Summary
The successful results seen after organ transplantation are largely attributable to the potency and specificity of modern immunosuppressive agents. Although drug-free unresponsiveness to graft alloantigens has not been routinely achieved in clinical practice, recent appreciation of the importance of cell chimerism, which develops after the migration from donor to host of leukocytes contained in solid organ grafts, has introduced a concept which may explain the mechanism of graft tolerance. Recent evidence has indicated that immunosuppressive drugs may have a common potential to induce graft tolerance, even though they act through diverse mechanisms, and that this potential may be mediated by a permissive effect on the migration and survival of donor-derived leukocytes. This review briefly examines the mechanisms by which immunosuppressive drugs function and analyses the different methods which these agents might use to induce chimerism associated with graft tolerance. Furthermore, we describe ongoing clinical studies in which the chimerism produced after solid organ transplantation is augmented with donor bone marrow in an attempt to facilitate the induction of tolerance.
Keywords: bone marrow transplantation, chimerism, immunosuppression, immunosuppressive agents, organ transplantation, tolerance
Introduction
The use of immunosuppressive drugs to prevent or control graft rejection has revolutionised the outcome of transplantation surgery. Even so, complications of immunosuppression and side-effects associated with these agents remain significant risks for transplant patients. The idea of inducing ‘drug-free’ actively acquired tolerance was initially proposed and demonstrated in animals by Billingham, Brent & Medawar in 1953 (1). Although the ability to routinely induce drug-free unresponsiveness to alloantigens expressed on organ grafts remains out of reach in clinical transplantation, some recently developed concepts have increased our understanding of the circumstances that may predispose to tolerance induction and provide a foundation on which techniques to induce tolerance might be designed.
The current advanced state of rejection control has been attributed to the distinct molecular actions of the newer immunosuppressive agents (2,3). In addition, a new hypothesis has been proposed based on the recent appreciation of donor cell chimerism persisting up to 30 years after solid organ transplantation (4–7). The essence of this hypothesis is the permissive effect of all immunosuppressants on leukocyte migration between graft and host, ultimately allowing the establishment of stable cell chimerism (8–11). This paper discusses possible mechanisms by which drugs and other xenobiotics might permit chimerism and tolerance to occur. While relating the actions of these chemically diverse pharmacological agents to the chimerism associated with allograft tolerance, we discuss the mechanisms which may be necessary for the induction of permanent donor-specific unresponsiveness.
We shall consider the concept that the first step in tolerance induction may be the initiation of a pharmacologically controlled two-way allogeneic response. Such attenuation of immune reactivity may permit the natural migration of passenger leukocytes from graft to host and their survival allowing the continuous representation of donor alloantigens to the recipient under, as yet, poorly understood conditions that abrogate rejection and allow donor-specific unresponsiveness to occur.
Pharmacological control of rejection
Because of the extreme heterogeneity of agents that permit cell migration and chimerism to occur and that induce tolerance induction in appropriate experimental models, we will begin by briefly describing the bewildering array of drugs and other therapeutic immunosuppressants. Rejection commences when an array of foreign antigens, especially products of the donor major histocompatibility complex (MHC), are presented to host T-helper (Th) cells by graft antigen-presenting cells (APC) (12). Although allografts disseminate a variety of ‘passenger’ cells (10,11), the cell primarily responsible for presentation of donor antigen has been shown to be the dendritic cell – the most potent of APC (13–15).
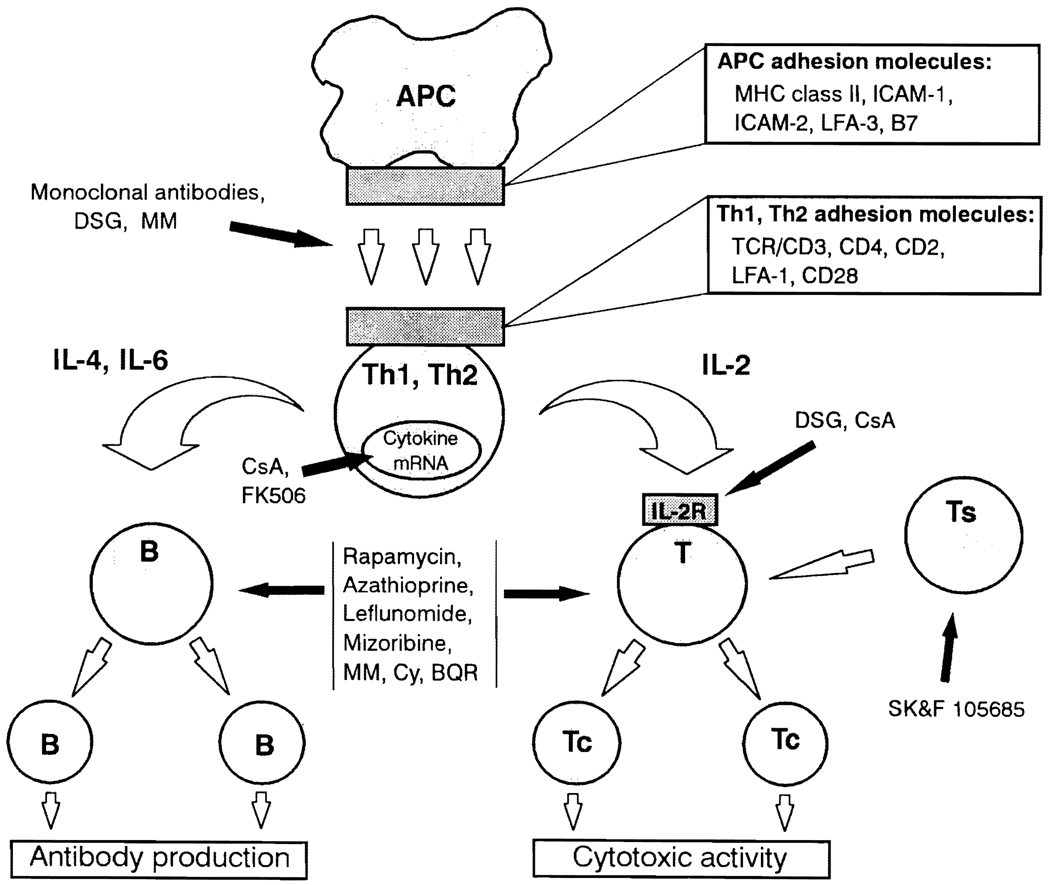
Antigen presentation and the ensuing cascade of events leading to graft rejection can be inhibited at various levels by immunosuppressive agents. The agents which can effect such immunosuppression and their levels of action are portrayed in Table 1 and Fig. 1. These drugs act at many different stages of the immune response to allogeneic stimulation. Deoxyspergualin (DSG) is thought to induce immunosuppression and tolerance by inhibition of APC function (16), although it may also inhibit B cell mitogenesis and depress IL-2 receptor expression on leukocytes. The subsequent induction of Th-cell activation and initiation of the rejection response requires that antigen is first presented by APC in the context of MHC class II gene products and then recognized by the T-cell receptor (TCR). This interaction is facilitated by numerous accessory molecules and costimulatory factors, including several intercellular adhesion molecules. A variety of monoclonal antibodies (mAb) has been used to control rejection and induce tolerance in experimental animals by blocking the recognition or interaction of these molecules (see Table 1) (17–23). Because of the T-cell-mediated nature of allograft rejection, potent antiT-cell agents such as cyclosporine A (CsA) (24–26) and FK506 (27–31) have demonstrated excellent results since being introduced to the field of transplantation. Unfortunately, neither agent has induced tolerance in humans with the frequency seen in experimental organ transplantation in rodents (26,27,32–34). Among their effects, both drugs inhibit the production of multiple cytokines (35–37) (but not IL-10 (38)), thereby contributing to control of rejection. Rapamycin is another powerful anti-T-cell agent capable of inducing tolerance in animals (39), however, results of ongoing phase I clinical trials are awaited before its wider introduction to clinical transplantation.
Table 1.
Immunosuppressive drugs capable of inducing tolerance
| Agent | Structure | Level of action | Reference |
|---|---|---|---|
| Deoxyspergualin | semisynthetic polyamine | macrophage function, cytotoxic T cells | 16 |
| Anti-CD4 | monoclonal antibody | adhesion/accessory molecule expression | 20 |
| Anti-LFA-1* | monoclonal antibody | adhesion/accessory molecule expression | 19 |
| Anti-ICAM-1† | monoclonal antibody | adhesion/accessory molecule expression | 22 |
| Cyclosporine A | cyclic peptide | inhibits IL-2 production | 26 |
| FK506 | carboxycyclic lactone | inhibits IL-2 production | 27 |
| Anti-IL-2R | monoclonal antibody | inhibits IL-2 action | 23 |
| Rapamycin | carboxycyclic lactone | inhibits IL-2 action | 39 |
| Azathioprine | 6-mercaptopurine derivative | inhibits DNA synthesis | 40 |
| Leflunomide | isoxazole derivative | ? B cell suppression | 46 |
| Mizoribine | imidazole nucleoside | inhibits DNA synthesis | 42 |
| Mycophenolate mofetil | mycophenolic acid derivative | inhibits DNA synthesis | 43 |
| Cyclophosphamide | nitrogen mustard derivative | inhibits DNA synthesis | 41 |
| Brequinar sodium | carboxylic acid derivative | inhibits DNA synthesis | 44 |
| SK&F 105685 | azaspirane analogue | ? induction of suppressor cells | 45 |
LFA-1 = lymphocyte function-associated antigen-1
ICAM-1 = intercellular adhesion molecule-1.
Fig. 1.
A diagrammatic representation of the immune response during rejection which demonstrates the principal sites of action of immunosuppressive drugs and monoclonal antibodies used to control anti-allograft responses. These agents also have the potential to induce tolerance. APC = antigen presenting cell; Thl/Th2 = T-helper 1 or T-helper 2 cell; T(c/s) = T-(cytotoxic/suppressor) cell; B = B-cell; IL-2R = IL-2 receptor. DSG = deoxyspergualin; MM = mycophenolate mofetil; CsA = cyclosporine A; Cy = cyclophosphamide; BQR = brequinar sodium.
Drugs such as azathioprine, cyclophosphamide, mizoribine, mycophenolate mofetil (previously known as RS61443) and brequinar sodium inhibit DNA synthesis and cause immunosuppression by reducing the clonal proliferation which occurs after lymphocyte stimulation (40–44). SK&F 105685 and leflunomide are two recently described immunosuppressants which have the potential to join the clinical armamentarium of antirejection drugs (45,46). Briefly, preliminary results obtained with these agents suggest that each can significantly prolong allograft survival in animal models (47,48) and may promote tolerance through the induction of suppressor cell activity and B-cell suppression, respectively (45,46,49).
Although the drugs described above show great chemical diversity, they are all powerful immunosuppressive agents which are either being investigated experimentally or used for the control of clinical graft rejection. Improvements in the control of rejection in the last decade have been attributed to newer and stronger immunosuppressive drugs acting at precise molecular levels (2,3,50). However, the purpose of this report is to suggest that allograft tolerance may be secondary to a common effect of these drugs that allows the migration and survival of donor-derived leukocytes which may predispose to the establishment of peripheral T-cell tolerance (8–11,51).
Donor-derived leukocyte migration – a phenomenon permitted by immunosuppressive agents
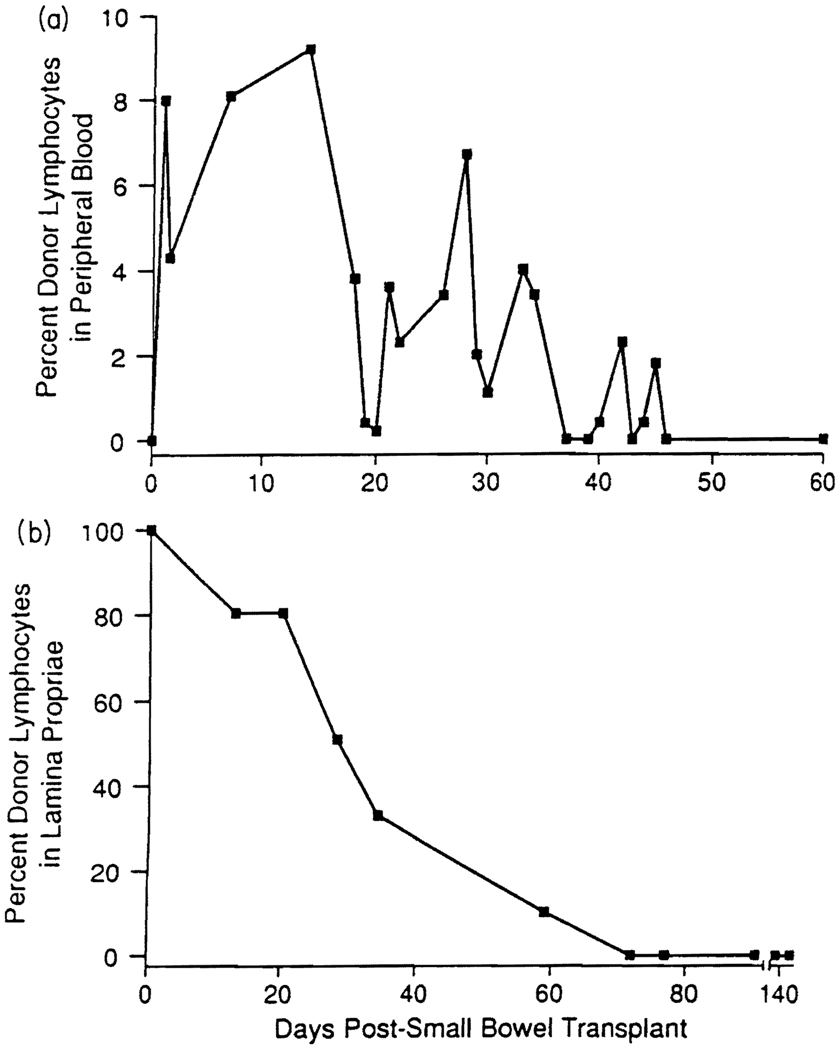
The importance of the widespread migration of donor-derived leukocytes and their survival after solid organ transplantation has only recently been appreciated (Fig. 2). The cell migration seen is now believed to be a natural event which occurs after the transplantation of all organs, and the survival of these cells may be critical to long term graft survival (8–11,52–54). Although cells move both from host to graft and from graft to host, it is the latter pathway that appears to be of primary importance for the induction of tolerance. Migration commences within hours of graft insertion (10,11,54) and cells appear to follow the ‘preprogrammed’ migratory routes for their particular lineage (e.g. dendritic cell (55,56)). During the first five days after surgery, leukocytes leave the graft and move to the spleen, lymph nodes, thymus and bone marrow (10,11). Two weeks later, donor leukocytes have undergone a second phase of movement and can be found in all other tissues examined throughout the recipient’s body, including lymph nodes, thymus, bone marrow, tongue and heart (10,11). With the exception of transplants between a limited number of experimental animal strains, immunosuppressive control is essential for the second phase of migration and the long-term survival of donor leukocytes (10,11), albeit at a low level (Fig. 3a). Such migration is a gradual process and, with sequential monitoring, the gradual depletion and replacement (by host cells) of donor leukocytes within the allograft can be measured (Fig. 3b).
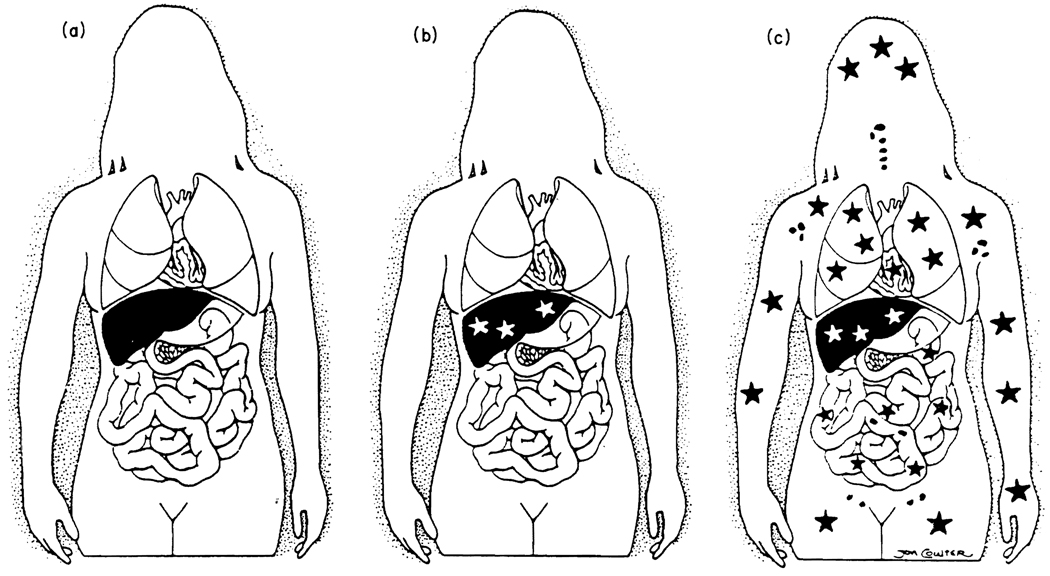
Fig. 2.
Progressive development of an understanding of liver transplantation: (a) historical view; (b) realisation (in 1969) that the liver graft became a genetic composite (chimera); (c) proof in 1992 of systemic chimerism. Stars represent the exchange of cells between graft and host.
Fig. 3.
Donor mononuclear cells appear in the blood of a human intestinal transplant recipient (a) and interstitial donor leukocytes are progressively replaced within the graft during the same time period (b).
When transplants are performed without immunosuppression, the first stage of migration occurs as predicted. After reaching the lymphoid organs, however, the donor leukocytes are progressively destroyed by rejection over a period of 14 days (11). When immunosuppressive agents are used, the passenger leukocytes persist and redistribute to widespread organs, as indicated above. The clinical data accumulated so far, using many different immunosuppressive regimes, strongly suggest that this effect is not drug-specific (4,6–9,57) (Table 2). Likewise, the permanent graft acceptance seen in experimental transplantation with every major immunosuppressant introduced in the last 30 years also indicates that drugs merely act as permissive agents which allow migration to occur (3,8).
Table 2.
Immunosuppressive regimes used in 44 long-term (10–30 years) survivors of liver transplantation. Data are expressed as means ± SD
| Group | No. of patients |
Drugs used | Amount administered (mg/day) |
|---|---|---|---|
| 1 | 12 | Azathioprine | 50 ± 26 |
| Prednisone | 8.7 ± 3.2 | ||
| 2 | 11 | Cydosporine | 266 ± 132 |
| Prednisone | 6.6 ± 3.4 | ||
| 3 | 5 | Cydosporine | 232 ± 132 |
| 4 | 3 | Azathioprine | 58 ± 14 |
| Cydosporine | 158 ±38 | ||
| Prednisone | 9.2 ± 1.4 | ||
| 5 | 2 | Azathioprine | 75,50 |
| 6 | 3 | Azathioprine/ Cydosporine |
25, 50/250, 100 |
| 7 | 1 | FK 506/Prednisone | 6/15 |
| 8 | 1 | FK 506 | 10 |
| 9 | 1 | Prednisone | 10 |
| 10 | 5 | Nothing | − |
Although the therapeutic agent used is not critical, it appears that prolonged continuous immunosuppression allows a greater percentage of donor leukocytes to survive than might otherwise be the case (11). As the majority of migrating cells are terminally differentiated (58), it is easy to understand how important it is that the maximum number of progenitor cells be allowed to engraft in the host to permit the indefinite survival of donor cells. Even so, in the majority of cases, the number of donor cells present after transplantation decreases to a small percentage (9–11), explaining the use of the term ‘microchimerism’, initially proposed by Liegeois et al. (59). Although the number of cells is sometimes too small to detect by flow cytometry (Fig. 3a), their presence can be demonstrated throughout the body after donor DNA amplification using either in situ hybridization or the polymerase chain reaction (Fig. 4 and Table 3).
Fig. 4.
Detection of chimerism by molecular HLA class II typing in various tissues after liver transplantation in a patient with type 1 Gaucher’s disease. Southern blot analysis of DR1-specific amplification of the DNA extracted from small bowel, skin, bone marrow, blood and liver. The denatured DNA present on the nylon membrane was hybridised to a radioactively labelled DR1 (donor) specific oligonucleotide probe (7001). In the case of the liver, only 1/100 of the amplification product was used.
Table 3.
Microchimerism in liver allograft recipients according to Y-chromosome detection with in situ hybridisation or PCR. All studies were completed between April and June of 1992. In addition to Y-chromosome detection, underlined patients were shown to demonstrate chimerism by immunocytochemical detection of donor HLA alleles (in cases 4, 6 and 9) or by PCR molecular typing (all patients except 6 and 8)
| Tissue distribution |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Liver allograft |
Blood |
Lymph node |
Skin |
||||||
| Patient number |
TP date (m/d/y) |
Age at TP (years) |
ISH | PCR | PCR | ISH | PCR | ISH | PCR |
| 1 | 2/18/73 | 3 | + + + | + + + | − | − | + | + | + |
| 2 | 1/21/76 | 30 | + + + | + + + | + | + | − | + | + |
| 3 | 1/04/78 | 2 | + + + | + + + | + | + | + | + | NT |
| 4 | 2/26/78 | 5 | + + + | + + + | + | + | + | + | NT |
| 5 | 9/09/78 | 35 | + + + | + + + | + | + | + | + | + |
| 6 | 3/09/80 | 29 | NT | + + + | NT | NT | − | NT | + |
| 7 | 3/21/80 | 34 | + + + | + + + | − | − | − | + | + |
| 8* | 8/29/80 | 28 | + + + | + + + | + | + | − | − | + |
| 9 | 12/28/81 | 45 | + + + | + + + | + | + | + | + | + |
TP = transplantation: ISH = in situ hybridisation: PCR = polymerase chain reaction: NT = not tested.
This patient also tested positive for Y chromosome in the intestine with ISH and in multiple other tissues (including aortic wall).
Although different tissues contain very different quantities of nonparenchymal cells, cell migration is now thought to occur after the transplantation of all solid organs (4–9, 11). The migrating cells are of multiple lineages and include B cells, T cells, NK cells, macrophages, mast cells and dendritic cells (10,11,58). These cells are postulated to modulate the recipient’s immune reaction and reduce donor-specific immune responsiveness (4–11).
Donor-specific blood transfusion (DST) may confer some benefit on allograft survival in a similar fashion, although the evidence supporting this concept has recently become less compelling. In fact transfused patients may actually have reduced graft survival (60). Many mechanisms to explain the ‘blood transfusion effect’ have been proposed and although various cellular- and antibody-mediated mechanisms have been considered, there is as yet no single defined mechanism to account for the DST effect (61,62).
Disruption of intercellular signalling concomitant with continuous donor alloantigen expression (Table 4)
Table 4.
Mechanisms by which xenobiotics might promote the induction of allograft tolerance
|
Since the pioneering experimental work on actively acquired tolerance in mice by Billingham et al. (1), the induction of transplantation tolerance in humans (generally after bone marrow transplantation (BMT)) has required ablation of the recipient’s immune system with drugs or radiation, thus avoiding graft rejection. The transplanted bone marrow stem cells are then able to mature throughout the recipient’s immune system, including the thymus, rendering the host tolerant to donor alloantigens (63,64). Much of the seminal work-concerning bone marrow chimerism has been performed by Sachs and his group. In studies on experimental bone marrow transplantation in xenogeneic and allogeneic models, they have emphasised the importance of mixed chimerism for the induction of allograft and xenograft tolerance (65,66). More recent studies show that such mixed chimerism, capable of inducing donor-specific tolerance, can be induced without myeloablation (53).
Under immunosuppressive therapy, or without this condition in several experimental liver transplant models, donor leukocytes survive. The donor APC, particularly dendritic cells, but possibly also other lineages, including activated B cells (10,67,68), may contribute to the induction of tolerance by continuously presenting donor-MHC to the recipient as they interact with the recipient’s immune system. Several factors are likely to be involved.
Based on the immune network theory initially proposed by Jerne (69–73), it is possible that anti-idiotypic clones of host T and B cells (anti-self), produced as part of the network response to donor alloantigen, may recognize a component of the class II MHC/T-cell receptor complex on the activated host CD4 (+) T cells and thereby control rejection (54). Development of such regulatory control would be dependent on a rejection response attenuated by careful pharmacological control. Excessive immunosuppression could be harmful and inhibit tolerance induction by abrogating both the recipient response to donor and the replication of donor cells (54,59,74). The influence of antilymphocytic agents on T-cell development deserves separate comment. The powerful T-cell-directed immunosuppressants FK506 and CsA, both of which induce tolerance in rodents, also alter the thymic micro-environment in which T cells mature (75,76). As a result, these drugs inhibit thymocyte maturation and cause a dispersal of immature T cells into the periphery (77,78). During immunosuppression, thymic recruitment of interdigitating dendritic cells also occurs (79). In the presence of a transplanted organ, both effects may increase the exposure of immature recipient T cells to donor APC – a process which closely parallels the concept of intrathymic antigen presentation which is used as the basis for allograft tolerance induction following intrathymic injection of donor cells (80,81). Furthermore, it has been demonstrated in such models that the presence of donor APC is essential for donor-specific tolerance to occur (68,82,83). These data reassert the importance of the chimeric state following organ transplantation in which the emigrant donor leukocytes may act as a ‘vaccine’ of donor MHC alloantigen which affects the maturation of the recipient immune system. The result envisaged is similar to that seen after T-cell vaccination for the inhibition or prevention of autoimmune disease (84).
The B-cell component of the donor-derived leukocytes may contribute to the induction of tolerance. Although not ‘professional’ APC, they have the ability to present antigen to T lymphocytes and induce unresponsiveness in these cells (85). Furthermore, polyclonal B-cell activation is known to occur in some experimental models of transplantation tolerance (86,87). This may contribute to an antibody-mediated component of the network control of rejection (54), a concept supported by early clinical reports of improved graft survival in patients with high levels of anti-Fab (anti-idiotypic) antibodies (88,89).
Finally, the two-signal model of lymphocyte activation initially proposed by Bretscher and Cohn provides further insight into possible mechanisms of tolerance induction (90). In this model, TCR occupation alone does not induce clonal proliferation. Instead, a second signal (either secretion of a cytokine or expression of a eostimulatory APC surface molecule such as B7/BB1 (91)) is required. The inhibition of APC function and APC–T-cell interaction that occurs with many immunosuppressants could block delivery of this second signal and perhaps induce T-cell anergy through the production of putative ‘anergy proteins’ and decreases in intracellular calcium within the T cells (91). The anergic state may be maintained by a defect in IL-2 accumulation in the presence of continuous antigenic stimulation supplied by the donor-derived APC (91,92). When the first and second signals are transmitted simultaneously, clonal proliferation occurs and rejection ensues. A prime candidate for second signal activation is the B7 ligand CD28, a T-cell surface molecule which promotes translation and stability of cytokine mRNA (including that of IL-2) (93). The continuous presence of donor MHC on leukocytes that have migrated from the graft is likely to induce chronic TCR occupancy in any cells that do divide. This chronic stimulation would be likely to generate more putative negative regulators, thus reinforcing the anergic state.
Stopping immunosuppressive therapy
The ideal clinical situation would obviously be the routine induction of donor-specific tolerance after organ transplantation, as evidenced by in vitro donor-specific hyporeactivity, followed by weaning from immunosuppressive medications. This would reduce exposure of the patient to the complications of immunosuppression and the side effects of antirejection therapy. But when can immunosuppressive treatment be stopped, if at all?
Given the conceptual basis for tolerance induction outlined above, the absolute requirement for immunosuppression is that it is in place at the time of transplantation to allow the establishment of a chimeric state. In many animal models of transplantation tolerance, drug therapy is only administered at or around the time of organ grafting; this is sufficient to induce both chimerism and tolerance, which are then self-maintained.
Although the problem is more complex in humans, some patients do achieve drug-free status after organ transplantation (Table 5). This has been shown to occur between 2 months and 11 years postoperatively, but in an unreliable and unpredictable way (8,9). The ease with which immunosuppression can be stopped appears to be dependent on the type of organ transplanted. It has been shown in many experimental animal models (34,94–99) and clinically (8,9,100) that liver grafts (which are rich in passenger leukocytes) are much more prone to induce tolerance than kidney or heart grafts (with their fewer passenger leukocytes) when immunosuppression is discontinued.
Table 5.
Features of six liver allograft recipients who had achieved drug-free graft tolerance
| Patient | Year of TP |
Year drug stopped |
Years drug free |
Prednisone (mg/day) |
Liver function |
|---|---|---|---|---|---|
| 1 | 1973 | 1982 | 10 | − | Normal |
| 2 | 1974 | 1985 | 7 | − | Normal |
| 3 | 1977 | 1987 | 5 | − | Normal |
| 4 | 1978 | 1979 | 13 | − | Normal |
| 5 | 1979 | 1981 | 11 | − | Normal |
| 6 | 1980 | 1985 | 7 | 10 | Failing* |
TP = transplantation
Recurrent chronic active hepatitis secondary to hepatitis C virus was the biopsy-proven diagnosis from the graft hepatectomy specimen obtained at retransplantation.
Although it is not known whether there is an optimal time to stop immunosuppression, the introduction of FK506 with its powerful ability to reverse episodes of acute rejection (28) makes the withdrawal of immunosuppressive therapy a real possibility with significant inherent advantages for the transplant patient (101). Clearly however, it is prudent to wait until the recipient has been stable without rejection for some time and has shown evidence of only minimal in vitro donor alloreactivity, before any attempt is made to wean drug therapy. A trial is currently underway at the University of Pittsburgh Medical Centre aimed, ultimately, at removing immunosuppressant therapy in patients with liver transplants who have been free of rejection for 5 years or more (8,101). The results of this study may have important implications for the future management of liver recipients, and perhaps recipients of other organ transplants as well.
Clinical augmentation of the ‘natural’ chimeric state
In an effort to augment the ‘natural’ chimerism that occurs after organ transplantation (caused by donor leukocytes of bone marrow origin), human recipients of whole organ grafts at this medical centre have received the simple expedient of donor bone marrow infusion at the time of transplantation. There has been no deviation from the standard initial immunosuppressive regime (routine immunosuppression with FK506 and prednisone and without cytoablation) (102). The expectation was that the acceptance of organs less tolerogenic than the liver, such as the heart and kidney (or even the liver itself) might be facilitated by augmenting the natural process of chimerism with the infusion of donor bone marrow (4,6,8,9).
The results to date are consistent with this hypothesis. Sixteen organ-bone marrow recipients (kidney (n = 9), liver (n = 6; 3 with islets), heart (n = 1)) have good graft function, 3–13 months postoperatively. All patients, except one whose nearly complete HLA match precludes examination, show readily identifiable chimerism by flow cytometric analysis and corroborative polymerase chain reaction quantitative testing. Rejection occurred in nine patients and graft-versus-host disease in two – both complications were successfully treated. This study reconfirms previous warnings that chimerism is not synonymous with tolerance, but is only a necessary precondition for its development (4,8,103). The pace of drug weaning, with the eventual goal of drug-free tolerance, is being determined individually for each patient with guidance from serial tests of in vitro immune reactivity to donor and third party alloantigens. Most patients show evidence of evolving donor-specific hyporesponsiveness, however, none have yet achieved a drug-free state. These results correlate with those of Barber et al. who, using the ‘Monaco model’ of adjuvant donor bone marrow administration (104), showed an increase in one-year renal allograft survival from 71 to 90% (105). The similar number of rejection episodes seen in the bone marrow-treated and the control groups was a source of some concern to the authors, however, we feel that a controlled recipient response to donor plays a crucial part in the initiation of ultimate graft tolerance.
Beyond an adjuvant role for whole organ transplantation, it will be important to determine if MHC mismatched bone marrow engrafted under the above conditions can be used without an accompanying organ in patients whose disease can be corrected with a mixed chimeric state. The potential list of such indications is long (106), and is exemplified by the lysosomal enzyme deficiencies (7).
Breaking tolerance with immunosuppressive agents
The tolerant state envisaged after organ transplantation is an active phenomenon, with a constant but stable interaction between the donor and recipient at many different levels (11,54,58,70,73,87,107). Such stability requires positive recognition of donor with integration of host and recipient immune systems secondary to mutual stimulation (70) – an active process which does not appear to involve either clonal deletion or anergy (58). In effect, this active process by the donor-derived leukocytes is an incipient graft-versus-host (GVH) reaction which is thought to be essential for tolerance to occur (9,54).
The stable and balanced chimerism that equates with tolerance may topple either towards graft rejection by the recipient or GVH disease mediated by donor-derived cells (108). Although the topics of rejection and GVH reactivity are too large to address in this paper, some drug-related phenomena related to the breaking of tolerance are important and will be addressed briefly.
Even though the cessation of immunosuppression is a desirable goal, there is experimental evidence indicating problems that may arise. In certain rat strain combinations for example, stable engraftment following liver or small bowel transplantation is seen until immunosuppression is stopped; GVH disease then ensues with fatal results (109,110). In these phenotype combinations, the two immune systems are able to mutually coexist while being controlled by drugs, but donor cells can overpower those of the recipient after pharmacological control has been terminated (109,110).
Notably, histocompatibility differences are not required for the development of GVH disease. Glazier et al. were the first to describe a model of syngeneic GVH disease in adult rats after lethal irradiation, syngeneic bone marrow transplantation and a temporary, 40-day period of CsA treatment (111). Although the pathogenesis of this disorder is not completely understood, autoreactive T lymphocytes are certainly involved (112). These cells appear to be produced in a CsA-damaged thymus which cannot eliminate potentially autoreactive lymphocytes during their differentiation (113). A key factor in the induction of this syndrome is the abolition of immune regulatory systems by a combination of irradiation and CsA (114). When normal mature T cells are allowed to remain in animals treated with CsA alone, the disease does not develop (24).
In a recent clinical case, reminiscent of the tolerance breaking experiments described in a classic article by Billingham, Brent and Medawar (115), stored graft-recipient bone marrow cells have been used to reverse graft-versus-host disease in a patient given a combined liver-(donor) bone marrow transplant. The tolerance breaking effect of the bone marrow infusion was incomplete – this allowed control of the GVHD without attendant allograft rejection. The case was that of a 56-year-old male with gastric leiomyosarcoma and liver metastases who underwent liver transplantation after upper abdominal exenteration (116). The patient received 5.5 Gy total lymphoid irradiation preoperatively followed immediately postoperatively by 19 × 109 nonpurged donor bone marrow cells. During the next few weeks (in the presence of 22–34% circulating donor phenotype lymphocytes), he developed a severe graft-versus-host disease, with >80% skin involvement, which did not respond to changes in immunosuppression. On the 42nd and 43rd postoperative days, 2.83 × 108 autologous stored bone marrow cells were infused. Over the subsequent two weeks, while the donor phenotype lymphocytes decreased to 3% of total, the skin rash resolved and the patient was discharged without evidence of rejection.
Other preliminary data have also been reported on the deliberate breaking of tolerance in a clinical model (117). Because of the beneficial antitumor effect of GVH disease after experimental allogeneic BMT, a trial was conducted to evaluate whether GVH reactivity could be induced in humans with CsA therapy. Studying a group of patients with lymphoma in resistant relapse, Jones et al. demonstrated that moderately severe GVH disease could indeed be induced in patients undergoing autologous BMT following total body irradiation and CsA therapy (117). Furthermore, preliminary results suggested that the presence of GVH disease in these patients promoted a significant antitumour effect (118).
Conclusion
The development of modern immunosuppressive regimens largely accounts for current successes in allogeneic organ transplantation. Although the mechanism of action of the diverse range of immunosuppressive agents in current use varies greatly, it appears that they have a common potential to induce graft tolerance. Adequate, but not complete immunosuppression is now believed to permit donor-derived leukocytes to migrate from the graft, disperse widely in the recipient’s tissues and establish multilineage microchimerism. Donor APC then continuously represent donor alloantigens to the recipient immune system. Under pharmacological immunosuppressive therapy, a minimal rejection process develops. Rejection, however, is limited by immunosuppression and may be converted to an active, network-regulated tolerant state.
In some organ-transplant patients, it is possible to cease immunosuppressive therapy and donor-specific tolerance is maintained spontaneously. Investigations are currently underway to define which organ allograft recipients are suitable candidates for this strategic approach without invoking excessive risk to the patient of developing graft rejection or GVH disease. Further investigations are examining whether the synchronous transplantation of donor bone marrow combined with a solid organ into recipients receiving routine immunosuppression, without cytoablation, can augment the chimeric state and facilitate allograft acceptance.
References
- 1.Billingham RE, Brent L, Medawar PB. ‘Actively acquired tolerance’ of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2.Sigal NH, Dumont FJ. Cyclosporin A, FK506 and rapamycin: pharmacologic probes of lymphocyte signal transduction. Ann Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- 3.Thomson AW, Starzl TE. New immunosuppressive drugs: mechanistic insights and potential therapeutic advances. Immunol Rev. 1993;136:71–98. doi: 10.1111/j.1600-065x.1993.tb00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, ehimerism and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Demetris AJ, Trucco M, et al. Systemic chimerism in female recipients of male livers. Lancet. 1992;340:876–877. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Demetris AJ, Trucco M, et al. Chimerism and donor-specific nonreactivity 27 to 29 years after kidney allotransplantation. Transplantation. 1993;55:1272–1277. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Demetris AJ, Trucco M, et al. Chimerism after liver transplantation for type IV glycogen storage disease and type I Gaucher’s disease. N Engl J Med. 1993;328:745–749. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Demetris AJ, Murase N, Thomson AW, Trucco M, Ricordi C. Donor cell chimerism permitted by immunosuppressive drugs: a new view of organ transplantation. Immunol Today. 1993;14:326–332. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 10.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demetris AJ, Murase N, Fujisaki S, Fung JJ, Rao AS, Starzl TE. Hematolymphoid cell trafficking, chimerism and tolerance after liver, bone marrow and heart transplantation. Transplant Proc. 1993;25:3337–3344. [PMC free article] [PubMed] [Google Scholar]
- 12.Steinmuller D. Immunization with skin isografts taken from tolerant mice. Science. 1967;158:127–129. doi: 10.1126/science.158.3797.127. [DOI] [PubMed] [Google Scholar]
- 13.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. 1. Morphology, quantification, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinman RM. The dendritic cell system and its role in immunogenicity. Ann Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 15.Demetris AJ, Qian S, Sun H, et al. Early events in liver allograft rejection. Am J Pathol. 1991;138:609–618. [PMC free article] [PubMed] [Google Scholar]
- 16.Engemann R, Gassel HJ, Lafrenz E, Stoffregen C, Thiede A. Transplantation tolerance after short-term administration of 15-deoxyspergualin in orthotopic rat liver transplantation. Transplant Proc. 1987;19:4241–4243. [PubMed] [Google Scholar]
- 17.Reinherz EL, Hussey RE, Schlossman SF. A monoclonal antibody blocking human T-cell function. Eur J Immunol. 1980;10:758–762. doi: 10.1002/eji.1830101006. [DOI] [PubMed] [Google Scholar]
- 18.Lenschow DJ, Zeng Y, Thistlethwaite JR, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 19.Nakakura EK, McCabe SM, Zheng B, et al. Potent and effective prolongation by anti-LFA-1 monoclonal antibody monotherapy of non-primarily vascularized heart allograft survival in mice without T-cell depletion. Transplantation. 1993;55:412–417. [PubMed] [Google Scholar]
- 20.Shizuru JA, Seydel KB, Flavin TF, et al. Induction of donor-specific unresponsiveness to cardiac allografts in rats by pretransplant anti-CD4 monoclonal antibody therapy. Transplantation. 1990;50:366–373. doi: 10.1097/00007890-199009000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Orosz CG, Ohye RG, Pelletier RP, et al. Treatment with anti-vascular cell adhesion molecule 1 monoclonal antibody induces long-term murine cardiac allograft acceptance. Transplantation. 1993;56:453–460. doi: 10.1097/00007890-199308000-00039. [DOI] [PubMed] [Google Scholar]
- 22.Isobe M, Yagita H, Okumuru K, Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992;255:1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- 23.Kirkman RL, Barrett LV, Kelley VE, Ythier A, Strom TB. Administration of an anti-interleukin-2 receptor monoclonal antibody prolongs cardiac allograft survival in mice. J Exp Med. 1985;162:358–366. doi: 10.1084/jem.162.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hess AD. Cellular immunobiology of cyclosporin. In: Thomson AW, Starzl TE, editors. Immunosuppressive Drugs: Developments in Anti-Rejection Therapy. London: Edward Arnold; 1994. pp. 47–62. [Google Scholar]
- 25.Keown PA. Influence of cyclosporin A on graft rejection. In: Thomson AW, Starzl TE, editors. Immunosuppressive Drugs: Developments in Anti-Rejection Therapy. London: Edward Arnold; 1994. pp. 63–82. [Google Scholar]
- 26.White DJ, Rolles K, Ottawa T, Turell O. Cyclosporin-A-induced long-term survival of fully incompatible skin and heart grafts in rats. Transplant Proc. 1980;12:261–265. [PubMed] [Google Scholar]
- 27.Ochiai T, Nakajima K, Nagata M, Hori S, Asano T, Isono K. Studies of the induction and maintenance of long-term graft acceptance by treatment with FK 506 in heterotopic cardiac allotransplantation in rats. Transplantation. 1987;44:734–738. doi: 10.1097/00007890-198712000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan R, Jain A. FK506 for liver, kidney and pancreas transplantation. Lancet. 1989;334:1001–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starzl TE, Todo S, Fung JJ, Groth CFK506. A potential breakthrough in immunosuppression - clinical implications. Transplant Proc. 1990;22:5–6. [PubMed] [Google Scholar]
- 30.Starzl TE, Thomson AW, Todo S, Fung JJ. Proceedings of the first international congress on FK 506. Transplant Proc. 1991;23:2709–3380. [PubMed] [Google Scholar]
- 31.Fung JJ, Shapiro R, Armitage J, Starzl TE. Influence of FK506 in clinical transplantation. In: Thomson AW, Starzl TE, editors. Immunosuppressive Drugs: Developments in Anti-Rejection Therapy. London: Edward Arnold; 1994. pp. 121–128. [Google Scholar]
- 32.Murase N, Kim DG, Todo S, Cramer DV, Fung JJ, Starzl TE. Suppression of allograft rejection with FK506. I. Prolonged cardiac and liver survival in rats following short-course therapy. Transplantation. 1990;50:186–189. doi: 10.1097/00007890-199008000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murase N, Kim DG, Todo S, Cramer DV, Fung JJ, Starzl TE. FK506 suppression of heart and liver allograft rejection. II. The induction of graft acceptance in rats. Transplantation. 1990;50:739–744. doi: 10.1097/00007890-199011000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murase N, Demetris AJ, Kim DG, Todo S, Fung JJ, Starzl TE. Rejection of the multivisceral allograft in rats: a sequential analysis with comparison to isolated orthotopic small bowel and liver grafts. Surgery. 1990;108:880–889. [PMC free article] [PubMed] [Google Scholar]
- 35.Tocci MJ, Matkovich DA, Collier KA, et al. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989;143:718–726. [PubMed] [Google Scholar]
- 36.Fruman DA, Burakoff SJ, Bierer BE. Molecular actions of cyclosporin A, FK506 and rapamycin. In: Thomson AW, Starzl TE, editors. Immunosuppressive Drugs: Developments in Anti-Rejection Therapy. London: Edward Arnold; 1994. pp. 15–35. [Google Scholar]
- 37.Kronke M, Leonard WJ, Depper JM, et al. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci (USA) 1984;81:5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang SC, Morel PA, Wang Q, Jordan ML, Simmons RL, Tweardy DJ. A dual mechanism of immunosuppression by FK506. Transplantation. 1993;56:978–985. doi: 10.1097/00007890-199310000-00038. [DOI] [PubMed] [Google Scholar]
- 39.Kahan BD, Chang JY, Seghal SN. Preclinical evaluation of a new potent immunosuppressive agent rapamycin. Transplantation. 1991;52:185–191. doi: 10.1097/00007890-199108000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Elion GB. The pharmacology of azathioprine. Ann NY Acad Sci. 1993;685:400–407. doi: 10.1111/j.1749-6632.1993.tb35897.x. [DOI] [PubMed] [Google Scholar]
- 41.Mayumi H, Good RA. Long-lasting skin allograft tolerance in adult mice induced across fully allogeneic (multi-major H-2 plus multiminor histocompatability) antigen barriers by a tolerance-inducing method using cyclophosphamide. J Exp Med. 1989;169:213–238. doi: 10.1084/jem.169.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amemiya H, Itoh H. Mizoribine (bredinin): mode of action and effects on graft rejection. In: Thomson AW, Starzl TE, editors. Immunosuppressive Drugs: Developments in Anti-Rejection Therapy. London: Edward Arnold; 1994. pp. 161–176. [Google Scholar]
- 43.Hao L, Calcinaro F, Gill RG, Eugui EM, Allison AC. Facilitation of specific tolerance induction in adult mice by RS61443. Transplantation. 1992;53:590–595. doi: 10.1097/00007890-199203000-00020. [DOI] [PubMed] [Google Scholar]
- 44.Cramer DV, Knoop M, Chapman FA, Wa GD, Jaffee BD, Makowka L. Prevention of liver allograft rejection in rats by a short course of therapy with brequinar sodium. Transplantation. 1992;54:752–753. doi: 10.1097/00007890-199210000-00042. [DOI] [PubMed] [Google Scholar]
- 45.Schmidbauer G, Hancock WW, Badger AM, Kupiec-Weglinski JW. Induction of non-specific x-irradiation-resistant suppressor cell activity in vivo and prolongation of vascularised allograft survival by SK&F 105685, a novel immunomodulatory azaspirane. Transplantation. 1993;55:1236–1243. [PubMed] [Google Scholar]
- 46.Schorlemmer HU, Seiler FR, Bartlett RR. Prolongation of allogeneic transplanted skin grafts and induction of tolerance by leflunomide, a new immunosuppressive isoxazole derivative. Transplant Proc. 1993;25:763–767. [PubMed] [Google Scholar]
- 47.Hancock WW, Schmidbauer G, Badger AM, Kupiec-Weglinski JW. SK&F 105685 suppresses allogeneically induced mononuclear and endothelial cell activation and cytokine production and prolongs rat cardiac allograft survival. Transplant Proc. 1992;24:231–232. [PubMed] [Google Scholar]
- 48.Kuchle CCA, Thoenes GH, Langer KH, Schorlemmer HU, Bartlett RR, Schleyerbach R. Prevention of kidney and skin graft rejection in rats by leflunomide, a new immunomodulating agent. Transplant Proc. 1990;23:1803–1806. [PubMed] [Google Scholar]
- 49.Xiao F, Chong ASF, Bartlett RR. Leflunomide: a promising immunosuppressant in transplantation. In: Thomson AW, Starzl TE, editors. Immunosuppressive Drugs: Developments in Anti-Rejection Therapy. London: Edward Arnold; 1994. pp. 203–212. [Google Scholar]
- 50.Thomson AW, Starzl TE, editors. Immunosuppressive Drugs: Developments in Anti-Rejection Therapy. London: Edward Arnold; 1994. [Google Scholar]
- 51.Thomson AW, Demetris AJ, Murase N, Starzl TE. Promotion of cell chimerism by immunosuppressive drugs: a possible basis for tolerance induction following organ transplantation. In: Thomson AW, Starzl TE, editors. Immunosuppressive Drugs: Developments in Anti-Rejection Therapy. London: Edward Arnold; 1994. pp. 221–230. [Google Scholar]
- 52.Russell PS. Modification of runt disease in mice by various means. In: Wolstenholme CEW, Cameron MP, London J, Churchill A, editors. Transplantation: Ciba Foundation Symposium. Boston: Little Brown and Co; 1962. pp. 350–383. [Google Scholar]
- 53.Sharabi Y, Abraham VS, Sykes M, Sachs DH. Mixed allogeneic chimeras prepared by a non-myeloablative regimen: requirement for chimerism to maintain tolerance. Bone Marrow Transplant. 1992;9:191–197. [PubMed] [Google Scholar]
- 54.Demetris AJ, Murase N, Rao AS, Starzl TE. The role of passenger leukocytes in rejection and ‘tolerance’ after solid organ transplantation: a potential explanation of a paradox. In: Touraine JL, et al., editors. Rejection and Tolerance. Netherlands: Kluwer Academic Publishers; 1994. pp. 325–392. [Google Scholar]
- 55.Austyn JM, Larsen CP. Migration patterns of dendritic leukocytes. Implications for transplantation. Transplantation. 1990;49:1–7. doi: 10.1097/00007890-199001000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Starzl TE, Murase N, Demetris AJ, Ildstad S, Ricordi C, Trucco M. Allograft and xenograft acceptance under FK506 and other immunosuppressive treatment. Ann NY Acad Sci. 1993;685:46–51. doi: 10.1111/j.1749-6632.1993.tb35849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dahmen U, Qian S, Rao AS, et al. Split tolerance induced by orthotopic liver transplantation in mice. Transplantation. doi: 10.1097/00007890-199407000-00001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liegeois A, Escourrou J, Ouvre E, Charriere J. Microchimerism: a stable state of low-ratio proliferation of allogeneic bone marrow. Transplant Proc. 1977;9:273–276. [PubMed] [Google Scholar]
- 60.Ahmed Z, Terasaki PI. Effect of transfusions. Clinical Transplants. 1991:305–312. [PubMed] [Google Scholar]
- 61.MacLeod AM. The blood transfusion effect: clinical aspects. Immunol Lett. 1991;29:123–126. doi: 10.1016/0165-2478(91)90212-s. [DOI] [PubMed] [Google Scholar]
- 62.Bradley JA. The blood transfusion effect: experimental aspects. Immunol Lett. 1991;29:127–132. doi: 10.1016/0165-2478(91)90213-t. [DOI] [PubMed] [Google Scholar]
- 63.Slavin S, Reitz B, Bieber CP, Kaplan HS, Strober S. Transplantation tolerance in adult rats using total lymphoid irradiation: permanent survival of skin, heart and marrow allografts. J Exp Med. 1978;147:700–707. doi: 10.1084/jem.147.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Slavin S, Fuks Z, Strober S, Kaplan HS. Transplantation tolerance across major histocompatibility complex barriers after total lymphoid irradiation. Transplantation. 1979;25:359–361. doi: 10.1097/00007890-197911000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Sykes M, Sachs DH. Bone marrow transplantation as a means of inducing tolerance. Semin Immunol. 1990;2:401–417. [PubMed] [Google Scholar]
- 66.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. J Exp Med. 1984;307:168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 67.Inaba M, Inaba K, Hosono M, et al. Distinct mechanisms of neonatal tolerance induced by dendritic cells and thymic B cells. J Exp Med. 1991;173:549–559. doi: 10.1084/jem.173.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campos L, Posselt AM, Deli BC, et al. The failure of intrathymic transplantation of nonimmunogenic islet allografts to promote induction of donor-specific unresponsiveness. Transplantation. 1994;57:950–953. doi: 10.1097/00007890-199403270-00030. [DOI] [PubMed] [Google Scholar]
- 69.Jerne NK. Towards a network theory of the immune system. Ann Immunol (Paris) 1974;125:373–389. [PubMed] [Google Scholar]
- 70.Coutinho A, Bandeira A. Tolerize one, tolerize them all: tolerance is self-assertion. Immunol Today. 1989;10:264–266. doi: 10.1016/0167-5699(89)90138-2. [DOI] [PubMed] [Google Scholar]
- 71.Coutinho A. Beyond clonal selection and network. Immunol Rev. 1989;110:63–87. doi: 10.1111/j.1600-065x.1989.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 72.Varela FJ, Coutinho A. Second generation immune networks. Immunol Today. 1991;12:159–166. doi: 10.1016/S0167-5699(05)80046-5. [DOI] [PubMed] [Google Scholar]
- 73.Arnold B, Schonrich G, Hammerling GJ. Multiple levels of peripheral tolerance. Immunol Today. 1993;14:12–14. doi: 10.1016/0167-5699(93)90317-E. [DOI] [PubMed] [Google Scholar]
- 74.Wood KJ. Alternative approaches for the induction of transplantation tolerance. Immunol Lett. 1991;29:133–138. doi: 10.1016/0165-2478(91)90214-u. [DOI] [PubMed] [Google Scholar]
- 75.Beschorner WE, Namnoum JD, Hess AD, Shinn CA, Santos GW. Cyclosporin A and the thymus: immunopathology. Am J Pathol. 1987;126:487–492. [PMC free article] [PubMed] [Google Scholar]
- 76.Pugh-Humphreys RGP, Ross CSK, Thomson AW. The influence of FK-506 on the thymus: an immunophenotypic and structural analysis. Immunology. 1990;70:398–404. [PMC free article] [PubMed] [Google Scholar]
- 77.Hosseinzadeh H, Goldschneider I. Recent thymic emigrants in the rat express a unique antigenic phenotype and undergo post-thymic maturation in peripheral lymphoid tissues. J Immunol. 1993;150:1670–1679. [PubMed] [Google Scholar]
- 78.Hosseinzadeh H, Goldschneider I. Demonstration of large-scale migration of cortical thymocytes to peripheral lymphoid tissues in cyclosporin A-treated rats. J Exp Med. 1993;178:285–293. doi: 10.1084/jem.178.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Waal EJ, Rademakers LH, Schuurman HJ, Van Loveren H. Interdigitating cells in the rat thymus during cyclosporin A treatment: ultrastructural observations. Thymus. 1992;20:163–170. [PubMed] [Google Scholar]
- 80.Posselt AM, Barker CF, Tomaszewski JE, Markmann JF, Choti MA, Naji A. Induction of donor-specific unresponsiveness by intrathymic islet transplantation. Science. 1990;249:1293–1295. doi: 10.1126/science.2119056. [DOI] [PubMed] [Google Scholar]
- 81.Odorico JS, Posselt AM, Naji A, Markmann JF, Barker CF. Promotion of rat cardiac allograft survival by intrathymic inoculation of donor splenocytes. Transplantation. 1993;55:1104–1107. doi: 10.1097/00007890-199305000-00032. [DOI] [PubMed] [Google Scholar]
- 82.Ketchum RJ, Moyer C, Pan F, Moore WV. Intrathymic transplantation of allogeneic nonimmunogenic perinatal islet tissue does not induce donor-specific tolerance. Transplantation. 1993;56:728–730. [PubMed] [Google Scholar]
- 83.Campos L, Alfrey EJ, Posselt AM, Odorico JS, Barker CF, Naji A. Prolonged survival of rat orthotopic liver transplants (OLT) following intrathymic (IT) inoculation of donor strain cells. Transplantation. 1993;55:866–870. doi: 10.1097/00007890-199304000-00034. [DOI] [PubMed] [Google Scholar]
- 84.Cohen IR, Weiner HL. T-cell vaccination. Immunol Today. 1988;9:332–335. doi: 10.1016/0167-5699(88)91330-8. [DOI] [PubMed] [Google Scholar]
- 85.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 86.Goldman M, Abramowicz D, Lambert P, Vandervorst P, Bruyns C. Hyperactivity of donor B cells after neonatal induction of lymphoid chimerism in mice. Clin Exp Immunol. 1988;72:79–83. [PMC free article] [PubMed] [Google Scholar]
- 87.Bandeira A, Coutinho A, Carnaud C, Jacquemart F, Forni L. Transplantation tolerance correlates with high levels of T- and B-lymphocyte activity. Proc Natl Acad Sci (USA) 1989;86:272–276. doi: 10.1073/pnas.86.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Susal C, Guo ZG, Terness P, Opelz G. Role of anti-IgG autoantibodies in kidney transplantation. Immunol Lett. 1990;26:121–125. doi: 10.1016/0165-2478(90)90133-b. [DOI] [PubMed] [Google Scholar]
- 89.Chauhan B, Phelan DL, Marsh JW, Mohanakumar T. Characterization of antiidiotypic antibodies to donor HLA that develop after liver transplantation. Transplantation. 1993;56:443–448. doi: 10.1097/00007890-199308000-00037. [DOI] [PubMed] [Google Scholar]
- 90.Bretscher P, Cohn M. A theory of self-non-self discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 91.Jenkins MK. The role of cell division in the induction of clonal anergy. Immunol Today. 1992;13:69–73. doi: 10.1016/0167-5699(92)90137-V. [DOI] [PubMed] [Google Scholar]
- 92.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal activation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Ann Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 93.Lindsten T, June CH, Ledbetter JA, Stella G, Thomson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244:339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- 94.Starzl TE, Marchioro TL, Porter KA, et al. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131–155. [PMC free article] [PubMed] [Google Scholar]
- 95.Valdivia LA, Fung JJ, Demetris AJ, Starzl TE. Differential survival of hamster-to-rat liver and cardiac xenografts under FK 506 immunosuppression. Transplant Proc. 1991;23:3269–3271. [PMC free article] [PubMed] [Google Scholar]
- 96.Gamier H, Clot J, Bertrand M, et al. Liver transplantation in the pig: surgical approach. Cr Acad Sci (Paris) 1965;260:5621–5623. [PubMed] [Google Scholar]
- 97.Peacock JH, Terblanche J. Orthotopic homotransplantation of the liver in the pig. In: Read AE, editor. The Liver. London: Butterworth & Co., Ltd; 1967. pp. 333–336. [Google Scholar]
- 98.Calne RY, White HJO, Yoffa DE, et al. Observations of orthotopic liver transplantation in the pig. Br Med J. 1967;2:478–480. doi: 10.1136/bmj.2.5550.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zimmerman FA, Butcher GW, Davies HS, Brons G, Kamada N, Turel O. Techniques for orthotopic liver transplantation in the rat and some studies of the immunologic responses to fully allogeneic liver grafts. Transplant Proc. 1979;11:571–577. [PubMed] [Google Scholar]
- 100.Starzl TE. In: Experience in Hepatic Transplantation. Starzl TE, Putnam CW, editors. Philadelphia: WB Saunders; 1969. pp. 203–207. [Google Scholar]
- 101.Reyes J, Tzakis A, Ramos HC, et al. The frequent achievement of a drug free state after orthotopic liver transplantation. Transplant Proc. 1993;25:3315–3319. [PMC free article] [PubMed] [Google Scholar]
- 102.Fontes P, Rao AS, Demetris AJ, et al. Augmentation with bone marrow of donor leukocyte migration for kidney, liver, heart and pancreas islet transplantation. Lancet. 1994;344:151–155. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Strober S, Modry DL, Hoppe RT, et al. Induction of specific unresponsiveness to heart allografts in mongrel dogs treated with total lymphoid irradiation and anti-thymocyte globulin. J Immunol. 1984;132:1013–1018. [PubMed] [Google Scholar]
- 104.Monaco AE, Wood ML, Russell PS. Studies of heterologous anti-lymphocyte serum in mice. III. Immunologic tolerance and chimerism produced across the H-2 locus with adult thymectomy and anti-lymphocyte serum. Ann NY Acad Sci. 1966;129:190–209. [Google Scholar]
- 105.Barber WH, Mankin JA, Laskow DA, et al. Long-term results of a controlled prospective study with transfusion of donor-specific bone marrow in 57 cadaveric renal allograft recipients. Transplantation. 1991;51:70–75. doi: 10.1097/00007890-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 106.Parkman R. The application of bone marrow transplantation to the treatment of genetic diseases. Science. 1986;232:1373–1378. doi: 10.1126/science.3520819. [DOI] [PubMed] [Google Scholar]
- 107.Streilein JW, Levy RB, Ruiz P, Matriano J, Socarras S. Multiple mechanisms induce and maintain neonatal transplantation tolerance. Transplant Sci. 1993;3:1–6. [Google Scholar]
- 108.Cooper MH, Nalesnik MA, Watkins SC, Hoffman RA, Ildstad ST. Cross-species graft-versus-host-disease is accompanied a donor-derived cellular immune response. Transplantation. 1993;56:934–940. doi: 10.1097/00007890-199310000-00030. [DOI] [PubMed] [Google Scholar]
- 109.Murase N, Demetris AJ, Woo J, et al. Graft-versus-host disease after Brown Norway-to-Lewis and Lewis-to-Brown Norway rat intestinal transplantation under FK506. Transplantation. 1993;55:1–7. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tanabe M, Murase N, Demetris AJ, et al. The influence of donor and recipient strains in isolated small bowel transplantation in rats. Transplantation. In press. [PMC free article] [PubMed] [Google Scholar]
- 111.Glazier A, Tutschka PJ, Farmer ER, Santos GW. Graft-versus-host disease in cyclosporin A treated rats after syngeneic and autologous bone marrow transplantation. J Exp Med. 1983;158:1–8. doi: 10.1084/jem.158.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hess AD, Horowitz L, Beschorner WE, Santos GW. Development of graft-versus-host disease-like syndrome in cyclosporin treated rats after syngeneic bone marrow transplantation. I. Development of cytotoxic T lymphocytes with apparent polyclonal anti-la specificity including autoreactivity. J Exp Med. 1985;161:718–730. doi: 10.1084/jem.161.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Parkman R. Graft-versus-host disease: an alternative hypothesis. Immunol Today. 1989;10:362–364. doi: 10.1016/0167-5699(89)90267-3. [DOI] [PubMed] [Google Scholar]
- 114.Hess AD, Fischer AC. Immune mechanisms in cyclosporin-induced syngeneic graft-versus-host disease. Transplantation. 1989;48:895–900. doi: 10.1097/00007890-198912000-00001. [DOI] [PubMed] [Google Scholar]
- 115.Billingham R, Brent L, Medawar P. Quantitative studies on tissue transplantation. III. Actively acquired tolerance. Philosophical Transcripts of the Royal Society of London (Biological) 1956;239:357–412. [Google Scholar]
- 116.Ricordi C, Tzakis AG, Demetris AJ, et al. Reversal of graft-versus-host disease with infusion of stored autologous bone marrow cells following combined liver-bone marrow allotransplantation in man. Transplantation Science. 1993;3:1. [PMC free article] [PubMed] [Google Scholar]
- 117.Jones RJ, Vogelsang GB, Hess AD, et al. Induction of graft-versus-host disease after autologous bone marrow transplantation. Lancet. 1989;i:754–757. doi: 10.1016/s0140-6736(89)92575-0. [DOI] [PubMed] [Google Scholar]
- 118.Hess AD, Jones RJ, Morris LE, Noga SJ, Vogelsang GB, Santos GW. Autologous graft-versus-host disease: a novel approach for antitumour immunotherapy. Human Immunology. 1992;34:219–224. doi: 10.1016/0198-8859(92)90115-4. [DOI] [PubMed] [Google Scholar]