Abstract
OBJECTIVE
To investigate whether oestrogen, selective oestrogen receptor modulators (SERMs), and growth hormone (GH) can prevent the development of voiding dysfunction in a postpartum postmenopausal rat model of voiding dysfunction.
MATERIALS AND METHODS
Immediately after spontaneous delivery, nine primiparous Sprague-Dawley rats served as uninjured controls (sham group) and 54 underwent intravaginal balloon dilation. On day 7, the 54 subject rats underwent bilateral ovariectomy. A week later, six treatment groups of nine rats were randomized to receive: normal saline (injured control group), 17β-oestradiol (E2), raloxifene, levormeloxifene, GH, or GH + E2. The treatment groups received daily subcutaneous injections for 3 weeks. The effects of hormone treatment were examined by conscious cystometry at the end of the study. Voiding dysfunction was defined to include overactive bladder and sphincter deficiency.
RESULTS
The sham rats had a mean (SD) voiding frequency of 3 (0.87) times in 10 min and a bladder capacity of 0.43 (0.13) mL with smooth cystometry curves. The number of rats in each treatment group (each group contained nine rats) that had voiding dysfunction was as follows: E2, three; raloxifene, six; levormeloxifene, four; and controls, four (P > 0.05 among the groups). Only one rat in the GH-treated group and no rats in the GH + E2-treated group had voiding dysfunction, which was significantly less in the GH + E2-treated group than in the controls (P = 0.041).
CONCLUSION
This functional data suggest that the development of voiding dysfunction can be prevented by short-term administration of GH and GH + E2 in our rat model. SERMs and E2 alone seem to have no therapeutic effect.
Keywords: oestrogen, SERMs, growth hormone, voiding dysfunction, incontinence, rat model, vaginal dilation
INTRODUCTION
Voiding dysfunction such as urinary incontinence (UI) and overactive bladder (OAB) is a pervasive problem with a global prevalence of >200 million people [1]. The impact of voiding dysfunction is profound, costing society billions of dollars and causing a significant decrease in patient quality of life [2].
Multiparity, prolonged second stage of labour and menopause are factors that contribute to the development of female UI [3]. Hormone replacement has been a mainstay of medical therapy for women with postmenopausal urinary dysfunction for several decades [4]. However, recently several studies have concluded that hormone replacement therapy actually increases the rate of voiding dysfunction, particularly UI. For instance, the Heart and Estrogen/Progestin Replacement Study (HERS) showed that oestrogen/progesterone therapy increased the rate of UI (39% vs 27% with placebo therapy) [5]. Similarly, in the Women’s Health Initiative (WHI), a multicentre double-blind, placebo-controlled, randomized clinical trial of menopausal hormone therapy in 27 347 postmenopausal women aged 50–79 years, oestrogen alone and oestrogen with medroxyprogesterone increased the risk of UI among continent women and worsened UI among already symptomatic women after 1 year. This led the authors to propose that oestrogen with or without progestin should not be prescribed for the prevention or relief of UI [6]. The pathophysiology of oestrogen and progestin’s effect on voiding dysfunction has not been fully elucidated.
While advances have been made in surgical and pharmacological treatments of voiding dysfunction [7–9], a poor understanding of the molecular mechanisms that underlie the condition continues to hamper development of new therapies. In the present study, we examine whether a short-term therapy with ultra-low dose oestrogen, selective oestrogen receptor modulators (SERMs), and growth hormone (GH) can prevent the development of voiding dysfunction in a postpartum, postmenopausal voiding dysfunction rat model. We aim to better understand the effect of these hormones on voiding and ultimately improve treatment and prevention of postmenopausal voiding dysfunction.
MATERIALS AND METHODS
In all, 63 pregnant nulliparous Sprague-Dawley rats (3 months old, 250–300 g) were obtained from Charles River Laboratories (Wilmington, MA, USA). After spontaneous delivery, rats were randomly divided into seven equal groups. Rats in group 1 served as postpartum uninjured controls (sham group) and did not undergo further intervention. The remaining 54 rats underwent intravaginal balloon dilation to simulate a prolonged second stage of labour and bilateral ovariectomy 1 week later to induce menopause. Group 2 served as injured controls, while the remaining groups were treated with 17β-oestradiol (E2, group 3), levormeloxifene (group 4), raloxifene (group 5), recombinant GH (group 6), and a recombinant rat GH + E2 combination (group 7). All experiments were approved by the University of California, San Francisco, Institutional Animal Care and Use Committee.
After spontaneous delivery, rats in groups of two to seven were anaesthetized with ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). The rats were placed on a heating pad to maintain isothermia at 37°C. The balloon of a transurethral catheter (18 F; Bard, Covington, GA, USA) was placed intravaginally and was filled with 3 mL water. A 130-g weight was placed on the freely suspended end of the catheter providing a constant pull to direct the force to the pelvic floor and this catheter was left in situ for 3 h. A week later, the rats were bilaterally ovariectomized under 2% isoflurane anaesthesia.
Treatment was started 1 week after bilateral ovariectomy. The experimental agents were dissolved in 0.1 mL normal saline and were injected s.c. once daily for 3 weeks. Injections contained normal saline (controls), E2 (0.01 mg/kg; Sigma-Aldrich, St. Louis, MO, USA), levormeloxifene (0.4 mg/kg; Novo Nordisk, Bagsværd, Denmark), raloxifene (1 mg/kg, Sigma-Aldrich), rat recombinant GH (1 mg; (NIDDK-Rat-GH-B-12/courtesy of NIDDK-NIH), or a combination of rat GH (0.5 mg) and E2 (0.005 mg/kg).
After 3 weeks of treatment, bladder function was assessed. Under isoflurane anaesthesia, a transvesical catheter was inserted into the rat bladder 24 h before conscious cystometry. The tip of a polyethylene-90 (PE-90) tubing (Clay-Adams, Parsippany, NJ, USA) was heated to create a collar. After a lower abdominal midline incision was made, the catheter tip was implanted at the bladder dome and a preset purse-string suture was tied to secure its position. Filling the bladder ensured that no leakage occurred at the implantation site. The tube was passed through the abdominal wall muscle and tunnelled s.c. to emerge at the dorsum of the neck.
For conscious cystometry, the rat was restrained in a custom-made tunnel attached to a metabolic cage grid (Braintree Scientific, Braintree, MA, USA). The bladder was filled through the implanted PE-90 tube with warm normal saline at 0.1 mL/min using an infusion pump (KD Scientific, Holliston, MA, USA). The PE-90 tube was connected to a Y-tube pressure transducer (Utah Medical Products, Midvale, UT, USA). Intravesical pressure changes were recorded by a computer with LabView 6.0 software (National Instruments, Austin, TX, USA) at 10 samples/s. The voided urine was also recorded by an electronic scale connected to the LabView software. After stabilization of the micturition cycle for 10 min, the bladder was emptied by aspiration and micturition cycles were recorded in each rat for 20 min without emptying the bladder in-between.
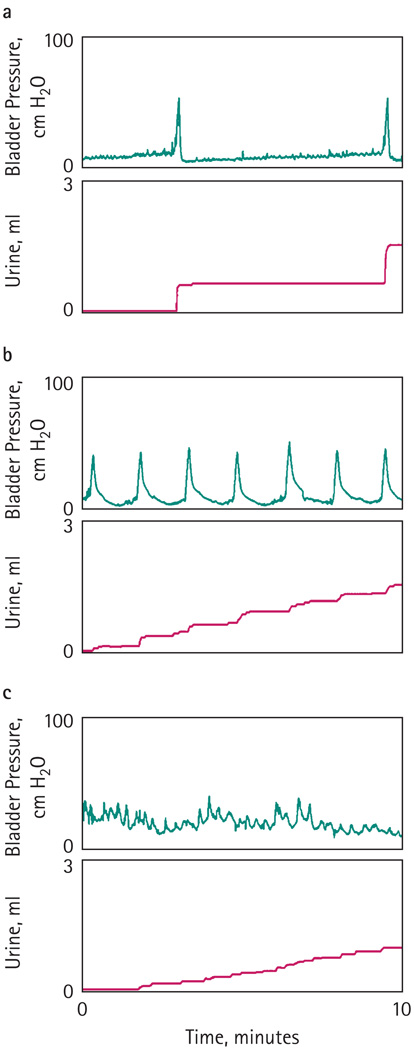
During cystometry, the frequency of bladder contractions per 10 min and the bladder capacity (mL) were measured. The modified leak-point pressure (mLPP, cmH2O) was calculated as the intravesical pressure when the first drop of urine was detected in the meatus. Normal micturition patterns were defined as pressure increases resulting in voiding with a frequency of ≤4 times in 10 min (Fig. 1a). Voiding dysfunction included OAB and sphincter deficiency. OAB was determined as contraction frequencies of >4 times in 10 min (Fig. 1b). Sphincter deficiency was assumed when urine leaked continuously or intermittently at low intravesical pressure (with or with no bladder contractions) during the filling phase (Fig. 1c). To exclude overflow incontinence, these rats also underwent ultrasound of the bladder with a 7.5 mHz small-port probe and had postvoid residual urine volumes (PVR) of <0.03 mL, similar to the sham rats.
FIG. 1.
Normal micturition patterns were defined as pressure increases resulting in voiding with a frequency of ≤4 times in 10 min (a). OAB was determined as contraction frequencies of >4 times in 10 min (b). Sphincter deficiency was assumed when urine leaked continuously or intermittently at low intravesical pressure (with or with no bladder contractions) during the filling phase (c).
The cystometric variables were analysed by comparing the groups using one-way ANOVA and Bonferroni t-tests. The Fisher’s exact test was used to analyse the micturition pattern ratio between each group. Results are given as the mean (SD) unless otherwise stated.
RESULTS
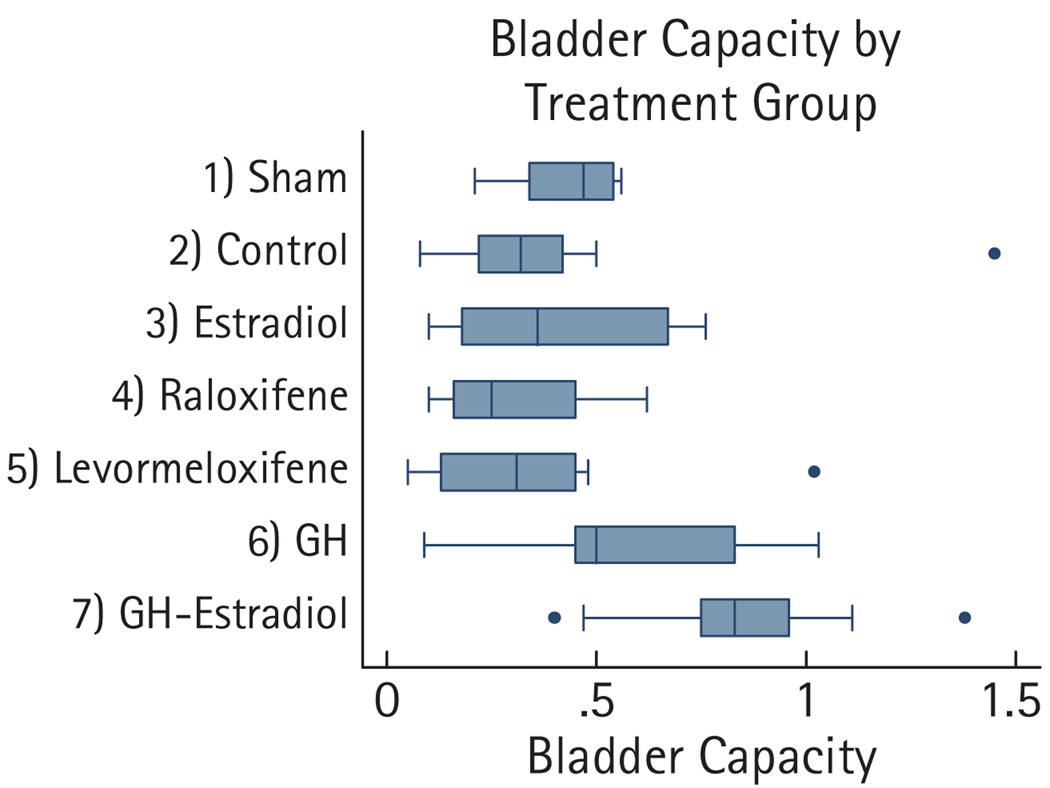
Postpartum sham rats had a mean (SD) voiding frequency of 3 (0.87) times in 10 min and a bladder capacity of 0.43 (0.13) mL with smooth cystometric curves. The number of rats in each treatment group (each group contained nine rats) that had voiding dysfunction was as follows: controls, four; E2, three; raloxifene, six; levormeloxifene, four; GH, one; and GH + E2, zero (Table 1). When compared with the control group, the GH + E2-treated group had significantly less voiding dysfunction (P = 0.041). When not combined with E2, the GH-treated group neared statistical significance compared with the controls for voiding dysfunction (P = 0.147). There was no significant difference in the mLPP among the experimental groups. The bladder capacity increased significantly in the GH + E2-treated group (Fig. 2, Table 2).
TABLE 1.
Comparison of two voiding patterns between treatment groups (nine rats in each group)
| Groups | Normal | OAB | Sphincter deficiency |
Voiding dysfunction (OAB + ISD) |
P |
|---|---|---|---|---|---|
| Group 1 (sham) | 9 | 0 | 0 | 0 | 0.041 |
| Group 2 (control) | 5 | 1 | 3 | 4 | – |
| Group 3 (E2) | 6 | 3 | 0 | 3 | 0.50 |
| Group 4 (raloxifene) | 3 | 4 | 2 | 6 | 0.319 |
| Group 5 (levormeloxifene) | 5 | 3 | 1 | 4 | 0.681 |
| Group 6 (GH) | 8 | 0 | 1 | 1 | 0.147 |
| Group 7 (GH + E2) | 9 | 0 | 0 | 0 | 0.041 |
| Total, n (%) | 45 (71.4) | 11 (17) | 7 (11) | 18 (28.6) |
Fisher’s exact used with all comparisons made with the control group.
FIG. 2.
Box and whisker plots of bladder capacities.
TABLE 2.
Conscious cystometric variables after treatment
| Mean (SD) |
||
|---|---|---|
| Groups | Bladder capacity, mL | mLPP, cmH2O |
| Group 1 (sham) | 0.43 (0.13) | 14.33 (7.73) |
| Group 2 (control) | 0.42 (0.41) | 18.78 (14.20) |
| Group 3 (E2) | 0.41 (0.26) | 14.67 (7.09) |
| Group 4 (raloxifene) | 0.30 (0.18) | 7.44 (6.46) |
| Group 5 (levormeloxifene) | 0.34 (0.30) | 12.56 (8.88) |
| Group 6 (GH) | 0.56 (0.32) | 16.22 (10.27) |
| Group 7 (GH + E2) | 0.84 (0.30)* | 20.00 (9.80) |
Using the two-sample Wilcoxon rank-sum Mann–Whitney test, bladder capacity was significantly larger in the GH + E2-treated group than in the control group, P = 0.012. The remaining bladder capacities and mLPP were not significantly different compared with the controls.
DISCUSSION
In the present pilot study, the effects of short-term use of hormones and SERMs on voiding in a rat model of prolonged second-stage labour and postmenopausal voiding dysfunction were assessed. Traumatic vaginal delivery is strongly correlated with later development of stress UI (SUI). Snooks et al. [10] showed evidence of partial denervation of the pelvic floor musculature with pudendal neuropathy, which was more marked in women with SUI. They also indicated that pudendal neuropathy from vaginal delivery persists and may increase with time. Pregnancy-related UI and persistent postpartum SUI has a prevalence of 28–50% and 5–20%, respectively [11]. Vaginal balloon inflation and pudendal nerve injury in rats have been used to simulate vaginal delivery and associate nerve injury [12–16]. These studies seem to conclude that injury to periurethral nerves and urethral smooth and striated muscle is the principal mechanism of voiding dysfunction.
With the establishment of conscious cystometry in our laboratory, we noted that some rats that underwent prolonged second-stage labour and postmenopausal voiding dysfunction also developed OAB with or with no urine leakage. The normal value of our conscious cystometry was established by analysing the voiding pattern of 10 3-month-old-nonpregnant female rats (not part of the present study) followed by small-port high-resolution ultrasound to detect PVR. In the postpartum sham rats, the cystometry was characterized by continuous filling of the bladder which resulted in regular micturition cycles, with each void being associated with a micturition event and urine flow [9,17]. The mean (SD) voiding frequency in this group was 3 (0.87) times in 10 min similar to the established normal value in nonpregnant female rats mentioned above.
Oestrogen therapy has been regarded as a mainstay for postmenopausal UI for over 50 years. Nevertheless, during the past decade there have been conflicting conclusions of oestrogen’s effect on voiding dysfunction. A meta-analysis of published studies suggests that oestrogen may improve subjective symptoms but not the objective amount of urine lost [18]. Conversely, HERS reported that long-term (4 years) oestrogen/progesterone therapy in 1228 women with coronary heart disease increased the rate of UI (64% vs 49% in placebo-treated group) [5,19]. The WHI study, a double-blind, placebo-controlled trial, also showed that all types of UI were increased by oestrogen [6]. Ultralow-dose oestrogens, alternatively, may not increase the frequency of UI symptoms. As part of the Ultra Low Dose Transdermal estRogen Assessment trial, Waetjen et al. [20] produced a multicentre, randomized, double-blinded, placebo-controlled trial of unopposed ultra-low dose transdermal oestradiol for prevention of osteoporosis in 417 postmenopausal women. There was not a significant change in UI frequency between groups at 2 years. Similarly, the oestrogen group in the present study received an ultralow dose (0.01 mg/kg) and did not develop significantly more voiding dysfunction.
SERMs such as tamoxifen, levormeloxifene, raloxifene, were originally developed for the treatment of breast cancer [21,22]. These agents modulate ERs in various tissues and have been used for treating osteoporosis. The definitive impact of SERMs on the urinary tract and on voiding dysfunction and UI is not well known. Goldstein et al. [23] reported that raloxifene did not increase the subjective complaint of UI at 3 years follow-up compared with placebo. Another study to show no increase in UI is the Multiple Outcomes of Raloxifene Evaluation trial, in which 963 women were randomly assigned to receive raloxifene or a placebo [24]. At 3 years, the subjects completed questionnaires about UI and there was no significant increase in UI. By contrast, Vardy et al. [25] found raloxifene associated with prolapse, although UI was no different from placebo. Another SERM, levormeloxifene, was also developed for the prevention and treatment of osteoporosis. However, its phase III clinical trial was aborted due to the high incidence of adverse events, including an increased rate of UI [26]. The short-term use of SERMs in the present study did not result in increased voiding dysfunction.
GH and its mediator IGF-1 have been used to treat GH deficiency syndrome. In animal studies, administration of GH has been shown to increase the recovery of nitric oxide synthase-containing nerves after cavernosal nerve injury [27], pudendal nerve regeneration after crush injury [28], and skeletal muscle regeneration in rat [29]. In the present report, the development of voiding dysfunction in our rat model was largely prevented in the groups that received recombinant rat GH and combined GH + E2. This provocative finding requires further investigation. Nevertheless, GH therapy might contribute to better recovery of neural and muscular function in the bladder and urethra and thus prevent voiding dysfunction in these rats.
The present study limitations include its small size and short duration of therapy.
In conclusion, the present pilot study with conscious cystometry, short-term therapy with E2, SERMs and GH suggest that, in the dosage and duration used in this study, GH and GH + E2 seem to prevent the development of voiding dysfunction while E2 alone and SERMs do not have significant effects. Further studies with various doses and duration are needed to identify the best regimen for prevention and treatment of voiding dysfunction in this rat model.
ACKNOWLEDGEMENTS
We wish to thank the National Hormone and Peptide Program, directed by Dr A.F. Parlow at Harbor UCLA Medical Center for providing the rat GH.
Abbreviations
- SERM
selective oestrogen receptor modulator
- E2
17β-oestradiol
- GH
growth hormone
- UI
urinary incontinence
- OAB
overactive bladder
- mLPP
modified leak-point pressure
- PVR
postvoid residual urine volume
- HERS
Heart and Estrogen/Progestin Replacement Study
- WHI
Women’s Health Initiative.
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Norton P, Brubaker L. Urinary incontinence in women. Lancet. 2006;367:57–67. doi: 10.1016/S0140-6736(06)67925-7. [DOI] [PubMed] [Google Scholar]
- 2.Huang AJ, Brown JS, Kanaya AM, et al. Quality-of-life impact and treatment of urinary incontinence in ethnically diverse older women. Arch Intern Med. 2006;166:2000–2006. doi: 10.1001/archinte.166.18.2000. [DOI] [PubMed] [Google Scholar]
- 3.Cheater FM, Castleden CM. Epidemiology and classification of urinary incontinence. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:183–205. doi: 10.1053/beog.1999.0071. [DOI] [PubMed] [Google Scholar]
- 4.Andersson KE. Drug therapy for urinary incontinence. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:291–313. doi: 10.1053/beog.1999.0075. [DOI] [PubMed] [Google Scholar]
- 5.Brown JS, Grady D, Ouslander JG, Herzog AR, Varner RE, Posner SF. Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Heart & Estrogen/Progestin Replacement Study (HERS) Research Group. Obstet Gynecol. 1999;94:66–70. doi: 10.1016/s0029-7844(99)00263-x. [DOI] [PubMed] [Google Scholar]
- 6.Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA. 2005;293:935–948. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- 7.Smith PP, McCrery RJ, Appell RA. Current trends in the evaluation and management of female urinary incontinence. CMAJ. 2006;175:1233–1240. doi: 10.1503/cmaj.060034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juma S, Brito CG. Transobturator tape (TOT): two years follow-up. Neurourol Urodyn. 2007;26:37–41. doi: 10.1002/nau.20353. [DOI] [PubMed] [Google Scholar]
- 9.Chapple CR, Gormley EA. Developments in pharmacological therapy for the overactive bladder. BJU Int. 2006;98 Suppl. 1:78–89. doi: 10.1111/j.1464-410X.2006.06381.x. [DOI] [PubMed] [Google Scholar]
- 10.Snooks SJ, Swash M, Mathers SE, Henry MM. Effect of vaginal delivery on the pelvic floor: a 5-year follow-up. Br J Surg. 1990;77:1358–1360. doi: 10.1002/bjs.1800771213. [DOI] [PubMed] [Google Scholar]
- 11.Groutz A, Gordon D, Keidar R, et al. Stress urinary incontinence: prevalence among nulliparous compared with primiparous and grand multiparous premenopausal women. Neurourol Urodyn. 1999;18:419–425. doi: 10.1002/(sici)1520-6777(1999)18:5<419::aid-nau2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Sievert KD, Bakircioglu ME, Tsai T, Dahms SE, Nunes L, Lue TF. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I. functional and structural change. J Urol. 2001;166:311–317. [PubMed] [Google Scholar]
- 13.Resplande J, Gholami SS, Graziottin TM, et al. Long-term effect of ovariectomy and simulated birth trauma on the lower urinary tract of female rats. J Urol. 2002;168:323–330. [PubMed] [Google Scholar]
- 14.Lin AS, Carrier S, Morgan DM, Lue TF. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52:143–151. doi: 10.1016/s0090-4295(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 15.Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol. 2003;170:1027–1031. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- 16.Pan HQ, Kerns JM, Lin DL, Liu S, Esparza N, Damaser MS. Increased duration of simulated childbirth injuries results in increased time to recovery. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1738–R1744. doi: 10.1152/ajpregu.00784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Streng T, Hedlund P, Talo A, Andersson KE, Gillespie JI. Phasic non-micturition contractions in the bladder of the anaesthetized and awake rat. BJU Int. 2006;97:1094–1101. doi: 10.1111/j.1464-410X.2006.06137.x. [DOI] [PubMed] [Google Scholar]
- 18.Andersson KE, Appell R, Cardozo LD, et al. The pharmacological treatment of urinary incontinence. BJU Int. 1999;84:923–947. doi: 10.1046/j.1464-410x.1999.00397.x. [DOI] [PubMed] [Google Scholar]
- 19.Steinauer JE, Waetjen LE, Vittinghoff E, et al. Postmenopausal hormone therapy: does it cause incontinence? Obstet Gynecol. 2005;106:940–945. doi: 10.1097/01.AOG.0000180394.08406.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waetjen LE, Brown JS, Vittinghoff E, et al. The effect of ultralow-dose transdermal estradiol on urinary incontinence in postmenopausal women. Obstet Gynecol. 2005;106:946–952. doi: 10.1097/01.AOG.0000182576.48290.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albertazzi P, Sharma S. Urogenital effects of selective estrogen receptor modulators: a systematic review. Climacteric. 2005;8:214–220. doi: 10.1080/13697130500117946. [DOI] [PubMed] [Google Scholar]
- 22.Cox DA, Helvering LM. Extracellular matrix integrity: a possible mechanism for differential clinical effects among selective estrogen receptor modulators and estrogens? Mol Cell Endocrinol. 2006;247:53–59. doi: 10.1016/j.mce.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein SR, Johnson S, Watts NB, Ciaccia AV, Elmerick D, Muram D. Incidence of urinary incontinence in postmenopausal women treated with raloxifene or estrogen. Menopause. 2005;12:160–164. doi: 10.1097/00042192-200512020-00010. [DOI] [PubMed] [Google Scholar]
- 24.Waetjen LE, Brown JS, Modelska K, et al. Effect of raloxifene on urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2004;103:261–266. doi: 10.1097/01.AOG.0000109429.67671.d1. [DOI] [PubMed] [Google Scholar]
- 25.Vardy MD, Lindsay R, Scotti RJ, et al. Short-term urogenital effects of raloxifene, tamoxifen, and estrogen. Am J Obstet Gynecol. 2003;189:81–88. doi: 10.1067/mob.2003.374. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187:521–527. doi: 10.1067/mob.2002.123938. [DOI] [PubMed] [Google Scholar]
- 27.Jung GW, Kwak JY, Yoon S, Yoon JH, Lue TF. IGF-I and TGF-beta2 have a key role on regeneration of nitric oxide synthase (NOS)-containing nerves after cavernous neurotomy in rats. Int J Impot Res. 1999;11:247–259. doi: 10.1038/sj.ijir.3900402. [DOI] [PubMed] [Google Scholar]
- 28.Kerns JM, Shott S, Brubaker L, et al. Effects of IGF-I gene therapy on the injured rat pudendal nerve. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:2–8. doi: 10.1007/s00192-002-0995-2. [DOI] [PubMed] [Google Scholar]
- 29.Ullman M, Oldfors A. Skeletal muscle regeneration in young rats is dependent on growth hormone. J Neurol Sci. 1991;106:67–74. doi: 10.1016/0022-510x(91)90196-e. [DOI] [PubMed] [Google Scholar]