Abstract
Interleukin-33 (IL-33) is a novel member of the interleukin-1 family that induces mucosal pathology in vivo and may drive fibrosis development and angiogenesis. To address its potential role in inflammatory bowel disease, we explored its tissue expression in biopsy specimens from untreated ulcerative colitis patients, observing a 2.6-fold up-regulation of IL-33 mRNA levels, compared to controls. Immunohistochemical analyses of surgical specimens showed that a prominent source of IL-33 in ulcerative colitis lesions were ulceration-associated myofibroblasts that co-expressed the fibroblast marker heat shock protein 47, platelet-derived growth factor receptor (PDGFR)β, and, in part, the myofibroblast marker α-smooth muscle actin (SMA). In contrast, IL-33-positive myofibroblasts were almost absent near the deep fissures seen in Crohn’s disease. A screen of known and putative activators of IL-33 in cultured fibroblasts revealed that the Toll-like receptor-3 agonist poly (I:C) was among the strongest inducers of IL-33 and that it synergized with transforming growth factor-β, a combination also known to boost myofibroblast differentiation. Experimental wound healing in rat skin revealed that the de novo induction of IL-33 in pericytes and the possible activation of scattered, tissue-resident IL-33+PDGFRβ+αSMA− fibroblast-like cells were early events that preceded the later appearance of IL-33+PDGFRβ+αSMA+ cells. In conclusion, our data point to a novel role for IL-33 in mucosal healing and wound repair and to an interesting difference between ulcerative colitis and Crohn’s disease.
Ulcerative colitis (UC) and Crohn’s disease (CD) constitute the two major forms of inflammatory bowel disease (IBD) and have a substantial impact on quality of life in a large number of patients worldwide.1 The introduction of tumor necrosis factor (TNF)α blocking antibodies has been welcomed as an effective treatment option for these patients, but shows side effects that are not negligible.2,3 Moreover, there is a substantial number of nonresponders to anti-TNF treatment, underlining the current opinion that our understanding of the complex cytokine networks active in IBD is far from complete.4,5
Interleukin (IL)−33 (C9ORF26, NF-HEV, DVS27, and IL-1F11) is a novel member of the IL-1 family which also includes the pro-inflammatory cytokines IL-1α, IL-1β, and IL-18.6,7,8 IL-33 was initially associated with the development of T helper (Th)2 immunity, based on the expression of its receptor ST2L (IL-1R4) in polarized Th2 lymphocytes and its ability to induce the production of Th2-associated cytokines (IL-5 and IL-13) in vivo.9 On the other hand, IL-33 appears to exacerbate arthritis,10,11 generally considered to be a Th1/Th17 lesion. Indeed, ST2L mediates the action of IL-33 on several other leukocyte subsets (reviewed in (ref. no) 8, 12, 13), as well as tissue-resident cells.14,15
Despite a large body of evidence describing the effects of recombinant IL-33 and/or the modulation of its receptor, much less is known about the cellular sources and secretion modes of IL-33. Immunohistochemical analysis of healthy organs has revealed that IL-33 expression is restricted to the nuclei of vascular endothelial cells,16,17 a subset of epithelial cells and fibroblast-like cells in lymph nodes.17,18,19 In fact, nuclear IL-33 appears to act as a transcriptional repressor20 and to bind to nucleosomal proteins,21 but how its nuclear function affects cellular behavior remains unknown. Strikingly, while pro-inflammatory IL-1β and TNFα leads to a strong down-regulation of IL-33 in endothelial cells,16 these cytokines and also ligands to Toll-like receptor (TLR)2, TLR3, or TLR4, as well as mechanical strain, lead to up-regulation or de novo expression of IL-33 in smooth muscle cells, astrocytes, fibroblasts, or hepatic stellate cells.9,10,11,22,23,24 Accordingly, induction of nuclear IL-33 has been observed in inflamed synovium, in cardiac failure, and in liver fibrosis.11,22,24
Low levels of IL-33 have also been found in the supernatant of several cell types22,23,25,26 and it can be released from necrotic27 and damaged cells.28 On the other hand, the mechanisms that allow secretion of IL-33 from intact cells remain unclear (reviewed in 29). Nevertheless, use of recombinant, bioactive IL-33 shows some features of particular interest to the present study: first, daily injections of IL-33 in murine skin leads to the development of cutaneous fibrosis30 and second, IL-33 appears to stimulate angiogenesis.14
In addition to a need to more fully understand the cytokine network of the intestine, there are several good reasons to map the expression of IL-33 in mucosal inflammation. First, intraperitoneal administration of recombinant IL-33 induced inflammatory infiltrates in the esophagus, hypertrophy of intestinal goblet cells, and increased intestinal mucus.9 Second, exogenous IL-33 also facilitated the expulsion of intestinal Trichuris infection, apparently by inducing IL-4, IL-9, and IL-13 and preventing an inappropriate parasite-specific Th1-polarized response. Moreover, infection triggered elevated mRNA levels of IL-33 in cecal tissue.31 Finally, while CD is a transmural, granulomatous, inflammatory process that shows features of Th1/Th17 disease,4 UC is considered an atypical Th2 disease characterized by high levels of IL-1332 and shows the pathological features of a more superficial disease in which mucosal damage is an overriding factor. Thus, UC and CD would appear suitable to compare the nature of IL-33 expression in two polarized cytokine environments within the same organ.
Here, we argue that that a prominent feature of IBD-associated IL-33 expression is the accumulation of fibroblasts and myofibroblasts in ulcerations of UC lesions. Moreover, we observed that the strongest single stimulus to induce IL-33 expression was via TLR3, a sensor of viral double-stranded RNA but also of mRNA released from damaged cells33 and that TLR3 ligation synergized with TGFβ to boost the expression of IL-33. Finally, we took advantage of a model of experimental wound healing to discover that pericytes were among the early cell populations to express nuclear IL-33 de novo.
Materials and Methods
Patient Specimens
A total of 41 IBD and 28 non-inflamed control (non-IBD) patients were included in the present study according to protocols approved by the Regional Committees for Research Ethics and the Norwegian Social Science Data Services. Endoscopic biopsy specimens from untreated UC patients (n = 25) and controls (n = 22) undergoing flexible sigmoidoscopy or colonoscopy for diagnostic purposes were used for quantitative PCR analysis. The diagnosis was based on established clinical, endoscopic, and histological criteria.34 The indication for colonoscopy in the control group was IBS without diarrhea. Subjects with normal colonoscopy and histology were included. The clinical characteristics of patients and controls are listed in Table 1. The disease activity for the UC patients included in the PCR analyses was evaluated using the scoring system Ulcerative Colitis Disease Activity Index, which is based on clinical signs (score 0–12) and on endoscopic evaluation of the distal colon during colonoscopy (grade 0–3).35 Biopsies were taken from the most severely inflamed colonic mucosa and immediately immersed in RNAlater (Applied Biosystems, Ambion Inc, Austin, TX).
Table 1.
Clinical Characteristics of Patient Specimens
| Number of patients | Sex (F/M) | Age (mean/range) | Endoscopic score (median/range) | UCDAI score (median/range) | Specimen position
|
Histological inflammation grade
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rectum | Left colon | Right colon | Mild chronic | Moderate chronic active | Severe chronic active | ||||||
| Biopsy specimens | |||||||||||
| - UC | 25 | 11/14 | 40.0 (17–71) | 2 (0–3) | 7 (0–12) | 15 | 7 | 3 | ND | ND | ND |
| - Controls | 22 | 13/9 | 48.5 (21–82) | 0 | ND | 8 | 7 | 7 | ND | ND | ND |
| Surgical specimens | |||||||||||
| - UC | 6 | 1/5 | 42.2 (19–77) | ND | ND | 2 | 2 | 2 | 0 | 1 | 5 |
| - CD | 10 | 4/6 | 36.5 (20–52) | ND | ND | 0 | 1 | 9 | 3 | 3 | 4 |
| - Controls | 6 | 4/2 | 69.3 (49–85) | ND | ND | 1 | 5 | 0 | 0 | 0 | 0 |
UC, ulcrative colitis; ND, not determined; CD, Crohn’s disease.
Surgical specimens from patients with either UC (n = 6) or CD (n = 10), as well as control specimens obtained from patients who underwent bowel resection for nonmalignant conditions (n = 6) were used as a source for immunohistochemical studies. The original pathology examination records of UC and CD patients ranged from mild chronic to severe chronic active inflammation (Table 1) and were reexamined by a pathologist (C.H.). Surgical specimens from each IBD patient included areas preferably ranging from mild to severe inflammation. Control samples showed no signs of macro- or microscopic inflammation.
Quantitative PCR Analysis
The real-time PCR procedures have been described in detail previously.36,37 Total RNA was extracted from biopsies according to the Trizol method (Invitrogen, Paisley, UK) and quantified at 260 nm in a U-1500 UV/Vis spectrophotometer (Hitachi Instruments Inc, San Jose, CA). RNA integrity was assessed in an Agilent 2100 Bioanalyzer on RNA 6000 Nano chips (Agilent Technology, Böblingen, Germany) according to the manufacturer’s instructions and all samples included were found to be RIN ≥8 (RNA integrity number) on a scale from 0 to 10. Reverse transcription of total RNA was performed by iScript (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Levels of mRNA for IL-33 and β-actin were determined in duplicates by real-time quantitative PCR using TaqMan chemistry (Applied Biosystems, Foster City, CA) and a standardized threshold value. Evaluations of pre-PCR steps and assays have been conducted earlier37 showing that the quantitative PCR method discriminates a difference of two samples with a variation of 24% or more with a probability of 95% over a 5log10 range.38 Primer sequences: IL-33 fwd: 5′ TGAGTCTCAACACCCCTCAAATG 3′, IL-33 rev: 5′ GGCATGCAACCAGAAGTCTTTT 3′, IL-33 probe: FAM 5′ CAGGTGACGGTGTTGATGGTAAGATGTTAATG 3′ BHQ; β-actin fwd: 5′ TGCCGACAGGATGCAGAAG 3′, β-actin rev: 5′ GCCGATCCACACGGAGTACT 3′; β-actin probe: FAM 5′ CAGGTGACGGTGTTGATGGTAAGATGTTAATG 3′ BHQ. The stability of β-actin as a housekeeping gene in the present context has been verified earlier.36 Cytokine transcript levels relative to those of β-actin were analyzed according to the comparative CT-method and expressed as 2−ΔCT × 10E4.39 The PCR efficiencies were close to 2 for both the IL-33 and the β-actin cDNA using serial dilutions of the templates. The laboratory investigators were blinded to the clinical data. A Pearson correlation coefficient was calculated from logarithmically transformed cytokine expression signals using SPSS 16 (SPSS Inc., Chicago, IL).
Immunohistochemical Analyses
The primary antibodies used are listed Table 2. Affinity purified IL-33Nter rabbit antibody was raised against the first 15 amino acids of human IL-33. Sections (3 μm) of formalin-fixed paraffin-embedded samples were deparaffinized before antigen retrieval (Tris-EDTA pH 9.0 buffer for 3 minutes at 750 W and 20 minutes at 90 W (Whirlpool Talent Microwave Oven) or for 20 minutes at 100°C in a water bath). For enzyme immunohistochemistry, the antibodies were diluted in Ventana Antibody Diluent (Ventana Medical Systems, Tucson, AZ, Cat no. 251-018). Primary antibodies were incubated on the slides for 30 minutes at 37°C. Detection on human tissue was performed using Ventana ultraView DAB (Cat no. 760-500) or Ventana ultraView Red (Cat. No. 760-501) detection kits according to instructions by the manufacturers (Ventana, Tucson, AZ). Detection on rat tissue was performed using Ventana iView DAB Detection Kit (760-091) or Ventana Enhanced V-Red Detection Kit (760-031) replacing the secondary antibody of the kits with biotinylated rabbit anti-mouse Ig (Dako E0464) or using horseradish peroxidase- or alkaline phosphatase-conjugated Mouse-on-Rat Polymer Kits (MRT511 and MRT515, Biocare Medical, Concord, CA). The slides were washed in Ventana APK detergent (Cat no. 250-042) between incubations. Detailed protocols are available on request. For immunofluorescent detection, primary antibodies were incubated over night (4°C), followed by secondary antibodies for 2 hours at room temperature and then stained with Hoechst nuclear counterstain for 5 minutes before mounting in polyvinyl alcohol mounting medium, as described elsewhere.16 Images were either generated from a Nikon Ellipse E800 microscope equipped with Nikon Plan-Fluor objectives and an F-VIEW digital camera controlled by AnalySIS 3.2 software (Soft Imaging System), or with an Olympus BX51 microscope with an Olympus U-TVO.5XC camera and Olympus CellR̂ image acquisition software.
Table 2.
Table of Primary Antibodies
| Specificity | Clone | Species and subclass | Working concentration | Source |
|---|---|---|---|---|
| IL-33 | Nessy-1 | Mouse IgG1 | 1 μg/ml | Alexis |
| IL-33 | IL-33Nter | Rabbit | 1 μg/ml | Eurogentec |
| IL-33 | AF3625 | Goat | 1:1000 | R&D |
| αSMA | 1A4 | Mouse IgG2a | 1:100 | DAKO |
| CD31 | JC/70A | Mouse IgG1 | 1:10 | DAKO |
| CD34 | QBend/10 | Mouse IgG1 | 1:100 | Novocastra |
| CD45 | 135-4C5 | Mouse IgG2b | 3 μg/ml | LabVision |
| CD45 | 2B11+ PD7/26 | Mouse IgG1 | 1:100 | DAKO |
| CD68 | PG-M1 | Mouse IgG3 | 1:100 | DAKO |
| Cytokeratin | AE1 & AE3 | Mouse IgG1 | 1:20 | DAKO |
| Cytokeratin | MNF116 | Mouse IgG1 | 1:100 | DAKO |
| Desmin | D33 | Mouse IgG1 | 1:100 | DAKO |
| HSP47 | sc-5293 | Mouse IgG2a | 1:200 | Santa Cruz |
| Mast cell tryptase | AA1 | Mouse IgG1 | 1:2000 | DAKO |
| PDGFRβ | 28E1 | Rabbit mAb | 0.15 μg/ml | Cell Signaling |
| VE-cadherin | ALX-210-232 | Rabbit | 1:10,000 | Alexis |
| Vimentin | 3B4 | Mouse IgG2a | 1:200 | DAKO |
| Irrelevant control | MOPC21 | Mouse IgG1 | Concentration matched | Sigma |
| Irrelevant control | 42/2 | Mouse IgG2a | Concentration matched | R. Burns, Edinburgh |
Cell numbers were related to ulcers or fissures by means of an ocular graticule calibrated to area in specimens and counted by two independent observers (J.S. and C.H.), yielding an intraclass correlation coefficient of 0.90, calculated in SPSS 16.
Fibroblast Culture
Human foreskin fibroblasts (NHDF-c, PromoCell, Heidelberg, Germany) were cultured in Fibroblast Growth Medium (C-23010, PromoCell) with SupplementMix (C-39315, PromoCell) containing insulin (5 μg/ml) and basic fibroblast growth factor (1 μg/ml) according to instructions of the vendor. Cells were seeded in gelatin-coated Lab-Tek chamber slides (Nalge Nunc International, Hereford, UK) and stimulated with recombinant human (rh) IL-1β, rhIL-17, rh-interferon (IFN)γ, rh-thymic stromal lymphopoietin (TSLP), rhTNFα, rh-vascular endothelial growth factor (VEGF)-165, or human platelet-derived, acid-activated, transforming growth factor (TGF)-β1 (all from R&D Systems, Minneapolis, MN). A panel of TLR ligands was obtained from InvivoGen (San Diego, CA). Anti-TLR3 (clone TLR3.7) was from eBiosciences (San Diego, CA). Cells were stained for immunofluorescent analysis with rabbit polyclonal anti-IL-33 (IL-33Nter) and Hoechst nuclear dye as counterstain.
Western Blot Analysis
Total protein was harvested from fibroblast cultures by lysing them in 2× Laemmli buffer (4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromophenol blue, 0.125 mol/L Tris-HCl) protease inhibitor cocktail (P-8340, 1:100, Sigma-Aldrich), diluting the lysates 1:1 in PBS and subsequently boiling them for 10 minutes. Protein concentrations were determined using the RC/DC Protein Assay kit (BioRad, Oslo, Norway) and up to 20 μg protein was loaded per lane. For visualization of protein loading, membranes were stained with Ponceau S solution (0.1% [w/v] Ponceau S, 5% acetic acid in ddH2O). The protein was blotted to a nitrocellulose membrane (Hybond-ECL, RPN303D, Amersham Biosciences). After blocking in PBS Tween 0.05% and 5% milk, IL-33 protein was detected by sequential incubation of blots with mouse anti-human IL-33 (Nessy-1, 1 μg/ml), biotinylated horse anti-mouse IgG (BA-2000, 3 μg/ml, Vector Laboratories), and horseradish peroxidase-conjugated streptavidin (21124, 0.04 μg/ml, Pierce, Cramlington, UK). After IL-33 detection, the blots were re-incubated with mouse anti-human actin (sc-8432, 1:500, Santa Cruz, Heidelberg, Germany) followed by the same detection method. Horseradish peroxidase signal was detected by enhanced chemiluminescence substrate (Pierce #32106) and analyzed on a Kodak Image Station 4000R.
Wound Healing Experiments
Inbred BD-IX rats were from Charles River Laboratories (Lyon, France) and handled according to national legislation and institutional guidelines. A combination of fentanyl/fluanisone (Hypnorm, Janssen) and midazolam (Dormicum, Roche) was used subcutanously for anesthesia. Bupivacaine (Marcain, AstraZeneca) was applied directly into the wound for additional local analgesia before suturing. The wound healing assay was performed by making a full-thickness, 10-mm, midline skin incision with scissors on the back of the neck and closed with two sutures (Vicryl, 3−0, Ethicon). A boat-shaped 10 × 5 mm full-thickness piece of the wounded skin was then harvested using scissors before resuturing.
Results
Levels of IL-33 mRNA in Colonic UC Biopsies Are Elevated
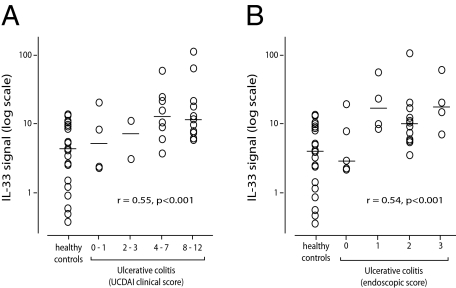
The mRNA expression level of IL-33 was analyzed by means of quantitative PCR on colonic biopsies from untreated UC patients (n = 25) and controls (n = 22). Patients were assigned to four Ulcerative Colitis Disease Activity Index subgroups and the transcript levels compared to those found in control biopsies (Figure 1A), showing that the median mRNA transcript level of IL-33 was 2.6-fold higher in the two subgroups of highest activity compared to controls. In addition, we observed a small number of patients in these subgroups who expressed up to 26-fold higher levels of IL-33 mRNA than the median of the control group. Moreover, analysis of log-transformed signal values showed a moderate correlation between relative mRNA IL-33 expression and increasing Ulcerative Colitis Disease Activity Index category (r = 0.55, P < 0.001, Figure 1A) and between IL-33 expression and increasing endoscopic grade of inflammation (r = 0.54, P < 0.001, Figure 1B).
Figure 1.
IL-33 mRNA up-regulation in UC correlates with clinical and endoscopic score. Quantitative PCR of IL-33 in biopsies from 25 patients with UC compared to 22 healthy controls showed a moderate but highly significant correlation between IL-33 signal and Ulcerative Colitis Disease Activity Index category (A) and between IL-33 and endoscopic score (B).
IBD-Associated IL-33-Positive Cells Accumulate in UC-Associated Ulcers but Not in CD Fissures
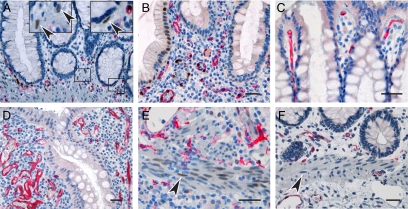
To explore the cellular sources of enhanced IL-33 expression levels in UC and compare them to CD lesions, we next immunostained resection samples from 6 UC patients, 10 CD patients, and 6 controls (Table 1). The patient samples contained areas ranging from mild chronic to severe chronic active inflammation. Immunostainings were performed with three different antibodies to IL-33 (see Table 2). All antibodies generated similar results but the monoclonal reagent performed with a better signal-to-noise ratio and all immunohistochemical data shown are generated with the monoclonal antibody. We first confirmed our recent findings16 and those of others,17 that endothelial cells of most blood vessels in the histologically healthy colon are the main IL-33-expressing cell population (Figure 2A) and that the vascular expression levels of IL-33 were generally similar in the IBD lesions (Figure 2B). In addition, we observed that the most superficial blood vessels in the normal colonic lamina propria facing the intestinal lumen expressed only weak or no nuclear IL-33 (Figure 2C). Moreover, we observed an increased density of such IL-33-negative vessels in IBD samples (Figure 2D), perhaps reflecting angiogenic activity.16
Figure 2.
IL-33 expression in endothelial, epithelial, and smooth muscle cells in healthy and inflamed intestine. Immunohistochemical staining of normal colonic mucosa (A, C, and F), as well as CD (B) and UC (D and E) lesions. Note that endothelial cells of most blood vessels expressed IL-33 in both normal intestine (A) and IBD lesions (B), although IL-33−negative vessels were also seen in luminal areas of healthy intestine (C) and focally in IBD samples (D). Moreover, left inset in panel A shows higher magnification of area with single non-endothelial cells expressing IL-33 and right inset in panel A shows IL-33−expressing, putative pericryptal myofibroblast. Panels E and F show focal induction of IL-33 in smooth muscle cell nuclei (arrowheads) of UC lesions (E) but not normal mucosa (F). All panels show IL-33 in brown and CD34 in red. Scale bars = 50 μm.
Interestingly, nuclear expression in enterocytes was not observed in the control samples and was seen only rarely in single crypts of diseased intestine (Figure 2B, but compare to other panels in Figures 2, 3, and 4), therefore contrasting the reported, generalized expression of IL-33 in IBD epithelia.40,41 In addition, we observed occasional IL-33 expression in single, nonendothelial cells of the lamina propria, some of which appearing to be pericryptal myofibroblasts based on shape and position (Figure 2A, inserts).
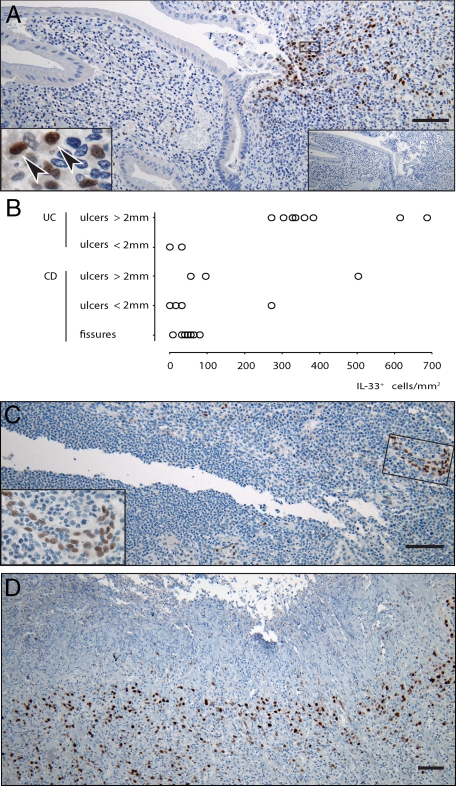
Figure 3.
IL-33−positive cells accumulate in UC-associated ulcers but not in CD fissures. Immunohistochemistry of UC (A), CD (C), and gastric mucosae (D) lesions and counts of IL-33−positive cells in IBD lesions (B). Panels A, C, and D show IL-33 in brown. Left inset in panel A shows higher magnification of area with solid frame in the main panel, arrowheads point to cells with cytoplasmic in addition to nuclear signal. Right inset in panel A shows subclass- and concentration-matched negative control in serial section. Inset in panel C shows IL-33−positive venule at higher magnification framed in the main panel. Scale bars = 200 μm.
Figure 4.
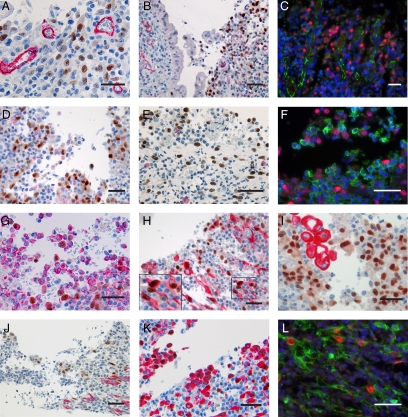
Identification of ulcer-associated IL-33−positive cells in UC. Immunohistochemistry (A, B, D, and E, and G−K) and immunofluorescence (C, F, and L) of UC ulcers. Panels A, B, D and E, and G−K show IL-33 in brown and CD34 (A), CD31 (B), CD68 (D), mast cell tryptase (E), vimentin (G), αSMA (H), pancytokeratin (I), desmin (J), or heat shock protein 47 (K) in red. Panels C, F, and L show IL-33 in red and VE-cadherin (C), CD45 (clone 135-4C5) (F), or PDGFRβ (L) in green and cell nuclei in blue. Left inset in panel H shows higher magnification of area with solid frame in the main panel. Scale bars = 50 μm.
Taken together, these findings appeared unsuitable to explain the enhanced level of IL-33-encoding mRNA in UC samples. However, smooth muscle cells of the muscularis mucosae showed a moderate to weak nuclear staining for IL-33 in three out of the six UC samples (Figure 2E). This induction was not observed in control samples (Figure 2F) and seen in only one out of ten CD samples. In addition, careful examination of the rather large surgical specimens revealed that another striking feature of UC samples was the focal accumulation of cells with large, strongly IL-33-positive nuclei underlying ulcerations (Figure 3A). These cells showed a shield-like distribution, restricted to areas where the mucosa was denuded and some cells also showed a cytoplasmic signal compatible with secretion of IL-33 (insert Figure 3A). Accumulations of IL-33 positive cells were seen in all ulcerations larger than 2 mm in four out of the six UC samples, but were less prominent in smaller ulcers (Figure 3B). Moreover, fissures seen in CD specimens were consistently devoid of such accumulations (Figure 3, B and C) and although aggregates of these large, IL-33 cells were also seen in two out of 10 CD samples, they were again observed in ulcer-like lesions (Figure 3B). Thus, given that their distribution appeared so strongly associated with the ulcerations, we analyzed a gastric ulcer, again finding abundant amounts of similar cells located luminally in the granulation tissue (Figure 3D).
Up-Regulation of IL-33 in UC Lesions May Predominantly Originate from Ulcer Infiltration by Fibroblasts/Myofibroblasts
In efforts to identify the ulceration-associated, IL-33-positive cells, we first assessed if they might be single, angiogenic endothelial cells. However, the lack of co-staining for cluster differentiation antigen (CD)34, CD31, or VE-cadherin (Figure 4, A−C) did not support this hypothesis. Given the large size of their nuclei, we then speculated if they might be macrophages or mast cells, but they failed to co-stain for CD68 or mast cell tryptase, as well as the hematopoietic cell marker CD45 (Figure 4, D−F), rather pointing to a stromal cell origin. Accordingly, virtually all IL-33-positive cells expressed vimentin, and a large fraction were α-smooth muscle actin (αSMA) positive (Figure 4, G and H). The expression of αSMA narrowed our focus to myoepithelial cells, smooth muscle cells, pericytes, or myofibroblasts. To this end, the lack of costaining for pancytokeratin or desmin (Figure 4, I and J) as well as their stellate morphology (insert Figure 4H) led to the conclusion that αSMA-positive myofibroblasts were a prominent population among the ulceration-associated IL-33-positive cells. Accordingly, we hypothesized that the population of αSMA-negative cells in this area may be activated fibroblasts/αSMA-negative myofibroblasts that had not yet differentiated to express αSMA. This hypothesis was supported by the observation that all of the IL-33-positive cells appeared to express heat shock protein 47 (Figure 4K), a chaperone for collagens that has been associated with fibroblasts in wound healing,42 as well as platelet-derived growth factor receptor β (PDGFRβ), essential for fibroblast and pericyte recruitment during wound healing (Figure 4L).43
The TLR3 Agonist Poly (I:C) and TGFβ Synergize to Induce IL-33 in Fibroblasts
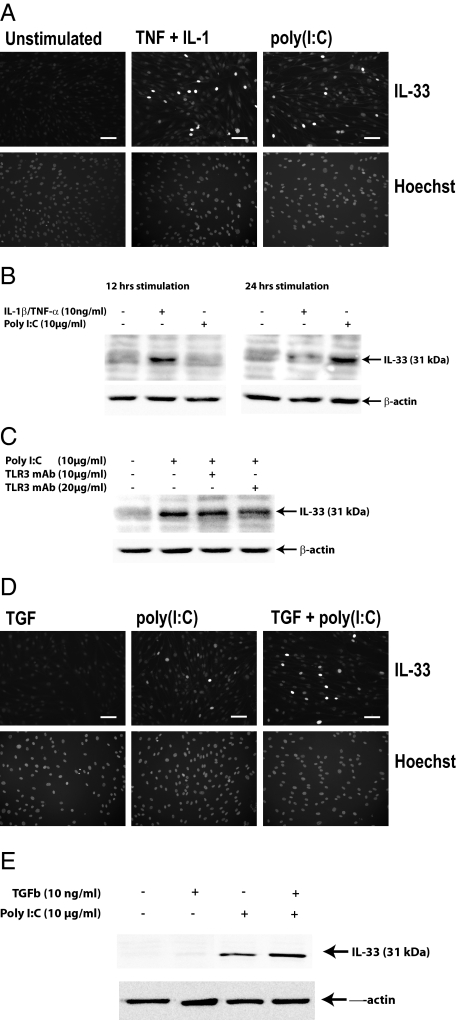
To map possible inducers of IL-33 in fibroblasts and myofibroblasts, we next analyzed the expression of IL-33 in human fibroblast cultures. We confirmed that IL-33 can be induced in fibroblasts upon activation with TNFα and/or IL-1β9,10 peaking at 12 hours (Figure 5, A and B). On the other hand, testing Th1-associated IFN-γ, we observed only weak induction at 100 ng/ml (Table 3). Moreover, Th17-associated IL-17 and Th2-associated IL-4 and IL-13 gave no response (Table 3). However, under the assumption that inflamed gut lesions generally contain elevated levels of these cytokines, it appeared that they might not be sufficient to drive in vivo expression of nuclear IL-33 in fibroblasts that are not associated with the base of ulcers. We therefore tested the effect of TGFβ, thymic stromal lymphopoietin, (TSLP) or VEGF, all known to be released from damaged epithelial barriers, observing that they also gave no response to the expression level of nuclear IL-33 (Figure 5D and Table 3). We then considered the possibility that an increased exposure to components of the gut flora could be involved in IL-33 induction and assessed the effect of ligands to TLRs known to be expressed in fibroblasts (TLR2, −3, −4, and −9), observing that the TLR4 ligand lipopolysaccharide and in particular the TLR3 ligand poly (I:C) induced IL-33, the latter to a level higher than that observed when exposing the cells to IL-1β or TNFα alone and similar to that seen in response to the combination of both cytokines (Figure 5A and Table 3) but in contrast to the cytokine response reaching maximum expression levels at 24 hours (Figure 5B). The TLR2 ligands heat-killed Listeria Monocytogenes (HKLM) or lipoteichoic acid, as well as the TLR9 ligand ODN2006 showed no induction over background levels. All stimulation data are summarized in Table 3.
Figure 5.
Pro-inflammatory regulation of nuclear IL-33 in fibroblasts. A: Immunocytochemistry of dermal fibroblasts expressing IL-33 in the absence or presence of TNFα and IL-1β (10 ng/ml and 10 ng/ml for 12 hours) or poly (I:C) (10 μg/ml for 24 hours). B: Western blot of dermal fibroblast lysates; time course of IL-33 expression in response to TNFα/IL-1β or poly (I:C), reagent concentrations as in A. C: Western blot of dermal fibroblast lysates; monoclonal antibody to TLR3 (clone TLR3.7, 10, or 20 μg/ml) was added to cultures 60 minutes before the addition of poly (I:C) and found to inhibit expression of IL-33. D and E: Synergistic effect of poly (I:C) (10 μg/ml) and TGFβ (10 ng/ml) on expression of IL-33 shown by immunocytochemistry (D) and Western blot (E) of dermal fibroblasts.
Table 3.
Summary of IL-33 Induction in Fibroblasts Exposed to Cytokines, Growth Factors, or TLR Ligands
| IL-1β (10 ng/ml)* | ++** | ||
| TNFα (10 ng/ml) | ++ | IL-1β/TNFα | +++ |
| LPS (10 μg/ml) | + | TNFα/LPS | ++ |
| HKLM (108 cells/ml) | − | ||
| LTA (100 μg/ml) | − | ||
| ODN 2006 (10 μmol/L) | − | ||
| poly(I:C) (10 μg/ml) | ++(+) | ||
| TGFβ (10 ng/ml) | − | poly(I:C)/TGFβ | ++++ |
| VEGF (100 ng/ml) | − | ||
| PDGF (100 ng/ml) | − | ||
| IL-4 (100 ng/ml) | − | ||
| IL-13 (100 ng/ml) | − | ||
| IL-17 (100 ng/ml) | − | ||
| IFNγ (100 ng/ml) | + | ||
| TSLP(100 ng/ml) | − |
Upper concentration of agonists tested in a range of three log levels in three independent experiments.
Expression levels were assessed by microscopic evaluation of immunostained cells or band intensity of Western blots on a scale from − to ++++.
Given the roles of newly discovered pathways for detecting double-stranded RNA (RIG-I and MDA-5)44 in addition to TLR3 ligation, we asked whether the effect of poly (I:C) was mediated via TLR3. Addition of a blocking antibody to TLR345 led to a reduction in IL-33 expression (Figure 5C). We then asked whether the IL-33−inducing effect of poly (I:C) might be potentiated by the addition of TGFβ, given the recent demonstration that TLR3 activation augments production of collagen and fibronectin in myofibroblast via TGFβ.46 Indeed, while addition of TGFβ alone had no effect on IL-33 expression and the effect of poly (I:C) was moderate, we found TGFβ to synergistically boost the effect of poly (I:C)-induced expression of IL-33 (Figure 5, D and E).
IL-33−Expressing Myofibroblasts in Wound Healing May Be Generated from Pericytes
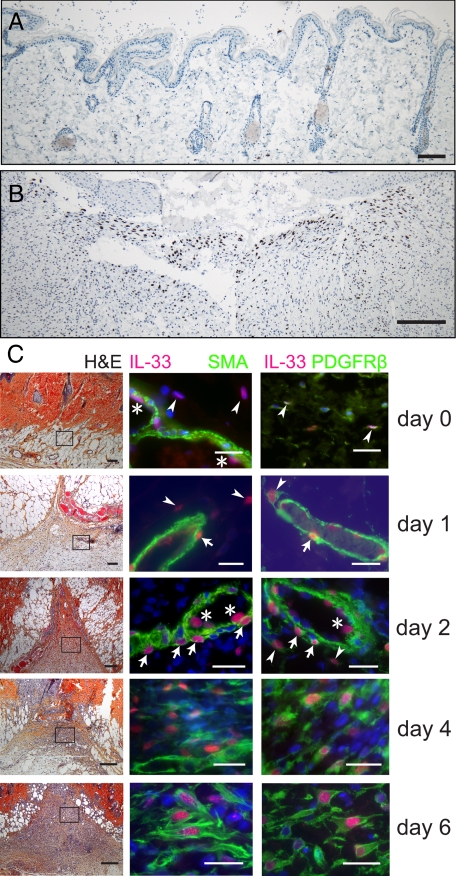
To assess if the recruitment of IL-33−positive fibroblasts and myofibroblasts to the granulation tissue of mucosal ulcers could be generalized to also include wound healing in other organs and to shed light on the origin of these cells, we analyzed samples collected from healing skin wounds in the rat. In control samples (Figure 6A) we found IL-33 in endothelial cell nuclei, as published elsewhere,16 and, at a generally lower expression level, in rare, scattered cells with chromatin-dense cigar-shaped nuclei compatible with being fibroblasts (Figure 6C, arrowheads, upper panel). By contrast, in the granulation tissue of healing wounds (Figure 6B) we observed a massive recruitment of cells that showed a strong nuclear signal for IL-33. Moreover, by analyzing a time course of biopsy specimens, we observed that nuclear IL-33 was induced in αSMA-positive pericytes (Figure 6C, arrows) as soon as 24 hours after wounding but also the occurrence of a strong nuclear IL-33 signal in αSMA-negative cells with large, round nuclei that were not in direct contact with the vessels (Figure 6C, arrowheads). To further dissect the phenotypes of IL-33−expressing cells, we co-stained for PDGFRβ, another pericyte-associated marker thought to be involved in endothelial cell-pericyte interaction.47 However, we observed that PDGFRβ was detectable not only on pericytes, but also to a lesser extent on the cigar-shaped IL-33−positive cells seen in control samples (Figure 6C). In the course of the following days, PDGFRβ expression appeared to increase on IL-33−positive cells, peaking at day 6. In addition, we observed co-expression of αSMA at day 4, also peaking at day 6 (Figure 6C).
Figure 6.
IL-33−positive fibroblasts and myofibroblasts accumulate in dermal wound repair. Immunohistochemistry of healthy skin (A) and healing, incisional wound at day 9 (B), showing IL-33 signal in brown. Panel C shows healthy control skin and wounds harvested at days 1, 2, 4, and 6 with H&E-stained overviews in the left column, IL-33 (red) and αSMA (green) in the middle column and IL-33 (red) and PDGFRβ (green) in the right column. Areas of immunofluorescent detection are outlined in the H&E images. Arrowheads point to single IL-33−positive cells and arrows to IL-33−positive pericytes. Some endothelial cell nuclei as labeled with an asterisk for clarity. Scale bars: panels A and B and left panel in C (200 μm); and middle and right panels in C (50 μm).
Discussion
The discovery of IL-33 and its association to Th2 immunity has raised the question of how it behaves in chronic inflammatory lesions. In this study, we observed that a dominant source of elevated mRNA IL-33 levels in UC lesions may be myofibroblasts in the granulation tissue of ulcers. This observation prompted us to also look in gastric ulceration and experimental wound healing of the skin, allowing us to conclude that the accumulation of IL-33−positive myofibroblasts may be a general feature of mucosal healing and wound repair.
The development of myofibroblasts from connective tissue fibroblasts in wound repair is a process driven by TGFβ and mechanical strain,48 in which the fibroblast acquires the αSMA-containing stress fibers thought necessary to achieve the contraction of the wound and successful healing.49 Our observation that TGFβ also acts to boost the TLR3-driven induction of IL-33 in cultured fibroblasts is intriguing because it appears to be well in line with the recent observation that TLR3 activation also drives myofibroblast differentiation.46 While TLR3 is commonly thought to signal the presence of virus-derived double stranded RNA, it can also be activated by host mRNA released from damaged or necrotic cells, a scenario compatible with loss of the epithelial barrier.33 Thus, if the recruitment of IL-33−positive fibroblasts to ulcers is a protective process (based on its similarity to the physiological wound healing seen in the skin), it is tempting to consider that such mechanisms may contribute to explaining why TLR3 activation protects against dextran sulphate sodium−induced colitis.50 Conversely, the lack of IL-33−positive myofibroblast accumulations near the deep fissures of CD-lesions may reflect a reduced response of fibroblasts to breaks in the epithelial barrier in CD. To this end, it is interesting to note that TLR3 expression is reportedly reduced in CD51 and TLR3, localized on chromosome 4 (q35), is bordering a linkage region of an IBD susceptibility gene.52 The inhibition of poly (I:C)-induced IL-33 expression by means of a monoclonal antibody to TLR3 implies that at least part of the effect is mediated via this receptor. Of note, this antibody inhibited only about 50% of the IFNβ production in lung-derived fibroblasts,45 pointing either to an incomplete blocking effect or the presence of for example RIG-I-like receptors,44 that also sense RNA and so could explain the remaining response to poly (I:C). It will therefore be interesting to further dissect the mechanisms underlying the response of IL-33 to poly (I:C).
It appears equally interesting that while lamina propria mononuclear cells from UC patients showed increased production of TGFβ compared to controls, lower levels were observed in CD samples,53 perhaps reflecting a suppressive role of CD-associated IFN-γ on the production of TGF β. In addition, IFN-γ inhibits TGFβ signaling54 and αSMA induction in fibroblasts,55 perhaps explaining the inhibitory role of IFN-γ in wound healing.56 Moreover, IFN-γ in combination with TNFα has been associated with the apparent loss of pericryptal intestinal myofibroblasts57 and reduced migration of intestinal fibroblasts.58
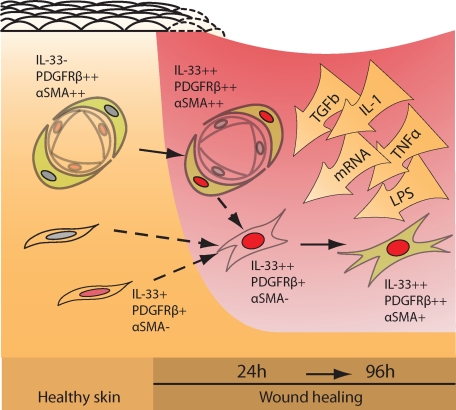
It is thought that myofibroblasts can develop from tissue-resident fibroblasts and to this end we observed nuclear IL-33 expression in fibroblast-like cells in intact skin and healthy colon. Moreover, it was interesting to observe in the wound model that nuclear IL-33 was also induced in pericytes. These pericytes were PDGFRβ-positive and although not formally proven in our study, their phenotypic change and positioning appear well in line with the recent observation that pericytes are a major source of myofibroblasts in kidney fibrosis,59,60 and that blocking PDGFRβ signaling inhibits myofibroblast recruitment in wound healing.43 On the other hand, it is equally possible that the IL-33/PDGFRβ double-positive, putative fibroblasts of intact rat skin were the origin of the IL-33/PDGFRβ double-positive cells with round enlarged nuclei seen after wounding. These possible chains of events are schematically summarized in Figure 7. In addition, we have not ruled out the possibility that smooth muscle cells, endothelial cells, and even epithelial cells transdifferentiating to a mesenchymal phenotype are among the sources of IL-33−positive myofibroblasts in skin wounds.
Figure 7.
Schematic representation of IL-33−induction in fibroblasts, pericytes, and myofibroblasts during wound healing. Stromal, tissue-resident fibroblasts (IL-33−positive or −negative) are activated by mRNA/TGFβ/lipopolysaccharide to express nuclear IL-33 (red nuclei) and may contribute to the pool of myofibroblasts invading the wound area. Likewise, pericytes become activated and may contribute to this pool. By contrast, vascular endothelial cells, which are strongly positive in healthy tissue, down-regulate IL-33 in the course of activation.16
Although we could not extend our observations of pericyte activation to the ulcerations of UC lesions, IL-33 appears well suited to enhance the resolution of myofibroblast characterization, not only in IBD, but also in fibroblast function of other organs. It is known that fibroblasts and myofibroblasts each show a spectrum of different phenotypes. To this end, our observations appear to establish nuclear IL-33 as an early marker for ulcer-associated activated fibroblasts and myofibroblasts and it may prove useful to link specific functions to specific subtypes of these cells.
The modest increase of IL-33 mRNA levels and association to UC disease activity that we observed in untreated UC patients is generally well in line with two recent reports on the subject.40,41 Moreover, protein levels of IL-33 of solubilized samples were similarly increased.41 On the other hand, our immunohistochemical analysis of IL-33 is at variance with the reported data because both reports conclude that there is a general IBD-associated signal from epithelial cells, whereas we only observed IL-33 in scattered crypts. These differences may possibly be attributed to different antibodies and/or different protocols, but it also appears likely that our use of surgical specimens that are substantially larger than biopsy specimens (20 mm vs. 2 mm), were indeed required to discover the highly focused expression of IL-33 in ulcerations.
The observation that IL-33 is associated with areas of broken barrier function also prompts a discussion of mucosal healing. Increased permeability and diarrhea are the virtually ever present symptoms in active IBD. In this situation, the epithelial barrier is dysfunctional, either disrupted in active IBD lesions, or as in chronic IBD showing signs of persistent mucosal leakiness.61,62 Accordingly, the concept of mucosal healing is getting increased attention as an optimal treatment goal for IBD patients, because such patients are more likely to stay in long-term remission.63 Conversely, the process of regeneration associated with chronic inflammation appears to be closely related to the generation of excess connective tissue and the development of fibrosis. It is therefore of high interest to more fully understand the cellular functions central to these repair processes and to analyze more carefully if the elevated serum levels of IL-33 observed by Beltran et al41 can be correlated to barrier function.
The function of IL-33 in fibroblasts, pericytes, and myofibroblasts is currently unknown. The transcriptional repressor activity of IL-33 in recombinant systems20 implies that IL-33 may affect their nuclear functions, but it is also possible that IL-33 is released from these cells. Indeed, we observed scattered cells in ulcers that also showed a signal for IL-33 in their cytoplasm but our efforts to measure IL-33 in the supernatant of cultured fibroblasts were not successful. Nevertheless, secretion of IL-33 may serve to recruit ST2-expressing leukocytes to the ulceration, but it is also possible that IL-33 release may promote angiogenesis,14 and perhaps scar formation,30 by acting directly or indirectly on stromal cells. It will be interesting to analyze the outcome of both experimental colitis and wound healing in IL-33−deficient animals.
Acknowledgments
We thank Linda Manley for expert technical assistance, Are H. Pripp for assistance with the statistical analysis, and Helge Scott, Finn-Eirik Johansen, Frode Jahnsen, Per Brandtzaeg, and Harald Torsvik for helpful discussions, and Robert Burns for providing antibodies.
Footnotes
Address reprint requests to Guttorm Haraldsen, M.D., Ph.D., Division of Pathology, Oslo University Hospital Rikshospitalet, Oslo, Sognsvannsveien 20, N-0027 Oslo, Norway. E-mail: gharalds@rr-research.no.
Supported by Helse Sør-Øst (2008139 and 2007044), the Norwegian Cancer Society (PK01-2006-0448 and PK01-2007-0199), the Research Council of Norway (133924/300), and the University of Oslo (131406). J.S. and A.M.K. are Ph.D. and Postdoctoral Fellows of Helse Sør-Øst, J.P. and J.B. are Postdoctoral Fellows of the Norwegian Cancer Society.
J.P. and T.O. contributed equally.
Disclosure: G.H. is inventor of patent WO 2004/056868 A2.
Current address for J.P.: Dept. of Obstetrics and Fetal-Maternal Medicine, Medical University of Vienna, Austria.
References
- Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeire S, Van Assche G, Rutgeerts P. Serum sickness, encephalitis and other complications of anti-cytokine therapy. Best Pract Res Clin Gastroenterol. 2009;23:101–112. doi: 10.1016/j.bpg.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Mackey AC, Green L, Liang LC, Dinndorf P, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:265–267. doi: 10.1097/MPG.0b013e31802f6424. [DOI] [PubMed] [Google Scholar]
- Shale M, Ghosh S. Beyond TNF. Th1 and Th2 in inflammatory bowel disease. Gut. 2008;57:1349–1351. doi: 10.1136/gut.2008.151563. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136:1182–1197. doi: 10.1053/j.gastro.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, Pitman N, Kurowska-Stolarska M, McKenzie AN, McInnes IB, Liew FY. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci USA. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G, Talabot-Ayer D, Lamacchia C, Toy D, Seemayer CA, Viatte S, Finckh A, Smith DE, Gabay C. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009;60:738–749. doi: 10.1002/art.24305. [DOI] [PubMed] [Google Scholar]
- Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- Murphy GE, Xu D, Liew FY, McInnes IB. Role of interleukin 33 in human immunopathology. Ann Rheum Dis. 2010;69 Suppl 1:i43–i47. doi: 10.1136/ard.2009.120113. [DOI] [PubMed] [Google Scholar]
- Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng YS, Park H, Kim J, Kim YM, Kwon YG. IL-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial NO production. Blood. 2009;114:3117–3126. doi: 10.1182/blood-2009-02-203372. [DOI] [PubMed] [Google Scholar]
- Nishida A, Andoh A, Imaeda H, Inatomi O, Shiomi H, Fujiyama Y. Expression of interleukin 1-like cytokine interleukin 33 and its receptor complex (ST2L and IL1RAcP) in human pancreatic myofibroblasts. Gut. 2010;59:531–541. doi: 10.1136/gut.2009.193599. [DOI] [PubMed] [Google Scholar]
- Kuchler AM, Pollheimer J, Balogh J, Sponheim J, Manley L, Sorensen DR, De Angelis PM, Scott H, Haraldsen G. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol. 2008;173:1229–1242. doi: 10.2353/ajpath.2008.080014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS ONE. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda H, Kasuya H, Takakura K, Hori T, Imaizumi T, Takeuchi T, Inoue I, Takeda J. Identification of genes differentially expressed in canine vasospastic cerebral arteries after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1999;19:1279–1288. doi: 10.1097/00004647-199911000-00013. [DOI] [PubMed] [Google Scholar]
- Baekkevold ES, Roussigne M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel L, Erard M, Cayrol C, Girard JP. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep. 2008;9:1006–1012. doi: 10.1038/embor.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. J Leukoc Biol. 2008;84:631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvie P, Lisbonne M, L‘Helgoualc'h A, Rauch M, Turlin B, Preisser L, Bourd-Boittin K, Theret N, Gascan H, Piquet-Pellorce C, Samson M. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J Cell Mol Med. 2010;14:1726–1739. doi: 10.1111/j.1582-4934.2009.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009;284:19420–19426. doi: 10.1074/jbc.M901744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, Lavelle EC, Martin SJ. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Kuchler AM. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227–233. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Rankin AL, Mumm JB, Murphy E, Turner S, Yu N, McClanahan TK, Bourne PA, Pierce RH, Kastelein R, Pflanz S. IL-33 induces IL-13-dependent cutaneous fibrosis. J Immunol. 2009;184:1526–1535. doi: 10.4049/jimmunol.0903306. [DOI] [PubMed] [Google Scholar]
- Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- Sands BE. From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology. 2004;126:1518–1532. doi: 10.1053/j.gastro.2004.02.072. [DOI] [PubMed] [Google Scholar]
- Sutherland LR, Martin F, Greer S, Robinson M, Greenberger N, Saibil F, Martin T, Sparr J, Prokipchuk E, Borgen L. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894–1898. doi: 10.1016/0016-5085(87)90621-4. [DOI] [PubMed] [Google Scholar]
- Olsen T, Goll R, Cui G, Husebekk A, Vonen B, Birketvedt GS, Florholmen J. Tissue levels of tumor necrosis factor-alpha correlates with grade of inflammation in untreated ulcerative colitis. Scand J Gastroenterol. 2007;42:1312–1320. doi: 10.1080/00365520701409035. [DOI] [PubMed] [Google Scholar]
- Cui G, Olsen T, Christiansen I, Vonen B, Florholmen J, Goll R. Improvement of real-time polymerase chain reaction for quantifying TNF-alpha mRNA expression in inflamed colorectal mucosa: an approach to optimize procedures for clinical use. Scand J Clin Lab Invest. 2006;66:249–259. doi: 10.1080/00365510600590472. [DOI] [PubMed] [Google Scholar]
- Goll R, Olsen T, Cui G, Florholmen J. Evaluation of absolute quantitation by nonlinear regression in probe-based real-time PCR. BMC Bioinformatics. 2006;7:107. doi: 10.1186/1471-2105-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Seidelin JB, Bjerrum JT, Coskun M, Widjaya B, Vainer B, Nielsen OH. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol Lett. 2010;128:80–85. doi: 10.1016/j.imlet.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Beltran CJ, Nunez LE, Diaz-Jimenez D, Farfan N, Candia E, Heine C, Lopez F, Gonzalez MJ, Quera R, Hermoso MA. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1097–1107. doi: 10.1002/ibd.21175. [DOI] [PubMed] [Google Scholar]
- Wang J, Dodd C, Shankowsky HA, Scott PG, Tredget EE. Deep dermal fibroblasts contribute to hypertrophic scarring. Lab Invest. 2008;88:1278–1290. doi: 10.1038/labinvest.2008.101. [DOI] [PubMed] [Google Scholar]
- Rajkumar VS, Shiwen X, Bostrom M, Leoni P, Muddle J, Ivarsson M, Gerdin B, Denton CP, Bou-Gharios G, Black CM, Abraham DJ. Platelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am J Pathol. 2006;169:2254–2265. doi: 10.2353/ajpath.2006.060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhaei P, Genin P, Civas A, Hiscott J. RIG-I-like receptors: sensing and responding to RNA virus infection. Semin Immunol. 2009;21:215–222. doi: 10.1016/j.smim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem Biophys Res Commun. 2002;293:1364–1369. doi: 10.1016/S0006-291X(02)00380-7. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Ichikawa T, Koarai A, Yanagisawa S, Minakata Y, Matsunaga K, Hirano T, Akamatsu K, Ichinose M. Activation of Toll-like receptor 3 augments myofibroblast differentiation. Am J Respir Cell Mol Biol. 2009;40:654–662. doi: 10.1165/rcmb.2008-0371OC. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M, Wu H, Aitken J, Kolachala VL, Neish AS, Sitaraman SV, Gewirtz AT. Activation of toll-like receptor 3 protects against DSS-induced acute colitis. Inflamm Bowel Dis. 2007;13:856–864. doi: 10.1002/ibd.20142. [DOI] [PubMed] [Google Scholar]
- Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe J, Schreiber S, Shaw SH, Lau KF, Bridger S, Macpherson AJ, Cardon LR, Sakul H, Harris TJ, Buckler A, Hall J, Stokkers P, van Deventer SJ, Nurnberg P, Mirza MM, Lee JC, Lennard-Jones JE, Mathew CG, Curran ME. A genomewide analysis provides evidence for novel linkages in inflammatory bowel disease in a large European cohort. Am J Hum Genet. 1999;64:808–816. doi: 10.1086/302294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Zotto B, Mumolo G, Pronio AM, Montesani C, Tersigni R, Boirivant M. TGF-beta1 production in inflammatory bowel disease: differing production patterns in Crohn’s disease and ulcerative colitis. Clin Exp Immunol. 2003;134:120–126. doi: 10.1046/j.1365-2249.2003.02250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Rubbia-Brandt L, Abdiu A, Walz T, Macieira-Coelho A, Gabbiani G. Alpha-smooth muscle actin is expressed in a subpopulation of cultured and cloned fibroblasts and is modulated by gamma-interferon. Exp Cell Res. 1992;201:64–73. doi: 10.1016/0014-4827(92)90348-c. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Takayasu T, Iwakura Y, Mukaida N. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J Immunol. 2004;172:1848–1855. doi: 10.4049/jimmunol.172.3.1848. [DOI] [PubMed] [Google Scholar]
- Francoeur C, Bouatrouss Y, Seltana A, Pinchuk IV, Vachon PH, Powell DW, Sawan B, Seidman EG, Beaulieu JF. Degeneration of the pericryptal myofibroblast sheath by proinflammatory cytokines in inflammatory bowel diseases. Gastroenterology. 2009;136:268–277 e263. doi: 10.1053/j.gastro.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Leeb SN, Vogl D, Gunckel M, Kiessling S, Falk W, Goke M, Scholmerich J, Gelbmann CM, Rogler G. Reduced migration of fibroblasts in inflammatory bowel disease: role of inflammatory mediators and focal adhesion kinase. Gastroenterology. 2003;125:1341–1354. doi: 10.1016/j.gastro.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderholm JD, Olaison G, Peterson KH, Franzen LE, Lindmark T, Wiren M, Tagesson C, Sjodahl R. Augmented increase in tight junction permeability by luminal stimuli in the non-inflamed ileum of Crohn’s disease. Gut. 2002;50:307–313. doi: 10.1136/gut.50.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitter AH, Wullstein F, Fromm M, Schulzke JD. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology. 2001;121:1320–1328. doi: 10.1053/gast.2001.29694. [DOI] [PubMed] [Google Scholar]
- Froslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]