Abstract
ERas activation and GKN2 reduction in gastric cancer has raised some notices in recent years, while nuclear beta-catenin positivity is considered as a tumoral marker. In this study, we compared immunohistochemistry of beta-catenin, GKN2 and ERas on tumoral and non-tumoral mucosae of 50 gastric carcinomas and 13 gastric samples of cancer-free patients. Nuclear positivity of beta-catenin was strong in 31 non-tumoral mucosae (62%) and 29 tumoral mucosae (58%). It was absent in samples of cancer-free patients. There was a correlation between non-tumoral and tumoral zones for nuclear beta-catenin positivity (P=0.013). ERas was positive in 35 non-tumoral tissues (70%) and 31 tumoral tissues (62%) but negatvie in samples of cancer-free patients. It was weak and spotty in non-tumoral mucosae but strong and diffuse in tumors. Positivity of ERas was age-related (P=0.028). However it had background staining effect. GKN2 was expressed in 33 non-tumoral mucosae (66%) and 35 tumoral mucosae (70%). Though GKN2 staining was moderate to strong in non-tumoral tissues and was comparatively weaker in tumors, their difference was minimal and difficult to discern. Conclusions: Beta-catenin nuclear location could be considered as a paraneoplastic pattern which is considerably tumor-related. ERas may be a potential biomarker for gastric cancer, but advanced studies are wanted. GKN2 reduction is indiscernible by immunostaining.
Keywords: Stomach neoplasm, gastric mucosa, beta Catenin, gastrokine 2, ERas
Introduction
Gastric carcinoma is one of the most frequent cancers in eastern Asia. Detection of the tumors through biopsy depends principally on dysplastic morphology. In some infiltrative cases, the tumor cells diffusely disperse under non-tumoral mucosae, while in surface the neoplastic protrusions or ulcers are minimal. It makes the biopsies difficult and usually negative in these cases [1]. On the other hand, in gastric cancers, the non-tumoral mucosae may have a high risk of tumoral developing, as it coexists with carcinoma in the same environmental hazard. Detection of neoplasm-related molecular changes in non-tumoral mucosae may help improving the diagnostic and better perceiving the early molecular changes in tumorigenesis. In this study, 3 candidatures were tested by immunohistochemistry: beta-catenin, gastrokine-2 (GKN2) and embryonic stem cell expressed Ras (ERas).
Nuclear accumulation is estimated as the activated state of beta-catenin. It is frequently detected in gastric carcinomas, and is thought being related with tumoral infiltration and worse prognostic [2]. As a component of Wnt signaling pathway, beta-catenin can promote cell growth through activation of cyclinD1 [3] and c-myc genes [4]. Mostly, it is expressed in cytoplasms and membranes of epithelial cells in normal mucosae [5]. Tumoral cells or mucosae with intestinal metaplasia may present nuclear expression [6]. Some reports have demonstrated that during Helicobacter pylori (Hp) infection the gastric epithelium may present a nuclear beta-catenin accumulation, stimulated by CagA and pro-inflammatory cytokines [7]. The explanations for nuclear distribution of beta-catenin are variable in different tumors. It may be regulated by interaction with other signaling pathways. Loss of components on membrane surface like E-cadherin and change of APC (Adenomatous Polyposis Coli) protein may favor beta-catenin to translocate from cytoplasm into nucleus [8]. DNA methylation of E-Cadherin (CDH1) gene could be frequently detected in gastric cancers and associated with loss of E-cadherin expression [9]. Alteration of APC gene has also been reported in gastric cancers [10]. Mutations of beta-catenin (CTNNB1) gene, mostly in exon 3, can be found in rat stomach adenocarcinomas and associated with its nuclear accumulation [11], but somatic changes of beta-catenin gene are rare in human gastric carcinomas [12] and its role in tumoral development is uncertain. Whether nuclear expression of beta-catenin is a neoplasm-related change is still waiting to be further explored.
Gastrokine-2 (GKN2) has lately been found being a potential biomarker for gastric cancer and gastritis. It was identified as a binding partner of gastric trefoil factor family-2 peptide (TFF2) [13], a mucin-associated protein containing trefoil domains and participating in homeostasis maintenance of gastrointestinal tract. The location of GKN2 is still to some extent unexplained. GKN2 is known being expressed in cytoplasm of superficial gastric foveolar cells [13-14], while its binding partner TFF2 is expressed in the deeper gastric glands [15]. It has been reported that the expression of GKN2 is reduced in Helicobacter-pylori-infected gastritis [16] and in gastric cancers [17]. GKN2 may be down-regulated by cytokines like TNF-alpha and IL-6 [18]. TNF-alpha could promote Wnt/beta-catenin signaling [19], and IL-6 is a member of LIF-JAK-STAT3 pathway which may converge with Wnt pathway on c-myc [20]. The modification of GKN2 may play a role during oncogenesis through interaction with its partners.
Embryonic stem cell expressed Ras (ERas) on-cogene has been recently detected expressed in gastric cancers. It is normally active when the cells are in viviparous phase of embryos. The protein contains structure identical to that in Ras and may cause tumoral transformation [21]. ERas expression could be detected in tumors of colons, pancreas and breasts but not in normal tissues [22]. In gastric carcinomas, ERas expression is found in 44% of tumors, and is associated with tumoral spreading and prognostic [23], yet the mechanism of ERas oncogene activation is not clarified. ERas interacts with phosphatidylinositol-3-OH kinase (PI3K) [21], which may lead to nuclear translocation of beta-catenin [24].
The study is focused on comparing the immu-nostains of beta-catenin, GKN2 and ERas in tumoral and non-tumoral mucosae of gastric cancer, and trying to conclude an expression pattern which may be tumor-associated.
Materials and methods
Gastric samples
The archival tissues were collected from 50 cases of gastric carcinoma, operated from 2008 to 2009 in Putuo Hospital affiliated to Shanghai University of Traditional Chinese Medicine (Table 1). The specimens were trimmed from the surgical pieces, then formalin-fixed and paraffin-embedded routinely. Patients were aged from 42 to 91 (64±11.7), including 28 men and 22 women. 13 Hp-negative gastric samples from neoplasm-free patients were also collected, and patients were followed during 2 years without tumoral diseases.
Table 1.
Patient clinicopathological features
| Beta-catenin nuclear positive n (%) | ERas positive n (%) | GKN2 positive n (%) | Total cases = 50 | ||
|---|---|---|---|---|---|
| Tumoral zones | Non-tumoral zones | Tumoral zones | Tumoral zones | ||
| Age, n [mean] | 29 [62.5] | 31 [62.6] | 31 [66.3] | 35 [65.9] | [64] |
| Gender | |||||
| Female | 12 (24) | 13 (26) | 14 (28) | 15 (30) | 22 |
| Male | 17 (34) | 18 (36) | 17 (34) | 20 (40) | 28 |
| Tumor average size (cm) | 4.7 | 4.8 | 5.0 | 4.9 | |
| Histological types | |||||
| Tubular adenocarcinoma | 22 (44) | 24 (48) | 26 (52) | 27 (54) | 39 |
| Well differentiated | 2(4) | 2(4) | 2(4) | 2(4) | 2 |
| Moderately differentiated | 9(18) | 10 (20) | 10 (20) | 11 (22) | 17 |
| Poorly differentiated | 11 (22) | 12 (24) | 14 (28) | 14 (28) | 20 |
| Signet-ring cell adenocarcinoma | 6(12) | 6(12) | 4(8) | 7(14) | 8 |
| Mucinous adenocarcinoma | 1(2) | 1(2) | 1(2) | 1(2) | 3 |
| Tumor-zone changes | |||||
| Lymphatic tumor emboli | 17 (34) | 17 (34) | 20 (40) | 19 (38) | 33 |
| blood vessel tumor emboli | 5(10) | 6(12) | 4(8) | 5(10) | 7 |
| Perineural invasions | 8(16) | 6(12) | 4(8) | 5(10) | 9 |
| Non-tumor-zone changes | |||||
| Intestinal metaplasia | 10 (20) | 9(18) | 10 (20) | 10 (20) | 17 |
| Helicobacter pylori colonies | 5(10) | 6(12) | 4(8) | 4(8) | 6 |
| Local infiltration* | |||||
| Tis | 0 | 0 | 1(2) | 1(2) | 1 |
| T1 | 1(2) | 1(2) | 2(4) | 2(4) | 2 |
| T2 | 4(8) | 4(8) | 2(4) | 3(6) | 4 |
| T3 | 10 (20) | 12 (24) | 10 (20) | 11 (22) | 16 |
| T4 | 13 (26) | 13 (26) | 14 (28) | 17 (34) | 26 |
| Tx | 1(2) | 1(2) | 1(2) | 1(2) | 1 |
| Lymphatic metastasis* | |||||
| N0 | 11 (22) | 13 (26) | 10 (20) | 11 (22) | 15 |
| N1 | 7(14) | 5(10) | 7(14) | 8(16) | 11 |
| N2 | 5(10) | 7(14) | 7(14) | 8(16) | 13 |
| N3 | 5(10) | 5(10) | 6(12) | 7(14) | 10 |
| Nx | 1(2) | 1(2) | 1(2) | 1(2) | 1 |
According to the 7th edition of TNM classification (UICC 2010).
Tissue microarray (TMA) construction
TMA block was constructed by a bone marrow biopsy needle (18-gauge, 1.2mm in diameter). The tip of the needle had been smoothed to be flat. The tissue cores were “biopsied” and transferred from donor blocks to recipient blocks on which the cylindrical holes had been previously arrayed by defined X-Y position. Two zones of non-tumoral mucosae were punched for each case: one was in the zones close to tumor (less than 0.5 cm), another was in the surgical sections which was distant from tumor (3 to 7 cm in average). For each zone, 2 tissue cores were collected. The representative areas on the donor block were selected through microscopic examination on the corresponding sections. Mucosae with intraepithelial neoplasm were shunned and the collected non-tumoral tissues should be graded in category 1 according to Padova international classification [25]. Tumoral zones in each case were also punched for comparing study, including intramucous dysplastic areas found in 3 cases. Sections of 4μm thickness were cut.
Immunohistochemistry (IHC)
The tissue sections were deparaffinized in xylene and rehydrated through graded alcohol to water. Antigen retrieval was performed by micro-waving the sections in 10 mmol/L sodium citrate buffer (pH 6.0) for 15 min at 800W. Primary antibodies (Mouse anti-beta-catenin, M-0545, Clone 17C2, 1:50 in dilution, Antibody Diagnos-tica, USA; Rabbit anti-GKN2, ab70480, and Rabbit anti-ERas, ab72401, polyclones, 1:100 in dilution, Abcam, USA) were applied and incubated overnight at 4°C. The slides were rinsed with PBS, and the secondary incubations were carried out by applying HRP-polymerized dextran -linked anti-mouse or anti-rabbit reagents (D-3001, D-3002, Antibody Diagnostica, USA) for 40 min at room temperature. Immunoreactivity was visualized with DAB and counterstained with hematoxylin.
Expressions of beta-catenin, GKN2 and ERas were considered as positive when at least 10% of cells were colored by DAB [26]. Weak, moderate or strong staining was assessed according to the intensity [14]. Negative controls were performed by replacing primary antibodies with PBS. Positivity of GKN2 or ERas was demonstrated by a cytoplasmic brown granular staining. Nuclear or membrano-cytoplasmic staining of beta-catenin was evaluated independently. The distribution of positive cells in different zones of mucosae (surface epithelium, fovea, neck, profound glands etc.) was noted.
Statistical analysis
The information of patients, the positivity of beta-catenin, GKN2 and ERas with IHC in tumoral and non-tumoral tissues were analyzed by using SPSS 10.0. Data were subjected to T test, Chi square, Mann-Whitney test and Spearmen method (2-tailed).
Results
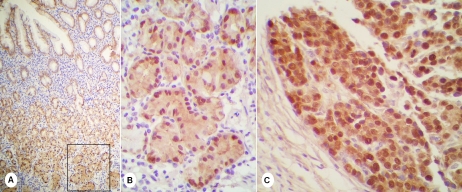
Beta-catenin expression could be seen in all the epithelial cells except one case in which it was negative in tumoral area. Strong nuclear positivity of beta-catenin was clearly observed in 29 cases of carcinomas (58%) and 31 cases of non -tumoral mucosae (62%) (Table 1), in which 22 non-tumoral tissues (44%) presented nuclear and cytoplasmic co-expression. In 2 non-tumoral mucosae and 7 tumors, only a few cells showed nuclear staining or the stains were so weak that the pattern was difficult to evaluate. Mostly, the nuclear expression of beta-catenin in non-tumoral mucosae was observed focally but strongly in profound glands (Figure 1A and 1B). The mucosae often had atrophic gastritis, but in some cases it might be “purely” normal. All the Hp-colonized samples (6 cases) showed nuclear location of beta-catenin, while the intestinal metaplasia was not indispensable for it, and there was no difference between tumor-closed and tumor-distanced mucosae on the pattern of beta-catenin expression. The stainings in 3 in-tramucous dysplastic samples and 1 carcinoma in situ were accordant with that in non-tumoral mucosae (Table 2). In tumoral zones, the nuclear staining of beta-catenin was usually massive (Figure 1C), and tumors with beta-catenin nuclear expression had more tendency to infiltrate the nerves (z = -1.986, P = 0.047) (Table 3). There was a co-relation between non-tumoral and tumoral zones for nuclear positivity of beta-catenin (r = 0.384, P = 0.013) (Table 4). Regarding these results, we furthered the IHC of beta-catenin on 13 Hp-negative gastric samples from cancer-free patients, and none of them presented nuclear positivity.
Figure 1.
IHC of beta-catenin in gastric cancer. (A, B) Beta-catenin was expressed in whole mucosa of non-tumoral zones with focal but strong nuclear positivity in deeper glands; (C, D) Nuclear stain of beta-catenin was strong and massive in tumoral zones.
Table 2.
Immunostains of beta-catenin in 4 preinvasive samples
| Non-tumoral zones | Intramucous dysplasia | Tumoral zones | Profundity* | |
|---|---|---|---|---|
| Case 1 | Nuclear | Nuclear | Cytoplasmic | T2 |
| Case 2 | Cytoplasmic | Cytoplasmic | Nuclear & Cytoplasmic | T3 |
| Case 3 | Cytoplasmic | Cytoplasmic | Cytoplasmic | T4 |
| Case 4 | Cytoplasmic | Cytoplasmic | Tis |
According to the 7th edition of TNM classification (UICC 2010).
Table 3.
Nuclear positivity of beta-catenin and perineural invasion in gastric cancer
| IHC of beta-catenin | ||||
|---|---|---|---|---|
| Nuclear positive | Nuclear negative* | Total** | P*** | |
| Perineural invasions | ||||
| Found | 8 | 1 | 9 | 0.047 |
| Not found | 21 | 19 | 40 | |
| Total** | 29 | 20 | 49 | |
Including 7 cases in which the nuclear positivity of beta-catenin was difficult to determine and in which 1 case presented perineural invasion.
Total 50 cases, 1 Carcinoma in situ was excluded.
Z = -1.986, Mann-Whitney test (2-tailed).
Table 4.
Correlation between non-tumoral and tumoral zones for nuclear positivity of beta-catenin
| Non-tumoral zones | ||||
|---|---|---|---|---|
| Nuclear positive | Nuclear negative | Total* | P** | |
| Tumoral zones | ||||
| Nuclear positive | 25 | 4 | 29 | 0.013 |
| Nuclear negative | 6 | 6 | 12 | |
| Total* | 31 | 10 | 41 | |
Total 50 cases, 9 pairs of samples were excluded as their nuclear positivities of beta-catenin were difficult to determine.
Correlation coefficient = 0.384, Spearman method (2-tailed).
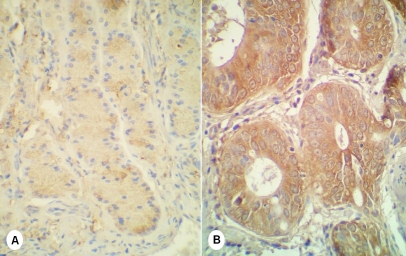
ERas was detected positive in 35 cases of non-tumoral mucosae (70%), and all were weak and spotty in the neck regions and deeper glands (Figure 2A). 31 tumors, with 3 intramucous dysplastic samples, were ERas positive (62%) (Table 1), in which 25 tumors (81%) presented strong and diffuse stainings (Figure 2B). The staining patterns were different between tumoral and non-tumoral tissues (z=-2.677, P=0.007) (Table 5). Patients of ERas-positive group had more advanced ages than that of ERas-negative group (66.3±12.7 VS 57.5±7.6; P=0.028, T test). However immunoreactions of ERas might extend around the glands into the lamina propria, and ERas positivity could not be ensured in 2 non-tumoral tissues and 2 tumors because of background staining effects. IHC of ERas was also tested on 13 gastric samples from cancer-free patients, in which few gland cells were DAB-stained that no sample could be considered as positive.
Figure 2.
IHC of ERas in gastric cancer. (A) ERas was weak and spotty in cytoplasms of middle and deeper glands cells in non-tumoral mucosae; (B) Expression of ERas was strong and diffuse in tumoral glands.
Table 5.
Immunostains of ERas in non-tumoral and tumoral zones of gastric cancers
| Negative | Positive | Total* | P** | ||
|---|---|---|---|---|---|
| Weak and spotty | Strong and diffuse | ||||
| Non-tumoral mucosae | 13 | 35 | 0 | 48 | |
| Tumoral mucosae | 17 | 6 | 25 | 48 | 0.007 |
| Total* | 30 | 41 | 25 | 96 | |
Total 50 cases, 2 non-tumoral samples and 2 tumoral-samples were excluded as their positivities of ERas could not be ensured because of background staining effects.
Z = -2.677, Mann-Whitney test (2-tailed).
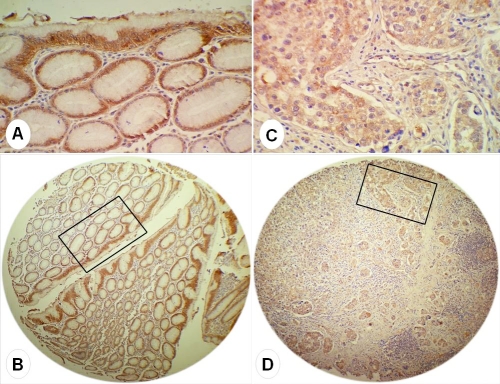
GKN2 was expressed in 33 non-tumoral tissues (66%) and 35 tumors (70%) (Table 1). In most of non-tumoral mucosae, GKN2 staining was generally moderate to strong in surface epithelium and weaker in deeper glands (Figure 3A and 3B). Comparatively, its positivity in tumors was moderate and slightly weaker (Figure 3C and 3D), and the staining difference between tumoral and non-tumoral mucosae was usually minimal and difficult to discern. Eight cases were negative for GKN2 in surface epithelium but showed a moderate GKN2 staining in profound glands of non-tumoral mucosae, while in these deeper zones, seven cases presented nuclear positivity of beta-catenin. Positivity of GKN2 could also be observed moderately in 4 gastric samples from cancer-free patients, all were on the surface epithelium.
Figure 3.
IHC of GKN2 in gastric cancer. (A, B) GKN2 staining was generally moderate to strong in surface epithelium and glands of non-tumoral zones; (C, D) GKN2 positivity was comparatively weaker in tumors, but the staining difference between tumoral and non-tumoral mucosae was minimal and difficult to discern.
Discussion
Tumors with beta-catenin nuclear expression may be relatively aggressive [9] as our results showed its tendency for nerve invasion. Nuclear staining is generally absent in normal gastric mucosae [27]. In this study, it was frequently observed in non-tumoral areas of gastric cancers. The mucosae may present a normal morphology without dysplasia or inflammatory changes. The pattern of beta-catenin distribution was independent of intestinal metaplasia. These results differ from the previous reports that few cells present nuclear beta-catenin expression in non-tumoral areas of gastric carcinomas [5]. The disagreement is perhaps due to using different clones of antibody for IHC or adopting different populations of patient for research. Nuclear accumulation of beta-catenin may present in mucosae which are far away from the tumor, probably because the tumoral and non-tumoral epithelium are under the same ambient stimuli of various agents, like Hpinfection. The expression of beta-catenin in nuclei was accordant between non-tumoral and tumoral areas, and it was usually observed focally in deeper glands of normal mucosa, which is known the most frequent site of gastric tumoral origin. Nuclear dislocation of beta-catenin has been reported as a precancerous change in some lesions like oral leukoplakia [28]. The correlation between non-tumoral and tumoral zones for nuclear beta-catenin expression in our study may conclude it as a paracancerous element: a tumor-indicating pattern in gastric mucosae. The finding of nuclear beta-catenin expression in normal mucosae should raise the attention for probable tumors in surrounding areas.
Previous researches reported that the changes of beta-catenin (CTNNB1) gene were rare in human gastric cancers. We have tested the CTTNNB1 gene amplification usingchromogenic in situ hybridization (CISH) on the gastric cancer samples of this study, and no amplification signal save chromosomal aneuploidy was noticed in some tumor cells (unpublished observations). The interaction between beta-catenin and other signaling partners may be the main cause for its nuclear distribution. Besides, there were 4 cases presented nuclear expression of beta-catenin in non-tumoral zones but not in carcinoma areas. It is possible that during cancer infiltration, beta-catenin may relocate from nuclei to cytoplasms sporadically [26, 29], or sometimes nuclear translocation of beta-catenin is not required in gastric tumorigenesis. Another 6 cases had no beta-catenin nuclear expression in non-tumoral mucosae, while it was observed in tumoral zones. The reason is probably that we employed TMA technique which was limited by manipulating small tissue cores and the nuclear expression of beta-catenin was focally dispersed in non-tumoral mucosae. It is also possible that beta-catenin occasionally begin to locate into nuclei at advanced stage of cancer.
Activation of ERas gene in normal mucosae is abnormal. In somatic cells, ERas oncogene is silenced by CpG island hypermethylation in promoter regions [30] or hindered by premature polyadenylation signal [31]. In this study, positiv-ity of ERas in gastric carcinomas is significantly age-related. Probably the age-dependent DNA methylation or demethylation may play an important role in the control of ERas expression [32]. ERas has tumor promoting effect through ERas/PI(3)K pathway [21] which activates c-myc and suppresses GSK3, a common activity with beta-catenin/Wnt pathway [20, 33]. Our results agreed that ERas may be a potential tumoral biomarker for gastric cancer, but immunostaining analysis was sometimes hampered by using the present antibody. A more specific monoclonal antibody is desirable for advanced study and diagnostic purpose.
GKN2 is reported reduced in gastric carcinoma compared with that in normal mucosae [17]. However, the intensities of GKN2 staining of our results were almost parallel between tumoral and non-tumoral zones. Though the former is slightly weaker, it is difficult to distinguish them by immunostaining technique. Surface mucosa is a preferential area for GKN2 expression [13-14]. Until now TFF2 is the only partner of GKN2, but TTF2 is reported expressed in profound glands and not in surface mucosae [15]. Infection of Helicobacter pylori, which is situated on surface and fovea, may inhibit the expression of GKN2 through activating pro-inflammatory cytokines [18]. In current study, apart from expression on surface area of non-tumoral mucosae, GKN2 was often found expressed in deeper glands, where nuclear positivity of beta-catenin was most frequently encountered. In some cases, expression of GKN2 was limited in profound glands, and it was often in these deeper zones of mucosae that GKN2 expression coexisted with beta-catenin nuclear accumulation, possibly there are some interactions between them. Though it seems that the regulation of GKN2 expression is different between surface epithelium and profound glands in non-tumoral mucosae, as the biopsy samples are usually convoluted and GKN2 expression is weak in deep glands, it is not practicable to analyze these immunostaining patterns routinely.
In conclusion, the nuclear expression of beta-catenin could be considered as a paraneoplas-tic element which is considerably associated with tumoral existence, although it does not reflect all the malignant transformation of gastric carcinoma. ERas may be a potential tumor biomarker for gastric cancer, but more efficient antibodies are wanted for advanced study.
Acknowledgments
This work was supported by Science Developing Foundation of Shanghai Health Bureau (No. 2008046), China.
References
- 1.Yamamoto T, Nagahama R, Nakashima H, Suyama M, Yoshida M, Ohkura Y, Watanabe N, Hirashima H, Miyamoto A. Basis of the diagnosis and feature of scirrhous gastric cancer. Stomach and Intestine (Tokyo) 2010;45:428–444. [Google Scholar]
- 2.Nabais S, Machado JC, Lopes C, Seruca R, Carneiro F, Sobrinho-Simoes M. Patterns of beta-catenin expression in gastric carcinoma: clinicopathological relevance and mutation analysis. Int J Surg Pathol. 2003;11:1–9. doi: 10.1177/106689690301100102. [DOI] [PubMed] [Google Scholar]
- 3.Utsunomiya T, Doki Y, Takemoto H, Shiozaki H, Yano M, Sekimoto M, Tamura S, Yasuda T, Fujiwara Y, Monden M. Correlation of beta-catenin and cyclin D1 expression in colon cancer. Oncology. 2001;61:226–233. doi: 10.1159/000055379. [DOI] [PubMed] [Google Scholar]
- 4.Brabletz T, Herrmann K, Jung A, Faller G, Kirchner T. Expression of nuclear beta-catenin and c-myc is correlated with tumor size but not with proliferative activity of colorectal adenomas. Am J Pathol. 2000;156:865–870. doi: 10.1016/s0002-9440(10)64955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grabsch H, Takeno S, Noguchi T, Hommel G, Gabbert HE, Mueller W. Different patterns of beta-catenin expression in gastric carcinomas: relationship with clinicopathological parameters and prognostic outcome. Histopathology. 2001;39:141–149. doi: 10.1046/j.1365-2559.2001.01177.x. [DOI] [PubMed] [Google Scholar]
- 6.Hung KH, Wu JJ, Yang HB, Su LJ, Sheu BS. Host Wnt/beta-catenin pathway triggered by Helicobacter pylori correlates with regression of gastric intestinal metaplasia after H. pylori eradication. J Med Microbiol. 2009;58:567–576. doi: 10.1099/jmm.0.007310-0. [DOI] [PubMed] [Google Scholar]
- 7.Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L, Perez-Perez GI, Hatakeyama M, Whitehead R, Gaus K, O'Brien DP, Romero-Gallo J, Peek RM., Jr Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barth AI, Näthke IS, Nelson WJ. Caderins, catenins and APC proteinterplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Zhang F, Wu PP, Jiang XC, Zheng L, Yu YY. Disordered beta-catenin expression and Ecadherin/CDH1 promoter methylation in gastric carcinoma. World J Gastroenterol. 2006;12:4228–4231. doi: 10.3748/wjg.v12.i26.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JY, Hsieh JS, Chen CC, Tzou WS, Cheng TL, Chen FM, Huang TJ, Huang YS, Huang SY, Yang T, Lin SR. Alterations of APC, c-met, and p53 genes in tumor tissue and serum of patients with gastric cancers. J Surg Res. 2004;120:242–248. doi: 10.1016/j.jss.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Tsukamoto T, Yamamoto M, Ogasawara N, Ushijima T, Nomoto T, Fujita H, Matsushima T, Nozaki K, Cao X, Tatematsu M. Beta-catenin mutations and nuclear accumulation during progression of rat stomach adenocarcinomas. Cancer Sci. 2003;94:1046–1051. doi: 10.1111/j.1349-7006.2003.tb01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki Y, Morimoto I, Kusano M, Hosokawa M, Itoh F, Yanagihara K, Imai K, Tokino T. Mutational analysis of the beta-catenin gene in gastric carcinoma. Tumour Biol. 2001;22:123–130. doi: 10.1159/000050606. [DOI] [PubMed] [Google Scholar]
- 13.Otto WR, Patel K, McKinnell I, Evans MD, Lee CY, Frith D, Hanrahan S, Blight K, Blin N, Kayademir T, Poulsom R, Jeffery R, Hunt T, Wright NA, McGregor F, Oien KA. Identification of blotta novel gastric trefoil factor family-2 binding protein. Proteomics. 2006;6:4235–4245. doi: 10.1002/pmic.200500911. [DOI] [PubMed] [Google Scholar]
- 14.Moss SF, Lee JW, Sabo E, Rubin AK, Rommel J, Westley BR, May FE, Gao J, Meitner PA, Tavares R, Resnick MB. Decreased expression of gastrokine1 and the trefoil factor interacting protein TFIZ1/GKN2 in gastric cancer: influence of tumor histology and relationship to prognosis. Clin Cancer Res. 2008;14:4161–4167. doi: 10.1158/1078-0432.CCR-07-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May FE, Griffin SM, Westley BR. The trefoil factor interacting protein TFIZ1 binds the trefoil protein TFF1 preferentially in normal gastric mucosael cells but the co-expression of these proteins is deregulated in gastric cancer. Int J Biochem Cell Biol. 2009;41:632–640. doi: 10.1016/j.biocel.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resnick MB, Sabo E, Meitner PA, Kim SS, Cho Y, Kim HK, Tavares R, Moss SF. Global analysis of the human gastric epithelial transcriptome altered by Helicobacter pylori eradication in vivo. Gut. 2006;55:1717–1724. doi: 10.1136/gut.2006.095646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du JJ, Dou KF, Peng SY, Wang WZ, Wang ZH, Xiao HS, Guan WX, Liu YB, Gao ZQ. Down-regulated full-length novel gene GDDR and its effect on gastric cancer. Zhonghua Yi Xue Za Zhi. 2003;83:1166–1168. [PubMed] [Google Scholar]
- 18.Baus-Loncar M, Lubka M, Pusch CM, Otto WR, Poulsom R, Blin N. Cytokine regulation of the trefoil factor family binding protein GKN2 (GDDR/TFIZ1/blottin) in human gastrointestinal epithelial cells. Cell Physiol Biochem. 2007;20:193–204. doi: 10.1159/000104166. [DOI] [PubMed] [Google Scholar]
- 19.Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M. Activated macrophages promote Wnt signaling through tumour necrosis factor-alpha in gastric tumor cells. EMBO J. 2008;27:1671–1681. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristensen DM, Kalisz M, Nielsen JH. Cytokine signaling in embryonic stem cells. APMIS. 2005;113:756–772. doi: 10.1111/j.1600-0463.2005.apm_391.x. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Mitsui K, Yamanaka S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature. 2003;423:541–545. doi: 10.1038/nature01646. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda K, Yashiro M, Sawada T, Ohira M, Hirakawa K. Clinical significance of ERas oncogene on cancer. 2007 ASCO Annual Meeting Proceedings (Post-Meeting Edition) J Clin Oncol. 2007;25(Suppl 18):S15083. [Google Scholar]
- 23.Kaizaki R, Yashiro M, Shinto O, Yasuda K, Matsuzaki T, Sawada T, Hirakawa K. Expression of ERas oncogene in gastric carcinoma. Anticancer Res. 2009;29:2189–2193. [PubMed] [Google Scholar]
- 24.Everly DN, Jr, Kusano S, Raab-Traub N. Accumulation of cytoplasmic beta-catenin and nuclear glycogen synthase kinase 3beta in Epstein-Barr virus-infected cells. J Virol. 2004;78:11648–11655. doi: 10.1128/JVI.78.21.11648-11655.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, Lewin K, Riddell RH, Sipponen P, Watanabe H. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24:167–176. doi: 10.1097/00000478-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Tsukashita S, Kushima R, Bamba M, Nakamura E, Mukaisho K, Sugihara H, Hattori T. Betacatenin expression in intramucosael neoplastic lesions of the stomach. Oncology. 2003;64:251–258. doi: 10.1159/000069310. [DOI] [PubMed] [Google Scholar]
- 27.D'Odorico A, Cassaro M, Grillo S, Lazzari R, Buda A, Cardellini P, Sturniolo Giacomo C, Rugge M. Trefoil peptides, E-cadherin, and betacatenin expression in sporadic fundic gland polyps: further evidence toward the benign nature of these lesions. Appl Immunohistochem Mol Morphol. 2009;17:431–437. doi: 10.1097/PAI.0b013e3181a03188. [DOI] [PubMed] [Google Scholar]
- 28.Ishida K, Ito S, Wada N, Deguchi H, Hata T, Hosoda M, Nohno T. Nuclear localization of betacatenin involved in precancerous changes in oral leukoplakia. Mol Cancer. 2007;6:62. doi: 10.1186/1476-4598-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyazawa K, Iwaya K, Kuroda M, Harada M, Serizawa H, Koyanagi Y, Sato Y, Mizokami Y, Matsuoka T, Mukai K. Nuclear accumulation of beta-catenin in intestinal-type gastric carcinoma: correlation with early tumor invasion. Virchows Arch. 2000;437:508–513. doi: 10.1007/s004280000283. [DOI] [PubMed] [Google Scholar]
- 30.Yashiro M, Yasuda K, Nishii T, Kaizaki R, Sawada T, Ohira M, Hirakawa K. Epigenetic regulation of the embryonic oncogene ERas in gastric cancer cells. Int J Oncol. 2009;35:997–1003. doi: 10.3892/ijo_00000414. [DOI] [PubMed] [Google Scholar]
- 31.Kameda T, Thomson JA. Human ERas gene has an upstream premature polyadenylation signal that results in a truncated, noncoding transcript. Stem Cells. 2005;23:1535–1540. doi: 10.1634/stemcells.2005-0054. [DOI] [PubMed] [Google Scholar]
- 32.Calvanese V, Lara E, Kahn A, Fraga MF. The role of epigenetics in aging and age-related diseases. Ageing Res Rev. 2009;8:268–276. doi: 10.1016/j.arr.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama M, Hisatsune J, Yamasaki E, Isomoto H, Kurazono H, Hatakeyama M, Azuma T, Yamaoka Y, Yahiro K, Moss J, Hirayama T. Helicobacter pylori VacA-induced inhibition of GSK3 through the PI3K/Akt signaling pathway. J Biol Chem. 2009;284:1612–1619. doi: 10.1074/jbc.M806981200. [DOI] [PMC free article] [PubMed] [Google Scholar]