Abstract
Anaplastic carcinoma (AC) of spindle cell type is an exceedingly rare ovarian malignant neoplasm. The histo-genesis of these tumors is still controversial. Although it was first thought to carry an invariably unfavorable prognosis, recent data indicates that this does not apply to stage Ia tumors. To date, there have been less than ten cases of anaplastic spindle cell carcinoma reported in the medical literature. Furthermore, our case is the first time this tumor has been described in a 40-year-old female with malignant spindle cells merging with conventional high grade adeno-carcinoma. The differential diagnosis of spindle cell proliferation in the ovary will be discussed and their distinction using a panel of immunohistochemical stains. This report demonstrated that the findings of malignant spindle cell proliferation does not imply this entity to be carcinosarcoma. The distinction of AC from true sarcomas is important because of the poorer prognosis of the later compared with the quite favorable behavior of AC. However, such existence necessitates a careful tissue sampling for the logical distinction between AC and carcinosarcoma, which is critical for planning further management and ultimately the predictor of prognosis.
Keywords: Ovary, mucinous neoplasm, anaplastic carcinoma, spindle cell, case report
Introduction
Ovarian AC was histologically described as carcinoma to include three different presentations: rhabdoid, spindled (sarcomatoid), and pleomor-phic. The foci of rhabdoid have a diffuse arrangement of cells with large, bright, eosino-philic cytoplasms, eccentric nuclei, and one or more prominent nucleoli. The sarcomatoid pattern is characterized by a spindle cell proliferation, with atypical and vesicular nuclei often with herringbone pattern, while the pleomorphic foci exhibit overlapping features of the first two categories [1]. ACs arising in other organs such as thyroid, lung, kidney or pancreas remains one of the most virulent of all cancers in humans. Most of such tumors have an unfavorable prognosis. Ovarian ACs with sarcomatoid features contain spindle cells that demonstrate an epithelial malignant nature in both their histopathology and immunohistochemistry [2]. Most commonly, AC has been reported as small foci within a mucinous tumor of the ovary [3, 4].
We report here a patient with ovarian anaplastic carcinoma, includingspindle cell type, arising in a background of a mucinous cystic tumor with areas of conventional mucinous adenocarci-noma of gland forming type. Immunohistochemical studies were performed to assess the proliferating activity of these spindle cells and their characteristics using antibodies against epithelial and non-epithelial markers. In addition, the differential diagnosis of a patient with these foci within a mucinous tumor will be explored. This report, with a documented case of an ovarian AC of spindled cell type, adds to our knowledge of the histogenesis of spindle cell proliferation in ovarian neoplasms.
Case Report
A 40-year-old female with a history of dysfunctional uterine bleeding presented with increased vaginal bleeding, low back pain, and increased abdominal fullness with nausea and vomiting. A CT of the abdomen showed a large complex adnexal mass on the left and she was scheduled for a total abdominal hysterectomy and bilateral salpingo-oophorectomy, with staging.
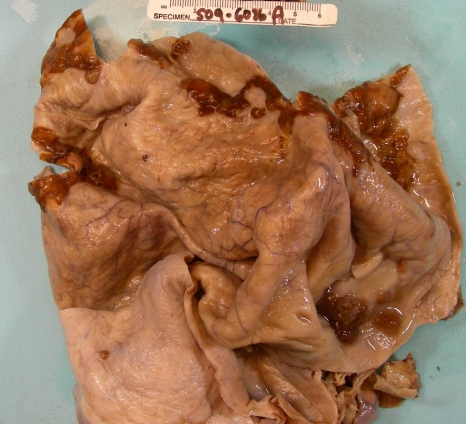
On gross examination, the left ovarian cystic mass measured 30.0 cm in diameter to which a normal fallopian tube was attached. The outer ovarian surface was smooth. The cut surface of the left ovary showed a multilocular cyst which was filled with dark-brown bloody fluid. There were several well circumscribed solid, raised, brown focally hemorrhagic nodular areas measuring up to 8.0 cm arising from the inner surface (Figure 1). The remianing inner cystic surface was smooth. The right adnexa and uterus were unremarkable.
Figure 1.
Multilocular cystic mass of the ovary with brown rasied nodules.
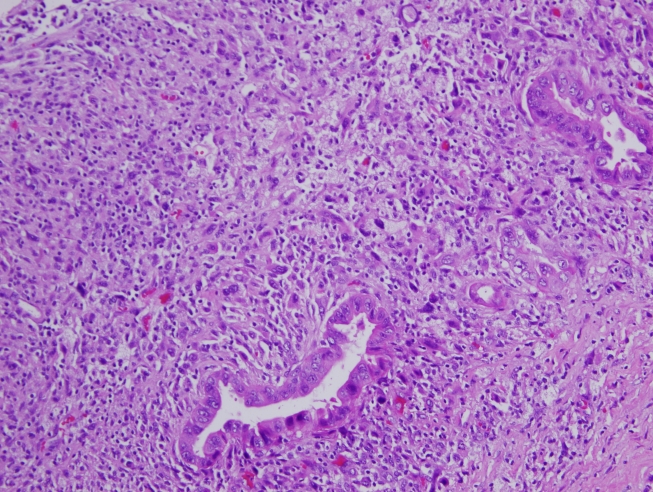
On microscopic examiantion, the cyst wall was lined by mucinous epithelium ranging from a mucinous cystadenoma to borderline mucinous neoplasm. The rasied nodules were made up of a spindle, pleomorphic, densely cellular population with a sarcomatous appearance. There were, in addition, foci of clearly recognizable conventional high grade adenocarcinoma in the form of glandular and acinar formation in intimate admixture, with an anaplastic spindle cell component (Figure 2). The individual spindle cells were made up of spindle-shaped hyper-chromatic nuclei with prominent nucleoli and abundant eosinophilic moderate cytoplasm with well-defined borders. The spindled cells were too atypical to be benign and contained identifiable abnormal mitotic figures (Figure 3). The uterus and the tissues from the cul-de-sac, mesentery, colic gutter, peritoneum, appendix and pelvic lymph nodes showed no evidence of tumor extension. The cytology of the peritoneal washing showed no tumor cells.
Figure 2.
Foci of conventional high grade adenocarcinoma in the form of glandular and acinar formation in intimate admixture with the anaplastic spindle cell component.
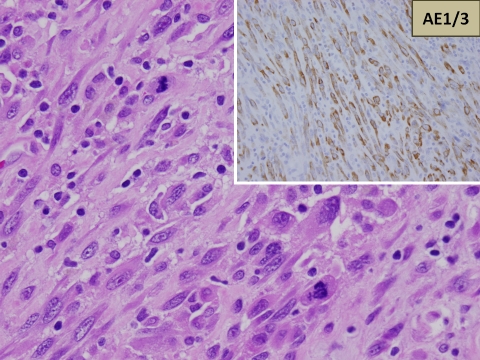
Figure 3.
The spindled cells were too atypical to be benign and contained identifiable abnormal mitotic figures. On the top right, the spindle cells show a strong cytoplasmic positivity for cytokeratin AE1/3.
On examination of the immunohistochemical stains, the spindle tumor cells in the nodules showed a diffuse cytoplasmic positivity for cytokeratin AE1/3 (Figure 3) and negative staining with CD68.
Following consultation with an experienced gynecologic pathologist at another institution, the diagnosis was confirmed to be anaplastic carcinoma, spindle cell type. The patient is alive and well 12 months after surgery.
Discussion
Anaplastic carcinoma within a mucinous ovarian tumor is a rare occurrence with only 17 cases reported in the literature [1]. When first reported in 1982, an AC focus carried a poor prognosis and was described as a single morphologic entity [5]. Additional prognostic information and histopathologic characteristics of these foci have since been described in the literature for these tumors.
The histopathology has been expanded to include three different presentations: rhabdoid, spindled (sarcomatoid), and pleomorphic. The rhabdoid subtype is described as cells with a bright eosinophilic cytoplasm and atypical, eccentric, ovoid nuclei diffusely oriented [1]. Grossly, these nodules appear to be poorly circumscribed with necrotic cut surfaces. Frequent tumor necrosis, ovarian stromal infiltration, and vascular invasion are all reported with the rhabdoid subtype. The spindle cell pattern demonstrates more of a hypercellular proliferation of spindle cells with less eosinophilic and ill -defined cytoplasm [1]. The arrangement of the spindle cells reflects a “herringbone pattern” rather than the more diffuse distribution displayed by the rhabdoid subtype. Necrosis is seen less frequently, but vascular invasion and stromal infiltration is observed. In addition, cases have described the spindle cells infiltrating glands were forming the conventional mucinous adenocarcinoma [1]. The pleomorphic subtype foci encompasses both the spindle cell and rhabdoid patterns. There also have been two case reports of sarcomatoid and one case of choriocarcinoma foci within the anaplastic carcinoma [1]. All reports of pleomorphic anaplastic carcinoma showed infiltration, in addition to hemorrhage, necrosis, and frequent mitosis.
New data has also pointed toward a more favorable prognosis in patients with AC foci, especially in those without metastasis or infiltration beyond the ovarian capsule [1]. When initially reported, these areas of AC were thought to carry a poor prognosis. Provenza et al. reviewed 34 cases of AC foci within mucinous tumors and found that neoplasms confined to the ovary (FIGO grade Ia) had no change in the prognosis of those patients [6]. In fact, the ultimate prognostic factors in a patient with mucinous adenocarcinoma were not altered by the presence of AC foci, but rather the staging of the primary mucinous tumor [6]. Additionally, the histologi-cal subtype of the tumor also had no adverse effects on prognosis [1].
Anaplastic carcinoma can be commonly confused with endometriosis, sarcomas, sarcoma-like nodules (SLMNs), and carinocarcinoma. Endometriosis can have spindle cells appearing as stromal cells, but usually have reactive changes including hemosiderin and endometrial glands. The SLMNs nodules tend to affect younger women and have a favorable prognosis [1,3,7,8]. There are usually multiple, well circumscribed nodules unlike AC foci which are usually solitary and infiltrative [1,3,7,8]. Furthermore, SLMNs have multi-nucleated giant cell inflammation within their sarcomatous his-tological appearance, differing from malignant sarcoma nodules, which lack an inflammatory component [3,7]. Differentiation between the two sarcomatous foci leans heavily on mitotic activity (frequent in sarcomas), cellular pleomor-phism (increased in sarcomas), and the presence of an inflammatory component (present in SLMNs, but absent in sarcomas).9 Carinocarcinoma is a mixed heterologous tumor with some areas of both chondrosarcoma or rhabdomyo-sarcoma making this tumor more distinctive from the other three neoplastic foci [1]. The importance of differentiating this tumor from AC is the altered prognosis, with the former having a poorer prognosis than the latter. The diagnosis of AC is further complicated by the possibility of multiple foci of different histopathological neoplasms that can be found within a single mucinous tumor. Immunohistochemistry can be beneficial since AC foci tend to stain strongly for cytokeratins, whereas sarcomas and SLMNs are negative for keratins, but positive for vimentin and CD68 immunostaining [2].
Summary
Anaplastic carcinoma within a mucinous ovarian tumor is rarely encountered and recently, the prognosis of these foci was not believed to be as poor as initially suggested. The case reported here stresses the importance of a careful histologic analysis of mural nodules in ovarian mucinous tumors. Classification of a mural nodule as sarcomatous or carcinomatous may determine the proper treatment and prognosis in these patients.
References
- 1.Rodriguez I, Prat J. Mucinous tumors of the ovary: a clinicopathologic analysis of 75 borderline tumors (of intestinal type) and carcinomas. Am J Surg Pathol. 2002;26(2):139–52. doi: 10.1097/00000478-200202000-00001. Feb. [DOI] [PubMed] [Google Scholar]
- 2.Nichols GE, Mills SE, Ulbright TM, Czernobilsky B, Roth LM. Spindle cell mural nodules in cystic ovarian mucinous tumors. A clinicopathologic and immunohistochemical study of five cases. Am J Surg Pathol. 1991;15(11):1055–62. doi: 10.1097/00000478-199111000-00004. Nov. [DOI] [PubMed] [Google Scholar]
- 3.Fox H, Langley FA. Chicago: Year Book Medical Publishers, Inc; 1976. Tumors of the Ovary. [Google Scholar]
- 4.Selye H. The ovary. Section VII. Montreal: Richardson bond and Wright; 1946. Encyclopedia of Endocrinology. [Google Scholar]
- 5.Prat J, Scully RH. Ovarian Mucinous Tumors with sarcoma-like mural nodules: A Report of Seven Cases. Cancer. 44(4):1332–1344. doi: 10.1002/1097-0142(197910)44:4<1332::aid-cncr2820440426>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Provenza C, Young RH, Prat J. Anaplastic carcinoma in mucinous ovarian tumors: a clinicopathologic study of 34 cases emphasizing the crucial impact of stage on prognosis, their histologic spectrum, and overlap with sarcoma-like mural nodules. Am J Surg Pathol. 2008;32(3):383–9. doi: 10.1097/PAS.0b013e3181451b93. Mar. [DOI] [PubMed] [Google Scholar]
- 7.Bague S, Rodriguez I, Prat J. Sarcoma-Like Mural Nodules in Mucinous Cystic Tumors of the Ovary Revisited.: A Clinicopathologic Analysis of 10 Additional Cases. Am J Surg Pathol. 2002;26(11):1467–1476. doi: 10.1097/00000478-200211000-00009. Nov. [DOI] [PubMed] [Google Scholar]
- 8.Vella J, Cracchiolo B, Heller DS. Anaplastic carcinoma arising in an ovarian mucinous cystadenocarcinoma in a 17-year-old female. J Pediatr Adolesc Gynecol. 2006;19(1):39–43. doi: 10.1016/j.jpag.2005.11.005. Feb. [DOI] [PubMed] [Google Scholar]
- 9.Prat J, Young RH, Scully RE. Ovarian mucinous tumors with foci of anaplastic carcinoma. Cancer. 1982;50(2):300–4. doi: 10.1002/1097-0142(19820715)50:2<300::aid-cncr2820500222>3.0.co;2-e. Jul 15. [DOI] [PubMed] [Google Scholar]