Abstract
Knowledge of the experience and outcomes of current paediatric antiretroviral treatment (ART) programmes in sub-Saharan Africa can inform new programmes in the region as well as enhance existing ones. This is urgently needed to facilitate the scale-up of treatment, which is needed to address the burden of paediatric HIV cases on the continent. We reviewed the characteristics and outcomes of programmes with clinical paediatric ART studies published prior to 1 January 2008. The outcomes of the studies were comparable to similar ones from developed countries; however, the duration of follow-up was relatively limited in almost all the studies reviewed. One-year survival probability was between 84% and 91%, and considerable improvement in the clinical, immunologic and viral status of the paediatric patients was generally recorded. Loss to follow-up was less than 10% in all but two studies. Adherence to treatment was good and few adverse events were reported. This is despite the fact that many programmes were subject to enormous constraints in terms of health services, and despite widespread use of adult fixed-dose combinations for paediatric patients, including young infants. While the majority of children commencing ART were severely ill, most children were old (median age > 5 years for almost all studies) with relatively few infants and young children (age < 2 years) receiving treatment. This is in contrast to knowledge of rapid disease progression in the majority of HIV-infected infants and despite the World Health Organization’s recent recommendations to commence ART in all HIV-infected infants less than one year old. There is an urgent need to address barriers to ART for infants. Studies of the outcomes of programmes treating infants as well as those with longer-term follow-up are also needed.
Keywords: children, growth monitoring, HIV/AIDS, infants, mortality, programme assessment, survival, treatment issues, treatment practices, WHO guidelines
Introduction
The burden of paediatric HIV infections is greatest in sub-Saharan Africa, where as of December 2007 almost 90% of the world’s two million HIV-infected children were living, with approximately 370 000 new paediatric infections having occurred in the preceding year (UNAIDS, 2008). In the absence of antiretroviral therapy (ART), HIV-related illnesses account for approximately 10% of childhood mortality across the continent and up to 50% childhood mortality in southern Africa (UNAIDS, 2006; Little, Thorne, Luo, Bunders, Ngongo, McDermott & Newell, 2007). ART is highly effective in reducing morbidity and mortality in HIV-infected children in the developed world; however, until very recently, its availability to children in Africa has been limited (Patel, Hernan, Williams, Seeger, McIntosh, Van Dyke & Seage, 2008). For example, according to the most recent available paediatric ART coverage estimates, in more than half of sub-Saharan African countries, less than 15% of children needing ART were receiving it at the end of 2006 (WHO/UNAIDS, 2006). Coverage was below 35% for the remainder of the sub-continent, apart from Namibia and Botswana (WHO/UNAIDS, 2006).
Encouragingly, access to ART for children in sub-Saharan Africa is increasing, with more than 2 500 facilities providing treatment and nearly 160 000 children receiving ART by December 2007 (WHO, UNICEF & UNAIDS, 2008). Nine of the ten countries in which the vast majority of HIV-infected children live are in sub-Saharan Africa, and access to ART in these countries increased dramatically between 2005 and 2007. For example, in South Africa, Kenya, Malawi and Zimbabwe, the numbers of children receiving ART increased 2.6 times, 3 times, 4 times and nearly 5 times in that period, respectively (WHO et al., 2008). The corresponding total numbers of children on ART in those countries by the end of 2007 were 32 000 (South Africa), 15 000 (Kenya), 10 000 (Malawi) and 8 000 (Zimbabwe) (WHO et al., 2008).
ART provision for children in sub-Saharan Africa —with its enormous health service constraints —needs to be tailored to local circumstances. Outcomes may also be different to those in the developed world due to different socio-economic circumstances and background burden of disease. Information from observational cohorts of children receiving ART in Africa is therefore vital to demonstrate programmatic issues, such as the nature of ART provision on the continent and the effectiveness of different models of care for children, as well as the outcomes of children receiving ART.
Since 2004, reports from isolated cohorts of children receiving ART in Africa have been published with progressively increasing frequency and greater numbers of children on treatment. To our knowledge, there is one published review of clinical studies in the medical literature demonstrating good outcomes, but with little emphasis on the characteristics of individual programmes (i.e. Sutcliffe, Van Dijk, Bolton, Persaud & Moss, 2008).
This study was funded by the International Epidemiological Databases to Evaluate AIDS (IeDEA) Southern Africa cohort collaboration. We aimed to review published paediatric ART clinical studies in order to contextualize future paediatric ART cohort analyses, assess the feasibility of ART for children in Africa, and inform context-specific guidelines on treatment for children, as well as highlight areas for additional relevant research.
Our specific objectives were to review the following:
The characteristics of paediatric ART programmes, in terms of funding, user fees, ART eligibility criteria and regimens used;
The characteristics o f patients at ART initiation across different programmes;
Outcomes among children receiving ART, specifically vital status and retention in care, as well as clinical and laboratory outcomes in those who remained in care; and,
Treatment durability and adherence.
Methods
We searched the literature through the PubMed database using the search terms ‘antiretroviral children Africa’ and ‘antiretroviral’ AND (‘paediatric’ OR ‘pediatric’) AND ‘Africa.’ Eligibility criteria were observational studies published prior to 1 January 2008 with a cohort including at least 25 children treated with three-drug ART and reporting on vital status outcomes. The children needed to be part of an observational cohort and not participants in an antiretroviral (ARV) drug trial. Data were extracted onto a standard form and summarised as described under the following subheadings.
Data on treatment programmes
Variables extracted from the studies included the number of facilities at which ART was provided, external funding sources, user fees, clinical care providers, availability of laboratory testing facilities, criteria used to determine patient eligibility for ART, and ART regimens used.
Patient characteristics at ART initiation
In addition to the age and gender profile of the participants in each study, disease severity was summarised using as many of the following measures as were reported: CD4 cell count and CD4 percentage (CD4%), anthropometric data reported as age- and sex-standardised z-scores, viral load, WHO clinical stage of disease, and opportunistic infections. The relationship of caregivers to the patients was also recorded.
Outcomes on ART
We extracted information on overall mortality and loss to follow-up (as defined by each study individually), and specifically mortality at one year in those studies where this was reported. We also noted changes in markers of disease severity, including clinical events, growth, immune and virological response. We describe treatment durability in terms of proportion of children requiring treatment changes and the reasons for this, and report on adherence for those studies where this was measured.
Results
Characteristics of the treatment programmes
Overview of the studies reviewed and their countries of origin
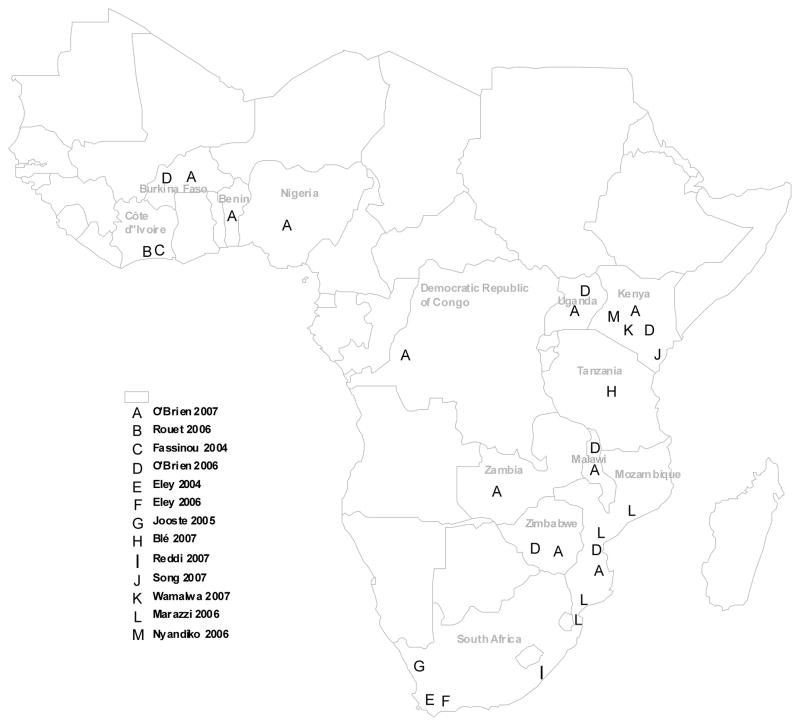
The characteristics of 18 studies (15 cohorts) that met eligibility criteria are shown in Table 1. Most (13 of 18; 60%) were reports from a single health facility, with three of the remainder being from multiple sites within a country and the other two being reports of the Médecins Sans Frontières (MSF) HIV programmes from multiple resource-limited countries (including non-African sites). Most reports are from southern Africa and East Africa (see Figure 1). The Malawi Paediatric Antiretroviral Treatment Group (2007) reports on national aggregate cohort data, not individualized outcomes. Two studies were reported by each of the following programmes: Agence Nationale de Recherches sur le SIDA, Abdijan, Cote d’Ivoire (i.e. Fassinou, Elenga, Rouet, Laguide, Kouakoussui, Timite et al., 2004; Rouet, Fassinou, Inwoley, Anaky, Kouakoussui, Rouzioux et al., 2006); Red Cross Children’s Hospital, Cape Town, South Africa (i.e. Eley, Nuttall, Davies, Smith, Cowburn, Buys & Hussey, 2004; Eley, Davies, Apolles, Cowburn, Buys, Zampoli et al., 2006), and the MSF multi-site cohort (i.e. O’Brien, Sauvageot, Zachariah & Humblet, 2006; O’Brien, Sauvageot, Olson, Schaeffer, Humblet, Pudjades et al., 2007).
Table 1.
Summary of the studies reviewed and the characteristics of the children at the start of antiretroviral therapy (ART) for each cohort (CD4% = CD4 percentage; IQR = Interquartile range; WAZ = Weight-for-age z-score; HAZ = Height-for-age z-score; NR = not reported)
| Study | Country | No. of sites |
Observation time |
No. of children on ART |
Percentage females |
Median (IQR) age (years) |
Percentage aged <18 months |
Median (IQR) CD4% |
Percentage CD4 <15% |
Percentage advanced stagea |
Median (IQR) WAZ score |
Median (IQR) HAZ score |
Median (IQR) log viral load |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fassinou et al., 2004 | Côte d’Ivoire | 1 | 2000–2002 | 78 | 44 | 7.2 (0.7; 15.2)b | 5c | 8.0 (1.9; 11.1) | NR | 13 | −2.02 | 2.03 | 5.4 (3.3; 7.0)b |
| Rouet et al., 2006 | Côte d’Ivoire | 1 | 2000–2004 | 78 | 44 | 6.5 (0.7; 15.2)b | NR | 7.5 (2.1; 11.1) | NR | NR | NR | NR | 5.4 (5.1; 6.0) |
| O’Brien et al., 2006 | 8 countries | multiple | 2001–2005 | 1–184 | 48 | 7 (4.6; 9.3) | 1 | NR | 85d | 39 | NR | −2.0 (−3.2; −1.1) | NR |
| O’Brien et al., 2007 | 14 countries | 26 | 2002–2006 | 568 | 45 | 3.2 (2.5; 4.1) | 0 | NR | 79 | 42 | NR | NR | NR |
| Eley et al., 2004 | South Africa | 1 | 2002–2003 | 80 | 39 | 4.2e (3.5; 4.9)f | NR | NR | NR | 94 | NR | NR | NR |
| Eley et al., 2006 | South Africa | 1 | 2002–2005 | 409 | 44 | 1.9 (0.7; 4.5) | 51c | 11.7 (7; 17.3) | 66 | 99 | −2.17 (−3.09;−1.12) | −2.51 (−3.41;−1.72) | 5.6 (5.1; 6.1) |
| Jooste et al., 2005 | South Africa | >1 throughout province | 2003–2004 | 100 | NR | 5.5e (0.3; 13)b | NR | NR | 96 | NR | NR | NR | NR |
| Nyandiko et al., 2006 | Kenya | 9 | 2002–2005 | 279 | 49 | 6 (0.4; 13.7)f | 7g | 10 (1; 38)b | NR | NR | −2.6 ( −9.6; 2.3)f | −2.4 ( 6.1;−4.2)f | NR |
| Marazzi et al., 2006 | Mozambi que | 6 | 2002 ? | 297 | NR | 5.1b (3.2)h | 22c | NR | 56 | NR | NR | NR | NR |
| Blé et al., 2007 | Tanzania | 1 | 2002–2005 | 59 | 34 | 4.5 (0.6; 10.6)b | 5g | 9.6 (0; 27.6)b | NR | NR | −2.13 (−5.49; 2.65)b | −2.07 ( 6.09;−4.37)b | NR |
| Reddi et al., 2007 | South Africa | 1 | 2003–2005 | 151 | 51 | 5.7 (0.3; 15.4)b | NR | 7.4 (2.1; 13.7) | 80 | 70 | −1.9 ( −3.6; 0.9) | −2.2 ( −3.0; 1.1) | NR |
| Song et al., 2007 | Kenya | 1 | 2003–2004 | 29 | 47 | 8.5e (3.4)h | 0 | NR | NR | NR | −1.61e (1.39)h | NR | 5.1 e (0.7) h |
| Wamalwa et al., 2007 | Kenya | 1 | 2004–2005 | 67 | 49 | 4.4 (2.4; 6.0) | NR | 6.2 (3.6; 10.3) | 82 | NR | −2.45 (−4.3; 1.54) | −2 (−3.32; 1.04) | 6.4 (6.0; 6.6)i |
| Kamya et al., 2007 | Uganda | 1 | 2004–2005 | 250 | 48 | 9.2e (4.5)h | NR | 8.6 (3.5; 12.7) | NR | 89 | NR | NR | 5.3e (0.8)h |
| Malawi Paediatric Antiretroviral Treatment Group, 2007 | Malawi | Multiple throughout country | 2004–2005 | 233 | NR | NR | 3g | NR | NR | NR | NR | NR | NR |
| Bolton-Moore et al., 2007 | Zambia | 18 | 2004–2007 | 2 938 | 52 | 6.8 (3; 10.4) | 10 | 11.8 (7.2; 17.4) | NR | 72 | −2.2 ( −3.4; 1.2) | NR | |
| Ellis & Molyneux, 2007 | Malawi | 1 | 2004–2005 | 238 | 46 | 7.3 (0.6; 17.6)b | 12 | NR | NR | 86 | −3.02 (−6.77; 0.69)b | −3.19 (−7.17; 0.99)b | NR |
| Bong et al., 2007 | Malawi | 1 | 2004–2006 | 439 | 50 | NR | 2g | (0.1; 43.6)b,j | NR | NR | NR | NR | NR |
CDC category C disease, or WHO stage 3 or 4 disease.
Range.
Percentage among those less than age 2 years.
CD4 percentage was only available for 34% of children and was only reported for children ≤59 months of age.
Mean value.
95% confidence interval.
Percentage among those less than age 1 year.
Standard deviation.
Children age 18 months to 3 years only.
CD4 percentage given for different subgroups only, not for all patients in the cohort.
Figure 1.
Map of paediatric ART sites with published studies included in the review
Funding and payment
Sixteen of the 18 studies report on funding sources, and 15 of these received external funding for the ART programme in addition to state health-service resources. The most common funders were the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund to Fight AIDS, Tuberculosis and Malaria (contributing to 25% and 31% of the studies, respectively). Eight studies acknowledged additional support for research activities.
Only McCord Hospital (in Durban, South Africa) did not provide free care and ART for HIV-infected children; rather a standard co-payment of approximately US$10 per month (irrespective of number of consultations, treatment or laboratory testing needed) was required per family receiving care (Reddi, Leeper, Grobler, Geddes, France, Dorse et al., 2007). However, the provision of laboratory testing as part of routine care varied widely in the programmes reviewed. Outside of South Africa and the research settings, viral-load testing was not provided, and in some of the Malawian studies CD4 testing needed to be paid for by the patients (e.g. Ellis & Molyneux, 2007).
Providers of medical care
Half of the studies provided information on the main providers of medical care. Doctors are the exclusive providers in four of nine (44%) studies, and doctors provided care in combination with clinical officers and/or primary care nurses in another four of nine studies (44%). In a single study from Zambia, the focus for care provision was specifically from nurses and clinical officers (i.e. Bolton-Moore, Mubiana-Mbewe, Cantrell, Chintu, Stringer, Chi et al., 2007). This was an explicit strategy of ‘task shifting’ aimed at extending the reach of HIV services and the programme successfully provided care to 4 975 HIV-infected children (2 938 on ART) via primary healthcare (PHC) facilities. This contrasts starkly to most of the other studies from hospital-based settings. Indeed, most studies that did not report on which cadre of staff provided clinical care are from hospital settings, suggesting a reliance on doctors.
Eligibility criteria for ART and the regimens used
Most of the studies used the World Health Organization (WHO) guidelines (e.g. WHO, 2004 and 2006) available at the time (or a local modification thereof) as criteria for ART commencement. Where ART was commenced prior to the publication of the 2003 WHO guidelines, the criteria in use were similar to those of WHO relating to clinical and/or immunological disease severity. In three studies, from Zambia and Malawi, access to virological testing to confirm diagnosis in children age ≤18 months was extremely limited, so presumptive diagnoses were made and treatment commenced in children with severe clinical and/or immunological disease (Bolton-Moore et al., 2007; Ellis & Molyneux, 2007; Malawi Paediatric Antiretroviral Treatment Group, 2007). Ellis & Molyneux (2007) noted that in Malawi CD4 testing had to be paid for by the patient, so immunological eligibility criteria could only be considered in those who could afford testing.
The main first-line ART regimens used are shown in Table 2. Notably, four studies exclusively used adult fixed-dose combination (FDC) tablets.
Table 2.
First-line ART regimen used (FDC = fixed-dose combination; NRTI = Nucleoside Reverse Transcriptase Inhibitor; NVP = Nevirapine; EFV = Efavirenz; ; PI = Protease Inhibitor; 3TC = Lamivudine; d4T = Stavudine )
| Main first-line regimen used | Study reference |
|---|---|
| Adult FDC d4T/3TC/NVP | O’Brien et al., 2006; Bong et al., 2007; Ellis & Molyneux, 2007; Malawi Paediatric Antiretroviral Treatment Group, 2007 |
| 2 NRTIs + NVP | Marazzi et al., 2006; Nyandiko et al., 2006; Bolton-Moore et al., 2007; O’Brien et al., 2007; Song et al., 2007; Wamalwa et al., 2007 |
| 2 NRTIs + EFV/PI | Eley et al., 2004; Eley et al., 2006; Fassinou et al., 2004; Rouet et al., 2006; Reddi et al., 2007; Jooste et al., 2008 |
| 2 NRTIs + NVP/PI | Blé et al., 2007 |
| 2 NRTIs + NVP/EFV | Kamya, Mayanja-Kizza, Kambugu, Bakeera-Kitaka, Semitala, Mwebaze- Songa et al., 2007 |
Patient characteristics at the start of ART
Considering the 18 studies reviewed, a total of 7 477 children commenced ART, with the median (IQR) number per study being 236 (Interquartile range: 79–381). Gender representation was essentially equal, with females comprising 49.1% of the total cohort.
Age
The median/mean age of the children ranged from 4.2 to 9.2 years, in all except two studies. One of these specifically included only children aged 18 months to 5 years (i.e. O’Brien et al., 2007), while the median age of 1.9 years for the Cape Town cohort (i.e. Eley et al., 2006) was markedly younger than all the others. Where age categories are reported, it is clear that there are few infants and young children (age <2 years) on ART. Less than 12% were under 18 months old in all studies— except in one study each from South Africa and Mozambique, where 50% and 22% of children, respectively, were under age 2 years (Eley et al., 2006; Marazzi, Germano, Liotta, Buonomo, Guidotti & Palombi, 2006). Overall, approximately 9.5% (650 of 6 777) of children in all cohorts of those studies reporting age category were under age 18 months.
Disease severity
The majority of children had severe disease at ART commencement (Table 1). In most studies more than 70% of children had advanced clinical HIV disease (WHO stage 3 or 4, or CDC category C) and their median/mean weight-for-age (WFA) and height-for-age (HFA) z-scores were well below 0. Only three studies provided data on weight-for-height (WFH) z-scores, with medians between −0.6 and −1.64. The median CD4% was below 10% in all but two studies, both of which included relatively larger numbers of young children (i.e. Eley et al., 2004; Bolton-Moore et al., 2007). Indeed, in the Zambian cohort, median CD4% declines with increasing age at the start of ART (Bolton-Moore et al., 2007). Similarly, the proportion with CD4% <15% is high (≥79%) in all studies except those including many young children (i.e. Eley et al., 2006; Marazzi et al., 2006). Only seven of the 18 studies report on baseline viral load, indicating poor access to this technology, and the median/mean log10 viral load ranges were high. Studies from the Northern Cape Province (South Africa) and from Mozambique reported 80% and 55% of children having >5log10 viral load, respectively.
Seven studies reported on current or past opportunistic infections at the start of ART. Prevalence of TB at entry into the programme was 33% in Durban, South Africa (Reddi et al., 2007), and 46% in Dodoma, Tanzania (Blé, Floridia, Muhale, Motto, Giuliano, Gabbuti et al., 2007). The Tanzanian cohort, however, comprised a small group of institutionalised children and it is unclear whether TB was ‘active’ or previous. Other studies, from Zambia, Malawi and Côte d’Ivoire, reported lower proportions (6–7%) of active TB at commencement of ART, with previous TB in 13–24% of the children (Fassinou et al., 2004; Bolton-Moore et al., 2007; Bong, Chen, Jong, Tok, Hsu, Schouten et al., 2007). Nutritional/gastrointestinal illnesses, fever, recurrent pneumonia and dermatitis were not uncommon.
Caregivers
Two studies from South Africa and one each from Malawi and Kenya reported on the child’s main caregiver (i.e. Eley et al., 2004; Reddi et al., 2007; Ellis & Molyneux, 2007; Wamalwa, Farquhar, Obimbo, Selig, Mbori-Ngacha, Richardson et al., 2007). This caregiver was the mother for between 38% and 79% of the children, with the greater proportion occurring in the studies that included younger children. Fathers were the primary caregivers for up to 21% of children in some studies, but for far fewer children in other settings (e.g. Eley et al., 2004). Grandparents (mostly grandmothers) and other family members comprised the third-most-common primary caregivers, respectively providing care for 6–21% and 7–26% of children. Only 2–7% of the children were cared for by an institution.
Response to ART
Survival and retention in care
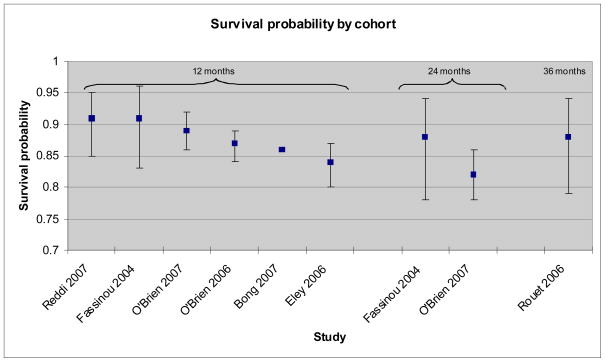
In all but four studies less than 10% of the children died during the follow-up period, with a maximum of 15.4% deaths in the study with the highest mortality (see Table 3). Loss to follow-up was also low (<10% in all but 2 studies). One-year survival probabilities are only reported by six studies and are all within a small range (0.84–0.91) (Figure 2). In general, lower probabilities of survival are seen in those studies where children were younger at commencement of ART.
Table 3.
Summary of the study outcomes (CD4% = CD4 percentage; IQR = Interquartile range; NR = not reported)
| Study | Median (IQR) follow-up duration (months) | Number of children on ART | Percentage deaths | Percentage loss to follow- up | Percentage transferred out | Percentage treatment stopped or withdrawn | Percentage outcomes unknown | Median (IQR) CD4% gain at one year | Percentage viral load <400 copies/ml at one year |
|---|---|---|---|---|---|---|---|---|---|
| Fassinou, 2004 | 21a | 78 | 11.5 | NR | NR | NR | NR | NR | 49.3 |
| Rouet, 2006 | 36 (30–42) | 78 | 11.5 | NR | NR | NR | NR | 15.6e | 46.5e |
| O’Brien, 2006 | 6 (2–12) | 1 184 | 3 | 8 | 0 | 0 | 3 | NR | NR |
| O’Brien, 2007 | 14 (7–20) | 568 | 6 | 8 | 4 | 2 | 1 | 16 (11–22.6)f | NR |
| Eley, 2004 | NR | 80 | 8.8 | 5 | 0 | 1.3 | 0 | NR | 75h |
| Eley, 2006 | 12b | 409 | 15.4d | 4.6 d | 11.2 d | 2.9 d | 0 d | NR | 69.7 |
| Jooste, 2005 | 6b | 100 | 9d | 3d | 1d | 3d | 0 d | NR | 67h |
| Nyandiko, 2006 | 8 (7–10)c | 279 | 3.6 | 11 | 0 | 0 | 0 | 11a | NR |
| Marazzi, 2006 | 10 (5–16) | 297 | 9.5 | 4.2 | 0 | 0 | 0 | NR | 46h |
| Blé, 2007 | 12b | 59 | 0d | 0d | 0d | 0d | 0d | 15a (10.3–19.7)c | NR |
| Reddi, 2007 | 8 (4–14) | 151 | 8.6 | 0 | 0 | 0 | 0 | 16.2 (9.6–20.3) | 80.3 |
| Song, 2007 | 15b | 29 | 1d | 3d | 0d | 0d | 0d | NR | 55i |
| Wamalwa et al., 2007 | 9 (3–15)j | 67 | 9 | lR | NR | NR | NR | 11.3 | 67h |
| Kamya et al., 2007 | 12b | 250 | 5d | 1d | 0d | 0d | 0d | 14.7;11.3g | 74 |
| Malawi Paediatric Antiretroviral Treatment Group, 2007 | 12b | 233 | 13d | 9d | 6d | 0d | 0d | NR | NR |
| Bolton-Moore et al., 2007 | 12 (5–23) | 2 938 | 6.7 | 13 | 5.4 | 0 | 0 | 14a (13.4–14.7)c | NR |
| Ellis & Molyneux, 2007 | 6b | 238 | 8d | 8d | 2d | 0d | 0d | NR | NR |
| Bong et al., 2007 | Variable up to 12 months | 439 | NR | NR | NR | NR | NR | NR | NR |
Mean value.
Only includes children with specified number of months potential follow-up duration; all children who were not lost to follow-up, transferred out, or deceased were followed up for this duration.
95% confidence interval.
Total percentage with given outcome in specified follow-up period.
At 36 months.
Children with baseline CD4 percentage between 5% and 15%.
Children with and without viral suppression, respectively.
At six months.
At nine months.
Range.
Figure 2.
Summary of survival probability as reported by nine paediatric ART studies from sub-Saharan Africa
Response to treatment in survivors
Clinical events
Fassinou et al. (2004) reported decreased incidence of pneumonia and diarrhoea after commencement of ART in children age <6.5 years from Cote d’Ivoire, with no change in older children, while Wamalwa et al. (2007) showed reduced hospitalisation during the six months after initiation of ART in a Kenyan programme. However, in that cohort, 58% of the children had been hospitalised during the six months preceding the start of ART.
Growth
The most consistent observation regarding growth in the 12 studies that reported this is improvement in weight-for-age (WFA) z-scores and/or reduced proportions in underweight, with the latter being reduced by 30–80% (Eley et al., 2006; Marazzi et al., 2006; Ellis & Molyneux, 2007; O’Brien et al., 2007). Improvement in weight-for-height (WFH) z-score (reduced proportion with wasting) also appears consistent across the studies, with the proportion wasted reduced to a similar degree (cf. Eley et al., 2006; Ellis & Molyneux, 2007). There is conflicting data with regard to height response: three studies showed improvement, albeit smaller than for other growth parameters, while three studies showed no change. It appears that while significant improvements in all growth parameters do occur after commencement of ART, it is rare for these to normalise.
Immune response
The median/mean increase in CD4% at 12 months was between 11% and 17.5% (Table 3). Rouet et al. (2006) found CD4% to increase during the first 18 months on treatment and then stabilise, with better immune recovery in those children on Nelfinavir as compared to Efavirenz-based regimens. Nyandiko, Ayaya, Nabakwe, Tenge, Sidle, Yiannoutsos et al. (2006) similarly found CD4% to increase rapidly during the first 30 weeks of ART, after which increases were much less pronounced.
Virological response
Ten studies reported on virological outcome at varying time intervals (see Table 3). This is a relatively high proportion as viral monitoring is not routinely available in sub-Saharan Africa outside South Africa and Botswana. At one year, the proportion of children with suppressed viral load (<400 copies/ml) in most studies was between 70% and 80%, but it was strikingly lower in the Côte d’Ivoire cohort at 49% (Fassinou et al., 2004). The Côte d’Ivoire cohort was the only one reporting on viral suppression beyond one year, with approximately half the children being suppressed at both age 24 months and age 36 months (Fassinou et al., 2004; Rouet et al., 2006).
Treatment changes, adverse effects and failure
No studies reported specifically on the criteria used to ascertain treatment failure; however, 13 studies reported the proportion of children requiring treatment changes due to failure, toxicity or other reasons. Changes due to toxicity were minimal (<10% of children) in most studies, except the Zambian cohort where single drug substitutions were required for 17.6% of the children. Reasons for these substitutions, however, included toxicity and dosing issues (Bolton-Moore et al., 2007). There were few adverse events reported in the six studies providing this data. Most common were skin rash, hepatotoxicity and anaemia. Two cases of Stephens-Johnson Syndrome occurred overall, one of which was fatal (Marazzi et al., 2006; Bolton-Moore et al., 2007). Switching treatment regimes due to failure was extremely rare (<5% in all studies that reported it) being highest in the Côte d’Ivoire cohort, with extended follow-up as expected (Rouet et al., 2006). This is in strong contrast to the proportion of children with viral suppression in that study by Rouet et al. (2006).: 50–70% throughout follow-up.
Adherence
Adherence to ART was measured in eight cohorts, mostly by self-report alone (four studies). Supplementary pill counts were used in two studies, while pill counts and drug pick-up information were the primary instruments for one study each. Overall, adherence was good, with significant interruptions (more than two missed doses per month) noted for less than 11% of children in any cohort.
Discussion
This review demonstrates overwhelmingly the feasibility and effectiveness of providing ART to children in sub-Saharan Africa despite enormous health-service and socio-economic constraints. Dramatic improvements in survival, and clinical, immune and virologic status were achieved. It is difficult to make valid comparisons with studies from the developed world due to differences in socioeconomic context, prior treatment experience, criteria for treatment initiation, ART regimens, and access to laboratory testing and specialist care (Patel et al., 2008). For example, while viral suppression rates of 70–80% in most of the cohorts with access to viral load testing were certainly at least as good as, if not better than, those from rich countries, this may be due to the relatively older age of the children commencing ART and the fact that most children in Africa are treatment naïve (Van Rossum, Fraaij & De Groot, 2002). Nevertheless, it is notable that the good outcomes seen in these studies are similar across a range of settings, including those with limited access to ART drugs and laboratory technology as well as those where ART is not provided by doctors and care is administered from PHC facilities (such as in Zambia and Malawi) (see Bolton-Moore et al., 2007; Ellis & Molyneux, 2007; Malawi Paediatric Antiretroviral Treatment Group, 2007). However, this review also highlights numerous aspects of the nature of current ART delivery in Africa, as described in the following sections.
Follow-up duration, mortality and retention in care
Due to the relatively recent availability of ART for children in Africa, follow-up periods are generally short, and long-term outcomes are as yet unexplored (except in the single small cohort from Côte d’Ivoire) (Rouet et al., 2006). Nonetheless, most deaths occurred within the first few months after commencing ART, thus estimates of mortality are not likely to increase significantly, at least in the medium term. This is supported by the single longer-term study reported by Fassinou et al. (2004) where at 42 months there were no deaths additional to those reported at 12–24 months (Rouet et al., 2006). Still, low mortality estimates should be viewed with caution in the five cohorts where short-term loss to follow-up (LFU) equaled or exceeded mortality (i.e. O’Brien et al., 2006; Nyandiko et al., 2006; Bolton-Moore et al., 2007; Ellis & Molyneux, 2007; O’Brien et al., 2007), as many of those classified as ‘LFU’ may have been deceased (Maskew, MacPhail, Menezes & Ruel, 2007; Krebs, Chi, Mulenga, Morris, Cantrell, Mulenga et al., 2008). It is notable that all except one of the studies with this limitation were from multi-site collaborations, suggesting that it may be easier to have definite outcome ascertainment within a single site— as these may have specific patient-tracing and follow-up systems in place, or else may have made a concerted effort to ensure outcome ascertainment for the purposes of publication. Notwithstanding this, LFU in children in Africa is far lower than that of adults (Stringer, Zulu, Levy, Stringer, Mwango, Chi et al., 2006). Interestingly, two cohorts (from Tanzania and Kenya, respectively) reported zero deaths and very low (3.4%) LFU (Blé et al., 2007; Song, Jelagat, Dzombo, Mwalimu, Mandaliya, Shikely & Essajee, 2007). Both of these were small cohorts (59 and 29 children, respectively). The first was a residential institution for orphaned HIV-infected children with a medical doctor on site daily, while the second, in addition to providing free ART, also provided transport re-imbursement to the clinic attendees.
Age at ART initiation
The overall low mortality reported by the studies reviewed also needs to be viewed in context of the children being relatively old at the start of ART, with less than 10% overall initiating treatment before 18 months of age. In contrast, we know that HIV disease progression to death or advanced disease occurs in 50% of perinatally HIV-infected children by age 2 years (Little et al., 2007). Many infants and young children in Africa clearly need but are not receiving ART. Indeed, ART-treated children in Africa represent a cohort of survivors with relatively slower progression of disease, and good outcomes are to be expected. It is not surprising that the study reporting the highest percentage of deaths after one year on treatment (15.6% mortality) is from a tertiary institution, with over 50% of that cohort having been less than age 2 years at ART commencement, and 99% of the children having advanced disease (Eley et al., 2006). Yet, even in this group of very ill children, mortality on ART was relatively low.
A number of factors explain the fact that in these studies children generally commenced ART at older ages than in developed countries (Patel et al., 2008). First, this reflects the difficulty in diagnosing HIV infection in children under 18 months old where access to virologic testing is limited (Bolton-Moore et al., 2007; Ellis & Molyneux, 2007; Malawi Paediatric Antiretroviral Treatment Group, 2007). This is also a consequence of poor access to appropriate ART formulations for children and the difficulty in dosing young children even where liquid formulations and refrigeration are available, due to the requirement for surface-area dosing. In fact, in Malawi, the site of three of the cohorts, all children were treated with split doses of the adult fixed-dose combination (FDC) Triomune (d4T/3TC/NVP) according to weight bands (Bong et al., 2007; Ellis & Molyneux, 2007; Malawi Paediatric Antiretroviral Treatment Group, 2007), while one of the multi-country MSF cohort studies had the specific aim of demonstrating feasibility and good outcomes with use of adult FDCs in children (O’Brien et al., 2006). . While the Malawian studies highlight the problems of dosing the smallest children (<8 kg) with tablet once daily (which is not recommended, as d4T should be given twice daily: (WHO, 2006), Ellis & Molyneux (2007) argue that the good outcomes reported support use of this regimen due to the benefits of low cost, availability and simplicity.
Many clinicians feel inadequately trained for the perceived complicated care of the very young (Eley & Nuttall, 2007; Meyers, Moultrie, Naidoo, Cotton, Eley & Sherman, 2007). In fact, the Malawian ART scale-up programme specifically treated young children only in health facilities where paediatricians or medical doctors skilled in paediatric care were available (Ellis & Molyneux, 2007). In South Africa, paediatric care, particularly of young infants, remains centered in tertiary-care institutions (Eley & Nuttall, 2007; Meyers et al., 2007).
The relative lack of young children receiving ART in Africa may also be an indication of the poor integration of HIV services for children and shortage of prevention-of-mother-to-child-transmission (PMTCT) programmes and general child health services, which although they clearly have overlapping target patient groups, are often run as separate vertical programmes within the health system. This may result in poor systems for detection and referral to ART care for HIV-infected infants and young children before disease progression. Many children with HIV infection are only diagnosed once they are severely ill, or die before an HIV diagnosis is made.
Severity of illness at ART initiation
Despite the relatively old age of children in these studies (age > 5 years) and the fact that they represent survivors with slower disease progression, most children in the total cohort were severely ill at commencement of therapy. At least half of the children commencing treatment had advanced clinical disease and the median CD4% at ART initiation was low. This is in keeping with the use of WHO guidelines for treatment eligibility by most programmes. However, in the multi-country MSF cohort, the final CD4% after one year remained lower in those with greater baseline immune suppression, suggesting that immune recovery may be limited if treatment is delayed until illness is advanced (O’Brien et al., 2007).
It has been suggested that the background burden of disease in Africa may exacerbate disease progression in HIV-infected children and thus complicate ART. In this regard, it is notable that two studies reported very high prevalence of TB at entry into the programme (i.e. Blé et al., 2007; Reddi et al., 2007). However, those figures may have been over-estimates. The difficulty of making a definitive diagnosis of TB in children, particularly when dually infected with HIV, may lead to over-diagnosis to avoid missed opportunities to treat a potentially curable opportunistic infection. Nevertheless, a high prevalence of TB in children is cause for concern. The medication burden and potential for TB immune reconstitution inflammatory syndrome (IRIS) may complicate ART (Smith, Kuhn, Coovadia, Meyers, Hu, Reitz et al., 2009). Furthermore, where lopinavir/ritonavir is used in the first-line regimen for young children, concomitant TB therapy requires additional ritonavir-boosting of the regimen. Notwithstanding, in Malawi, there was no difference in survival between children commencing ART with active TB, previous TB or a non-TB diagnosis (Bong et al., 2007); however, only Nevirapine (NVP) -based regimens were used, and ART was delayed until the continuation phase of TB treatment to avoid Rifampicin–NVP interactions and the risk of IRIS. (Bong et al., 2007).
Treatment durability
Treatment was well-tolerated with few adverse effects overall and few drug substitutions required. The rate of switching to second-line regimens was very low. This, however, needs to be viewed in the context of many of the cohorts not having access to virologic testing and/or second-line medication for children, as well as there being no current virologic guidelines for switching therapies in children (WHO, 2006). It is clear that a number of children are being maintained on first-line therapy despite ongoing viraemia, and the effects of this need to be examined in the long term. Furthermore, it would be useful to determine whether access to virologic testing has any implications for programme outcomes.
Growth response to treatment
Growth response as measured by scores for weight-for-age (WFA) and weight-for-height (WFH) was consistently good, despite probable poorer access to food in developing-country contexts, with very few programmes providing nutritional supplementation (Marazzi et al., 2006). Still, the failure to attain normal z-score values for these parameters may reflect the residual insult of advanced disease. This is possibly also the reason for conflicting measures for growth response in terms of height-for-age (HFA), a reflection of chronic illness.
Representativeness
An important limitation of this review is that these studies may not be representative of all routine programmes across Africa. Although all are observational cohorts, many are from research settings, where care, monitoring, access to drugs and laboratory testing may be markedly better than what is available in routine services. The relatively high proportion of studies with follow-up information on patients’ viral load supports this. In addition, there is a probable publication bias in favour of successful programmes; hence, the restriction of this review to published clinical studies may have selected programmes with better outcomes. It is plausible that country-level data, programme reports, and social-science literature may portray a different picture. Furthermore, all but one of the cohorts reporting on funding had received some form of external support in addition to that from state health services, albeit through the national programme in some cases. Nevertheless, two of the studies reported on the outcomes of routine national programmes (i.e. Malawi Paediatric Antiretroviral Treatment Group, 2007; Jooste, Van Zyl, Baker, Crawford & Jassen, 2008), with one using only routinely collected data, yet their findings are consistent with those from research settings.
In addition to financial programme support, there was evidence of considerable international academic collaboration within these studies. While one-third of these published studies have both local senior and lead authorship, in an almost equal proportion (five of 16), both these roles are taken by international academics, and all except four of the publications (three cohorts) included at least one international collaborator. It is therefore possible that patient care at these sites may have been enhanced through the involvement of research institutions from wealthy countries, and thus may not be replicable on a massive scale. This also demonstrates the need to develop research and analytic capacity in sub-Saharan Africa so that lessons learned can be disseminated.
Conclusions
The feasibility and benefits of ART provision for children in sub-Saharan Africa have been clearly shown in a range of settings; however, infants and young children remain largely excluded from ART programmes, and commencement of ART occurs mostly in older children with advanced HIV disease. Emerging evidence of rapid progression of disease in children failing PMTCT regimens, and the survival benefit with early initiation of ART, has led to the revision of WHO guidelines to recommend ART commencement for all HIV-infected children under age 1 year (Mphatswe, Blanckenberg, Tudor-Williams, Prendergast, Thobakgale, Mkhwanazi et al., 2007; Violari, Cotton, Gibb, Babiker, Steyn, Madhi et al., 2008; WHO, 2008). There is an urgent need both to address barriers to the care of young children with HIV infection and to acquire a better understanding of the outcomes for children on ART and the determinants in this group.
Acknowledgments
Mary-Ann Davies, Andrew Boulle and Matthias Egger received support from the International Epidemiological Databases to Evaluate AIDS in Southern Africa (IeDEA-SA), a collaboration funded by the National Institute of Allergy and Infectious Diseases (NIAID) (USA) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (USA) (grant 1 U01 AI069924–01).
Biographies
Mary-Ann Davies is a registrar in public health, with an interest in paediatric HIV and TB. She has been involved with the antiretroviral treatment programme at the Red Cross Children’s Hospital in Cape Town, South Africa.
Matthias Egger is a professor of epidemiology and public health with a long-standing interest in HIV/AIDS. He is one of the principal investigators of the International epidemiological Databases to Evaluate AIDS (IeDEA).
Olivia Keiser has a master’s degree in biology. She was the epidemiologist in the data centre of the Swiss HIV Cohort Study. Currently, she is doing research towards a PhD, comparing treatment approaches and outcomes of HIV-positive patients in high-income and low-income countries.
Andrew Boulle is a public health specialist with an interest in the routine monitoring of HIV-treatment interventions. Since 2002, he has worked closely with the Khayelitsha and Western Cape Provincial antiretroviral treatment programmes in South Africa.
References
- Blé C, Floridia M, Muhale C, Motto S, Giuliano M, Gabbuti A, Giuman L, Mazzotta F. Efficacy of highly active antiretroviral therapy in HIV-infected, institutionalized orphaned children in Tanzania. Acta Paediatrica. 2007;96(7):1090–1094. doi: 10.1111/j.1651-2227.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, Chintu N, Stringer EM, Chi BH, Sinkala M, Kankasa C, Wilson CM, Wilfert CM, Mwango A, Levy J, Abrams EJ, Bulterys M, Stringer JS. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298(16):1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- Bong CN, Chen SC, Jong YJ, Tok TS, Hsu CF, Schouten EJ, Makombe SD, Yu JK, Harries AD. Outcomes of HIV-infected children with tuberculosis who are started on antiretroviral therapy in Malawi. International Journal of Tuberculosis and Lung Disease. 2007;11(5):534–538. [PubMed] [Google Scholar]
- Eley B, Davies M-A, Apolles P, Cowburn C, Buys H, Zampoli M, Finlayson H, King S, Nuttall J. Antiretroviral treatment for children. South African Medical Journal. 2006;96(9):988–993. [PubMed] [Google Scholar]
- Eley B, Nuttall J. Antiretroviral therapy for children: challenges and opportunities. Annals of Tropical Paediatrics. 2007;27:1–10. doi: 10.1179/146532807X170448. [DOI] [PubMed] [Google Scholar]
- Eley B, Nuttall J, Davies MA, Smith L, Cowburn C, Buys H, Hussey G. Initial experience of a public-sector antiretroviral treatment programme for HIV-infected children and their infected parents. South African Medical Journal. 2004;94(8):643–646. [PubMed] [Google Scholar]
- Ellis J, Molyneux EM. Experience of anti-retroviral treatment for HIV-infected children in Malawi: the 1st 12 months. Annals of Tropical Paediatrics. 2007;27(4):261–267. doi: 10.1179/146532807X245643. [DOI] [PubMed] [Google Scholar]
- Fassinou P, Elenga N, Rouet F, Laguide R, Kouakoussui KA, Timite M, Blanche S, Msellati P. Highly active antiretroviral therapies among HIV-1-infected children in Abidjan, Côte d’Ivoire. AIDS. 2004;18(14):1905–1913. doi: 10.1097/00002030-200409240-00006. [DOI] [PubMed] [Google Scholar]
- Jooste JP, Van Zyl AJM, Baker A, Crawford W, Jassen A. Antiretroviral treatment in the Northern Cape. South African Medical Journal. 2008;95(11):812. [PubMed] [Google Scholar]
- Kamya MR, Mayanja-Kizza H, Kambugu A, Bakeera-Kitaka S, Semitala F, Mwebaze-Songa P, Castelnuovo B, Schaefer P, Spacek LA, Gasasira AF, Katabira E, Colebunders R, Quinn TC, Ronald A, Thomas DL, Kekitiinwa A. Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2007;46(2):187–193. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- Krebs DW, Chi BH, Mulenga Y, Morris M, Cantrell RA, Mulenga L, Levy J, Sinkala M, Stringer JS. Community-based follow-up for late patients enrolled in a district-wide programme for antiretroviral therapy in Lusaka, Zambia. AIDS Care. 2008;20:311–317. doi: 10.1080/09540120701594776. [DOI] [PubMed] [Google Scholar]
- Little K, Thorne C, Luo C, Bunders M, Ngongo N, McDermott P, Newell ML. Disease progression in children with vertically-acquired HIV infection in sub-Saharan Africa: reviewing the need for HIV treatment. Current HIV Research. 2007;5(2):139–153. doi: 10.2174/157016207780077002. [DOI] [PubMed] [Google Scholar]
- Malawi Paediatric Antiretroviral Treatment Group. Antiretroviral therapy for children in the routine setting in Malawi. Transcripts of the Royal Society of Tropical Medicine and Hygiene. 2007;101:511–516. doi: 10.1016/j.trstmh.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Marazzi MC, Germano P, Liotta G, Buonomo E, Guidotti G, Palombi L. Pediatric highly active antiretroviral therapy in Mozambique: an integrated model of care. Minerva Pediatrica. 2006;58(5):483–490. [PubMed] [Google Scholar]
- Maskew M, MacPhail P, Menezes C, Ruel D. Lost to follow-up — contributing factors and challenges in South African patients on antiretroviral therapy. South African Medical Journal. 2007;97:853–857. [PubMed] [Google Scholar]
- Meyers T, Moultrie H, Naidoo K, Cotton M, Eley B, Sherman G. Challenges to pediatric HIV care and treatment in South Africa. Journal of Infectious Diseases. 2007;196(supplement 3):S474–S481. doi: 10.1086/521116. [DOI] [PubMed] [Google Scholar]
- Mphatswe W, Blanckenberg N, Tudor-Williams G, Prendergast A, Thobakgale C, Mkhwanazi N, McCarthy N, Walker BD, Kiepiela P, Goulder P. High frequency of rapid immunological progression in African infants infected in the era of perinatal HIV prophylaxis. AIDS. 2007;19(10):1253–1261. doi: 10.1097/QAD.0b013e3281a3bec2. [DOI] [PubMed] [Google Scholar]
- Nyandiko WM, Ayaya S, Nabakwe E, Tenge C, Sidle JE, Yiannoutsos CT, Musick B, Wools-Kaloustian K, Tierney WM. Outcomes of HIV-infected orphaned and non-orphaned children on antiretroviral therapy in western Kenya. Journal of the Acquired Immune Deficiency Syndromes. 2006;43(4):418–425. doi: 10.1097/01.qai.0000243122.52282.89. [DOI] [PubMed] [Google Scholar]
- O’Brien DP, Sauvageot D, Olson D, Schaeffer M, Humblet P, Pudjades M, Ellman T, Zachariah R, Szumilin E, Arnould L, Read T. Treatment outcomes stratified by baseline immunological status among young children receiving non-nucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in resource-limited settings. Clinical Infectious Diseases. 2007;44(9):1245–1248. doi: 10.1086/513433. [DOI] [PubMed] [Google Scholar]
- O’Brien DP, Sauvageot D, Zachariah R, Humblet P. In resource-limited settings good early outcomes can be achieved in children using adult fixed-dose combination antiretroviral therapy. AIDS. 2006;20(15):1955–1960. doi: 10.1097/01.aids.0000247117.66585.ce. [DOI] [PubMed] [Google Scholar]
- Patel K, Hernan MA, Williams PL, Seeger JD, McIntosh K, Van Dyke RB, Seage GR., III Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clinical Infectious Diseases. 2008;46(4):507–515. doi: 10.1086/526524. [DOI] [PubMed] [Google Scholar]
- Reddi A, Leeper SC, Grobler AC, Geddes R, France KH, Dorse GL, Vlok WJ, Mntambo M, Thomas M, Nixon K, Holst HL, Karim QA, Rollins NC, Coovadia HM, Giddy J. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatrics. 2007;7(13):13. doi: 10.1186/1471-2431-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouet F, Fassinou P, Inwoley A, Anaky MF, Kouakoussui A, Rouzioux C, Blanche S, Msellati P. Long-term survival and immuno-virological response of African HIV-1-infected children to highly active antiretroviral therapy regimens. AIDS. 2006;20(18):2315–2319. doi: 10.1097/QAD.0b013e328010943b. [DOI] [PubMed] [Google Scholar]
- Smith K, Kuhn L, Coovadia A, Meyers T, Hu CC, Reitz C, Barry G, Strehlau R, Sherman G, Abrams EJ. Immune reconstitution inflammatory syndrome among HIV-infected South African infants initiating antiretroviral therapy. AIDS. 2009;23(9):1097–1107. doi: 10.1097/QAD.0b013e32832afefc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Jelagat J, Dzombo D, Mwalimu M, Mandaliya K, Shikely K, Essajee S. Efficacy of highly active antiretroviral therapy in HIV-1 infected children in Kenya. Pediatrics. 2007;120(4):e856–e861. doi: 10.1542/peds.2006-1122. [DOI] [PubMed] [Google Scholar]
- Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, Mtonga V, Reid S, Cantrell RA, Bulterys M, Saag MS, Marlink RG, Mwinga A, Ellerbrock TV, Sinkala M. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296(7):782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- Sutcliffe CG, Van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. The Lancet Infectious Diseases. 2008;8:477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- UNAIDS. AIDS Epidemic Update, December 2006. Geneva: World Health Organization (WHO); 2006. [Google Scholar]
- UNAIDS. 2008 Report on the Global AIDS Epidemic. Geneva: World Health Organization (WHO); 2008. [Google Scholar]
- Van Rossum AMC, Fraaij PLA, De Groot R. Efficacy of highly active antiretroviral therapy in HIV-1 infected children. The Lancet Infectious Diseases. 2002;2:93–102. doi: 10.1016/s1473-3099(02)00183-4. [DOI] [PubMed] [Google Scholar]
- Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, Jean-Philippe P, McIntyre JA. Early antiretroviral therapy and mortality among HIV-infected infants. New England Journal of Medicine. 2008;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamalwa DC, Farquhar C, Obimbo EM, Selig S, Mbori-Ngacha DA, Richardson BA, Overbaugh J, Emery S, Wariua G, Gichuhi C, Bosire R, John-Stewart G. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. Journal of Acquired Immune Deficiency Syndromes. 2007;45(3):311–317. doi: 10.1097/QAI.0b013e318042d613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Scaling Up Antiretroviral Therapy in Resource-Limited Settings: Treatment Guidelines for a Public Health Approach, 2003 Revision. Geneva: World Health Organization (WHO); 2004. [Google Scholar]
- WHO. Antiretroviral Therapy of HIV Infection in Infants and Children: Towards Universal Access. Geneva: World Health Organization (WHO); 2006. [PubMed] [Google Scholar]
- WHO. Report of the WHO Technical Reference Group meeting, Paediatric HIV/ART Care Guideline Meeting; 10–11 April 2008; Geneva: World Health Organization (WHO); 2008. Available at: < http://www.who.int/hiv/pub/meetingreports/art_meeting_april2008/en/index.html>. [Google Scholar]
- WHO/UNAIDS. WHO Global Health Atlas. Geneva: World Health Organization (WHO); 2006. [Accessed 4 June 2009]. Available at: < http://apps.who.int/globalatlas/dataQuery/default.asp>. [Google Scholar]
- WHO, UNICEF & UNAIDS. Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector. Geneva: World Health Organization (WHO); 2008. [Google Scholar]