Abstract
Solute transport in biological tissues is a fundamental process necessary for cell metabolism. In connective soft tissues, such as articular cartilage, cells are embedded within a dense extracellular matrix that hinders the transport of solutes. However, according to a recent theoretical study (Mauck et al., 2003, J. Biomech. Eng. 125, 602–614), the convective motion of a dynamically loaded porous solid matrix can also impart momentum to solutes, pumping them into the tissue and giving rise to concentrations which exceed those achived under passive diffusion alone. In this study, the theoretical predictions of this model are verified against experimental measurements. The mechanical and transport properties of an agarose–dextran model system were characterized from independent measurements and substituted into the theory to predict solute uptake or desorption under dynamic mechanical loading for various agarose concentrations and dextran molecular weights, as well as different boundary and initial conditions. In every tested case, agreement was observed between experiments and theoretical predictions as assessed by coefficients of determination ranging from R2=0.61 to 0.95. These results provide strong support for the hypothesis that dynamic loading of a deformable porous tissue can produce active transport of solutes via a pumping mechanisms mediated by momentum exchange between the solute and solid matrix.
Keywords: Solute transport, Agarose, Dextran, Dynamic loading
1. Introduction
Solute transport in biological tissues is a fundamental process necessary for cell metabolism. Solute transport in a fluid occurs by diffusion, in the presence of a solute concentration gradient, and by convection, as a result of the motion of the solvent, typically under the action of a pressure gradient (Crank, 1979). Both of these transport mechanisms are modulated by frictional interactions between the solute and the surrounding solvent. In diffusion, solutes are hindered by friction against the solvent molecules; in convection, solvent molecules drag solutes along with their motion. Diffusion is generally modeled using Fickian theory (Fick, 1855), where the frictional interaction between the solute and solvent is represented by the diffusion coefficient.
In connective soft tissues, such as articular cartilage, cells are embedded within a dense extracellular matrix that hinders the transport of solutes. This hindrance results from additional frictional interactions between the solute and the solid matrix (Deen, 1987). This is a well-recognized mechanism, generally modeled with Fickian theory using a solute diffusion coefficient smaller than that in free solution (Deen, 1987; Johnson et al., 1995, 1996; Kosto and Deen, 2005). Arguably the most common application that takes advantage of this hindrance mechanism against a porous solid matrix is gel electrophoresis, where charged proteins or DNA fragments can be separated according to their molecular weight, which relates inversely to their diffusivity.
In addition to hindering solute diffusion, the convective motion of a porous solid matrix can similarly impart momentum to solutes (Ateshian et al., 2006; Mauck et al., 2003), in analogy to convective mechanisms occurring between solvent and solute. This convective momentum transfer between solid matrix and solute may be particularly significant in connective soft tissues that undergo significant deformations under cyclical loading conditions, such as articular cartilage. Though the influence of solid matrix hindrance to solute diffusion has long been recognized (Ferry, 1936), the effect of convective solute transport by cyclical solid matrix deformation remains a current topic of investigation.
Past experimental and theoretical studies have investigated the role of dynamic mechanical loading on nutrient uptake in biological systems, and release kinetics in drug delivery devices (Evans and Quinn, 2006; Lee et al., 2000; O'Hara et al., 1990; Urciuolo et al., 2008; Wang et al., 2000). These investigations have shown that dynamic loading can increase the rate of uptake or desorption of solutes in biological tissues or hydrogels and this response has been typically attributed to oscillatory fluid convection induced by dynamic loading.
In a theoretical study by Mauck et al. (2003), the additional momentum transfer between solid matrix and solute was explicitly modeled to investigate how it might influence solute uptake in dynamically loaded soft tissues and gels, in comparison to modeling only the effects of momentum transfer between solvent and solute. The theoretical predictions from that study challenged the conventional view by predicting that solid–solute momentum transfer could pump the solute into the tissue to achieve concentrations far exceeding the passive equilibrium concentration. The theory suggested that this pumping mechanism would be effective only for certain combinations of mechanical and transport properties of the tissue–solute system, which could be summarized by non-dimensional numbers whose range determined the effectiveness of this pumping mechanism.
Since Mauck's theoretical study posited predictions not yet observed, experimental studies were conducted using an agarose gel–dextran solute model system that would allow varying mechanical and transport properties (using different gel concentrations and solute molecular weights) without altering the underlying molecular composition of the system (Albro et al., 2008). Though agarose and dextran are not biological materials, the purpose of those experimental studies was to investigate the validity of a theoretical framework that is highly relevant to biological transport in connective soft tissues, in analogy to prior studies using similar model systems (Johnson et al., 1995, 1996; Laurent and Killander, 1964; Lazzara and Deen, 2004; Leddy and Guilak, 2003, 2008; Leddy et al., 2006; Pluen et al., 1999). Furthermore, since agarose hydrogels have been used extensively in cartilage cell culture and tissue-engineering studies (Buschmann et al., 1995, 1992; Leddy et al., 2004; Mauck et al., 2000; O'Driscoll et al., 1997; Sittinger et al., 1999), where solute transport plays a critical role, the results of such a model system could be directly applicable to the investigation of methods for enhancing nutrient supply in tissue-engineering scaffolds. While dextran is a linear molecule, unlike globular proteins, it has been shown previously to diffuse in agarose according to standard Fickian behavior (Albro et al., 2009; Chahine et al., 2009; Leddy and Guilak, 2003).
These experimental studies (Albro et al., 2008) demonstrated that dynamic loading of hydrogels did in fact enhance the uptake of dextran molecules to concentrations that far exceeded those achieved under passive diffusion. Upon termination of loading, the solute concentration in the hydrogels reverted to the equilibrium values achieved under passive diffusion, confirming that the observed uptake was solely due to dynamic loading. These results were qualitatively consistent with the theoretical predictions of Mauck et al. (2003), and could be explained by the mechanism of convective momentum transfer between the solid matrix and the solute.
The objective of this study was to investigate whether the theoretical model of Mauck et al. (2003) is in quantitative agreement with the earlier experimental results of Albro et al. (2008), as well as additional experimental results reported here. This model validation was performed by measuring the mechanical and transport properties of the agarose–dextran system, for various combinations of gel concentrations and solute molecular weights. These measured properties were then substituted into the governing equations for dynamic loading of a cylindrical gel, and the predicted responses were compared to the experimental results. The purpose of this validation was to provide further support for the hypothesis that enhanced solute uptake during dynamic loading results from momentum transfer between the solute and solid matrix, and increase our confidence that the theoretical framework of Mauck et al. (2003) may be applied to other geometries and initial and boundary conditions of interest for solute transport in tissues and tissue constructs.
2. Methods
This study examined the experimental and theoretical responses of the average concentration of dextran in a cylindrical disk of agarose subjected to cyclical loading. To investigate the quantitative predictive ability of the theoretical framework over a broad range of conditions, the following were varied: initial conditions for solute concentration inside the gel; boundary conditions for solute concentration outside the gel; agarose concentration; and dextran molecular weight. The specific combinations are summarized in Table 1. Some of the experimental results for these various combinations were reported in our previous study (Albro et al., 2008), as indicated in the table. The remaining experimental results represent new measurements obtained for the current study, using the same methods as before.
Table 1.
List of dextran solute transport tests performed on agarose disks, describing agarose gel concentration (agarose), dextran molecular weight (dextran), solute initial condition [c*(0) as per Eq. (9)] and boundary condition [c*(t) for t > 0, as per Eq. (7)] ( = 7 and 50μM for 70 and 10 kDa dextran, respectively). All tests included a dynamic loading (DL) group, and a passive diffusion (PD) group. The last column displays the coefficient of determination for the correlation between experimental measurements and theoretical predictions (DL case, unless specified).
| Test no. | Agarose (%) | Dextran (kDa) | c*(0) | c*(t) t > 0 | Comments | Study | R2 | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 70 | 0 |
|
Uptake (no pre-incubation) | Albro et al. (2008) | 0.61 | ||
| 2 | 7 | 70 | 0 |
|
Uptake (no pre-incubation) | Albro et al. (2008) | 0.74 | ||
| 3 | 7 | 10 | 0 |
|
Uptake (no pre-incubation) | Albro et al. (2008) | 0.80 | ||
| 4 | 9 | 70 | 0 |
|
Uptake (no pre-incubation) | Albro et al. (2008) | 0.74 | ||
| 5 | 7 | 70 |
|
|
24 h pre-incubation, uptake | Current | 0.67 | ||
| 6 | 7 | 70 |
|
0 | 24 h pre-incubation, desorption | Current | 0.95 (PD) 0.92 (DL) |
The theoretical predictions were obtained by first measuring the mechanical and transport properties of the dextran and agarose gels, then substituting these values into the theory, and comparing the predicted average solute concentration in the gel to the experimental results. The measurement of these mechanical and transport properties was performed in the current study, as summarized below.
A summary of the governing equations from the prior theoretical framework (Mauck et al., 2003) is also provided to identify the relevant material properties needed for this problem.
2.1. Theoretical analysis
The general equations for the mixture theory framework employed here were formulated succinctly in a recent study (Ateshian et al., 2006). When applied to the problem of unconfined compression of a homogeneous disk of material, with only one neutral solute species in an uncharged gel, loaded axially under displacement control using rigid impermeable platens, these reduce to a coupled system of nonlinear partial differential equations,
| (1) |
| (2) |
where
| (3) |
subject to the boundary conditions
| (4) |
| (5) |
| (6) |
| (7) |
and initial conditions
| (8) |
| (9) |
In these expressions, t is the time variable, r represents the radial coordinate in a cylindrical coordinate system and r0 is the disk radius; c is the solute concentration and ur is the radial component of the solid matrix displacement; D0 is the solute diffusivity in free solution (which accounts for frictional interactions between solvent and solute), and D is its diffusivity in the mixture (including frictional interactions with the solid matrix); HA is the aggregate modulus of the solid matrix and v is its Poisson's ratio; k is the hydraulic permeability of the solvent through the porous solid matrix, and k̃ is the permeability of the solution through the matrix; κ is the partition coefficient for the solute in the mixture (0 ≤ κ ≤ 1), representing steric volume exclusion from pores that are too small; R is the universal gas constant and θ is the absolute temperature; c*(t) is the prescribed solute concentration history in the external bath; ε(t) is the prescribed axial strain history and ε̇ (t) is its time-derivative.
To predict the solute concentration c(r,t) inside the disk from theory, the following material coefficients needed to be determined from experiments: D0, D, HA, v, k and κ. Since agarose has been shown to exhibit a significantly higher modulus in tension than compression (Huang et al., 2008), and since the temporal response for solvent flux in unconfined compression is regulated by a time constant dependent on the tensile modulus (Soltz and Ateshian, 2000), the value of HA used in the above equations was obtained from tensile testing.
To better understand how Eq. (2) represents a generalization of the classical diffusion–convection equation, it may be noted that it was derived from the equation of conservation of mass for the solute [Eq. (14) in Ateshian et al. (2006), with ϕw assumed constant under small strains],
| (10) |
where vw and vs represent the solvent and solid velocities, respectively. When frictional interactions between the solute and the solid are neglected, D reduces to D0 and Eq. (10) produces the classical diffusion–convection equation. Thus, the contribution to the convective term that represents solute–solid interactions becomes evident.
2.2. Materials and experimental methods
The detailed description of materials used in this study, and methods for measuring the necessary material properties, are described in Appendix A: Supplementary data. In summary, type VII hydroxyethyl agarose was prepared at nominal concentrations of 6%, 7%, and 9% w/v. The diffusivity of tracer solutions of 70 and 10 kDa fluorescein-conjugated dextran in phosphate buffered saline (PBS) was measured using fluorescence recovery after photo bleaching (FRAP), in free solution, D0, as well as in agarose hydrogels, D. The aggregate modulus, HA, and Poisson's ratio, v, were measured in a tensile testing device using optical measurements of the axial and lateral strains. The permeability of agarose hydrogels to PBS, k, was measured in a permeation device. The equilibrium partition coefficient of dextran in agarose, κ, and all solute concentration measurements in general, were obtained with a fluorescent plate reader.
3. Results
3.1. Material properties
Experimental measurements demonstrated that agarose gels subjected to dynamic loading exhibited a dextran diffusivity that depended on the loading history. With increasing loading duration (0–48 h), the diffusivity D decreased markedly (dynamically loaded or DL range in Fig. 1). Once loading was terminated, the diffusivity no longer varied with time when gels were further incubated under free swelling conditions, as verified from diffusivity measurements at 100 and 200 h (p>0.8), in 7% agarose disks, using 70 kDa dextran (FS range in Fig. 1). This dependence on loading history varied with agarose concentration and dextran molecular weight (Fig. 2). For most cases, the average diffusivity in the gel decreased exponentially with increasing loading duration; in the case of 70 kDa dextran in 6% agarose, the diffusivity exhibited a linear decrease (Fig. 2).
Fig. 1.
Experimentally measured diffusivity of 70 kDa dextran in 7% agarose disks subjected to an initial 48 h of dynamic loading (DL), followed by a free swelling (FS) period of 152 h. Measurements were performed using FRAP, on disks collected at specific time points over the DL or FS periods. The FS period demonstrated that no further significant decrease in diffusivity occurred after loading was terminated (p>0.8 when comparing values at t=100 and 200 h against t=48 h).
Fig. 2.
Experimentally measured diffusivity of (A) 70 kDa and (B) 10 kDa dextran in agarose disks subjected to dynamic loading. Dashed curves represent exponential and linear fits to the experimental data, described in Table 2.
To account for this dependence on loading history, either an exponential or a linear function was best fit to the experimentally observed temporal response of D (Fig. 2) with the corresponding equations reported in Table 2. Unloaded (free swelling or FS) gels did not exhibit a variation in D over time (results not shown). The values of D for FS gels can be recovered from the formulas in Table 2, when evaluated at t=0.
Table 2.
Dextran diffusivity and partition coefficient, in agarose gels of various concentrations and in free solution (0% agarose, where κ=1 by definition). The functional dependence D(t) on loading history was obtained from experimental measurements performed at various time points following dynamic loading (Fig. 2). These properties were used in theoretical predictions corresponding to the tests listed in the last column.
| Agarose (%) | Dextran (kDa) | D(×10−12m2 /s) | κ | Tests |
|---|---|---|---|---|
| 0 | 70 | 37.2±2.7 | 1.0 | 1,2,4–6 |
| 6 | 70 | 6.5−2.1 × 10−5t | 0.36±0.03 | 1 |
| 7 | 70 | 4 6e−t/49072 + 1 5 | 0.21±0.02 | 2 |
| 7 (24 h) | 70 | 1.9e−t/66564+3.8 | 0.17±0.01 | 5,6 |
| 9 | 70 | 3 1e−t/21713 +1.2 | 0.08±0.01 | 4 |
| 0 | 10 | 126.8 ± 1.5 | 1.0 | 3 |
| 7 | 10 | 9.9e−t/39582 + 37.2 | 0.67±0.06 | 3 |
The effect of loading history on the diffusivity was also observed to depend on the agarose gelation time prior to initiation of loading. For 70 kDa dextran in 7% agarose, initiating the loading immediately after casting decreased the diffusivity by 75% after 48 h of loading; in contrast, when loading was initiated 24 h after casting, the diffusivity only decreased 30% (Fig. 2).
The dextran diffusivities in free solution are also reported in Table 2, and were observed to be significantly greater than in agarose, implying significant hindrance by the gels (Pluen et al., 1999). These reported values are consistent with prior literature findings (Lebrun and Junter, 1993; Perry et al., 2006).
The partition coefficient κ of dextran in agarose was measured from the equilibrium response of FS gels (Table 2). κ was observed to decrease with increasing agarose concentration and dextran molecular weight.
Measurements of the tensile Young's modulus EY, Poisson's ratio v, and hydraulic permeability k did not exhibit a dependence on loading history. Corresponding values are reported in Table 3, showing that EY and v increased, whereas k decreased, with increasing agarose concentration. Furthermore, no significant difference was found between the tensile modulus of freshly cast agarose, or agarose tested 24 h after casting (p>0.35). Values of EY and k were consistent with prior literature reports (Gu et al., 2003; Huang et al., 2008; Kosto and Deen, 2005). HA was calculated from EY and v as described in the caption for Table 3.
Table 3.
Agarose gel properties, for various gel concentrations. For 7% gels, EY and v were measured in freshly cast gels as well as 24 h after casting. HA was calculated from HA =(1−v)EY / (1 + v)(1 − 2v). These properties were used in theoretical predictions corresponding to the tests listed in the last column.
| Agarose (%) | φw | EY (kPa) | v | k(× 10−15 m4/N s) | Tests |
|---|---|---|---|---|---|
| 6 | 0.934±0.006 | 166±28 | 0.20±0.14 | 10.3±2.9 | 1 |
| 7 | 0.918±0.004 | 212±11 | 0.31±0.09 | 6.7±1.1 | 2,3 |
| 7 (24 h) | 0.918±0.004 | 236±48 | 0.11±0.09 | 6.7±1.1 | 5,6 |
| 9 | 0.902±0.005 | 428±125 | 0.42±0.02 | 3.6±0.8 | 4 |
3.2. Solute concentration in loaded disks
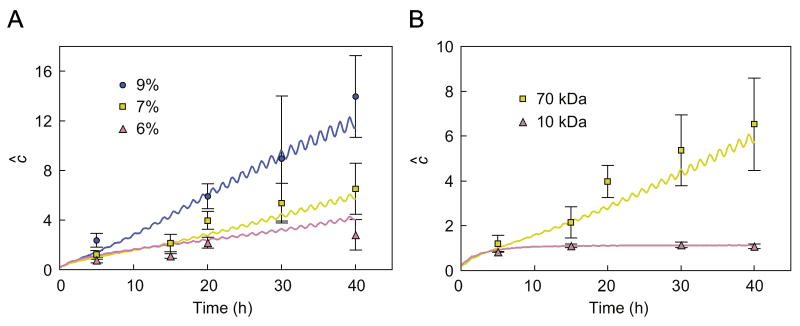
Experimental measurements of the average dextran concentration in agarose disks under dynamic loading (DL) and passive diffusion (PD) were normalized to the dextran bath concentration . For PD tests, the equilibrium value of the normalized average concentration represented the partition coefficient κ (Table 2). In the case of desorption studies, where the external bath concentration was zero (Test 6 in Table 1), the concentration in the disk was normalized to the dextran concentration in the prior incubation bath. To illustrate the potential enhancement of DL conditions over PD, results for DL were plotted as the ratio , where cavg is the average concentration in the disks (Figs. 3–5). Experimental results from the prior study (Albro et al., 2008) appear in Fig. 3 (Tests 1–4), and those from the current study appear in Fig. 4 (Test 5) and Fig. 5 (Test 6).
Fig. 3.
Enhancement of solute uptake in agarose disks subjected to dynamic loading. The experimentally measured ratio is given for (A) 70 kDa dextran and three agarose gel concentrations (Tests 1, 2 and 4, Table 1); and (B) 7% agarose and two dextran molecular weights (Tests 2 and 3, Table 1). Solid curves represent numerical predictions based on the theoretical framework. Experimental data are from an earlier study (Albro et al., 2008), except for the last time point of 70 kDa dextran in 6% agarose.
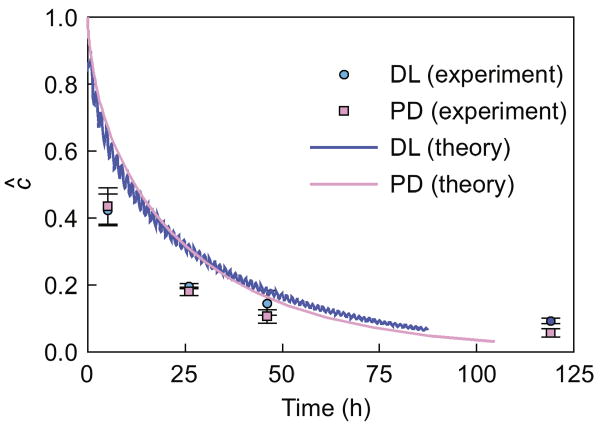
Fig. 5.
Desorption response of 70 kDa dextran out of 7% agarose disks under static (PD) and dynamic (DL) loading (Test 6, Table 1). Solid curves represent numerical predictions based on the theoretical framework.
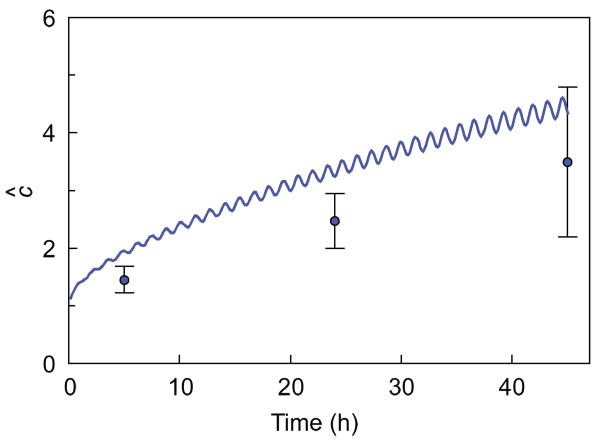
Fig. 4.
Enhancement ratio of 70 kDa dextran in 7% agarose disks subjected to dynamic loading, following a 24-h pre-incubation period in 70 kDa dextran solution (Test 5, Table 1). The solid curve represents the numerical prediction based on the theoretical framework.
In Test 5, the normalized concentration at the end of the initial 24 h pre-incubation period was ĉ = 0.16 ± 0.01; this value was not statistically different (p > 0.9) from the corresponding partition coefficient κ measured in the PD group after an additional 45 h incubation (Table 2), indicating that fully equilibrated conditions were achieved at the end of pre-incubation. Dynamic loading significantly increased the concentration of 70 kDa dextran in these gels relative to passive diffusion at all measured time points (p<0.001, Fig. 4). The corresponding enhancement ratio following 45 h of dynamic loading, ĉ = 3.5 ± 1.3, was significantly lower (p < 0.01) than the enhancement ratio in dynamically loaded freshly cast 7% agarose with no pre-incubation ĉ = 6.5 ± 2.1, Test 2).
In Test 6, after 24 h of pre-incubation in 70 kDa dextran, 7% agarose disks exhibited a normalized equilibrium concentration of ĉ =0.18 ± 0.004. Upon replacement of the bath solution with dextran-free PBS, the concentration in these disks decreased, reaching ĉ = 0.017 ± 0.001 under dynamic loading, and ĉ = 0.010 ± 0.002 under static loading, after 125 h (Fig. 5). No statistical difference between static and dynamic loading was observed at any time point (p > 0.45).
3.3. Theoretical predictions
Theoretical predictions were obtained by substituting the material and transport properties of Tables 2 and 3 into the governing Eqs. (1)–(9). The average concentration cavg in the disk was evaluated by suitably integrating c(r,t) over the range of r and the resulting values were normalized to to produce ĉ(t) for DL and PD cases. The resulting enhancement ratio under dynamic loading is presented with the corresponding experimental results (Figs. 3–5). A correlation analysis between theory and experimental results produced coefficients of determination R2 summarized in Table 1.
4. Discussion
The results of this study served as a direct experimental validation of the theoretical framework of Mauck et al. (2003) for solute transport in deformable porous media, which first suggested that dynamic loading can actively pump solutes into the porous medium, yielding concentrations beyond the physical limit under passive diffusion (Albro et al., 2008). Using independent experimental measurements of the mechanical and transport properties of agarose hydrogels and dextran polysaccharides, the theory was able to faithfully and consistently predict the enhanced solute uptake experienced by this transport system under dynamic loading. These predictions proved accurate for a wide range of agarose concentrations and dextran molecular weights, successfully accounting for scenarios of modest enhancement, with ĉ = 1.2, as well as scenarios of more considerable enhancement, with ĉ = 14.1 (Fig. 3). Predictions were also accurate for different sets of initial and boundary conditions, producing loading-enhanced uptake with (Fig. 3) or without (Fig. 4) pre-incubation of the tissue in the solute bath, and demonstrating no loading-enhanced desorption (Fig. 5).
This successful agreement was achieved without any adjustments to the theory or measured material properties, by direct substitution of experimentally acquired properties into the governing equations, and prediction of solute uptake and desorption measurements from independent experiments. Considering the number of idealizations adopted in the theory (frictionless platens, strain-independent modulus and permeability for agarose, ideal solution behavior of dextran in physiologically buffered saline), the observed agreement attested by the near-unity coefficients of determination (Table 1) provided strong support for the hypothesis that the observed enhancements with dynamic loading resulted from the momentum exchange (the pumping mechanism) between solid and solute embodied in the theoretical framework. In contrast, using the classical diffusion–convection equation (obtained from the above relations by letting D=D0) could not predict any enhanced solute uptake, as described in the earlier theoretical study (Mauck et al., 2003).
In the experimental findings of Fig. 4, disks were pre-incubated in the dextran solutions for 24 h to ensure that uptake by passive diffusion had equilibrated prior to loading. Thus, any subsequent increase in uptake observed after initiation of dynamic loading could be attributed exclusively to the effect of loading. These novel results complement our earlier experimental findings (Albro et al., 2008) (shown in Fig. 3) where disks were loaded in a dextran bath without pre-incubation.
In the experimental findings of Fig. 5, boundary conditions were modified to examine the effect of dynamic loading on solute desorption instead. It was found that dynamic loading of 7% agarose had no significant effect on the desorption of 70 kDa dextran from the gel. This result was confirmed in theoretical simulations that either accounted for the effect of loading history on solute diffusivity, or employed a constant value (Fig. 5). Considering that the agarose–dextran combination employed in this desorption test was able to induce a substantial 3.5-fold concentration enhancement under uptake conditions (Fig. 4), it became evident that both theory and experiment demonstrated asymmetric responses to dynamic loading for solute uptake and desorption: uptake from an external solute bath was enhanced by dynamic loading, but solute desorption from the disk was not affected. These experimental and theoretical results strongly suggest that dynamic loading plays no significant role in promoting the desorption of solutes. From a physiological perspective, this outcome suggests that desorption of endogenous soluble factors (such as growth factors or fragments of matrix products) will not be enhanced by mechanical loading. However, these findings differ from earlier literature reports that dynamic loading enhances the rate of desorption of solutes from a gel or tissue, relative to statically loaded controls (Evans and Quinn, 2006; Lee et al., 2000; O'Hara et al., 1990). These differences might be attributable to variations in initial and boundary conditions, loading conditions, or tissue–solute combinations. Therefore, future investigations may be needed to resolve these differences.
An unanticipated experimental observation of this study was that the diffusivity of large dextran molecules in dense agarose gels was affected by the loading history (Figs. 1 and 2). While many past experimental studies have used hydrogels to investigate various transport phenomena (Kosto and Deen, 2005; Lebrun and Junter, 1993; Pluen et al., 1999), none had to consider the possibility of microstructural alterations under dynamic loading, since they were performed under unloaded conditions. This dependence of diffusivity on loading history was unrelated to the theoretical framework of Mauck et al. (2003); thus it played no particular role in explaining the momentum transfer between solid and solute. This interesting experimental observation (akin to strain-hardening in metals) had to be incorporated in the evaluation of the theoretical response to produce accurate predictions.
The main conclusion of this study is that the theoretical framework of Mauck et al. (2003) was supported directly from experiments. Whereas prior frameworks of solute transport in rigid or deformable porous media accounted for the increased hindrance to solute diffusion by the solid matrix (e.g., gel electrophoresis), and for convective transport from solute interactions with the solvent (classical diffusion–convection), the current framework also accounts for convective transport from solute interactions with the deforming solid, as shown explicitly in Eq. (10). As described in our earlier studies (Albro et al., 2008; Mauck et al., 2003) and illustrated in movie animations of the theoretical solution for the case corresponding to Test 5 (Table 1) in Appendix A: Supplementary data (Figs. S2 and S3), the downstroke portion of the sinusoidal loading profile ejects more solvent than solute at the disk periphery, thus producing a small spike in solute concentration there. During the upstroke, as the solid matrix recoils, it drags this solute spike inward due to the momentum exchange between the solute and solid. At the next downstroke, though the spike is similarly pushed toward the periphery, it gets reinforced by the same mechanism that initially produced it. Over time, this growing spike produces a concentration gradient that enhances the inward transport of solutes, leading to a net uptake from the external bath.
The potential implication of this study is that the mechanism of enhanced solute uptake under dynamic loading may be pervasive in biological tissues such as articular cartilage, intervertebral disc, skeletal and cardiac muscle, skin, lung, etc. The large uptake enhancements found in this study were based on continuous dynamic loading for a period up to 45 h, though considerable enhancement was also observed at earlier time points. In future studies, it would be interesting to investigate whether shorter loading durations repeated over multiple days, more representative of loading histories in connective tissues, also produce significant uptake enhancements. Therefore, additional experimental investigations are needed to explore the extent of this mechanism, and its potential biological implications, in these various systems.
Furthermore, in the field of tissue engineering, dynamic mechanical stimulation is currently used as a means of enhancing biosynthetic activity. Cell-seeded constructs for cartilage and tendon tissue have all shown greater matrix elaboration when subjected to such stimulation (Mauck et al., 2000; Shearn et al., 2007). While the beneficial effects of dynamic loading have been well characterized for a variety of tissue types, their underlying mechanisms are still unclear. The results from this study suggest that large nutrients and macromolecules can potentially transport actively in these dynamically loaded tissues, and that the current framework can be used to predict these phenomena reliably.
Supplementary Material
Acknowledgments
This study was supported with funds from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the US National Institutes of Health (AR46532).
Footnotes
Conflict of interest statement: The authors have no conflicts of interest with regard to this study and the materials contained herein.
Appendix A. Supplementary Data: Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jbiomech.2010.04.041.
References
- Albro MB, Chahine NO, Li R, Yeager K, Hung CT, Ateshian GA. Dynamic loading of deformable porous media can induce active solute transport. J Biomech. 2008;41:3152–3157. doi: 10.1016/j.jbiomech.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro MB, Rajan V, Li R, Hung CT, Ateshian GA. Characterization of the concentration-dependence of solute diffusivity and partitioning in a model dextran–agarose transport system. Cell Mol Bioeng. 2009;2:295–305. doi: 10.1007/s12195-009-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ateshian GA, Likhitpanichkul M, Hung CT. A mixture theory analysis for passive transport in osmotic loading of cells. J Biomech. 2006;39:464–475. doi: 10.1016/j.jbiomech.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108(Part 4):1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745–758. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- Chahine NO, Albro MB, Lima EG, Wei VI, Dubois CR, Hung CT, Ateshian GA. Effect of dynamic loading on the transport of solutes into agarose hydrogels. Biophys J. 2009;97:968–975. doi: 10.1016/j.bpj.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crank J. The Mathematics of Diffusion. Clarendon Press; Oxford, England: 1979. [Google Scholar]
- Deen WM. Hindered transport of large molecules in liquid-filled pores. AIChE Journal. 1987;33:1409–1425. [Google Scholar]
- Evans RC, Quinn TM. Solute convection in dynamically compressed cartilage. J Biomech. 2006;39:1048–1055. doi: 10.1016/j.jbiomech.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Ferry JD. Statistical evaluation of sieve constants in ultrafiltration. J Gen Physiol. 1936;20:95–104. doi: 10.1085/jgp.20.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick A. Über Diffusion. Ann Phys. 1855;94:59–86. [Google Scholar]
- Gu WY, Yao H, Huang CY, Cheung HS. New insight into deformation-dependent hydraulic permeability of gels and cartilage, and dynamic behavior of agarose gels in confined compression. J Biomech. 2003;36:593–598. doi: 10.1016/s0021-9290(02)00437-2. [DOI] [PubMed] [Google Scholar]
- Huang AH, Yeger-McKeever M, Stein A, Mauck RL. Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels. Osteoarthritis Cartilage. 2008;16:1074–1082. doi: 10.1016/j.joca.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EM, Berk DA, Jain RK, Deen WM. Diffusion and partitioning of proteins in charged agarose gels. Biophys J. 1995;68:1561–1568. doi: 10.1016/S0006-3495(95)80328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EM, Berk DA, Jain RK, Deen WM. Hindered diffusion in agarose gels: test of effective medium model. Biophys J. 1996;70:1017–1023. doi: 10.1016/S0006-3495(96)79645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosto KB, Deen WM. Hindered convection of macromolecules in hydrogels. Biophys J. 2005;88:277–286. doi: 10.1529/biophysj.104.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent TC, Killander J. A theory of gel filtration and its experimental verification. J Chromatogr. 1964;14:317–330. [Google Scholar]
- Lazzara MJ, Deen WM. Effects of concentration on the partitioning of macromolecule mixtures in agarose gels. J Colloid Interface Sci. 2004;272:288–297. doi: 10.1016/j.jcis.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Lebrun L, Junter GA. Diffusion of sucrose and dextran through agar gel membranes. Enzyme Microb Technol. 1993;15:1057–1062. doi: 10.1016/0141-0229(93)90054-6. [DOI] [PubMed] [Google Scholar]
- Leddy HA, Awad HA, Guilak F. Molecular diffusion in tissue-engineered cartilage constructs: effects of scaffold material, time, and culture conditions. J Biomed Mater Res B Appl Biomater. 2004;70:397–406. doi: 10.1002/jbm.b.30053. [DOI] [PubMed] [Google Scholar]
- Leddy HA, Guilak F. Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Ann Biomed Eng. 2003;31:753–760. doi: 10.1114/1.1581879. [DOI] [PubMed] [Google Scholar]
- Leddy HA, Guilak F. Site-specific effects of compression on macromolecular diffusion in articular cartilage. Biophys J. 2008;95:4890–4895. doi: 10.1529/biophysj.108.137752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leddy HA, Haider MA, Guilak F. Diffusional anisotropy in collagenous tissues: fluorescence imaging of continuous point photobleaching. Biophys J. 2006;91:311–316. doi: 10.1529/biophysj.105.075283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Hung CT, Ateshian GA. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J Biomech Eng. 2003;125:602–614. doi: 10.1115/1.1611512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- O'Driscoll SW, Fitzsimmons JS, Commisso CN. Role of oxygen tension during cartilage formation by periosteum. J Orthop Res. 1997;15:682–687. doi: 10.1002/jor.1100150509. [DOI] [PubMed] [Google Scholar]
- O'Hara BP, Urban JP, Maroudas A. Influence of cyclic loading on the nutrition of articular cartilage. Ann Rheum Dis. 1990;49:536–539. doi: 10.1136/ard.49.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry PA, Fitzgerald MA, Gilbert RG. Fluorescence recovery after photobleaching as a probe of diffusion in starch systems. Biomacromolecules. 2006;7:521–530. doi: 10.1021/bm0507711. [DOI] [PubMed] [Google Scholar]
- Pluen A, Netti PA, Jain RK, Berk DA. Diffusion of macromolecules in agarose gels: comparison of linear and globular configurations. Biophys J. 1999;77:542–552. doi: 10.1016/S0006-3495(99)76911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearn JT, Juncosa-Melvin N, Boivin GP, Galloway MT, Goodwin W, Gooch C, Dunn MG, Butler DL. Mechanical stimulation of tendon tissue engineered constructs: effects on construct stiffness, repair biomechanics, and their correlation. J Biomech Eng. 2007;129:848–854. doi: 10.1115/1.2800769. [DOI] [PubMed] [Google Scholar]
- Sittinger M, Perka C, Schultz O, Haupl T, Burmester GR. Joint cartilage regeneration by tissue engineering. Z Rheumatol. 1999;58:130–135. doi: 10.1007/s003930050162. [DOI] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. A conewise linear elasticity mixture model for the analysis of tension-compression nonlinearity in articular cartilage. J Biomech Eng. 2000;122:576–586. doi: 10.1115/1.1324669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urciuolo F, Imparato G, Netti PA. Effect of dynamic loading on solute transport in soft gels implication for drug delivery. AIChE J. 2008;54:824–834. [Google Scholar]
- Wang L, Cowin SC, Weinbaum S, Fritton SP. Modeling tracer transport in an osteon under cyclic loading. Ann Biomed Eng. 2000;28:1200–1209. doi: 10.1114/1.1317531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.