Abstract
Acute ascorbic acid (AA) administration increases muscle blood flow during dynamic exercise in older adults, and this is associated with improved endothelium-dependent vasodilation. We directly tested the hypothesis that increase in muscle blood flow during AA administration is mediated via endothelium-derived vasodilators nitric oxide (NO) and prostaglandins (PGs). In 14 healthy older adults (64 ± 3 yr), we measured forearm blood flow (FBF; Doppler ultrasound) during rhythmic handgrip exercise at 10% maximum voluntary contraction. After 5-min steady-state exercise with saline, AA was infused via brachial artery catheter for 10 min during continued exercise, and this increased FBF ∼25% from 132 ± 16 to 165 ± 20 ml/min (P < 0.05). AA was infused for the remainder of the study. Next, subjects performed a 15-min exercise bout in which AA + saline was infused for 5 min, followed by 5 min of the nitric oxide synthase (NOS) inhibitor NG-monomethyl-l-arginine (l-NMMA) and then 5 min of the cyclooxygenase inhibitor ketorolac (group 1). The order of inhibition was reversed in eight subjects (group 2). In group 1, independent NOS inhibition reduced steady-state FBF by ∼20% (P < 0.05), and subsequent PG inhibition had no impact on FBF (Δ 3 ± 5%). Similarly, in group 2, independent PG inhibition had little effect on FBF (Δ −4 ± 4%), whereas subsequent NO inhibition significantly decreased FBF by ∼20% (P < 0.05). In a subgroup of five subjects, we inhibited NO and PG synthesis before AA administration. In these subjects, there was a minimal nonsignificant improvement in FBF with AA infusion (Δ 7 ± 3%; P = nonsignificant vs. zero). Together, our data indicate that the increase in muscle blood flow during dynamic exercise with acute AA administration in older adults is mediated primarily via an increase in the bioavailability of NO derived from the NOS pathway.
Keywords: blood flow, aging, muscle contractions
the regulation of blood flow and oxygen delivery to contracting muscle is a complex response that involves mechanical factors, the sympathetic nervous system, and local metabolic and endothelium-derived substances that can influence vascular tone (28). In aging humans, an imbalance occurs in these regulatory mechanisms that typically results in impaired vasodilation and attenuated muscle blood flow during exercise (17, 19, 25, 26). Investigators have hypothesized that impaired endothelial vasodilator function contributes to the age-related impairment in muscle blood flow during exercise (8, 27). Recently, our laboratory demonstrated (17) that improving endothelium-dependent vasodilation via the potent antioxidant ascorbic acid also improved muscle blood flow during rhythmic handgrip exercise in older adults, providing the first direct experimental support for this hypothesis. Addressing the specific vasodilatory pathways by which ascorbic acid improves muscle blood flow was beyond the purpose of this initial study.
The contribution of various endothelium-derived vasodilators in the regulation of muscle blood flow during exercise has been studied by many investigators. Nitric oxide [NO; synthesized via nitric oxide synthase (NOS)] and vasodilating prostaglandins [PGs; synthesized via cyclooxygenase (COX)] are two endothelium-derived vasodilatory substances that contribute to the hyperemic response during dynamic knee-extensor exercise in young healthy humans (1, 14, 21, 22). In the forearm model, combined inhibition of NO and PG synthesis during exercise in young adults results in a significant decrease in forearm blood flow (FBF; ∼20%), primarily mediated through NOS inhibition (30). However, in healthy older individuals, the reduction in FBF during exercise due to NOS inhibition is significantly less than that in young adults, indicating an age-associated loss of NO-mediated hyperemia during exercise (29). These findings are consistent with observations of an age-related decline in NO bioavailability in response to the endothelium-dependent agonist acetylcholine (33), an idea also tested by utilizing intrabrachial infusion of a NOS inhibitor. Related to vasodilating PGs, COX inhibition during exercise in aging humans has essentially no impact on FBF (29), whereas in young adults inhibition of PG synthesis results in a transient ∼12% reduction in FBF during rhythmic handgrip exercise (30). Together, these data indicate that older adults demonstrate an attenuated contribution of endothelial vasodilatory pathways (e.g., NO and PGs) to exercise hyperemia compared with young healthy humans.
In older humans, ascorbic acid infusion has been shown to improve endothelial vasodilator function in response to both pharmacological (acetylcholine) and physiological (flow mediated) stimuli (9, 17, 33), whereas it has no effect in young healthy subjects (17, 32, 33). In the case of an acetylcholine stimulus, the improvement due to ascorbic acid was directly related to a restoration of NO bioavailability (33). In vitro experiments indicate that antioxidant treatment of endothelial cells can acutely increase vasodilatory PG production as well (37). To date, the exact mechanism by which ascorbic acid improves endothelial function and muscle blood flow during the metabolic stimulus of exercise in vivo, and whether this is through the NO and PG vasodilatory pathways, is currently unknown.
With this information as background, the purpose of the present investigation was to extend our original observations (17) and directly test the hypothesis that the ascorbic acid-mediated improvement in muscle blood flow during exercise in older humans is primarily via a NO and PG mechanism. To test this hypothesis, we measured forearm hemodynamics (Doppler ultrasound) during rhythmic forearm handgrip exercise before and during ascorbic acid infusion followed by independent and combined inhibition of NO and PG synthesis in healthy older humans. To investigate whether intact NOS and COX pathways are necessary to observe an improvement in exercise hyperemia with ascorbic acid, we first performed NO and PG blockade before ascorbic acid administration in a subgroup of older adults.
METHODS
Subjects
With Institutional Review Board approval and after written informed consent, a total of 17 healthy older adults participated in the present study. Two subjects participated in the study on two occasions, each time in a different experimental group. All subjects were sedentary to moderately active, nonobese, normotensive (resting blood pressure <140/90 mmHg) nonsmokers and were not taking any medications including antioxidants. Subjects were further evaluated for clinical evidence of cardiopulmonary disease with a physical examination and resting and graded exercise electrocardiograms. Studies were performed after a 4-h fast and 24-h abstention from caffeine and exercise, with subjects in the supine position and the experimental arm abducted to 90° and slightly elevated above heart level on a tilt-adjustable table (2). All studies were performed according to the Declaration of Helsinki.
Arterial Catheterization
A 20-gauge, 7.6-cm catheter was placed in the brachial artery of the nondominant arm under aseptic conditions after local anesthesia (2% lidocaine) for local administration of study drugs. The catheter was connected to a three-port connector as well as a pressure transducer for mean arterial pressure (MAP) measurement and continuously flushed at 3 ml/h with heparinized saline (6, 17). The two side ports were used for infusions of vasoactive drugs.
Blood Samples
Measurements of total cholesterol, low- and high-density lipoproteins (LDL and HDL), and triglycerides were performed via conventional methods by the clinical laboratory of the Poudre Valley Hospital (Fort Collins, CO).
Body Composition and Forearm Volume
Body composition was determined by dual-energy X-ray absorptiometry (DEXA; Hologic, Bedford, MA). Total forearm volume and fat-free mass (FFM) were calculated from regional analysis of the experimental forearm (from proximal to distal radioulnar joint) from whole body DEXA scans with QDR series software for normalization of the dose of ascorbic acid (17). Body mass index was calculated as body weight (kg) divided by height (m) squared.
Forearm Blood Flow and Vascular Conductance
A 12-MHz linear-array ultrasound probe (Vivid 7, General Electric, Milwaukee, WI) was used to determine brachial artery mean blood velocity (MBV) and brachial artery diameter ∼2–3 in. proximal to the catheter site. A holder for the probe was securely fixed to the skin over the brachial artery to ensure that measurements were being taken in the same anatomic position for each trial. For blood velocity measurements, the probe insonation angle was maintained at <60° and the frequency used was 5 MHz. The Doppler shift frequency spectrum was analyzed via a Multigon 500V TCD (Multigon Industries, Mt. Vernon, NY) spectral analyzer from which mean velocity was determined as a weighted mean of the spectrum of Doppler shift frequencies. Brachial artery diameter measurements were made in triplicate in duplex mode at end diastole and between contractions during steady-state conditions. FBF was calculated as
where FBF is in milliliters per minute, MBV is in centimeters per second, the brachial artery diameter is in centimeters, and 60 was used to convert from milliliters per second to milliliters per minute. Forearm vascular conductance (FVC) was calculated as (FBF/MAP) × 100 and expressed as milliliters per minute per 100 mmHg.
Rhythmic Handgrip Exercise
Maximal voluntary contraction (MVC) was determined for the experimental arm as the average of three maximal squeezes of a handgrip dynamometer (Stoelting, Chicago, IL) that were within 3% of each other. Forearm exercise during the trials was performed with weight corresponding to 10% MVC attached to a pulley system and lifted 4–5 cm over the pulley at a duty cycle of 1 s of contraction-2 s of relaxation (20 contractions/min) with the use of both visual and auditory feedback to ensure the correct timing as described previously (6). We chose this mild-intensity rhythmic handgrip exercise to limit the contribution of systemic hemodynamics to forearm hyperemic responses and eliminate reflex activation of the sympathetic nervous system (2, 31). Additionally, this workload and mode of exercise have been shown to be tolerated well by older subjects over a prolonged time course and do not evoke progressive increases in heart rate and arterial pressure (17). Thus our experimental model aimed to isolate the local effects of muscle contractions on forearm hyperemia without engaging potentially confounding systemic influences on vascular tone. Finally, our lab has demonstrated (17) that ascorbic acid acutely improves muscle blood flow during this type of exercise in a similar population of older humans.
Vasoactive Drug Administration
As a method of acutely improving exercise hyperemia, the potent antioxidant ascorbic acid (vitamin C, American Regent, Shirley, NY) was infused at 8 mg·100 ml forearm volume−1·min−1 for 10 min during handgrip exercise as a loading dose and at 40% of this loading dose for maintenance infusion throughout the remainder of the experiment (17).
To determine the role of NO in the improvement in exercise hyperemia via ascorbic acid, the NOS inhibitor NG-monomethyl-l-arginine (l-NMMA; Clinalfa/Bachem, Weil am Rhein, Germany) was administered intra-arterially to inhibit the production of NO. A loading dose totaling 25 mg (5 mg/min for 5 min) was given during rhythmic handgrip, and a maintenance dose (1.0 mg/min) was infused for the duration of the study to ensure continuous blockade. This dose of l-NMMA has been shown previously to significantly reduce basal vascular tone and also the vasodilatory effects of acetylcholine (5, 6), consistent with effective NOS inhibition (35). Furthermore, our dose of l-NMMA is within the range of incremental doses that were previously shown to lower venous plasma nitrite concentrations at rest and inhibit NO release in response to intrabrachial acetylcholine infusion, both of which were associated with corresponding reductions in FBF (18).
The contribution of PGs to the ascorbic acid-mediated improvement in muscle blood flow during handgrip was determined by performing regional COX inhibition. Ketorolac (trade name Toradol; Hospira, Lake Forest, IL), a nonselective COX inhibitor, was administered intra-arterially to inhibit the synthesis of PGs. A loading dose totaling 6 mg (1.2 mg/min for 5 min) and a maintenance dose (240 μg/min) were infused for the duration of the study to ensure continuous blockade. This dose of ketorolac is twice that which was previously demonstrated to reduce circulating PGF1α (a stable breakdown product of PGs) at rest and during handgrip exercise (7) as well as reduce FBF during handgrip exercise in humans (30).
Experimental Protocols
Protocol 1.
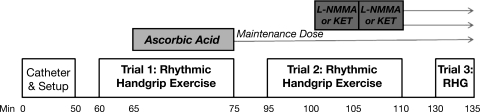
A timeline of the experimental study is depicted in Fig. 1. Two minutes of resting data was acquired before the start of all exercise trials. To test the effect of ascorbic acid on exercise hyperemia (trial 1), subjects performed rhythmic forearm exercise at 10% MVC with saline infusion for the first 5 min of exercise, followed by 10 min of ascorbic acid infusion during continued exercise. The total exercise duration was 15 min. Upon completion of exercise, the ascorbic acid dose was reduced and infused continuously at 40% of the original dose for the remainder of the experiment. After 20 min of rest, trial 2 was performed. The maintenance dose of the ascorbic acid was infused throughout the entire trial. After 5 min of exercise, one of the two inhibitors, l-NMMA or ketorolac, was administered at the previously stated doses for 5 min. The specific inhibitor that was not infused first was then given over the last 5 min of exercise. Again, the total exercise duration was 15 min. The order of inhibitors was randomized across subjects. Upon completion of trial 2, the doses of l-NMMA and ketorolac were reduced to their respective maintenance doses and infused for the remainder of the experiment. Another 20 min of rest was allowed before trial 3 was performed. This trial consisted of 5 min of rhythmic handgrip exercise that was performed with concomitant infusion of ascorbic acid, l-NMMA, and ketorolac, all at maintenance dose rates. Trial 3 was completed in order to assess the effects of ascorbic acid and combined NOS and COX inhibition on the hemodynamic changes from rest to steady-state exercise.
Fig. 1.
Experimental timeline. After placement of the brachial artery catheter and general setup, subjects performed 15 min of rhythmic handgrip exercise (RHG) at 10% maximal voluntary contraction (MVC). Noted as trial 1, exercise was performed for 5 min with saline infusion to achieve “steady-state” hemodynamics. Ascorbic acid was administered concurrently throughout the next 10 min of exercise. The dose of ascorbic acid was then reduced to 40% of the original dose and infused for the remainder of the experiment. After 20 min of rest, trial 2 was performed, which consisted of another 15-min bout of exercise. After 5 min, either NG-monomethyl-l-arginine (l-NMMA) or ketorolac (Ket) was infused for 5 min. The other drug was infused for the last 5 min of exercise. Both l-NMMA and Ket were reduced to maintenance doses after trial 2. Subjects again rested for 20 min before trial 3, which consisted of 5 min of exercise. See text for further details.
Protocol 2.
To test whether intact NO and PG vasodilating pathways are requisite to observe a significant improvement in hyperemia due to ascorbic acid infusion as well as to confirm that NO and PGs play a minor role in exercise hyperemia under control (without ascorbic acid) conditions (29), in five additional subjects we reversed trials 1 and 2 of protocol 1. Specifically, NOS and COX inhibition was performed during the first exercise trial, and ascorbic acid was administered during the second exercise trial. Trial 3 was a similar 5-min exercise bout with maintenance doses of ascorbic acid, l-NMMA, and ketorolac being infused throughout.
Protocol 3.
Time-control experiments were performed in four subjects who returned to the laboratory on a separate occasion to study the effects of repeated exercise without pharmacological infusion. These subjects performed three trials of rhythmic handgrip exercise at 10% MVC, each 20 min apart. The first two exercise trials lasted 15 min each, and data were analyzed for ∼30 s at rest and at minutes 5, 10, and 15 of exercise. The third exercise trial was 5 min in duration, and data were analyzed at rest and end of exercise. In these subjects, MAP was measured noninvasively over the brachial artery of the control arm (Cardiocap/5; Datex-Ohmeda, Louisville, CO) at rest and on a beat-to-beat basis during exercise via finger photoplethysmography (Finapres; FMS, Amsterdam, The Netherlands) on the middle finger of the control hand (2).
Data Acquisition and Analysis
Data were collected and stored on a computer at 250 Hz and were analyzed off-line with signal-processing software (WinDaq, DATAQ Instruments, Akron, OH). MAP was determined from the arterial pressure waveform. FBF, heart rate, and MAP represent an average of the last 30 s of steady-state conditions (e.g., baseline rest, steady-state exercise, ascorbic acid exercise). To quantify the impact of vasoactive drugs on FBF and take into account individual differences in hemodynamics, the percent increase in FBF due to ascorbic acid infusion was calculated as
Similarly, the percent change in FBF due to vasodilator inhibition was calculated as
Changes in FVC were calculated in a similar manner.
Statistics
Data are presented as means ± SE. Differences within and between trials and conditions for each group were determined via repeated-measures analysis of variance (ANOVA). Post hoc analysis was performed by the Student-Newman-Keuls method when significance was observed. Specific hypothesis testing related to differences between experimental groups was analyzed with independent two-tailed Student's t-tests. Significance was set a priori at P < 0.05.
RESULTS
Subject Characteristics
Subject characteristics for the groups in protocols 1 and 2 are presented in Table 1. There were no significant differences in subject characteristics between the experimental groups. In protocol 1, six subjects received l-NMMA followed by ketorolac during the second exercise trial (group 1). Eight subjects received ketorolac followed by l-NMMA (group 2).
Table 1.
Subject characteristics and baseline hemodynamics
|
Protocol 1: Drug Order |
|||
|---|---|---|---|
| Variable | l-NMMA + Ket | Ket + l-NMMA | Protocol 2: Combined Inhibition Before AA |
| Male:female | 3:3 | 4:4 | 2:3 |
| Age, yr | 66 ± 3 | 62 ± 2 | 64 ± 2 |
| Body mass index, kg/m2 | 24.3 ± 0.5 | 26.0 ± 1.0 | 23.3 ± 0.7 |
| Body fat, % | 24.5 ± 2.0 | 31.0 ± 2.3 | 28.9 ± 4.2 |
| Forearm FFM, g | 740 ± 87 | 746 ± 105 | 737 ± 105 |
| Forearm volume, ml | 933 ± 84 | 951 ± 92 | 782 ± 92 |
| MVC, kg | 38 ± 4 | 36 ± 4 | 32 ± 4 |
| 10% MVC, kg | 3.8 ± 0.4 | 3.6 ± 0.4 | 3.2 ± 0.4 |
| Total cholesterol, mmol/l | 4.1 ± 0.1 | 4.6 ± 0.2 | 4.7 ± 0.3 |
| LDL cholesterol, mmol/ | 2.4 ± 0.2 | 2.8 ± 0.2 | 3.1 ± 0.3 |
| HDL cholesterol, mmol/l | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 |
| Triglycerides, mmol/l | 1.0 ± 0.2 | 1.2 ± 0.3 | 0.9 ± 0.1 |
| Mean arterial pressure, mmHg | 93 ± 5 | 93 ± 4 | 91 ± 4 |
| HR, beats/min | 60 ± 3 | 63 ± 3 | 54 ± 3 |
| Forearm blood flow, ml/min | 33 ± 6 | 43 ± 6 | 36 ± 8 |
| Forearm VC, ml•min−1•100 mmHg−1 | 36 ± 7 | 36 ± 7 | 40 ± 8 |
Data are presented as means ± SE. l-NMMA, NG-monomethyl-l-arginine; Ket, ketorolac; AA ascorbic acid; FFM, fat-free mass; MVC, maximal voluntary contraction; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HR, heart rate; VC, vascular conductance.
Protocol 1: Effect of Ascorbic Acid on Forearm Hemodynamics During Rhythmic Handgrip Exercise (Trial 1)
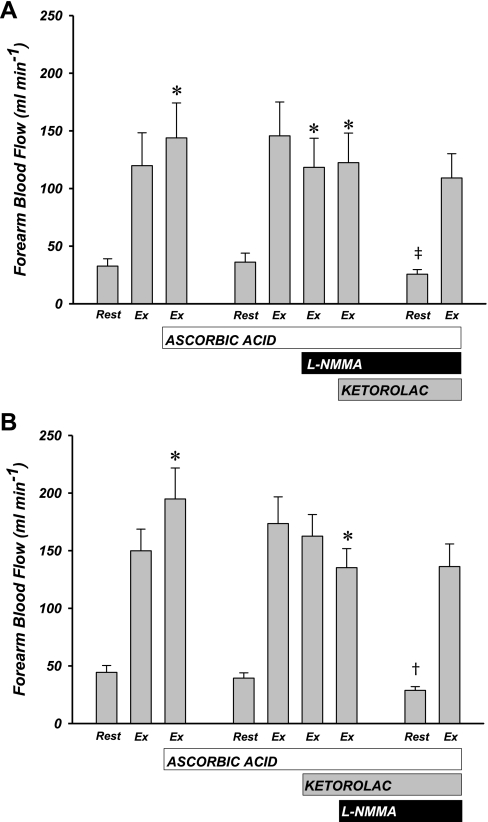
Consistent with previous findings from our laboratory (17), ascorbic acid infusion significantly improved muscle blood flow during rhythmic handgrip exercise (group 1: 120 ± 29 vs. 144 ± 30 ml/min, group 2: 150 ± 19 vs. 195 ± 27 ml/min; for both P < 0.05; Fig. 2). Data were similar when analyzed as FVC (Table 2). When both experimental groups in this protocol were combined, the mean improvement in FBF due to ascorbic acid infusion was ∼25% (137 ± 16 vs. 173 ± 21 ml/min; P < 0.05; see Fig. 4). Rhythmic forearm exercise significantly increased MAP from rest; however, ascorbic acid had no impact on MAP (Table 2), and thus the increase in FBF was due to local vasodilation.
Fig. 2.
Forearm blood flow (FBF) responses to ascorbic acid infusion and local inhibition of nitric oxide (NO) and prostaglandins (PGs). FBF is presented during rest and steady-state exercise (Ex). A: subjects in whom NO (l-NMMA) was inhibited before PG (ketorolac). B: subjects in whom PG inhibition occurred before NO inhibition. Respective drug infusions occurred as indicated. Ascorbic acid significantly increased FBF in both groups. Independent NO inhibition did cause a significant decrease in FBF, and subsequent PG inhibition had no further effect (A). Independent PG inhibition had no effect on FBF and combined PG-NO inhibition significantly attenuated FBF (A). *P < 0.05 vs. within-trial steady-state exercise condition. †P < 0.05 vs. previous trial resting condition. ‡P = 0.09 vs. previous trial resting condition.
Table 2.
Systemic hemodynamics and forearm vascular conductance across all experimental conditions in protocol 1
|
l-NMMA + Ket |
Ket + l-NMMA |
|||||
|---|---|---|---|---|---|---|
| HR, beats/min | MAP, mmHg | FVC, ml•min−1•100 mmHg−1 | HR, beats/min | MAP, mmHg | FVC, ml•min−1•100 mmHg−1 | |
| Trial 1 | ||||||
| Rest | 60 ± 3 | 93 ± 5 | 36 ± 7 | 62 ± 2 | 96 ± 5 | 45 ± 5 |
| SS Ex | 64 ± 3* | 99 ± 4* | 124 ± 31* | 65 ± 3 | 101 ± 5 | 150 ± 21* |
| SS Ex + AA | 62 ± 3 | 99 ± 4* | 148 ± 31*† | 65 ± 3 | 101 ± 5 | 196 ± 31*† |
| Trial 2 | ||||||
| Rest | 58 ± 3 | 96 ± 5 | 39 ± 8 | 57 ± 2‡ | 97 ± 5 | 40 ± 4 |
| SS Ex (AA) | 61 ± 3 | 98 ± 5 | 153 ± 32* | 62 ± 2* | 100 ± 5 | 175 ± 25* |
| SS Ex + single inhibition | 59 ± 3 | 103 ± 6* | 121 ± 29*† | 62 ± 2* | 104 ± 5* | 157 ± 19* |
| SS Ex + combined inhibition | 59 ± 3 | 104 ± 4*† | 122 ± 26*† | 62 ± 2* | 105 ± 6* | 131 ± 18*† |
| Trial 3 | ||||||
| Rest | 56 ± 3 | 94 ± 5 | 28 ± 5‡ | 56 ± 1 | 104 ± 5‡ | 27 ± 2‡ |
| SS Ex (AA + l-NMMA + Ket) | 59 ± 3 | 98 ± 5 | 115 ± 24* | 60 ± 2* | 106 ± 6 | 131 ± 21* |
Data are presented as means ± SE. MAP, mean arterial pressure; FVC, forearm vascular conductance; SS, steady state; Ex, exercise;
P < 0.05 vs. rest;
P < 0.05 vs. within-trial SS Ex;
P < 0.05 rest vs. previous trial resting condition
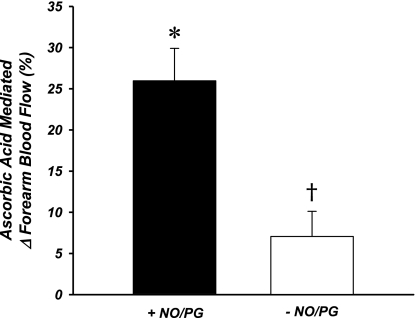
Fig. 4.
Improvement in exercise hyperemia due to ascorbic acid infusion. When ascorbic acid was infused with NO and PG synthesis pathways intact (+NO/PG), FBF improved ∼25%. When NO and PG synthesis were inhibited before ascorbic acid (protocol 2 subgroup; −NO/PG), the improvement due to ascorbic acid was abolished. *P < 0.05 vs. zero. †P < 0.05 vs. +NO/PG.
Protocol 1: Effect of NO and PG Inhibition on Forearm Hemodynamics During Rhythmic Handgrip Exercise with Prior Ascorbic Acid (Trial 2)
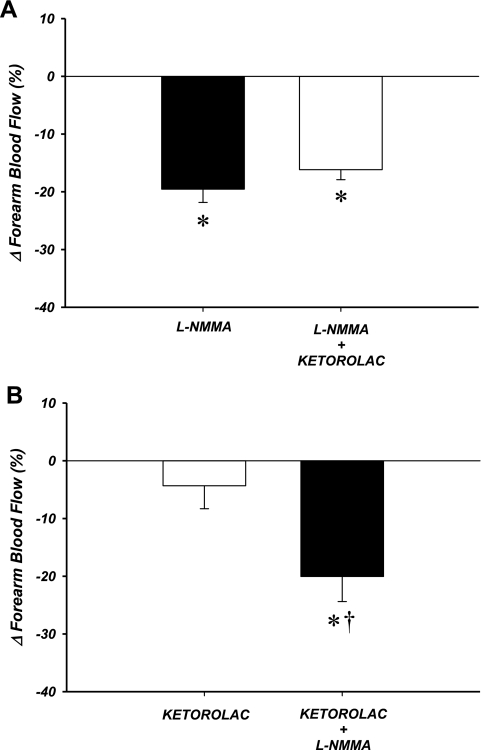
Similar to our previous findings (17), resting blood flow was unaffected by ascorbic acid infusion in all experimental subjects in this protocol (39 ± 4 vs. 38 ± 4 ml/min; P = 0.46). When NO synthesis was inhibited independently (via l-NMMA) during exercise, there was a significant decrease in FBF (146 ± 29 vs. 118 ± 25 ml/min; P < 0.05; Figs. 2A and 3A). The addition of PG inhibition (via ketorolac) had no further effect on FBF (122 ± 26 ml/min; P = 0.17). The overall decrease in exercise hyperemia due to combined inhibition of NO and PGs was ∼20% (Fig. 3A). When PG synthesis was inhibited independently during exercise there was no effect on FBF (174 ± 23 vs. 163 ± 19; P = 0.21; Figs. 2B and 3B). Subsequent inhibition of NO synthesis resulted in a significant reduction in FBF (135 ± 16 ml/min; P < 0.05). In this experimental group, similar to those subjects that received l-NMMA first followed by ketorolac, the combined effect of PG and NO inhibition was a ∼20% reduction in FBF (Fig. 3B). Minor increases in MAP were observed in this trial (Table 2). When the data were analyzed as FVC, all significant changes were similar to those observed for FBF (Table 2).
Fig. 3.
Relative change in FBF after ascorbic acid infusion due to local inhibition of NO and/or PG. Independent NO inhibition decreased FBF ∼20% (A). In contrast, there was no significant change in exercise hyperemia with independent PG inhibition (B). Combined inhibition of NO and PG attenuated FBF ∼20% in both groups. *P < 0.05 vs. zero. †P < 0.05 vs. single-inhibition condition.
Protocol 1: Hemodynamic Response from Rest to Steady-State Rhythmic Handgrip Exercise with Prior Ascorbic Acid and Combined NO and PG Inhibition (Trial 3)
Under the condition of maintenance doses of ascorbic acid, l-NMMA, and ketorolac, resting blood flow was reduced compared with baseline blood flow during trial 1 and trial 2 (27 ± 2 ml/min; P < 0.001 vs. trial 1, P < 0.001 vs. trial 2). Handgrip exercise significantly increased FBF to levels similar to those achieved before any drug infusions in trial 1 [group 1: 109 ± 21 ml/min; group 2: 136 ± 20 ml/min; P = not significant (NS); Fig. 2]. FVC after 5 min of steady-state exercise was also similar to predrug conditions (Table 2).
Protocol 2: Inhibition of NO and PG Synthesis Before Ascorbic Acid Infusion
A subgroup of subjects were studied to determine the effect of ascorbic acid infusion with prior blockade of NOS and COX, as well as the contribution of NO and PGs to exercise hyperemia without prior ascorbic acid infusion. In these subjects, there was no independent effect of l-NMMA infusion (n = 3; 128 ± 27 vs. 133 ± 25 ml/min) or ketorolac infusion (n = 2; 132 ± 24 vs. 133 ± 36 ml/min). Furthermore, there was no significant effect of combined NO and PG inhibition on exercise hyperemia (n = 5; 130 ± 19 vs. 130 ± 16 ml/min; P = NS). Resting FBF was significantly reduced after inhibition of NO and PGs (36 ± 8 vs. 28 ± 6 ml/min; P < 0.05). Combined blockade of NO and PG also had no effect on the ability of these subjects to attain similar levels of hyperemia from rest to steady-state exercise (124 ± 14 ml/min). Subsequent infusion of ascorbic acid during this exercise trial did not significantly impact FBF (133 ± 14 ml/min; P = NS), and the percent increase in FBF from steady-state exercise (∼7%) was significantly lower than in protocol 1 (∼25%; Fig. 4). Resting blood flow before the third exercise trial was not different from that during trial 2 (28 ± 5 ml/min; P = NS) but was still lower than during trial 1 (P < 0.05). Additionally, in the third exercise trial when all drugs were being infused at maintenance doses, FBF was similar to that achieved before any drug infusions (128 ± 17 ml/min), consistent with our observations in the first protocol. Data were similar when analyzed as FVC (data not shown).
Protocol 3: Time Controls
A subgroup of subjects (n = 4) performed three exercise trials with no drug infusion. Average MVC in these subjects was 36 ± 6 kg, and average exercise workload was 3.6 ± 0.6 kg. Resting blood flow did not vary across the three trials (trial 1: 54 ± 11, trial 2: 53 ± 9, trial 3: 54 ± 9 ml/min; P = 0.82). Exercising FBF was not different at minutes 5, 10, and 15 of trial 1 (156 ± 29, 156 ± 31, and 158 ± 29 ml/min, respectively) or at minutes 5, 10, or 15 of trial 2 (155 ± 28, 158 ± 28, and 158 ± 30 ml/min, respectively). In the final exercise trial that lasted 5 min, exercising FBF was also similar to that the previous trials (155 ± 26 ml/min; P = NS within and between trials). Small increases in pressure (Δ 5 ± 2 mmHg) were observed with exercise; thus the data were similar when expressed as FVC (data not shown). These data indicate steady forearm hemodynamics over the three experimental trials, and thus changes in FBF documented in the invasive protocols were due to vasoactive drug infusions.
DISCUSSION
In the present study, we determined whether the improvement in exercise hyperemia in older humans that occurs with local ascorbic acid infusion is through NO and/or PG pathways. The primary novel findings of the present study are as follows. First, inhibition of NO synthesis abolished the observed improvement in FBF due to local infusion of ascorbic acid. In contrast, inhibition of PG synthesis did not significantly affect muscle blood flow during exercise after local ascorbic acid infusion. Second, when the endothelial vasodilating pathways of NO and PG synthesis were inhibited before ascorbic acid infusion, no significant improvement in FBF was observed. Together, our data indicate that the primary mechanism by which ascorbic acid improves muscle blood flow during exercise in older adults is through an increase in the bioavailability of NO derived from the NOS pathway.
Aging and Exercise Hyperemia: Ascorbic Acid, NO, and PGs
In the present study, we infused ascorbic acid into the brachial artery during rhythmic handgrip exercise and observed a significant improvement in muscle blood flow (∼25%) similar to our previous experimental findings (17). When we inhibited the NOS-mediated synthesis of the vasodilator NO, blood flow was significantly reduced from the previous ascorbic acid-improved level and was no longer greater than the pre-ascorbic acid condition. The addition of PG inhibition had no effect on FBF. Our finding of a significant role for NO as well as the lack of a role for PGs in the mechanism of ascorbic acid improvement was also evident when the order of inhibition was reversed. Further evidence of an obligatory role for NOS-derived NO in the ascorbic acid-mediated improvement in exercise hyperemia was obtained in protocol 2. When NO and PG synthesis were inhibited prior to ascorbic acid infusion, no significant increase in FBF was observed during exercise.
Under normal conditions (without prior ascorbic acid infusion), the contribution of NO and PGs to exercise hyperemia in older adults is substantially impaired compared with that in young healthy humans (29, 30). In the present study, we confirmed a minimal role of these endothelium-derived dilators during exercise hyperemia in our own subgroup of older subjects (see Protocol 2 results). In fact, our data demonstrate no effect of NOS inhibition on FBF during exercise, whereas Schrage and colleagues (29) reported a significant reduction. However, given our small subject group (n = 3) in this secondary protocol, we recommend some caution in the interpretation of our findings. The reason for our slight discrepancy from the data of Schrage et al. is somewhat unclear but may be due to differences in study design, NOS inhibitor used [l-NMMA vs. NG-nitro-l-arginine methyl ester (l-NAME)], and subject populations. Despite this difference, it is important to note that in the present study acute infusion of ascorbic acid restored a significant contribution of NO (Fig. 3) to exercise hyperemia in aged humans at a level (∼20%) similar to that previously observed in young subjects (30). Furthermore, we would like to reemphasize that the subgroup we studied was mainly intended to determine the effects of ascorbic acid infusion after NOS and COX inhibition, and the findings were consistent with the main experimental protocol, indicating that the NOS pathway is mechanistically linked with the improvement in exercise hyperemia via ascorbic acid in older adults.
In addition to exercise, aging humans demonstrate a diminished endothelium-mediated component of vasodilation to other stimuli. Taddei and colleagues have shown an impaired NO-mediated component of vasodilation to the endothelial agonist acetylcholine in older humans (33) and, importantly, that intrabrachial infusion of ascorbic acid can restore this NO-mediated component of vasodilation to acetylcholine in sedentary older adults (32). Furthermore, COX inhibition during acetylcholine infusion increases vasodilation in older healthy humans, suggesting a possible shift toward vasoconstricting PGs (e.g., thromboxane) (34). Collectively, these data and the present experimental findings are consistent with the existing evidence indicating that the contribution of endothelium-derived dilators to vascular control is impaired in aging humans to a variety of stimuli.
Attenuated responsiveness to acetylcholine classically defines “endothelial dysfunction.” While we did not directly test endothelial function in this sense in the present study protocol, we can likely reason that this population, which is similar in all characteristics to older adults previously studied by our group (16, 17), would demonstrate some level of endothelial dysfunction. As we have established a strong link between endothelial function and altered vascular control during forearm exercise, it is reasonable to hypothesize that the mechanisms for improvement in endothelial function via ascorbic acid may be similar to the mechanisms for improvement in exercise blood flow via ascorbic acid. The results from the present study confirm this hypothesis. Through local infusion of ascorbic acid, we were able to partially restore a significant contribution of NO to the hyperemic response to exercise not normally present in this population of older subjects.
We observed little impact of COX inhibition in the present study both in the experimental group in which inhibition occurred after ascorbic acid and in the control group in which we confirmed the normal contribution of PGs to exercise hyperemia. Given that the effect of COX inhibition was similar both in the presence and the absence of ascorbic acid, there is little reason to believe that the ascorbic acid-mediated improvements in muscle blood flow during exercise are via the COX-initiated PG pathway. Recent evidence suggests that ∼30–40% of exogenous PG (prostacyclin)-mediated vasodilation is NO dependent and older adults exhibit blunted vasodilation to prostacyclin due to a loss of the NO component (23). Potential interactions of the NO and PG pathways in the regulation of vascular function are of interest, especially in aging humans; however, our data from the present study do not appear to provide much evidence for any direct interactions between these two vasodilating pathways during exercise or in response to ascorbic acid infusion in older humans.
Potential Mechanisms
As stated above, our data indicate that the improvement in exercise hyperemia by ascorbic acid is mediated via increased NO bioavailability. Given that we essentially eliminated the improvement in exercise hyperemia due to ascorbic acid with inhibition of NOS (via l-NMMA), it appears that NO bioavailability is being modulated primarily through the NOS enzymatic pathway as opposed to any other source of NO that may contribute to vasodilation [e.g., S-nitroso-hemoglobin, nitrite reduction (15)]. The pharmacological inhibitor of NOS that we utilized in this study is a nonspecific NOS inhibitor, and, as such, we are unable to make any conclusion related to the specific contributions of the various isoforms of NOS (endothelial, neuronal, or inducible) to the responses we observed. A general consensus is that l-NMMA is more specific for endothelial NOS (eNOS) rather than neuronal or inducible NOS (11). Thus we conservatively propose that eNOS is the isoform likely mediating the changes we observed with infusion of l-NMMA, but recognize we have little direct evidence to support this claim. Future studies will be needed to fully address this issue.
The biochemical basis for ascorbic acid improving NO bioavailability via NOS in humans in vivo is not entirely clear, but several studies have attempted to address this issue. It is possible that ascorbic acid, at the supraphysiological dose we administered, may directly scavenge free radicals [e.g., superoxide (O2−)] (13, 24), which would preserve NO bioavailability by decreasing the uncoupling of NOS. One specific mechanism by which this may occur is through stabilization of tetrahydrobiopterin (BH4), an important cofactor for NOS (12). In this context, increased BH4 concentrations significantly improved endothelium-dependent vasodilation in older animals and humans (4, 10). Whether increased BH4 concentrations may also improve vascular control during exercise in older or diseased humans who demonstrate endothelial dysfunction has yet to be studied.
Experimental Considerations
Some experimental considerations exist for the present study. First, we did not directly test the efficacy of our pharmacological inhibitors in the present study. Related to our findings that PG inhibition had little effect in any condition, it could be argued we did not completely inhibit the COX enzymes. However, we administered doses of drugs similar to those in previous studies (see methods) and maintenance doses of drugs were infused throughout the protocol, and thus we are confident that we achieved successful inhibition. On the basis of our findings that l-NMMA infusion 1) significantly attenuated the improvement in exercise hyperemia due to ascorbic acid infusion, 2) abolished any increase in exercise hyperemia with ascorbic acid infusion when in combination with PG inhibition, and 3) significantly reduced FBF at rest in all blockade conditions, we are confident that we successfully caused nonselective inhibition of the NOS enzyme and NO synthesis. Furthermore, had we not achieved full inhibition of this vasodilator pathway, our results would underestimate the contribution of NO to the improvement.
Second, we did not study a young group of subjects in the present study, as we have previously demonstrated that ascorbic acid infusion has no effect on exercise hyperemia in young subjects (17). However, previous studies indicate that there are significant impairments in the NO and PG pathways in older compared with young healthy humans during exercise (29, 30). These collective observations provided the rationale for pursuing the role these endothelium-derived dilators might have in the observed improvement in exercise hyperemia with ascorbic acid infusion we previously reported in older adults only.
Third, our exercise modality was of mild-to-moderate intensity with a small muscle mass (forearm). We chose this approach because of our previous findings that ascorbic acid can improve muscle blood flow during this stimulus that elicits little confounding systemic nervous system activation (17). During higher-intensity exercise or larger-muscle mass exercise (e.g., cycling), whether ascorbic acid can improve muscle blood flow and, if so, whether the underlying mechanism is similar has yet to be determined.
Perspectives and Conclusions
Our finding that the mechanism by which ascorbic acid improves muscle blood flow during exercise is primarily NO mediated (via NOS) is important and relevant to our comprehensive understanding of vasoregulation in older adults. The improvement due to ascorbic acid is important, as oxygen delivery is thereby increased to the exercising tissue. Given that an increase in NOS-derived NO bioavailability appears to be mediating this response, the vasoprotective characteristics of NO beyond its vasodilatory capacity are likely also increased. NO is known to inhibit smooth muscle cell proliferation, leukocyte adhesion, and platelet aggregation and adhesion (3). Thus, in addition to acute improvements in muscle blood flow and therefore oxygen delivery during exercise, the synthesis of NO is also being acutely improved, which may lead to additional cardioprotective effects of this intervention.
The local dose of ascorbic acid we administered intra-arterially is supraphysiological and was chosen on the basis of our previous findings and that of others that this dose successfully restores endothelial vasodilator function in older humans (17, 32, 33) as well as acutely improving muscle blood flow during rhythmic handgrip exercise (17). Interestingly, an acute oral dose (2 g) of ascorbic acid has been shown to cause increases in plasma ascorbate levels that peak 2 h after administration and improve endothelial function in patients with coronary artery disease (20). Furthermore, an oral ascorbic acid-containing antioxidant cocktail improved calf muscle perfusion at the end of plantar-flexion exercise in older healthy humans (36). Whether or not we could achieve the same improvements in exercise hyperemia with an oral dose of ascorbic acid is an interesting question with potential clinical applications that we plan to pursue in our laboratory in the future.
The results from the present investigation demonstrate that the primary mechanism by which ascorbic acid infusion can improve muscle blood flow during exercise in aging humans is via increased NO bioavailability through NOS. We present little evidence for a significant role of PG synthesis mediating the response to ascorbic acid. Future research will need to focus on what oral dosing strategy of ascorbic acid or another antioxidant can produce NOS-mediated improvements in exercise hyperemia similar to those we observed with our supraphysiological dose.
GRANTS
This research was supported by National Institutes of Health Grants AG-022337, AG-027150, and HL-087952 (F. A. Dinenno).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the subjects who volunteered to participate in this study as well as Julia A. Davis for her administrative assistance.
REFERENCES
- 1.Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol 543: 691–698, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson RE, Kirby BS, Voyles WF, Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol 294: H1963–H1970, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Cooke JP, Dzau VJ. Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med 48: 489–509, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol 586: 1161–1168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 553: 281–292, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287: H2576–H2584, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Drexler H, Hornig B. Importance of endothelial function in chronic heart failure. J Cardiovasc Pharmacol 27, Suppl 2: S9–S12, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frandsen U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with NG-nitro-l-arginine methyl ester in humans. J Physiol 531: 257–264, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. l-Ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem 276: 40–47, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Jackson TS, Xu A, Vita JA, Keaney JF., Jr Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res 83: 916–922, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Kalliokoski KK, Langberg H, Ryberg AK, Scheede-Bergdahl C, Doessing S, Kjaer A, Kjaer M, Boushel R. Nitric oxide and prostaglandins influence local skeletal muscle blood flow during exercise in humans: coupling between local substrate uptake and blood flow. Am J Physiol Regul Integr Comp Physiol 291: R803–R809, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol 26: 697–705, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kirby BS, Voyles WF, Crecelius AR, Dinenno FA. ATP-mediated vasodilation is not reduced in aging humans. FASEB J 23: 777–774, 2009 [Google Scholar]
- 17.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA 98: 12814–12819, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF, Jr, Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 93: 1107–1113, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 53: 993–999, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension 53: 973–978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishikimi M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun 63: 463–468, 1975 [DOI] [PubMed] [Google Scholar]
- 25.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol 95: 1963–1970, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation 13: 315–327, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162: 421–436, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc Sport Sci Rev 19: 313–349, 1991 [PubMed] [Google Scholar]
- 32.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension 29: 736–743, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 2: 997–1000, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil SS, Carlier PG, Richardson RS. Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am J Physiol Heart Circ Physiol 297: H1870–H1875, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu D, Liu L, Meydani M, Meydani SN. Effect of vitamin E on prostacyclin (PGI2) and prostaglandin (PG) E2 production by human aorta endothelial cells: mechanism of action. Ann NY Acad Sci 1031: 425–427, 2004 [DOI] [PubMed] [Google Scholar]