Abstract
E proteins are basic helix-loop-helix transcription factors that regulate many key aspects of lymphocyte development. Thymocytes express multiple E proteins that are thought to provide cooperative and compensatory functions crucial for T cell differentiation. Contrary to that, we report here that the E protein HEB was uniquely required at the CD4+CD8+ double-positive (DP) stage of T cell development. Thymocytes lacking HEB showed impaired survival, failed to make rearrangements of variable-α (Vα) segments to distal joining-α (Jα) segments in the gene encoding the T cell antigen receptor α-chain (Tcra) and had a profound, intrinsic block in the development of invariant natural killer T cells (iNKT cells) at their earliest progenitor stage. Thus, our results show that HEB is a specific and essential factor in T cell development and in the generation of the iNKT cell lineage, defining a unique role for HEB in the regulation of lymphocyte maturation.
The E protein family of transcriptional regulators mediate lymphocyte differentiation by binding to DNA at E-box sites, controlling the expression of genes essential to lineage commitment, targeting antigen-receptor gene rearrangement and enforcing key developmental checkpoints1,2. In mammals, E proteins include E12 and E47 (E2A; splice variants encoded by Tcf3, called ‘Tcfe2a’ here), HEB and E2-2, which in lymphocytes typically bind as homo- or heterodimers to consensus E-box sequences. The Id proteins are highly related but lack a DNA-binding domain and thus function to inhibit E protein activity when they are incorporated into E–Id heterodimers. E2A, HEB and E2-2 have all been linked to the regulation of thymocyte development. However, E2-2 functions mainly in the specification of precursors to the plasmacytoid dendritic cell lineage3. The combined activity of both E2A and HEB promote T cell antigen receptor (TCR) rearrangements and control the developmental progression, survival and proliferation of developing T cells, accounting for the majority of E protein function in the thymus2. E2A (E47) supports the expression of molecules involved in pre-TCR- and Notch-mediated signaling at the earliest stages of thymocyte development4 and directly regulates rearrangements of the gene encoding TCRβ (Tcrb)5. Furthermore, E2A is needed to restrain CD4−CD8− double-negative (DN) cells from proliferating or progressing to the double-positive (DP) stage until productive Tcrb rearrangement occurs1,6. Similarly, E proteins enforce the checkpoint for Tcra rearrangements, and the absence of the expression of both E2A and HEB by DP thymocytes results in the generation of CD8 single-positive (SP) T cells lacking rearranged TCRα chains and thus TCR expression7. In contrast to E2A deficiency, loss of HEB results in fewer thymocytes owing to a role for HEB in the transition from the DN stage to the DP stage8, but this defect is partially overcome by the compensatory activity of E2A8,9.
Although thymocytes lacking either E2A or HEB demonstrate notable perturbations in development, substantial numbers of T cells are nonetheless generated8,10. However, a block in thymocyte development is observed at the DN stage when a dominant negative mutation of HEB is introduced9 or both E2A and HEB are deleted at the DN stage11, which demonstrates that a minimum of E protein activity is necessary for T cell development. In the context of other lineages that require E proteins for specification, the effective replacement of Tcfe2a, which encodes E2A, with Tcf12, which encodes HEB, suggests that the E proteins encoded by these genes function interchangeably in ‘rescuing’ B cell development and embryonic lethality12. Similarly, in neurogenesis, for which E proteins are also necessary, all of the E proteins are equally effective partners for neurogenic differentiation factor 2, which suggests similar activity among the family members13. Thus, E2A and HEB have been thought to compensate for each other during lymphocyte development.
Natural killer T (NKT) cells rapidly produce many cytokines after activation, which affects the recruitment and function of cells that participate in subsequent innate and adaptive immune responses14–16. The majority of NKT cells express an invariant TCRα chain that mediates the recognition of glycolipid antigens presented by the major histocompatibility complex class I–like molecule CD1d (invariant NKT (iNKT) cells)14–16. Such glycolipids can be derived from microorganisms and mediate activation of iNKT cells during infection, and the absence of iNKT cells exacerbates pathology17. The iNKT cells mature in the thymus, differentiating from DP thymocytes and proceeding through defined developmental stages at which their maturation is influenced by many signaling molecules, transcription factors and cytokines that are not essential for conventional T cells14. In mice, iNKT cells can first be detected in the thymus as CD24+CD69+ cells bearing the canonical variable α-segment 14–to–joining α-segment 18 (Vα14-Jα18) Tcra rearrangement after their positive selection mediated by interactions with signaling lymphocytic-activation molecule receptors and CD1d molecules expressed by other DP thymocytes. Thus, mutations that affect either signaling through these receptors or CD1d-mediated antigen presentation result in the absence of iNKT cells at this early stage in development. As CD24 is downregulated, the cells proliferate and progress through three more developmental stages identified by the upregulation of CD44 and then NK1.1 (refs. 14,18,19). Progression through the early expansion phase is dependent on the transcription factor c-Myc20,21. During this maturation process, iNKT cells acquire the expression of molecules associated with T cell activation, NK cell receptors and the ability to produce cytokines and lyse target cells.
Many transcription factors have been linked to iNKT maturation. The earliest stage of iNKT cell development absolutely requires the transcription factor RORγt to induce upregulation of the antiapoptotic molecule Bcl-xL, which supports the survival of DP thymocytes22,23. The prolonged survival of DP thymocytes allows distal Tcra V-to-J rearrangements, to produce the canonical iNKT TCRα chain22–24. Thus, iNKT cell development is restored in RORγt-deficient thymocytes by transgenic expression of Bcl-xL or a rearranged Vα14-Jα18 TCR22,23. DP thymocytes deficient for the transcription factor Runx1 also fail to give rise to iNKT cells; however, in this case the canonical rearrangements are detected, which suggests a requirement for differentiation or expansion23. The T-box transcription factor T-bet also affects iNKT cell development, although at a later stage of thymic development25,26, probably by regulating interleukin 15 responsiveness27. The transcription factor Egr2 has also been found to be necessary for the generation of a normal iNKT cell compartment, and Egr2 deficiency alters both proliferation and survival28. Notably, the promyelocytic leukemia zinc-finger transcription factor PLZF has been shown to regulate the acquisition of the characteristic activated phenotype and effector functions of iNKT cells29,30. How these many transcription factors are coordinated to promote commitment to the iNKT lineage and subsequent maturation is not well defined.
Although the role of E proteins in conventional T cell development has been studied extensively, how these important transcriptional regulators affect the development of iNKT cells has not been investigated. Here we studied the development of iNKT cells from DP thymocytes lacking HEB and/or E2A and made the unexpected finding that HEB ‘instructs’ a unique gene-expression program and regulates both survival and Tcra rearrangements in a manner distinct from E2A. Our results identify HEB as an essential regulator of thymocyte development and highlight previously unknown distinctions in the functions of E protein family members.
RESULTS
E proteins are expressed during iNKT cell development
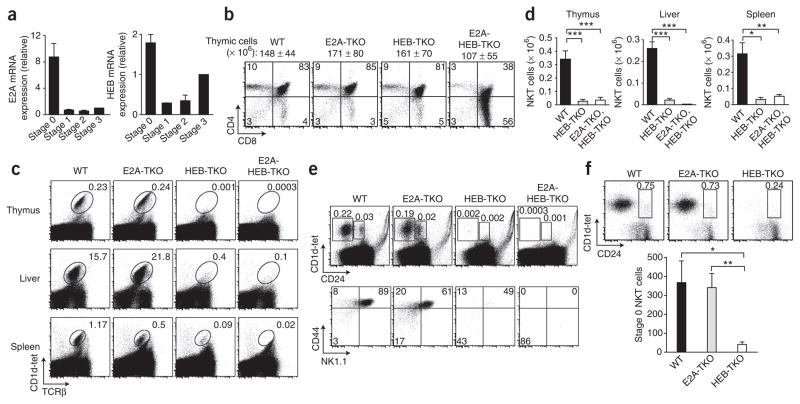
Although it is well established that E proteins are expressed by thymocytes and are necessary for the development of conventional αβ T cells2, it was unknown whether they are expressed by or function in thymocytes that commit to the iNKT cell lineage. We initially examined whether E protein transcripts were expressed during iNKT cell development. Many strategies have been used to characterize the developmental progression of iNKT cells2,5; for convenience, we used the following criteria to stage these subsets using α-galactosylceramide-loaded CD1d tetramers (CD1d-tet) to identify cells bearing the canonical Vα14-Jα18 TCR rearrangement: CD1d-tet+CD24+ (stage 0), CD1d-tet+CD24loCD44loNK1.1− (stage 1), CD1d-tet+CD24loCD44hiNK1.1− (stage 2) and CD1d-tet+CD24loCD44hi NK1.1+ (stage 3). We sorted each subset of iNKT cells from wild-type thymocytes and compared their expression of E2A and HEB mRNA. Notably, both E2A and HEB transcripts were most highly expressed at stage 0 and were abruptly downregulated by stage 1 (Fig. 1a). HEB showed the most dynamic mRNA changes, with low expression at stage 1 and a progressive increase in expression during stages 2 and 3 (Fig. 1a), whereas E2A mRNA abundance remained low. Of note, E2-2 was expressed at stage 0; however, further analysis showed that E2-2 deficiency had no effect on iNKT cell development (data not shown). These data demonstrated regulation of E-protein mRNA during development of iNKT cells and suggested they could potentially affect the earliest commitment to the iNKT cell lineage (stage 0).
Figure 1.
HEB is essential for iNKT cell development. (a) Expression of Tcfe2a (E2A) mRNA and Tcf12 (HEB) mRNA by sorted wild-type thymic CD1d-tet+TCRβ+ iNKT cells, normalized to stage-1 expression. Data are representative of three independent experiments (average of two or three separate samples). (b) Flow cytometry of the surface expression of CD4 and CD8 by wild-type (WT), E2A-TKO, HEB-TKO and E2A-HEB-TKO thymocytes. Thymocyte number above plots (average ± s.d.); numbers in quadrants indicate percent cells in each. Data are representative of three experiments. (c) Expression of CD1d-tet and TCRβ by lymphocytes from thymus, liver and spleen. Numbers adjacent to outlined areas indicate percent CD1d+TCRβ+ iNKT cells among lymphocytes. Data are representative of five independent experiments. (d) Total CD1d-tet+TCRβ+ iNKT cells in thymus, liver and spleen. Data are representative of seven experiments (average and s.e.m. of seven to nine mice per group). (e) Maturation subsets of thymocytes defined by expression of CD1d-tet and CD24 (top) or CD44 and NK1.1 (bottom) on CD1d-tet-gated iNKT cells. Numbers adjacent to outlined areas or in quadrants indicate percent cells in each. Data are representative of three or more independent experiments. (f) Expression of CD1d-tet and CD24 by thymocytes after enrichment for CD1d-tet+ cells by magnetic-activated cell sorting. Numbers adjacent to outlined areas indicate percent CD1d+CD24+ iNKT cells. Below, total CD1d+CD24+ iNKT cells (average and s.e.m. of four to six mice per group). Data are representative of four experiments. *P < 0.05, **P < 0.005, and ***P < 0.0005 (unpaired two-tailed t-test).
HEB is essential for iNKT cell development
We next investigated the role of E proteins in iNKT cell development in mouse models of T lineage–specific deletion of Tcfe2a and/or Tcf12 in DP thymocytes. We crossed mice with loxP-flanked alleles of these genes (Tcfe2af/f or Tcf12f/f) with a mouse line transgenic for expression of Cre recombinase driven by the promoter of the mouse gene encoding CD4 (Cd4-Cre+)7,11,31,32 to delete these genes specifically in DP thymocytes and thus subsequently maturing T cells. We generated the following mouse lines: Tcfe2af/fCd4-Cre+ (E2A–T cell knockout (E2A-TKO)), Tcf12f/fCd4-Cre+ (HEB–T cell knockout (HEB-TKO)) and Tcfe2af/fTcf12f/fCd4-Cre+ (E2A– and HEB–T cell knockout (E2A-HEB-TKO)). Initially, we examined by flow cytometry conventional αβ T cell development in these lines with conditional gene deletion (Fig. 1b). Total thymocyte numbers and subset composition, determined by surface expression of CD4 and CD8, were similar in wild-type, E2A-TKO and HEB-TKO mice, whereas thymocyte development in E2A-HEB-TKO mice was considerably perturbed, with a higher frequency of the CD8 SP subset, as reported before7 (Fig. 1b). The number of thymocytes in additional developmental stages was unchanged in E2A-TKO and HEB-TKO mice relative to that in wild-type mice but was altered in E2A-HEB-TKO mice (Supplementary Fig. 1). Thus, we observed no gross alterations in total cell number or thymic subsets due to single deficiency in E2A or HEB occurring at the DP stage, which indicated that each of these E proteins can compensate for the loss of the other at this stage.
We evaluated the iNKT cell populations in thymus, liver and spleen identified by staining for CD1d-tet and TCRβ (Fig. 1c). Loss of E2A alone did not affect the iNKT cell compartment at any stage (Fig. 1). In contrast, we found that a much lower frequency of iNKT cells resulted from HEB ablation (Fig. 1). Furthermore, analysis of iNKT cell numbers showed a similar paucity of iNKT cells in both HEB-TKO and E2A-HEB-TKO mice (Fig. 1d). Closer analysis of mice with heterozygous deficiency in E protein suggested that loss of E2A may have affected frequency of iNKT cells slightly, but this did not result in loss of overall iNKT cell numbers (Supplementary Fig. 2a,b). To rule out the possibility that the unchanged iNKT cell compartment observed in E2A-TKO mice was due to incomplete deletion of the loxP-flanked Tcfe2a allele, we reconstituted wild-type mice with fetal liver cells from E2A-deficient or HEB-deficient embryos (germline deletion) and analyzed the iNKT cell compartment. We observed normal frequencies of iNKT cells in mice reconstituted with E2A-deficient cells, but a complete loss of iNKT cells resulted in mice reconstituted with HEB-deficient cells (Supplementary Fig. 3).
To more accurately pinpoint the stage at which HEB was required during iNKT cell maturation, we analyzed the developmental subsets on the basis of expression of CD24, CD44 and NK1.1 in the presence or absence of HEB or E2A or both, before and after enrichment for CD1d-tet+ cells (Fig. 1e,f). This analysis showed a nearly complete loss of iNKT cells in the absence of HEB at the earliest stage of development (stage 0), as well as in subsequent developmental stages (Fig. 1e,f), suggesting a block in development before stage 0 and the expansion phase. These data define a unique and essential role for HEB during iNKT cell development, for which E2A is unable to compensate.
The HEB-TKO iNKT cell defect is cell intrinsic
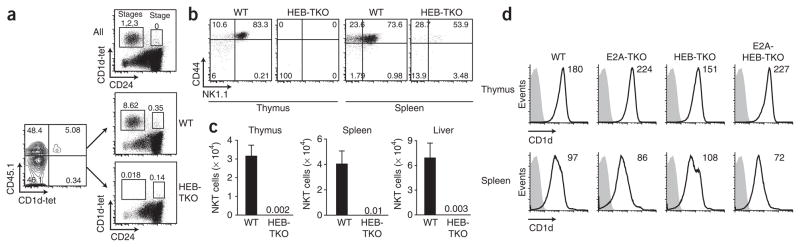
As iNKT cells are positively selected by CD1d molecules expressed by cortical thymocytes14,18,19 and the Cd4-Cre transgene induces deletion of the loxP-flanked Tcfe2a and Tcf12 alleles in DP thymocytes, we wanted to address whether the phenotype observed for HEB-TKO cells was cell intrinsic or was due to a defect in the ability of the cortical thymocytes to select developing iNKT cells. We generated mixed–bone marrow chimeras in which irradiated recipients were reconstituted with a 1:1 mixture of wild-type and HEB-TKO donor bone marrow, each distinguishable by their congenic expression of CD45 alleles. After reconstitution, we enriched for CD1d-tet+ thymocytes and determined the percentage of iNKT cells in both the wild-type and HEB-TKO fractions (Fig. 2a). We observed that HEB-TKO donor cells were unable to generate CD1d-tet+ iNKT cells (even in the presence of wild-type cells) and that this defect was at the earliest stage of iNKT cell development (as noted in HEB-TKO mice; Fig. 2a). Furthermore, HEB deficiency led to a loss of iNKT cells at all other stages of development (stages 1, 2 and 3) in the thymus and periphery even in the presence of wild-type thymocytes (Fig. 2b). Comparison of absolute numbers of iNKT cells from these mixed chimeras confirmed that HEB deficiency did not solely affect frequency, as we observed extremely small numbers of iNKT cells in the thymus, spleen and liver of HEB-TKO mice relative to the numbers in their wild-type counterparts (Fig. 2c). Mixed–bone marrow chimeras generated with wild-type and E2A-TKO, HEB-TKO donor cells showed similar results (Supplementary Fig. 4). We also examined expression of CD1d by wild-type and E protein–deficient lymphocytes and found no differences (Fig. 2d). Thus, we conclude that there is no evidence of a processing or presentation defect by cortical thymocytes, as wild-type cells were unable to ‘rescue’ the HEB-TKO iNKT cell developmental defect.
Figure 2.
The defect in iNKT cell development by HEB-TKO thymocytes is cell intrinsic. (a) Expression of CD45.1 and CD1d-tet by donor thymocytes (left) and expression of CD1d-tet and CD24 by total thymocytes and wild-type (CD45.1+) and HEB-TKO thymocytes in reconstituted recipients (right). Data are representative of two experiments. (b) Surface expression of NK1.1 and CD44 by CD1d-tet+ donor cells from thymus and spleen of bone marrow chimeras. Data are representative of two experiments with five chimeras total. Numbers adjacent to outlined areas or in quadrants (a,b) indicate percent cells in each. (c) Total CD1d-tet+TCRβ+ cells among wild-type or HEB-TKO donor cell populations from thymus, spleen and liver. Numbers above HEB-TKO indicate average number of cells (bars not visible). Data are representative of QQ experiments (average and s.e.m. of five mice). (d) CD1d surface expression (black lines) by thymocytes and splenocytes from wild-type, E2A-TKO, HEB-TKO and E2A-HEB-TKO mice. Gray-filled histograms, unstained cells. Numbers in plots indicate mean fluorescence intensity. Data are representative of three independent experiments.
HEB regulates the survival and proliferation of DP thymocytes
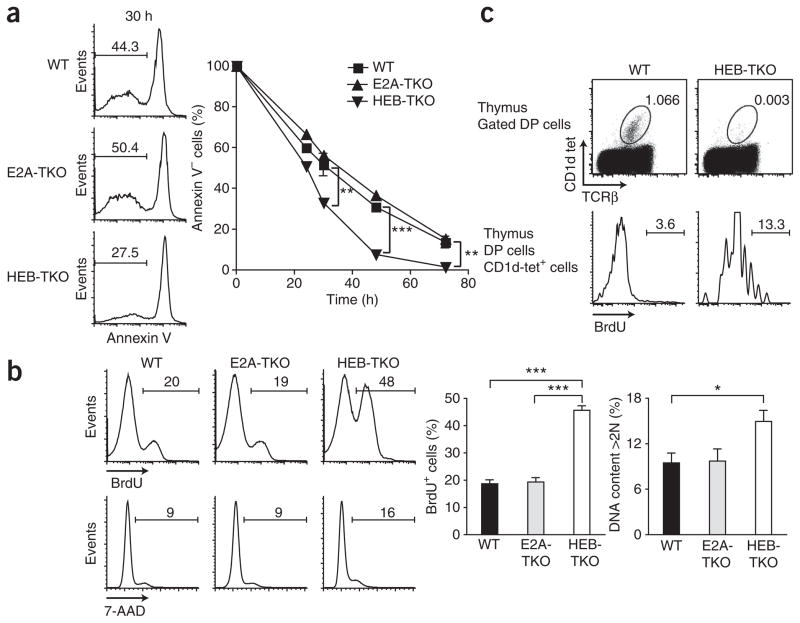
To address how HEB affects iNKT cell selection from the DP population, we examined the survival and proliferation of thymocytes. Notably, we found that HEB deficiency at the DP stage accelerated cell death in vitro (Fig. 3a), which suggested a defect in survival. After 30 h in culture, <30% of the HEB-TKO cells were negative for annexin V, compared with ~50% of the wild-type and E2A-TKO cells, and by 50 h only ~5% of the HEB-TKO cells were still alive, compared with ~35% of the wild-type and E2A-TKO cells (Fig. 3a). Similarly, E2A-deficient thymocytes (germline deletion) survived in vitro as well as wild-type and E2A-TKO cells did (Supplementary Fig. 5). Notably, we also found that HEB-TKO DP thymocytes showed greater proliferation and DNA content than did their wild-type counterparts (Fig. 3b). Approximately twice as many HEB-TKO thymocytes as wild-type thymocytes incorporated 5-bromodeoxyu-ridine (BrdU) after a 12-hour pulse, and significantly more had >2N DNA content (P = 0.04), which indicated that they were in S-G2 phases of the cell cycle. Consistent with the results obtained with the DP population, the very few HEB-TKO CD1d-tet+ cells incorporated more BrdU (Fig. 3c), which indicated that the failure to accumulate HEB-TKO iNKT cells was not due to a defect in the proliferative burst after their positive selection. Thus, HEB deficiency affects both the survival and proliferation of developing thymocytes.
Figure 3.
HEB deficiency influences the survival and proliferation of thymocytes. (a) Annexin V expression by wild-type, E2A-TKO or HEB-TKO thymocytes cultured for 0–72 h. Numbers above bracketed lines (left) indicate annexin V–negative cells. Data are representative of five independent experiments. (b) BrdU incorporation and 7-amino-actinomycin D (7-AAD) staining of wild-type, E2A-TKO or HEB-TKO DP thymocytes after a 12-hour in vivo pulse of BrdU; numbers above bracketed lines indicate BrdU+ cells (top) or 7-AAD+ cells (middle). Data are representative of four independent experiments (average and s.e.m.). (c) BrdU incorporation by wild-type or HEB-TKO CD1d-tet+ DP thymocytes after a 3-hour pulse of BrdU. Numbers adjacent to outlined areas (top) indicate percent CD1d-tet+TCRα+ cells; numbers above bracketed lines (bottom) indicate BrdU+ cells. Data are representative of two independent experiments. *P < 0.05, **P < 0.005, ***P < 0.0005 (unpaired two-tailed t-test).
HEB controls a unique gene-expression profile in thymocytes
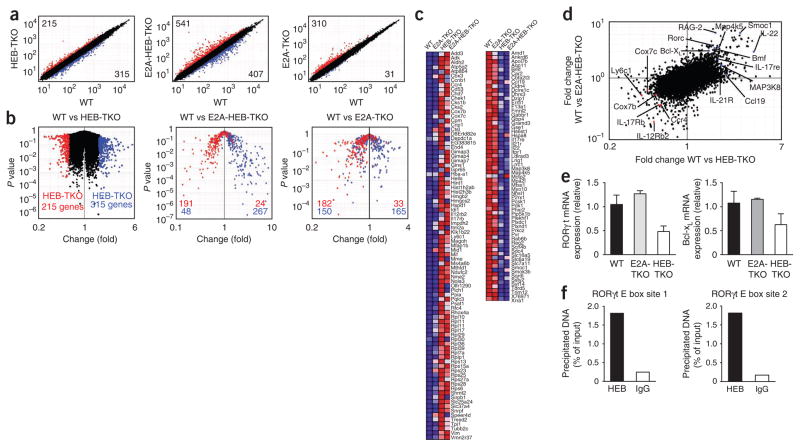
We next addressed whether HEB controlled a larger subset of genes distinct from the related E protein and frequent binding partner E2A. We compared the transcript profiles of wild-type, E2A-TKO, HEB-TKO and E2A-HEB-TKO DP thymocytes by microarray analysis. Loss of HEB at the DP stage affected the expression of many genes, with 215 genes upregulated more than 1.7-fold and 315 genes downregulated relative to their wild-type counterparts (Fig. 4a). In contrast, when we compared E2A-TKO and wild-type DP thymocytes, we found that a similar number of genes were upregulated by the E2A-TKO thymocytes (310 genes) but fewer were downregulated (31 genes; Fig. 4a), which suggested that HEB could compensate for many of the targets positively regulated by E2A when deletion occurred at the DP stage. Not unexpectedly, loss of both E2A and HEB resulted in more extensive changes in gene expression, with 541 genes upregulated and 407 genes downregulated compared with expression in their wild-type counterparts (Fig. 4a), which indicated that these two E proteins do act together in regulating many targets and that loss of both is not compensated for by the activity of other E proteins. Studies focusing on the HEB-regulated genes showed that E2A-HEB-TKO DP cells had a high correlation: 89% of the genes upregulated by HEB-TKO cells also descended to the left on a P value–versus–fold change ‘volcano’ plot, and 85% of the genes downregulated by HEB-TKO cells also descended to the right (Fig. 4b). Furthermore, those genes that were positively regulated by HEB did not show a similar pattern of expression in E2A-TKO cells, with only 52% also down-regulated (Fig. 4b), which indicated no correlation and supported the observation of a unique profile of genes regulated specifically by HEB. Notably, those genes upregulated because of HEB deficiency were similarly upregulated (84%) by the E2A-TKO thymocytes (Fig. 4b).
Figure 4.
Unique gene-expression profile of HEB-TKO DP cells. Affymetrix microarray analysis of mRNA from DP thymocytes sorted from wild-type, E2A-TKO, HEB-TKO and E2A-HEB-TKO mice. (a) Normalized expression values for wild-type versus HEB-TKO (left), E2A-HEB-TKO (middle) or E2A-TKO (right) DP thymocytes. Numbers in corners indicate number of genes with a difference in expression of 1.7-fold or more (upregulation, top left (red dots); downregulation, bottom right (blue dots)). (b) ‘Volcano’ plots of the gene-expression data of wild-type versus HEB-TKO (left), E2A-HEB-TKO (middle) or E2A-TKO (right) DP cells. Numbers in plots indicate genes with a difference in expression of 1.7-fold or more in HEB-TKO DP cells versus wild-type cells (upregulation, red; downregulation, blue). Data are representative of experiments with two or three data sets per group. (c) Annotated gene expression of transcripts with a difference in expression of 1.7-fold or more (upregulation, red; downregulation, blue). (d) Comparison of the gene-expression changes in wild-type versus E2A-HEB-TKO DP cells (horizontal axis) and wild-type versus E2A-HEB-TKO DP cells (vertical axis). Red and blue dots indicate transcripts uniquely up- and downregulated, respectively, by both HEB-TKO and E2A-HEB-TKO DP cells. (e) Expression of RORγt and Bcl-xL mRNA by E2A-TKO and HEB-TKO DP cells, normalized to the expression of GAPDH (glyceraldehyde phosphate dehydrogenase) and presented relative to their expression in wild-type cells. Data are representative of three experiments (average and s.e.m. of three or four individual samples). (f) Chromatin immunoprecipitation from wild-type thymocytes with antibody to HEB or control immunoglobulin G antibody (IgG), followed by quantitative PCR analysis of input or precipitated DNA containing RORγt E-box site 1 (left) or 2 (right).
In an additional analysis, we visualized the mean expression of a group of annotated genes whose transcripts were regulated differently by both HEB-TKO and E2A-HEB-TKO DP cells relative to wild-type DP cells (Fig. 4c) and defined clusters of genes that were regulated together after loss of HEB but not after loss of E2A. Notably, many molecules encoded by the genes upregulated after HEB deletion were involved in metabolic processes, including eleven ribosomal proteins and five that function in oxidative phosphorylation. We summarized changes in gene expression in HEB-TKO and E2A-HEB-TKO cells relative to the expression in wild-type DP cells and highlighted genes up- or downregulated in both (Fig. 4d). Many genes encoding cytokines and chemokines (and their receptors), such as Il17rb, Il12rb, Il22, Ccr4 and Ccl19, as well as genes encoding molecules that influence survival and cell death, such as Rorc, Bcl2l1, Cox7b, Cox7c and Bmf, showed similar trends. Thus, HEB specifically regulates a discrete subset of genes during thymocyte development, potentially affecting many biological pathways.
Using quantitative PCR, we confirmed some of the targets identified and also asked specifically whether HEB was required to support the expression of molecules known to be necessary for the development of iNKT cells from DP cells. Transcripts of many genes encoding molecules known to regulate iNKT cell development, including Sh2d1a, Tox, Gata3, Runx1, Zbtb16, Myc and Slamf6, were not altered considerably in the absence of E2A or HEB or of both E2A and HEB (Supplementary Fig. 6). However, consistent with the microarray results, mRNA for RORγt and Bcl-xL was less abundant in HEB-TKO DP cells than in wild-type DP cells (Fig. 4e). As RORγt is known to promote Bcl-xL expression, and thus thymo-cyte survival, this finding provided a possible mechanism for the enhanced death of the HEB-TKO thymocytes. To determine whether HEB was potentially regulating RORγt directly, we did chromatin immunoprecipitation of wild-type thymocytes with an HEB-specific antibody and found that HEB was indeed bound to two E-box sites33 in the promoter of the gene encoding RORγt (Fig. 4f). This binding was lower in HEB-TKO cells (Supplementary Fig. 7) and was more prominent than E2A binding (Supplementary Fig. 7). Thus, we concluded that HEB regulates a unique gene-expression profile distinct from that of its sister protein E2A.
Impairment of distal Jα rearrangements in the absence of HEB
During rearrangement of the Tcra locus, the first rearrangements tend to use proximal Jα genes, whereas distal Jα gene segments are included during secondary rearrarangements34. The canonical iNKT TCR rearrangement joins Vα14 to the distal Jα18 region and thus requires such secondary rearrangements and extended survival, which provides time for this to occur. We wanted to ascertain if the impaired survival of HEB-TKO thymocytes, mediated by lower expression of RORγt and Bcl-xL, resulted in fewer distal rearrangements by DP cells, which would in turn lead to fewer iNKT cells and skewing of the TCR repertoire.
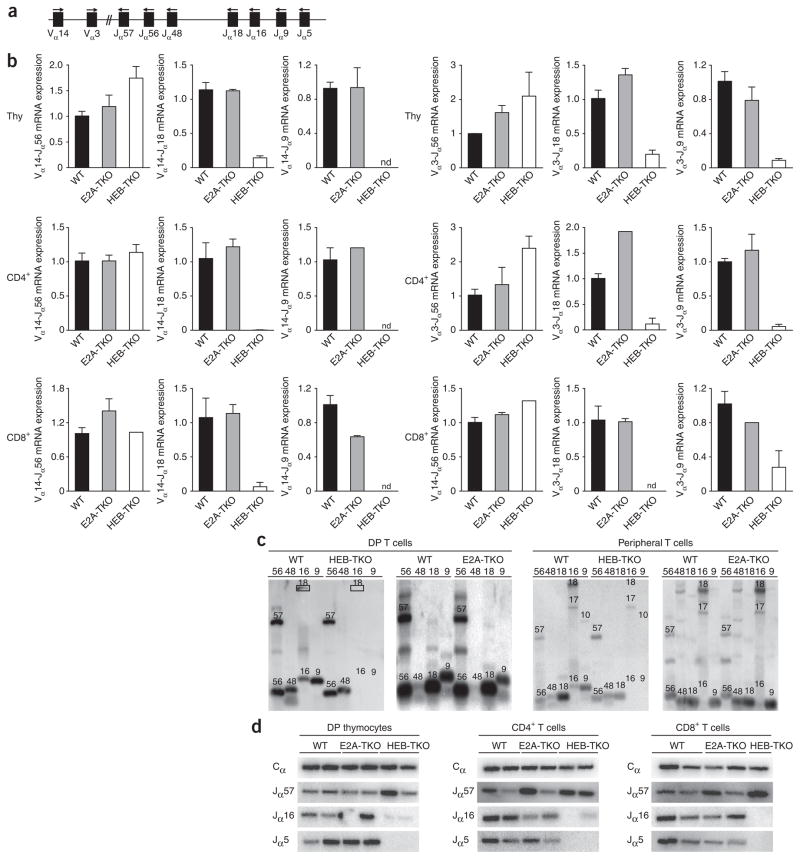
First, using quantitative PCR to detect Vα14 or Vα3 rearrangements to specific J segments, we found that HEB-TKO thymocytes and CD4+ and CD8+ splenic T cells showed a profound lack of rearrangements using distal J segments (Fig. 5a,b). This result was in contrast to results obtained with E2A-TKO cells, in which there was no difference in rearrangements compared with those of wild-type cells. Next we extracted genomic DNA from sorted wild-type or HEB-TKO DP thymocytes and CD4+ splenocytes and analyzed Vα14 rearrangements with proximal Jα56 and Jα48 and distal Jα16 and Jα9 rearrangements (Fig. 5a). We analyzed Vα14-to-Jα(x) rearrangements (where ‘(x)’ is region 56, 48, 16 or 9) by multiplex PCR and Southern blot analysis with a Vα14-specific probe. We observed that Vα14-Jα18 rearrangements were largely absent (Fig. 5c), and if this rearrangement was detected in HEB-TKO DP samples, the efficiency was low (data not shown), in agreement with the quantitative PCR analysis. Notably, even more distal rearrangements, such as those to Jα16 and Jα9, were completely absent in HEB-TKO thymocytes and peripheral T cells (Fig. 5c). In contrast, when we examined Vα14-to-Jα(x) gene rearrangements in the absence of E2A, we observed no differences in distal Jα gene rearrangements. Finally, we also examined this issue by analyzing products of PCR-amplified Vα3-to-Cα (constant α-segment) cDNA with probes specific for proximal and distal J segments34 and found loss of distal J segment use only among the HEB-TKO populations (Fig. 5d). Although greater proliferation of DP cells (Fig. 3) can result in degradation of the RAG-2 recombinase protein35,36, which could in turn impair rearrangements37, we found that wild-type and HEB-TKO thymocytes expressed similar amounts of RAG-2 protein (Supplementary Fig. 8). Thus, HEB-TKO DP thymocytes and mature T cells show substantially impaired use of distal Jα segments during rearrangement, consistent with their impaired survival and failure to generate iNKT cells.
Figure 5.
Rearrangements of Vα to distal Jα segments are impaired in the absence of HEB. (a) TCRα locus; arrows above indicate primers used for PCR. (b) Quantitative PCR analysis of transcripts of Vα14 and Vα3 rearrangements to Jα56, Jα18 or Jα9 segments by sorted thymocytes (Thy; top) or CD4+ (middle) or CD8+ (bottom) splenocytes, presented relative to the expression of Cα transcripts. Data are representative of two experiments (average and s.e.m. of two individual samples). (c) Southern blot analysis of multiplex PCR products of genomic DNA from sorted wild-type, HEB-TKO or E2A-TKO DP cells or CD4+ splenocytes. DNA was amplified with Vα14 forward primers and various Jα(x) reverse primers (numbers above lanes indicate ‘x’); numbers above bands indicate the amplified rearrangement (for example, ‘57’ indicates Vα14-Jα57). Data are representative of three different experiments with three wild-type and HEB-TKO pairs, two wild-type and E2A-TKO pairs for thymocytes and two per genotype for CD4+ cells. (d) RT-PCR analysis of DP, CD4+ or CD8+ splenic T cells from pairs of wild-type, E2A-TKO or HEB-TKO mice with primers for Vα3-Cα, followed by immunoblot analysis with a Jα-specific oligonucleotide probe. Top, internal Cα oligonucleotide probe used for normalization of the template. Data are representative of one experiment.
‘Rescue’ of HEB deficiency by expression of TCR or Bcl-xL
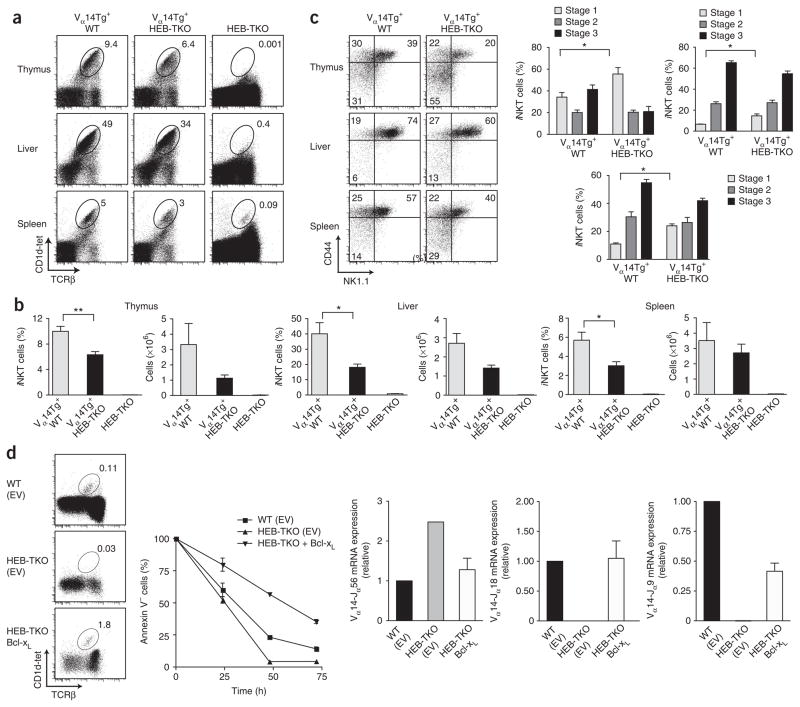
If the main role of HEB in iNKT cell development is to promote distal Tcra rearrangements, we reasoned that provision of a rearranged iNKT TCR Vα14-Jα18 transgene (Vα14Tg) would ‘rescue’ the iNKT cell phenotype observed in the absence of HEB. Thus, we crossed Tcf12f/fCd4-Cre+ mice with the Vα14Tg line and found that expression of the transgene supported significant iNKT cell development, even in the absence of HEB (Fig. 6). The frequency and cell number of iNKT cells in Vα14Tg+ HEB-TKO mice was largely restored in the thymus, liver and spleen compared with that of HEB-TKO mice (Fig. 6a,b), which suggested that HEB-mediated regulation of DP cell survival and Tcra rearrangements govern iNKT cell maturation. We did observe a slight accumulation of cells at stage 1 (Fig. 6c). Notably, we found that HEB transcripts were re-expressed as iNKT cells progressed to stages 2 and 3 (Fig. 1), which suggested that HEB may influence these later stages of maturation as well. However, the rescued iNKT cells that developed in the absence of HEB were both phenotypically and functionally mature in terms of downregulation of CD24, proliferation, expression of activation markers and ability to produce interferon-γ after stimulation compared with wild-type cells (Supplementary Fig. 9). Similarly, we evaluated expression of the panel of iNKT cell–specific genes and found that the Vα14Tg+ HEB-TKO iNKT cells did not show altered abundance of any of the transcripts compared with that of their wild-type counterparts (data not shown). Thus, the rescue of iNKT cells in the absence of HEB by expression of the canonical TCRα provides evidence that the defect originated from the failure of HEB-TKO thymocytes to survive and undergo successful distal rearrangements.
Figure 6.
Expression of a rearranged Vα14-Jα18 TCR transgene restores iNKT cell development in the absence of HEB. (a) Expression of CD1d-tet and TCRβ by Vα14tg+ wild-type, Vα14tg+ HEB-TKO and HEB-TKO lymphocytes from thymus, liver and spleen. Numbers adjacent to outlined areas indicate percent CD1d-tet+TCRβ+ cells in gate. Data are representative of five independent experiments. (b) Frequency (left) and absolute number (right) of CD1d-tet+TCRβ+ iNKT cells in thymus, liver and spleen of Vα14tg+ wild-type, Vα14tg+ HEB-TKO and HEB-TKO mice. Data are representative of two experiments (average and s.e.m.). (c) Surface expression of CD44 and NK1.1 by iNKT cells in thymus, liver and spleen of Vα14tg+ wild-type and Vα14tg+ HEB-TKO mice (left); numbers in quadrants indicate percent cells in each. Right, frequency of iNKT cells at developmental stages 1–3. *P < 0.05, **P < 0.005, ***P < 0.0005 (unpaired two-tailed t-test). Data are representative of three experiments (right, average and s.e.m. of four mice). (d) Expression of CD1d-tet and TCRβ (top left) by infected donor (CD45.2+Thy-1.1+) thymocytes in irradiated CD45.1+ hosts reconstituted with wild-type or HEB-TKO bone marrow infected with empty vector (EV; control) or HEB-TKO bone marrow infected with retrovirus encoding Bcl-xL (bottom). Right, annexin V expression by DP thymocytes obtained from the recipients described above and cultured for 0–72 h. Below, quantitative PCR analysis of transcripts of Vα14 rearrangements to Jα56, Jα18 or Jα9 segments by sorted thymocytes, presented relative to Cα transcripts. Data are representative of two experiments (top left), two independent experiments (top right) or two experiments (below; average and s.e.m. of two individual samples).
We reasoned that if HEB regulates RORγt expression, then introduction of Bcl-xL, reported to be regulated by RORγt and to be essential for thymocyte survival, would restore iNKT cell development even in the absence of HEB. Using a retroviral vector expressing Bcl-xL, we infected HEB-TKO bone marrow stem cells and used these cells to reconstitute sublethally irradiated hosts. After reconstitution, we observed that the provision of Bcl-xL restored the development of iNKT cells from HEB-TKO bone marrow, whereas empty vector did not (Fig. 6d). Furthermore, expression of Bcl-xL restored the survival and distal Jα Tcra gene rearrangements of HEB-TKO thymocytes (Fig. 6d). These data provide support for the idea that HEB regulates thymocyte survival, Tcra rearrangements and iNKT cell development through its control of the expression of RORγt and Bcl-xL.
DISCUSSION
Although E proteins are broadly recognized as important regulators of the development of conventional αβ T cells, our study has highlighted the unexpected and specific roles of HEB at the DP thymocyte stage in the control of a unique gene-expression program, survival, Tcra rearrangement and the development of iNKT-lineage cells. Notably, we found that HEB regulated the expression of a cohort of genes by DP cells independently of the related E protein E2A. HEB deficiency led to lower expression of RORγt and Bcl-xL, diminished survival and greater proliferation of DP thymocytes and specifically affected distal Jα gene rearrangements of the Tcra locus, altering the entire TCR repertoire. Additionally, HEB was expressed early in iNKT commitment and HEB deficiency abolished iNKT cell development. Thus, our data define previously unappreciated roles for HEB during T cell development.
Published studies have shown that HEB acts as a heterodimer with E2A, influencing T cell development at both the DN and DP stages7,9,11. However, these blocks are incomplete, and substantial αβ T cell development does occur in the absence of HEB, which supports the idea of compensatory roles for E2A and HEB. Furthermore, conditional deletion of both E2A and HEB in DP thymocytes leads to a failure of the DP-to-SP checkpoint for TCR expression, but normal T cell development was thought to proceed when only one E protein is deleted at this stage, which indicates that HEB and E2A also act together to control the DP-to-SP transition7. Here we found that conditional deletion of HEB in DP thymocytes resulted in a nearly complete loss of differentiation of thymocytes to the iNKT lineage but conditional deletion of E2A did not. This block was cell intrinsic and was not ‘rescued’ by wild-type thymocytes, which precludes the idea of a role for HEB in regulating proper processing and presentation of the CD1d-mediated positive selection. HEB-TKO thymocytes showed a block at the earliest detectable stage of iNKT commitment, similar to RORγt-, Bcl-xL- and Runx1-deficient mice and apparently before Sap-, c-Myc-, PLZF- and Egr2-dependent iNKT development20–23,28,29,38,39. The extreme paucity of cells at stage 0 and the greater proliferation of HEB-TKO DP cells and stage-0 cells challenge the idea of an expansion defect, which occurs during the later transitions from stage 0 through stage 2 (refs. 20,38).
The survival of HEB-TKO thymocytes was substantially impaired in vitro. Consistent with their impaired survival, HEB-TKO DP thymocytes had lower expression of RORγt and its target Bcl-xL and demonstrated greater proliferation, as do RORγt-deficient thymocytes40. Notably, HEB directly binds E-box sites in the promoter regions of the gene encoding RORγt, which suggests direct regulation of RORγt33. RORγt, through its regulation of Bcl-xL, is known to support prolonged survival and thus distal Tcra rearrangements33,34,41. The development of iNKT cells is thought to be specifically impaired due to mutations that affect thymocyte survival, as the canonical Tcra rearrangement uses the Jα18 segment, which is quite distal from the Vα gene segments and thus requires secondary rearrangements found only in cells that can survive long enough for this to occur. Consistent with that idea, HEB-TKO DP thymocytes demonstrated a profound defect in rearrangements using the Jα segments most distal to the V segments regardless of whether these Jα segments combined with Vα14 or with Vα3 gene segments. The failure to generate distal rearrangements also affected conventional CD4+ and CD8+ T cell populations, further emphasizing the role of HEB in influencing TCR repertoire. Notably, the expression of a rearranged iNKT TCR transgene, which bypasses the need for prolonged survival at the DP stage for secondary rearrangements, resulted in considerable restoration of iNKT cell development by HEB-TKO thymocytes. We found that the Vα14Tg+ HEB-TKO iNKT cells reached percentages and numbers near those of their Vα14Tg+ wild-type control cells and were phenotypically and functionally mature. Likewise, the development, survival and distal Tcra rearrangements of HEB-TKO iNKT cells were restored by Bcl-xL expression, which provides further support for the idea that HEB–RORγt–Bcl-xL controls T cell development.
Our microarray gene-expression profiling studies showed that HEB uniquely controls expression of a substantial subset of genes at the DP stage. Among these targets are those encoding many cytokines, cytokine receptors, signaling molecules and regulators of metabolism and cell survival. Notably, loss of HEB did not alter transcript abundance in DP cells for many molecules that are key during iNKT cell development, including PLZF, c-Myc, Sap, Tox and Runx1. However, the DP defect resulting from HEB deficiency probably precedes the requirement for those molecules. We did observe moderately lower expression of Fyn and Slamf1 mRNA by HEB-TKO DP thymocytes, which indicates that signaling maybe affected. However, even complete loss of either Fyn or SlamF1 did not generate a block in iNKT cell development as substantial as we observed after HEB deletion. Thus, our global gene-expression analysis of DP thymocytes has identified a subset of targets specifically regulated by HEB at this stage of development that are involved in a range of functions, which supports the idea of a unique role for HEB in T cell development. We have shown here that one specific role of HEB is to support thymocyte survival, allowing sustained Tcra rearrangements, affecting the total TCR repertoire and controlling iNKT cell development. These observations fit into a broader view of E-protein function, whereby this family of transcription factors, which are expressed throughout hematopoiesis, is able to regulate specific aspects of lineage commitment, such as E2-2 in the development of plasmacytoid dendritic cells3, E2A in B cell commitment2 and now HEB in thymocyte survival and iNKT cell development.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureimmunology/.
Supplementary Material
Acknowledgments
We thank C. Murre (University of California San Diego) for discussions, reagents and mice; Y. Zhuang (Duke University) for Tcf12−/−, Tcf12f/f and Tcfe2af/f mouse lines; A. Bendelac (Howard Hughes Medical Institute) and M. Kronenberg (La Jolla Institute for Allergy and Immunology) for Vα14-Jα18 TCR–transgenic mice; A. Abbas (University of California San Francisco) for the constructs MSCV2.2–Bcl-xL–IRES–Thy-1.1 and MSCV2.2–empty–IRES–Thy-1.1; M. Kronenberg for advice; I. Ch’en for advice and assistance; and J. Hamerman, G. Barton, E. Zuniga and S. Hedrick and members of the Goldrath laboratory for critical review of the manuscript. Supported by the Cancer Research Institute, the Pew Charitable Trusts, the National Institutes of Health (AI067545 and AI072117 to A.W.G.), the Leukemia & Lymphoma Society (L.M.D.) and University of California San Diego undergraduate research scholarship program (J.K.F.).
Footnotes
Accession codes. UCSD-Nature Signaling Gateway (http://www.signaling-gateway.org): E2A; GEO: microarray data, GSE19923.
Note: Supplementary information is available on the Nature Immunology website.
AUTHOR CONTRIBUTIONS
L.M.D. designed the study, did experiments, analyzed data and wrote the manuscript; J.K. and J.K.F. did experiments and analyzed data; and A.W.G. designed the study, analyzed data and wrote the manuscript.
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Engel I, Johns C, Bain G, Rivera RR, Murre C. Early thymocyte development is regulated by modulation of E2A protein activity. J Exp Med. 2001;194:733–745. doi: 10.1084/jem.194.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murre C. Helix-loop-helix proteins and lymphocyte development. Nat Immunol. 2005;6:1079–1086. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- 3.Cisse B, et al. Transcription factor E2–2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikawa T, Kawamoto H, Goldrath AW, Murre C. E proteins and Notch signaling cooperate to promote T cell lineage specification and commitment. J Exp Med. 2006;203:1329–1342. doi: 10.1084/jem.20060268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolf J, et al. Molecular profiling reveals distinct functional attributes of CD1d-restricted natural killer (NK) T cell subsets. Mol Immunol. 2008;45:2607–2620. doi: 10.1016/j.molimm.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Engel I, Murre C. E2A proteins enforce a proliferation checkpoint in developing thymocytes. EMBO J. 2004;23:202–211. doi: 10.1038/sj.emboj.7600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones ME, Zhuang Y. Acquisition of a functional T cell receptor during T lymphocyte development is enforced by HEB and E2A transcription factors. Immunity. 2007;27:860–870. doi: 10.1016/j.immuni.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barndt R, Dai MF, Zhuang Y. A novel role for HEB downstream or parallel to the pre-TCR signaling pathway during αβ thymopoiesis. J Immunol. 1999;163:3331–3343. [PubMed] [Google Scholar]
- 9.Barndt RJ, Dai M, Zhuang Y. Functions of E2A-HEB heterodimers in T-cell development revealed by a dominant negative mutation of HEB. Mol Cell Biol. 2000;20:6677–6685. doi: 10.1128/mcb.20.18.6677-6685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bain G, et al. E2A deficiency leads to abnormalities in αβ T-cell development and to rapid development of T-cell lymphomas. Mol Cell Biol. 1997;17:4782–4791. doi: 10.1128/mcb.17.8.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wojciechowski J, Lai A, Kondo M, Zhuang Y. E2A and HEB are required to block thymocyte proliferation prior to pre-TCR expression. J Immunol. 2007;178:5717–5726. doi: 10.4049/jimmunol.178.9.5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuang Y, Barndt RJ, Pan L, Kelley R, Dai M. Functional replacement of the mouse E2A gene with a human HEB cDNA. Mol Cell Biol. 1998;18:3340–3349. doi: 10.1128/mcb.18.6.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravanpay AC, Olson JM. E protein dosage influences brain development more than family member identity. J Neurosci Res. 2008;86:1472–1481. doi: 10.1002/jnr.21615. [DOI] [PubMed] [Google Scholar]
- 14.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 15.Geissmann F, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 18.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 19.Kronenberg M, Engel I. On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Curr Opin Immunol. 2007;19:186–193. doi: 10.1016/j.coi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Dose M, et al. Intrathymic proliferation wave essential for Vα14+ natural killer T cell development depends on c-Myc. Proc Natl Acad Sci USA. 2009;106:8641–8646. doi: 10.1073/pnas.0812255106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mycko MP, et al. Selective requirement for c-Myc at an early stage of Vα14i NKT cell development. J Immunol. 2009;182:4641–4648. doi: 10.4049/jimmunol.0803394. [DOI] [PubMed] [Google Scholar]
- 22.Bezbradica JS, Hill T, Stanic AK, Van Kaer L, Joyce S. Commitment toward the natural T (iNKT) cell lineage occurs at the CD4+8+ stage of thymic ontogeny. Proc Natl Acad Sci USA. 2005;102:5114–5119. doi: 10.1073/pnas.0408449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egawa T, et al. Genetic evidence supporting selection of the Vα14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Hager E, Hawwari A, Matsuda JL, Krangel MS, Gapin L. Multiple constraints at the level of TCRα rearrangement impact Vα14i NKT cell development. J Immunol. 2007;179:2228–2234. doi: 10.4049/jimmunol.179.4.2228. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda JL, et al. T-bet concomitantly controls migration, survival, and effector functions during the development of Vα14i NKT cells. Blood. 2006;107:2797–2805. doi: 10.1182/blood-2005-08-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Townsend MJ, et al. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 27.Ohteki T, Ho S, Suzuki H, Mak TW, Ohashi PS. Role for IL-15/IL-15 receptor β-chain in natural killer 1.1+ T cell receptor-αβ+ cell development. J Immunol. 1997;159:5931–5935. [PubMed] [Google Scholar]
- 28.Lazarevic V, et al. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat Immunol. 2009;10:306–313. doi: 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovalovsky D, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattner J, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304–315. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan L, Hanrahan J, Li J, Hale LP, Zhuang Y. An analysis of T cell intrinsic roles of E2A by conditional gene disruption in the thymus. J Immunol. 2002;168:3923–3932. doi: 10.4049/jimmunol.168.8.3923. [DOI] [PubMed] [Google Scholar]
- 32.Wolfer A, et al. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nat Immunol. 2001;2:235–241. doi: 10.1038/85294. [DOI] [PubMed] [Google Scholar]
- 33.Xi H, Schwartz R, Engel I, Murre C, Kersh GJ. Interplay between RORγt, Egr3, and E proteins controls proliferation in response to pre-TCR signals. Immunity. 2006;24:813–826. doi: 10.1016/j.immuni.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Guo J, et al. Regulation of the TCRα repertoire by the survival window of CD4+CD8+ thymocytes. Nat Immunol. 2002;3:469–476. doi: 10.1038/ni791. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Dordai DI, Lee J, Desiderio S. A conserved degradation signal regulates RAG-2 accumulation during cell division and links V(D)J recombination to the cell cycle. Immunity. 1996;5:575–589. doi: 10.1016/s1074-7613(00)80272-1. [DOI] [PubMed] [Google Scholar]
- 36.Lin WC, Desiderio S. Cell cycle regulation of V(D)J recombination-activating protein RAG-2. Proc Natl Acad Sci USA. 1994;91:2733–2737. doi: 10.1073/pnas.91.7.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yannoutsos N, et al. The role of recombination activating gene (RAG) reinduction in thymocyte development in vivo. J Exp Med. 2001;194:471–480. doi: 10.1084/jem.194.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nichols KE, et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savage AK, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J Exp Med. 2005;202:485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Z, et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.