Abstract
We investigated the tolerance of the insulin-resistant diabetic heart to ischemic injury in the male Goto-Kakizaki (GK) rat, a model of type 2 diabetes. Changes in energy metabolism, nitric oxide (NO) pathway, and cardiac function were assessed in the presence of physiological substrates. Age-matched control Wistar (n = 19) and GK (n = 18) isolated rat hearts were perfused with 0.4 mM palmitate, 3% albumin, 11 mM glucose, 3 U/l insulin, 0.2 mM pyruvate, and 0.8 mM lactate for 24 min before switching to 1.2 mM palmitate (11 rats/group) during 32 min low-flow (0.5 ml·min−1·g wet wt−1) ischemia. Next, flow was restored with 0.4 mM palmitate buffer for 32 min. A subset of hearts from each group (n = 8 for control and n = 7 for GK groups) were freeze-clamped for determining baseline values after the initial perfusion of 24 min. ATP, phosphocreatine (PCr), and intracellular pH (pHi) were followed using 31P magnetic resonance spectroscopy with simultaneous measurement of contractile function. The NO pathway was determined by nitric oxide synthase (NOS) isoform expression and total nitrate concentration (NOx) in hearts. We found that coronary flow was 26% lower (P < 0.05) during baseline conditions and 61% lower (P < 0.05) during reperfusion in GK vs. control rat hearts. Rate pressure product was lower during reperfusion in GK vs. control rat hearts (P < 0.05). ATP, PCr, and pHi during ischemia-reperfusion were similar in both groups. Endothelial NOS expression was increased in GK rat hearts during baseline conditions (P < 0.05). NOx was increased during baseline conditions (P < 0.05) and after reperfusion (P < 0.05) in GK rat hearts. We report increased susceptibility of type 2 diabetic GK rat heart to ischemic injury that is not associated with impaired energy metabolism. Reduced coronary flow, upregulation of eNOS expression, and increased total NOx levels confirm NO pathway modifications in this model, presumably related to increased oxidative stress. Modifications in the NO pathway may play a major role in ischemia-reperfusion injury of the type 2 diabetic GK rat heart.
Keywords: type 2 diabetes, cardiac ischemia-reperfusion, Goto-Kakizaki rats, nitric oxide, energy metabolism, endothelial nitric oxide synthase
cardiovascular disease is a long-term complication of type 2 diabetes mellitus, with a twofold increased risk of heart failure and greater mortality after myocardial infarction than nondiabetic patients (64). Clinical studies and experimental animal models have shown that diabetes mellitus is associated with a specific cardiomyopathy independent of hypertension, coronary artery disease, or hyperlipidemia. The underlying mechanisms of diabetic cardiomyopathy remain incompletely understood. Vascular complications play a vital and crucial role in the morbidity and mortality of patients with diabetes mellitus. Nitric oxide (NO), generated from l-arginine by NO synthase (NOS), is an important endogenous vasodilator and has been involved in the regulation of blood flow in diabetes (65). Impaired NO bioactivity is a pathogenic factor in various forms of cardiac disease, but little is known of the complexities of the biological actions of NO on the vasculature and heart in type 2 diabetes. Also, data from various animal models of insulin resistance with or without obesity reveal differing susceptibility of the type 2 diabetic heart to ischemia depending on the model, the severity of the diabetic state, the degree of ischemia (low flow vs. no flow), and the substrates (1, 14, 34, 43). This inconsistency is particularly striking in light of the clear clinical evidence that, following myocardial infarction, the outcome for diabetic patients is substantially worse than for nondiabetic patients (35).
Type 2 diabetes mellitus is a complex multifactorial genetic syndrome that is determined by several different genes and environmental factors. Identification of the genes responsible for the development of the disease is difficult, and >60 potential genes may be involved in its susceptibility (4). Consequently, polygenic animal models of type 2 diabetes have proven invaluable for candidate gene identification and for the development of the most effective treatments. Six independent genetic loci are responsible for defects in glucose and insulin metabolism in the Goto-Kakizaki (GK) rat, a highly inbred strain derived from outbred, glucose-intolerant Wistar rats that spontaneously develop type 2 diabetes within the first few weeks of age (6). The GK rat is one of the best-characterized animal models of spontaneous type 2 diabetes mellitus (33, 55, 56). GK rats exhibit mild basal hyperglycemia, hyperinsulinemia, hepatic and peripheral insulin resistance, and evidence of vascular complications but are not obese (6, 15, 56). We have previously demonstrated that the heart of 10-mo-old male GK rats is insulin resistant (24). Significantly, this model allows one to study the effect of diabetes on the heart without other complications such as obesity.
Here, we aim to define the cardiovascular alterations associated with insulin resistance of the GK rat heart in the presence of physiological substrates. We investigated the tolerance to ischemia-reperfusion injury of male control and GK rat hearts perfused with a physiological substrate mixture containing lactate and pyruvate as well as glucose and palmitate. This physiological substrate mixture was chosen because, although it is known that the metabolism of the diabetic heart is typically characterized by reduced glucose use and increased fatty acid oxidation, the effect of fatty acids on experimental models may be different when glucose and palmitate are not the only substrates, and the potential importance of lactate and pyruvate on the relationship between glycolysis and glucose oxidation has been underlined (12). Furthermore, our objective was specifically to investigate energy metabolism and the NO pathway in parallel to cardiac function in GK rat hearts. Experiments were conducted in aging GK and age-matched control rat hearts, since insulin resistance of the GK rat hearts was shown in 10-mo-old GK rats with a decreased insulin-stimulated glucose uptake and an impaired insulin signaling pathway (24). High-energy phosphates (HEP) and intracellular pH (pHi) were measured during the experimental time course by 31P magnetic resonance spectroscopy. Rate pressure product was used as an index of myocardial performance. Myocardial tissue content of creatine kinase was also used as a marker of myocardial damage. The expression of the three NOS isoforms and total nitrate concentration (NOx) were determined in freeze-clamped hearts as markers of the NO pathway.
MATERIALS AND METHODS
Animals.
Age-matched male control Wistar (n = 19) and Goto-Kakizaki [GK/Par subline (55)] (n = 18) (9–14 mo, weight 396–628 g) rats were used in the experiments. All procedures involving animals were approved by the Institutional Ethic Committee for animal research of the Medical School La Timone of Marseille. All animals received humane care in compliance with the Principle of Laboratory Animal Care formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 1996). All investigations in this project were conducted under a license for animal research granted by the French Ministry of Agriculture. Animals were fed ad libitum with a commercial pelleted chow (diet 113; SAFE, Augy, France).
Heart perfusion and experimental protocol.
Rats were anesthetized by intraperitoneal injection of 35 mg/kg pentobarbital sodium. After removal of the heart, blood samples were taken from the chest cavity and immediately centrifuged, and the supernatant was kept on ice for determination of plasma glucose and free fatty acids (FFAs). Hearts were cannulated and perfused in the Langendorff mode at constant pressure as described previously (22). Left ventricular developed pressure and heart rate were monitored as previously reported (19). The rate pressure product (product of left ventricular developed pressure and heart rate) was used as an index of cardiac function. Coronary flow was measured by time collection of the coronary venous effluent.
For establishing preischemic baseline values, control (n = 8) and GK (n = 7) rat hearts were perfused with a physiological recirculating Krebs-Henseleit buffer containing 0.4 mM palmitate, 3% albumin, 11 mM glucose, 3 U/l insulin, 0.8 mM lactate, and 0.2 mM pyruvate for 24 min.
For ischemia-reperfusion experiments, control (n = 11) and GK (n = 11) rat hearts were perfused with 250 ml of a physiological recirculating Krebs-Henseleit buffer containing 0.4 mM palmitate, 3% albumin, 11 mM glucose, 3 U/l insulin, 0.8 mM lactate, and 0.2 mM pyruvate for 24 min. The perfusion buffer was changed 4 min before low-flow ischemia to 150 ml of fresh, nonrecirculating Krebs-Henseleit buffer containing 1.2 mM palmitate, 3% albumin, 11 mM glucose, 3 U/l insulin, 0.8 mM lactate, and 0.2 mM pyruvate. Hearts were subjected to 32 min of low-flow ischemia (0.5 ml·min−1·g wet wt−1) in the same buffer. A higher concentration of palmitate (1.2 mM) was provided during ischemia because it causes maximal damage during ischemia and reperfusion and is a relevant concentration during a myocardial infarction, which is what we are modeling with a low-flow ischemic model (48, 54). Next, regular flow was restored with 0.4 mM palmitate buffer for 32 min.
31P magnetic resonance spectroscopy.
Perfused rat hearts were placed in a 20-mm magnetic resonance sample tube and inserted in a 31P probe that was seated in the bore of a superconducting wide-bore (89-mm) 4.7-Tesla magnet (Oxford Instruments, Oxford, UK) interfaced with a Bruker Avance WB 200 spectrometer (Bruker, Karlsruhe, Germany). The appropriate conditions for acquiring 31P magnetic resonance spectra have been detailed previously (5, 19). The quantification of phosphorus metabolites and the determination of pHi were described previously (19).
Collection of data.
Heart function and 31P magnetic resonance spectra were monitored before ischemia, during low-flow ischemia, and during reperfusion. Blood samples were collected immediately after excising the heart. For biochemical analyses, hearts were rapidly freeze-clamped with a Wollenberger clamp precooled in liquid nitrogen either after preischemic baseline conditions (n = 8 for control group and n = 7 for GK group) or after reperfusion (n = 11 for control group and n = 11 for GK group) and kept at −80°C before analysis.
Biochemical analyses in plasma.
Plasma glucose was measured by using an assay kit (Randox Laboratories, Crumlin, Antrim, UK). Plasma FFAs were determined using a NEFA kit (Roche Diagnostics, Roche Applied Science, Mannheim, Germany).
Biochemical analyses in freeze-clamped hearts.
For determination of adenine nucleotides and malondialdehyde (MDA) contents by ion-exchange high-performance liquid chromatography, perchloric acid extraction of freeze-clamped cardiac tissue was adapted from Lazzarino et al. (44) as described previously (22). Separation of adenine nucleotides and MDA was performed by the modified ion-pairing reverse-phase technique of Lazzarino et al. (44) at, respectively, 254 and 268 nm. Qualitative and quantitative analysis was carried out using adenine nucleotide standards and thymidine monophosphate (Sigma, Poole, Dorset, UK) as internal standard. MDA was used to evaluate lipid peroxidation and oxidative stress (45). Myocardial creatine kinase content was measured as previously described (21). Endothelial (e), neuronal (n), and inducible (i) nitric oxide synthase (NOS) isoform expression was determined by Western blot in portions of whole hearts as previously described (19). A control rat heart homogenate was run as an internal standard for quantification in each assay. Tissue NOx was determined according to the method described by Cross et al. (17) using a nitrate/nitrite colorimetric assay kit (Cayman Chemical, Ann Arbor, MI).
Expression of results and statistical analyses.
Data are expressed as means ± SE. Function and 31P magnetic resonance spectroscopy data are presented as absolute values. Significant differences between groups were determined using two-way ANOVA with repeated measures over time for the time-dependent variables (function and 31P magnetic resonance spectroscopy data) followed by Bonferroni post hoc test. For biochemical data, the effects of time and group were analyzed with two-way ANOVA followed by Bonferroni post hoc test. Unpaired Student's t-test was used for other parameters. A P value ≤0.05 was considered significant.
RESULTS
Physiological characteristics of experimental animals.
Plasma glucose was 78% higher in GK vs. control rats (P < 0.05, Table 1). Plasma FFAs were similar in both groups (Table 1). GK rats weighed significantly less than their age-matched controls (P < 0.05), and, although heart wet weights were the same, heart-to-body weight ratio was 36% higher in GK compared with control rats (P < 0.05, Table 1).
Table 1.
Physiological parameters of control and GK rats
| Control | GK | |
|---|---|---|
| Glucose, mM | 10.3 ± 0.5 | 17.0 ± 1.0* |
| Free fatty acids, mM | 0.2 ± 0.0 | 0.3 ± 0.0 |
| Body wt, g | 628 ± 15 | 396 ± 11* |
| Heart wt, g | 2.0 ± 0.1 | 1.8 ± 0.1 |
| Heart-to-body wt ratio (×1,000) | 3.1 ± 0.1 | 4.5 ± 0.1* |
Values are means ± SE. GK, Goto-Kakizaki.
P < 0.05 vs. control.
Myocardial function and coronary flow.
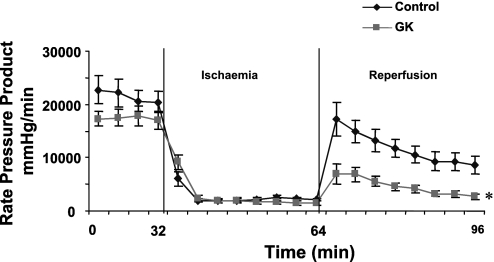
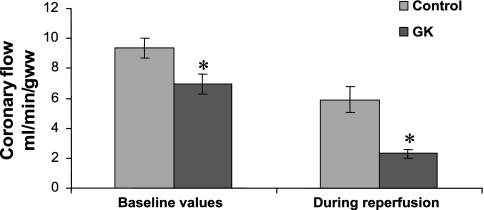
Functional parameters, rate pressure product, and coronary flow rates are shown, respectively, in Table 2 and Figs. 1 and 2. Analysis of baseline myocardial function as assessed by the rate pressure product in control and GK rats indicated no significant differences in preischemic function (Fig. 1). The rate pressure product was significantly lower during reperfusion in GK compared with control rat hearts (P < 0.05, Fig. 1), with a 69% lower postischemic functional recovery in GK rat hearts (P < 0.05) due to a lower heart rate during reperfusion in GK vs. control rat hearts (P < 0.05, Table 2). Developed pressure was not different between groups during the experimental time course (Table 2). End diastolic pressure was the same during the experimental time course in both groups (Table 2). Coronary flow rates were 26% lower (P < 0.05) during preischemic baseline conditions and 61% lower (P < 0.05) during reperfusion in GK vs. control rat hearts (Fig. 2). In addition, compared with baseline values, the percentage of reduction of postischemic coronary flow rates was higher in GK (67 ± 4%) than in control (37 ± 8%, P < 0.05) rat hearts.
Table 2.
Functional parameters of control and GK rat hearts
| Control |
GK |
|||||
|---|---|---|---|---|---|---|
| DP, mmHg | HR, beats/min | EDP, mmHg | DP, mmHg | HR, beats/min | EDP, mmHg | |
| Before ischaemia | 76 ± 7 | 267 ± 14 | 8 ± 1 | 63 ± 6 | 271 ± 8 | 10 ± 1 |
| End ischaemia | 17 ± 3 | 154 ± 17 | 4 ± 1 | 15 ± 4 | 84 ± 17 | 6 ± 1 |
| End reperfusion | 38 ± 7 | 246 ± 24 | 3 ± 1 | 24 ± 4 | 116 ± 14* | 2 ± 1 |
Values are means ± SE. DP, developed pressure; HR, heart rate; EDP, end-diastolic pressure.
P < 0.05 vs. control.
Fig. 1.
Rate pressure product during the experimental time course in control and Goto-Kakizaki (GK) rat hearts. *P < 0.05 vs. control. Results are expressed as means ± SE in mmHg/min.
Fig. 2.
Coronary flow rates during preischemic baseline conditions and during reperfusion in control and GK rat hearts. Results are expressed as means ± SE in ml·min−1·g wet wt−1. *P < 0.05 vs. control.
Measurements of myocardial energy metabolism and pHi.
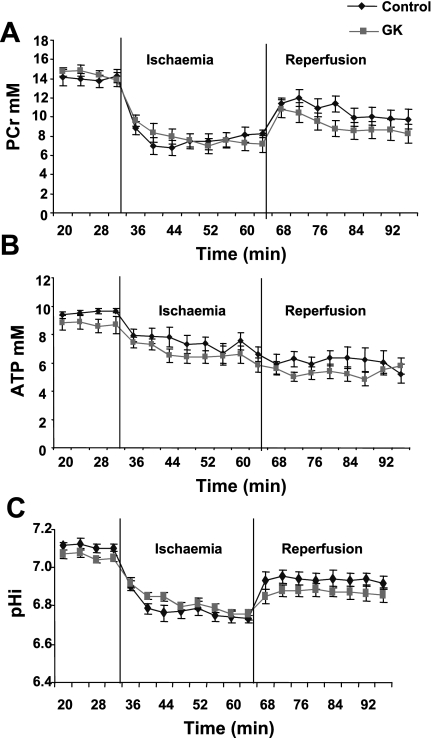
Kinetics of PCr (A), ATP (B), and pHi (C) as measured by 31P magnetic resonance spectroscopy during the experimental time course are shown in Fig. 3.
Fig. 3.
Kinetics of phosphocreatine (PCr) (A), ATP (B), and intracellular pH (pHi) (C) during the experimental time course in control and GK rat hearts. Results are expressed in mM except pHi and are means ± SE.
Preischemic values for PCr and ATP were similar in all hearts. No difference in PCr decrease during ischemia was observed in control and GK rat hearts. During reperfusion, PCr recovery was similar in both groups. The loss of ATP was similar during low-flow ischemia, and no difference in ATP content was observed during reperfusion in all hearts.
No significant difference was found in total adenine nucleotides (TAN, ATP + ADP + AMP, expressed as μmol/g protein) content between control and GK rat hearts during preischemic baseline conditions (38.3 ± 1.3 and 43.3 ± 2.8, respectively) and after reperfusion (27.2 ± 1.1 and 26.1 ± 2.0, respectively). Ischemia-reperfusion involved a significant decrease in TAN content after reperfusion compared with baseline values in control (27.2 ± 1.1 vs. 38.3 ± 1.3, P < 0.05) and GK (26.1 ± 2.0 vs. 43.3 ± 2.8, P < 0.05) rat hearts. In addition, TAN loss after reperfusion was not significantly different between the two groups.
The pHi before ischemia was the same in all hearts, and the decrease in pHi was similar during ischemia in control and GK rat hearts. During reperfusion, pHi recovered to the initial value in both groups without any significant difference.
NOx concentration.
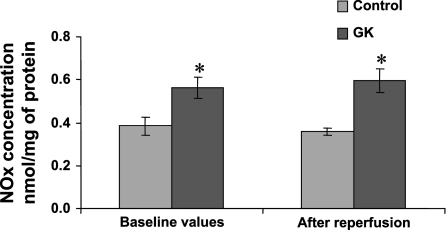
Tissue NOx during preischemic baseline conditions and after reperfusion in control and GK rat hearts is given in Fig. 4. Determination of NOx was used to reflect in vivo NO production as previously reported (20, 32, 70, 71). A good correlation has been found between this measure and the determination of NOS activity in vitro by measuring the conversion of l-arginine to l-citrulline (71). NOx concentration was increased by 32% during preischemic baseline conditions and 40% after reperfusion in GK compared with control rat hearts (P < 0.05 and P < 0.05, respectively).
Fig. 4.
Total nitrate concentration (NOx) in freeze-clamped hearts during preischemic baseline conditions and after reperfusion in control and GK rat hearts. Results are expressed as means ± SE in nmol/mg protein. *P < 0.05 vs. control.
NOS isoform expression.
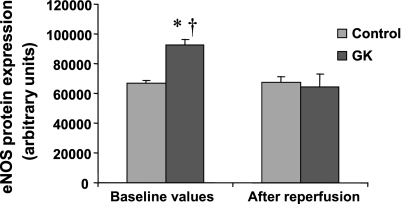
eNOS and nNOS protein expression during preischemic baseline conditions and after reperfusion in control and GK rat hearts are shown, respectively, in Figs. 5 and 6. eNOS expression was significantly higher in GK compared with control rat hearts during preischemic baseline conditions (P < 0.05), but no significant difference was found after reperfusion between the two groups. nNOS protein expression was not significantly different during preischemic baseline conditions or after reperfusion in control and GK rat hearts. eNOS and nNOS proteins expression did not change during ischemia-reperfusion compared with preischemic baseline conditions in control rat hearts. Ischemia-reperfusion decreased eNOS protein expression compared with preischemic baseline conditions in GK rat hearts (P < 0.05) without any change in nNOS protein content. iNOS protein expression was not detected in any condition despite maximal loading and high iNOS antibody concentration.
Fig. 5.
Endothelial nitric oxide synthase (eNOS) expression in freeze-clamped hearts during preischemic baseline conditions and after reperfusion in control and GK rat hearts. Equal protein loading was checked using Ponceau red staining. The bar graphs show results from six control and six GK rat hearts. P < 0.05 vs. control (*) and vs. after reperfusion (†). Results are expressed as means ± SE in arbitrary units.
Fig. 6.
Neuronal nitric oxide synthase (nNOS) expression in freeze-clamped hearts during preischemic baseline conditions and after reperfusion in control and GK rat hearts. Equal protein loading was checked using Ponceau red staining. The bar graphs show results from six control and six GK rat hearts. Results are expressed as means ± SE in arbitrary units.
Malondialdehyde.
MDA was used to evaluate lipid peroxidation and consequently oxidative stress (45) and expressed as micromoles per gram of protein. MDA content was significantly higher in GK rat hearts compared with their control during preischemic baseline conditions (0.20 ± 0.03 vs. 0.09 ± 0.02, respectively, P < 0.05), but no significant difference was found after reperfusion between the two groups (0.06 ± 0.01 and 0.07 ± 0.03, respectively, for control and GK groups). MDA content was significantly lower in GK rat hearts after reperfusion compared with preischemic baseline conditions (P < 0.05).
Creatine kinase content.
During preischemic baseline conditions, myocardial creatine kinase content (expressed as mmol/g protein) was similar in control and GK rat hearts (6.06 ± 0.32 and 5.56 ± 0.20, respectively, for control and GK groups). Ischemia-reperfusion involved a loss of creatine kinase in GK rat hearts (4.56 ± 0.13) compared with control rat hearts (5.50 ± 0.37) (P < 0.05) and vs. preischemic baseline conditions (P < 0.05).
DISCUSSION
No difference in cardiac function was found before ischemia in GK rat hearts compared with their controls, as reported either in younger GK rats (11, 43) or in older GK rats (36, 37). The existence of contractile dysfunction has been shown in some animal models of type 2 diabetes and insulin resistance (1, 18, 53) but not in all (13). Data from various animal models of insulin resistance with or without obesity reveal differing results with increased (1, 34) or decreased (14, 43) susceptibility of the type 2 diabetic rat heart to ischemia depending on the model, the severity of the diabetic state, the degree of ischemia, and the substrates. Previously, we reported lower cardiac function associated with lower insulin-stimulated glucose uptake rates during the preischemic period but similar postischemic functional recovery in male GK rat hearts compared with control rat hearts perfused with glucose alone (23). Here, we found a lower postischemic functional recovery in GK compared with control rat hearts perfused with a mixture of physiological substrates, including glucose, palmitate, pyruvate, and lactate. Increased susceptibility was also shown by reduced coronary flow during reperfusion and alteration of tissue creatine kinase content. Although the experimental setting was different in this previous study compared with the present study, impeding a direct comparison, we may hypothesize that differences are at least partly related to the different substrates. Here, our objective was specifically to investigate the tolerance to ischemia-reperfusion injury of male type 2 diabetic GK rat hearts by simultaneous measurement of energy metabolism using 31P magnetic resonance spectroscopy combined with a functional evaluation and a measure of the NO pathway.
The effect of diabetes on cardiac metabolism is typically characterized by decreased glucose utilization and increased fatty acid oxidation (12), and it has been proposed that these metabolic changes may contribute to the development of diabetic cardiomyopathy (48). However, the effect of fatty acids on experimental models may be different when glucose and palmitate are not the only substrates. It is well established in vitro and in vivo that, in heart, lactate is more readily oxidized than glucose (47, 69). So, the relationship between glycolysis and glucose oxidation may not be that important when physiologically relevant carbohydrate mixtures are used. Consequently, in the present study, hearts were perfused with a physiological substrate mixture, including glucose, palmitate, lactate, and pyruvate. A higher concentration of palmitate (1.2 mM) was provided during ischemia because it was reported to cause maximal damage during ischemia and reperfusion (48, 54). In addition, this is a relevant concentration occurring during myocardial infarction, which is what we are modeling here with a low-flow ischemic model. Interestingly, Wang et al. (68, 69) showed that, although cardiac function was slightly depressed under baseline conditions in the Zucker diabetic fatty rats, postischemic functional recovery was increased after low-flow ischemia in Zucker diabetic fatty rats with a mixture of glucose, insulin, palmitate, lactate, and pyruvate. The concentration of palmitate used by Wang et al. (68, 69) was lower (0.32 mM) than in our study, and the authors cannot rule out that the use of higher fatty acids or glucose concentration could have affected either baseline cardiac function or the response to ischemia.
We have investigated if altered postischemic function of GK rat hearts was related to HEP modifications. HEP changes may be expected as a consequence of glucolipotoxicity, which leads to a variety of deleterious effects such as increased formation of reactive oxygen species and mitochondrial dysfunction (2, 3, 62). Despite altered substrate supply and utilization in diabetic hearts, it is important to underline that there were no differences in ATP and PCr content during baseline, low-flow ischemia and reperfusion between control and GK rat hearts. HEP metabolism has been evaluated in patients with type 2 diabetes (25, 59, 61). Diamant et al. (25) found a decreased PCr/ATP in type 2 diabetic patients compared with control subjects but did not confirm this finding in a subsequent study with a group of well-controlled uncomplicated type 2 diabetic patients showing unchanged HEP metabolism despite altered myocardial substrate metabolism (59). On the other hand, the PCr-to-ATP ratio has been shown to be modified in type 2 diabetic patients with no evidence of coronary artery disease or impaired cardiac function (61). These different results in type 2 diabetic patients in the literature have been related to differences in patient characteristics (59). Diabetic patients in these studies did not have signs of cardiac ischemia. In the present study, the animal model allows us to explore the energy metabolism not only during baseline conditions but also during ischemia and reperfusion. However, we did not observe modifications of HEPs in any condition; thus, energy metabolism is not a major determinant in the increased susceptibility of GK hearts to ischemia-reperfusion. Interestingly, Bollano et al. (8) suggested that cardiac remodeling rather than disturbed myocardial energy metabolism (as evaluated by the PCr-to-ATP ratio) was associated with cardiac dysfunction in streptozotocin-induced diabetes. In addition, other factors may be also involved in cardiac dysfunction of GK rats, such as myocardial triglyceride content (60), inflammation (10, 26), and age (66). For instance, myocardial triglyceride content can be detected noninvasively using proton magnetic resonance spectroscopy, and this method has been used successfully for studying myocardial steatosis in type 2 diabetic patients (39, 46, 51, 60), in obese patients (30), and in Zucker rat heart (63). Interestingly, increased myocardial triglyceride content has been associated with impaired left ventricular diastolic function in type 2 diabetic patients (60) and elevated left ventricular mass in obese patients (63). On the other hand, McGavock et al. (51) reported that cardiac steatosis precedes the onset of type 2 diabetes mellitus and left ventricular systolic dysfunction.
GK rats have a significant decreased coronary flow before ischemia and after reperfusion compared with control rats. This is consistent with our previous results obtained in GK rat hearts perfused with glucose alone (23). In addition, we have shown that female GK rats had defective myocardial blood flow associated with altered left ventricular function in vivo (38). This observation is consistent with the reduced coronary flow observed in type 2 diabetic patients (31, 73). There is evidence in the literature that the NO pathway is altered in various models of diabetes. Changes in expression of NOS isoforms or changes in their activity may lead to coronary dysfunction and myocyte injury in the type 2 diabetic GK rat heart. Consequently, we have studied the NO pathway in cardiac tissue by measuring NOx as an evaluation of in vivo NO production, as previously reported (20, 32, 70, 71), as well as the three NOS isoforms expression before ischemia and after reperfusion. NOx concentration was significantly increased before ischemia and after reflow in GK compared with control rat hearts. eNOS protein expression was upregulated in GK hearts during baseline conditions, whereas no difference was seen after reperfusion in either group. nNOS protein expression was similar in all conditions, and we did not observe iNOS expression in type 2 diabetic rat hearts. Consequently, myocardial NO production in the GK rat may be due to both eNOS and nNOS protein expression. Increased expression of eNOS has been reported in heart and in aorta in various animal models of diabetes. Bitar et al. (7) have shown increased eNOS expression in the aortas of young GK rats compared with Wistar rats. Interestingly, Kazuyama et al. (41) showed an increase in eNOS and a decrease in nNOS mRNA expression in 12-wk-old GK rat aortas compared with age-matched controls. Jesmin et al. (40) reported increased eNOS and iNOS protein expression and downregulation of nNOS protein in the heart of early streptozotocin-induced diabetic rats. It has been hypothesized that upregulation of eNOS was a consequence of an enhanced oxidative stress induced by hyperglycemia (7, 9, 40) and inactivation of NO by the formation of reactive oxygen intermediates. In agreement with this hypothesis, total NOx concentration has been shown to be increased in endothelial cells subjected to high glucose and has been related to increased O2− and ONOO− concentrations in parallel to decreased NO concentration (57). These authors have then shown that NO bioavailability decreased in high-glucose conditions even though NO production increased. We also found increased total NOx content in GK rat hearts. Surprisingly, some other studies have rather shown decreased NOx content in the hearts (9) or in the aortas of young GK rats (7) together with upregulation of eNOS. In contrast to upregulation of eNOS, a significant decrease of eNOS, iNOS, and nNOS mRNA expressions was observed in the 70-wk-old GK rat aortas compared with those in the younger GK rats (41). In the same animal model, El-Omar et al. (27) have shown that eNOS expression was similar in control and diabetic hearts, whereas only diabetic hearts expressed iNOS protein.
To assess the status of increased generation of reactive oxygen species in our model of type 2 diabetes, which may react with NO to form peroxynitrite, we have determined the MDA content in control and GK rat hearts, as an index of lipid peroxidation (45, 67). Interestingly, we found increased MDA content in GK rat hearts before ischemia, which is in agreement with findings from cardiomyocytes isolated from diabetic mice (72), reflecting increased lipid peroxidation. Impaired functioning of reactive oxygen species defence mechanisms may also be involved. Ye et al. (72) found that catalase overexpression reduces diabetes-induced oxidative stress via the reduction of reactive oxygen species. Interestingly, Fujita et al. (29) suggested an impaired compensatory upregulation of the mRNA expression of antioxidant enzymes in the streptozotocin-induced diabetic mice. mRNA expression of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, or catalase has not been performed here but would be necessary to understand the mechanisms involved in increased MDA content in type 2 diabetic GK rat heart. The decreased MDA content in GK rat hearts after reflow is puzzling and difficult to explain. It is important to note that MDA content was not increased after reperfusion in control rat hearts in contrast to other studies performed under different experimental conditions reporting increased MDA content after reperfusion in isolated rat hearts following no-flow global ischemia (74) or after ligation of the left descending coronary artery (52).
Changes in the NO pathway may explain the alterations in coronary flow in diabetes. However, the effects of NO bioactivity on the heart go well beyond its vasodilatory and antithrombotic actions in the endothelium, since all three NOS isoforms (eNOS, nNOS, and iNOS) are expressed in cardiac myocytes themselves. The various cell types comprising cardiac muscle contain one or more of the three NOS isoforms (49). Studies suggest multiple intracardiac roles of NO in mediating coronary artery vasodilation, systolic and diastolic left ventricular function, chronotropic function, ischemic heart disease, cardiac remodeling, and chronic heart failure (28, 42, 50, 58). Although the findings support the potential use of NO-targeted therapies for treatment of cardiac disease, little is known on the complexities of the biological actions of NO in the vasculature and heart, particularly in type 2 diabetes, and the development of therapies is still largely in the preliminary stages. On the other hand, alterations in coronary flow may also be related to endothelium-independent relaxation mechanisms as was recently reported in type 2 diabetic human patients (16). Assessing endothelium-dependent and -independent relaxation has not been performed in this study, but it would be interesting to go further on the link between the modifications of the NO pathway and coronary flow alterations in the type 2 diabetic GK rat heart.
In conclusion, we report increased susceptibility of type 2 diabetic GK rat heart to ischemic injury that is not associated with impaired energy metabolism. Decreased coronary flow, upregulation of eNOS expression, and increased total NOx levels confirm NO pathway modifications in this model, which are presumably related to increased oxidative stress. Modifications in the NO pathway may play a major role in ischemia-reperfusion injury in the type 2 diabetic GK rat heart. The mechanisms involved in NO pathway modifications and the relationship with ischemia susceptibility of the type 2 diabetic GK rat heart need to be clarified in the future for a better understanding of the cardiovascular complications observed in type 2 diabetes.
GRANTS
This work was supported by Centre National de la Recherche Scientifique Unité Mixte de Recherche 6612, Programme Multidisciplinaire Centre National de la Recherche Scientifique-Commissariat à l'Énergie Atomique (Imagerie du Petit Animal), Assistance Publique-Hôpitaux de Marseille, and the Bristish Heart Foundation and Alliance Partnership Programme.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Danielle Bailbé from Laboratoire Biologie et Pathologie du Pancréas Endocrine, Paris.
REFERENCES
- 1.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes 52: 434–441, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Ghani MA, DeFronzo RA. Mitochondrial dysfunction, insulin resistance, type 2 diabetes mellitus. Curr Diab Rep 8: 173–178, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Ghani MA, Muller FL, Liu Y, Chavez AO, Balas B, Zuo P, Chang Z, Tripathy D, Jani R, Molina-Carrion M, Monroy A, Folli F, Van Remmen H, DeFronzo RA. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab 295: E678–E685, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Almind K, Dorio A, Kahn CR. Putting the genes for type II diabetes on the map. Nat Med 7: 277–279, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bernard M, Caus T, Sciaky M, Lan C, Cozzone PJ. Optimized cardiac graft preservation: a comparative experimental study using P-31 magnetic resonance spectroscopy and biochemical analyses. J Heart Lung Transplant 18: 572–581, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Bisbis S, Bailbe D, Tormo MA, Picarel-Blanchot F, Derouet M, Simon J, Portha B. Insulin resistance in the GK rat: decreased receptor number but normal kinase activity in liver. Am J Physiol Endocrinol Metab 265: E807–E813, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol 511: 53–64, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bollano E, Omerovic E, Svensson H, Waagstein F, Fu M. Cardiac remodeling rather than disturbed myocardial energy metabolism is associated with cardiac dysfunction in diabetic rats. Int J Cardiol 114: 195–201, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Bulhak AA, Jung C, Ostenson CG, Lundberg JO, Sjoquist PO, Pernow J. PPAR-alpha activation protects the type 2 diabetic myocardium against ischemia-reperfusion injury: involvement of the PI3-Kinase/Akt and NO pathway. Am J Physiol Heart Circ Physiol 296: H719–H727, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Caglayan E, Stauber B, Collins AR, Lyon CJ, Yin F, Liu J, Rosenkranz S, Erdmann E, Peterson LE, Ross RS, Tangirala RK, Hsueh WA. Differential roles of cardiomyocyte and macrophage peroxisome proliferator-activated receptor gamma in cardiac fibrosis. Diabetes 57: 2470–2479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandler MP, Morgan EE, McElfresh TA, Kung TA, Rennison JH, Hoit BD, Young ME. Heart failure progression is accelerated following myocardial infarction in type 2 diabetic rats. Am J Physiol Heart Circ Physiol 293: H1609–H1616, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Chatham JC. The effect of diabetes on myocardial glucose metabolism. In: The Heart in Diabetes, edited by Chatham JC, Forder JR, McNeill JH. Norwell, MA: Kluwer, 1996, p. 215–251 [Google Scholar]
- 13.Chatham JC, Seymour AML. Cardiac carbohydrate metabolism in Zucker diabetic fatty rats. Cardiovasc Res 55: 104–112, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Higashino H, Kamenov ZA, Azuma M, Lee WH, Yang XQ, Zhou DJ, Yuan WJ. Preserved postischemic heart function in sucrose-fed type 2 diabetic OLETF rats. Life Sci 72: 2839–2851, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Cheng ZJ, Vaskonen T, Tikkanen I, Nurminen K, Ruskoaho H, Vapaatalo H, Muller D, Park JK, Luft FC, Mervaala EM. Endothelial dysfunction and salt-sensitive hypertension in spontaneously diabetic Goto-Kakizaki rats. Hypertension 37: 433–439, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Clements RT, Sodha NR, Feng J, Boodhwani M, Liu Y, Mieno S, Khabbaz KR, Bianchi C, Sellke FW. Impaired coronary microvascular dilation correlates with enhanced vascular smooth muscle MLC phosphorylation in diabetes. Microcirculation 16: 193–206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cross HR, Murphy E, Steenbergen C. Ca2+ loading and adrenergic stimulation reveal male/female differences in susceptibility to ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 283: H481–H489, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Deng JY, Huang JP, Lu LS, Hung LM. Impairment of cardiac insulin signaling and myocardial contractile performance in high-cholesterol/fructose-fed rats. Am J Physiol Heart Circ Physiol 293: H978–H987, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Desrois M, Caus T, Belles PM, Dalmasso C, Lan C, Cozzone PJ, Bernard M. Limitation of myocardial and endothelial injury of the rat heart graft after preservation with Centre de Resonance Magnetique Biologique et Medicale (CRMB) solution. Transpl Int 21: 276–283, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Desrois M, Caus T, Dalmasso C, Lan C, Cozzone PJ, Bernard M. Expression of the three nitric oxide synthase isoforms and nitric oxide level in the rat heart during cold storage and blood reperfusion. Cell Mol Biol Suppl 55: OL1208–1214, 2009 [PubMed] [Google Scholar]
- 21.Desrois M, Sciaky M, Lan C, Cozzone PJ, Bernard M. l-Arginine during long-term ischemia: effects on cardiac function, energetic metabolism and endothelial damage. J Heart Lung Transplant 19: 367–376, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Desrois M, Sciaky M, Lan C, Cozzone PJ, Bernard M. Preservation of amino acids during long term ischaemia and subsequent reflow with supplementation of l-arginine, the nitric oxide precursor, in the rat heart. Amino Acids 24: 141–148, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Desrois M, Sidell RJ, Gauguier D, Davey CL, Radda GK, Clarke K. Gender differences in hypertrophy, insulin resistance and ischemic injury in the aging type 2 diabetic rat heart. J Mol Cell Cardiol 37: 547–555, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Desrois M, Sidell RJ, Gauguier D, King LM, Radda GK, Clarke K. Initial steps of insulin signaling and glucose transport are defective in the type 2 diabetic rat heart. Cardiovasc Res 61: 288–296, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, Romijn JA, de Roos A, Radder JK. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol 42: 328–335, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Diamant M, Lamb HJ, Smit JW, de Roos A, Heine RJ. Diabetic cardiomyopathy in uncomplicated type 2 diabetes is associated with the metabolic syndrome and systemic inflammation. Diabetologia 48: 1669–1670, 2005 [DOI] [PubMed] [Google Scholar]
- 27.El-Omar MM, Lord R, Draper NJ, Shah AM. Role of nitric oxide in posthypoxic contractile dysfunction of diabetic cardiomyopathy. Eur J Heart Fail 5: 229–239, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Elrod JW, Greer JJ, Bryan NS, Langston W, Szot JF, Gebregzlabher H, Janssens S, Feelisch M, Lefer DJ. Cardiomyocyte-specific overexpression of NO synthase-3 protects against myocardial ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol 26: 1517–1523, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Fujita A, Sasaki H, Ogawa K, Okamoto K, Matsuno S, Matsumoto E, Furuta H, Nishi M, Nakao T, Tsuno T, Taniguchi H, Nanjo K. Increased gene expression of antioxidant enzymes in KKAy diabetic mice but not in STZ diabetic mice. Diabetes Res Clin Pract 69: 113–119, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Gaborit B, Kober F, Moro P, Jacquier A, Vignaud A, Dadoun F, Cozzone PJ, Bernard M, Dutour A. CMR assessment of epicardial fat volume in human morbid obesity at 3 T: relationship to cardiac function and morphology (Abstract). J Cardiovasc Magnetic Reson 12: 268, 2010 [Google Scholar]
- 31.Galderisi M, Capaldo B, Sidiropulos M, D'Errico A, Ferrara L, Turco A, Guarini P, Riccardi G, de Divitiis O. Determinants of reduction of coronary flow reserve in patients with type 2 diabetes mellitus or arterial hypertension without angiographically determined epicardial coronary stenosis. Am J Hypertens 20: 1283–1290, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Gill RM, Braz JC, Jin N, Etgen GJ, Shen W. Restoration of impaired endothelium-dependent coronary vasodilation in failing heart: role of eNOS phosphorylation and CGMP/cGK-I signaling. Am J Physiol Heart Circ Physiol 292: H2782–H2790, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Goto YKM, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med 119: 85–90, 1976 [DOI] [PubMed] [Google Scholar]
- 34.Greer JJ, Ware DP, Lefer DJ. Myocardial infarction and heart failure in the db/db diabetic mouse. Am J Physiol Heart Circ Physiol 290: H146–H153, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in non diabetic subjects with and without prior myocardial infarction. N Engl J Med 339: 229–234, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Howarth FC, Jacobson M, Shafiullah M, Adeghate E. Long-term effects of type 2 diabetes mellitus on heart rhythm in the Goto-Kakizaki rat. Exp Physiol 93: 362–369, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Howarth FC, Shafiullah M, Qureshi MA. Chronic effects of type 2 diabetes mellitus on cardiac muscle contraction in the Goto-Kakizaki rat. Exp Physiol 92: 1029–1036, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Iltis I, Kober F, Desrois M, Dalmasso C, Lan C, Portha B, Cozzone PJ, Bernard M. Defective myocardial blood flow and altered function of the left ventricle in type 2 diabetic rats: a noninvasive in vivo study using perfusion and cine magnetic resonance imaging. Invest Radiol 40: 19–26, 2005 [PubMed] [Google Scholar]
- 39.Iozzo P, Lautamaki R, Borra R, Lehto HR, Bucci M, Viljanen A, Parkka J, Lepomaki V, Maggio R, Parkkola R, Knuuti J, Nuutila P. Contribution of glucose tolerance and gender to cardiac adiposity. J Clin Endocrinol Metab 94: 4472–4482, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Jesmin S, Zaedi S, Maeda S, Yamaguchi I, Goto K, Miyauchi T. Effects of a selective endothelin a receptor antagonist on the expressions of iNOS and eNOS in the heart of early streptozotocin-induced diabetic rats. Exp Biol Med (Maywood) 231: 925–931, 2006 [PubMed] [Google Scholar]
- 41.Kazuyama E, Saito M, Kinoshita Y, Satoh I, Dimitriadis F, Satoh K. Endothelial dysfunction in the early- and late-stage type-2 diabetic Goto-Kakizaki rat aorta. Mol Cell Biochem 332: 95–102, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res 79: 363–380, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Kristiansen SB, Lofgren B, Stottrup NB, Khatir D, Nielsen-Kudsk JE, Nielsen TT, Botker HE, Flyvbjerg A. Ischaemic preconditioning does not protect the heart in obese and lean animal models of type 2 diabetes. Diabetologia 47: 1716–1721, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Lazzarino G, Pierro DD, Tavazzi B, Cerroni L, Giardina B. Simultaneous separation of malondialdehyde, ascorbic acid, and adenine nucleotide derivatives from biological samples by ion-pairing high-performance liquid chromatography. Anal Biochem 197: 191–196, 1991 [DOI] [PubMed] [Google Scholar]
- 45.Lefevre G, Beljean-Leymarie M, Beyerle F, Bonnefont-Rousselot D, Cristol JP, Therond P, Torreilles J. Evaluation of lipid peroxidation by measuring thiobarbituric acid reactive substances. Ann Biol Clin (Paris) 56: 305–319, 1998 [PubMed] [Google Scholar]
- 46.Lingvay I, Raskin P, Szczepaniak LS. The fatty hearts of patients with diabetes. Nat Rev Cardiol 6: 268–269, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Lloyd SG, Wang P, Zeng H, Chatham JC. Impact of low-flow ischemia on substrate oxidation and glycolysis in the isolated perfused rat heart. Am J Physiol Heart Circ Physiol 287: H351–H362, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Lopaschuk GD. Fatty acid metabolism in the heart following diabetes . In: The Heart in Diabetes, edited by Chatham JC, Forder JR, McNeill JH. Norwell, MA: Kluwer, 1996, p. 215–251 [Google Scholar]
- 49.Massion PB, Dessy C, Desjardins F, Pelat M, Havaux X, Belge C, Moulin P, Guiot Y, Feron O, Janssens S, Balligand JL. Cardiomyocyte-restricted overexpression of endothelial nitric oxide synthase (NOS3) attenuates beta-adrenergic stimulation and reinforces vagal inhibition of cardiac contraction. Circulation 110: 2666–2672, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res 93: 388–398, 2003 [DOI] [PubMed] [Google Scholar]
- 51.McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 116: 1170–1175, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Ozer MK, Parlakpinar H, Cigremis Y, Ucar M, Vardi N, Acet A. Ischemia-reperfusion leads to depletion of glutathione content and augmentation of malondialdehyde production in the rat heart from overproduction of oxidants: can caffeic acid phenethyl ester (CAPE) protect the heart? Mol Cell Biochem 273: 169–175, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Panagia M, Schneider JE, Brown B, Cole MA, Clarke K. Abnormal function and glucose metabolism in the type-2 diabetic db/db mouse heart. Can J Physiol Pharmacol 85: 289–294, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Paulson DJ. The diabetic heart is more sensitive to ischemic injury. Cardiovasc Res 34: 104–112, 1997 [DOI] [PubMed] [Google Scholar]
- 55.Portha B. Programmed disorders of beta-cell development and function as one cause for type 2 diabetes? The GK rat paradigm. Diabetes Metab Res Rev 21: 495–504, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Portha B, Serradas P, Bailbe D, Suzuki K, Goto Y, Giroix MH. Beta-cell insensitivity to glucose in the GK rat, a spontaneous nonobese model for type II diabetes. Diabetes 40: 486–491, 1991 [DOI] [PubMed] [Google Scholar]
- 57.Potdar S, Kavdia M. NO/peroxynitrite dynamics of high glucose-exposed HUVECs: chemiluminescent measurement and computational model. Microvasc Res 78: 191–198, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raij L. Nitric oxide in the pathogenesis of cardiac disease. J Clin Hypertens (Greenwich) 8: 30–39, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, Bax JJ, de Roos A, Twisk JW, Heine RJ, Lammertsma AA, Smit JW, Diamant M. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol 54: 1524–1532, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 52: 1793–1799, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 107: 3040–3046, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Schrauwen P, Schrauwen-Hinderling V, Hoeks J, Hesselink MK. Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta 1801: 266–271, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D'Ambrosia G, Arbique D, Vongpatanasin W, Unger R, Victor RG. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med 49: 417–423, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation 105: 1727–1733, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 2: 997–1000, 1989 [DOI] [PubMed] [Google Scholar]
- 66.van der Meer RW, Rijzewijk LJ, Diamant M, Hammer S, Schar M, Bax JJ, Smit JW, Romijn JA, de Roos A, Lamb HJ. The ageing male heart: myocardial triglyceride content as independent predictor of diastolic function. Eur Heart J 29: 1516–1522, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Vincent HK, Powers SK, Dirks AJ, Scarpace PJ. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int J Obes Relat Metab Disord 25: 378–388, 2001 [DOI] [PubMed] [Google Scholar]
- 68.Wang P, Chatham JC. Onset of diabetes in Zucker diabetic fatty (ZDF) rats leads to improved recovery of function after ischemia in the isolated perfused heart. Am J Physiol Endocrinol Metab 286: E725–E736, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 288: H2102–H2110, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Xiao DS, Jiang L, Che LL, Lu L. Nitric oxide and iron metabolism in exercised rat with l-arginine supplementation. Mol Cell Biochem 252: 65–72, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Xuan YT, Tang XL, Qiu Y, Banerjee S, Takano H, Han H, Bolli R. Biphasic response of cardiac NO synthase isoforms to ischemic preconditioning in conscious rabbits. Am J Physiol Heart Circ Physiol 279: H2360–H2371, 2000 [DOI] [PubMed] [Google Scholar]
- 72.Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes 53: 1336–1343, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Yokoyama I, Ohtake T, Momomura S, Yonekura K, Woo-Soo S, Nishikawa J, Sasaki Y, Omata M. Hyperglycemia rather than insulin resistance is related to reduced coronary flow reserve in NIDDM. Diabetes 47: 119–124, 1998 [DOI] [PubMed] [Google Scholar]
- 74.Zhang LP, Yang CY, Wang YP, Cui F, Zhang Y. Protective effect of polydatin against ischemia/reperfusion injury in rat heart. Sheng Li Xue Bao 60: 161–168, 2008 [PubMed] [Google Scholar]