Abstract
Acute increases in O-linked β-N-acetylglucosamine (O-GlcNAc) levels of cardiac proteins exert protective effects against ischemia-reperfusion (I/R) injury. One strategy to rapidly increase cellular O-GlcNAc levels is inhibition of O-GlcNAcase (OGA), which catalyzes O-GlcNAc removal. Here we tested the cardioprotective efficacy of two novel and highly selective OGA inhibitors, the NAG-thiazoline derivatives NAG-Bt and NAG-Ae. Isolated perfused rat hearts were subjected to 20 min global ischemia followed by 60 min reperfusion. At the time of reperfusion, hearts were assigned to the following four groups: 1) untreated control; 2) 50 μM NAG-Bt; 3) 100 μM NAG-Bt; or 4) 50 μM NAG-Ae. All treatment groups significantly increased total O-GlcNAc levels (P < 0.05 vs. control), and this was significantly correlated with improved contractile function and reduced cardiac troponin I release (P < 0.05). Immunohistochemistry of normoxic hearts showed intense nuclear O-GlcNAc staining and higher intensity at Z-lines with colocalization of O-GlcNAc and the Z-line proteins desmin and vinculin. After I/R, there was a marked loss of both cytosolic and nuclear O-GlcNAcylation and disruption of normal striated Z-line structures. OGA inhibition largely preserved structural integrity and attenuated the loss of O-GlcNAcylation; however, nuclear O-GlcNAc levels remained low. Immunoblot analysis confirmed ∼50% loss in both nuclear and cytosolic O-GlcNAcylation following I/R, which was significantly attenuated by OGA inhibition (P < 0.05). These data provide further support for the notion that increasing cardiac O-GlcNAc levels by inhibiting OGA may be a clinically relevant approach for ischemic cardioprotection, in part, by preserving the integrity of O-GlcNAc-associated Z-line protein structures.
Keywords: hexosamine biosynthesis, protein O-GlcNAcylation, O-GlcNAcase, ischemia-reperfusion, cardioprotection
the posttranslational modification of nucleocytoplasmic proteins with the monosaccharide O-linked β-N-acetylglucosamine (O-GlcNAc) is increasingly recognized as a critical signaling mechanism regulating a diverse range of biological processes in mammalian cells (3, 19, 44). This attachment of O-GlcNAc to Ser/Thr residues of proteins is frequently considered to be analogous to protein phosphorylation in that it is a highly dynamic, reversible, and tightly regulated enzyme-catalyzed process. Sustained activation of the hexosamine biosynthesis pathway (HBP) and the resulting increase in O-GlcNAcylation has been implicated in the etiology of glucotoxicity and insulin resistance. However, there is rapidly emerging evidence demonstrating that acute activation of these pathways affords protection against a wide range of injury, including cardioprotection against ischemia-reperfusion (I/R) injury in different biological systems (4, 5, 17, 21).
We have previously reported that pretreatment with high glucose or glucosamine attenuated cell death following hypoxia-reoxygenation in isolated cardiomyocytes (4, 5). We also demonstrated that administration of glucosamine and glutamine before ischemia resulted in enhanced functional recovery and decreased tissue injury following ischemia in the perfused heart (11, 20–22). One common mechanism by which glucose, glucosamine, and glutamine all mediate cardioprotection is via activation of the HBP with a subsequent increase in O-GlcNAc modification of proteins. The level of O-GlcNAcylation is regulated by O-GlcNAc transferase (OGT; uridine diphospho-N-acetylglucosamine: polypeptide β-N-acetylglucosaminyl-transferase), which catalyzes O-GlcNAc synthesis and attachment, and O-GlcNAcase (OGA; β-N-acetyl-d-glucosaminidase), which catalyzes its removal. Thus, an alternative approach to manipulate O-GlcNAc levels is via modulation of OGT or OGA activity. While OGT has been shown to be subject to both phosphorylation and O-GlcNAcylation, to date, there are no specific pharmacological approaches for increasing OGT activity. On the other hand, O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc), an O-GlcNAc analog that is a potent competitive inhibitor of OGA, has been widely used to increase O-GlcNAc levels. Indeed, we have shown that the protection against I/R injury seen with glucosamine is mimicked by OGA inhibition with PUGNAc (4, 21). However, PUGNAc is not a selective OGA inhibitor [inhibitory constant (KI) = 0.046 μM]; it also inhibits other glycoside hydrolases such as the lysosomal β-hexosaminidases (KI = 0.036 μM) and β-N-acetylglucosaminidases (KI = 6 μM) (24, 25). PUGNAc is also known to have poor permeability in aqueous solutions and caused decreased growth in several cell lines (34).
Thus, the lack of specificity of PUGNAc may not only complicate the interpretation of results but also limit its therapeutic potential. Indeed, we have found that, at the cellular level, PUGNAc was less effective in protecting against hypoxia-reoxygenation than glucosamine (4). Macauley and coworkers (25) have recently developed novel OGA inhibitors, analogous derivatives of the parent NAG-thiazoline (1,2 dideoxy-2′-methyl-α-d-glycopyranoso-[2,1-d]-Δ2′-thiazoline), that have been shown to be more selective inhibitors of eukaryotic OGA over the functionally related lysosomal β-hexosaminidases than PUGNAc. For example, NAG-Bt (1,2 dideoxy-2′-propyl-α-d-glycopyranoso-[2,1-d]-Δ2′-thiazoline) is a potent OGA inhibitor (KI = 0.23 μM) and highly selective over the β-hexosaminidases (KI = 340 μM) with an ∼1,500-fold greater specificity than PUGNAc (12, 25); NAG-Ae (1,2 dideoxy-2′-ethylamino-α-d-glucopyranoso-[2,1-d]-Δ2′-thiazoline) was found to be a stable and more potent and selective inhibitor of OGA (∼30-fold; KI = 0.021 μM) than NAG-Bt (12, 43). NAG-Bt has been shown to successfully increase O-GlcNAc levels in a small number of cell lines, including isolated cardiomyocytes (5, 24); however, neither NAG-Bt nor NAG-Ae has been tested to determine their efficacy against I/R injury at the whole organ level.
The development of reperfusion therapy to reduce ischemic injury has been a critical factor in decreasing the mortality associated with acute myocardial infarction; however, restoration of blood flow leads to further cardiomyocyte cell death, accounting for up to 50% of the final infarct size (42). This has yielded considerable interest in the development of cell protection strategies that could be given immediately before and following reperfusion or revascularization to attenuate cardiomyocyte death (37). However, although considerable efforts have been invested in developing novel cardioprotective strategies, many promising therapeutic agents, which are effective when applied before ischemia, are often ineffective when used only during reperfusion (2, 23). We have previously reported that administration of glucosamine and PUGNAc during the first 20 min of reperfusion improved functional recovery and decreased injury following ischemia (21); however, as noted above, both glucosamine and PUGNAc have limitations with regard to specificity. Therefore, in this study, we set out to establish whether selective inhibition of OGA at the time of reperfusion with the NAG-thiazoline analogs NAG-Bt and NAG-Ae attenuated I/R injury in the isolated perfused heart and to compare the effectiveness of NAG-Bt and NAG-Ae in mediating functional recovery.
Elevated O-GlcNAc levels have typically been perceived as detrimental (7); the notion that acute increases in protein O-GlcNAcylation represented an endogenous cellular stress response that could potentially confer a survival advantage was first reported in 2004 (45). It has also been shown that a standard ischemic preconditioning protocol, which markedly attenuates infarct size, also increases cardiac O-GlcNAc levels (17). Interestingly, however, while we have reported that cardiac O-GlcNAc levels increased during ischemia, we observed a subsequent decrease during reperfusion (4, 11). Thus, it is clear that our understanding of the effects of I/R on cardiac O-GlcNAc levels remains limited. Furthermore, while it is well established that nuclear proteins are highly O-GlcNAcylated (3, 44), the distribution of O-GlcNAc in the intact adult heart is not known. Consequently, in addition to testing the cardioprotective effects of OGA inhibition, we used immunohistochemistry to examine the effects of I/R on intracellular O-GlcNAc distribution in the heart.
MATERIALS AND METHODS
Materials.
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. The OGA inhibitors of NAG-Bt and NAG-Ae were kind gifts from Dr. David Vocadlo (Simon Fraser University, Burnaby, BC, Canada).
Animals.
Animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham and conformed to the Guide for the Care and Usage of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). Nonfasted, male Sprague-Dawley rats (Charles Rivers Laboratories, Wilmington, MA) weighing 337 ± 6 g were employed.
Experimental protocol.
After 30 min equilibration, isolated hearts were randomly assigned to either a time-control, untreated normoxia group or the following four I/R groups: 1) I/R, untreated (control); 2) I/R, 50 μM NAG-Bt (NBt50); 3) I/R, 100 μM NAG-Bt (NBt100); 4) I/R, 50 μM NAG-Ae (NAe). There were three to seven replicates in each group as indicated in the legends for Table 1 and Fig. 1–8.
Table 1.
Baseline cardiac functions before ischemia in control, untreated hearts and hearts subsequently treated with NAG-thiazolines during reperfusion
| Groups | n | Coronary Flow, ml/min | Heart Rate, beats/min | LVDP, mmHg | +dP/dt, mmHg/s | −dP/dt, mmHg/s | RPP, mmHg/min |
|---|---|---|---|---|---|---|---|
| Control | 6 | 10.1 ± 0.7 | 324 ± 13 | 96.6 ± 10.0 | 3,374 ± 174 | 2,166 ± 308 | 30,688 ± 1,725 |
| NBt50 | 7 | 12.3 ± 0.9 | 323 ± 9 | 89.9 ± 9.9 | 3,451 ± 313 | 2,078 ± 253 | 28,905 ± 2,881 |
| NBt100 | 5 | 13.2 ± 0.9 | 335 ± 18 | 83.6 ± 6.5 | 3,428 ± 259 | 1,966 ± 146 | 27,744 ± 1,610 |
| NAe | 3 | 14.2 ± 1.0 | 359 ± 29 | 74.6 ± 5.5 | 3,930 ± 375 | 1,796 ± 125 | 27,029 ± 3,596 |
Values are means ± SE ; n, no. of replicates/group, LVDP, left ventricular developed pressure; +dP/dt, maximum rate of change in left ventricular pressure; −dP/dt, minimum rate of change in left ventricular pressure; RPP, rate-pressure product. Baseline cardiac function was assessed after 30 min normoxic equilibration before 20 min zero-flow ischemia. There were no significant differences in preischemic cardiac parameters between untreated, ischemia-reperfusion hearts (control) and hearts treated with 50 μM 1,2 dideoxy-2′-propyl-α-d-glycopyranoso-[2,1-d]-Δ2′-thiazoline [NAG-Bt (NBt50)], 100 μM NAG-Bt (NBt100), and 50 μM 1,2 dideoxy-2′-ethylamino-α-d-glucopyranoso-[2,1-d]-Δ2′-thiazoline (NAe).
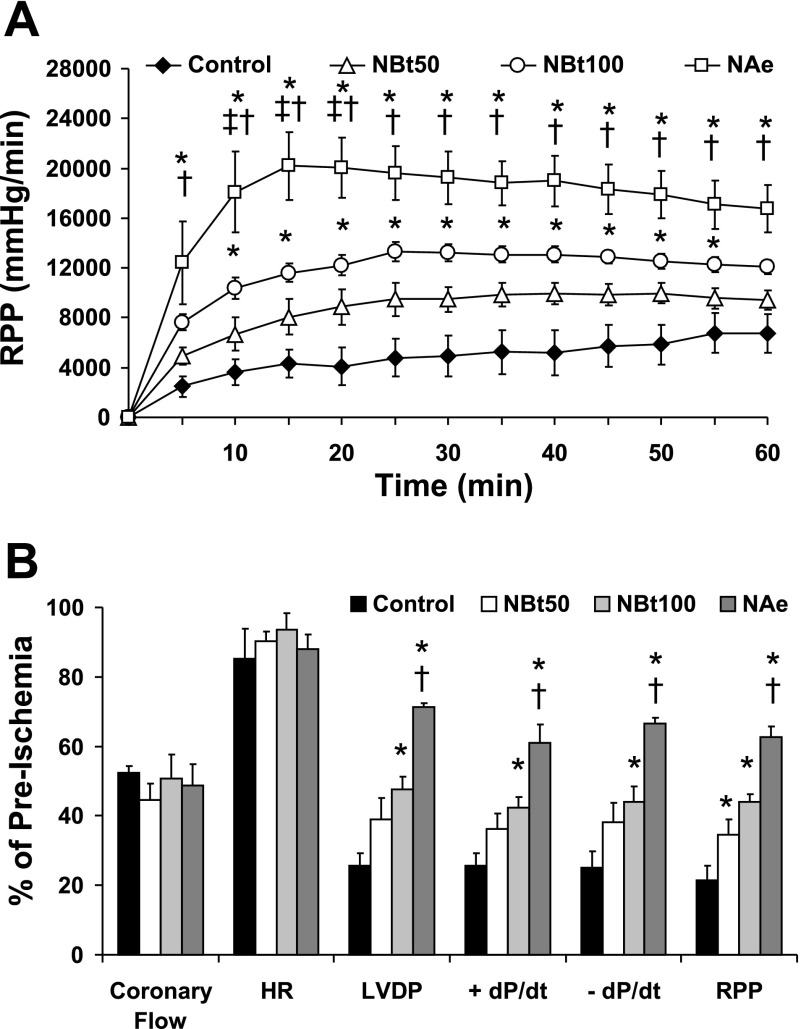
Fig. 1.
Effect of 1,2 dideoxy-2′-methyl-α-d-glycopyranoso-[2,1-d]-Δ2′-thiazoline (NAG-thiazoline) treatment during reperfusion on functional recovery after 20 min zero-flow ischemia. A: time course of changes in rate-pressure product (RPP) during 60 min reperfusion. B: functional recoveries of coronary flow, heart rate (HR), left ventricular developed pressure (LVDP), positive and negative rates of left ventricular pressure change (±dP/dt), and RPP following 60 min reperfusion as a percent of preischemic values in untreated ischemia-reperfusion hearts (control; n = 7) and hearts treated with 50 μM 1,2 dideoxy-2′-propyl-α-d-glycopyranoso-[2,1-d]-Δ2′-thiazoline [NAG-Bt (NBt50); n = 7], 100 μM NAG-Bt (NBt100; n = 5), and 50 μM 1,2 dideoxy-2′-ethylamino-α-d-glucopyranoso-[2,1-d]-Δ2′-thiazoline [NAG-Ae (NAe); n = 3]. P < 0.05 vs. control (*), vs. NBt50 (†), and vs. NBt100 (‡).
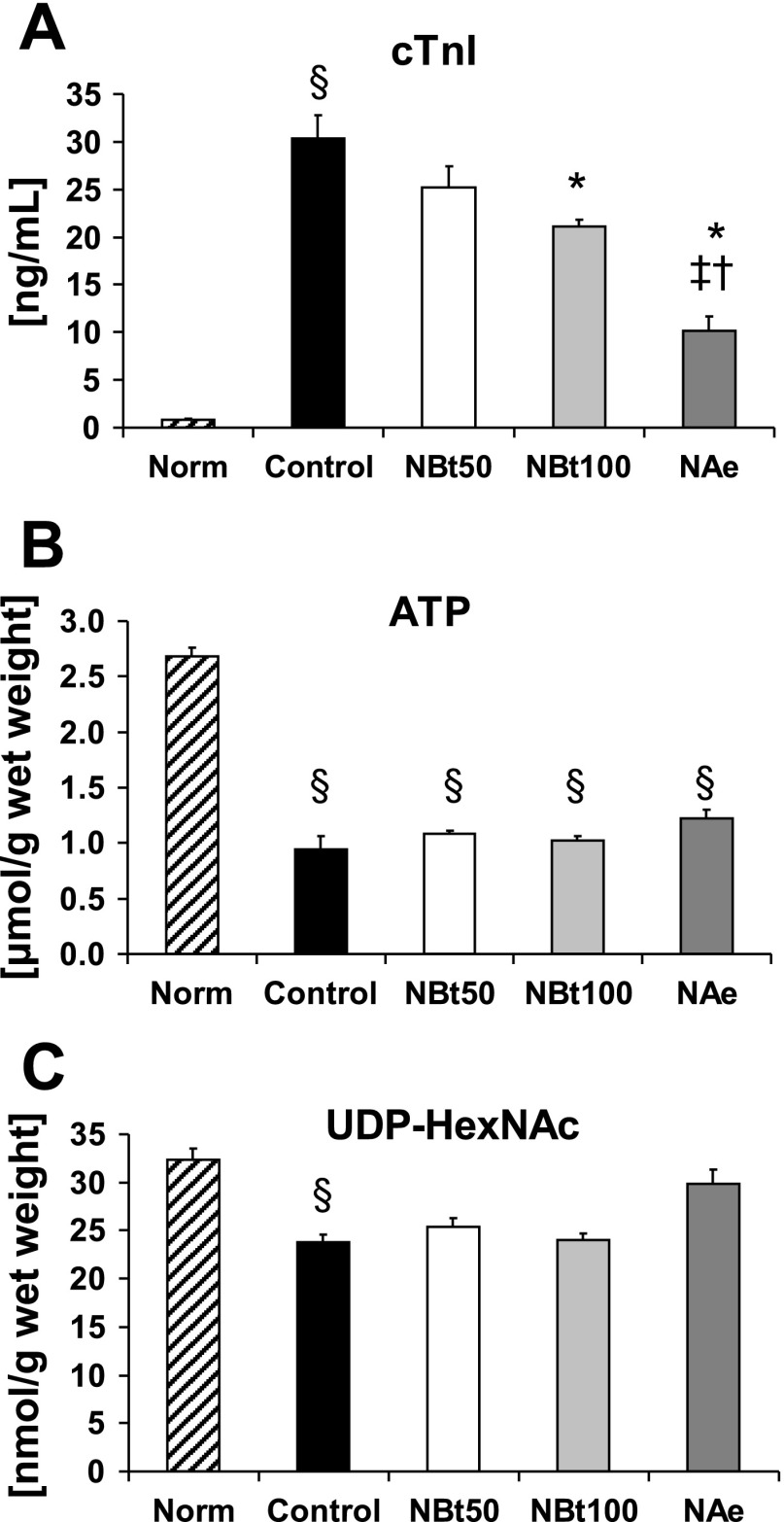
Fig. 2.
Effect of NAG-thiazoline treatment on cardiac troponin I (cTnI) release (A), ATP levels (B), and UDP-N-acetylhexosamine (HexNAc) concentrations (C) in untreated, time-control, normoxic hearts (Norm, n = 5) at the end of 110 min normoxic perfusion and at the end of 60 min reperfusion after 20 min zero-flow ischemia in untreated ischemia-reperfusion hearts (control, n = 7) and NBt50 (n = 7), NBt100 (n = 5), and NAe (n = 3) hearts. P < 0.05 vs. control (*), vs. NBt50 (†), vs. NBt100 (‡), and vs. Norm (§).
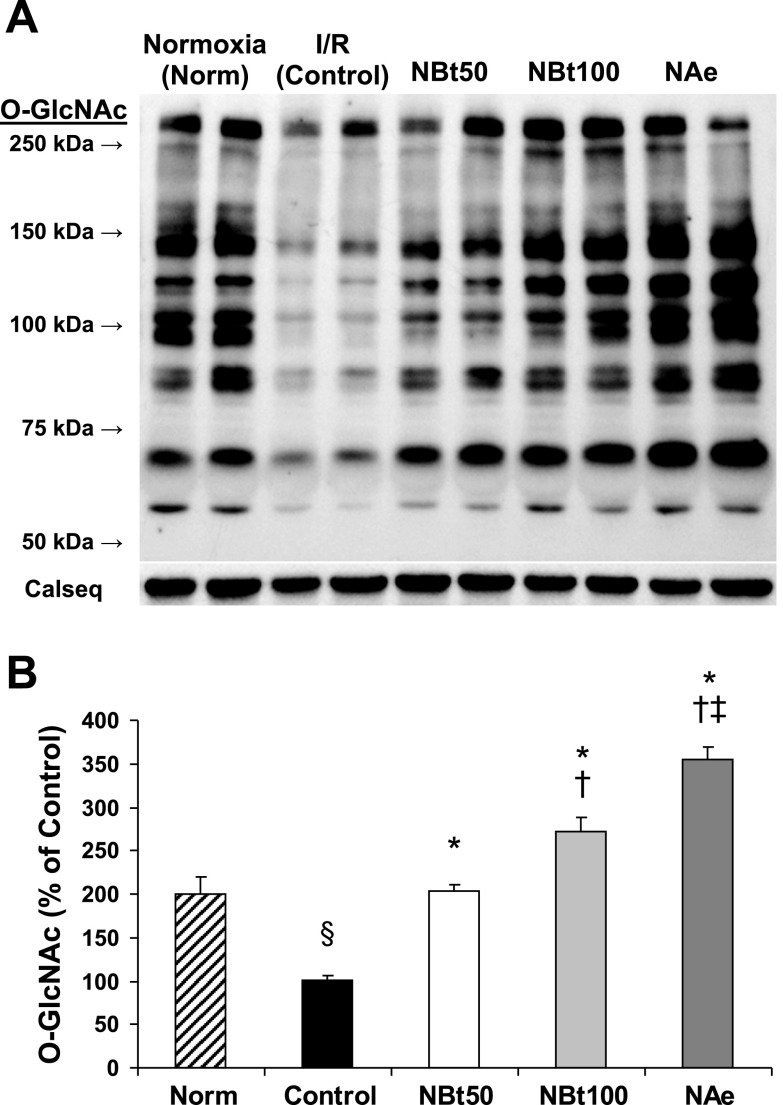
Fig. 3.
Effect of NAG-thiazoline treatment on O-linked β-N-acetylglucosamine (O-GlcNAc) levels of the perfused rat heart. Representative anti-O-GlcNAc (CTD110.6) immunoblot (A) and densitometric results of protein-associated cardiac O-GlcNAc levels (B) after time-control, normoxic perfusions (Norm); and after 20 min zero-flow ischemia and 60 min reperfusion (I/R) in the absence (control) and in the presence of NBt50, NBt100, and NAe treatments. Densitometric analyses were performed using original anti-O-GlcNAc immunoblots to compare all experimental groups (n = 3–5 hearts/group). The entire lane mean intensities are normalized to calsequestrin levels shown as protein loading control, and are relative to the control group. P < 0.001 vs. control (*), vs. NBt50 (†), vs. NBt100 (‡), and vs. Norm (§).
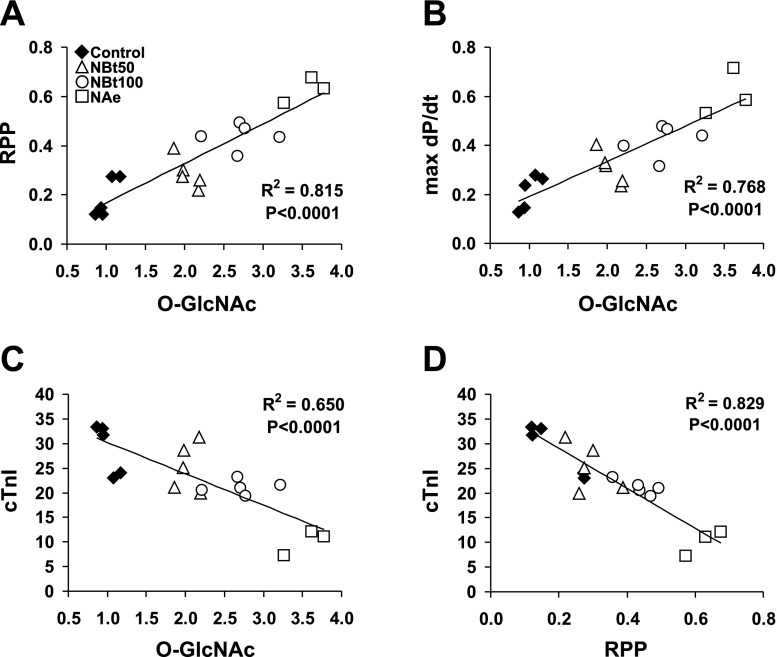
Fig. 4.
Correlations of cardiac O-GlcNAc levels and RPP (A), maximum rate of left ventricular pressure changes (max dP/dt; B), and cTnI release (C) at the end of reperfusion. D: correlation of cTnI levels and RPP after reperfusion. Data of cardiac functions are expressed as %preischemic values and are presented for all ischemia-reperfusion hearts in the untreated group (control; n = 7) and following treatments in the NBt50 (n = 7), NBt100 (n = 5), and NAe (n = 3) groups.
Fig. 5.
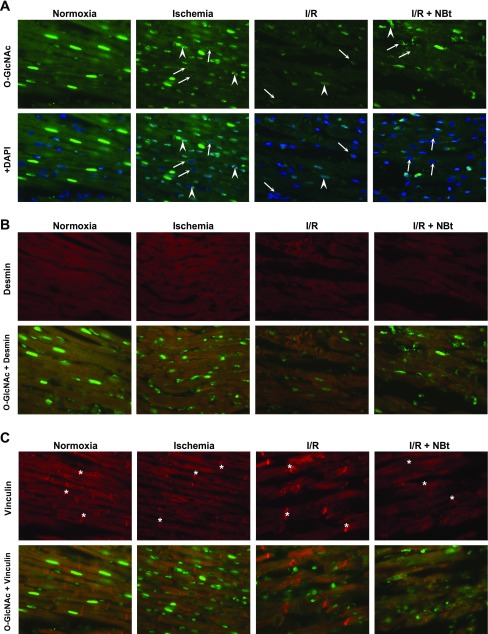
Immunohistochemistry of rat myocardium after normoxic perfusions (Normoxia); after 20 min zero-flow ischemia (Ischemia); at the end of 60 min reperfusion without treatment (I/R); and after reperfusion following treatment with 50 μM NAG-Bt (I/R + NBt). A: O-GlcNAc immunohistochemistry (green) and merged images with nuclei staining (blue; 4′,6-diamidino-2-phenylindole, DAPI); note that ischemia and I/R result in alterations in nuclear O-GlcNAc distribution with appearance of punctate staining (arrowheads) and perinuclear staining of otherwise O-GlcNAc-negative nuclei (arrows). B: desmin immunohistochemistry (red) and its cross-striated colocalization with O-GlcNAc (green) at Z-line. C: vinculin immunohistochemistry (red) and colocalization of O-GlcNAc (green) with vinculin at Z-line, but not intercalated disc (*) (×40 magnification).
Fig. 6.
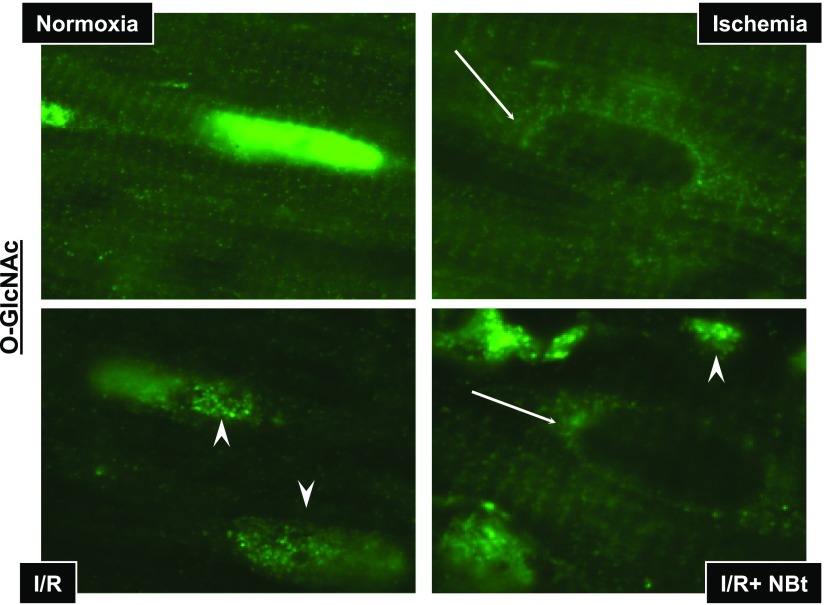
Immunohistochemistry of nuclear O-GlcNAcylation in response to ischemia, I/R, I/R + NBt. In normoxic hearts (Normoxia), intensity of O-GlcNAc (green) staining is predominantly concentrated in the nuclei of cardiomyocytes. Ischemia leads to prominent changes in nuclear O-GlcNAc distribution with increased punctate staining (arrowheads) and perinuclear staining of otherwise O-GlcNAc-negative nuclei (arrows).
Fig. 7.
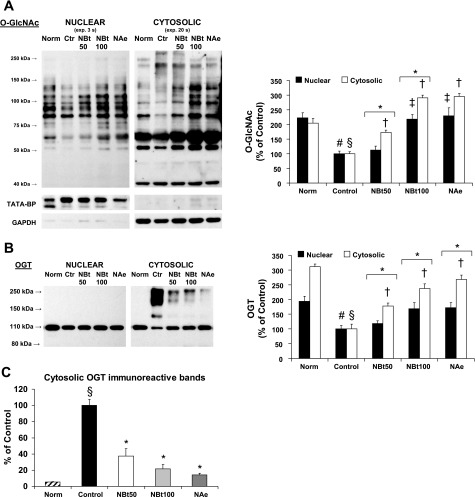
Effect of I/R and NAG-thiazoline treatment on O-GlcNAc levels and O-GlcNAc transferase (OGT) protein content in nuclear and cytosolic compartments of the perfused rat heart. A: representative anti-O-GlcNAc (CTD110.6) immunoblots, and densitometric analyses, demonstrate the changes in nuclear and cytosolic levels of O-GlcNAc. Note that, given the marked differences in overall O-GlcNAc levels between nuclear and cytosolic fractions, different exposure times as indicated were used for the gel images. Consequently, the mean data are shown normalized to the intensity of the untreated group [control (Ctr)] for each fraction; thus, comparison of O-GlcNAc levels between the two fractions cannot be made. B and C: OGT (B) and high-molecular-weight OGT (C) immunoreactive bands in the cytosolic fraction in hearts after time-control, normoxic perfusions (Norm) and after I/R in the untreated group (control) and the treated NBt50, NBt100, and NAe groups (n = 3–4 hearts/group). TATA-binding protein and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are shown as purity controls. P < 0.05, nuclear vs. cytosolic (*), vs. cytosolic control (†), vs. nuclear control (‡), vs. cytosolic Norm (§), and vs. nuclear Norm (#).
Fig. 8.
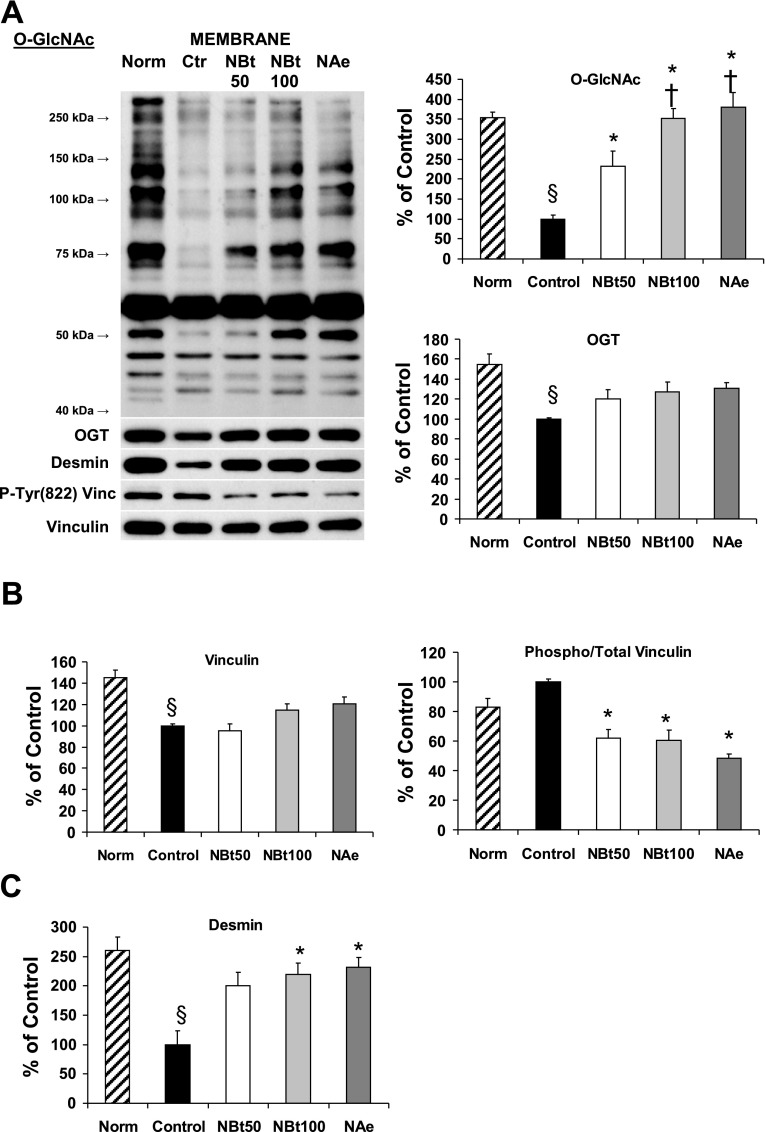
Immunoblot analyses of cardiac O-GlcNAc and OGT (A), phospho-Tyr822 vinculin and total vinculin (B), and desmin (C) levels in the crude membrane fraction (n = 3 hearts/group) after time-control, normoxic perfusions (Norm) and after I/R in untreated hearts (control) and hearts from NBt50, NBt100, and NAe groups. Data are expressed as %control group. P < 0.05 vs. control (*), vs. NBt50 (†), and vs. Norm (§).
In all I/R groups, hearts were subjected to 20 min global, no-flow ischemia followed by 60 min of reperfusion. In the treated I/R groups, hearts were perfused with NAG-thiazolines starting immediately at reperfusion and continuing throughout the 60-min reperfusion period. The starting concentration of NAG-Bt was chosen based on preliminary dose-response studies that demonstrated an EC50 of ∼30 μM for increasing O-GlcNAc levels in the normoxic heart perfused with NAG-Bt for 60 min (data not shown). Because of limited availability of NAG-Ae at the time of these studies, we were able to examine only a single concentration; therefore, to enable direct comparison with NAG-Bt, 50 μM NAG-Ae was used. In the time-control groups, hearts were perfused for a total of 110 min under normoxic conditions.
To determine whether inhibition of OGA alone had any effect on cardiac function, an additional group of rat hearts was perfused with NAG-Bt (50 μM) that was added 20 min after the initial 30-min equilibration period, and was present for the remaining 60 min of normoxic perfusion; thus, total perfusion time and exposure to NAG-Bt was the same as that for I/R treatment groups.
Heart perfusion.
Sprague-Dawley rats were anesthetized with intraperitoneal ketamine-hydrochloride injection (100 mg/kg; Vedco, St. Joseph, MO) and killed by decapitation, and hearts were rapidly excised. Isolated hearts were arrested in ice-cold perfusion buffer and then cannulated via the aorta for retrograde perfusion in a modified Langendorff model, as previously described (11, 20–22, 29, 38, 39). The Krebs-Henseleit perfusion buffer (pH 7.4) contained (in mM) 118 NaCl, 25 NaHCO3, 4.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.25 CaCl2, 11 glucose, and 3% BSA (fatty acid free; Millipore, Billerica, MA) gassed with 95% O2 and 5% CO2. The solution was filtered through a 0.45-μm filter to remove contaminants, and NAG-thiazolines were dissolved in the perfusion buffer immediately before use.
Cardiac function was monitored via a fluid-filled balloon inserted in the left ventricle via the left atrium, connected to a TXD-310 pressure transducer, and registered with a heart performance analyzer (HPA-410; Micro-Med, Louisville, KY). End-diastolic pressure (EDP) was set to 5 mmHg by adjusting balloon volume. Consistent with our earlier studies (11, 20–22, 29, 38, 39), coronary flow was controlled by a perfusion pump and was adjusted to maintain a constant perfusion pressure of 75 mmHg; myocardial temperature was monitored throughout the experiment at the pulmonary artery using a thermoelectrode and was maintained constant at ∼37°C by adjusting the temperature of the heating system consisting of a water-filled compartment circulating around the perfusion apparatus. Coronary flow was assessed by timed collections of right ventricle effluent in a graduated cylinder, and volume was measured. Hearts were allowed to beat spontaneously and were nonpaced throughout the experiment. Left ventricular developed pressure (LVDP = systolic pressure − EDP), maximum and minimum rate of left ventricular pressure development (±dP/dt) as the indexes of contraction and relaxation, respectively, rate-pressure product (RPP = LVDP × heart rate), and coronary flow were used to evaluate cardiac performance.
Global ischemia was induced by stopping coronary flow for 20 min, followed by 60 min of reperfusion at 75 mmHg. To determine whether NAG-thiazoline treatments have beneficial effects on I/R-induced arrhythmias, ventricular pressure signals were analyzed for the incidence of ventricular tachycardia (VT) and/or fibrillation (VF) during reperfusion (16, 32). VT was defined as a run of four or more consecutive ectopic beats, and VF was defined as fast mechanical activity with barely discernible beat and minimal LVDP (<5 mmHg) (16). At the end of reperfusion, hearts were either freeze-clamped in liquid nitrogen and stored at −80°C or perfused with 4% paraformaldehyde and preserved in 70% ethanol.
Cardiac troponin I release.
As a marker of tissue injury, cardiac troponin I (cTnI) concentration was determined in pooled coronary effluent collected at 15, 30, 45, and 60 min of reperfusion using a commercially available high-sensitivity cTnI ELISA kit (Life Diagnostics, West Chester, PA).
Measurement of ATP and UDP-N-acetylhexosamine levels.
ATP levels were determined using HPLC analysis of perchloric acid extracts of frozen tissue as previously described (10, 11, 21, 28). Briefly, heart tissue (70 mg) was precipitated in 1 ml 0.3 M perchloric acid and centrifuged (15,000 g, 15 min, at 4°C). The supernatants were neutralized with trioctylamine-1,1,2-trichlorotrifluoroethane mixture (1:4 ratio), loaded on a Partisil 10 SAX anion-exchange column (Beckman, Fullerton, CA), and then eluted with a linear salt (ammonium dihydrogen phosphate from 5 to 750 mM) and pH (from pH 2.8 to 3.7) gradient. Nucleotide sugar concentrations were determined using ultraviolet detection (262 nm) after calibration with appropriate standards. This method also detects UDP-GlcNAc levels; however, it cannot separate completely UDP-GlcNAc from uridine diphosphate-N-acetylgalactosamine (UDP-GalNAc), thus the results are presented as the sum of UDP-GlcNAc and UDP-GalNAc [uridine diphosphate-N-acetylhexosamine (UDP-HexNAc)].
Western blot analysis.
Heart tissue was homogenized (PowerGen homogenizer; Fisher Scientific, Pittsburgh, PA) in T-PER (Pierce, Rockford, IL) supplemented with 40 μM PUGNAc (Toronto Research Chemicals), 1 mM sodium orthovanadate, 20 mM sodium fluoride, and 5% protease inhibitor cocktail and lysed for 60 min on ice. Consistent with our earlier studies, PUGNAc was present throughout the tissue lysis protocol to inhibit O-GlcNAcase, thereby preventing loss of O-GlcNAc (10, 11, 21, 28); this is akin to using phosphatase inhibitors to prevent loss of protein phosphorylation. Although PUGNAc is less specific than NAG-thiazoline compounds, this may be a potential advantage in this setting, since it may also inhibit loss of O-GlcNAc due to hexosaminidase activity.
Whole tissue lysates (10% wt/vol) were centrifuged at 15,000 g for 15 min, and protein concentration of the supernatant was assessed using the Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA). Solubilized proteins were suspended in reducing loading buffer (Pierce), boiled, separated by SDS-PAGE, and then transferred to polyvinylidene difluoride membrane (Millipore) at a constant voltage of 100 V for 75 min. Equal protein loading (20 μg) was confirmed by Ponceau-S staining and cardiac specific calsequestrin (Abcam, Cambridge, MA) immunostaining as loading control. Immunoblots were probed with mouse monoclonal anti-O-GlcNAc IgM antibody (1:2,500; CTD110.6; Epitope Recognition and Immunodetection Facility, University of Alabama at Birmingham) and mouse monoclonal anti-vinculin IgG1 (1:4,000; CloneV284; Upstate, Billerica, MA), rabbit polyclonal anti-phospho-Tyr822 vinculin (1:1,000), and rabbit polyclonal anti-desmin (1:4,000; Abcam) antibodies diluted in 1% casein-PBS blocking buffer (Bio-Rad) overnight at 4°C. Membranes were washed three times with PBS and then incubated with appropriate horseradish peroxidase-conjugated secondary antibodies of goat anti-mouse IgM (Calbiochem, San Diego, CA), goat anti-rabbit IgG (Cell Signaling, Danvers, MA), and goat anti-mouse IgG (Upstate) diluted in 1% casein-PBS for 60 min at room temperature. After further washing, blots were visualized with enhanced chemiluminescence (Pierce). Densitometric data performed with Scion Image for Windows software are expressed as mean intensities of protein bands, including the entire lane for O-GlcNAc levels.
Subcellular fractionation of heart tissue.
Nuclear and cytoplasmic fractions of cardiac tissue were prepared with a commercially available kit of nuclear and cytoplasmic protein extraction reagents according to the manufacturer's instructions (Pierce). Briefly, heart tissue was homogenized in cytoplasmic extraction reagent I (10% wt/vol) and then incubated on ice for 10 min, followed by the addition of cytoplasmic reagent II. After being vortexed, lysates were centrifuged at 16,000 g for 5 min to obtain the cytoplasmic fraction in the supernatant. The pellet was resuspended in nuclear extraction reagent, incubated on ice for 40 min, and centrifuged at 16,000 g for 10 min to obtain the nuclear fraction in the supernatant. Equal protein amounts of fractions were separated and immunoblotted as described above. To determine changes in OGT levels, rabbit polyclonal anti-OGT antibody (1:2,000; DM-17; Sigma) was used. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and TATA-binding protein antibodies (Abcam) were used as purity controls for the cytoplasmic and nuclear fractions, respectively.
For preparation of the membrane compartment, heart tissue was homogenized in ice-cold lysis buffer containing (in mM) 20 Tris (pH 7.4), 5.0 EDTA, 250 sucrose, 1.0 phenylmethanesulfonyl fluoride, and 2.5% protease inhibitor cocktail. Tissue homogenates (20% wt/vol) were centrifuged at 1,000 g for 10 min to remove nuclei and debris, and supernatant was ultracentrifuged at 110,000 g for 75 min at 4°C to pellet the crude membrane fraction (both the sarcolemmal and microsomal subfractions). The resulting pellet was resuspended in solubilization buffer [50 mM Tris (pH 7.4), 100 mM sodium chloride, 50 mM lithium chloride, 5 mM EDTA, 0.5% (vol/vol) Triton X-100, 0.5% (wt/vol) sodium deoxycholate, 0.05% (wt/vol) SDS, and 0.02% (wt/vol) sodium azide] using a glass homogenizer. After incubation for 30 min on ice, remaining insoluble material was collected by centrifugation (14,000 g, 10 min, 4°C). Equal protein amounts of the supernatant (particulate membrane fraction) were suspended in 2× Laemmli buffer (Bio-Rad), boiled for 5 min, and then immunoblotted and visualized as described above. Pan-cadherin and GAPDH antibodies (Abcam) were used to verify the purity of membrane and cytoplasmic fractions, respectively.
O-GlcNAc, desmin, and vinculin immunofluorescence.
At the end of the perfusion, hearts were perfusion-fixed with 4% paraformaldehyde, stored in 70% ethanol until paraffin embedding, and sectioned at 5 μm before being mounted on slides; deparaffinized in xylene; rehydrated in ethanol; and blocked with 5% goat serum in 1% bovine serum for 1 h at room temperature. Sections were incubated with primary antibodies against O-GlcNAc (1:50, CTD110.6), desmin (1:300; Abcam), and vinculin (1:100; Abcam) diluted in 5% goat serum in 1% bovine serum overnight at 4°C; appropriate secondary antibodies conjugated to either Alexa Fluor 488 (green) or 594 (red) (Invitrogen, Carlsbad, CA) were used to visualize the specific proteins with 4′,6-diamidino-2-phenylindole (DAPI) to identify nuclei (350 nm; blue). Image acquisition was performed on a Leica DM6000B epifluorescence microscope (Leica Microsystems, Bannockburn, IL) with a Hamamatsu ORCA ER cooled CCD camera and SimplePCI software (Compix, Cranberry Township, PA).
For the comparison of ischemic and I/R-induced changes in O-GlcNAc, desmin, and vinculin cellular distribution, an additional series of perfusions was carried out where hearts were subjected to 20 min ischemia without reperfusion, before perfusion fixation.
Data analysis.
All data are expressed as means ± SE. All of the examined variables showed normal distribution evaluated by Kolmogorov-Smirnov test. Statistical analyses were performed using two-way and one-way ANOVA followed by Bonferroni's multiple-comparison test, Pearson's correlations, χ2-test, and unpaired t-test for data as appropriate using Prism 4.0 (GraphPad Software, La Jolla, CA). Statistically significant differences between experimental groups were defined as P < 0.05.
RESULTS
Effects of OGA inhibitors NAG-Bt and NAG-Ae on recovery following I/R.
There were no significant differences in baseline cardiac function between any of the groups before ischemia (Table 1). Furthermore, in time-control (110 min) normoxic perfusions, there was no significant difference in RPP between untreated hearts and those treated with 50 μM NAG-Bt for the same duration as the ischemic hearts (normoxia: 19,621 ± 2,285; +NBt: 20,688 ± 1,311 mmHg/min), demonstrating that NAG-Bt alone has no effects on contractile function under normoxic conditions.
As shown in Fig. 1A, during reperfusion, NBt100 and NAe groups had significantly higher RPP values than the untreated control group. Two-way repeated measurements of ANOVA indicated an overall significant treatment effect with both NAG-Bt and NAG-Ae compounds on RPP. Increased recovery of RPP was apparent as early as 5 min after reperfusion in the NAe group, and this difference was maintained throughout reperfusion. Recoveries of LVDP and +dP/dt during reperfusion closely mirrored those seen with RPP [Supplemental Fig. 1 (Supplemental data for this article may be found on the American Journal of Physiology: Heart and Circulatory Physiology website.)]. At the end of reperfusion, RPP, LVDP, and ±dP/dt were all significantly higher in NBt and NAe groups compared with untreated controls; however, there were no significant differences in either coronary flow or heart rate between any of the groups (Fig. 1B). It is noteworthy that LVDP, ±dP/dt, and RPP were all significantly higher in the NAe group compared with both NBt50 and NBt100 groups.
In addition to improved contractile function, treatment with NAG-Bt and NAG-Ae significantly attenuated the appearance of arrhythmic activity during reperfusion. Ventricular premature beats could be observed in all hearts during the initial 10 min of reperfusion. Severe VT or VF (i.e., VT and/or VF) was seen in 86% of control untreated hearts, whereas the incidence of VT and/or VF was only 14% in hearts from the NBt50 group and were absent in both NBt100 and NAe groups (P < 0.05; χ2-test).
As shown in Fig. 2A, cTnI release during reperfusion was significantly lower in hearts from NBt100 and NAe groups compared with untreated control hearts, indicating reduced tissue injury in these two treatment groups. Similar to recovery of function, the level of cTnI release was significantly lower in the NAe group compared with both NBt50 and NBt100 groups. In the time-control normoxic perfusions, basal cTnI release remained very low and was unaffected by NAG-Bt (normoxia: 0.8 ± 0.1; +NBt: 0.8 ± 0.1 ng/ml).
ATP levels were significantly depressed in all ischemic groups at the end of reperfusion compared with the time-control normoxic perfusions; however, there were no differences in ATP levels between any of the I/R groups regardless of treatment (Fig. 2B). Thus the increased functional recovery and reduction in tissue injury is clearly not a consequence of improved bioenergetic status.
UDP-GlcNAc is the essential sugar donor for O-GlcNAc synthesis and is increased by activation of the HBP. Following I/R, UDP-HexNAc levels were significantly reduced compared with the normoxic group (Fig. 2C); however, there were no significant differences in UDP-HexNAc concentrations between any of the I/R groups regardless of treatment, demonstrating that inhibition of OGA during reperfusion had no upstream effects on precursors for O-GlcNAc synthesis.
Effect of NAG-Bt and NAG-Ae on cardiac O-GlcNAc levels following I/R.
In the untreated control I/R group, overall protein O-GlcNAc levels were ∼50% lower compared with the time-matched normoxic group (Fig. 3); however, in all treatment groups, protein O-GlcNAc levels were significantly higher compared with the untreated control I/R group (Fig. 3B). O-GlcNAc levels were also significantly higher in the NBt100 group compared with the NBt50 group, and the NAe group exhibited significantly higher O-GlcNAc levels compared with both NBt50 and NBt100 groups. (The original immunoblots used to derive the mean data shown in Fig. 3B are shown in Supplemental Fig. 2.) There were also significant correlations between overall O-GlcNAc levels and RPP (r2 = 0.82; P < 0.05; Fig. 4A), +dP/dt (r2 = 0.77; P < 0.05; Fig. 4B), and cTn (r2 = 0.65; P < 0.05; Fig. 4C). There was also a significant relationship between RPP and cTnI (r2 = 0.83; P < 0.05; Fig. 4D). Taken together, these data support the notion that preventing the loss of O-GlcNAc that occurs on reperfusion, by treatment with OGA inhibitors, contributes to the improved functional recovery and decreased tissue injury.
A number of studies have demonstrated overall changes in O-GlcNAc levels in response to I/R and other stresses in cardiomyocytes; however, there are no reports of the distribution of O-GlcNAc proteins in the heart or how this is affected by I/R. In Fig. 5A, we show O-GlcNAc distribution in the normoxic heart, at the end of ischemia and I/R, as well as the effect of NAG-Bt treatment. Consistent with previous reports in other cells/tissue (1, 9, 33), O-GlcNAc staining was particularly intense in cardiomyocyte nuclei. Surprisingly, we also observed that O-GlcNAc staining exhibited a clear cross-striated pattern through the cytoplasm consistent with Z-line structures in cardiomyocytes (Fig. 5A). The striated O-GlcNAc staining is consistent with the patterns seen with both desmin and vinculin, two cytoskeletal proteins associated with the Z-lines in cardiomyocytes (13, 18, 31), confirming localization of O-GlcNAc to the Z-line regions in the normoxic heart (Fig. 5, B and C). Vinculin is present at both Z-lines and intercalated disc; the lack of O-GlcNAc-modified proteins at the intercalated disc is clearly evident in Fig. 5C. Further confirmation of colocalization of O-GlcNAc staining with desmin and vinculin was obtained by analysis of changes in relative fluorescence intensity spatially across the cell; these data, included as Supplemental Fig. 3, clearly demonstrate coincidence of the peaks of O-GlcNAc staining with those for both desmin and vinculin, confirming the localization of O-GlcNAc-modified proteins at the Z-lines.
At the end of ischemia, the cross-striated arrangement of O-GlcNAc-modified proteins was maintained; however, ischemia-induced changes in nuclear O-GlcNAc distribution were very striking with increased punctate staining and the appearance of O-GlcNAc-negative nuclei with O-GlcNAc staining in the perinuclear region of cardiomyocytes (Figs. 5A and 6). O-GlcNAc-negative nuclei with perinuclear staining were not seen in normoxic hearts. After reperfusion, there was a loss of overall fluorescence in O-GlcNAc staining both in nuclear and cytoplasmic compartments, consistent with the lower O-GlcNAc levels seen in the immunoblots (Fig. 3A). There was also a loss of striated pattern after reperfusion (Figs. 5A and 6); however, the ischemia-induced punctate and perinuclear O-GlcNAc staining was still observed in reperfused hearts.
Administration of NAG-Bt during reperfusion resulted in augmented O-GlcNAc fluorescence compared with that seen in untreated, reperfused hearts (Fig. 5A); this is also consistent with the O-GlcNAc immunoblot data (Fig. 3). In the NAG-Bt-treated hearts, striated O-GlcNAc staining was preserved; however, the nuclear O-GlcNAc staining remained low, and punctate and perinuclear O-GlcNAc staining was still evident (Figs. 5A and 6). Thus, while NAG-Bt treatment appeared to maintain localization of O-GlcNAc to the Z-lines, it did not restore the loss of nuclear O-GlcNAc that occurred in response to ischemia alone.
The loss of nuclear O-GlcNAc levels was confirmed by immunoblot analyses on nuclear and cytosolic fractions from all experimental groups (Fig. 7A), indicating ∼50% loss of O-GlcNAc levels in both compartments at the end of reperfusion. Consistent with the immunohistochemistry, 50 μM NAG-Bt treatment had no significant effect on nuclear O-GlcNAc levels, although cytosolic O-GlcNAc levels were significantly increased. Higher concentrations of NAG-Bt and NAG-Ae increased both nuclear and cytosolic O-GlcNAc levels; however, their effects on nuclear O-GlcNAc levels were blunted compared with cytosolic levels. The decrease in O-GlcNAc levels with reperfusion was associated with a marked reduction in OGT (Fig. 7B) in both nuclear and cytoplasmic fractions; however, this was particularly evident in the cytosolic fraction. Interestingly, although treatment with OGA inhibitors attenuated the loss of OGT in the cytosolic fraction, there was no significant effect on nuclear OGT levels. In the immunoblots from the nuclear fraction as well as the cytosolic fraction from the normoxic group, OGT is apparent as a single band at ∼110 kDa; however, following I/R, intense OGT immunoreactive bands were observed between 150 and 250 kDa (Fig. 7B). Of note, following OGA inhibitor treatment, the intensity of these high-molecular-weight bands decreased in parallel with the increase in the 110-kDa band (Fig. 7, B and C). The absence of the high-molecular-weight OGT bands in the nuclear fraction and their decrease in the treatment group strongly suggest that these are high-molecular-weight OGT aggregates rather than nonspecific staining (see original immunoblots in Supplemental Fig. 4).
OGA inhibition attenuates I/R-induced changes of Z-line proteins.
We showed that, in response to I/R, there was a loss of overall intensity in desmin fluorescence, and disruption of the distinct Z-line pattern was apparent (Fig. 5B). In contrast, although there was evidence of overall structural disruption, vinculin localization and intensity remained relatively unchanged following I/R (Fig. 5C). However, OGA inhibition substantially attenuated the disruption of myocardial structure helping to maintain the striated pattern of both desmin and vinculin and maintaining colocalization with O-GlcNAc (Fig. 5, B and C). Immunoblot analyses of whole tissue lysates showed that I/R resulted in an approximately twofold loss of total desmin, which was significantly attenuated by OGA inhibition during reperfusion (Supplemental Fig. 5A). Consistent with the immunohistochemistry, there were no changes in overall vinculin levels in any of the groups; however, OGA inhibition blunted the I/R-induced increase in vinculin phosphorylation (Tyr822) (Supplemental Fig. 5, B and C). At the end of I/R, there was a marked loss (>3-fold) of overall O-GlcNAc in the membrane fraction and a more than twofold decrease in desmin, both of which were reversed by treatment with OGA inhibitors during reperfusion (Fig. 8). In the membrane fraction, there was also modest but significant loss of both OGT and vinculin in response to I/R; however, these changes were not reversed in the treatment groups. In contrast, phosphorylation of vinculin at Tyr822 was significantly decreased in the membrane fraction by treatment with OGA inhibitors during reperfusion (Fig. 8).
DISCUSSION
The posttranslational modification of proteins by O-GlcNAc is rapidly emerging as a new signaling mechanism that regulates diverse cellular functions, and in particular plays a critical role in modulating the responses of cells to pathophysiological stress conditions (3, 19, 44). In earlier studies, we have shown that acute increases in O-GlcNAcylation, either by the addition of glucosamine or inhibiting OGA with PUGNAc, increased tolerance of the perfused heart to I/R injury (11, 21, 22). However, glucosamine is effective only at high concentrations and thus could have effects independent of its increase in O-GlcNAcylation (26, 35), and PUGNAc can inhibit other lysosomal hexosaminidases, thereby affecting glycoconjugates in addition to O-GlcNAc (12). Thus, although these earlier studies provided strong support for the notion that acute augmentation of O-GlcNAc levels is cardioprotective, the lack of specificity and other off-target effects of these interventions may give rise to disparate physiological effects, thereby limiting their potential clinical applications. Recently Macauley and colleagues (25) developed new NAG-thiazoline derivatives, NAG-Bt and NAG-Ae, that have markedly higher selectivity for OGA than PUGNAc. Here we demonstrated for the first time that both of these OGA inhibitors exhibited significant cardioprotective effects as indicated by increased functional recovery, reduced tissue injury, and mechanical arrhythmic activity following I/R. Importantly, the cardioprotection associated with these inhibitors was evident even though they were administered only at the time of reperfusion; furthermore, improved functional recovery was evident as early as 5 min after the beginning of reperfusion in the NAe treatment group. The observation of significant correlations between O-GlcNAc levels, functional recovery, and tissue injury further supports the concept that inhibition of OGA at the time of reperfusion exerts its cardioprotective effect by increasing protein O-GlcNAc levels.
It was evident that, although OGA inhibition clearly attenuated tissue injury and improved functional recovery, it had no effect on tissue ATP levels. This is perhaps not surprising, since OGA inhibitors were administered at reperfusion and the majority of ATP depletion occurs during the ischemic period; furthermore, the majority of ATP breakdown products are lost on reperfusion, thereby preventing any substantial resynthesis of ATP. Thus, these data clearly indicate that the protection observed with OGA inhibitors cannot be attributed to an ATP-sparing effect or increased ATP salvage on reperfusion. A number of studies have also shown that modulation of glycogen levels influences the response of the heart to I/R (27). Furthermore, because glycogen synthase is a target for O-GlcNAc modification (30), in principle, the protection observed with the OGA inhibitors could be mediated via altered glycogen metabolism. However, most, if not all, studies investigating the effects of glycogen on the response to ischemia and I/R have focused on modulating glycogen levels before the ischemic event; consequently, since isolated hearts from ad libitum-fed rats were randomly assigned to experimental groups and interventions here were only during reperfusion after no-flow ischemia, it seems most unlikely that changes in glycogen metabolism could be the major contributor to the protection seen with OGA inhibitors.
To obtain a better insight into the effects of ischemia and I/R on O-GlcNAcylation, we used immunohistochemistry to examine tissue distribution of protein O-GlcNAcylation. Under normoxic conditions, there was a high level of O-GlcNAc staining in the nucleus, consistent with other cell systems (1, 9, 33). We also demonstrated, for the first time, a cross-striated pattern throughout the cytoplasm, consistent with O-GlcNAc enrichment of cardiomyocyte Z-line proteins (Figs. 5A and 6). Ischemia alone had relatively little effect on overall O-GlcNAc immunofluorescence, and the overall striated pattern remained unchanged; however, it led to the appearance of O-GlcNAc-negative nuclei and perinuclear O-GlcNAc staining (Figs. 5A and 6). Following I/R, there was a marked loss of overall O-GlcNAc immunofluorescence accompanied by an increased number of O-GlcNAc-negative nuclei and a loss of structural integrity. OGA inhibitor treatment increased overall O-GlcNAc staining and helped maintain structural integrity as well as the striated pattern of O-GlcNAc staining; however, nuclear O-GlcNAc levels remained depressed (Figs. 5A and 6). This differential response of subcellular O-GlcNAc location following I/R, which was modulated by OGA inhibition, was also confirmed by the immunoblot analyses (Figs. 3 and 7A). In both nuclear and cytosolic fractions, there was ∼50% loss of O-GlcNAc, and, although OGA inhibitors increased O-GlcNAc levels in both compartments, the relative increases in nuclear O-GlcNAc levels in response to treatment were consistently lower than the increase in cytosolic levels regardless of the treatment group, which may reflect the fact that OGA is predominantly localized in the cytosol (3).
Previous studies have demonstrated that, in multiple cell lines, increased protein O-GlcNAcylation represented an endogenous cellular stress response and that inhibition of this response decreased cell survival, whereas augmentation increased cellular stress tolerance (45). Ischemic preconditioning, which is a potent cardioprotective strategy, also increased cardiac O-GlcNAc levels (17). Thus, the observation here that there was a marked loss in protein O-GlcNAcylation following I/R in untreated hearts appears contrary to these earlier studies. We have previously reported that cardiac O-GlcNAc levels do indeed increase during ischemia (11) and during simulated I/R in cardiomyocytes there was a further increase in O-GlcNAc during early reperfusion (4). However, in both cardiomyocytes and whole heart models of I/R, there was a subsequent decrease in O-GlcNAc during reperfusion (4, 11). We have also observed similar phenomenon in vivo following hemorrhagic shock and resuscitation, where the loss of tissue O-GlcNAcylation was sustained for up to 24 h following resuscitation (28). The mechanisms underlying this loss of O-GlcNAc following I/R have not been previously identified; however, it is of note that Jones et al. (17) showed that treatment of cardiomyocytes with H2O2 resulted in a loss of O-GlcNAcylation, which was attenuated by OGA inhibition with PUGNAc. This suggests that oxidative stress, which is a key component of reperfusion injury, could be a contributing factor in the decrease in O-GlcNAc that occurs during reperfusion.
Here we found that the decrease in O-GlcNAc levels was associated with a significant decrease in OGT protein, which was particularly prominent in the cytosolic fraction (Fig. 7B); furthermore, this was accompanied by the appearance of high-molecular-weight OGT immunoreactive bands in the cytosolic fraction (Fig. 7C and Supplemental Fig. 4). Thus, I/R appears to result in covalent modifications to OGT, thereby decreasing the amount of the active, 110-kDa form of OGT and potentially contributing to the reduction in cytosolic O-GlcNAc levels. These high-molecular-weight bands could be inactive aggregates or multimers of OGT; however, the presence of a band around 140–150 kDa also suggests some form of posttranslational modification, such as polyubiquitination. Because the decrease in O-GlcNAc seems to be particularly evident under conditions such as reperfusion, resuscitation, as well as exposure to H2O2, this raises the possibility that the high-molecular-weight OGT-positive bands are a result of oxidative stress-induced damage to OGT. Clearly, further studies are warranted to characterize the nature of these high-molecular-weight OGT isoforms and to better understand the effect of I/R on OGT modifications, function, and activity.
Our novel finding of O-GlcNAc localization to the Z-line regions was confirmed by demonstrating its colocalization with desmin and vinculin, two proteins well known to be associated with the Z-lines (13, 18) (Fig. 5, B and C, and Supplemental Fig. 3). The Z-line region is frequently viewed as playing primarily a passive mechanical role in force transmission (13); however, given the numerous signaling-related proteins that are localized to this region, it is increasingly apparent that the Z-line is a critical component in regulating cardiomyocyte function by mediating the responses to hemodynamic and mechanical stresses (13, 31). Previous studies have shown a progressive loss of vinculin with ischemia (36, 41) and that this has been linked to increased cardiomyocyte injury and the onset of irreversible injury. Although we found no change in total tissue levels of vinculin, even in the untreated control I/R group (Supplemental Fig. 5), there was a significant decrease in membrane-associated vinculin (Fig. 8B). Interestingly, while OGA inhibition had little or no effect on total tissue levels of vinculin, there was a significant decrease in vinculin phosphorylation in the treated groups. Although vinculin is one of the best-characterized interacting proteins of the focal adhesion complex and is known to be activated by phosphatidylinositol 4,5-bisphosphate, its specific role remains poorly identified (8). The physiological consequences of vinculin phosphorylation in the heart are unclear; however, disruption of interacting proteins at the focal adhesion complex in cardiomyocytes has been shown to exacerbate ischemic injury (40). Thus, it is possible that the decrease in vinculin phosphorylation seen with OGA inhibitors could help to prevent disruption of the focal adhesion complex and attenuate the loss of structural integrity observed following I/R.
In contrast to vinculin, there was marked overall decrease in desmin immunofluoresence following I/R as well as an apparent reorganization into a longitudinal pattern and a loss of association with the Z-line (Fig. 5B). This was also apparent in immunoblot analysis of whole cell lysates (Supplemental Fig. 5) and particularly evident in the membrane fraction (Fig. 8C), both of which were largely attenuated by NAG-Bt and NAG-Ae treatment. Given the role of desmin in maintaining structural integrity and its involvement in linking extracellular mechanical stress to the nucleus (18, 31), the fact that increased O-GlcNAc levels attenuated the loss of desmin as well as prevented its structural disorganization could be an important contributing factor to the improved functional recovery and decreased injury. However, it remains to be determined whether this is a direct effect of O-GlcNAc modification of desmin or a secondary consequence of an overall increased O-GlcNAcylation.
A major goal of this study was to demonstrate the efficacy of OGA inhibition at the time of reperfusion as a cardioprotective strategy. We have previously demonstrated that augmenting O-GlcNAc levels with glucosamine or PUGNAc at reperfusion improved recovery and decreased tissue injury (11, 21, 22); the lack of specificity of both interventions raised questions as to whether the protection could be due, at least in part, to off-target effects. The demonstration here that new OGA inhibitors with greater specificity than PUGNAc were indeed cardioprotective, and that the degree of protection appears to be related both to the specificity of the inhibitor (i.e., NAG-Ae > NAG-Bt at equivalent concentrations) and the overall level of O-GlcNAcylation, strongly supports the notion that OGA inhibition has the potential to be a valuable approach for ameliorating I/R injury. In this study, we only examined the effectiveness of OGA inhibition during reperfusion. We felt this was important because new therapeutic agents, which are effective before ischemia, are often ineffective when used only during reperfusion (2, 23). Although we have previously reported that glucosamine is effective when present before and after ischemia as well as just at reperfusion (21, 22), we have not directly determined whether there is added benefit from pretreatment protocols. Consequently, further studies are needed to assess whether there would be additional benefit by inhibiting OGA before ischemia as well as during reperfusion; if so, this might indicate potential utility in the cardiac surgery setting where pretreatment protocols are practical. Nevertheless, there are clearly potential limitations in extrapolating to potential success in an in vivo environment, since we used an ex vivo, isovolumic perfused heart model in this study with glucose as the sole carbon substrate. However, we have previously reported that increasing O-GlcNAc levels with glucosamine was cardioprotective where glucose was the sole carbon substrate (21, 22) as well as where a more physiological substrate mixture was present (11). In addition, PUGNAc improved 24-h survival rates and decreased tissue injury in an in vivo rodent model of hemorrhagic shock and resuscitation (28).
More importantly, potential mechanisms of ischemic cardioprotection associated with increased O-GlcNAc levels have yet to be identified. It has been attributed to increased synthesis of heat shock protein (HSP) 40 and HSP70 (45) and increased p38 phosphorylation (11), which could lead to activation of prosurvival pathways through downstream effectors, such as αB-crystallin and HSP27. Increased levels of O-GlcNAc have also been reported to inhibit protein degradation, most likely because of inhibition of the proteasome (3, 44), which may also contribute to enhanced cell survival. It is noteworthy, however, that functional recovery was enhanced within the first 5–10 min of reperfusion in NBt and NAe groups (Fig. 1A). Because NAG-Bt and NAG-Ae were present only during reperfusion, these data suggest that the protection resulting from OGA inhibition is likely mediated via transcriptionally independent mechanisms. One such mechanism could be attenuation of calcium ion overload, which is a key contributing factor to early reperfusion injury (27, 42). This would be consistent with previous obervations that increased O-GlcNAc levels were associated with a decrease in calcium influx as a result of I/R in isolated cardiomyocytes (4) and attenuation of the I/R-induced activation calpain-mediated proteolysis with glucosamine or PUGNAc (21).
It should be also noted that much of our understanding of the role of O-GlcNAcylation in regulation of cell function is the context of chronic disease states, including cancer, neurodegenerative diseases, and diabetes (3, 7, 19). Indeed, studies by Clark et al. (6) and Hu and colleagues (14, 15) have linked increased O-GlcNAc levels to cardiomyocyte and cardiac dysfunction in diabetes (6, 14) and impaired cardiomyocyte mitochondrial function (15). Such adverse effects of increased O-GlcNAcylation would appear to be at odds with cardioprotective effects seen here and elsewhere. It is possible, however, that the beneficial effect of increased O-GlcNAcylation could be a consequence of short-term increases in O-GlcNAc levels, whereas the adverse effects are a result of chronic increases in O-GlcNAcylation. For example, a short-term increase in overall O-GlcNAcylation may result in different target proteins being modified compared with those that are O-GlcNAcylated in response to a sustained increase in flux through OGT. However, to date, there is no information regarding the temporal relationship between increased OGT flux and target protein specificity.
In conclusion, we have shown that administration of OGA inhibitors at the time of reperfusion significantly increased cardiac O-GlcNAc levels, improved functional recovery, and attenuated tissue injury following I/R compared with untreated hearts. The degree of functional recovery and the reduction of tissue injury were directly proportional to the effectiveness of these agents at increasing O-GlcNAc levels. Immunohistochemistry showed, for the first time, that cardiac proteins at the Z-line regions are highly enriched in O-GlcNAcylated proteins and that one consequence of ischemia and reperfusion is a redistribution of nuclear and cytoplasmic O-GlcNAcylation. Consistent with our earlier studies, O-GlcNAc levels were significantly lower in untreated control hearts following I/R than normoxic hearts (11), and this loss was prevented by OGA inhibition. We found that OGA inhibition significantly reduced disruption of structural integrity and attenuated the loss of desmin, a key Z-line protein that occurred following I/R, but did not prevent the loss of nuclear O-GlcNAcylation. However, given the large number of proteins subject to O-GlcNAcylation, it is unlikely that O-GlcNAc modification of a single protein could account for the observed cardioprotection. Future studies using new and emerging techniques for purification of O-GlcNAcylated proteins combined with “shot-gun” proteomics will be needed to elucidate the cardioprotective mechanism associated with OGA inhibition and increased O-GlcNAcylation.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-079364 HL-067464 to J. C. Chatham.
DISCLOSURES
John C. Chatham is co-inventor on pending US Patent Application 10/593,417 “Activators of Hexosamine Biosynthesis as Inhibitors of Injury Induced by Ischemia or Hemorrhagic Shock.”
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. David Vocadlo (Simon Fraser University, Burnaby, Canada) for providing the NAG-thiazoline OGA inhibitors; Mary Ann Accavitti (Epitope Recognition and Immunodetection Facility, University of Alabama at Birmingham) for the production of CTD 110.6 monoclonal antibody; Dr. László G. Nöt and Dr. Norbert Fülöp for methodological assistance; and Dr. Richard B. Marchase for continued enthusiasm and support for this work.
Current address for S. A. Marsh: Program in Nutrition and Exercise Physiology, Washington State University, Spokane, WA.
REFERENCES
- 1. Akimoto Y, Kawakami H, Yamamoto K, Munetomo E, Hida T, Hirano H. Elevated expression of O-GlcNAc-modified proteins and O-GlcNAc transferase in corneas of diabetic Goto-Kakizaki rats. Invest Ophthalmol Vis Sci 44: 3802–3809, 2003. [DOI] [PubMed] [Google Scholar]
- 2. Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res 95: 125–134, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Butkinaree C, Park K, Hart GW. O-linked beta-N-acetylglucosamine (O-GlcNAc): extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta 1800: 96–106, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol 292: C178–C187, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol 294: C1509–C1520, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte cycling through increased nuclear O-GlcNAcylation. J Biol Chem 278: 44230–44237, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am J Physiol Endocrinol Metab 295: E17–E28, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Critchley DR. Cytoskeletal proteins talin and vinculin in integrin-mediated adhesion. Biochem Soc Trans 32: 831–836, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Degrell P, Cseh J, Mohas M, Molnar GA, Pajor L, Chatham JC, Fulop N, Wittmann I. Evidence of O-linked N-acetylglucosamine in diabetic nephropathy. Life Sci 84: 389–393, 2009. [DOI] [PubMed] [Google Scholar]
- 10. Fulop N, Mason MM, Dutta K, Wang P, Davidoff AJ, Marchase RB, Chatham JC. The impact of type 2 diabetes and aging on cardiomyocyte function and O-Linked N-acetylglucosamine levels in the heart. Am J Physiol Cell Physiol 292: C1370–C1378, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Fulop N, Zhang Z, Marchase RB, Chatham JC. Glucosamine cardioprotection in perfused rat heart associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am J Physiol Heart Circ Physiol 292: H2227–H2236, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gloster TM, Vocadlo DJ. Mechanism, structure, and inhibition of O-GlcNAc processing enzymes. Curr Signal Transduct Ther 5: 74–91, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol 290: H1313–H1325, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res 96: 1006–1013, 2005. [DOI] [PubMed] [Google Scholar]
- 15. Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, Han W, Hu Y, Oyeleye MO, Dillmann WH. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J Biol Chem 284: 547–555, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huggins CE, Bell JR, Pepe S, Delbridge LM. Benchmarking ventricular arrhythmias in the mouse–revisiting the “Lambeth Conventions” 20 years on. Heart Lung Circ 17: 445–450, 2008. [DOI] [PubMed] [Google Scholar]
- 17. Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 117: 1172–1182, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Kostin S, Hein S, Arnon E, Scholz D, Schaper J. The cytoskeleton and related proteins in the human failing heart. Heart Fail Rev 5: 271–280, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Laczy B, Hill BG, Wang K, Paterson AJ, White CR, Xing D, Chen YF, Darley-Usmar V, Oparil S, Chatham JC. Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system. Am J Physiol Heart Circ Physiol 296: H13–H28, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol 42: 177–185, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu J, Marchase RB, Chatham JC. Increased O-GlcNAc levels during reperfusion lead to improved functional recovery and reduced calpain proteolysis. Am J Physiol Heart Circ Physiol 293: H1391–H1399, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol 40: 303–312, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Ludman AJ, Yellon DM, Hausenloy DJ. Cardiac preconditioning for ischaemia: lost in translation. Dis Model Mech 3: 35–38, 2010. [DOI] [PubMed] [Google Scholar]
- 24. Macauley MS, Bubb AK, Martinez-Fleites C, Davies GJ, Vocadlo DJ. Elevation of global O-GlcNAc levels in 3T3–L1 adipocytes by selective inhibition of O-GlcNAcase does not induce insulin resistance. J Biol Chem 283: 34687–34695, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ. O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J Biol Chem 280: 25313–25322, 2005. [DOI] [PubMed] [Google Scholar]
- 26. Masson E, Wiernsperger N, Lagarde M, El Bawab S. Glucosamine induces cell-cycle arrest and hypertrophy of mesangial cells: implication of gangliosides. Biochem J 388: 537–544, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88: 581–609, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Not LG, Brocks CA, Vamhidy L, Marchase RB, Chatham JC. Increased O-linked beta-N-acetylglucosamine levels on proteins improves survival, reduces inflammation and organ damage 24 hours after trauma-hemorrhage in rats. Crit Care Med 38: 562–571, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pang Y, Bounelis P, Chatham JC, Marchase RB. The hexosamine pathway is responsible for the inhibition by diabetes of phenylephrine-induced inotropy. Diabetes 53: 1074–1081, 2004. [DOI] [PubMed] [Google Scholar]
- 30. Parker GJ, Lund KC, Taylor RP, McClain DA. Insulin resistance of glycogen synthase mediated by o-linked N-acetylglucosamine. J Biol Chem 278: 10022–10027, 2003. [DOI] [PubMed] [Google Scholar]
- 31. Pyle WG, Solaro RJ. At the crossroads of myocardial signaling: the role of Z-discs in intracellular signaling and cardiac function. Circ Res 94: 296–305, 2004. [DOI] [PubMed] [Google Scholar]
- 32. Ravingerova T, Tribulova N, Slezak J, Curtis MJ. Brief, intermediate and prolonged ischemia in the isolated crystalloid perfused rat heart: relationship between susceptibility to arrhythmias and degree of ultrastructural injury. J Mol Cell Cardiol 27: 1937–1951, 1995. [DOI] [PubMed] [Google Scholar]
- 33. Rex-Mathes M, Werner S, Strutas D, Griffith LS, Viebahn C, Thelen K, Schmitz B. O-GlcNAc expression in developing and ageing mouse brain. Biochimie 83: 583–590, 2001. [DOI] [PubMed] [Google Scholar]
- 34. Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW. Perturbations in O-linked beta-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem 280: 32944–32956, 2005. [DOI] [PubMed] [Google Scholar]
- 35. Stanley P. A method to the madness of N-glycan complexity? Cell 129: 27–29, 2007. [DOI] [PubMed] [Google Scholar]
- 36. Steenbergen C, Hill ML, Jennings RB. Cytoskeletal damage during myocardial ischemia: changes in vinculin immunofluorescence staining during total in vitro ischemia in canine heart. Circ Res 60: 478–486, 1987. [DOI] [PubMed] [Google Scholar]
- 37. Theroux P. Myocardial cell protection: a challenging time for action and a challenging time for clinical research. Circulation 101: 2874–2876, 2000. [DOI] [PubMed] [Google Scholar]
- 38. Wang P, Fraser H, Lloyd SG, McVeigh JJ, Belardinelli L, Chatham JC. A comparison between ranolazine and CVT-4325, a novel inhibitor of fatty acid oxidation, on cardiac metabolism and left ventricular function in rat isolated perfused heart during ischemia and reperfusion. J Pharmacol Exp Ther 321: 213–220, 2007. [DOI] [PubMed] [Google Scholar]
- 39. Wang P, Lloyd SG, Chatham JC. The impact of high glucose/high insulin and dichloroacetate treatment on carbohydrate oxidation and functional recovery following low flow ischemia. Circulation 111: 2066–2072, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Wei H, Campbell W, Vander Heide RS. Heat shock-induced cardioprotection activates cytoskeletal-based cell survival pathways. Am J Physiol Heart Circ Physiol 291: H638–H647, 2006. [DOI] [PubMed] [Google Scholar]
- 41. Wei H, L'Ecuyer T, Vander Heide RS. Effect of increased expression of cytoskeletal protein vinculin on ischemia-reperfusion injury in ventricular myocytes. Am J Physiol Heart Circ Physiol 284: H911–H918, 2003. [DOI] [PubMed] [Google Scholar]
- 42. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007. [DOI] [PubMed] [Google Scholar]
- 43. Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ, Vocadlo DJ. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol 4: 483–490, 2008. [DOI] [PubMed] [Google Scholar]
- 44. Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta 1761: 599–617, 2006. [DOI] [PubMed] [Google Scholar]
- 45. Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 279: 30133–30142, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.