Abstract
Human aldo-keto reductase (AKR) 1C3, type 2 3α-hydroxysteroid dehydrogenase (HSC)/ type 5 17β-HSD, is known to be involved in steroids, prostaglandins, and lipid aldehydes metabolism. The expression of AKR1C3 has been demonstrated in hormone-dependent normal tissues such as breast, endometrium, prostate, and testis; and de -regulated AKR1C3 expression has been shown in breast carcinoma, endometrial hyperplasia, endometrial carcinoma, and prostate carcinoma. AKR1C3 expression has also been demonstrated in hormone-independent normal tissues (renal tubules and urothelium) and neoplastic tissues (renal cell carcinoma, Wilm's tumor, and urothelial cell carcinoma). Extensive expression of AKR1C3 in normal and neoplastic as well as hormone-dependent and hormone-independent tissues indicates that AKR1C3 may have functions beyond steroid hormone metabolism. In this report, we describe a widespread expression of AKR1C3 in glial neoplasms and meningiomas, with limited expression in medulloblastoma and no expression in Schwannoma. These tumors, except meningioma, are not classically considered to be sex hormone-dependent or related brain tumors. The current results corroborate our earlier observations that AKR1C3 is expressed in both sex hormone-dependent and hormone-independent malignancies. Similar to AKR1C3 distribution in Wilm’s tumor, we also demonstrate that expression of AKR1C3 is reduced in tumors with embryonic phenotypes.
Keywords: AKR, aldo-keto reductase; AR, androgen receptor; ER, estrogen receptor; HSD, hydroxysteroid dehydrogenase; PG, prostaglandin; PR, progesterone receptor; PPAR, peroxisome proliferator activating receptor
Introduction
The aldo-keto reductases (AKRs) comprise a functionally diverse 15-gene family [1]. Members of the AKR superfamily are generally monomeric (37 kD), cytosolic, and NAD(P)(H)-dependent oxidoreductases that share a common (α/β)8-barrel structural motif (http://www.med.upenn.eud/akr). This family of enzymes convert carbonyl groups to primary or secondary alcohols [2]. Four human AKR1C isoforms have been cloned and characterized; they are known as AKR1C1 [20α(3α)-hydroxysteroid dehydrogenase (HSD)] [3], AKR1C2 (type 3 3α-HSD) [4, 5], AKR1C3 (type 2 3α/type 5 17P-HSD) [6, 7], and AKR1C4 (type 1 3α-HSD) [5]. Natural substrates for these enzymes include steroids, prostaglandins (PGs), and lipid aldehydes [8].
Based on enzyme kinetics, AKR1C3 possesses 3α-hydroxysteroid dehydrogenase (HSD), 3p-HSD, 17P -HSD, and 11-ketoprostaglandin reductase activities, and catalyzes estrogen, progesterone, androgen, and PG metabolism [7, 9-11]. As a result, AKR1C3 is capable of indirectly governing ligand access to various nuclear receptors, including estrogen receptor (ER), progesterone receptor (PR), androgen receptor (AR), and peroxisome proliferator-activated receptor (PPAR), and regulating trans-activation activities of these nuclear receptors through intracrine actions [12]. The presence of AKR1C3 has been demonstrated in steroid hormone-dependent cells including breast cells [13], endometrial cells [14], prostate cells, and Leydig cells [15]. De-regulated expression of AKR1C3 has been demonstrated in multiple types of hormone-related cancers, including breast cancer [16], endometrial cancer [14, 17], and prostate cancer [18-21].
AKR1C3 expression has also been identified in classical hormone-independent cells including urothelial epithelium [19] and epithelium of the renal tubules [22]. In addition, abnormal AKR1C3 expression has been demonstrated in non-hormone-related cancers, including lung cancer [23] and Wilms’ tumor [24]. The role of AKR1C3 in the development and progress of these non-hormone-related cancers remain unknown.
Studies of AKR1C isoforms in the brain have been focused on neurosteroid biosynthesis [25, 26] that is related to anxiolytic steroid production [27] or neurodegenerative diseases [28]. In the brain, AKR1C3 mRNA has been detected using isoform-selective PCR primers [10]. AKR1C3-mediated neurosteroid conversion in brain tumors remains undefined. In this communication, AKR1C3 distributions in different brain tumor types including medulloblastoma, glial neoplasm (astrocytic, oligodendroglial, and ependymal), Schwannoma, and meningioma are described.
Materials and methods
Materials
Mouse anti-AKR1C3 monoclonal antibody was produced in our laboratory [13]. Stable diami-nobenzidine tetrahydrochloride (DAB) and goat serum were purchased from Invitrogen (Carlsbad, CA). Hematoxylin and permount mounting media were obtained from Sigma-Aldrich (St. Louis, MO). Biotinylated goat-anti mouse IgG antibody and horseradish peroxidase (HRP)-conjugated streptavidin were obtained from Vector Laboratories (Burlingame, CA).
Human tissues
A total of 72 cases of archival, formalin fixed, paraffin surgical pathology brain tumor specimens, all from different patients, and 4 autopsy brains with no neuropathologic findings, were obtained from the Department of Pathology at the University of Oklahoma Health Sciences Center with Institutional Review Board (IRB) approval for this study. This consortium included 10 cases of medulloblastoma, 17 cases of astrocytic neoplasms including glioblastoma, 9 cases of oligodendroglial neoplasms, 11 cases of ependymal neoplasms, 7 cases of schwannomas, and 18 cases of meningeal neoplasms. The results of deletion of 1p and 19q of oligodendroglial tumors are obtained from the original pathology report.
Immunohistochemistry of tissue sections
Immunohistochemistry of human tissue sections followed our previously reported procedures [19] and were performed in duplicates. Briefly, tissue sections cut at 4-6 μm were mounted and baked at 60 °C for 1 hr. Sections were de-paraffinized with xylene and re-hydrated in graded ethanol followed by rinses with 0.1 M Tris-HCI (pH 7.6). Endogenous peroxidase activity was blocked by incubating the tissue sections with 1.6% H2O2 in methanol for 30 min. Antigen retrieval was performed with 0.01 M sodium citric acid buffer (pH 6.0) at 95 °C for 1 hr. Non-specific binding was blocked by incubating the tissue sections with 0.1 M Tris -HCI containing 10% goat serum for 2 hr. AKR1C3 was then detected by incubated the tissue sections with mouse anti-AKR1C3 monoclonal antibody (clone NP6G6.A6) at a 1:200 dilution in the above blocking solution, and incubating in a moist chamber at 4 °C overnight. Negative controls were performed in parallel in the absence of primary antibody. After washes with 0.1 M Tris-HCI, the tissue sections were treated with 1:400 dilution of biotinylated horse anti-mouse secondary antibody and incubated at room temperature for 2 hr. Following another rinse with 0.1 M Tris-HCI, antibody binding was detected by incubating the tissue sections with HRP-conjugated streptavidin at room temperature for 30 min. DAB-H2O2 substrate was then added to the slides and incubated at room temperature for an additional 4 min. Tissue sections were counter stained lightly with hematoxylin, dehydrated in graded alcohol, cleared in xylene, and mounted with Permount Mounting Media for visualization by light-microscopy.
Immunohistochemical evaluation and scoring
The stained sections were evaluated with a conventional light microscope and digital photomicrographs were taken. The percentage of positive cells within the entire population of tumor cells were evaluated and allocated to one of the following categories: negative to positivity < 5%, positivity >5% but ≤ 25%, positivity >25% but ≤ 75%, >75% but ≤100% positivity, and 100% positivity. The tumors were then segregated into 4 categories: Diffuse immunoreactivity (positivity >75%), widespread immunoreactivity (positivity >25% but < 75%), focal immunoreactivity (positivity >5% but < 25%), sporadic or no reactivity (negative to positivity ≤ 5%). The intensity of staining is also evaluated for being weak, moderate, and strongfor every case.
Results
Medulloblastomas
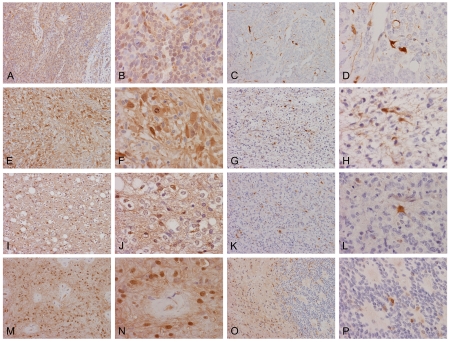
A total of 10 biopsy specimens were studied (Table 1). One of the cases (10%) showed extensive and strong positive immunoreactivity (Figure 1A and 1B) and fell into the diffuse immunoreactivity group. Both cytoplasmic immunoreactivity and nuclear immunoreactivity were demonstrated. There were 2 cases (20%) in the widespread immunoreactivity group and both demonstrated only moderate to weak immunoreactivity in tumor cells. The neuropils tended to be positive. There were also 3 cases (30%) that demonstrated sporadic, isolated positive cells and 4 cases (40%) that were entirely negative in tumor cells. The immunoreactivity was low in medulloblastoma with 7 of the 10 cases (70%) belonged to the sporadic or no immunoreactivity group (Table 2).
Table 1.
Intensity and percentage of positive immunoreactivity
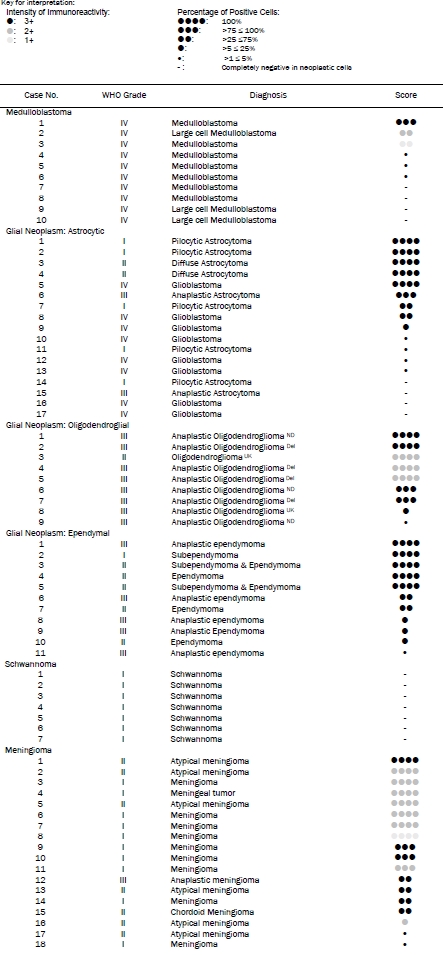 |
Del: Chromosome 1p and 19q are deleted as per pathology report.
ND: No deletion of chromosome 1p and 19q as per pathology report.
UK: The status of 1p and 19q are unknown, no test was performed as per pathology report.
Figure 1.
Distribution of AKR1C3 in medulloblastomas and glial neoplasms. Widespread nuclear and cytoplasmic immunoreactivities are present in this medulloblastoma (A and B). Sporadic but strong positive immunoreactivity is present in another medulloblastoma (C and D). In this anaplastic astrocytoma (E and F), practically all neoplastic cells show nuclear and cytoplasmic immunoreactivities. In contrast, the immunoreactivities in this astrocytoma (G and H) are present in only some cells and their stellate cytoplasmic processes are outlined. In this oligodendroglioma (I and J), the immunoreactivities is both nuclear and cytoplasmic. In another case of anaplastic oligodendroma (K and L), there are sporadic immunoreactive cells with strong nuclear and cytoplasmic immunoreactivities. In this case of ependymoma (M and N), practically all the cytoplasmic processes and nuclei are immunoreactive. In another case (0 and P), immunoreactivities are present in the less cellular area while only sporadic positive cells are noted in the more cellular area. (Original magnification: A, C, E, G, I, K, M, and 0 are 20×, B, D, F, H, J, L, N, and P are 60×)
Table 2.
Summary of immunoreactivities
| Diagnosis | No. of | Diffuse | Wide spread | Focal | Sporadic or no |
|---|---|---|---|---|---|
| Medulloblastoma | 10 | 1*(10%) | 2 (20%) | 0 (0%) | 7 (70%) |
| Glial neoplasm | 37 | 18 (48.6%) | 4 (10.8%) | 5 (13.6%) | 10(27%) |
| Schwannoma | 7 | 0 (%) | 0 (%) | 0 (%) | 7 (100%) |
| Meningioma | 18 | 11 (61.2%) | 4 (22.2%) | 1 (5.5%) | 2 (11.1%) |
This number indicates the number of positive cases.
Glial Neoplasms
A total of 37 glial neoplasms were studied (17 astrocytic neoplasms and glioblastomas, 9 oli-godendroglial neoplasms, and 11 ependymal neoplasms) (Table 1).
In astrocytic tumors and glioblastomas, 6 of the 17 cases (35.2%) demonstrated widespread immunoreactivity and belong to the diffuse immunoreactivity group. Both cytoplasmic and nuclear immunoreactivity were demonstrated in these tumors (Figure 1E and 1F). There were 2 cases (11.8%) with widespread immunoreactivity, 1 case (5.9%) with focal immunoreactivity, and 8 cases (47.1%) with very low or no immunoreactivity. In the cases with sporadic or focal immunoreactivity, sporadic or small clusters of positive cells are scattered within the tumor (Figure 1G and 1H).
In oligodendroglial neoplasms, 7 of the 9 cases (77.8%) demonstrated diffuse immunoreactivity. There was 1 case that fell into the focal immunoreactivity group (11.1%) and 1 case in the sporadic or no immunoreactivity group (11.1%) (Table 1). In contrast to the astrocytic tumors, it was more common to see cytoplasmic immunoreactivity only in many tumor cells but combined nuclear and cytoplasmic immunoreactivity were also readily seen (Figure 1I and 1J). In tumor with focal immunoreactivity, sporadic positive cells were present. Some of these cells had morphologic features astrocytic cells (Figure 1K and 1L).
Out of the 7 cases with widespread immunoreactivity, 4 of the cases had deletion of chromosome 1p and 19q. There was no deletion of chromosome 1p and 19q in 2 of the cases and the status of one of the cases is not known. One of the cases that belonged to the sporadic or no immunoreactivity group did not have deletion and the status of the other is uncertain. Our sample size is too small to conclude on the relationship of AKR1C3 and co-deletion of chromosome 1p and 19q.
In ependymal neoplasms, 5 of the 11 cases (45.5%) demonstrated widespread and strong immunoreactivity and belonged to the diffuse immunoreactivity group. There were 2 cases (18.2%) that fell into the widespread immunoreactivity group, 3 cases (27.2%) in the focal immunoreactivity group and 1 case (9.1%) in the sporadic or no immunoreactivity group. Combined nuclear and cytoplasmic immunoreactivity was the most common pattern (Figure 1M and 1N). Sporadic positive cells appeared to be mostly genuine ependymal tumor cells.
In the glial neoplasms (Table 2), slightly less than half of all the cases belonged to the diffuse immunoreactivity group, slightly under one quarter of the cases belonged to the widespread or focal immunoreactivity group, and slightly over one quarter of the cases belonged to the sporadic or no immunoreactivity group. The expression of AKR1C3 does not appear to correlate with histologic grade (Table 1).
Schwannoma
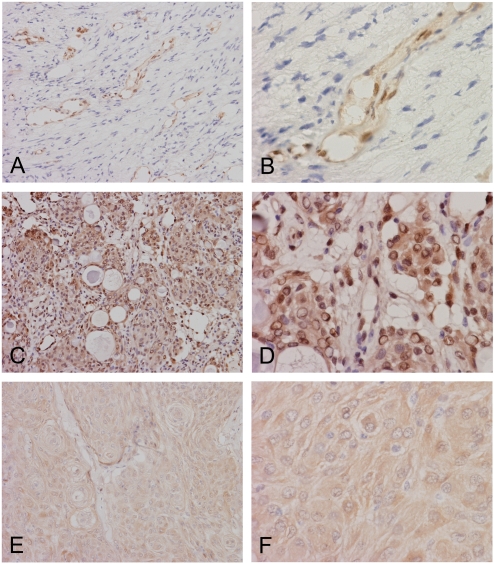
A total of 7 intracranial schwannoma were studied and they were uniformly negative for AKR1C3 immunoreactivity in the tumor cells (Table 1). As we described before, however, the endothelial cells were strongly positive and served as an internal control (Figure 2A and 2B).
Figure 2.
Expression of AKR1C3 in schwannomas and meningiomas. Neoplastic cells in this schwannoma (A and B) are completely negative. Note that the endothelial cells are strongly immunoreactive which serve as internal positive control. In this meningioma (C and D), there are widespread and strong cytoplasmic and nuclear immunoreactivities in tumor cells. In another meningioma (E and F), there is weak cytoplasmic immunoreactivities and very little nuclear immunoreactivities. Although the immunoreactivities are weak, it appears definitive when compared to the schwannoma cells. (Original magnification: A, C, and E are 20×, B, D, and F are 60×)
Meningioma
A total of 18 intracranial meningiomas were studied (Table 1). There were 11 cases (61.1%) that fell into the diffuse immunoreactivity group. There were 4 cases (22.2%) that showed wide spread immunoreactivity, 1 case with focal immunoreactivity (5.6%) and 2 cases with sporadic immunoreactivity (11.1%). Over three quarters of the cases showed diffuse or widespread immunoreactivity (Table 2). Similar to the other tumors being studied, combined nuclear and cytoplasmic immunoreactivities were very common (Figure 2C and 2D) particularly for those that diffusely and strongly express AKR1C3. In some of the cases that had less than strong immunoreactivity of AKR1C3, it is common to demonstrate predominantly cytoplasmic immunoreactivity without nuclear reactivity (Figure 2C and 2D). There would be a concern that these immunoreactivities were back-ground. However, there were clearly totally negative areas without this type of low intensity immunoreactivity in other tumors in this study such as the meduloloblastomas and schwnno-mas (Figure 1C, 1D and Figure 2A and 2B). We concluded that these low intensity immunoreactivities were genuine positive immunoreactivity.
Control Brains from Post Mortem and Reactive Astrocytes
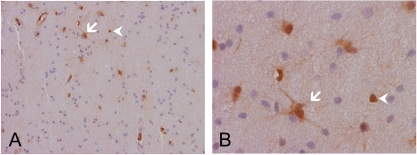
Four adult post mortem brains with no significant gross or microscopic pathologic findings were studied. No immunoreactivities were detected in these brains. In particular, there were no immunoreactivities in the endothelial cells. The immunoreactivity of residual brain tissue in the tumor cases of this study that were largely uninvolved by diffuse astrocytomas (WHO grade II) was studied. We demonstrated a low number of strongly immunoreactive cells with morphologic features highly suggestive of reactive astrocytes (Figure 3, arrow). There are also a few cells with strongly immunoreactive nuclei but no cytoplasmic immunoreactivity. It was not certain whether these are neoplastic astrocytes or reactive astrocytes (Figure 3, arrowheads). The brain parenchyma in these fragments, however, did not appear to be immunoreactive.
Figure 3.
AKR1C3 expression in normal brain tissues. These low and high magnification images are residual tissue largely uninvolved by tumor cells taken from a case of astrocytoma. Note that the residual brain parenchymal tissue is largely negative for nuclear and cytoplasmic immunoreactivities. There are some cells that have the morphologic features of reactive astrocytes (arrow) and also isolated positive nuclei (arrow head) without accompanying cytoplasmic immunoreactivity.
Discussion
The immunoreactivity for AKR1C3 of 72 surgically removed brain tumors and 4 normal control autopsy brains was studied. There were 10 medulloblastomas, 17 astrocytic neoplasms including glioblastomas, 9 oligodendrogliomas, 11 ependymal tumors, 7 schwannomas, and 18 meningiomas. AKR1C3 immunoreactivity of these tumors fell into three major groups. The first is the non-expression group with schwan-noma as the best example and no positive immunoreactivity was detected in all the schwannomas cases. The second is the low expression group with medulloblastoma as the best example with 70% of the cases belonging to the sporadic or no immunoreactivity group. Astrocytic neoplasms and glioblastomas also have 8 out of the 17 cases (47.1%) belonging to the sporadic or no immunoreactivity group. The third is the high AKR1C3-positive group which includes the glial neoplasm as a whole and meningiomas.
There was no detectible AKR1C3 immunoreactivity in brain tissues obtained from autopsy. In particular, there was no AKR1C3 expression in endothelial cells which have been used as internal controls in all specimens analyzed. We suspect that this enzyme could have been degraded rapidly at post-mortem; and, therefore, the negative immunoreactivity could not be explained as an evidence of the absence of AKR1C3 in normal brain tissue. In cases where residual brain tissues not involved or only minimally involved by the astrocytic tumors are present, AKR1C3 immunoreactivity was present in a few cells that are either reactive astrocytes or sporadic infiltrating astrocytoma cells. The brain parenchyma is largely negative. These observations suggest, but would not confirm, that AKR1C3 is absent in normal brain parenchyma. The number of cases and the amount of residual parenchyma are very limited. More extensive studies are needed for a definitive conclusion, although normal surgical specimens of the brain would not be easy to obtain to perform the screening.
AKR1C3 expression is less widespread in me-dulloblastoma, a tumor that recapitulates phe-notypes of the embryonic brain, in comparison to glial neoplasms which demonstrate features of the mature central nervous system. This result is similar to one of our other studies where we demonstrated reduced expression of AKR1C3 in Wilms’ tumor which recapitulate phenotypes of embryonic kidney as compared to renal cell carcinoma [29]. Based on these results, the expression of AKR1C3 may be different at different stages in development of organs.
Both schwann cells and meningothelial cells originate from neural crest; and schwannomas and meningiomas display schwannian and meningothelial cell-specific features. Although schwannomas lack AKR1C3 immunoreactivity in this study, meningiomas are highly AKR1C3 immunoreactive. The shared neural crest origin of schwannomas and meningiomas does not seem to provide a corresponding link in AKR1C3 expression. We speculate that the partial epithelial phenotypes of meningiomas may contribute to the wide spread expression of AKR1C3, as AKR1C3 is extensively expressed in epithelium lining ducts and hollow organs including urothelial epithelium [19], endometirum [30], and other lining epithelium such as ducts of the salivary gland, pancreas, duodenal mu-cosa, and gastric mucosa (data not shown).
Intratumoral steroid hormone biosynthesis and metabolism is important in the etiology and progression of hormone-related cancers. In situ estrogen metabolism, including biosynthesis and degradation, has recently been thought to play a very important role in the development and progression of various human estrogen-dependent neoplasms. Based on enzyme kinetics, AKR1C is capable of metabolizing multiple steroid hormones (estrogen, progesterone, an-drogen, and PG). AKR1C3 can catalyze inter-conversions of estrone and 17β-estrodiol, progesterone and 20α-hydroxyprogesterone, various androgen metabolism [7, 10], as well as 9α, 11β-PGF2α through its 11-ketoprostaglandin reductase activities [11]. As a result, this enzyme can reduce or accumulate these steroid metabolites and may have significant impacts on intratumoral hormone balance with various levels of AKR1C3 expression in brain tumors.
AKR1C3-mediated steroids and PG metabolism has been observed in cultured brain tumor cells and in brain tumor tissues. For example, human astrocytomas can transform steroids to compounds with modified hormonal activity [31]. An in vitro metabolism study using homogenates from astrocytomas has shown 17β-estradiol to estrone, pregnenolone to progesterone, and testosterone to either andros-tenedione or estradiol steroid conversions, although these reactions might be disease- and age-dependent. In addition, PGF2α is produced in C-6 glioma cells and N18TG2 neuroblastoma cells following arachidonic acid stimulation [32]. The formation of AKR1C3-related metabolic compounds in brain tumor cells may affect physiology and pathophysiology of the brain and may be of clinical significance in brain tumori-genesis or progression.
Distribution and function of steroid hormone receptors have been studied in tumors of the central nervous system. Urbanska et al. reported that ERβ trans-activation can contribute to the development of genomic instability in medulloblastomas [33]. Suppression of ER signaling significantly inhibits growth and migration of medulloblastoma in vitro and in vivo [34]. Progressively decreased ERβ expression has shown to parallel the progression of astrocytic neoplasms: moderate to strong nuclear ERβ immunoreactivity in non-neoplastic astrocytes and low-grade astrocytomas, but immunonega-tive to weak immunoreactivity in high-grade tumors [35]. In addition, majority of meningiomas possess the ER (both ERα and ERβ), PR, and AR [36-39]; and many studies have been performed to examine the role of steroids and steroid receptors in the growth of meningiomas [40-42]. Although patterns of ER, PR, and AR expression have been shown to be different between schwannomas and meningiomas [43], there is conflicting information regarding the presence of steroid hormone receptors in schwannomas [44-46]. The relationship between steroid hormone expression and clinical stages of schwannomas remains unsettled. PR isoforms have also been studied in neurogenic tumors. Both PR-A and PR-B are equally expressed in meningioma, but PR-B is the predominant isoform compared with PR-A in astro-cytic tumors and Schwannomas. There is a statistically significant inverse correlation between PR-A expression and proliferation indices in as-trocytic and meningiomas tumors. AKR1C3 has been implicated as a stress-related gene and as a hypoxia-induced gene in the malignant human glioma U251 cell line [47]. Ragel et al. proposed that hypoxia-induced AKR1C3 may modulate progesterone production necessary for tumor growth and/or angiogenesis in brain tumor. These observations suggest that steroid hormones are locally synthesized and exert their actions through different nuclear receptors in the human central nervous system. AKR1C3 may be involved in regulation of intracellular hormone balance, and the growth and development of neurogenic tumors via a variety of nuclear steroid receptors [40].
Glioblastomas are typically associated with epidermal growth factor receptor (EGFR) amplification (usually associated with chromosome 10 loss and gain of chromosome 7); and oligoden-droglial tumors are often associated with deletion of chromosome 1p and 19q. These two molecular features are mutually exclusive [48, 49]. Using microarray analysis, Ducray et al. reported that AKR1C3 is among a set of 22 genes that can distinguish gliomas with EGFR amplification and oligodendroglimas with 1p19q codeletion [50]. Based on multidimensional genomic profiles and glioma patient survival, Bredel et al. also reported that AKR1C3, along with another 6 nonrandom landscape genes, is statistically significantly associated with the duration of overall survival in patients with gliomas [51]. In this study, we demonstrated the expression of AKR1C3 in both glioblastomas and oligodendroglioma. However, our sample size was too small to conclude on the relationship of AKR1C3 with glioblastomas or oligodendroglioma. The role of AKR1C3 in these tumors should be further investigated.
Although brain tumors are not classically classified as a type of hormone-related cancer, recent studies clearly show a relationship between hormone metabolism and tumor progression. In contrast to the classical belief, some brain tumors may be hormonal responsive in analogy to other hormone-related cancers. AKR1C3-mediated steroid hormones metabolism and their consequences of nuclear receptor trans-activation as well as tumorigenesis and progression in medulloblastoma, glial neoplasm, schwannoma, and meningioma deserves further study. Equally important, the involvement of AKR1C3 in cell differentiation and epithelial lining ducts and hollow organs needs to be studied in medulloblastomas as well as in schean-nomasand meningiomas.
Acknowledgments
This work was supported in part by an internal grant awarded to Aubrey Park from the Department of Pathology of the University of Oklahoma Health Sciences Center, and Department of Veterans Affairs Merit Review Award to HKL
References
- 1.Jez JM, Flynn TG, Penning TM. A new nomenclature for the aldo-keto reductase superfamily. Biochem Pharmacol. 1997;54:639–647. doi: 10.1016/s0006-2952(97)84253-0. [DOI] [PubMed] [Google Scholar]
- 2.Jez JM, Bennett MJ, Schlegel BP, Lewis M, Penning TM. Comparative anatomy of the aldoketo reductase superfamily. Biochem J. 1997;326:625–636. doi: 10.1042/bj3260625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hara A, Matsuura K, Tamada Y, Sato K, Miyabe Y, Deyashiki Y, Ishida N. Relationship of human liver dihydrodiol dehydrogenases to hepatic bile-acid-binding protein and an oxidoreductase of human colon cells. Biochem J. 1996;313:373–376. doi: 10.1042/bj3130373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dufort I, Soucy P, Labrie F, Luu-The V. Molecular cloning of human type 3 3αhydroxysteroid dehydrogenase that differs from 20α-hydroxysteroid dehydrogenase by seven amino acids. Biochem Biophy Res Communication. 1996;228:474–479. doi: 10.1006/bbrc.1996.1684. [DOI] [PubMed] [Google Scholar]
- 5.Deyashiki Y, Ogasawara A, Nakayama T, Nakanishi M, Miyabe Y, Sato K, Hara A. Molecular cloning of two human liver 3α-hydroxysteroid/dihydrodiol dehydrogenase isoenzymes that are identical with chlordecone reductase and bile-acid binder. Biochem J. 1994;299:545–552. doi: 10.1042/bj2990545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khanna M, Qin KN, Wang RW, Cheng KC. Substrate specificity, gene structure, and tissue-specific distribution of multiple human 3αhydroxysteroid dehydrogenases. J Biol Chem. 1995;270:20162–20168. doi: 10.1074/jbc.270.34.20162. [DOI] [PubMed] [Google Scholar]
- 7.Lin HK, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. Expression and characterization of recombinant type 2 3αhydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3α/17b-HSD activity and cellular distribution. Mol Endocrinol. 1997;11:1971–1984. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- 8.Hyndman D, Bauman DR, Heredia VV, Penning TM. The aldo-keto reductase superfamily homepage. Chem Biol Interact. 2003;143-144:621–631. doi: 10.1016/s0009-2797(02)00193-x. [DOI] [PubMed] [Google Scholar]
- 9.Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3αhydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3βhydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem. 2004;279:10784–10795. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- 10.Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuura K, Shiraishi H, Hara A, Sato K, Deyashiki Y, Ninomiya M, Sakai S. Identification of a principal mRNA species for human 3αhydroxysteroid dehydrogenase isoform (AKR1C3) that exhibits high prostaglandin D2 11ketoreductase activity. J Biochem (Tokyo) 1998;124:940–946. doi: 10.1093/oxfordjournals.jbchem.a022211. [DOI] [PubMed] [Google Scholar]
- 12.Penning TM, Steckelbroeck S, Bauman DR, Miller MW, Jin Y, Peehl DM, Fung KM, Lin HK. Aldo-keto reductase (AKR) 1C3: Role in prostate disease and the development of specific inhibitors. Mol Cell Endocrinol. 2006;248:182–191. doi: 10.1016/j.mce.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Lin HK, Steckelbroeck S, Fung KM, Jones AN, Penning TM. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3α-hydroxysteroid dehydrogenase/type 5 17β-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids. 2004;69:795–801. doi: 10.1016/j.steroids.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Rizner TL, Smuc T, Rupreht R, Sinkovec J, Penning TM. AKR1C1 and AKR1C3 may determine progesterone and estrogen ratios in endometrial cancer. Mol Cell Endocrinol. 2006;248:126–135. doi: 10.1016/j.mce.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Ashley RA, Yu Z, Fung KM, Frimberger D, Kropp BP, Penning TM, Lin HK. Developmental evaluation of aldo-keto reductase 1C3 expression in the cryptorchid testis. Urology. 2009 doi: 10.1016/j.urology.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 16.Lewis MJ, Wiebe JP, Heathcote JG. Expression of progesterone metabolizing enzyme genes (AKR1C1, AKR1C2, AKR1C3, SRD5A1, SRD5A2) is altered in human breast carcinoma. BMC Cancer. 2004;4:27. doi: 10.1186/1471-2407-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito K, Utsunomiya H, Suzuki T, Saitou S, Akahira J, Okamura K, Yaegashi N, Sasano H. 17βhydroxysteroid dehydrogenases in human endometrium and its disorders. Mol Cell Endocrinol. 2006;248:136–140. doi: 10.1016/j.mce.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura Y, Suzuki T, Nakabayashi M, Endoh M, Sakamoto K, Mikami Y, Moriya T, Ito A, Takahashi S, Yamada S, Arai Y, Sasano H. In situ androgen producing enzymes in human prostate cancer. Endocr Relat Cancer. 2005;12:101–107. doi: 10.1677/erc.1.00914. [DOI] [PubMed] [Google Scholar]
- 19.Fung KM, Samara ENS, Wong C, Metwalli A, Krlin R, Bane B, Liu CZ, Yang JT, Pitha JT, Culkin DJ, Kropp BP, Penning TM, Lin HK. Increased expression of type 2 3α-hydroxysteroid dehydrogenase/type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocr Relat Cancer. 2006;13:169–180. doi: 10.1677/erc.1.01048. [DOI] [PubMed] [Google Scholar]
- 20.Stanbrough M, Bubley G, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 21.Wako K, Kawasaki T, Yamana K, Suzuki K, Jiang S, Umezu H, Nishiyama T, Takahashi K, Hamakubo T, Kodama T, Naito M. Expression of androgen receptor through androgen-converting enzymes is associated with biological aggressiveness in prostate cancer. J Clin Pathol. 2008;61:448–454. doi: 10.1136/jcp.2007.050906. [DOI] [PubMed] [Google Scholar]
- 22.Azzarello J, Fung KM, Lin HK. Tissue distribution of human AKR1C3 and rat homolog in adult genitourinary system. J Histochem Cytochem. 2008;56:853–861. doi: 10.1369/jhc.2008.951384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan Q, Mumford JL, Shen M, Demarini DM, Bonner MR, He X, Yeager M, Welch R, Chanock S, Tian L, Chapman RS, Zheng T, Keohavong P, Caporaso N, Rothman N. Oxidative damage-related genes AKR1C3 and OGG1 modulate risks for lung cancer due to exposure to PAH-rich coal combustion emissions. Carcinogenesis. 2004;25:2177–2181. doi: 10.1093/carcin/bgh240. [DOI] [PubMed] [Google Scholar]
- 24.Azzarello JT, Lin H-K, Gherezghiher A, Zakharov V, Yu Z, Kropp BP, Culkin DJ, Penning TM, Fung K-M. Expression of AKR1C3 in renal cell carcinoma, papillary urothelial carcinoma, and Wilms’ tumor. Int J Clin Expt Pathol. 2009;3:147–155. [PMC free article] [PubMed] [Google Scholar]
- 25.Schule C, Baghai TC, di Michele F, Eser D, Pasini A, Romeo E, Rupprecht R. Mirtazapine does not influence tetrahydrodeoxycorticosterone levels in depressed patients. World J Biol Psychiatry. 2010;11:308–313. doi: 10.3109/15622970701639429. [DOI] [PubMed] [Google Scholar]
- 26.Higashi T, Nagura Y, Shimada K, Toyo'oka T. Studies on neurosteroids XXVI. Fluoxetineevoked changes in rat brain and serum levels of neuroactive androgen, 5α-androstane-3α,17βdiol. Biol Pharm Bull. 2009;32:1636–1638. doi: 10.1248/bpb.32.1636. [DOI] [PubMed] [Google Scholar]
- 27.Edinger KL, Frye CA. Testosterone's antianxiety and analgesic effects may be due in part to actions of its 5α-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418–430. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Luchetti S, Bossers K, Frajese GV, Swaab DF. Neurosteroid biosynthetic pathway changes in substantia nigra and caudate nucleus in Parkinson's disease. Brain Pathol. 2010;20:945–951. doi: 10.1111/j.1750-3639.2010.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azzarello JT, Lin H-K, Gherezghiher A, Zakharov V, Yu Z, Kropp BP, Culkin DJ, Penning TM, Fung K-M. Expression of AKR1C3 in renal cell carcinoma, papillary urothelial carcinoma, and Wilms’ tumor. Int J Clin Expt Pathol. 2009;3:147–155. [PMC free article] [PubMed] [Google Scholar]
- 30.Zakharov V, Lin HK, Azzarello J, McMeekin S, Moore KN, Penning TM, Fung KM. Suppressed expression of type 2 3α/type 5 17βhydroxysteroid dehydrogenase (AKR1C3) in endometrial hyperplasia and carcinoma. Int J Clin Exp Pathol. 2010;3:608–617. [PMC free article] [PubMed] [Google Scholar]
- 31.Weidenfeld J, Schiller H. Metabolism of steroids by human brain tumors. Clin Neuropharmacol. 1984;7:395–397. doi: 10.1097/00002826-198412000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Chan PH, Fishman RA. Alterations of membrane integrity and cellular constituents by arachidonic acid in neuroblastoma and glioma cells. Brain Res. 1982;248:151–157. doi: 10.1016/0006-8993(82)91156-8. [DOI] [PubMed] [Google Scholar]
- 33.Urbanska K, Pannizzo P, Lassak A, Gualco E, Surmacz E, Croul S, Del Valle L, Khalili K, Reiss K. Estrogen receptor β-mediated nuclear interaction between IRS-1 and Rad51 inhibits homologous recombination directed DNA repair in medulloblastoma. J Cell Physiol. 2009;219:392–401. doi: 10.1002/jcp.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belcher SM, Ma X, Le HH. Blockade of estrogen receptor signaling inhibits growth and migration of medulloblastoma. Endocrinology. 2009;150:1112–1121. doi: 10.1210/en.2008-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batistatou A, Stefanou D, Goussia A, Arkoumani E, Papavassiliou AG, Agnantis NJ. Estrogen receptor beta (ERβ) is expressed in brain astrocytic tumors and declines with dedifferentiation of the neoplasm. J Cancer Res Clin Oncol. 2004;130:405–410. doi: 10.1007/s00432-004-0548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll RS, Zhang J, Black PM. Expression of estrogen receptors α and β in human meningiomas. J Neurooncol. 1999;42:109–116. doi: 10.1023/a:1006158514866. [DOI] [PubMed] [Google Scholar]
- 37.Blankenstein MA, Blaauw G, Lamberts SW, Mulder E. Presence of progesterone receptors and absence of oestrogen receptors in human intracranial meningioma cytosols. Eur J Cancer Clin Oncol. 1983;19:365–370. doi: 10.1016/0277-5379(83)90134-7. [DOI] [PubMed] [Google Scholar]
- 38.Carroll RS, Glowacka D, Dashner K, Black PM. Progesterone receptor expression in meningiomas. Cancer Res. 1993;53:1312–1316. [PubMed] [Google Scholar]
- 39.Maxwell M, Galanopoulos T, Neville-Golden J, Antoniades HN. Expression of androgen and progesterone receptors in primary human meningiomas. J Neurosurg. 1993;78:456–462. doi: 10.3171/jns.1993.78.3.0456. [DOI] [PubMed] [Google Scholar]
- 40.Inoue T, Akahira J, Suzuki T, Darnel AD, Kaneko C, Takahashi K, Hatori M, Shirane R, Kumabe T, Kurokawa Y, Satomi S, Sasano H. Progesterone production and actions in the human central nervous system and neurogenic tumors. J Clin Endocrinol Metab. 2002;87:5325–5331. doi: 10.1210/jc.2002-012096. [DOI] [PubMed] [Google Scholar]
- 41.Carroll RS, Brown M, Zhang J, DiRenzo J, Font De Mora J, Black PM. Expression of a subset of steroid receptor cofactors is associated with progesterone receptor expression in meningiomas. Clin Cancer Res. 2000;6:3570–3575. [PubMed] [Google Scholar]
- 42.Jay JR, MacLaughlin DT, Riley KR, Martuza RL. Modulation of meningioma cell growth by sex steroid hormones in vitro. J Neurosurg. 1985;62:757–762. doi: 10.3171/jns.1985.62.5.0757. [DOI] [PubMed] [Google Scholar]
- 43.Carroll RS, Zhang JP, Black PM. Hormone receptors in vestibular schwannomas. Acta Neurochir. 1997;139:188–192. doi: 10.1007/BF01844749. [DOI] [PubMed] [Google Scholar]
- 44.Jaiswal S, Agrawal V, Jaiswal AK, Pandey R, Mahapatra AK. Expression of estrogen and progesterone receptors in vestibular schwannomas and their clinical significance. J Negat Results Biomed. 2009;8:9. doi: 10.1186/1477-5751-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cafer S, Bayramoglu I, Uzum N, Yilmaz M, Memis L, Uygur K. Expression and clinical significance of Ki-67, oestrogen and progesterone receptors in acoustic neuroma. J Laryngol Otol. 2008;122:125–127. doi: 10.1017/S0022215107000229. [DOI] [PubMed] [Google Scholar]
- 46.Markwalder TM, Waelti E, Markwalder RV. Estrogen and progestin receptors in acoustic and spinal neurilemmomas. Clinicopathologic correlations. Surg Neurol. 1986;26:142–148. doi: 10.1016/0090-3019(86)90366-6. [DOI] [PubMed] [Google Scholar]
- 47.Ragel BT, Couldwell WT, Gillespie DL, Jensen RL. Identification of hypoxia-induced genes in a malignant glioma cell line (U-251) by cDNA microarray analysis. Neurosurg Rev. 2007;30:181–187. doi: 10.1007/s10143-007-0070-z. [DOI] [PubMed] [Google Scholar]
- 48.Idbaih A, Marie Y, Lucchesi C, Pierron G, Manie E, Raynal V, Mosseri V, Hoang-Xuan K, Kujas M, Brito I, Mokhtari K, Sanson M, Barillot E, Aurias A, Delattre JY, Delattre O. BAC array CGH distinguishes mutually exclusive alterations that define clinicogenetic subtypes of gliomas. Int J Cancer. 2008;122:1778–1786. doi: 10.1002/ijc.23270. [DOI] [PubMed] [Google Scholar]
- 49.Jenkins RB, Blair H, Ballman KV, Giannini C, Arusell RM, Law M, Flynn H, Passe S, Felten S, Brown PD, Shaw EG, Buckner JC. A t(1;19) (q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852–9861. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- 50.Ducray F, Idbaih A, de Reynies A, Bieche I, Thillet J, Mokhtari K, Lair S, Marie Y, Paris S, Vidaud M, Hoang-Xuan K, Delattre O, Delattre JY, Sanson M. Anaplastic oligodendrogliomas with 1p19q codeletion have a proneural gene expression profile. Mol Cancer. 2008;7:41. doi: 10.1186/1476-4598-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bredel M, Scholtens DM, Harsh GR, Bredel C, Chandler JP, Renfrow JJ, Yadav AK, Vogel H, Scheck AC, Tibshirani R, Sikic BI. A network model of a cooperative genetic landscape in brain tumors. JAMA. 2009;302:261–275. doi: 10.1001/jama.2009.997. [DOI] [PMC free article] [PubMed] [Google Scholar]