Abstract
Primary Central nervous system lymphoma (PCNSL) is most frequently a diffuse large B cell lymphoma (DLBCL), which is confined to the Central nervous system (CNS). We performed an experiment in which lymphoma cells from a PCNSL patient were implanted subcutaneously in an athymic mouse. The lymphoma cells were shown to home to the CNS with histologic evaluations of the brain showing multiple large B cells in blood vessels consistent with intravascular large B cell lymphoma (IVL). We did not find any evidence of lymphoma at the site of implantation or other locations. The findings are consistent with highly selective tropism of PCNSLforthe CNS and its vasculature.
Keywords: Lymphoma, central nervous system, tropism
Introduction
Primary CNS lymphoma is most frequently a diffuse large B cell lymphoma with a tropism for the CNS microenvironment and is usually confined to the CNS. It is an aggressive lymphoma with poor prognosis and remains incurable in most patients [1].
It is an interesting B cell lymphoma because it is located in the CNS where very few B lymphocytes, if any, are found under normal circumstances [2]. Immunophenotypically, PCNSL has features of activated B cell (ABC) subtype of DLBCL [3]. Some studies have indicated that PCNSL is of germinal center B cell origin [4, 5]. All these findings indicate that PCNSL develops from a B cell that has been exposed to a germinal center influence outside the CNS. The biological mechanism for selective CNS tropism is not known. It appears that this biological property is essential for pathogensis of PCNSL.
In this report, we showed that PCNSL cells from a human patient homed back selectively to the brain when they were implanted in subcutaneous space in an athymic mouse, giving an in vivo confirmation of their selective CNS tropism. To our knowledge, CNS tropism of PCNSL has not been replicated in an experimental setting.
Materials and methods
Patient
The patient is a thirty year old female who developed gait instability and dysarthria during her third trimester of pregnancy. One month after the delivery, a stereotactic biopsy of a periven-tricular lesion revealed DLBCL. A staging evaluation including computerized axial tomography (CT scans) of chest, abdomen, and pelvis, bone marrow biopsy, opthalmologic evaluation, and cerebrospinal fluid (CSF) analysis were negative. Human immunodeficiency virus (HIV) was negative. She was diagnosed with PCNSL. She had poor response to high-dose Methotrexate therapy; and eventually received whole brain radiation with excellent response; but unfortunately developed severe neurotoxicity. Nine months after the initial diagnosis, she developed abdominal pain. Imaging scans revealed multiple lesions in liver with CT-guided needle biopsy confirming DLBCL. She deteriorated quickly and passed away. No autopsy was performed.
Tumor implantation
Fresh PCNSL sample from the initial diagnostic biopsy from the patient’ brain was implanted together with Matrigel in a 6 week-old athymic mouse subcutaneously. We did not perform any processing of the tissue such as tissue cultures. The tissue sample was rather small and we decided on implantation in only one mouse. The study was approved by Institutional Animal Care and Use Committe. Our objective was to grow a tumor for in vivo expansion to develop a PCNSL cell line. Initially, there was a lump at the site of implantation. It gradually disappeared over 6 weeks. At about 16 weeks after the implantation, the mouse became ill and died. Thorough autopsy examination of the whole body including all the internal organs did not reveal any obvious tumor at any sites including the subcutaneous implantation site. We could not determine the cause of death based on gross examination.
Immunohistochemical (IHC) studies
IHC analysis of CNS biopsy sample from the patient and murine brain was performed.
Single antibody staining
The single antibody stain was performed with the mouse monoclonal antibody for CD20 (Dako, Inc, Carpinteria, CA; 1:800). Sections (5 μm) were cut from paraffin-embedded tissue blocks. Paraffin was removed with xylene. The sections were equilibrated with absolute ethanol; then rehydrated with 95% ethanol and tap water. Sections were equilibrated with phosphate-buffered saline containingTween 20 for 5 minutes, then placed into hot antigen retrieval solution at pH 6.0 for 25 minutes and cooled to room temperature. Endogenous peroxidases were blocked by treatment with 3% hydrogen peroxide. Slides were incubated with anti-CD20 antibody. A DAKOCytomation Immunostainer Plus unit was used for immunostaining. Antibody was detected with Envision + Labelled Polymer (Dako). Sections were counterstained with Gill 1 Hematoxylin (Richard Allen Scientific/Thermo Fisher), and cover-slipped using Cyto-seal mounting media.
The CD20 primary antibody is from mouse and the secondary antibody is anti-murine. To clarify whether or not the positive CD20 stain in our case is related to contamination from intrinsic IgH in plasma within the murine blood vessels, we performed IHC study with same antibody for CD20 on normal murine brain as a negative control.
Double staining
Dual immunohistochemical studies were performed with the combination of CD20 (Dako; 1:800)/Osteopontin (SPP1; R&D Systems, Inc, Minneapolis, Minnesota; 1:10). The CD20 IHC was performed as described above. After visualization of antibody expression with diamino-benzidine, sections were rewashed in buffer. The procedure was then repeated and SPP1 antibodies applied. Sections were treated with a Biocare Mach 4 Universal AP (alkaline phos-phatase) kit (Biocare Medical, LLC, Concord, California) following manufacturer instruction. Expression of SPP1 was visualized with Vulcan Fast Red chromogen (Biocare Medical). All sections were then counterstained with hematoxylin.
Results
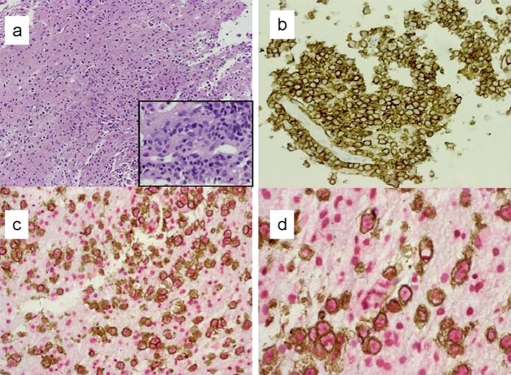
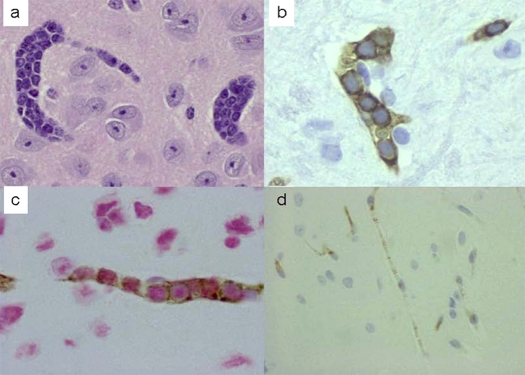
The Patient's brain biopsy showed large neo-plastic lymphocytes in the brain parenchyma with expression of CD20 in membranous pattern and SPP1 with predominant nuclear localization. The brain cells in the background are positive for osteopontin (Figure 1). IHCs on murine brain demonstrated intravascular large lymphoma cells with a similar pattern of CD20 and SPP1 expression as the patient's brain sample (Figure 2 a-c). We did not find any area of in-farcts in the murine brain. Immunohistochemistry studies were not performed in other organs. As for the negative control, although we did see some background stain in the normal murine brain, the differences are quite obvious. We did not detect any CD20 positive lymphocytes within the normal murine brain (Figure 2 d).
Figure 1.
IHC findings in patient's brain biopsy. The Patient's brain biopsy show the patchy infiltrate of neoplastic lymphocytes in the brain parenchyma (a, H&E × 10); immunostain of CD20 confirms the B cell lineage (b, × 20). The infiltrate demonstrates perivascular distribution (a, inlet × 40; and b). The dual stain of CD20 (brown, membranous pattern) and osteopontin (red, nuclear pattern) shows that lymphoma cells express osteopontin (c, × 10; d, × 40). The glial cells in the background are positive for osteopontin.
Figure 2.
IHC findings in murine brain. H and E stained section of murine brain demonstrates diffuse intravascular infiltrate of lymphoma cells (a, ×40); CD 20 stain shows multiple blood vessels loaded with CD20 positive lymphoma cells (b, ×60); while dual immunostain of CD20 and OPN show that lymphoma cells express both CD 20 (brown, complete circular membranous pattern) and OPN (red, predominantly nuclear pattern) (c, ×60). In contrast, the normal murine brain with anti-CD20 IHC is completely negative (d, × 40).
There is no tumor found in other organs and a general autopsy was performed.
Discussion
Our implantation experiment led to highly selective homing of PCNSL cells to the CNS vasculature with pathologic findings consistent with intravascular lymphoma. Given more time, there might be progression to parenchymal tumors. However, IVL may be lethal to mice and there may not be adequate time for parenchymal tumors to develop. Certain conclusions may be drawn from the study findings. It appears that PCNSL cells possess a molecular guidance system, which guides them back to the CNS micro-environment. They migrated back to the brain hematogenously after vascular invasion. Their CNS tropism was so selective that they all disappeared from the subcutaneous implantation site and homed to the CNS. They did not appear to be able to survive and thrive in a non-CNS mi-croenvironmentsuch as subcutaneous compartment.
One implication is in the development of PCNSL cell line from patients. Currently, there is no PCNSL cell line. It is apparent that murine sub-cutaneous compartment is not appropriate for growing these cells in vivo. Direct implantation in murine brain may be the only way to grow these cells in-vivo. It appears that subcutaneous implantation of PCNSL cells is a good model to study lymphoma cell migration and homing to the CNS. However, one should be aware of a long time lag of 16 weeks for CNS homing to become manifest.
Lymphomagenesis in PCNSL is not completely understood. Our hypothesis is that lymphoma cells originate in extracranial sites and then home completely to the CNS due to selective CNS tropism. The findings in secondary CNS lymphoma in which systemic lymphoma secondarily invades the CNS, suggest that lymphoma cells can home to the CNS from extracranial sites, possibly due to development of CNS tropism. There have been reports of coexistence of intravascular DLBCL in brain and PCNSL which would also support our hypothesis [6]. A study on HIV-infected individuals concluded that B cell infiltrates in the brain do not represent a pre-malignant PCNSL state [2]. The recurrence of lymphoma in the liver in our patient nine months after diagnosis with PCNSL would also suggest that the lymphoma developed outside the CNS.
The molecular biology of PCNSL is not well-understood. Our group has published a ‘CNS’ signature of PCNSL, consisting of a unique single gene expression and pathway signature, by comparing PCNSL to non-CNS DLBCL [7]. SPP1 (osteopontin) was found to be the most upregu-lated gene [7-10] and ECM-related pathways were down-regulated [7]. We suggest that these biologic alterations are part of a transitional process that B cells undergo to develop into PCNSL and may play an important role in CNS tropism. In this study, PCNSL cells express SPP1 as shown in our previous studies [7, 10]. The precise role of SPP1 in PCNSL needs to be further elucidated. CXCL13, a B cell attracting chemokine, has been shown to be upregulated in PCNSL [7, 11] and may play a role in CNS tropism of PCNSL.
In conclusion, B lymphoma cells in PCNSL seem to possess highly selective CNS tropism. Due to lack of tissue specimen, we were able to perform experiment in only one mouse. We recommend that the experiment should be repeated on a greater number of mice, using tissue specimen from a number of patients, to confirm our findings.
Acknowledgments
This work is supported by the Young Investigator Award and Scholarly Opportunity Award (Mayo Clinic internal funding). We would like to thank Heather Wikstrom, Melissa Walker, and Kelly Viola for their editorial assistance.
References
- 1.Deangelis LM, Iwamoto FM. An update on therapy of primary central nervous system lymphoma. Hematol Oncol Clin North Am. 2006;20:1267–85. doi: 10.1016/j.hoc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Anthony IC, Crawford DH, Bell JE. B lymphocytes in the normal bracontrasts with HIV-associated lymphoid infiltrates and lymphomas. Brain. 2003;126(Pt 5):1058–67. doi: 10.1093/brain/awg118. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri-Broet S, Criniere E, Broet P, Delwail V, Mokhtari K, Moreau A, Kujas M, Raphaël M, Iraqi W, Sautes-Fridman C, Colombat P, Hoang-Xuan K, Martin A. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107:190–6. doi: 10.1182/blood-2005-03-1024. [DOI] [PubMed] [Google Scholar]
- 4.Braaten KM, Betensky RA, de Leval L, Okada Y, Hochberg FH, Louis DN, Harris NL, Batchelor TT. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003;9:1063–9. [PubMed] [Google Scholar]
- 5.Larocca LM, Capello D, Rinelli A, Nori S, Antinori A, Gloghini A, Cingolani A, Migliazza A, Saglio G, Cammilleri-Broet S, Raphael M, Carbone A, Gaidano G. The molecular and phenotypic profile of primary central nervous system lymphoma identifies distinct categories of the disease and is consistent with histogenetic derivation from germinal center-related B cells. Blood. 1998;92:1011–9. [PubMed] [Google Scholar]
- 6.Imai H, Kajimoto K, Taniwaki M, Miura I, Hatta Y, Hashizume Y, Watanabe M, Shiraishi T, Nakamura S. Intravascular large B-cell lymphoma presenting with mass lesions in the central nervous system: a report of five cases. Pathol Int. 2004;54:231–6. doi: 10.1111/j.1440-1827.2004.01613.x. [DOI] [PubMed] [Google Scholar]
- 7.Tun HW, Personett D, Baskerville KA, Menke DM, Jaeckle KA, Kreinest P, Edenfield B, Zubair AC, O'Neill BP, Lai WR, Park PJ, McKinney M. Pathway analysis of primary central nervous system lymphoma. Blood. 2008;111:3200–10. doi: 10.1182/blood-2007-10-119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mrugala MM, Rubenstein JL, Ponzoni M, Batchelor TT. Insights into the biology of primary central nervous system lymphoma. Curr Oncol Rep. 2009;11:73–80. doi: 10.1007/s11912-009-0012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubenstein JL, Shen A, Batchelor TT, Kadoch C, Treseler P, Shuman MA. Differential gene expression in central nervous system lymphoma. Blood. 2009;113:266–7. doi: 10.1182/blood-2008-04-152835. author reply 267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tun H. Differential gene expression of central nervous system lymphoma. Blood. 2009;113:267. doi: 10.1182/blood-2008-04-152835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JR, Braziel RM, Paoletti S, Lipp M, Uguccioni M, Rosenbaum JT. Expression of B-cellattracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101:815–21. doi: 10.1182/blood-2002-05-1576. [DOI] [PubMed] [Google Scholar]