Abstract
The retinal pigment epithelium (RPE) is indispensable for photoreceptor function, not only because it provides functional photopigments to photoreceptors, but also because it eliminates oxidatively damaged materials from photoreceptors. Maintaining homeostatic antioxidative programs that support a healthy RPE is therefore important for the normal functioning of the eye. These homeostatic mechanisms, however, often fail in aged RPE cells that have been exposed repeatedly to excessive oxidative stress. When RPE cells succumb to oxidative stress, their death contributes to the development of retinal degenerative diseases such as age-related macular degeneration. Recent studies have highlighted the importance of reciprocal phosphoinositide signaling events orchestrated by phosphoinositide 3-kinase (PI3K) and phosphatase and tensin homolog (PTEN) in the homeostatic programs that protect RPE cells against oxidative stress. Here, we discuss the role of PI3K signaling pathways in RPE cells and suggest that they might be crucial targets of oxidative molecules that initiate early pathological events in retinal degenerative diseases.
Introduction
Light and oxygen are both essential ingredients for life but, ironically, these factors are also constant threats to the health of an organism. Retinal photoreceptors are especially susceptible to light-induced oxidative damage because reactive oxygen species (ROS) are constantly generated in the course of photochemical reactions that convert light energy to chemoelectrical signals [1–3]. Spontaneously generated ROS influence cellular reduction–oxidation (redox) status by interfering with the homeostatic regulation of the permeability both of plasma membranes and of intracellular organellar membranes, or by directly oxidizing intracellular molecules, including nucleic acids, proteins and lipids [2–6]. These oxidized molecules tend to aggregate and form deposits that can damage photoreceptors.
However, a series of cellular homeostatic mechanisms protect photoreceptors from light-induced oxidative damage. These include the intracellular antioxidation machinery, which enables photoreceptors to recover from oxidative insults, and the external support provided by the neighboring retinal pigment epithelium (RPE) [3,7]. The latter process, however, cannot fully eliminate oxidative stresses from the retina, but instead can enable transfer of toxic deposits from photoreceptors to RPE cells. Thus, RPE cells have not only evolved antioxidative processes that efficiently eliminate oxidatively damaged materials but also have developed self-protective programs that rescue the RPE cells themselves from oxidative stress [2–5,8].
Here, we discuss the ways in which RPE cells use their cellular protective programs to manage light-induced oxidative stress. We focus on recent studies describing the roles of phosphoinositide 3-kinase (PI3K) and its opposing partner phosphatase and tensin homolog (PTEN) in RPE cellular homeostatic mechanisms that protect against such stresses. Furthermore, we discuss the physiological importance of the balance between PI3K and PTEN activity in the RPE in maintaining intercellular junctional structures, which are usually disrupted in retinal degenerative diseases such as age-related macular degeneration (AMD).
Light-induced oxidative damage in photoreceptors
Eyes respond to light by using various light-excitable retinal photopigments, which are bound to photosensory proteins [9,10]. Rhodopsin, for example, which is the best known photosensory protein prevalent in vertebrate rod photoreceptors, carries 11-cis-retinaldehyde as a photopigment [9,10]. In response to light, retinal 11-cis-retinaldehyde is isomerized to all-trans-retinaldehyde, which is released from rhodopsin and subsequently reduced to all-trans retinol. Meanwhile, rhodopsin, which is activated by release of all-trans-retinaldehyde, triggers the phototransduction process. The all-trans-retinol is then secreted from photoreceptors and transferred to RPE cells, in which it is isomerized to 11-cis-retinol and oxidized to regenerate 11-cis-retinaldehyde; this is then delivered back to photoreceptors for another visual cycle [9,10]. This series of reduction, isomerization and oxidation processes spontaneously generates ROS as a byproduct, which can oxidize many cellular molecules in both photoreceptors and RPE cells [1–5].
Perhaps not surprisingly, photoreceptors have developed self-protective programs to defend themselves against the oxidative threats posed by ROS. First, photoreceptors activate antioxidation machinery that promptly eliminates ROS [11,12]. The elements of this machinery include: small antioxidant molecules such as thioredoxin [13]; nutrients, such as vitamin C, vitamin E, retinoids and carotenoids [2,3,14]; enzymes, such as glutathione peroxidase [15], NADPH-dependent retinol reductase [16], superoxide dismutase (SOD) [17], and catalase [3,13]; and photopigment carriers, such as cellular retinaldehyde-binding protein (CRALBP) [18]. These molecules capture ROS and convert them into less reactive forms before they have an opportunity to act on cellular targets [4,5,19,20]. Second, photoreceptors can induce the expression of molecular chaperones, such as heat shock proteins (HSPs) and crystallins, for example upon experimental autoimmune uveitis (EAU)-induced retinal inflammation or exposure to intense light that has been shown to generate ROS [21,22]. These chaperone proteins recognize proteins that have been denatured by ROS-mediated oxidization and prevent them from developing into toxic protein aggregates. Third, photoreceptors also maintain the activity of cell survival programs during cellular anti-oxidation responses to ROS. For example, B-cell lymphoma 2 (Bcl2)-related anti-apoptotic proteins, which maintain mitochondrial membrane potential, and the PI3K–Akt (also called protein kinase B) intracellular signaling pathway, which activates expression of anti-apoptotic genes, are known to contribute to the protection of photoreceptors from oxidative-stress-induced cell death [23,24].
A unique feature of photoreceptors that enables them to escape from oxidative insults is their ability to transfer damaged photoreceptor outer segments and oxidized molecules to RPE cells, which are known for their large anti-oxidative capacity [2,3,8,25]. Thus, the fate of photoreceptors lies not only in the successful operation of their own survival programs, but also depends on the supporting roles of RPE cells. RPE, therefore, can be included as an essential part of the retina owing to its indispensable roles in photoreceptor function and survival.
The RPE is indispensable for photoreceptor cell survival
RPE cells form a single epithelial layer that spans the outer surface of the photoreceptor layer [26,27]. One RPE cell normally contacts >100 photoreceptors through its apical microvilli, which are responsible for material exchange between photoreceptors and RPE cells [26,28,29]. This cell-to-cell transfer is accomplished by different mechanisms, depending on the size of the particles.
Small molecules, such as ions, chemicals and proteins, are secreted from photoreceptors or RPE cells into the subretinal space and then diffuse through channels or are captured by specific receptors in RPE microvilli or photoreceptor outer segments. The best-known example of secretion-dependent intercellular transfer is retinol cycling, as described earlier [10,30].
Larger photoreceptor structures, even whole photoreceptors themselves, are transferred to the RPE through microvilli-mediated phagocytosis [2,26,28,31]. The light-damaged distal ends of photoreceptor outer segments are engulfed by RPE cells and are replaced with freshly grown outer segment membranes [28]. RPE cells can also ingest dead photoreceptors on a daily basis to maintain a constant, functionally active photoreceptor pool [2,28,31,32]. After uptake, damaged photoreceptor components are guided by intracellular sorting pathways in RPE cells to lysosomes for degradation or are recycled into other cellular components [2,31].
Damaged photoreceptor outer segments and dead photoreceptors are enriched with oxidized materials, which accumulate as a result of repeated photochemical reactions [33]. RPE cells also contain their own intracellular light-induced oxidative materials, such as malondialdehyde and hydroxynonenal [1,32,34]. Thus, cellular redox homeostatic mechanisms of RPE cells must be maintained to protect against both externally and internally generated oxidative stresses [1,8,28,32]. These homeostatic mechanisms, however, are often not sufficient to rid the cell completely of oxidative molecules, especially in aged animals. As a result, RPE cells are impaired in their support for photoreceptors and even frequently die because of the toxic effects of accumulated oxidative materials [28,35] (Figure 1). The functional impairment or death of RPE cells eventually results in the degeneration of photoreceptors, which depend on the RPE for their survival. One leading hypothesis suggests that AMD is primarily caused by the degeneration of RPE cells owing to the toxicity of accumulated oxidized materials, and is based on this phenomenon [2–5,8,19,20,25,32].
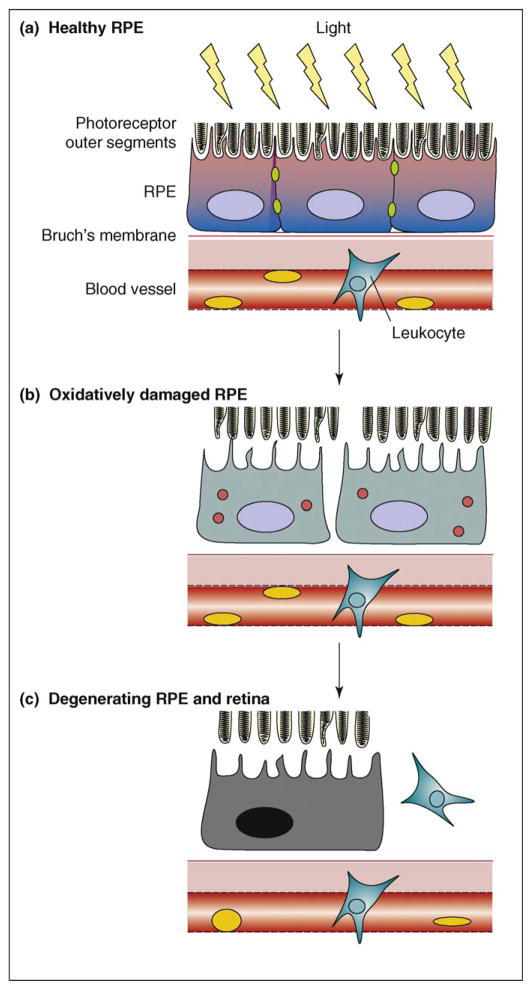
Figure 1.
Progression of oxidative-stress-induced RPE degeneration. (a) Light-induced oxidation of photoreceptor components is processed normally by RPE cells in young animals. The healthy RPE layer maintains intact adhesion with photoreceptor, with visual cycling of photopigments and polarized transport of nutrients and waste. (b) However, cellular metabolic activities wanes as animals grow older, so oxidative metabolites gradually accumulate in RPE cells. There is persistent elevation of ROS, dissolution of junction complexes and loss of cell-to-cell adhesion among the oxidatively damaged RPE cells. Oxidized molecules also start to form toxic aggregates (red circles) that eventually kill RPE cells. (c) Upon the loss of RPE cells, capillary leukocytes invade through the disrupted BRB into the retina and induce inflammatory reactions and neovascularization, resulting in retinal degeneration and consequent diminution of sight, as in AMD.
The blood–retinal barrier is composed of polarized RPE cells
RPE cells can successfully support photoreceptors only when they form a semipermeable epithelial layer [26,27,36]. Within this layer, RPE cells exhibit typical characteristics of epithelial cells, including the polarized distribution of cellular structures and the presence of intercellular junctions between adjacent cells. Junctions located along the lateral side of RPE cells divide the photoreceptor-associating apical microvilli from the basal folds, which adhere to Bruch’s membrane [26,27,36] (Figure 1). Adherens junctions (AJs), which include cad-herins and their cytoplasmic adaptor β-catenin, and the desmosome, which is composed of desmoglein and its adaptor desmoplakin, are localized to the lateral side of RPE cells through associations with the actin microfilament and intermediate filament cytoskeletal network, respectively [36,37]. The components of tight junctions (TJs), such as zona occludens 1 (ZO1), Par-3, and junctional adhesion molecule-A (JAM-A), are concentrated at the marginal zone of apical and lateral domains and seal off the circumference of RPE cells [36,38,39].
Because of these junctional structures, the free movement of plasma membrane components, such as proteins and lipids, across the junctional structures is limited in fully differentiated RPE cells [40,41]. The resultant asymmetric distribution of many membrane components into apical or basolateral domains contributes to the distinct cellular functions of each domain. Proteins involved in the interactions between RPE cells and photoreceptors, such as integrin αvβ5 and neural cell adhesion molecule (N-CAM), or the components of microvilli, such as ezrin and Na+–K+-ATPase, are associated only with the apical surface of the RPE [29,42–44]. By contrast, the proteins that anchor RPE cells to Bruch’s membrane, such as integrin α5β1, localize to the basal folds [42,45].
Another group of basal-targeting proteins are secretory factors that control the choroidal capillary network located outside Bruch’s membrane. These secretory factors include pro-inflammatory cytokines, such as interleukin (IL)-6 and IL-8, and angiogenic vascular endothelial growth factors (VEGFs) [46–48]. If inappropriately secreted apically into the retinal space, these RPE-produced proinflammatory cytokines and angiogenic factors induce inflammatory reactions and aberrant neovascularization, respectively, in the retina [46–48]. Even choroidal blood cells can sometimes also induce these devastating retinal inflammatory reactions when they intrude into the retina through spaces left by degenerating RPE [32,39,49]. Therefore, together with Bruch’s membrane, healthy RPE cells serve as a blood–retinal barrier (BRB) that blocks access of cytokines and blood cells to the retina [36,49] (Figure 1).
RPE degeneration and AMD
The retinal inflammation and angiogenesis described earlier can be observed during the course of AMD, which is the leading retinal degenerative disease in aged human populations [8,50]. More than 85% of AMD is the so-called ‘dry-type’, which is initiated by the death of RPE cells, whereas the remaining ~15% of AMD is categorized as ‘wet-type’, featuring aberrant neovascularization in the central retina and the intact RPE layer [8,50]. However, ‘dry-type’ AMD does not only involve the development of local atrophy, but often progresses to develop inflammatory responses and neovascularization in the retina [8,50,51]. Accordingly, the ‘dry-type’ AMD is sometimes difficult to discriminate from the ‘wet-type’ at later stages of the disease.
Although the ‘dry-type’ is the dominant form of AMD, to date, no effective therapy has been developed to treat it. By contrast, anti-angiogenic therapies are currently available for the treatment of ‘wet-type’ AMD patients [51,52]. Attempts to combat ‘dry-type’ AMD have so far focused on prevention rather than on curing symptoms [8,50,51]. Dietary antioxidant supplements have been broadly used to reduce the risk of AMD development, supporting the possibility that the toxicity of oxidized molecules in RPE cells is the primary triggering factor for ‘dry-type’ AMD [50–52,14,53].
Oxidative modification of lipids and proteins in the RPE
Several biochemical features of oxidatively damaged RPE cells have been characterized [3,8,19,50,51,54]. The best-known example is the accumulation of autofluorescent lipofuscin, which comprises peroxidized polyunsaturated fatty acids (PUFAs) and toxic lipophilic cation N-retinyl-N-retinylidene (A2E) [3,35]. Lipofuscins contribute to the oxidation of molecular substrates in RPE cells through ROS production, and also directly interfere with oxidative respiration in mitochondria [3,5,6,35,55]. The extensive oxidation and subsequent aggregation of PUFAs in the mitochondrial membrane disrupt the balance of the mitochondrial membrane potential, resulting in a respiratory burst that eventually leads to the death of RPE cells [5,6,55] (Figure 2).
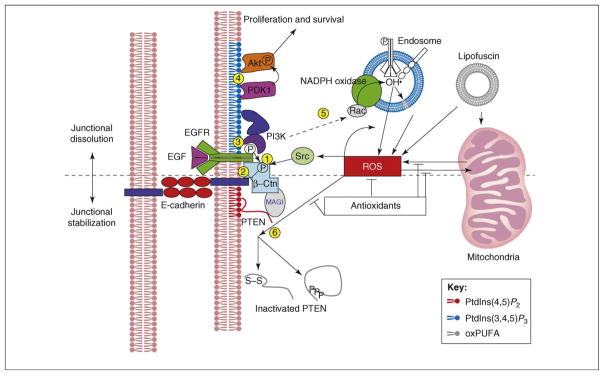
Figure 2.
Model for negative regulation of junctional stability in RPE by the PI3K–ROS circuit. Intact AJs are composed of multiple proteins. E-cadherin–β-catenin complexes localize to the center of AJs and recruit structural proteins and enzymes that provide the functionalities responsible for junctional dynamics. E-cadherin–β-catenin can also interact with the EGFR, which, like Src tyrosine kinase, can phosphorylate β-catenin ( ), causing it to dissociate from E-cadherin (
), causing it to dissociate from E-cadherin ( ). EGFR also exerts a junction-destabilizing signal through PI3K (
). EGFR also exerts a junction-destabilizing signal through PI3K ( ), which not only activates Akt-dependent cell proliferation and survival signaling cascades (
), which not only activates Akt-dependent cell proliferation and survival signaling cascades ( ) but also stimulates ROS production, at least in part through NADPH oxidase (
) but also stimulates ROS production, at least in part through NADPH oxidase ( ). Elevated levels of ROS might inactivate junctional protein tyrosine phosphatases (PTPases) or PTEN directly, by oxidizing them, or indirectly, by inducing their phosphorylation (
). Elevated levels of ROS might inactivate junctional protein tyrosine phosphatases (PTPases) or PTEN directly, by oxidizing them, or indirectly, by inducing their phosphorylation ( ). Through this series of events, EGFR has the potential to dissociate local AJ structures (above dashed line). However, cellular antioxidants might act to antagonize the persistent elevation of ROS and stop the spread of junctional dissolution. PTEN also reduces the levels of PtdIns(3,4,5)P3, thus inhibiting the PI3K-activated signaling cascades (below dashed line). This push-and-pull dynamic between PI3K and PTEN results in a localized equilibrium of junctional remodeling in cells of the RPE. However, this balance could start to unravel as ROS accumulate as a result of mitochondrial metabolic dysfunctions or an overload of oxidized photoreceptor components. Upon ROS-induced inactivation of PTEN and the consequent accumulation of PtdIns(3,4,5)P3, the PI3K signaling pathways could enter a positive-feedback loop, causing continuous generation of ROS. Once the balance is disrupted, the junction-destabilizing signaling pathway might be amplified and all junctions at the lateral zone of cell–cell adhesions might become involved in the dissociation process, leading to disruption of the RPE-supported BRB. Abbreviations: MAGI, membrane-associated guanylate kinase, WW- and PDZ-domain-containing; oxPUFA, oxidized polyunsaturated fatty acid.
). Through this series of events, EGFR has the potential to dissociate local AJ structures (above dashed line). However, cellular antioxidants might act to antagonize the persistent elevation of ROS and stop the spread of junctional dissolution. PTEN also reduces the levels of PtdIns(3,4,5)P3, thus inhibiting the PI3K-activated signaling cascades (below dashed line). This push-and-pull dynamic between PI3K and PTEN results in a localized equilibrium of junctional remodeling in cells of the RPE. However, this balance could start to unravel as ROS accumulate as a result of mitochondrial metabolic dysfunctions or an overload of oxidized photoreceptor components. Upon ROS-induced inactivation of PTEN and the consequent accumulation of PtdIns(3,4,5)P3, the PI3K signaling pathways could enter a positive-feedback loop, causing continuous generation of ROS. Once the balance is disrupted, the junction-destabilizing signaling pathway might be amplified and all junctions at the lateral zone of cell–cell adhesions might become involved in the dissociation process, leading to disruption of the RPE-supported BRB. Abbreviations: MAGI, membrane-associated guanylate kinase, WW- and PDZ-domain-containing; oxPUFA, oxidized polyunsaturated fatty acid.
In addition to modifying lipids, ROS can affect the activity of proteins that have key roles in the cellular homeostasis of the RPE [5,28,35,56]. ROS mainly target thiol groups in cysteine or methionine residues, and thereby change the biochemical activities of proteins [56–58]. Many phosphatases, including protein tyrosine phosphatase 1B (PTP1B), cdc25B, low molecular weight protein tyrosine phosphatase (LMW-PTP) and PTEN, share the common feature of a cysteine residue present in the active site that, when oxidized, causes enzymatic inactivation [58–60]. The oxidation-induced inactivation of phosphatases influences a variety of phosphorylation-dependent signaling events that are important for growth, survival and motility of epithelial cells [56–58,61]. Importantly, these phosphatases often serve as components of cell junctions, where they act to maintain these structures against junction-destabilizing signals such as growth factors and oxidative stresses [62–65]. The loss of junctions is known to be one of the common features of degenerating RPE cells of AMD patients, implying that the oxidation-induced inactivation of junctional phosphatases could be involved in the pathological events of AMD [66]. Supporting this idea, a recent study has shown that the inactivation of PTEN in RPE cells is one of the early pathological events leading to AMD-like retinal degeneration [67].
AMD-like retinal degeneration initiated by the inactivation of PTEN in the RPE
PTEN has been recognized as a tumor suppressor that opposes the proliferative signal transduction cascade induced by PI3K through its phosphoinositide 3-phosphatase activity [68,69]. Recent studies, however, have also emphasized that PTEN has important functions that are independent of its classical phosphoinositide 3-phosphatase activity. These include not only the dephosphorylation of protein (i.e. non-lipid) substrates such as focal adhesion kinase (FAK) but also the support lent to genomic stability by its interaction with the tumor-suppressor cellular tumor antigen p53 and centromere protein C (CENP-C) [69–74]. On account of these diverse anti-tumorigenic activities, genetic mutations of PTEN (e.g. in Cowden’s disease and Bannayan-Zonana syndrome) or epigenetic inactivation by DNA methylation at the PTEN gene promoter (e.g. in glioblastoma, sporadic thyroid carcinoma, and melanoma) causes various cancers in humans and mouse models [75,76]. The tumor-suppressor function of PTEN has been known to be carried out through the inhibition of cell proliferation, and by antagonizing the anti-apoptotic activity of the PI3K–Akt signaling pathway [77,78]. Loss of PTEN therefore has been predicted to facilitate cell survival but not to lead to the death of cells.
Surprisingly, the specific deletion of Pten in mouse RPE cells induced retinal degeneration and involved several AMD-like retinal pathological events, such as age-dependent degeneration of RPE cells and photoreceptors, retinal invasion by macrophages and consequent inflammatory reactions, and retinal neovascularization [67]. These events were not only caused by genetic inactivation of Pten, but were also reproduced by inducing RPE cell degeneration through oxidative damage. Degenerating RPE cells damaged by oxidative agents contained inactivated PTEN, which was phosphorylated at its C-terminal Ser380, Thr382 and Thr383 residues [79]. Furthermore, these events were observed in RPE cells derived from AMD mouse models in which the genes encoding chemokine) or the chemokine C C motif C–C motif receptor 2 (ccr2−/−–ligand 2 (ccl2−/−) were knocked out, strongly suggesting that PTEN inactivation can be a common event induced by AMD-triggering stimuli [67,80].
PI3K targets PTEN through ROS
The inactivation of PTEN protein by oxidative stress would be expected to affect a variety of cellular signaling events that are influenced by its lipid or protein phosphatase activity, including the activation of Akt, which is under the control of the opposing enzymatic activities of PI3K and PTEN [77,78]. The PI3K–Akt signaling pathway is known to have crucial roles in signal transduction cascades triggered by many growth factors [77,78]. The adaptor subunits of heterodimeric PI3K bind to activated growth factor receptors, and the activity of associated catalytic subunits subsequently increases the local concentration of phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3] [77,78]. The accumulation of PtdIns(3,4,5)P3 at the membrane in the vicinity of the growth factor receptors results in activation of downstream phosphoinositide-dependent kinase-1 (PDK1) and Akt, both of which contain the pleckstrin-homology (PH) domain capable of specifically binding to PtdIns(3,4,5)P3 [77,78]. Thus, activated PDK1 catalyzes the activating phosphorylation of Ser308 in Akt, which then contributes to cell growth, proliferation and survival [77,78] (Figure 2).
The classic view of phosphoinositide signaling in growth factor signaling pathways focuses on the active role of PI3K, which increases the level of cellular PtdIns(3,4,5)P3 drastically by phosphorylating phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] at its 3′ phosphate in response to the stimuli [68,77]. PTEN, by contrast, has been recognized for its passive role in returning the PI3K-depleted PtdIns(4,5)P2 to basal levels at a constant rate. Recent studies, however, have revisited the roles of PTEN in growth-factor-induced accumulation of PtdIns(3,4,5)P3, by showing that growth factor signals are able to increase PtdIns(3,4,5)P3 levels not only by activating PI3K but also by inactivating PTEN [81,82]. Remarkably, ROS have been shown to act as second messengers involved in mediating the inactivation of PTEN in response to growth factor stimulation (Figure 2). As a consequence of ROS-mediated inactivation of PTEN, the recovery of PtdIns(4,5)P2 from PtdIns(3,4,5)P3 is retarded, resulting in prolonged activation of Akt [61,83].
Interestingly, PI3K proteins, especially those of the IB class, are able to increase intracellular levels of ROS through the activation of NADPH oxidase in neutrophils [84,85]. The PI3K-induced activation of NADPH oxidase is accomplished by activation of the small GTPase Rac, or by the direct binding of the NADPH oxidase component p40phox to PtdIns(3,4,5)P3 [84–86] (Figure 2). In this context, it is conceivable that PI3K-induced elevation of PtdIns(3,4,5)P3 could also inactivate PTEN through NADPH-oxidase-mediated ROS generation. Support for this mechanism operating in the RPE has been provided by the observation that chemical inhibition of PI3K by LY294002 suppresses the phosphorylation inactivation of PTEN and the activation of Akt in oxidatively damaged RPE cells (K.H. Kang et al., unpublished). Thus, the rapid local conversion of PtdIns(4,5)P2 to PtdIns(3,4,5)P3 in RPE cells could be synergistically accomplished through the activation of PI3K and the concomitant oxidative inactivation of PTEN.
Junctional failures in the RPE upon PTEN inactivation
Interestingly, the distributions of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 in many epithelial cells are highly polarized along the apical–basal axis, with PtdIns(3,4,5)P3 concentrated at lateral regions and PtdIns(4,5)P2 enriched in apical domains [40,87–89]. This distinct localization of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 contributes to the polarization of developing epithelial cells [40,87,89]. In particular, the role of PTEN and its enzymatic product, PtdIns(4,5)P2, in constructing apical structures has been repeatedly demonstrated [90]. PTEN, for example, contributes to the formation of the apical lumen of epithelial tubes in Madin-Darby canine kidney (MDCK) cells by increasing the local concentration of PtdIns(4,5)P2, which preserves TJs [87].
In RPE cells, PI3K and PTEN and their respective enzymatic products, PtdIns(3,4,5)P3 and PtdIns(4,5)P2, exhibit overtly similar distribution patterns to those in MDCK cells [67]. However, the genetic deletion of mouse Pten and the oxidative inactivation of PTEN in mouse RPE cells unexpectedly revealed a requirement for PTEN in the basolateral domain rather than in the apical region [67]. In the absence of PTEN, the lateral junctional structures in RPE cells were severely affected, whereas the TJs and the apical microvilli remained intact. The contribution of PTEN to basolateral cell–cell adhesions is not completely unexpected, however, because PTEN had already been observed in AJ complexes in other epithelial cells [65,91,92] (Figure 2). PTEN was also detected in the basolateral junctional structures of the RPE, although at much lower levels than in other parts of the cells [67], thereby suggesting that the requirement for PTEN in the basolateral domain is greater than the need for PTEN in the apical domain, regardless of the relative distributions of PTEN in each compartment.
ROS balances the tug-of-war between junctional PI3K and PTEN
Neither the contribution of PI3K to the junctional structures in RPE cells, nor that of PTEN, correlates with the steady-state concentration of PtdIns(3,4,5)P3. Based on the distribution of PtdIns(3,4,5)P3 in RPE cells, PI3K activity should be compatible with maintenance of junctional structures [40,87–89]. However, PI3K has been shown to destabilize junctional structures in RPE cells and to act downstream of epidermal growth factor receptor (EGFR) and the c-Met hepatocyte growth factor (HGF) receptor to further stimulate RPE cell migration [93]. These two receptor tyrosine kinases (RTKs) are also known to disrupt AJs and desmosomes in RPE cells through tyrosine phosphorylation of junctional proteins [94–96] (Figure 2).
Interestingly, both PI3K and PTEN have been shown to associate with β-catenin and E-cadherin, both of which are also able to interact with EGFR and c-Met [91,94]. Thus, it is possible that these proteins might all localize to the same complex in the lateral zone of RPE cells. In this context, RTKs are able to activate PI3K to initiate migratory signaling events, and also phosphorylate β-catenin, causing it to dissociate from E-cadherin [94–96] (Figure 2). In addition, the cytoplasmic protein tyrosine kinases (PTKs), such as Src, Fyn, and Abl, can also act synergistically to increase β-catenin phosphorylation, facilitating junctional dissociation in response to growth factor signals [97]. Nevertheless, these destabilizing events would normally be insufficient to induce rapid and complete dissociation of entire junctional complexes because protein tyrosine phosphatases (PTPases) including LMW–PTPase, PTP-μ, PTPκ, PTP-lB-like phosphatase, leukocyte antigen-related protein (LAR)–PTP, and Pez, also associate with AJs through β-catenin, providing a counterbalancing dephosphorylation activity that supports junctional maintenance [97–99]. Furthermore, PTEN in the complex would be expected to quickly convert PI3K-produced PtdIns(3,4,5)P3 to PtdIns(4,5)P2, enabling signaling events downstream of PI3K to persist only temporarily. This ‘tug-of-war’ between PTKs and PTPases, and that between PI3K and PTEN, might enable RPE cells to preserve their junctional structures by enabling only a limited, localized, remodeling dynamic to occur.
However, junction-destabilizing signals could potentially overcome the PTPase- and PTEN-supported antagonizing effects if the signals are amplified by ROS [84–86]. Upon ROS-induced inactivation of PTEN and the consequent accumulation of PtdIns(3,4,5)P3, the PI3K signaling pathways could enter a positive feedback loop, causing continuous generation of ROS (Figure 2). As cellular ROS levels continuously increase, the population of oxidatively inactivated PTPases might also grow, consequently causing an accumulation of phosphorylated junctional proteins that gradually dissociate from the complexes [58–60,100]. At the same time, the ROS signal might also activate Src, thus facilitating β-catenin phosphorylation [97]. Therefore, ROS might drastically increase the phosphorylation of junctional components, both by activating PTKs and by inactivating PTPases, and thus might hasten junctional dissolution.
Fortunately, physiological levels of ROS can be rapidly neutralized by RPE that is enriched in antioxidants so, under normal circumstances, ROS-stimulated inactivation of PTPases or PTEN might be only transient [5,19,57]. However, in aged RPE cells, the antioxidation machinery might not be efficient enough to keep pace with the continuous generation of ROS from accumulated oxidative materials and thus might fail to protect PTEN and PTPases from oxidative inactivation, eventually resulting in the disruption of junctions [3,35]. Accordingly, retinal degeneration could be initiated in RPE cells when the balance between PTEN and PI3K crosses a crucial threshold that is beyond the recoverable range, and ROS-induced junctional destabilization signals become amplified.
Concluding remarks
The RPE layer is an essential part of the retina because of both its functional support for photoreceptors and its structural importance for the retina. Just as a levee prevents a river from flooding the surrounding land, RPE cells protect the retina from inflammatory cytokines from extraocular blood cells. And, just as a levee supplies water to a village by selectively opening floodgates, RPE cells nurture photoreceptors by selectively transporting nutrients, survival factors and active visual photopigments. When the RPE layer degenerates, the retina is therefore irreversibly damaged by inflammatory reactions induced by inappropriately delivered cytokines. We have discussed how PTEN might protect the RPE from physiological stressors. Somatic mutations or physiological inactivation of PTEN in RPE cells, for example owing to oxidative stress, would remove this protection, resulting in failures in junctional maintenance.
Further studies will be needed to investigate the role of PTEN in retinal function, and the molecular mechanisms that underlie the junctional maintenance supported by PTEN. It is also unclear whether or not PTEN inactivation is a common factor in epithelial junctional failures, events that in other epithelial cells might result in an entirely different physiological outcome, such as cancer development.
Acknowledgments
We appreciate the efforts of Walton Jones (KAIST), who read and edited the manuscript. This work was supported by a grant from the Korean Health Technology Research and Development Project, Ministry of Health, Welfare and Family Affairs (A084337), and by the Research Program for New Drug Target Discovery (M10748000222–08N4800–22210) and Stem Cell Research (M10641000055–07N4100–05510) grants from the Korean Ministry of Education, Science and Technology.
References
- 1.Algvere PV, et al. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006;84:4–15. doi: 10.1111/j.1600-0420.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel A, et al. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Winkler BS, et al. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty S, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 5.Cai J, et al. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 6.Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 7.Pacione LR, et al. Progress toward understanding the genetic and biochemical mechanisms of inherited photoreceptor degenerations. Annu Rev Neurosci. 2003;26:657–700. doi: 10.1146/annurev.neuro.26.041002.131416. [DOI] [PubMed] [Google Scholar]
- 8.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 9.Arshavsky VY, et al. G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 10.Rando RR. Molecular mechanisms in visual pigment regeneration. Photochem Photobiol. 1992;56:1145–1156. doi: 10.1111/j.1751-1097.1992.tb09739.x. [DOI] [PubMed] [Google Scholar]
- 11.Noell WK. Possible mechanisms of photoreceptor damage by light in mammalian eyes. Vision Res. 1980;20:1163–1171. doi: 10.1016/0042-6989(80)90055-3. [DOI] [PubMed] [Google Scholar]
- 12.Komeima K, et al. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213:809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- 13.Tanito M, et al. Cytoprotective effect of thioredoxin against retinal photic injury in mice. Invest Ophthalmol Vis Sci. 2002;43:1162–1167. [PubMed] [Google Scholar]
- 14.Hogg R, Chakravarthy U. AMD and micronutrient antioxidants. Curr Eye Res. 2004;29:387–401. doi: 10.1080/02713680490517890. [DOI] [PubMed] [Google Scholar]
- 15.Gosbell AD, et al. Retinal light damage: structural and functional effects of the antioxidant glutathione peroxidase-1. Invest Ophthalmol Vis Sci. 2006;47:2613–2622. doi: 10.1167/iovs.05-0962. [DOI] [PubMed] [Google Scholar]
- 16.Winkler BS. An hypothesis to account for the renewal of outer segments in rod and cone photoreceptor cells: renewal as a surrogate antioxidant. Invest Ophthalmol Vis Sci. 2008;49:3259–3261. doi: 10.1167/iovs.08-1785. [DOI] [PubMed] [Google Scholar]
- 17.Imamura Y, et al. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2006;103:11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saari JC, et al. Reduction of all-trans-retinal limits regeneration of visual pigment in mice. Vision Res. 1998;38:1325–1333. doi: 10.1016/s0042-6989(97)00198-3. [DOI] [PubMed] [Google Scholar]
- 19.Decanini A, et al. Changes in Select Redox Proteins of the Retinal Pigment Epithelium in Age-related Macular Degeneration. Am J Ophthalmol. 2007;143:607–615. doi: 10.1016/j.ajo.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnstable CJ, Tombran-Tink J. Molecular mechanisms of neuroprotection in the eye. Adv Exp Med Biol. 2006;572:291–295. doi: 10.1007/0-387-32442-9_40. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi H, et al. Intense light exposure changes the crystallin content in retina. Exp Eye Res. 2003;76:131–133. doi: 10.1016/s0014-4835(02)00249-x. [DOI] [PubMed] [Google Scholar]
- 22.Rao NA, et al. Elevated retina-specific expression of the small heat shock protein, αA-crystallin, is associated with photoreceptor protection in experimental uveitis. Invest Ophthalmol Vis Sci. 2008;49:1161–1171. doi: 10.1167/iovs.07-1259. [DOI] [PubMed] [Google Scholar]
- 23.Doonan F, Cotter TG. Apoptosis: a potential therapeutic target for retinal degenerations. Curr Neurovasc Res. 2004;1:41–53. doi: 10.2174/1567202043480215. [DOI] [PubMed] [Google Scholar]
- 24.Li G, et al. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J Neurosci. 2007;27:203–211. doi: 10.1523/JNEUROSCI.0445-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Algvere PV, Seregard S. Age-related maculopathy: pathogenetic features and new treatment modalities. Acta Ophthalmol Scand. 2002;80:136–143. doi: 10.1034/j.1600-0420.2002.800204.x. [DOI] [PubMed] [Google Scholar]
- 26.Marmorstein AD, et al. Morphogenesis of the retinal pigment epithelium: toward understanding retinal degenerative diseases. Ann N Y Acad Sci. 1998;857:1–12. doi: 10.1111/j.1749-6632.1998.tb10102.x. [DOI] [PubMed] [Google Scholar]
- 27.Bharti K, et al. The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Res. 2006;19:380–394. doi: 10.1111/j.1600-0749.2006.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarangarajan R, Apte SP. Melanization and phagocytosis: implications for age related macular degeneration. Mol Vis. 2005;11:482–490. [PubMed] [Google Scholar]
- 29.Bonilha VL, et al. Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J Cell Biol. 1999;147:1533–1548. doi: 10.1083/jcb.147.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin M, et al. The role of interphotoreceptor retinoid-binding protein on the translocation of visual retinoids and function of cone photoreceptors. J Neurosci. 2009;29:1486–1495. doi: 10.1523/JNEUROSCI.3882-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosch E, et al. Phagocytosis of outer segments by retinal pigment epithelium: phagosome-lysosome interaction. J Histochem Cytochem. 1993;41:253–263. doi: 10.1177/41.2.8419462. [DOI] [PubMed] [Google Scholar]
- 32.Schraermeyer U, Heimann K. Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment Cell Res. 1999;12:219–236. doi: 10.1111/j.1600-0749.1999.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 33.Bergmann M, et al. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:562–564. doi: 10.1096/fj.03-0289fje. [DOI] [PubMed] [Google Scholar]
- 34.Rozanowska M, et al. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem. 1995;270:18825–18830. doi: 10.1074/jbc.270.32.18825. [DOI] [PubMed] [Google Scholar]
- 35.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Rizzolo LJ. Polarity and the development of the outer blood-retinal barrier. Histol Histopathol. 1997;12:1057–1067. [PubMed] [Google Scholar]
- 37.McKay BS, et al. Cell-cell adhesion molecules and the development of an epithelial phenotype in cultured human retinal pigment epithelial cells. Exp Eye Res. 1997;65:661–671. doi: 10.1006/exer.1997.0374. [DOI] [PubMed] [Google Scholar]
- 38.Luo Y, et al. Expression of JAM-A, AF-6, PAR-3 and PAR-6 during the assembly and remodeling of RPE tight junctions. Brain Res. 2006;1110:55–63. doi: 10.1016/j.brainres.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 39.Tornquist P, et al. Permeability of ocular vessels and transport across the blood-retinal-barrier. Eye. 1990;4:303–309. doi: 10.1038/eye.1990.41. [DOI] [PubMed] [Google Scholar]
- 40.Comer FI, Parent CA. Phosphoinositides specify polarity during epithelial organ development. Cell. 2007;128:239–240. doi: 10.1016/j.cell.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Boulan E, et al. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 42.Finnemann SC, Rodriguez-Boulan E. Macrophage and retinal pigment epithelium phagocytosis: apoptotic cells and photoreceptors compete for αvβ3 and αvβ5 integrins, and protein kinase C regulates αvβ5 binding and cytoskeletal linkage. J Exp Med. 1999;190:861–874. doi: 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gundersen D, et al. Apical polarity of Na, K-ATPase in retinal pigment epithelium is linked to a reversal of the ankyrin-fodrin submembrane cytoskeleton. J Cell Biol. 1991;112:863–872. doi: 10.1083/jcb.112.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gundersen D, et al. Apical polarization of N-CAM in retinal pigment epithelium is dependent on contact with the neural retina. J Cell Biol. 1993;121:335–343. doi: 10.1083/jcb.121.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mousa SA, et al. Role of hypoxia and extracellular matrix-integrin binding in the modulation of angiogenic growth factors secretion by retinal pigmented epithelial cells. J Cell Biochem. 1999;74:135–143. [PubMed] [Google Scholar]
- 46.Holtkamp GM, et al. Retinal pigment epithelium-immune system interactions: cytokine production and cytokine-induced changes. Prog Retin Eye Res. 2001;20:29–48. doi: 10.1016/s1350-9462(00)00017-3. [DOI] [PubMed] [Google Scholar]
- 47.Blaauwgeers HG, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155:421–428. doi: 10.1016/S0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slomiany MG, Rosenzweig SA. IGF-1-induced VEGF and IGFBP-3 secretion correlates with increased HIF-1 α expression and activity in retinal pigment epithelial cell line D407. Invest Ophthalmol Vis Sci. 2004;45:2838–2847. doi: 10.1167/iovs.03-0565. [DOI] [PubMed] [Google Scholar]
- 49.Boulton M, Dayhaw-Barker P. The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye. 2001;15:384–389. doi: 10.1038/eye.2001.141. [DOI] [PubMed] [Google Scholar]
- 50.Cook HL, et al. Age-related macular degeneration: diagnosis and management. Br Med Bull. 2008;85:127–149. doi: 10.1093/bmb/ldn012. [DOI] [PubMed] [Google Scholar]
- 51.Petrukhin K. New therapeutic targets in atrophic age-related macular degeneration. Expert Opin Ther Targets. 2007;11:625–639. doi: 10.1517/14728222.11.5.625. [DOI] [PubMed] [Google Scholar]
- 52.Lien S, Lowman HB. Therapeutic anti-VEGF antibodies. Handb Exp Pharmacol. 2008;181:131–150. doi: 10.1007/978-3-540-73259-4_6. [DOI] [PubMed] [Google Scholar]
- 53.Coleman H, Chew E. Nutritional supplementation in age-related macular degeneration. Curr Opin Ophthalmol. 2007;18:220–223. doi: 10.1097/ICU.0b013e32814a586b. [DOI] [PubMed] [Google Scholar]
- 54.Montezuma SR, et al. Review of genetics in age related macular degeneration. Semin Ophthalmol. 2007;22:229–240. doi: 10.1080/08820530701745140. [DOI] [PubMed] [Google Scholar]
- 55.Suter M, et al. Age-related macular degeneration. The lipofusion component N-retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cells. J Biol Chem. 2000;275:39625–39630. doi: 10.1074/jbc.M007049200. [DOI] [PubMed] [Google Scholar]
- 56.Cross JV, Templeton DJ. Regulation of signal transduction through protein cysteine oxidation. Antioxid Redox Signal. 2006;8:1819–1827. doi: 10.1089/ars.2006.8.1819. [DOI] [PubMed] [Google Scholar]
- 57.Rhee SG, et al. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Xu D, et al. Oxidants painting the cysteine chapel: redox regulation of PTPs. Dev Cell. 2002;2:251–252. doi: 10.1016/s1534-5807(02)00132-6. [DOI] [PubMed] [Google Scholar]
- 59.Chiarugi P. The redox regulation of LMW-PTP during cell proliferation or growth inhibition. IUBMB Life. 2001;52:55–59. doi: 10.1080/15216540252774775. [DOI] [PubMed] [Google Scholar]
- 60.Meng TC, et al. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J Biol Chem. 2004;279:37716–37725. doi: 10.1074/jbc.M404606200. [DOI] [PubMed] [Google Scholar]
- 61.Ross SH, et al. Differential redox regulation within the PTP superfamily. Cell Signal. 2007;19:1521–1530. doi: 10.1016/j.cellsig.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, et al. Myotubularin phosphoinositide phosphatases, protein phosphatases, and kinases: their roles in junction dynamics and spermatogenesis. J Cell Physiol. 2005;204:470–483. doi: 10.1002/jcp.20303. [DOI] [PubMed] [Google Scholar]
- 63.Herve JC, Sarrouilhe D. Modulation of junctional communication by phosphorylation: protein phosphatases, the missing link in the chain. Biol Cell. 2002;94:423–432. doi: 10.1016/s0248-4900(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 64.Alema S, Salvatore AM. p120 catenin and phosphorylation: Mechanisms and traits of an unresolved issue. Biochim Biophys Acta. 2007;1773:47–58. doi: 10.1016/j.bbamcr.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Kotelevets L, et al. The lipid phosphatase activity of PTEN is critical for stabilizing intercellular junctions and reverting invasiveness. J Cell Biol. 2001;155:1129–1135. doi: 10.1083/jcb.200105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bailey TA, et al. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:675–684. doi: 10.1167/iovs.03-0351. [DOI] [PubMed] [Google Scholar]
- 67.Kim JW, et al. Retinal degeneration triggered by inactivation of PTEN in the retinal pigment epithelium. Genes Dev. 2008;22:3147–3157. doi: 10.1101/gad.1700108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maehama T, Dixon JE. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 1999;9:125–128. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- 69.Salmena L, et al. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 70.Tamura M, et al. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 71.Shen WH, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 72.Freeman DJ, et al. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell. 2003;3:117–130. doi: 10.1016/s1535-6108(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 73.Puc J, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7:193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 74.Yin Y, Shen WH. PTEN: a new guardian of the genome. Oncogene. 2008;27:5443–5453. doi: 10.1038/onc.2008.241. [DOI] [PubMed] [Google Scholar]
- 75.Stiles B, et al. PTENless means more. Dev Biol. 2004;273:175–184. doi: 10.1016/j.ydbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki A, et al. Portrait of PTEN: messages from mutant mice. Cancer Sci. 2008;99:209–213. doi: 10.1111/j.1349-7006.2007.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 78.Fruman DA, et al. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 79.Vazquez F, et al. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ambati J, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 81.Kohli R, et al. Mitochondrial reactive oxygen species signal hepatocyte steatosis by regulating the phosphatidylinositol 3-kinase cell survival pathway. J Biol Chem. 2007;282:21327–21336. doi: 10.1074/jbc.M701759200. [DOI] [PubMed] [Google Scholar]
- 82.Seo JH, et al. The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol Biol Cell. 2005;16:348–357. doi: 10.1091/mbc.E04-05-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leslie NR, et al. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ellson C, et al. PtdIns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation. EMBO J. 2006;25:4468–4478. doi: 10.1038/sj.emboj.7601346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hawkins PT, et al. The role of PI3Ks in the regulation of the neutrophil NADPH oxidase. Biochem Soc Symp. 2007;74:59–67. doi: 10.1042/BSS0740059. [DOI] [PubMed] [Google Scholar]
- 86.Dinauer MC. Regulation of neutrophil function by Rac GTPases. Curr Opin Hematol. 2003;10:8–15. doi: 10.1097/00062752-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 87.Martin-Belmonte F, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mostov KE, Cardone MH. Regulation of protein traffic in polarized epithelial cells. Bioessays. 1995;17:129–138. doi: 10.1002/bies.950170208. [DOI] [PubMed] [Google Scholar]
- 89.Pinal N, et al. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr Biol. 2006;16:140–149. doi: 10.1016/j.cub.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 90.von Stein W, et al. Direct association of Bazooka/PAR-3 with the lipid phosphatase PTEN reveals a link between the PAR/aPKC complex and phosphoinositide signaling. Development. 2005;132:1675–1686. doi: 10.1242/dev.01720. [DOI] [PubMed] [Google Scholar]
- 91.Kotelevets L, et al. Implication of the MAGI-1b/PTEN signalosome in stabilization of adherens junctions and suppression of invasiveness. FASEB J. 2005;19:115–117. doi: 10.1096/fj.04-1942fje. [DOI] [PubMed] [Google Scholar]
- 92.Vogelmann R, et al. TGFβ-induced downregulation of E-cadherin-based cell-cell adhesion depends on PI3-kinase and PTEN. J Cell Sci. 2005;118:4901–4912. doi: 10.1242/jcs.02594. [DOI] [PubMed] [Google Scholar]
- 93.Xu KP, Yu FS. Cross talk between c-Met and epidermal growth factor receptor during retinal pigment epithelial wound healing. Invest Ophthalmol Vis Sci. 2007;48:2242–2248. doi: 10.1167/iovs.06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takahashi K, et al. Induction of tyrosine phosphorylation and association of β-catenin with EGF receptor upon tryptic digestion of quiescent cells at confluence. Oncogene. 1997;15:71–78. doi: 10.1038/sj.onc.1201160. [DOI] [PubMed] [Google Scholar]
- 95.Gaudry CA, et al. Tyrosine-phosphorylated plakoglobin is associated with desmogleins but not desmoplakin after epidermal growth factor receptor activation. J Biol Chem. 2001;276:24871–24880. doi: 10.1074/jbc.M102731200. [DOI] [PubMed] [Google Scholar]
- 96.Monga SP, et al. Hepatocyte growth factor induces Wnt-independent nuclear translocation of β-catenin after Met-β-catenin dissociation in hepatocytes. Cancer Res. 2002;62:2064–2071. [PubMed] [Google Scholar]
- 97.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of β-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 98.Taddei ML, et al. β-catenin interacts with low-molecular-weight protein tyrosine phosphatase leading to cadherin-mediated cell-cell adhesion increase. Cancer Res. 2002;62:6489–6499. [PubMed] [Google Scholar]
- 99.Wadham C, et al. The protein tyrosine phosphatase Pez is a major phosphatase of adherens junctions and dephosphorylates β-catenin. Mol Biol Cell. 2003;14:2520–2529. doi: 10.1091/mbc.E02-09-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chiarugi P. PTPs versus PTKs: the redox side of the coin. Free Radic Res. 2005;39:353–364. doi: 10.1080/10715760400027987. [DOI] [PubMed] [Google Scholar]