Abstract
Efforts to regenerate the cavernous nerve (CN), which provides innervation to the penis, have been minimally successful, with little translation into improved clinical outcomes. We propose that, Sonic hedgehog (SHH), is critical to maintain CN integrity, and that SHH delivered to the CN by novel peptide amphiphile (PA) nanofibers, will promote CN regeneration, restore physiological function, and prevent penile morphology changes that result in erectile dysfunction (ED). We performed localization studies, inhibition of SHH signaling in the CN, and treatment of crushed CNs with SHH protein via linear PA gels, which are an innovative extended release method of delivery. Morphological, functional and molecular analysis revealed that SHH protein is essential to maintain CN architecture, and that SHH treatment promoted CN regeneration, suppressed penile apoptosis and caused a 58% improvement in erectile function in less than half the time reported in the literature. These studies show that SHH has substantial clinical application to regenerate the CN in prostatectomy and diabetic patients, that this methodology has broad application to regenerate any peripheral nerve that SHH is necessary for maintenance of its structure, and that this nanotechnology method of protein delivery may have wide spread application as an in vivo delivery tool in many organs.
Introduction
Peripheral nerve injury of the cavernous nerve (CN) is a significant concern to diabetic, aging, metabolic syndrome and prostate cancer patients who develop erectile dysfunction (ED) as a result of denervation of the penis. ED affects ~ 50% of the male population between the ages of 40 and 70 and has a high impact on men’s health since ED is an early warning sign for cardiovascular disease [1]. Loss of innervation causes profound and irreversible morphological changes in the penis including induction of abundant apoptosis in penile smooth muscle, primarily in the first week after CN injury [2]. Current therapies for ED, including phosphodiesterase type 5 (PDE5) inhibitors, target relaxation of penile smooth muscle by elevating cGMP. These therapies become ineffective with corpora cavernosal remodeling that results from 10 CN injury and are thus effective in only a minority of patients with neuropathy of the CN (16–82% ineffective in prostatectomy patients [3], and 56–59% ineffective in diabetic patients [4]). Thus new therapies are required that address the under lying causes of ED by promoting CN regeneration and suppressing penile apoptosis.
As is the case with other peripheral nerves, efforts to regenerate the CN have so far been minimally successful, with little translation into improved clinical outcomes. Acellular nerve grafts [5], use of Schwann cell seeded guidance tubes [6–7] and alginate supports to bridge the injury gap [8], and treatment with assorted growth factors, including growth hormone [9], vascular endothelial growth factor [10], brain-derived neurotrophic factor [10] (BDNF), erythropoietin [11], and neuturin [12], are state of the art treatments however they have been only partially successful in regenerating the CN in animal models. In other peripheral nerves, such as the sciatic nerve [13–14] and facial nerve [15], it has been suggested that the Sonic hedgehog (SHH) pathway may play a significant role in nerve regeneration after injury. This is supported by improvement in nerve function by SHH treatment in diabetic models of neuropathy [16–17]. In our previous studies we have shown that SHH is an essential regulator of penile morphology that can suppress smooth muscle apoptosis caused by CN injury [18–20] and SHH signaling in the adult penis is mediated by SHH signaling in the CN, by neural activity and trophic factors in the CN [20–22], thus emphasizing the importance of regenerating the CN for preventing penile apoptosis and ED. SHH protein is abundant in neuronal nitric oxide synthase (NOSI) positive neurons of the pelvic ganglia that innervate the penis [21] and in Schwann cells of the CN [21], and SHH positive Schwann cells are recruited to the site of CN injury for repair [21]. These results show that SHH in Schwann cells is essential to maintain CN integrity and suggest that SHH is required for CN regeneration. Thus we propose the innovative approach of delivering SHH protein locally to the CN at the time of CN injury/prostatectomy in order to promote CN regeneration and prevent penile apoptosis. We hypothesize that SHH protein delivered to the CN via monodomain gels containing aligned peptide amphiphile (PA) nanofibers [23], will speed CN regeneration. The advantage of using a gel of aligned PA nanofibers (Figure 1) for SHH delivery is that they are biodegradable, provide directional guidance to regenerating axons and could deliver proteins directionally and over extended periods. If SHH protein delivered by aligned PA nanofibers promotes CN regeneration, then there is substantial clinical application to humans to regenerate the CN after prostatectomy and in diabetic patients where SHH protein is also decreased [19]. There is also the potential for broad application of this methodology to any peripheral nerve that SHH signaling is necessary for regeneration.
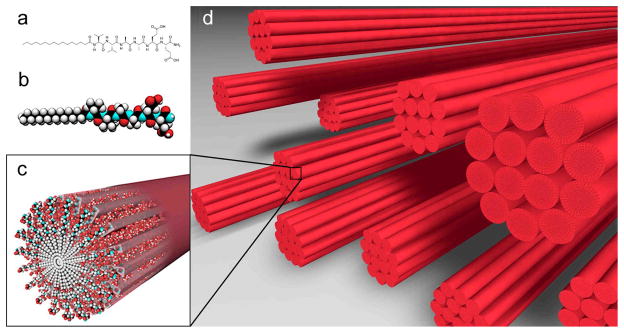
Figure 1.
Chemical structure of the (C16)-V2A2E2-(NH2) PA used to form monodomain noodle gels (a), molecular graphics representation of the PA molecule (b), self-assembly of PA molecules into a nanofiber (c), and schematic representation of nanofiber bundles oriented in a common orientation after the noodle gel is formed (d).
Methods
Animals
Sprague Dawley rats postnatal day 115–120 (P115–P120) were obtained from Charles River. Animals were cared for in accordance with institutional IACUC approval and the National Research Council publication Guide for Care and Use of Laboratory Animals.
Peptide amphiphile synthesis
PAs were synthesized at the Northwestern Institute for BioNanotechnology in Medicine Chemistry Core Facility. PAs were synthesized by standard methods via FMoc-based solid phase synthesis on Rink resins [23]. Through standard deprotection and coupling procedures, the VVAAEE sequence was synthesized on the resin beads and terminated in a palmitic acid (C16) alkyl tail. Batches were cleaved from the resin, precipitated in cold ethyl ether, and lyophilized to a powder. PAs were then purified by preparative high performance liquid chromatography and lyophilized to a powder. Each dry powder was redissolved in PBS, adjusted to pH 7 using dilute sodium hydroxide, and dialyzed against de-ionized water. The PA solutions were then re-lyophilized, and stored at −80°C as a dry powder until needed. The final PA is shown in Figure 1 as ((C16)-V2A2E2-(NH2), molecular weight=854 g/mol). Compositions were confirmed by mass spectrometry.
Anti-kinesin treatment of the pelvic ganglia/CN
Affi-Gel beads (100–200 mesh, Bio-Rad) were equilibrated with mouse anti-kinesin (1.8 mg/mL, n=4, Sigma, St. Louis, MO, USA) and PBS (control, n = 3) overnight at 4°C. Approximately 10–20 beads were injected directly under the pelvic ganglia bilaterally in adult Sprague Dawley rats. Rats were sacrificed at 3 days following bead injection/anti-kinesin treatment and the penis tissue was frozen in liquid nitrogen or fixed in 4% paraformaldehyde. Semi-quantitative immunohistochemical analysis was performed on penis tissue assaying for SHH protein.
5E1 SHH inhibitor treatment of the pelvic ganglia
Affi-Gel beads (100–200 mesh, Bio-Rad, Hercules, CA) were equilibrated with 5E1 SHH inhibitor (378 μg/ml, Jessel, Hybridoma Bank at the University of Iowa) or mouse IgG (control) overnight at 4°C. Approximately 10–20 beads with SHH inhibitor (n=3) or mouse IgG (n=3) were injected directly under the pelvic ganglia bilaterally in adult Sprague Dawley rats. Injection was not made directly into the pelvic ganglia itself since this could damage the pelvic ganglia. Rats were sacrificed at 6 days following bead injection/SHH inhibition and the CN/pelvic ganglia were fixed in 2.5% glutaraldehyde for electron microscopy.
CN crush and SHH protein treatment via Affi-Gel beads implanted under the pelvic ganglia
The CN/pelvic ganglia were exposed in adult Sprague Dawley rats. Microforceps (size 0.02 × 0.06mm) were used to perform bilateral CN crush as described previously [24,25]. Forceps were closed on the CN and the pressure maintained for 30 seconds [24,25]. Affi-Gel beads (100–200 mesh, Bio-Rad, Hercules, CA) were equilibrated over night with SHH protein (recombinant mouse SHH peptide [26] (R & D Systems), or BSA (control) at 4°C. Approximately 10–20 beads equilibrated with either 0.125μg/μl SHH protein (n=8 rats) or BSA (n=7 rats), were injected directly under the pelvic ganglia bilaterally in CN crushed rats. Penes were harvested from euthanized males by sharp dissection 2 or 6 weeks after CN crush and were frozen or fixed in 4% paraformaldehyde and embedded in paraffin.
CN crush and SHH protein or BSA (control) treatment via PAs
The CN/pelvic ganglia were exposed in adult Sprague Dawley rats. Microforceps (size 0.02 × 0.06mm) were used to perform bilateral CN crush. Forceps were closed on the CN and the pressure maintained for 30 seconds [24,25]. PA was rehydrated to a 100 mM concentration in distilled water and was heated at 80°C for 30 minutes in a water bath. The water bath was turned off and the PA was slowly allowed to cool to room temperature. 20mM CaCl2 (500μl) was added to a glass slide. 8μl of 100mM PA plus either 2.27μg SHH or BSA proteins (dissolved in 1.5 μl water) were pipetted onto the glass slide to form the linear PA. Final PA concentration was 10 mM and final SHH protein concentration was 2.27 μg per linear PA. The PA was then transferred with forceps on top of the crushed CNs bilaterally in Sprague Dawley rats so that each rat received 4.54μg SHH protein or BSA protein. Sixteen rats were treated bilaterally with SHH PA and 13 rats were treated with BSA PA. Penes and pelvic ganglia/CNs were harvested from euthanized males by sharp dissection 4 and 6 weeks after CN crush and were either fixed in 4% paraformaldehyde and embedded in paraffin, fixed in 2.5% glutaraldehyde for EM or frozen in liquid nitrogen for immunohistochemical analysis.
Electron microscopy (EM)
EM was performed as described previously [27] on CN from adult Sprague Dawley rats that had their pelvic ganglia treated with mouse IgG as a control (n=3) or 5E1 SHH inhibitor (n=3, 378 μg/ml, Jessel, Hybridoma Bank at the University of Iowa) via Affi-Gel beads for 6 days (n=3). EM was also performed on control BSA PA treated CN (n=3, 4) and SHH PA treated CN (n=3, 4) after 4 and 6 weeks of treatment. Isolated CNs were fixed in 2.5% glutaraldehyde, postfixed in 1% OsO4, dehydrated, and embedded in Epon resin. These sections were cut and stained with 2% uranyl acetate and 3% lead citrate. EM was performed using a Zeiss Electron Microscope 900 to examine CN morphology.
Immunohistochemical (IHC) analysis
IHC was performed as previously outlined [18–20] on pelvic ganglia/CN tissue assaying for goat polyclonal SHH (1/100, N-19, SC-1194, Santa Cruz) and HIP (1/100, D-15, Santa Cruz), rabbit GFAP (1/100, Z0334, DAKO, Carpinteria, CA), and mouse monoclonal CD3 (1/50, SC-52382, Santa Cruz). Secondary antibodies used were Alexa Fluor 488 chicken anti-goat (1/300, Molecular Probes, Carlsbad, CA), Alexa Fluor 488 chicken anti-rabbit (1/600, Molecular Probes, Carlsbad, CA), and Alexa Fluor 488 chicken anti-mouse (1/200, Molecular Probes, Carlsbad, CA). Negative controls were performed with secondary only (without primary) and with serum in place of primary antibodies, to test for non-specific staining and autofluorescence. Sections were mounted using Pro-Tex Mounting Medium (Baxter Diagnostics, Inc., Pittsburgh, PA). Microscopy was performed using a fluorescent microscope (Leitz) and photographed using a Nikon digital camera. Quantification of SHH and HIP proteins was performed using the Image J program [28]. Total fluorescence was measured in five fields from each section and five sections for each tissue.
TUNEL assay for apoptosis
TUNEL assay was performed using the Apoptag kit (Chemicon International) on isolated penis tissue fixed over night at 4°C in 4% paraformaldehyde, embedded in paraffin and sectioned 16μM in thickness as described previously [20]. All cells were stained for comparison using DAPI (0.005 μg/ml). Fluorescent apoptotic cells were observed under a fluorescent microscope (Leitz) and photographed using a Nikon digital camera. Quantification of apoptosis was performed by counting the total number of cells and the number of apoptotic cells in a given field selected at random by visual observation. The number of apoptotic cells/all cells (apoptotic index) in five fields from each section and five sections for each penis were counted. The apoptotic index was reported ± the standard error of the mean (SEM).
Intracavernosal pressure (ICP) measurements
ICP was measured as described previously [29,18]. Nerves were stimulated (intensity of 6 volts) by placing them on bipolar platinum stimulating electrodes connected to an electrical stimulator (Grass Instruments, Quincy, MA) delivering a series of square wave pulses (1 msec duration at 30 Hz). The cavernous nerve was unilaterally stimulated at a distance of 3 and 5 mm from the major pelvic ganglion. Stimulation lasted 40 sec. A resting interval of at least 5 min separated two consecutive stimulation procedures. The ICP was measured by inserting a 23-ga needle into the corpora cavernosa. A catheter was inserted into the carotid artery for measurement of arterial pressure. These instruments were connected to a pressure transducer. The data were reported as the peak ICP/average blood pressure.
SHH protein dissociation from the PA in vitro
PA was rehydrated to a 100 mM concentration and was heated at 80°C for 30 minutes in a water bath. The water bath was turned off and the PA was slowly allowed to cool to room temperature. 200 mM CaCl2 (500μl) was added to a glass slide and 8μl of 100mM PA was added to 2.27μg of SHH protein (R&D Systems, Minneapolis, MN) in 1.5μl water. The PA SHH mixture was pipetted onto the glass slide containing CaCl2 to form the linear PA. Three linear PAs were transferred with forceps into a single well of a 96 well plate and 75μl of a modified Ringer’s solution (100ml of a solution containing=600mg NaCl, 30mg KCl, and 20mg CaCl2 in 1X PBS) was added to the well on top of the PA to mimic the in vivo Chloride concentration. Six wells containing SHH and PA and two wells containing PA only (used as a blank for spectrophotometric analysis) were performed. Each well contained a total of 6.8μg SHH protein. 55 μl was removed from each well and replaced with fresh solution at 1, 5, 22, 29, 47, 52 and 75 hours. Absorbance was measured at 280 in a Beckman Spectrophotometer (DU 640, Fullerton, California) using a cuvette designed to measure small volumes of sample (Starna Cells, Inc, Atascadero, CA).
In situ hybridization of Shh in the CN
In situ hybridization was performed as described previously [30,18–19] on CNs (n=13) taken from adult Sprague Dawley rats (n=9). CNs were fixed in 4% paraformaldehyde overnight. A mouse Shh RNA 165 probe was obtained from Andrew McMahon [26].
Fluorescent labeling of SHH protein
Fluorescent labeling of SHH protein (R&D Systems, Minneapolis, MN) was performed using the Alexa Fluor 488 Microscale Protein Labeling Kit (Molecular Probes) according to manufacturer’s instructions. Briefly, 25μg SHH protein was dissolved in 25μl water. 2.5μl of a 1M sodium bicarbonate buffer was added to SHH protein. 2.9μl of reactive dye was added to the reaction tube and the solution was incubated for 25 minutes at room temperature. The reaction mixture was added to a previously prepared spin column and spun at 14,000×g for one minute. Labeled SHH protein was collected and the total protein and fluorescence were quantified by measuring the absorbance at 280 and 494 nm on a Beckman Spectrophotometer (DU 640, Fullerton, CA).
Fluorescent labeled SHH protein delivered by PA monodomain gel to the CN
P120 Sprague-Dawley rats under went bilateral CN crush for 30 seconds using forceps and labeled SHH PA was placed bilaterally on top of crushed CNs (n=3) as described above. To make the SHH PA a 100 mM solution of PA (8.5μl) was added to 3μl of a solution of Alexa Fluor 488 labeled SHH protein in water (0.24μg/μl, R&D Systems, Minneapolis, MN). The PA was formed on a glass slide containing 500μl of a 200 mM CaCl2 solution. Each linear PA contained 0.72μg labeled SHH protein. The amount of labeled SHH protein delivered by linear PA in vivo (0.24μg/μl) differed from the unlabeled PA (1μg/μl), because the labeling process diluted SHH protein so that if the same amount was added, the PA would not gel properly. Rats were sacrificed after four hours and the CN and pelvic ganglia were frozen in OCT prior to sectioning on a cryostat.
Quantification of CN and pelvic ganglia NosI and Gfap RNA expression by real time RT-PCR
RNA was isolated and Real time RT-PCR performed as previously described [22] assaying for NosI in sham control (n=3) and bilateral CN crushed (n=3) pelvic ganglia and CN tissue, 9 days after CN crush. Real time RT-PCR was also performed in no surgery controls (n=3), BSA PA controls (n=3) and SHH PA (n=3) treated CNs 6 weeks after CN crush. The specificity of primers was verified by melt curve analysis and sequencing at the Northwestern University Center for Genetic Medicine Sequencing Core Facility. NosI and CGfap were normalized to the Ribosomal subunit L-19 (Rpl19) housekeeper by the 2−ΔΔCT method [31]. Assays were performed in triplicate, the results averaged and the product ratios were reported as the mean plus or minus the standard error of the mean. The primers used were: NosI-F:5′-ACCTCGATGGCAAATCGCACAAAG-3′, NosI-R:5′-ACGGGTTGTTAAGGATCACAGGAA-3′, Rpl19-F:5′-ACAAGCGGATTCTCATGGAGCACA-3′, Rpl19-R:5′-TGATCTCCTCCTTCTTGGCTTGGA-3′, Gfap-F:5′-TGGCCACCAGTAACATGCAA-3′, Gfap-R:5′-CAGTTGGCGGCGATAGTCAT-3′.
Statistics
Statistics were performed using the Excel program and the results reported ± the standard error of the mean (SEM). A t-test was performed to determine significant differences. P-values ≤0.05 were considered significant.
Results
Anti-kinesin treatment of the CN
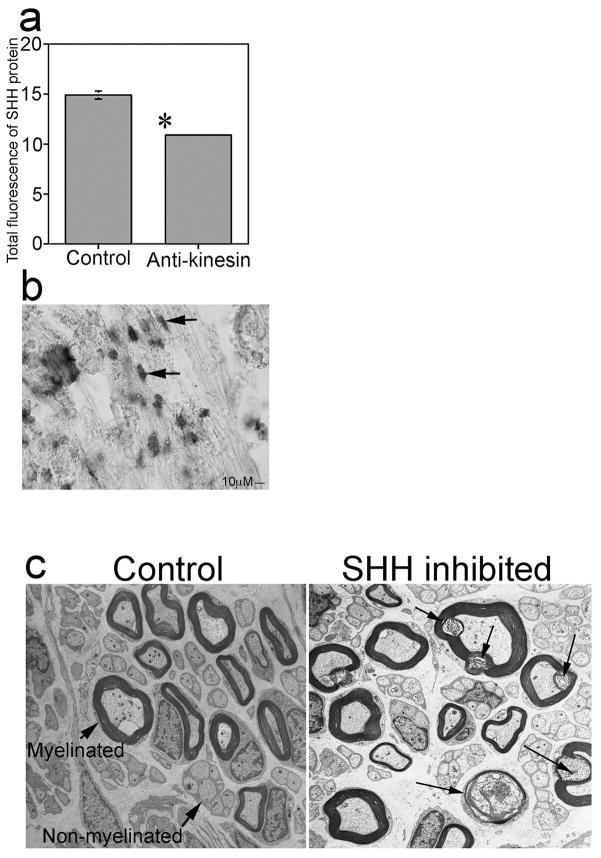
Little is known about how anterograde transport of the CN affects penile morphology. Blockade of anterograde transport was performed in order to show the effect of CN integrity and transport on SHH signaling in the penis and on penile morphology. CN’s of adult Sprague Dawley rats were treated bilaterally with anti-kinesin antibody for 3 days and SHH protein was quantified in penis tissue of the treated rats. SHH protein was decreased 27% in penis tissue of anti-kinesin treated rats (n=4, p=0.0001, Figure 2a) by comparison to controls (n=3), indicating that CN transport is necessary to maintain penile SHH protein abundance.
Figure 2.
(a) Quantification of SHH protein by semi-quantitative IHC analysis of penis tissue from Sprague Dawley rats that were treated with anti-kinesin or PBS in the pelvic ganglia via Affi-Gel beads. SHH protein was decreased 27% after interruption of anterograde transport in the CN by anti-kinesin. (b) In situ hybridization of Shh mRNA expression in adult Sprague Dawley rats shows Shh is localized in Schwann cells of the CN. Arrows indicate Schwann cells. 400X. (c) EM of control and SHH inhibited CN shows de-myelination and axonal degeneration of CN fibers after SHH inhibition. Arrows indicate myelinated and 15 non-myelinated fibers in control CN and myelin ovoids in SHH inhibited CN, where myelin is being broken down. 30,000X.
Localization of Shh RNA expression in Schwann cells of the CN
We previously showed that SHH protein is abundant in Schwann cells of the CN [21], however it was unclear whether SHH was synthesized in the Schwann cells, so in situ hybridization assaying for Shh mRNA localization was performed on CNs (n=13) isolated from normal adult Sprague Dawley rats (n=9). Shh RNA 220 was expressed in Schwann cells of the CN (Figure 2b) indicating that Shh is synthesized in Schwann cells, which play a critical role in CN regeneration. Aside from forming myelin in myelinated fibers, Schwann cells regulate the formation of the perineurium, offer trophic support to nerves, are essential to the survival and function of neurons, offer directional guidance to neurons and eliminate cellular debris, and are able to block apoptosis by participating in an autocrine circuit by releasing growth factors. Thus the expression of Shh in Schwann cells supports the hypothesis that SHH is essential for CN regeneration.
5E1 SHH inhibitor treatment of the pelvic ganglia
While SHH protein has been identified in NOSI positive neurons of the pelvic ganglia [21] that innervate the penis, the function of SHH in these tissues remains unclear. Studies in other nerves suggest a role for the SHH pathway in myelination of nerve fibers [32] and in neuronal survival [32,15]. In order to examine the function of SHH in the pelvic ganglia and CN, electron microscopy (EM) was performed on CN’s of adult Sprague Dawley rats that had their pelvic ganglia treated bilaterally with either 5E1 SHH inhibitor (n=3, disrupts binding of SHH to its receptor Patched) or mouse IgG (n=3), which was used as a control, via Affi-Gel beads. Control CN morphology consisted of both myelinated and un-myelinated fibers depending on the distance from the pelvic ganglia [33] (Figure 2c). The myelinated fibers are distinguished from non-myelinated fibers visually by the dark myelin sheath, which surrounds the fibers and appears as a circle in cross-section. Rats treated with SHH inhibitor displayed axonal degeneration and demyelination of CN fibers in comparison to controls after 6 days of treatment (Figure 2c). Axonal degeneration of CN fibers was identified by the presence of myelin ovoids, which are small oval compartments formed by Schwann cells as they catabolize myelin and engulf axon fragments [34]. These results show that SHH is necessary to maintain CN integrity.
Bilateral CN crush and SHH protein treatment via Affi-Gel beads in the pelvic ganglia
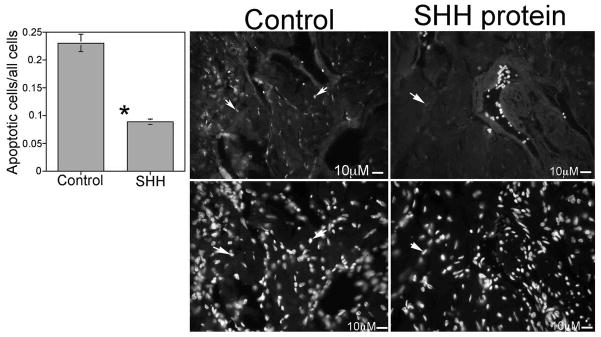
Studies in the literature suggest that the SHH pathway may play a role in peripheral nerve regeneration [13–15] and the importance of maintaining CN integrity and regenerating the CN after injury have been demonstrated previously [18–20,21]. In order to examine if SHH protein treatment could promote CN regeneration and prevent penile apoptosis, adult Sprague Dawley rats had their CNs crushed bilaterally and their pelvic ganglia/CN treated with either SHH protein (n=4) or bovine serum albumin (BSA, control, n=3) protein via Affi-Gel beads for two weeks. BSA was chosen as a control since it is a protein with no known function in the CN. TUNEL assay showed a 63% decrease in the apoptotic index in penis tissue of the SHH treated rats (p=0.0002, Figure 3). SHH and hedgehog interacting protein (HIP) abundance were also quantified in the penis. These are markers of normal penile morphology that decrease with nerve injury [22,20]. SHH protein remained unchanged (Control=11.2±0.11, SHH protein treated=11.66±0.15, p=0.06) in the penis however HIP protein was increased 6% in rats that were treated with SHH protein in the pelvic ganglia/CN at the time of CN crush (Control=11.51±0.12, SHH protein treated=12.19±0.10, p=0.008). Since HIP protein is transported by the CN [22] this suggests that SHH protein treatment is neuroprotective during crush injury.
Figure 3.
TUNEL assay (Top) and DAPI staining (Bottom) for all cells of penis tissue from Sprague Dawley rats that under went bilateral CN crush and were treated with either mouse IgG (control) or SHH protein by Affi-Gel beads for two weeks. SHH protein treatment of crushed CNs suppressed the apoptotic index in the penis by 53% (250X). Arrows indicate apoptotic cells.
At six weeks after bilateral CN crush and SHH protein (n=3) or BSA (n=3) treatment of the PG via Affi-Gel beads, there was no improvement in erectile function as measured by intracavernosal pressure (ICP)/blood pressure (BP, SHH treated=0.157±0.044, Control=0.157±0.059, p=0.5), indicating that the Affi-Gel bead delivery methodology is not optimal, either because it does not provide a surface for regenerating axons to grow along, as is the case with nerve guides which facilitate regeneration [6,8,5], the beads do not deliver SHH protein directly to the crush site, or the concentration and duration of SHH protein treatment were insufficient to promote regeneration.
Verification of CN crush model
In order to verify the extent and reproducibility of the CN crush injury, NosI and glial fibrillary acidic protein (Gfap) RNA expression were quantified in comparison to the housekeeping gene ribosomal protein L19 (RPL19), in the CN and pelvic ganglia using real time RT-PCR. NosI is abundant in neurons of the pelvic ganglia and CN that innervate the penis [35] and NosI decreases in neuronal and penis tissue after CN injury [36]. Gfap is an intermediate filament protein that is involved in maintaining structure and function of the cytoskeleton. Gfap increases in peripheral nerves in response to injury and decreases with regeneration. NosI expression was decreased in crushed CN tissue by 69% nine days after injury (Sham=1.31±0.034, Crushed=0.41±0.025, p=1.46E-05). Gfap expression was increased 88% in crushed CN tissue nine days after injury (Sham=0.0087±0.001, Crushed=0.075±0.002, p=1.68E-06). These results verify the injury to the CN upon crushing.
Treatment of CN crushed rats with SHH protein via monodomain gels of aligned PA nanofibers
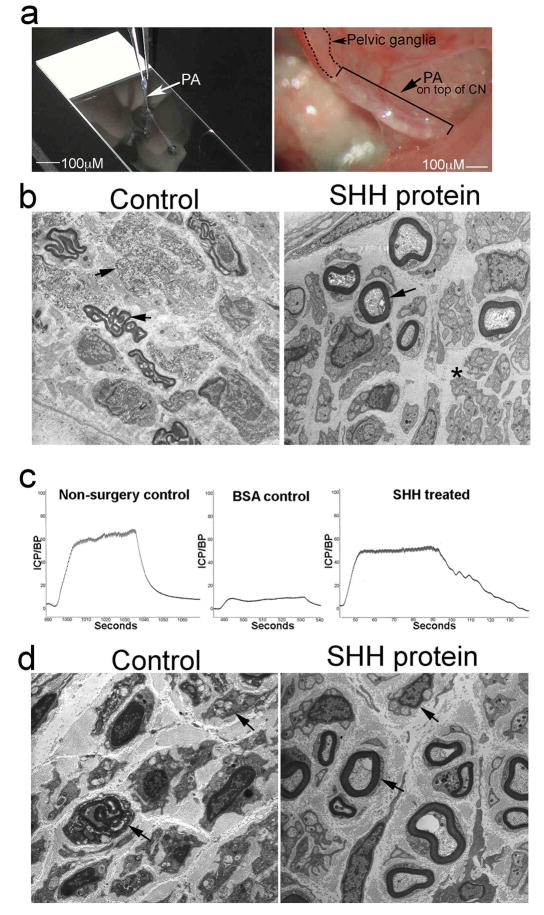
Biodegradable PA nanofibers are a non-invasive and effective method to deliver proteins in vivo [37–40] and to promote regeneration through display of bioactive signals [40]. The nanofibers proposed for SHH protein delivery to the CN in this study, are peptide amphiphiles (PA) which form bundles of nanofibers by self-assembly that can encapsulate the protein. Classical PAs [41–42] consist of a peptidic segment covalently grafted to a hydrophobic tail (e.g. palmitic acid). PA structures that form high aspect ratio cylindrical nanofibers were first reported by Stupp et al [41–42] and are composed of an interior β-sheet domain and a terminal domain that is charged or contains a biological signal. The amphiphilic nature of the molecules encourages aggregation in aqueous environments, while the β-sheet forming segment specifically drives self-assembly into high aspect ratio nanofibers [43]. At appropriate concentrations, the bundling and entanglement of these nanofibers leads to the formation of a hydrogel that mimics the architecture of extracellular matrices, with tunable mechanical properties [44]. The charged PA molecules remain largely soluble in aqueous solution, but can be triggered to self-assemble into nanofiber networks at exceedingly low concentrations (1% by weight or lower) by screening their charge with electrolytes or changes in pH [41–42]. The advantage of this methodology for protein delivery in vivo is that nanofibers are non-invasive, biodegradable, elicit no immune response [45] and form structures that can serve as a scaffold for regenerating axons [46,39]. Recently, Zhang et al., 2010 [23] have reported on their ability to create dense, directionally-aligned PA bundles with more robust physical properties, forming gels that consist of a single orientational domain of nanofibers. These linear PA “noodles” can be formed within seconds and laid directly upon the site of interest to deliver protein. In order to examine if PA methodology is effective in delivering SHH protein to the CN to promote CN regeneration, SHH protein was delivered to crushed CNs in adult Sprague Dawley rats using linear PAs for 4 and 6 weeks. Figure 4a shows a SHH PA being made and its placement in vivo on the CN. Regeneration is occurring in the SHH PA treated CNs at four weeks after injury as is shown by the presence of many axonal sprouts in EM of the SHH treated group (Figure 4b). Controls (Figure 4b) still show abundant demyelination and axonal degeneration of myelinated and non-myelinated fibers. Although EM shows improvement in CN morphology with SHH PA treatment, this is insufficient to restore CN function at 4 weeks after injury as shown by no change in ICP/BP after SHH PA treatment (n=3) in comparison to controls (n=3, Control=0.493±0.115, SHH treated=0.465±0.143, p=0.44). SHH and HIP proteins were increased by 9.3% (Control=11.0±0.42, SHH treated=12.13±0.27, p=0.028) and 13.3% (Control=13.51±0.51, SHH treated=15.59±0.19, p=0.002) in the penis after SHH PA treatment, thus supporting the observed improvement in CN morphology. The normal regeneration time for the CN in the rat is 10–12 weeks [25] and four weeks is very early to expect innervation to be restored.
Figure 4.
(a) Linear PA made on a slide and placed in vivo on top of a crushed CN. 100X. (b) EM of Sprague Dawley rats that had bilateral CN crush and were treated with BSA PA (control) or SHH PA for 4 weeks. Myelinated fibers are intact in the SHH treated CN and axonal sprouts are evident in non-myelinated fibers (asterisk). Control CNs still show abundant break down of myelin in myelinated fibers and axonal degeneration in non-myelinated fibers (arrows). 30,000X and 44,000X. (c) ICP analysis of CN crushed Sprague Dawley rats that were treated with BSA PA (control) or SHH PA for 6 weeks, shows a 58% improvement in erectile function in the SHH treated rats. (d) EM of Sprague Dawley rats that had bilateral CN crush and were treated with BSA PA (control) or SHH PA for 6 weeks. The SHH PA treated rats show significant regeneration by comparison to controls as indicated by intact myelinated and non-myelinated fibers (arrows) and axonal sprouts visible in non-myelinated fibers. Control rats show the break down of myelin in myelinated fibers and degeneration of non-myelinated fibers (arrows). 70,000X and 44,000X.
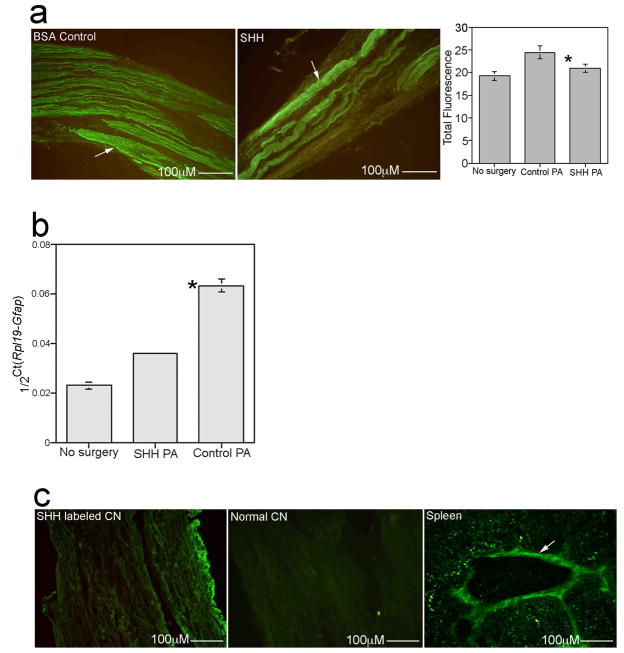
After 6 weeks of SHH PA (n=5) treatment, erectile function was improved 58% as measured by ICP/BP in comparison to controls (n=5, Figure 4c, Control=0.107±0.094, SHH treated=0.253±0.068, p=0.05), indicating significant reinnervation and regeneration. EM shows improved CN morphology 6 weeks after CN crush in the SHH treated group while control CNs still show abundant demyelination and axonal degeneration (Figure 4d). Semi-quantitative IHC analysis of crushed CNs treated with SHH PA (n=8) or control BSA PA (n=7) showed a significant 22% increase in GFAP in control CNs (p=0.004) that was not present in SHH PA treated CNs (p=0.1, Figure 5a). GFAP increases in response to injury and decreases with regeneration, showing that the SHH treated CNs have undergone regeneration. Quantification of GFAP was performed in this manner since the amount of tissue isolated from the CNs is too small to allow for Western analysis. This result was supported by Real time RT-PCR which showed a 64% increase in Gfap RNA expression in controls (Figure 5b, p=0.0006) and only a 36% increase in Gfap in the SHH treated group (Figure 5b, p=7.97 × 10−5).
Figure 5.
(a) Quantification of GFAP protein by semi-quantitative IHC analysis in CNs from Sprague Dawley rats that under went bilateral CN crush and were treated with BSA PA (control) or SHH PA for 6 weeks, shows a 22% increase in GFAP in control BSA treated tissues that was not present in SHH PA treated CNs. GFAP increases in peripheral nerves in response to injury and decreases with regeneration. Arrows indicate GFAP protein. 160X. (b) Real time RT-PCR of Gfap RNA expression in CNs from Sprague Dawley rats that under went bilateral CN crush and were treated with BSA PA (control) and SHH PA for 6 weeks, shows a 64% increase in Gfap expression in controls and only a 36% increase in Gfap in the SHH PA treated CNs, indicating significant CN regeneration. (c) IHC assayed for CD3 (immune response marker) shows no staining present in CNs treated with SHH PA (left), or in normal CN (middle), however CD3 protein was abundant in spleen tissue (right), which was used as a positive control. 160X, 160X and 250X.
Cluster differentiation protein three (CD3) forms part of the T cell receptor complex of a mature T lymphocyte and is considered a good, general immune response marker. CD3 IHC of SHH PA treated CNs showed no staining (Figure 5c), as did normal CNs (Figure 5c), indicating the absence of an immune response to the PA or SHH protein. Positive CD3 protein was observed in spleen tissue (Figure 5c), which was used as a positive control for CD3. By 6 weeks after CN crush and BSA PA and SHH PA treatment, there was no difference in SHH protein (SHH PA=9.05±1.03, BSA PA= 10.63±0.45, p=0.11) or in the apoptotic index (SHH PA=0.28±0.005, BSA PA= 0.29±0.015, p=0.18) in the penis, indicating that improvement in erectile function with SHH treatment is a result of early regenerative events that SHH has a positive influence on.
Quantification of SHH protein diffusion from linear PA in vitro
In order to determine the release rate of SHH protein from the PA in vitro, total protein was quantified by spectrophotometric analysis at 280 nm, in fluid taken from on top of SHH PA gelled in wells of a 96 well plate. Protein was quantified at 1, 5, 22, 29, 47, 52 and 75 hours after the SHH PA was formed (n=6 wells). 0.23 percent of SHH protein diffused from the PA in the first hour (Figure 6a). By 5 hours, 48% of SHH protein had eluted from the PA (Figure 6a). By 22 hours, 74% of SHH protein diffused from the PA (Figure 6a). By 75 hours 90% of the protein had diffused from the PA (Figure 6a). These results show that a short duration of SHH treatment (~3.1 days) at the time of CN injury is sufficient to speed CN regeneration.
Figure 6.
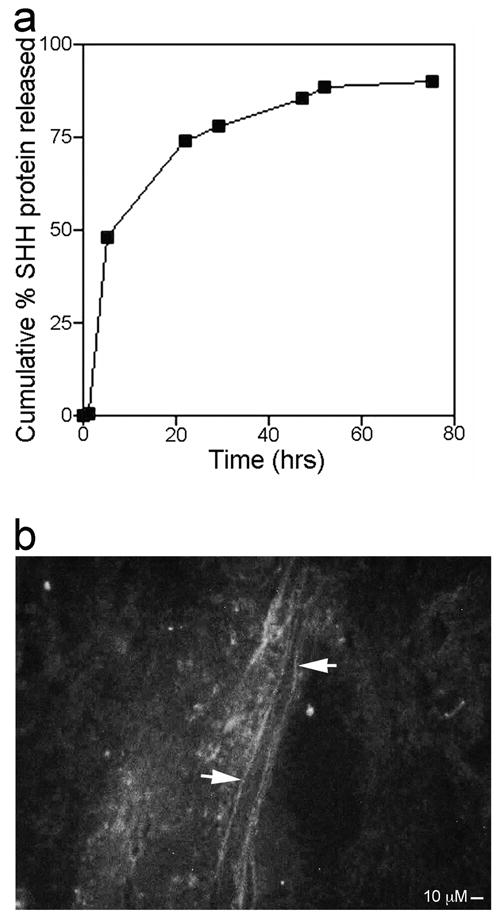
(a) Graph of cumulative percent SHH protein release from linear PA in vitro versus time (hours). Error for each point of the graph is too small for error bars to appear in the diagram. (b) Pelvic ganglia and CN from adult Sprague Dawley rats that under went bilateral CN crush and were treated with Alexa Fluor 488 labeled SHH protein via linear PA show axons between the crush site and pelvic ganglia which stain with labeled SHH protein, indicating retrograde transport of SHH protein from the crush site. 250X.
Localization of Alexa-fluor labeled SHH protein delivered by linear PA to the CN
Alexa Fluor 488 labeled SHH protein was incorporated into linear PAs which were placed in vivo on top of CNs from rats that under went bilateral CN crush (n=3). Rats were sacrificed after four hours in order to determine where SHH protein was incorporated in the CN. Fluorescent label was observed in axons of the CN between the crush site and the pelvic ganglia (Figure 6b), indicating retrograde transport of SHH protein from the crush site. SHH is likely transported to the neuronal cell bodies in the pelvic ganglia and CN, where it may initiate a signaling cascade, which facilitates regeneration.
Discussion
These studies show that SHH signaling in the CN is essential to maintain CN integrity, that SHH is a critical part of the regeneration process in the nerve and that SHH treatment is efficacious in speeding CN regeneration in the rat. The PA nanofibers utilized for these studies are effective in delivering SHH protein to the CN in a manner that does not generate an immune response. Up-regulating SHH signaling via ShRNA or viral gene delivery is unlikely to increase SHH protein since there is a blockade in synthesizing/processing SHH protein from Shh mRNA after CN injury [20], and therefore delivery of SHH protein itself is a better strategy. Although over expression of SHH has not been shown to cause any adverse reaction, over expression of SHH targets is linked to some forms of cancer, therefore local rather than systemic delivery of SHH is advantageous and would avoid potential complications in prostatectomy patients. So what do these studies imply for potential clinical application? There is significant translational potential to treat prostatectomy patients at the time of surgery with SHH protein via aligned monodomain gels of PA nanofibers applied directly to the CN. This would have the two-fold benefit of promoting CN regeneration and suppressing penile apoptosis, which leads to long term ED development. Although the CN in humans is more diffuse than that of the rat, the width of the monodomain gel can be adjusted to accommodate the more diffuse anatomical distribution of the CN in humans. There is substantial potential to utilize this methodology in diabetic patients with ED since SHH protein is decreased in the penis of diabetic rat models [19] in a very similar manner to rat prostatectomy models [20]. There is also the potential to apply SHH PA to promote regeneration of any peripheral nerve where SHH signaling plays a role in maintaining nerve integrity and regeneration, such as the sciatic [13] and facial [15] nerves.
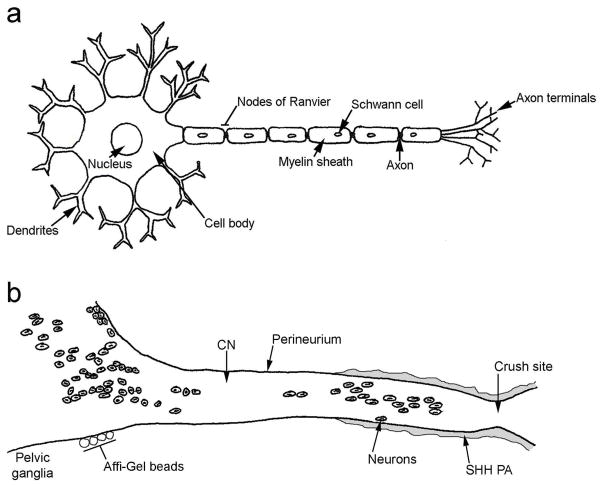
Affi-Gel bead delivery of SHH protein to the pelvic ganglia does not improve erectile function at six weeks after CN crush as does the PA methodology, indicating that the PA may be beneficial by acting as a scaffold for regenerating axons and/or by allowing for delivery of SHH protein directly to the crush site in the CN. Previous studies in the literature have demonstrated that similar PAs, used in a model of spinal cord injury, inhibit glial scar formation and promote axon elongation [39]. It is clear that the improvement in erectile function observed after SHH PA treatment does not only result as a benefit of the PA acting as a scaffold for regenerating axons, since PA with BSA instead of SHH showed significantly less improvement in CN morphology and erectile function (58%). Thus delivery of SHH directly to the crush site may be critical for facilitating regeneration. This makes sense when thinking about neuronal structure (Figure 7a) and transport and where SHH is synthesized. Neurons are composed of several dendrites (receive signals from other cells), a cell body (where proteins are synthesized), and a single axon (which conducts nerve impulses to other cells and transports proteins to and from the axon terminals). Neurons, which innervate the penis are located in the pelvic ganglia and are also dispersed down the CN. SHH protein is synthesized in pelvic ganglia neurons whose cell bodies can be quite distant from the crush site (Figure 7b). Previous studies have shown that SHH protein does not undergo anterograde transport [21]. Thus treatment of the pelvic ganglia with SHH protein via Affi-Gel beads does not likely result in delivery of SHH protein to the crush site (Figure 7b). In this study treatment of the crushed CN with fluorescently labeled SHH protein via PA resulted in retrograde transport of SHH to neuronal cell bodies between the crush site and pelvic ganglia. SHH treatment, in this manner, may result in upregulation of other factors either in the axon terminals or in the cell bodies that are critical for regeneration. It is also possible that the concentration of SHH protein delivered to the pelvic ganglia/CN by the Affi-Gel beads was insufficient to promote regeneration. Other investigators have demonstrated that SHH may have multiple effects on a tissue depending on the concentration [47].
Figure 7.
(a) Diagram of a neuron. (b) Diagram of the pelvic ganglia and cavernous nerve. CN=cavernous nerve. PA=peptide amphiphile.
SHH treatment at the crush site may mimic the effects of Schwann cells in the CN. SHH is synthesized by Schwann cells, which play a critical role in the regeneration process. They migrate towards an injury site to aid in repair by helping to phagocytize the damaged end of axons and then form a scaffold to guide regenerating axons. Schwann cells attract injured neurons by secreting neurotrophic factors [48], produce extracellular matrix components, and re-myelinate regenerating axons to reestablish the conductive properties of nerve fibers, ultimately leading to reinnervation of the target and functional recovery [6]. Other authors have shown that application of Schwann cells to CN cut models via tubing can decrease CN regeneration time [7]. It is thought that the Schwann cells upregulate factors critical for regeneration such as BDNF [14]. We have previously shown that Schwann cells migrate to the site of CN injury after crush and these Schwann cells stain abundantly for SHH protein [21], indicating that SHH is critical for regeneration. SHH application to the crush site may thus mimic, at least in part, one or more functions of Schwann cells, such as upregulating other essential regeneration factors, or by acting as a guide for regenerating axons, as was shown previously to occur with commissural axons in the spinal cord [49].
The in vitro release studies of SHH protein from the PA indicate that SHH is delivered by the PA only 405 for a relatively short period of time (~3.1 days) however SHH protein has a profound and sustained effect on CN morphology, function and the speed of regeneration. How this may occur remains unclear but clues as to how it is possible may be gleaned from known SHH signaling mechanisms which suggest that SHH is a switch that once it is turned on, activates a large signaling cascade including both positive and negative effectors of growth and much feed back regulation on SHH itself and its receptors. Studies from the literature suggest that BDNF is a target of SHH signaling as was shown by enhanced BDNF expression after treatment of cultured Schwann cells with exogenous SHH protein [14]. BDNF has been shown to promote CN regeneration [50], thus suggesting one avenue of SHH influence in the CN. Another potential target of SHH signaling in the CN is HIP, which is upregulated in response to exogenous SHH treatment of the CN [22]. HIP protein has the unique feature of being transported by the CN and can thus affect penile morphology [22] as was demonstrated by apoptosis induction in the penis in response to disturbed HIP signaling in the CN [22]. While it is possible to optimize PA structure to increase SHH retention and slow SHH release from the PA, our studies suggest that a bolus release is sufficient to initiate and speed CN regeneration and to suppress penile apoptosis. Since the majority of penile apoptosis takes place in the first week after CN injury [2], only a short duration of apoptosis suppression may be required to preserve penile morphology while the CN regenerates and can again provide innervation to the tissue.
Previous studies suggest that once CN morphology has been altered through apoptosis and induction of fibrosis, that these changes are irreversible irrespective of CN regeneration, due to the change in the responsiveness of the corpora cavernosa to neuronal signals [25]. Thus emphasizing the importance of maintaining CN morphology and suppressing penile apoptosis while the CN regenerates. We have shown in past studies, that SHH protein is effective in suppressing CN injury induced apoptosis when applied directly to the penis at the time of CN injury [20]. In this study we show that SHH applied to the pelvic ganglia/CN can suppress penile apoptosis when the CN is injured and that when SHH is delivered directly to the crush site, it can promote CN regeneration in roughly half the time previously reported in the literature [25]. Thus a two-pronged approach is necessary for penile rehabilitation, which treats both the acute injury to the nerve and prevents morphological changes in the target organ of innervation.
Conclusions
SHH protein is essential to maintain CN architecture. SHH treatment of the CN promoted regeneration, suppressed penile apoptosis and caused a 58% improvement in erectile function in less than half the time reported in the literature. These studies show that SHH treatment via aligned peptide amphiphile nanofibers has substantial clinical application to regenerate the CN in prostatectomy and diabetic patients where SHH is decreased, that this methodology has broad application to regenerate any peripheral nerve that SHH is necessary for maintenance of its structure, and that this nanotechnology method of protein delivery may have wide spread application as an in vivo delivery tool in many organs.
Acknowledgments
Grant Sponsor: National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, Grant numbers: DK068507 and DK079184.
Footnotes
Summary sentence: SHH plays a significant role in peripheral nerve regeneration and has clinical potential to be used as a regenerative therapy for the CN in prostatectomy patients and in other patients with neuropathy of peripheral nerves.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Montorsi P, Ravagnani PM, Galli S, Salonia A, Briganti A, Werba JP, et al. Association between erectile dysfunction and coronary artery disease: matching the right target with the right test in the right patient. Eur Urol. 2006;50:721–731. doi: 10.1016/j.eururo.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 2.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;169:1175–1179. doi: 10.1097/01.ju.0000048974.47461.50. [DOI] [PubMed] [Google Scholar]
- 3.Kendirci M, Hellstrom WJ. Current concepts in the management of erectile dysfunction in men with prostate cancer. Clin Prostate Cancer. 2004;3:87–92. doi: 10.3816/cgc.2004.n.017. [DOI] [PubMed] [Google Scholar]
- 4.Perimenis P, Markou S, Gyftopoulos K, Athanasopoulos A, Giannitsas K, Barbalias G. Switching from long-term treatment with self-injections to oral sildenafil in diabetic patients with severe erectile dysfunction. Eur Urol. 2002;41:387–391. doi: 10.1016/s0302-2838(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SS, Yoo JJ, Abouheba M, Soker S, McDougal WS, Atala A. Cavernous nerve regeneration using acellular nerve grafts. World J Urol. 2008;26:333–339. doi: 10.1007/s00345-008-0283-y. [DOI] [PubMed] [Google Scholar]
- 6.May F, Weidner N, Matiasek K, Caspers C, Mrva T, Vroemen M, et al. Schwann cell seeded guidance tubes restore erectile function after ablation of cavernous nerves in rats. J Urol. 2004;172:374–377. doi: 10.1097/01.ju.0000132357.05513.5f. [DOI] [PubMed] [Google Scholar]
- 7.May F, Matiasek K, Vroemen M, Caspers C, Mrva T, Arndt C, et al. GDNF-transduced schwann cell grafts enhance regeneration of erectile nerves. Eur Urol. 2008;54:1179–1187. doi: 10.1016/j.eururo.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Matsuura S, Obara T, Suchiya N, Suzuki Y, Habuchi T. Cavernous nerve regeneration by biodegradable alginate gel sponge sheet placement without sutures. Urology. 2006;68:1366–1371. doi: 10.1016/j.urology.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 9.Jung GW, Spencer EM, Lue TF. Growth hormone enhances regeneration of nitric oxide synthase-containing penile nerves after cavernous nerve neurotomy in rats. J Urol. 1998;160:1899–1904. [PubMed] [Google Scholar]
- 10.Lin CS, Lue TF. Growth factor therapy and neuronal nitric oxide synthase. Int J Impot Res. 2004;16 (Suppl 1):S38–39. doi: 10.1038/sj.ijir.3901214. [DOI] [PubMed] [Google Scholar]
- 11.Allaf ME, Hoke A, Burnett AL. Erythropoietin promotes the recovery of erectile function following cavernous nerve injury. J Urol. 2005;174:2060–2064. doi: 10.1097/01.ju.0000176808.94610.dd. [DOI] [PubMed] [Google Scholar]
- 12.Kato R, Wolfe D, Coyle CH, Wechuck JB, Tyagi P, Tsukamoto T, et al. Herpes simplex virus vector-mediated delivery of neurturin rescues erectile dysfunction of cavernous nerve injury. Gene Thr. 2009;16:26–33. doi: 10.1038/gt.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepinsky RB, Shapiro RI, Wang S, Chakraborty A, Gill A, Lepage DJ, et al. Long-acting forms of sonic hedgehog with improved pharmacokinetic and pharmacodynamic properties are effacious in a nerve injury model. J Pharm Sci. 2002;91:371–387. doi: 10.1002/jps.10052. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto M, Ishii K, Nakamura Y, Watabe K, Kohsaka S, Akazawa C. Neuroprotective effect of sonic hedgehog up-regulated in schwann cells following sciatic nerve injury. J Neurochem. 2008;107:918–927. doi: 10.1111/j.1471-4159.2008.05666.x. [DOI] [PubMed] [Google Scholar]
- 15.Akazawa C, Tsuzuki H, Nakamura Y, Sasaki Y, Ohsaki K, Nakamura S, et al. The upregulated expression of sonic hedgehog in motor neurons after rat facial nerve axotomy. J Neurosci. 2004;24:7923–7930. doi: 10.1523/JNEUROSCI.1784-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusano KF, Allendoerfer KL, Munger W, Pola R, Bosch-Marce M, Kirchmair R, et al. Sonic hedgehog induces arteriogenesis in diabetic vasa nervorum and restores function in diabetic neuropathy. Anterscler Thromb Vasc Biol. 2004;24:2102–2107. doi: 10.1161/01.ATV.0000144813.44650.75. [DOI] [PubMed] [Google Scholar]
- 17.Luo JD, Hu TP, Wang L, Chen MS, Liu SM, Chen AF. Sonic hedgehog improves delayed wound healing via enhancing cutaneous nitric oxide function in diabetes. Am J Physiol Endocrinol Metab. 2009;297:E525–E531. doi: 10.1152/ajpendo.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podlasek CA, Zelner DJ, Jiang HB, Tang Y, Houston J, McKenna KE, et al. Sonic hedgehog 45 cascade is required for penile postnatal morphogenesis, differentiation, and adult homeostasis. Biol Reprod. 2003;68:423–438. doi: 10.1095/biolreprod.102.006643. [DOI] [PubMed] [Google Scholar]
- 19.Podlasek CA, Zelner DJ, Harris JD, Meroz CL, Tang Y, McKenna KE, et al. Altered sonic hedgehog signaling is associated with morphological abnormalities in the penis of the BB/WOR diabetic rat. Biol Reprod. 2003;69:816–827. doi: 10.1095/biolreprod.102.013508. [DOI] [PubMed] [Google Scholar]
- 20.Podlasek CA, Meroz CL, Tang Y, McKenna KE, McVary KT. Regulation of cavernous nerve injury-induced apoptosis by sonic hedgehog. Biol Reprod. 2007;76:19–28. doi: 10.1095/biolreprod.106.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bond C, Tang Y, Podlasek CA. Neural influences on sonic hedgehog and apoptosis in the rat penis. Biol Reprod. 2008;78:947–956. doi: 10.1095/biolreprod.107.064766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angeloni NL, Bond CW, Monsivais D, Tang Y, Podlasek CA. Hedgehog-interacting protein in maintaining cavernous nerve integrity and adult penile morphology. J Sexual Medicine. 2009;6:2480–2493. doi: 10.1111/j.1743-6109.2009.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, Greenfield MA, Mata A, Palmer LC, Bitton R, Mantei JR, et al. A self-assembly pathway to aligned monodomain gels. Nat Mater. 2010 Jun 13; doi: 10.1038/nmat2778. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullerad M, Donohue JF, Li PS, Scardino PT, Mulhall JP. Functional sequelae of cavernous nerve injury in the rat: is there model dependency. J Sex Med. 2006;3:77–8342. doi: 10.1111/j.1743-6109.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 25.Nangle MR, Keast JR. Reduced efficacy of nitrergic neurotransmission exacerbates erectile dysfunction after penile nerve injury despite axonal regeneration. Exp Neurol. 2007;207:30–41. doi: 10.1016/j.expneurol.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 27.Tang Y, Rampkin O, Calas A, Facchinetti P, Giuliano F. Oxytocinergic and serotonergic innervation of identified lumbosacral nuclei controlling penile erection in the male rat. Neuroscience. 1998;82:241–254. doi: 10.1016/s0306-4522(97)00290-x. [DOI] [PubMed] [Google Scholar]
- 28.Rasband WS. Image J. Bethesda, MD: U.S. National Institutes of Health; 1997–2007. World Wide Web ( http://rsb.info.nih.gov/ij/) [Google Scholar]
- 29.Giuliano F, Rampin O, Bernabé J, Rousseau J-P. Neural control of penile erection in the rat. J Auton Nerv Syst. 1995;55:36–44. doi: 10.1016/0165-1838(95)00025-s. [DOI] [PubMed] [Google Scholar]
- 30.Podlasek CA, Zelner D, Bervig TR, Gonzalez CM, McKenna KE, McVary KT. Characterization and localization of NOS isoforms in BB/WOR diabetic rat. J Urology. 2001;166:746–755. [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and 75 the 2−ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Bambakidis NC, Wang RZ, Franic L, Miller RH. Sonic hedgehog-induced neural precursor proliferation after adult rodent spinal cord injury. J Neurosurg. 2003;99:70–75. doi: 10.3171/spi.2003.99.1.0070. [DOI] [PubMed] [Google Scholar]
- 33.Schaumburg HH, Zotova E, Cannella B, Raine CS, Arezzo J, Tar M, Melman A. Structural and functional investigations of the murine cavernosal nerve: a model system for serial spatio-temporal study of autonomic neuropathy. BJU International. 2007;99:916–924. doi: 10.1111/j.1464-410X.2006.06726.x. [DOI] [PubMed] [Google Scholar]
- 34.Cotran RS, Kumar V, Collins T. Robbins pathologic basis of disease. Philadelphia, PA: W.B. Saunders Company; 1999. p. 1274. [Google Scholar]
- 35.Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 36.Podlasek CA, Gonzalez GM, Zelner DJ, Jiang HB, McKenna KE, Mcvary KT. Analysis of NOS isoform changes in a post radical prostatectomy model of erectile dysfunction. International Journal of Impotence Research. 2001;13(Suppl 5):S1–S15. doi: 10.1038/sj.ijir.3900772. [DOI] [PubMed] [Google Scholar]
- 37.Rajangam K, Behanna HA, Hui MJ, Han X, Hulvat JF, Lomasney JW, et al. Heparin binding nanostructures to promote growth of blood vessels. Nanoletters. 2006;6:2086–2090. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 38.Capito RM, Azevedo HS, Velichko YS, Mata A, Stupp SI. Self-assembly of large and small molecules into hierarchically ordered sacs and membranes. Science. 2008;319 (5871):1812–1816. doi: 10.1126/science.1154586. [DOI] [PubMed] [Google Scholar]
- 39.Tysseling-Mattiace VM, Shni V, Niece KL, Birch D, Czeisler C, Fehlings MG, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neuroscience. 2008;28:3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah RN, Shah NA, Del Rosario Lim MM, Hsieh C, Nuber G, Stupp SI. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc Natl Acad Sci USA. 2010;107 (8):3293–3298. doi: 10.1073/pnas.0906501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 42.Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci USA. 2002;99:5133. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velichko YS, Stupp SI, de la Cruz MO. Molecular simulation study of peptide amphiphile self-assembly. Journal of Physical Chemistry B. 2008;112:2326–2334. doi: 10.1021/jp074420n. [DOI] [PubMed] [Google Scholar]
- 44.Pashuck ET, Cui HG, Stupp SI. Tuning supramolecular rigidity of peptide fibers through molecular structure. Journal of the American Chemical Society. 2010;132:6041–6046. 105. doi: 10.1021/ja908560n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guler MO, Hsu L, Soukasene S, Harrington DA, Hulvat JF, Stupp SI. Presentation of RGDS epitopes on self-assembled nanofibers of branched peptide amphiphiles. Biomacromolecules. 2006;7 (6):1855–1863. doi: 10.1021/bm060161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 47.Kolpak A, Zhang J, Bzo ZZ. Sonic hedgehog has a dual effect on the growth of retinal ganglion axons depending on its concentration. J Neurosci. 2005;25:3432–3441. doi: 10.1523/JNEUROSCI.4938-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhatheja K, Field J. Schwann cells: origins and role in axonal maintenance and regeneration. International Journal of Biochemistry & Cell Biology. 2006;38:1995–1999. doi: 10.1016/j.biocel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Parra LM, Zou Y. Sonic hedgehog induces response of commissural axons to semaphoring repulsion during midline crossing. Nature Neurosci. 2010;13:29–35. doi: 10.1038/nn.2457. [DOI] [PubMed] [Google Scholar]
- 50.Bella AJ, Lin G, Garcia MM, Tantiwongse K, Brant WO, Lin CS, et al. Upregulation of penile brain-derived neurotrophic factor (BDNF) and activation of the JAK/STAT signaling pathway in the major pelvic glanglion of the rat after cavernous nerve transcection. Eur Urol. 2007;52:574–580. doi: 10.1016/j.eururo.2006.10.043. [DOI] [PubMed] [Google Scholar]