (n = 3, *P < 0.01) (25) NKA membrane expression in wild-type and ankyrin-B+/− mouse ventricular cells. H: simulated and experimentally measured (n = 17, P = not significant) (28) AP duration (APD) at 90% repolarization. I: simulated and experimentally measured (n = 18, *P < 0.001) peak Ca2+ transients. In both the simulation and experiment, the cell was pulsed four times to test potential of +0 mV from a holding potential of −40 mV (28).
Mathematical model of isoproterenol effects.
The effects of the β-adrenergic receptor agonist isoproterenol (saturating concentration ≥ 0.1 μM) on the AP and Ca2+ transient were simulated according to previously published formulations (9, 40). Specifically, isoproterenol effects on ICaL, the slow component of the delayed rectifier K+ current (IKs), and sarco(endo)plasmic reticulum Ca2+-ATPase current (Iup) were incorporated into the models to simulate β-adrenergic stimulation (Supplemental Material) (9, 40). The modified equations and parameters may be found in the Supplemental Material.
Pacing protocol.
Models were paced from rest to steady state over a range of pacing cycle lengths (CLs; from 2,000 to 200 ms, stimulus amplitude = −60 μA/μF for the mouse and −52 μA/μF for the human; stimulus duration = 0.5 ms for the mouse and 1 ms for the human).
RESULTS
Ankyrin-B deficiency promotes Ca2+ overload at baseline.
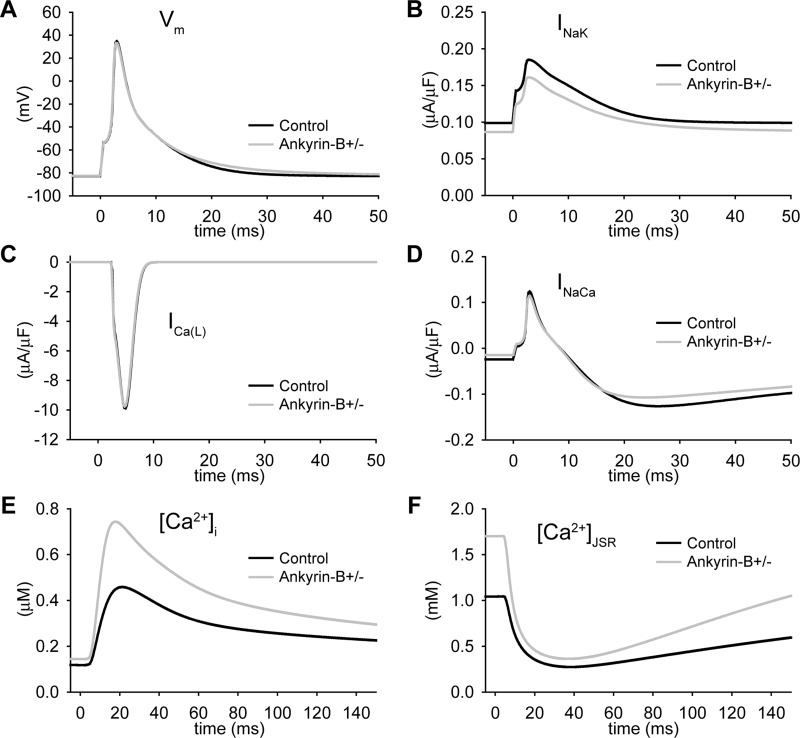
To determine the electrophysiological consequences of abnormal NCX and NKA membrane expression in ankyrin-B+/− cardiomyocytes, we paced the control and ankyrin-B+/− cell models (CL = 1,000 ms; Fig. 2). Consistent with experimental data (28), the simulated AP from the ankyrin-B+/− mouse myocyte was slightly longer than the control AP (Figs. 1H and 2A). Whereas the AP morphology and AP duration (APD) were similar between the ankyrin-B+/− and control model, loss of NCX and NKA targeting led to dramatic Ca2+ and Na+ accumulation in the cytosol and SR of the ankyrin-B+/− cell (Fig. 2, E and F, and Table 1). Specifically, ankyrin-B-deficient cells displayed an increase in intracellular Na+ concentration (Table 1), Ca2+ concentration in the junctional SR ([Ca2+]JSR), and Ca2+ transient amplitude (Fig. 2, E and F), consistent with experimental measurements from ankyrin-B+/− mice (28). Ca2+ bound to SR calsequestrin ([Ca2+-CSQN]) was also increased in ankyrin-B+/− cells but remained below the threshold for eliciting spontaneous Ca2+ release (not shown).
Fig. 2.
Ca2+ accumulation at baseline in the ankyrin-B+/− cell. A–F: simulated steady-state AP (A), INaK (B), ICaL (C), INaCa (D), Ca2+ transient (E), and JSR Ca2+ concentration ([Ca2+]JSR; F) in control and ankyrin-B+/− mouse ventricular cells (CL = 1,000 ms). Vm, membrane potential.
Table 1.
Intracellular Na+ concentration in a computational model of ankyrin-B syndrome
| Cycle Length | Control | Ankyrin-B Deficient | Na+/Ca2+ Exchanger Deficient | Na+-K+-ATPase Deficient |
|---|---|---|---|---|
| Rest | ||||
| Without Iso | 11.84 | 13.72 | 11.53 | 14.03 |
| 200 ms | ||||
| Without Iso | 12.3 | 14.24 | 12.01 | 14.53 |
| With Iso | 12.25 | 14.18 | 11.96 | 14.48 |
| 1,000 ms | ||||
| Without Iso | 11.95 | 13.85 | 11.64 | 14.15 |
| With Iso | 11.88 | 13.79 | 11.58 | 14.09 |
Values are in mM. Iso, isoproterenol.
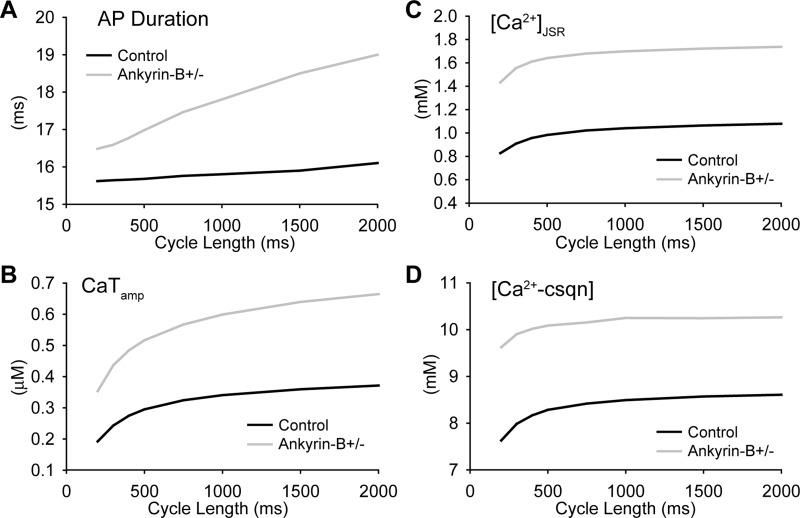
Arrhythmias in ankyrin-B+/− mice and human patients with ankyrin-B syndrome are commonly observed with increased heart rate (e.g., after stress or exercise) (28). To determine whether loss of NCX and NKA altered the response of the ankyrin-B+/− myocyte to changes in pacing rate, the ankyrin-B+/− and control models were paced over a CL range from 2,000 to 200 ms. APD in the ankyrin-B+/− model was slightly longer than control at all pacing CLs (Fig. 3A). Ca2+ transient amplitude, [Ca2+]JSR, and [Ca2+-CSQN] were also greater at every CL (Fig. 3, B–D). Consequently, the levels of Ca2+ bound to troponin were greater in ankyrin-B+/− cells compared with control (not shown), consistent with increased contractility at baseline (absence of β-adrenergic stimulation) in ankyrin-B-deficient myocytes (25). Importantly, spontaneous release was not observed in the ankyrin-B+/− cell even during rapid pacing (CL = 200 ms; not shown). Spontaneous release was not observed during rapid pacing in the human model either (CL = 500 ms; not shown). These results indicate that while loss of NCX and NKA targeting promoted Ca2+ accumulation in ankyrin-B+/− cells, it was not sufficient to trigger spontaneous release under basal conditions (absence of β-adrenergic stimulation).
Fig. 3.
Rate dependence of the AP and Ca2+ transient in wild-type and ankyrin-B+/− cells. A–D: APD (A), Ca2+ transient amplitude (CaTamp; B), [Ca2+]JSR (C), and concentration of Ca2+ bound to calsequestrin ([Ca2+-CSQN]; D) in control and ankyrin-B+/− cells paced to steady state at CLs of 200, 400, 500, 750, 1,000, 1,500, and 2,000 ms.
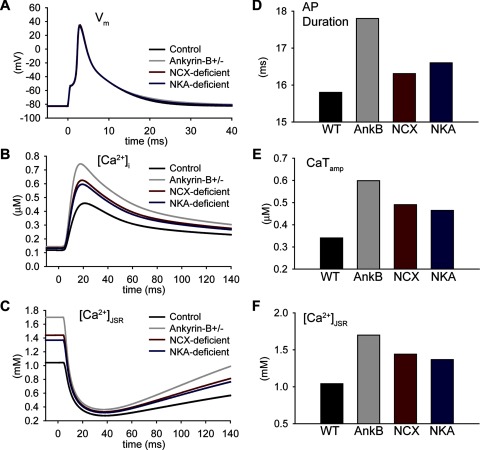
Role of NCX and NKA in Ca2+ overload in ankyrin-B+/− cells.
To identify the molecular mechanism responsible for Ca2+ accumulation in ankyrin-B+/− cells, we determined APs and Ca2+ transients in the ankyrin-B+/− cell with NKA (Fig. 4, NCX deficient, red lines) or NCX restored to normal levels (Fig. 4, NKA deficient, blue lines). Restoring normal NCX targeting had little impact on APD (compare ankyrin-B and NKA deficient in Fig. 4, A and D; CL = 1,000 ms) in the ankyrin-B+/− cell but dramatically reduced Ca2+ transient amplitude (Fig. 4, B and E) and [Ca2+]JSR toward control levels (Fig. 4, C and F). In contrast, restoring normal NKA targeting in the ankyrin-B+/− cell had a greater impact on APD (Fig. 4, A and D), with a lesser effect on Ca2+ transient amplitude (Fig. 4, B and E) or [Ca2+]JSR (Fig. 4, C and F) compared with restoring NCX targeting. NCX loss was also the dominant mechanism responsible for increased Ca2+ transient amplitude and [Ca2+]JSR in the human ankyrin-B-deficient cell (Supplemental Fig. S2). Together, these data indicate that NKA and NCX loss play distinct but important roles in ankyrin-B deficient cells. Whereas loss of NKA targeting is primarily responsible for changes in APD in ankyrin-B+/− cells, loss of NCX drives the accumulation of cytosolic and SR Ca2+.
Fig. 4.
Role of NCX and NKA in Ca2+ accumulation in the ankyrin-B+/− cell. A–C: simulated steady-state AP (A), Ca2+ transient (B), and [Ca2+]JSR (C) in control, ankyrin-B+/−, NCX-deficient (NKA restored to normal levels), and NKA-deficient (NCX restored to normal levels) cells. D–F: summary data showing steady-state APD (D), CaTamp (E), and [Ca2+]JSR (F) in control, ankyrin-B+/− (AnkB), NCX-deficient, and NKA-deficient cells (CL = 1,000 ms).
Increased levels of Ca2+ with isoproterenol.
Human patients with ANK2 mutations show increased likelihood of stress-induced cardiac arrhythmias (28, 34). Similarly, ankyrin-B+/− mice display lethal ventricular arrhythmias in response to catecholamine injection after exercise (28). Based on these observations, we next determined the response of the ankyrin-B+/− and control models to treatment with the β-adrenergic receptor agonist isoproterenol (Fig. 5). Isoproterenol treatment resulted in a dramatic increase in the Ca2+ transient amplitude in both control and ankyrin-B+/− cells (Fig. 5, B and E) due to increased ICaL (Fig. 5, C and F) and SR Ca2+ uptake (not shown). Spontaneous Ca2+ release from the SR did not occur in either the control or ankyrin-B+/− models during slow pacing (CL = 1,000 ms) even in the presence of isoproterenol.
Fig. 5.
Isoproterenol (Iso) increases intracellular Ca2+ in control and ankyrin-B+/− cells. A–F: simulated steady-state AP (A and D), Ca2+ transient (B and E), and ICaL (C and F) for control (A–C) and ankyrin-B+/− (D–F) mouse ventricular cardiomyocytes at baseline and in the presence of a saturating concentration of Iso (> 0.1 μM, CL = 1,000 ms).
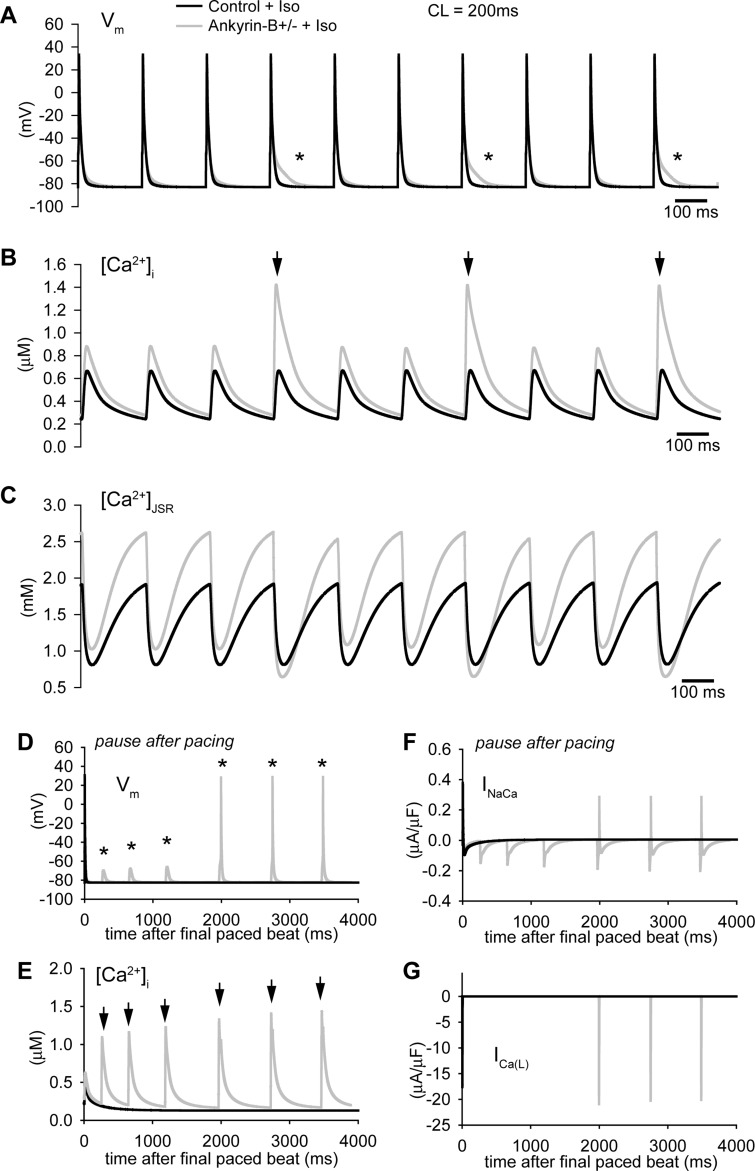
We next subjected the control and ankyrin-B+/− models to rapid pacing in the presence of isoproterenol. Interestingly, multiple spontaneous SR Ca2+-release events were observed in the ankyrin-B+/− model during rapid pacing (CL = 200 ms; arrows in Fig. 6B) due to the increased SR Ca2+ load (Fig. 6C). These spontaneous release events altered AP morphology during pacing (asterisks in Fig. 6A) and even produced afterdepolarizations that successfully triggered APs during a subsequent pause (Fig. 6D). Spontaneous release and afterdepolarizations did not occur in the control model during rapid pacing (Fig. 6, A and B) or a subsequent pause (Fig. 6, D and E) in the presence of isoproterenol. These simulations indicate that ankyrin-B reduction increases the likelihood of spontaneous release and AP afterdepolarizations. Consistent with experimental observations, afterdepolarizations were only observed during rapid pacing rates (such as exercise) and in the presence of isoproterenol (β-adrenergic stimulation) (28). We observed similar behavior in simulations with the human model (Supplemental Fig. S3). Specifically, spontaneous release was observed during rapid pacing in the presence of isoproterenol in the ankyrin-B+/− cell but not in the control cell (Supplemental Fig. S3A). In fact, spontaneous release produced afterdepolarizations that generated APs during pacing in the human model (asterisk in Supplemental Fig. S3A).
Fig. 6.
Spontaneous Ca2+ release and afterdepolarizations in the ankyrin-B+/− cell during rapid pacing in the presence of Iso. A–C: simulated AP (A), Ca2+ transient (B), and [Ca2+]JSR (C) in control and ankyrin-B+/− mouse ventricular cardiomyocytes during rapid pacing to steady state (CL = 200 ms) in the presence of Iso. Frequent spontaneous release events (arrows in B) led to abnormal repolarization (* in A) in the ankyrin-B-deficient cell. D–G: simulated AP (D), Ca2+ transient (E), INaCa (F), and ICaL (F) in control and ankyrin-B+/− cells during a subsequent pause after rapid pacing to steady state. Note the spontaneous release (arrows) and afterdepolarizations (*) that ultimately produced an AP.
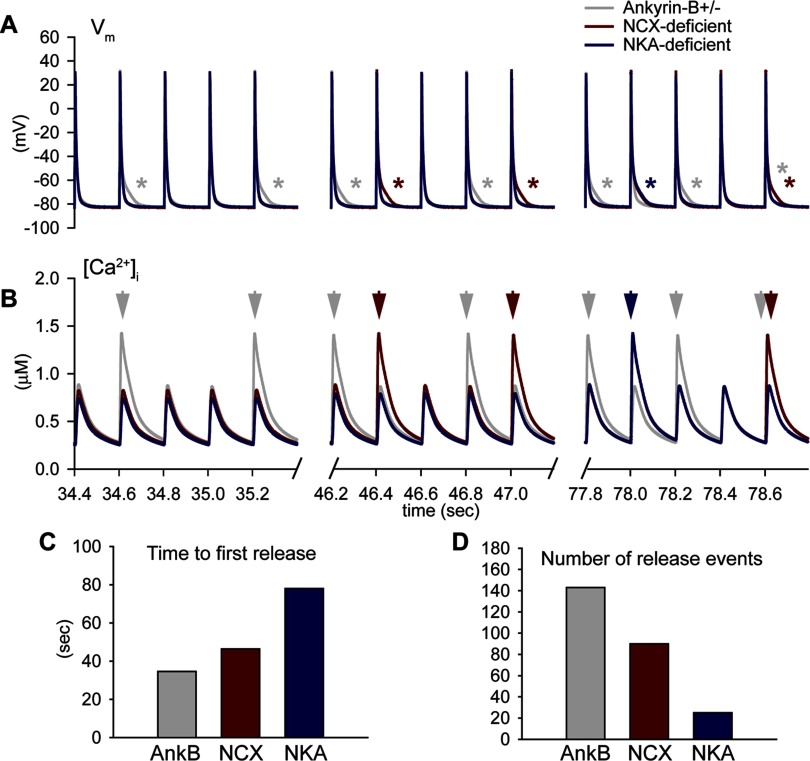
To determine the relative contributions of NCX and NKA loss to Ca2+ overload, spontaneous release, and afterdepolarizations, we subjected the ankyrin-B+/− cell with normal NCX targeting or with normal NKA targeting to rapid pacing in the presence of isoproterenol (Fig. 7). Restoring NCX targeting greatly reduced the occurrence of spontaneous release and afterdepolarizations in ankyrin-B+/− cells (number of events decreased >5-fold in NKA-deficient compared with ankyrin-B+/− cells; Fig. 7, A, B, and D). While restoring normal NKA targeting had a lesser impact on spontaneous release, it reduced the number of events and delayed the time to first spontaneous release (compare ankyrin-B- and NCX-deficient cells in Fig. 7, C and D). Consistent with these findings, loss of NCX resulted in more frequent and earlier spontaneous release events than NKA loss in the human model (Supplemental Fig. S3, B and C). These data indicate that while both NCX and NKA loss contribute to SR Ca2+ overload and afterdepolarizations in ankyrin-B deficient cells, loss of NCX is the dominant mechanism.
Fig. 7.
Role of NCX and NKA in spontaneous Ca2+ release and afterdepolarizations in the ankyrin-B+/− cell. A and B: simulated AP (A) and Ca2+ transient (B) in ankyrin-B+/−, NCX-deficient, and NKA-deficient cells during rapid pacing to steady state (CL = 200 ms). Spontaneous Ca2+ release (arrows in B) and abnormal repolarization (asterisks in A) were observed in NCX-deficient and NKA-deficient cells, although with decreased frequancy and delayed onset compared with the ankyrin-B+/− cell. C and D: summary data showing the time to first spontaneous release of Ca2+ from the SR (C) and number of spontaneous release events during rapid pacing (D) in ankyrin-B+/−, NKA-deficient, and NCX-deficient cells.
DISCUSSION
NKA and NCX are functionally coupled transporters for the regulation of ion homeostasis and contractility in the heart (10, 31, 37). Specifically, NKA maintains the Na+ gradient across the membrane necessary for NCX to remove Ca2+ from the cell. For centuries, this coupling has been exploited for the treatment of heart failure symptoms through the use of digitalis (18). However, only in the past 50 yr has the direct link between digitalis, NKA, and contractility been established. Recent studies (23, 30) have revealed that functional coupling of NKA and NCX in fact depends on physical coupling of the transporters. In fact, ankyrin-B binds to both NKA and NCX and is required for normal membrane expression of NKA and NCX (23). Loss of ankyrin-B disrupts targeting of NCX and NKA as well as InsP3 receptor and PP2A and is linked to arrhythmia in humans and mice (2, 7, 23–25, 28–30). Our data indicate that loss of NCX and NKA targeting in ankyrin-B+/− cells promotes intracellular Ca2+ accumulation at baseline and spontaneous Ca2+ release and afterdepolarizations with rapid pacing in the presence of isoproterenol. These findings agree with experimental observations in both ankyrin-B+/− mice and human patients (26, 28, 29). Whereas NKA mistargeting is primarily responsible for AP prolongation in ankyrin-B+/− cells, loss of NCX is the dominant mechanism for intracellular Ca2+ accumulation, spontaneous release, and afterdepolarizations. In light of these findings, it is surprising that, in contrast to ankyrin-B+/− mice, NCX1 knockout mice have normal Ca2+ transients with a modest decrease in contractility and normal lifespan (13). However, unlike ankyrin-B-deficient mice, NCX1 knockout mice show a compensatory decrease in ICaL, which limits Ca2+ influx and likely mitigates the detrimental effects of NCX loss (13). Future studies are required to determine the mechanism for ICaL downregulation in NCX1 mice and the reason for differential regulation in NCX1 knockout and ankyrin-B-deficient mice.
It is important to note that additional factors not accounted for in the model may regulate triggered activity in ankyrin-B deficiency. One possibility includes an altered phosphorylation state of SR and/or membrane target proteins due to loss of PP2A activity (2). In fact, reduced expression of the B56α regulatory subunit of PP2A has been shown to cause Ca2+/calmodulin-dependent kinase II-dependent hyperphosphorylation of ryanodine receptor 2 and afterdepolarizations (39). Moreover, Ca2+ overload itself due to loss of NKA and NCX may result in abnormal kinase activity (33). Thus, perturbation of kinase/phosphatase regulation in ankyrin-B deficiency has the potential to exacerbate dysfunction due to loss of NKA and NCX targeting.
While not a consistent clinical finding, a subpopulation of patients with ankyrin-B syndrome present with prolongation of the QT interval on the electrocardiogram (26, 28, 34). The mechanism responsible for this phenotype is unclear. Our simulation results indicate that loss of NKA is responsible for the slight prolongation of APD in ankyrin-B+/− cells. Nonetheless, it is unclear whether QT prolongation is due to AP prolongation or another mechanism [e.g., abnormal conduction (28)]. Future studies are required to address QT prolongation in the subpopulation of ankyrin-B syndrome patients with long QT.
A recent study (11) from our group has identified Eps15 homology domain (EHD) proteins as a new class of cardiac proteins involved in ankyrin-B-based targeting of NCX. EHD knockdown in wild-type neonatal cardiomyocytes reduces NCX membrane expression and function. Conversely, EHD overexpression increases cell surface NCX and current. The results from the present study support the idea that restoring NCX function by targeting ankyrin and/or EHD may be an effective strategy for preventing cardiac arrhythmia and sudden death in patients with congenital or acquired ankyrin-B deficiency.
Limitations.
Our model of the ankyrin-B+/− cardiomyocyte is based closely on cellular data from an animal model of ankyrin-B+/− that phenocopies human patients with ankyrin-B syndrome. While this effort is an important first step, it is important to note that our model is limited by the available experimental data. While loss of current and surface expression of NCX and NKA are well documented in ankyrin-B+/− mice, data on NCX and NKA biophysical properties are limited. However, it has been shown that while there is a loss of ouabain-binding sites (parallels loss of NKA) in ankyrin-B-deficient cardiomyocytes, the affinity for ouabain is unchanged for residual sites (25). These data, while far from conclusive, suggest that the population of NKA that targets successfully in the absence of ankyrin-B functions normally. Furthermore, while the model addresses loss of NCX and NKA targeting in ankyrin-B-deficient cells, it does not account for defects in the localization of the InsP3 receptor (or PP2A, as discussed above) in ventricular cardiomyocytes. In fact, the role of the InsP3 receptor in cardiomyocytes is unclear, particularly in the ventricle, where Ca2+ release is controlled by ryanodine receptor Ca2+-release channels. We expect our model to serve as a quantitative framework into which new data on InsP3 function (and PP2A) in ventricular cardiomyocytes may be incorporated to determine the consequences of InsP3 mislocalization in ankyrin-B+/− cells.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-096805 (to T. J. Hund) and HL-084583 and HL-083422 (to P. J. Mohler), the Pew Scholars Trust (to P. J. Mohler), and a Fondation Leducq Award to the Alliance for Calmodulin Kinase Signaling in Heart Disease.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Footnotes
Supplemental Material for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1. Ayalon G, Davis JQ, Scotland PB, Bennett V. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135: 1189– 1200, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Bhasin N, Cunha SR, Mduannayake M, Gigena MS, Rogers TB, Mohler PJ. Molecular basis for PP2A regulatory subunit B56α targeting in cardiomyocytes. Am J Physiol Heart Circ Physiol 293: H109– H119, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Bondarenko VE, Rasmusson RL. Transmural heterogeneity of repolarization and Ca2+ handling in a model of mouse ventricular tissue. Am J Physiol Heart Circ Physiol 299: H454– H469, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bondarenko VE, Szigeti GP, Bett GC, Kim SJ, Rasmusson RL. Computer model of action potential of mouse ventricular myocytes. Am J Physiol Heart Circ Physiol 287: H1378– H1403, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci USA 104: 20990– 20995, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christensen MD, Dun W, Boyden PA, Anderson ME, Mohler PJ, Hund TJ. Oxidized calmodulin kinase II regulates conduction following myocardial infarction: A computational analysis. PLoS Comput Biol 5: e1000583, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cunha SR, Bhasin N, Mohler PJ. Targeting and stability of Na/Ca exchanger 1 in cardiomyocytes requires direct interaction with the membrane adaptor ankyrin-B. J Biol Chem 282: 4875– 4883, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Eber SW, Gonzalez JM, Lux ML, Scarpa AL, Tse WT, Dornwell M, Herbers J, Kugler W, Ozcan R, Pekrun A, Gallagher PG, Schroter W, Forget BG, Lux SE. Ankyrin-1 mutations are a major cause of dominant and recessive hereditary spherocytosis. Nat Genet 13: 214– 218, 1996. [DOI] [PubMed] [Google Scholar]
- 9. Faber GM, Rudy Y. Calsequestrin mutation and catecholaminergic polymorphic ventricular tachycardia: a simulation study of cellular mechanism. Cardiovasc Res 75: 79– 88, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujioka Y, Matsuoka S, Ban T, Noma A. Interaction of the Na+-K+ pump and Na+-Ca2+ exchange via [Na+]i in a restricted space of guinea-pig ventricular cells. J Physiol 509: 457– 470, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gudmundsson H, Hund TJ, Wright PJ, Kline CF, Snyder JS, Qian L, Koval OM, Cunha SR, George M, Rainey MA, Kashef FE, Dun W, Boyden PA, Anderson ME, Band H, Mohler PJ. EH domain proteins regulate cardiac membrane targeting. Circ Res 107: 84– 95, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hashemi SM, Hund TJ, Mohler PJ. Cardiac ankyrins in health and disease. J Mol Cell Cardiol 47: 203– 209, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, Frank JS, Jordan MC, Roos KP, Ross RS, Philipson KD. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res 95: 604– 611, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Hund TJ, Decker KF, Kanter E, Mohler PJ, Boyden PA, Schuessler RB, Yamada KA, Rudy Y. Role of activated CaMKII in abnormal calcium homeostasis and INa remodeling after myocardial infarction: Insights from mathematical modeling. J Mol Cell Cardiol 45: 420– 428, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hund TJ, Wright PJ, Dun W, Snyder JS, Boyden PA, Mohler PJ. Regulation of the ankyrin-B-based targeting pathway following myocardial infarction. Cardiovasc Res 81: 742– 749, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kline CF, Kurata HT, Hund TJ, Cunha SR, Koval OM, Wright PJ, Christensen M, Anderson ME, Nichols CG, Mohler PJ. Dual role of KATP channel C-terminal motif in membrane targeting and metabolic regulation. Proc Natl Acad Sci USA 106: 16669– 16674, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, Marionneau C, Chen B, Wu Y, Demolombe S, Song LS, Le Marec H, Probst V, Schott JJ, Anderson ME, Mohler PJ. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci USA 105: 15617– 15622, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee CO. 200 years of digitalis: the emerging central role of the sodium ion in the control of cardiac force. Am J Physiol Cell Physiol 249: C367– C378, 1985. [DOI] [PubMed] [Google Scholar]
- 19. Lehnart SE, Ackerman MJ, Benson DW, Jr, Brugada R, Clancy CE, Donahue JK, George AL, Jr, Grant AO, Groft SC, January CT, Lathrop DA, Lederer WJ, Makielski JC, Mohler PJ, Moss A, Nerbonne JM, Olson TM, Przywara DA, Towbin JA, Wang LH, Marks AR. Inherited arrhythmias: a National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation 116: 2325– 2345, 2007. [DOI] [PubMed] [Google Scholar]
- 20. London B, Michalec M, Mehdi H, Zhu X, Kerchner L, Sanyal S, Viswanathan PC, Pfahnl AE, Shang LL, Madhusudanan M, Baty CJ, Lagana S, Aleong R, Gutmann R, Ackerman MJ, McNamara DM, Weiss R, Dudley SC., Jr Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation 116: 2260– 2268, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res 74: 1071– 1096, 1994. [DOI] [PubMed] [Google Scholar]
- 22. Medeiros-Domingo A, Kaku T, Tester DJ, Iturralde-Torres P, Itty A, Ye B, Valdivia C, Ueda K, Canizales-Quinteros S, Tusie-Luna MT, Makielski JC, Ackerman MJ. SCN4B-encoded sodium channel β4 subunit in congenital long-QT syndrome. Circulation 116: 134– 142, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol 3: e423, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mohler PJ, Davis JQ, Davis LH, Hoffman JA, Michaely P, Bennett V. Inositol 1,4,5-trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin-B. J Biol Chem 279: 12980– 12987, 2004. [DOI] [PubMed] [Google Scholar]
- 25. Mohler PJ, Healy JA, Xue H, Puca AA, Kline CF, Allingham RR, Kranias EG, Rockman HA, Bennett V. Ankyrin-B syndrome: enhanced cardiac function balanced by risk of cardiac death and premature senescence. PLoS One 2: e1051, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mohler PJ, Le Scouarnec S, Denjoy I, Lowe JS, Guicheney P, Caron L, Driskell IM, Schott JJ, Norris K, Leenhardt A, Kim RB, Escande D, Roden DM. Defining the cellular phenotype of “ankyrin-B syndrome” variants: human ANK2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation 115: 432– 441, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, Bennett Nav1 V. 5. E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci USA 101: 17533– 17538, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 421: 634– 639, 2003. [DOI] [PubMed] [Google Scholar]
- 29. Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, Priori SG, Keating MT, Bennett V. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci USA 101: 9137– 9142, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moore ED, Etter EF, Philipson KD, Carrington WA, Fogarty KE, Lifshitz LM, Fay FS. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature 365: 657– 660, 1993. [DOI] [PubMed] [Google Scholar]
- 31. Reuter H, Henderson SA, Han T, Ross RS, Goldhaber JI, Philipson KD. The Na+-Ca2+ exchanger is essential for the action of cardiac glycosides. Circ Res 90: 305– 308, 2002. [DOI] [PubMed] [Google Scholar]
- 32. Rudy Y, Silva JR. Computational biology in the study of cardiac ion channels and cell electrophysiology. Q Rev Biophys 39: 57– 116, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sapia L, Palomeque J, Mattiazzi A, Petroff MV. Na+/K+-ATPase inhibition by ouabain induces CaMKII-dependent apoptosis in adult rat cardiac myocytes. J Mol Cell Cardiol 49: 459– 468, 2010. [DOI] [PubMed] [Google Scholar]
- 34. Schott JJ, Charpentier F, Peltier S, Foley P, Drouin E, Bouhour JB, Donnelly P, Vergnaud G, Bachner L, Moisan JP, Le Marec H, Pascal O. Mapping of a gene for long QT syndrome to chromosome 4q25–27. Am J Hum Genet 57: 1114– 1122, 1995. [PMC free article] [PubMed] [Google Scholar]
- 35. Sedlacek K, Stark K, Cunha SR, Pfeufer A, Weber S, Berger I, Perz S, Kaab S, Wichmann HE, Mohler PJ, Hengstenberg C, Jeron A. Common genetic variants in ANK2 modulate QT interval: results from the KORA study. Circ Cardiovasc Genet 1: 93– 99, 2008. [DOI] [PubMed] [Google Scholar]
- 36. Stagg MA, Carter E, Sohrabi N, Siedlecka U, Soppa GK, Mead F, Mohandas N, Taylor-Harris P, Baines A, Bennett P, Yacoub MH, Pinder JC, Terracciano CM. Cytoskeletal protein 4.1R affects repolarization and regulates calcium handling in the heart. Circ Res 103: 855– 863, 2008. [DOI] [PubMed] [Google Scholar]
- 37. Su Z, Zou A, Nonaka A, Zubair I, Sanguinetti MC, Barry WH. Influence of prior Na+ pump activity on pump and Na+/Ca2+ exchange currents in mouse ventricular myocytes. Am J Physiol Heart Circ Physiol 275: H1808– H1817, 1998. [DOI] [PubMed] [Google Scholar]
- 38. Ten Tusscher KH, Noble D, Noble PJ, Panfilov AV. A model for human ventricular tissue. Am J Physiol Heart Circ Physiol 286: H1573– H1589, 2004. [DOI] [PubMed] [Google Scholar]
- 39. Terentyev D, Belevych AE, Terentyeva R, Martin MM, Malana GE, Kuhn DE, Abdellatif M, Feldman DS, Elton TS, Gyorke S. miR-1 overexpression enhances Ca2+ release and promotes cardiac arrhythmogenesis by targeting PP2A regulatory subunit B56α and causing CaMKII-dependent hyperphosphorylation of RyR2. Circ Res 104: 514– 521, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Terrenoire C, Clancy CE, Cormier JW, Sampson KJ, Kass RS. Autonomic control of cardiac action potentials: role of potassium channel kinetics in response to sympathetic stimulation. Circ Res 96: e25– e34, 2005. [DOI] [PubMed] [Google Scholar]
- 41. Ueda K, Valdivia C, Medeiros-Domingo A, Tester DJ, Vatta M, Farrugia G, Ackerman MJ, Makielski JC. Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proc Natl Acad Sci USA 105: 9355– 9360, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 114: 2104– 2112, 2006. [DOI] [PubMed] [Google Scholar]