Abstract
Cardiac hypertrophy in response to hypertension or myocardial infarction is a pathological indicator associated with heart failure (HF). A central component of the remodeling process is the loss of cardiomyocytes via cell death pathways regulated by the mitochondrion. Recent evidence has indicated that exercise training can attenuate or reverse pathological remodeling, creating a physiological phenotype. The purpose of this study was to examine left ventricular (LV) function, remodeling, and cardiomyocyte mitochondrial function in aortic-banded (AB) sedentary (HFSED; n = 6), AB exercise-trained (HFTR, n = 5), and control sedentary (n = 5) male Yucatan miniature swine. LV hypertrophy was present in both AB groups before the start of training, as indicated by increases in LV end-diastolic volume, LV end-systolic volume (LVESV), and LV end-systolic dimension (LVESD). Exercise training (15 wk) prevented further increases in LVESV and LVESD (P < 0.05). The heart weight-to-body weight ratio, LV + septum-to-body weight ratio, LV + septum-to-right ventricle ratio, and cardiomyocyte cross-sectional area were increased in both AB groups postmortem regardless of training status. Preservation of LV function after exercise training, as indicated by the maintenance of fractional shortening, ejection fraction, and mean wall shortening and increased stroke volume, was associated with an attenuation of the increased LV fibrosis (23%) and collagen (36%) observed in HFSED animals. LV mitochondrial dysfunction, as measured by Ca2+-induced mitochondrial permeability transition, was increased in HFSED (P < 0.05) but not HFTR animals. In conclusion, low-intensity interval exercise training preserved LV function as exemplified by an attenuation of fibrosis, maintenance of a positive inotropic state, and inhibition of mitochondrial dysfunction, providing further evidence of the therapeutic potential of exercise in a clinical setting.
Keywords: mitochondria, hypertrophy and fibrosis, cardiac function
the progression to terminal decompensated heart failure (HF) is characterized by pathological remodeling of the left ventricle (LV) and, as such, has become a target for therapeutic treatment. Interestingly, pathological cardiomyocyte growth in HF is observed concurrently with an increase in cardiomyocyte death (37, 38, 40). The loss of cardiomyocytes via apoptotic and necrotic signaling pathways plays a significant role in the transition from compensated hypertrophy to LV dilation (for reviews, see Refs. 10, 12, 17, 31, and 50). Conditions of myocardial stress, i.e., ischemia-reperfusion, HF, etc., have been show to stimulate increases in mitochondrial permeability and volume, known as mitochondrial permeability transition (MPT) (for a review, see Ref. 1). MPT has been implicated as a key mediator of cardiomyocyte cell death and is regulated by the MPT pore (MPTP). Well-documented increases in ROS production, altered Ca2+ regulation, and impaired energy metabolism are characteristics of HF and are capable of exerting considerable influence on the MPTP, which is redox, Ca2+, and nucleotide sensitive. As a result, the prevention of cardiomyocyte loss has been a target of potential treatment in HF (7).
Recent evidence has suggested that exercise training is a feasible treatment in both animal models and human HF, as illustrated by improved survival and delayed onset of decompensated HF, reversed or attenuated pathological LV remodeling, and improved quality of life (4, 11, 54). However, the precise frequency and intensity of exercise required to elicit cardiovascular benefits have not been fully elucidated. Recent studies by Schultz et al. (43), Emter et al. (11), and Chicco et al. (4) in the spontaneously hypertensive HR rat have illustrated this issue, as repeated bouts of high-intensity exercise in an untreated, hypertensive state increased LV chamber dilation, reduced cardiac function, and accelerated the progression to decompensated HF, whereas, in contrast, low-intensity exercise training positively influenced morbidity and mortality. A similar effect of exercise intensity was observed in humans by Wisloff et al. (54), where aerobic interval training, which allowed patients to exercise at greater intensities for brief periods of time, was found to have superior benefits to moderate continuous training on LV remodeling in HF patients. These studies clearly suggest that exercise training in HF can both exacerbate or prevent the progression toward decompensated HF depending on the frequency and intensity of training. The mechanisms of improved survival and attenuated pathological remodeling remain unclear in regard to exercise of different intensities in a setting of HF.
The purpose of this study was to assess the effects of chronic low-intensity interval exercise training on LV function, remodeling, and cardiomyocyte cell death in a miniature swine model of pressure overload HF. We hypothesized that low-intensity interval exercise training would attenuate pathological LV remodeling and maintain mitochondrial function, thus limiting cardiomyocyte death. Briefly, our results indicate that aortic banding resulted in pathological LV remodeling as characterized by increased fibrosis and LV mitochondrial dysfunction as indicated by increased Ca2+-induced MPT, which was not associated with increased apoptosis. Low-intensity interval exercise training preserved LV function, was associated with indicators of LV hypertrophy typically considered physiological, and prevented the increased sensitivity to MPT observed in sedentary failing hearts.
METHODS
Aortic banding and exercise training.
Before being banded, intact male Yucatan miniature swine (20–22 kg, 8 mo old) were matched for body mass and cardiac function and then assigned into three groups: nonsham-operated sedentary control (SED; n = 5), banded HF sedentary (HFSED; n = 6), and banded HF exercise trained (HFTR; n = 5). A left fourth space intercostal thoracotomy was performed, isolating the aortic arch, and a band was placed distal to the subclavian artery at the beginning of the descending aorta. Heart rate (HR) was raised to ≈140 beats/min using a dobutamine infusion (0.05 mg/ml, 6–12 ml/h) while aortic mean arterial pressure was monitored via a fluid-filled 6-Fr guide catheter (carotid artery insertion, Boston Scientific). The band was constricted at this HR until an ≈100-mmHg transtenotic gradient was achieved (HFSED: 94 ± 6 mmHg and HFTR: 99 ± 5 mmHg). Exercise training consisted of treadmill running 3 days/wk, 55 min/day, for 15 wk and was started 2 mo postbanding (≈10 mo old). A low-intensity interval exercise training regimen based on a combination of previously established rat, swine, and human protocols (11, 30, 54) was created with gradually increasing intensity as tolerated until it finally consisted of the following: 1) a 5-min warmup at 2 mph, 2) six 5-min sessions at 3 mph with five 3-min intervals at 4 mph in between, and 3) a 5-min cooldown at 2 mph. Animals were fed an average of 15–20 g/kg once daily, and water was provided ad libitum. Dissection of vital tissues and removal of the deltoid muscle for an analysis of citrate synthase activity (46) occurred at the time of death. All animal protocols were in accordance with the “Principles for the Utilization and Care of Vertebrate Animals Used in Testing Research and Training” and were approved by the Animal Care and Use Committee of the University of Missouri.
Transthoracic echocardiography.
Transthoracic echocardiography was performed under inhaled isoflurane anesthesia (0.5%) in the supine/right lateral position before aortic banding and at 2 and 6 mo postbanding. Short-axis two-dimensional and M-mode images were recorded at the midpapillary level using a 2.5-MHz transducer on a Toshiba PowerVision Ultrasound system. Ventricular chamber dimensions [LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), LV end-diastolic volume (LVEDV), and LV end-systolic volume (LVESV)] were calculated from M-mode recordings using previously established methods (27, 41, 47, 48). Ejection fraction (EF; in %) was calculated using the following equation: EF = (EDV − ESV)/EDV × 100. From an apical four-chamber view, the velocity time integral (VTI), a measure directly proportional to stroke volume (SV), was measured at the level of the aortic annulus using pulse-wave Doppler. SV was determined using the following equation: SV = π(r2) × VTI, where r is the radius. Mean wall shortening (MWS; in %) was calculated according to previously established methods (33) using the following equation: MWS = 100 × [(LVIDd + PWT) − (LVIDs + 2a)]/(LVIDd + PWT), where LVIDd is the LV internal dimension at diastole, PWT is the posterior wall thickness, and LVIDs is LV internal dimension at systole.
LV gross and cellular morphology.
LV morphology was determined using postmortem weights and immunohistology. Postmortem gross morphology was assessed according to previous methods established by Turk et al. (49). Cardiomyocyte morphology was determined by tracing the cell border in formalin-fixed, paraffin-embedded, and immunohistology-stained LV cross-sections. Cross-sectional area was measured from a minimum of 50 cells and 3 cross-sectional views/animal in two-dimensional images using Image J (NIH).
Tissue fractionation and mitochondrial swelling.
Mitochondria were isolated by differential centrifugation. Briefly, LV tissue was homogenized using a Dounce homogenizer in buffer containing 250 mM sucrose, 10 mM Tris (pH 7.4), and 1 mM EDTA. The homogenate was centrifuged at 1,000 g for 5 min to remove nuclei and debris. The supernatant was then centrifuged at 10,000 g for 10 min. The resultant supernatant was centrifuged at 100,000 g for 30 min to yield the cytosolic fraction. The 10,000-g pellet, corresponding to the mitochondrial fraction, was resuspended in homogenizer buffer without EDTA and centrifuged again at 10,000 g for 10 min. Washed mitochondria were then resuspended in either swelling buffer [120 mM KCl, 10 mM Tris (pH7.4), and 5 mM KH2PO4] or lysis buffer (see below). Protein concentrations were determined using the Bradford method. For the swelling experiments, mitochondria were resuspended at a final concentration of 0.25 mg/ml. Absorbance was then measured spectrophotometrically at 520 nm, and swelling was induced by the addition of CaCl2.
Western blot analysis.
Heart tissue and mitochondria were lysed in buffer containing 150 mM NaCl, 10 mM Tris (pH 7.4), 1 mM EDTA, and 1% Triton X-100. Proteins were resolved by SDS-PAGE using 10–15% acrylamide, transferred onto polyvinylidene difluoride membranes, and blotted using the following commercially available antibodies: anti-adenine nucleotide translocase (ANT), anti-Bax, and anti-GAPDH from Santa Cruz Biotechnology; anti-cyclophilin D (CypD) from Mitosciences; anti-cleaved caspase-3 and anti-poly(ADP-ribose) polymerease (PARP) from Cell Signaling; and anti-cytochrome c from BD Biosciences. The polyclonal anti-mitochondrial phosphate carrier antibody was custom made for us by Yenzyme. Membranes were then incubated with the appropriate alkaline phosphatase-linked secondary antibody (Santa Cruz Biotechnology) and visualized by enhanced chemifluorescence (Amersham).
DNA laddering.
Genomic DNA was extracted from LV tissue by standard procedures, and the degree of apoptosis-induced DNA laddering was assessed using a PCR-based kit (Maxim Biochemicals).
Histology and immunohistochemistry.
Cross-sections of LV were formalin fixed, embedded in paraffin, and immunohistochemistry stained for the assessment of fibrosis and collagen. Briefly, total fibrosis was visualized from 4-μm-thick sections of the LV using Masson's trichrome stain, and total collagen was visualized using Picrosirius red staining with previously established methods (23). Fibrosis and collagen were quantified from 4 separate fields/animal using Image-Pro Plus analysis software (version 6.2, MediaCybernetics, Bethesda, MD) and expressed as the percent area stained and density of the stain.
Statistical analysis.
All data analysis was performed using SPSS version 13.0 or SigmaStat version 3.5. All comparisons between groups were made using either one-way ANOVA or a two-tailed independent samples t-test. No differences in echocardiographic measures of morphology or function existed between HFSED and HFTR groups (P = not significant by an independent samples t-test) at the preband and 2-mo time points; therefore, data from both aortic-banded groups (HFSED and HFTR groups) were combined for within-group comparisons using a paired samples t-test before the start of exercise training. All data are means ± SE, and significance is reported at P < 0.10 and P < 0.05 levels (6, 53).
RESULTS
Low-intensity exercise training results in LV hypertrophy that appears more physiological than pathological after aortic banding.
Body weight (BW) was significantly decreased in HFSED animals (26 ± 1, 30 ± 1, and 32 ± 1 kg for HFSED, HFTR, and SED animals, respectively, P < 0.05 by one-way ANOVA); therefore, lung and heart morphology measures were normalized to BW. An increased lung weight-to-BW ratio (indicative of pulmonary congestion) was observed in HFSED compared with SED animals (9.3 ± 1.0 vs. 7.3 ± 0.03 g/kg, P = 0.06 by an independent samples t-test) and was attenuated by exercise training (8.0 ± 0.8 g/kg in HFTR animals). Echocardiography data summarizing the effect of aortic banding and exercise on LV remodeling are shown in Table 1. Two months postsurgery, aortic banding significantly increased LVEDV, LVEDD, LVESV, and LVESD. This effect (not observed in the SED group) indicates aortic banding-induced LV hypertrophy (an observation commonly associated with the progression of HF) was present before the onset of exercise training. At 6 mo, LVEDV was significantly greater in HFSED and HFTR animals compared with SED animals. The ratio of the percent increase in LVEDV (3.5, 2.0, and 1.8 for HFSED, SED, and HFTR animals, respectively) and LVEDD (2, 1.3, and 1.0 for HFSED, SED, and HFTR animals, respectively) to the percent increase in weight, an indicator of the relationship between cardiac growth and normal age-related growth, was greatest in HFSED animals. Six months postbanding, further increases in LVESV and LVESD in HFSED animals were attenuated with low-intensity exercise training, as these values were similar in HFTR and SED groups. Septal and LV systolic wall thickness were significantly lower in HFSED animals compared with both HFTR and SED groups.
Table 1.
Impact of aortic banding and exercise training on LV morphology and function
| Time Points |
|||||
|---|---|---|---|---|---|
| PreBand | 2 mo | 6 mo | Percent Increase in LVEDV/Percent Increase in Weight | Percent Increase in LVEDD/Percent Increase in Weight | |
| LVEDV, ml | |||||
| SED | 9.1 ± 0.9 | 11.0 ± 0.9 | 12.6 ± 0.6 | 28/14 | |
| HFSED | 8.3 ± 0.3 | 12.5 ± 0.6* | 15.1 ± 0.6† | 46/13 | |
| HFTR | 16.0 ± 0.4† | 48/27 | |||
| LVEDD, mm3 | |||||
| SED | 37.0 ± 1.9 | 40.7 ± 1.6 | 45.2 ± 1.8 | 18/14 | |
| HFSED | 35.4 ± 0.7 | 43.2 ± 1.1* | 47.6 ± 0.9 | 26/13 | |
| HFTR | 49.0 ± 0.6 | 28/27 | |||
| LVESV, ml | |||||
| SED | 3.4 ± 0.4 | 3.6 ± 0.6 | 4.2 ± 0.6 | ||
| HFSED | 3.1 ± 0.2 | 4.9 ± 0.6* | 6.4 ± 0.4† | ||
| HFTR | 4.5 ± 0.4 | ||||
| LVESD, mm3 | |||||
| SED | 23.0 ± 1.4 | 23.7 ± 1.8 | 25.4 ± 1.6 | ||
| HFSED | 22.1 ± 0.9 | 27.1 ± 1.6* | 31.4 ± 1.0† | ||
| HFTR | 26.4 ± 1.2 | ||||
| Septal wall thickness at systole, mm | |||||
| SED | 11.5 ± 0.3 | 13.5 ± 0.8 | 14.8 ± 0.5 | ||
| HFSED | 12.4 ± 0.8 | 12.7 ± 0.5 | 12.8 ± 0.3† | ||
| HFTR | 16.6 ± 0.3 | ||||
| Septal wall thickness at diastole, mm | |||||
| SED | 7.4 ± 0.2 | 8.0 ± 0.3 | 8.5 ± 0.2 | ||
| HFSED | 7.3 ± 0.3 | 7.6 ± 0.3 | 7.8 ± 0.4‡ | ||
| HFTR | 9.0 ± 0.4 | ||||
| LV wall thickness at systole, mm | |||||
| SED | 12.9 ± 0.4 | 15.0 ± 0.5 | 16.1 ± 0.8 | ||
| HFSED | 12.0 ± 0.3 | 14.1 ± 0.6 | 12.6 ± 0.4† | ||
| HFTR | 16.9 ± 0.1 | ||||
| LV wall thickness at diastole, mm | |||||
| SED | 6.8 ± 0.5 | 6.5 ± 0.4 | 5.8 ± 0.4 | ||
| HFSED | 6.7 ± 0.2 | 6.2 ± 0.3 | 4.7 ± 0.4 | ||
| HFTR | 5.7 ± 0.6 | ||||
| Fractional shortening, % | |||||
| SED | 38 ± 2 | 42 ± 2 | 44 ± 2 | ||
| HFSED | 38 ± 1 | 38 ± 3 | 34 ± 1† | ||
| HFTR | 46 ± 3 | ||||
| Ejection fraction, % | |||||
| SED | 63 ± 2 | 68 ± 3 | 69 ± 3 | ||
| HFSED | 63 ± 2 | 62 ± 3 | 58 ± 2† | ||
| HFTR | 72 ± 3 | ||||
| Stroke volume, ml | |||||
| SED | 39 ± 6 | 57 ± 6 | 60 ± 5 | ||
| HFSED | 32 ± 3 | 52 ± 3 | 56 ± 3 | ||
| HFTR | 72 ± 5§ | ||||
| MWS, % | |||||
| SED | 19 ± 1 | 21 ± 1 | 22 ± 1 | ||
| HFSED | 20 ± 1 | 19 ± 2 | 18 ± 1† | ||
| HFTR | 23 ± 2 | ||||
Values are means ± SE. Animals were divided into the following three groups: sedentary control (SED), aortic-banded sedentary (HFSED), and aortic-banded exercise-trained (HFTR) group. LV, left ventricular; LVEDV, LV end-diastolic volume; LVEDD, LV end-diastolic dimension; LVESV, LV end-systolic volume; LVESD, LV end-systolic dimension; FS, fractional shortening; EF, ejection fraction, SV, stroke volume; MWS, mean wall shortening.
P < 0.05 vs. the preband value (by a paired samples t-test);
P < 0.05 vs. the 6-mo SED value (by one-way ANOVA);
P < 0.10 vs. the 6-mo HFTR value (by one-way ANOVA);
P < 0.10 vs. 6-mo HFSED and SED values (by one-way ANOVA).
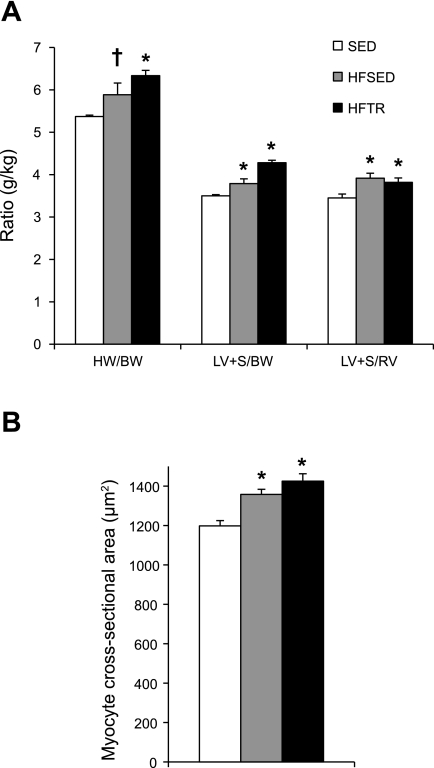
Postmortem assessment of whole heart, LV, and cardiomyocyte morphology support our echocardiography measures. Aortic banding significantly increased the heart weight-to-BW ratio, LV + septum (LV + S)-to-BW ratio, and LV + S-to-right ventricle (RV) ratio in the HFSED and HFTR groups (Fig. 1A). Cellular hypertrophy paralleled gross morphological remodeling as cardiomyocyte cross-sectional area was significantly greater in the HFTR and HFSED groups compared with their SED counterparts (Fig. 1B). Compared with SED animals, increases in the myocyte cross-sectional area of ∼12% and 16% closely mimicked increases in the LV + S-to-BW ratios of ∼8% and 18% in the HFSED and HFTR groups, respectively, and illustrate the coherence of our gross and cellular findings. Hypertrophy at the gross and cellular levels occurred in all aortic-banded groups regardless of training status.
Fig. 1.
Aortic banding generates left ventricular (LV) hypertrophy regardless of training status. Animals were divided into the following three groups: sedentary control (SED), aortic-banded sedentary (HFSED), and aortic-banded exercised-trained (HFTR) groups. A: LV hypertrophy, as indicated by an increased heart weight-to-body weight (BW) ratio (HW/BW), LV + septum (LV + S)-to-BW ratio (LV + S/BW), and LV+S-to-right ventricle ratio (LV + S/RV), was present in all aortic-banded groups. *P < 0.05 and †P = 0.06 vs. SED animals (by one-way ANOVA). B: myocyte cross-sectional area was increased in both HFSED and HFTR animals, indicating that cellular morphology paralleled gross observations. *P < 0.05, HFSED and HFTR vs. SED animals (by one-way ANOVA).
Citrate synthase activity was significantly elevated in the deltoid muscles of HFTR animals, indicative of exercise-induced training adaptations to our low-intensity exercise protocol (18.3 ± 1.1, 23.8 ± 0.9, and 19.0 ± 1.8 μmol·g wet wt muscle−1·min−1 for the HFSED, HFTR, and SED groups, respectively, P < 0.05 by one-way ANOVA).
Low-intensity exercise training is associated with improved LV function after aortic banding-induced LV hypertrophy.
Indicators of LV function derived from echocardiography measures are shown in Table 1. Fractional shortening, EF, and MWS in HFTR animals at 24 wk were similar to SED control values and significantly greater compared with HFSED animals after 15 wk of low-intensity exercise training. SV was greater at 24 wk in HFTR animals compared with HFSED and SED animals.
Low-intensity interval exercise training limits aortic banding-induced fibrotic LV remodeling.
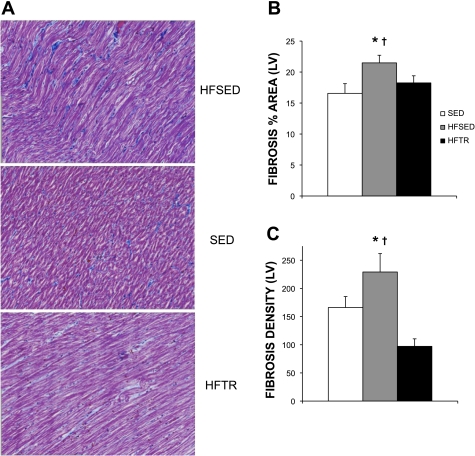
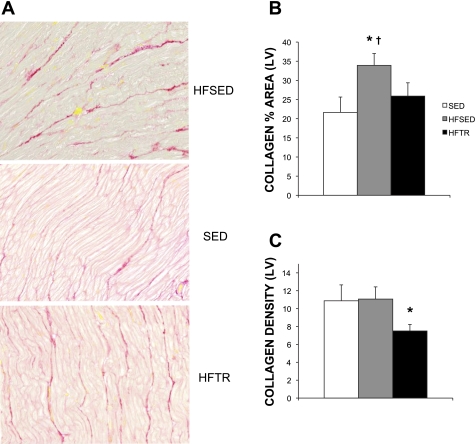
Aortic banding significantly increased general LV fibrosis (Fig. 2) and LV collagen deposition (Fig. 3). Representative histological sections of the LV from HFSED, SED, and HFTR animals showing the increases in overall LV fibrosis and collagen are shown in Figs. 2A and 3A, respectively. As evident in Fig. 3A, collagen expression in HFSED animals was more disorganized and articulated in thicker bands compared with SED and HFTR animals. When expressed as the percent area of LV stained, trichrome and Picrosirius red staining, indicating general fibrosis (Fig. 2B) and collagen (Fig. 3B), were significantly elevated in HFSED animals compared with SED control animals. Low-intensity exercise training attenuated this response in HFTR animals. Training was also associated with a significant reduction in the density of LV fibrosis (Fig. 2C) that was paralleled by a reduction in collagen density compared with both HFSED and SED animals (Fig. 3C).
Fig. 2.
Low-intensity exercise training attenuates aortic-banding induced increases in LV fibrosis. A: representative histological sections of trichrome-stained LVs, showing the increased fibrosis in HFSED animals. Magnification: ×40. B and C: exercise training attenuated the increases in LV fibrosis, as indicated by the assessment of both the percent area stained (B) and fibrosis density (C). B: *P < 0.05, HFSED vs. SED animals; †P = 0.10, HFSED vs. HFTR animals (by one-way ANOVA). C: *P < 0.05, HFSED vs. HFTR animals; †P < 0.10, HFSED vs. SED animals (by one-way ANOVA).
Fig. 3.
Low-intensity exercise training attenuates aortic banding-induced increases in LV collagen deposition. A: representative histological sections of Picrosirius red-stained LVs showing the increased collagen in HFSED animals. Magnification: ×40. B: increased LV collagen in HFSED animals was attenuated by low-intensity exercise training. *P < 0.10 (by one-way ANOVA); †P < 0.05, HFSED vs. SED animals (by a two-tailed independent samples t-test). C: decreases in collagen density were observed in HFTR animals. *P < 0.10, HFTR vs. HFSED and SED animals (by one-way ANOVA).
Aortic banding-induced mitochondrial dysfunction is prevented by low-intensity exercise training.
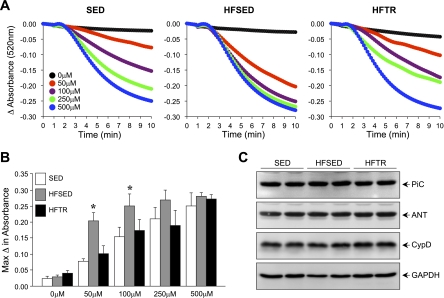
Mitochondrial dysfunction was evident in HFSED animals, as indicated by Ca2+-induced mitochondrial swelling (Fig. 4). The graded mitochondrial swelling response to increases in Ca2+ concentration seen in the SED control group was lost in the HFSED group, as application of 50 μM Ca2+ induced near-maximal mitochondrial swelling (Fig. 4A). Low-intensity exercise training in HFTR animals attenuated this response. Quantification of these measures indicated that the maximal change in absorbance was significantly greater at 50 and 100 μM Ca2+ concentrations in the HFSED group compared with the SED group (Fig. 4B). The observed alterations in MPT were not due to changes in the protein expression of components thought to comprise the MPTP. Western blots performed on LV tissue for the mitochondrial phosphate carrier, ANT, and CypD indicated no differences between groups (Fig. 4C).
Fig. 4.
Increased sensitivity to mitochondrial permeability transition (MPT) in failing hearts is attenuated by low-intensity exercise. A: Ca2+-induced mitochondrial swelling, an index of MPT, in SED, HFSED, and HFTR pigs. Each data point represents the mean absorbance for 4–5 pigs/group. B: quantification of the maximum changes in absorbance observed for each concentration of Ca2+ in each group. The maximum change in absorbance was greater at 50 and 100 μM Ca2+ concentrations in HFSED animals. *P < 0.05, HFSED vs. SED animals (by one-way ANOVA). C: Western blot analysis for phosphate carrier (PiC), adenine nucleotide translocase (ANT), and cyclophilin D (CypD) in LV tissue from SED, HFSED, and HFTR pigs, showing no differences in the protein expression of components thought to comprise the MPT pore. GAPDH was used to demonstrate equivalent loading.
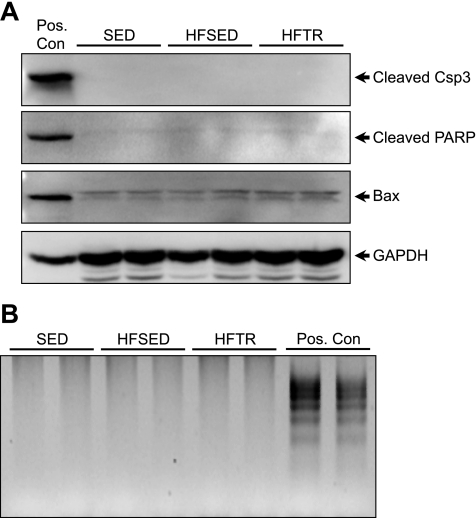
Mitochondrial dysfunction in HFSED animals is not associated with increased apoptosis.
The increased sensitivity to MPT in HFSED animals was not associated with an increase in the protein expression of well-established apoptotic markers. Western blots using cytosolic extracts for Bax, cleaved caspase-3, and cleaved PARP indicated no differences between groups (Fig. 5A). Furthermore, laddering of genomic DNA extracted from LV tissue indicated no increase in DNA fragmentation among groups (Fig. 5B).
Fig. 5.
Increased sensitivity to MPT in HFSED miniature swine is not associated with increased apoptosis. A: Western blot analysis for Bax, cleaved caspase-3 (Csp3), and cleaved poly(ADP-ribose) polymerase (PARP) in cytosolic extracts from SED, HFSED, and HFTR pigs. GAPDH was used to demonstrate equivalent loading. Pos. Con indicates human embryonic kidney-293 cells treated with 1 μM staurosporine for 4 h. B: DNA laddering in LV tissue from SED, HFSED, and HFTR pigs. Pos. Con indicates the positive control supplied with the PCR-based kit.
DISCUSSION
The results of our study illustrate several novel findings: 1) low-intensity interval exercise training attenuates pathological LV remodeling while preserving LV function, including reductions in LV fibrosis and collagen deposition, in a large animal model of compensated HF and 2) pathological LV hypertrophy in aortic-banded sedentary animals is associated with an increased sensitivity to Ca2+-induced mitochondrial swelling, an index of MPT that is attenuated by low-intensity exercise training.
Although low- to moderate-intensity exercise is currently recommended and considered safe for patients with stable HF, the intensity and volume of exercise required to optimize health benefits in this setting remain unclear, as evident by recent studies (4, 11, 43, 54) illustrating disparate results to exercise of varying frequency and intensity. Thus, a debate exists regarding the correct balance of exercise necessary to improve the prognosis of patients with HF against placing further demands on an already overstressed myocardium. Our exercise protocol was designed similarly to that previously published by Wisloff et al. (54), which allowed HF patients to exercise at higher intensities using brief intervals of increased work, thus providing a greater exercise stimulus while taking into account the exercise intolerance prevalent in HF. Previous work in humans (15, 19, 54) and in numerous small animal models of HF and/or hypertension (14, 25, 29, 34, 39) have demonstrated that exercise training attenuates or reverses pathological LV remodeling. Many of these studies showed concurrent increases in markers of peripheral mitochondrial markers of biogenesis (54) or inhibition of proapoptotic signaling pathways (14, 25). To address the lack of studies in large animal models regarding the mechanisms underlying the effects of exercise training on LV remodeling in HF, we conducted this study in a miniature swine model of compensated HF using novel exercise protocols of tolerable intensity to determine the impact on LV function, remodeling, and cardiomyocyte mitochondrial function.
The key finding of this study demonstrates that chronic low-intensity exercise attenuated pathological remodeling and preserved LV function. In the present study, exercise training did not prevent LV remodeling, as evident by our echocardiography, postmortem, and histological data. LVEDD, heart weight-to-BW ratio, LV + S-to-BW ratio, LV + S-to-RV ratio, and myocyte cross-sectional area were significantly increased in aortic-banded animals regardless of training status. However, the hypertrophy observed in exercise-trained animals was associated with indicators of hypertrophy typically considered physiological. The continuous increase in LVESV and LVESD in HFSED animals observed from 8 to 24 wk postbanding was attenuated in HFTR animals, suggesting an improvement in the positive inotropic state of the LV after low-intensity interval exercise training. The rate of normal age-related growth, as indicated by the ratios of LVEDV and LVEDD to weight, was disproportionate in HFSED animals compared with HFTR and SED animals. Furthermore, the increased lung weight-to-BW ratios present in HFSED animals was prevented in the HFTR group, suggesting that low-intensity exercise was adequate to preserve LV function and prevent lung congestion. Our findings are largely in agreement with human HF exercise studies that found an attenuation or a reversal of LV end-systolic measures of dimension and volume (15, 54), which, in one case, was associated with a decline in pulmonary vascular resistance (19).
In addition to its effects on LV morphology, low-intensity interval exercise training preserved LV function, as indicated by the maintenance of fractional shortening, EF, and MWS accompanied by a training-induced increase in SV. Our findings echo previously published human studies (15, 19, 54) showing increased cardiac output, SV, and EF after 3–6 mo of exercise training. The preservation of function in HFTR animals may in part be due to the attenuation of LV fibrosis, as previous studies in human patients with aortic stenosis have shown that EF is negatively correlated to the amount of LV fibrosis (21) and that SV significantly decreases in parallel with fibrosis severity (51). Interestingly, improvements in resting hemodynamics were observed after protocols using interval training and/or higher-intensity exercise based on patient tolerance (15, 19, 54) but not in patients that performed moderate continuous training in which subjects were instructed to “walk continuously…without breathing heavily” (54), illustrating the importance of appropriate training intensity in obtaining health benefits in a HF patient population. The combination of an increase in LVEDV, attenuation of pathological increases in LVESV and LVESD, and preservation of load-dependent LV function in HFTR animals suggests that the training-induced increase in SV is the result of an increase in the positive inotropic state of the heart after exercise training.
To this point, the majority of studies examining the underlying mechanisms involved in the prevention or reversal of LV hypertrophy after exercise in HF have been performed in rodent models. One variable thought to impact LV stiffness and potentially function in HF is an increase in LV fibrosis, a hallmark feature of pathological LV remodeling (9, 21). Reductions in LV fibrosis and collagen have been found following several modes of exercise (14, 25, 29, 34, 39). In the present study, we show, for the first time to the best of our knowledge, similar findings in a large animal model of compensated HF. Low-intensity interval exercise training attenuated increases in fibrosis and total collagen, results suggesting that exercise of a tolerable intensity for HF patients may be able to prevent the fibrotic phenotype and extracellular matrix disarray associated with pathological LV remodeling.
The progression from compensated hypertrophy to LV dilation is accompanied by increases in myocyte death, of which the mitochondrion is a key regulator. Apoptosis rates 50–100% greater than in control subjects have been observed in human end-stage dilated cardiomyopathy (16, 40, 42) and 10% increases in apoptosis rates over 2–6 mo generated dilated cardiomyopathy in transgenic mice overexpressing caspase-8 (12, 52). The prevention of cardiomyocyte loss and elucidation of the underlying molecular mechanisms have recently gained attention as a potential clinical treatment of HF (2, 7). The MPTP, a nonspecific channel thought to span both mitochondrial membranes, mediates the increases in mitochondrial permeability associated with cell death (18, 26). Increases in matrix Ca2+ and ROS induce MPTP opening, whereas adenine nucleotides inhibit the pore; indeed, many cardiac diseases, including HF, are associated with increases in MPT activators (Ca2+ and ROS) and reductions in MPT inhibitors (ATP/ADP). Studies in rodents have shown that inhibition of the MPTP blunts the loss of cardiac myocytes that underlies several cardiac pathologies, including myocardial ischemia-reperfusion injury (3, 5, 8, 20, 35) and Ca2+-induced cardiomyopathy (36). Considerable evidence exists demonstrating the anticell death effects of exercise, including inhibition of MPT (13, 22, 24, 28, 44, 45). Our results demonstrate that low-intensity interval exercise training prevented the mitochondrial dysfunction observed in HFSED animals, as indicated by an increase in Ca2+-induced MPT. Although a recent study by Matas et al. (32) showed an increase in the protein expression of CypD in rat model of chronic volume overload hypertrophy, we did not observe a change in any of our experimental groups regarding the protein expression of CypD, ANT, or phosphate carrier, molecular components thought to comprise the MPTP. Furthermore, we did not observe an increase in several proteins considered as markers of apoptosis, including Bax, cleaved caspase-3, cleaved PARP, cytochrome c (data not shown), or DNA fragmentation despite the increase in MPT sensitivity. Importantly, we provide evidence that early mitochondrial dysfunction likely plays a causal role in the development of terminal decompensated HF, a question of considerable interest given that previous observations of mitochondrial defects have been in observed in human hearts with end-stage HF.
In conclusion, our results demonstrate that chronic low-intensity interval exercise training prevents pathological LV remodeling and mitochondrial dysfunction in HF. Specifically, LV hypertrophy induced by aortic banding was characterized by disproportionate growth, increased fibrosis, and enhanced sensitivity to Ca2+-induced MPT. Low-intensity interval exercise training preserved LV function, as exemplified by an attenuation of fibrosis, maintenance of a more positive inotropic state, and inhibited increased susceptibility to MPT. Our results indicate that exercise may slow or prevent the loss of cardiomyocytes, an effect of great significance given the observation of mitochondrial dysfunction in early compensated HF and its probable role in the development of decompensated HF. We believe that the use of a large animal model and exercise paradigm of tolerable intensity found to have beneficial effects in humans strengthen the relevance of our findings and provide further evidence of the therapeutic utility of exercise in a clinical setting.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-52490 (to D. K. Bowles), HL-093982-01 (to C. A. Emter), and HL-094404 (to C. P. Baines).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Steve (Hsiao-Tung) Yang for teaching us to perform the aortic banding surgeries, Rumi Faizer for the gift of Gore-Tex, Jennifer Casati-Zajicek and Alexa Bermudez for the work on LV histology, Jan Ivey, Allison McGee, Corinne Mann, Rebecca Shaw, Isabelle Masseau, Erin O'Connor, and Hope Gole for considerable technical assistance, and Darla Tharp and Douglas Bowles for critical assessment of the study.
REFERENCES
- 1.Baines CP. The cardiac mitochondrion: nexus of stress. Annu Rev Physiol 72: 61–80, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Baines CP. The mitochondrial permeability transition pore as a target of cardioprotective signaling. Am J Physiol Heart Circ Physiol 293: H903–H904, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Chicco AJ, McCune SA, Emter CA, Sparagna GC, Rees ML, Bolden DA, Marshall KD, Murphy RC, Moore RL. Low-intensity exercise training delays heart failure and improves survival in female hypertensive heart failure rats. Hypertension 51: 1096–1102, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem 277: 34793–34799, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Curran-Everett D, Benos DJ. Guidelines for reporting statistics in journals published by the American Physiological Society. Am J Physiol Regul Integr Comp Physiol 287: R247–R249, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Das M. Apoptosis as a therapeutic target in heart failure. Am J Physiol Heart Circ Physiol 293: H1322–H1323, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem 276: 2571–2575, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Diwan A, Dorn GW., 2nd Decompensation of cardiac hypertrophy: cellular mechanisms and novel therapeutic targets. Physiology (Bethesda) 22: 56–64, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Dorn GW., 2nd Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res 81: 465–473, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emter CA, McCune SA, Sparagna GC, Radin MJ, Moore RL. Low-intensity exercise training delays onset of decompensated heart failure in spontaneously hypertensive heart failure rats. Am J Physiol Heart Circ Physiol 289: H2030–H2038, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest 115: 565–571, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French JP, Hamilton KL, Quindry JC, Lee Y, Upchurch PA, Powers SK. Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. FASEB J 22: 2862–2871, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garciarena CD, Pinilla OA, Nolly MB, Laguens RP, Escudero EM, Cingolani HE, Ennis IL. Endurance training in the spontaneously hypertensive rat: conversion of pathological into physiological cardiac hypertrophy. Hypertension 53: 708–714, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Giannuzzi P, Temporelli PL, Corra U, Tavazzi L. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure: results of the Exercise in Left Ventricular Dysfunction and Chronic Heart Failure (ELVD-CHF) trial. Circulation 108: 554–559, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami CA, Kajstura J, Anversa P. Myocyte death in the failing human heart is gender dependent. Circ Res 85: 856–866, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res 77: 334–343, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc Res 61: 372–385, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, Schoene N, Schuler G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA 283: 3095–3101, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res 60: 617–625, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, Bauer EP, Klovekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 107: 984–991, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Jin H, Yang R, Li W, Lu H, Ryan AM, Ogasawara AK, Van Peborgh J, Paoni NF. Effects of exercise training on cardiac function, gene expression, and apoptosis in rats. Am J Physiol Heart Circ Physiol 279: H2994–H3002, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11: 447–455, 1979 [DOI] [PubMed] [Google Scholar]
- 24.Kavazis AN, McClung JM, Hood DA, Powers SK. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol 294: H928–H935, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Konhilas JP, Watson PA, Maass A, Boucek DM, Horn T, Stauffer BL, Luckey SW, Rosenberg P, Leinwand LA. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ Res 98: 540–548, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 87: 99–163, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kronik G, Slany J, Mosslacher H. Comparative value of eight M-mode echocardiographic formulas for determining left ventricular stroke volume. A correlative study with thermodilution and left ventricular single-plane cineangiography. Circulation 60: 1308–1316, 1979 [DOI] [PubMed] [Google Scholar]
- 28.Kwak HB, Song W, Lawler JM. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J 20: 791–793, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Lachance D, Plante E, Bouchard-Thomassin AA, Champetier S, Roussel E, Drolet MC, Arsenault M, Couet J. Moderate exercise training improves survival and ventricular remodeling in an animal model of left ventricular volume overload. Circ Heart Fail 2: 437–445, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Laughlin MH, Overholser KA, Bhatte MJ. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol 67: 1140–1149, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis 14: 536–548, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Matas J, Young NT, Bourcier-Lucas C, Ascah A, Marcil M, Deschepper CF, Burelle Y. Increased expression and intramitochondrial translocation of cyclophilin-D associates with increased vulnerability of the permeability transition pore to stress-induced opening during compensated ventricular hypertrophy. J Mol Cell Cardiol 46: 420–430, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Mayet J, Ariff B, Wasan B, Chapman N, Shahi M, Poulter NR, Sever PS, Foale RA, Thom SA. Improvement in midwall myocardial shortening with regression of left ventricular hypertrophy. Hypertension 36: 755–759, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Miyachi M, Yazawa H, Furukawa M, Tsuboi K, Ohtake M, Nishizawa T, Hashimoto K, Yokoi T, Kojima T, Murate T, Yokota M, Murohara T, Koike Y, Nagata K. Exercise training alters left ventricular geometry and attenuates heart failure in dahl salt-sensitive hypertensive rats. Hypertension 53: 701–707, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest 117: 2431–2444, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA. Apoptosis in myocytes in end-stage heart failure. N Engl J Med 335: 1182–1189, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Narula J, Hajjar RJ, Dec GW. Apoptosis in the failing heart. Cardiol Clin 16: 691–710 and ix, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Oliveira RS, Ferreira JC, Gomes ER, Paixao NA, Rolim NP, Medeiros A, Guatimosim S, Brum PC. Cardiac anti-remodelling effect of aerobic training is associated with a reduction in the calcineurin/NFAT signalling pathway in heart failure mice. J Physiol 587: 3899–3910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med 336: 1131–1141, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58: 1072–1083, 1978 [DOI] [PubMed] [Google Scholar]
- 42.Saraste A, Pulkki K, Kallajoki M, Heikkila P, Laine P, Mattila S, Nieminen MS, Parvinen M, Voipio-Pulkki LM. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur J Clin Invest 29: 380–386, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Schultz RL, Swallow JG, Waters RP, Kuzman JA, Redetzke RA, Said S, de Escobar GM, Gerdes AM. Effects of excessive long-term exercise on cardiac function and myocyte remodeling in hypertensive heart failure rats. Hypertension 50: 410–416, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Siu PM, Bryner RW, Martyn JK, Alway SE. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. FASEB J 18: 1150–1152, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Siu PM, Bryner RW, Murlasits Z, Alway SE. Response of XIAP, ARC, and FLIP apoptotic suppressors to 8 wk of treadmill running in rat heart and skeletal muscle. J Appl Physiol 99: 204–209, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Srere PA. Citrate synthase. Methods Enzymol 13: 3–5, 1969 [Google Scholar]
- 47.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol 37: 7–11, 1976 [DOI] [PubMed] [Google Scholar]
- 48.Troy BL, Pombo J, Rackley CE. Measurement of left ventricular wall thickness and mass by echocardiography. Circulation 45: 602–611, 1972 [DOI] [PubMed] [Google Scholar]
- 49.Turk J, Turk M, Root C. Necropsy of the canine heart: a simple technique for quantifying ventricular hypertrophy and valvular alterations. Compend Contin Educ Practicing Vet 5: 905–912, 1983 [Google Scholar]
- 50.van Empel VP, Bertrand AT, Hofstra L, Crijns HJ, Doevendans PA, De Windt LJ. Myocyte apoptosis in heart failure. Cardiovasc Res 67: 21–29, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, Lange V, Beer M, Gattenlohner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 120: 577–584, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest 111: 1497–1504, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams JL, Hathaway CA, Kloster KL, Layne BH. Low power, type II errors, and other statistical problems in recent cardiovascular research. Am J Physiol Heart Circ Physiol 273: H487–H493, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115: 3086–3094, 2007 [DOI] [PubMed] [Google Scholar]