Abstract
In clinical studies, postnatal weight gain is strongly associated with retinopathy of prematurity (ROP). However, animal studies are needed to investigate the pathophysiological mechanisms of how postnatal weight gain affects the severity of ROP. In the present study, we identify nutritional supply as one potent parameter that affects the extent of retinopathy in mice with identical birth weights and the same genetic background. Wild-type pups with poor postnatal nutrition and poor weight gain (PWG) exhibit a remarkably prolonged phase of retinopathy compared to medium weight gain or extensive weight gain pups. A high (r2 = 0.83) parabolic association between postnatal weight gain and oxygen-induced retinopathy severity is observed, as is a significantly prolonged phase of proliferative retinopathy in PWG pups (20 days) compared with extensive weight gain pups (6 days). The extended retinopathy is concomitant with prolonged overexpression of retinal vascular endothelial growth factor in PWG pups. Importantly, PWG pups show low serum levels of nonfasting glucose, insulin, and insulin-like growth factor-1 as well as high levels of ghrelin in the early postoxygen-induced retinopathy phase, a combination indicative of poor metabolic supply. These differences translate into visual deficits in adult PWG mice, as demonstrated by impaired bipolar and proximal neuronal function. Together, these results provide evidence for a pathophysiological correlation between poor postnatal nutritional supply, slow weight gain, prolonged retinal vascular endothelial growth factor overexpression, protracted retinopathy, and reduced final visual outcome.
Although retinopathy of prematurity (ROP) in human infants develops postnatally, birth weight and short gestational age at birth have long been considered the most potent predictors for ROP.1 Postnatal oxygen incubation was recognized as an additional risk factor, but with modern perinatal care, the detrimental effects of oxygen incubation have been minimized.2 Postnatal weight gain, in contrast, has only recently received growing attention among clinicians as an independent, postnatal variable that can reliably predict ROP.3,4,5,6,7 To understand the pathophysiology of how postnatal weight gain affects ROP, we must identify and investigate the individual components of postnatal weight gain that can have an impact on pathological angiogenesis in the developing retina. This can best be achieved in the established animal models of oxygen-induced retinopathy (OIR), where the hallmarks of human ROP are replicated and retinal neovascularization (NV) can be studied in a well controlled setting.8,9
Similar to human ROP where the prematurely born infant is initially exposed to an environment that is relatively hyperoxic in comparison to the intrauterine environment,10 the OIR model exposes mouse pups to hyperoxic conditions (75% O2 from postnatal day (P)7 to P12) to induce vaso-obliteration (VO) of immature retinal capillaries. Also analogous to human ROP, where the second phase is characterized by tissue hypoxia of the avascular retina and subsequent up-regulation of proangiogenic factors, mouse pups in the second phase of the OIR model (P12–P17) reactivate retinal angiogenesis, leading to both functional vessel regrowth as well as pathological retinal NV.8,11
Our results demonstrate that mice with poor postnatal weight gain (PWG) exhibit a delayed and remarkably prolonged phase of retinopathy compared with medium weight gain (MWG) or extensive weight gain (EWG) pups. Associated with the differences in postnatal weight gain are distinct patterns for serum markers of metabolic supply. Increasing metabolic supply in litter-matched experiments isolates postnatal nutritional intake as one potent parameter affecting both postnatal weight gain and the severity of ROP. Locally, in the retina, these differences in nutritional supply and weight gain translate into distinct patterns of vascular endothelial growth factor (VEGF) expression: In PWG pups, retinal VEGF overexpression is significantly prolonged compared with MWG or EWG pups, matching the prolonged time course of active retinopathy in these pups. This prolongation of retinopathy, in turn, results in electroretinographic (ERG) deficits in adult PWG mice, demonstrating that insufficient nutritional supply and poor postnatal weight gain in association with changes in retinal VEGF expression profoundly alter the course and outcome of ROP.
Materials and Methods
Animals
All studies adhered to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Children’s Hospital Boston Animal Care and Use Committee. C57BL/6 (Taconic stock no. B6-F, B6-M) were purchased from Taconic Farms (Germantown, NY) and used for all experiments. The deciding factor for grouping the mice was weight at P17 (PWG <5 g, MWG 5–7.5 g and EWG >7.5 g at P17). Weights collected before P17 were entered retrospectively after the group assignment was decided based on P17 weights. Not all EWG mice were kept with two dams. We observed spontaneous EWG also in pups with one dam and small litter size.
O2-Induced Retinopathy
Mice were exposed to 75% oxygen from P7 to P12. On return to room air, hypoxia-driven NV is initiated and becomes morphologically visible from P15 onward.8,11 To quantify NV and VO, at age of analysis, mice were given a lethal dose of Avertin, and eyes were enucleated and fixed in 4% paraformaldehyde for 1 hour at room temperature. Retinas were dissected and stained overnight with fluoresceinated Isolectin B4 (Alexa Fluor 594 – I21413; Molecular Probes, Eugene, OR) in 1 mmol/L CaCl2 in PBS. Lectin-stained retinas were flat-mounted with the photoreceptor side down, and 20 images of each flat-mounted retina were obtained at 5× magnification on a Zeiss AxioObserver.Z1 microscope and merged to form one image using AxioVision 4.6.3.0 software. VO was quantified by manually outlining the avascular area using Adobe Photoshop.11 NV was analyzed using the SWIFT_NV method.12 SWIFT_NV consists of a set of macros that was developed to run on the National Institutes of Health’s free ImageJ platform. In brief, SWIFT_NV isolates the red channel from a lectin-stained retinal whole mount, divides the image into four quadrants, and removes background fluorescence to allow for the NV structures to stand out clearly against the background fluorescence of normal vessels. Using a slide bar to either increase or decrease a particular quadrant’s fluorescence threshold, the SWIFT_NV user designates a threshold that marks NV structures but not normal vessels to each quadrant. After setting the appropriate threshold, artifacts like remaining hyaloid vessels, cellular debris, or hyperfluorescent retinal edges can be manually marked and excluded from quantification. SWIFT_NV then analyzes all pixels in the image that lie above the chosen intensity threshold and are part of an object that has a defined minimum size. By setting this fixed cut-off in object size, small artifacts like vessel branch points are automatically removed. After measuring all four quadrants, SWIFT_NV creates a composite from all four NV quadrants and calculates the total NV pixel number.12
Serum Marker Measurements
Blood was obtained from mice at the indicated postnatal days. Care was taken to obtain blood samples at the same hour of the day to control for circadian fluctuations.13 Blood glucose was measured using Accu-Chek (Roche, Mannheim, Germany). The remaining blood was allowed to clot at room-temperature for 2 hours. After centrifugation (20 minutes at 2000 × g), the serum was collected, frozen at −80°C, and later analyzed using Insulin ELISAs (Crystal Chem.), insulin-like growth factor (IGF)-1 ELISAs (R&D Systems, Minneapolis, MN), and ghrelin ELISAs (Millipore, Bedford, MA) following the manufacturers’ instructions.
Retinal VEGF Gene and Protein Expression
Retinas were isolated at the indicated time points, frozen in liquid nitrogen, and stored at −80°C. For gene expression analysis, RNA was isolated using tissue homogenizer columns and RNA mini columns (Qiagen, Chatsworth, CA). Samples were treated with DNase I (Qiagen, Chatsworth, CA) to remove any contaminating genomic DNA and converted into cDNA using reverse transcriptase (Invitrogen, Carlsbad, CA). PCR primers targeting VEGF and an unchanging control gene, cyclophilin A, were designed using Primer Bank and National Center for Biotechnology Information Primer Blast software (sequences available on request). Quantitative analysis of gene expression was performed using an ABI Prism 7700 Sequence Detection System with the SYBR Green Master Mix kit. Gene expression was calculated relative to cyclophilin A using the ΔcT method. For protein analysis, retinas were homogenized and sonicated in 1% Nonidet P-40 in PBS (Roche Diagnostics) containing an array of phosphatase and protease inhibitors (Sigma-Aldrich, St. Louis, MO). Samples were normalized using a bicinchoninic acid assay (Pierce, Rockford, IL) and quantified for VEGF protein using a commercially available ELISA system (R&D Systems).
H&E Stain
Cryosections were prepared at 12-μm thickness, and staining was performed following standard protocols using H&E reagents from Sigma-Aldrich.
Measurement of Retinal Vessel Density
Retinas from P70 mice were obtained, and lectin-stained flat-mounts prepared as described above. Using ImageJ’s threshold algorithm, the area covered by lectin-positive vessels was quantified relative to the whole retinal area. Data are presented as the mean + SD.
Electroretinography
Retinal function was assessed at P70. Mice were presented with a series of “green” flashes of doubling intensity from 0.000500 to 2.05 cd · s · m−2 and then “white” flashes from 8.19 to 1050 cd · s · m−2; the white flash was found to be half as efficient (per cd · s · m−2) at eliciting a b-wave.14 The response to a 1.024 cd · s · m−2 light flickering at 8 Hz was also studied. The amplitude (RmP3) and sensitivity (S) of the rod photoresponse (PIII) were estimated from the ERG by ensemble fit of the parameters of the Hood and Birch15 formulation of the Lamb and Pugh16,17 model of the biochemical processes involved in the activation of phototransduction to the a-waves elicited by the white flashes. “Ensemble” means the mean square error across all included traces was minimized simultaneously. Fitting of the model was restricted to the leading edge of the a-wave. The amplitude (RmP2) and sensitivity (Log kP2) of the bipolar cell response was derived by fit of the Naka-Rushton function to the response versus intensity relationship of PII, the putatively pure postreceptor potential obtained by subtraction of PIII from the intact ERG waveform. The oscillatory potentials (OPs) were analyzed, ensemble, in the frequency domain following discreet Fourier transform of the first 100 ms of PII.18 The saturating energy in the OPs (Em) was derived, similarly to RmP2, by fit of the Michaelis-Menton equation to the response versus intensity relationship of OP energy. The trough-to-peak amplitude of the flicker response (R8) was also measured. All data were described as the log change from the EWG group; by expressing the data in log values, changes in observations of fixed proportion, either up or down, become linear, consistent with a constant fraction for physiologically meaningful changes in parameter values.
Statistics
Differences between groups were detected using analysis of variance corrected for multiple testing with Tukey-Kramer honestly significant difference. Comparisons between two groups were performed using unpaired Student’s t-test.
Results
Postnatal Weight Gain Determines Time Course and Severity of Retinopathy
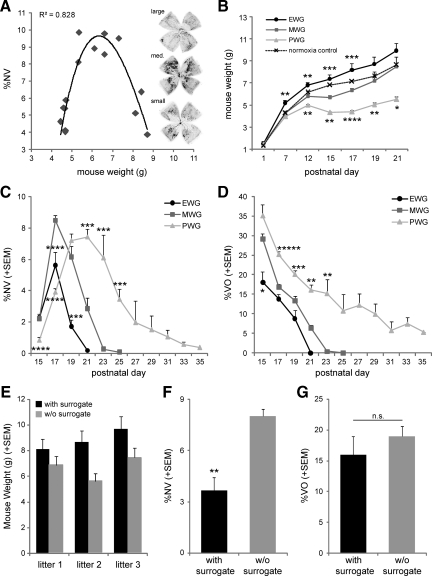
Inherent differences in weight gain in pups during the OIR model have been noted before, both in mice and rats.19,20 However, a stringent analysis of the correlation between weight gain and severity of retinopathy in rodents during OIR remained to be performed. We therefore initiated our study by screening a cohort of 15 litters of C57BL/6 wild-type mice undergoing OIR for correlations between postnatal body weight and extent of NV at P17, the standard time point to investigate NV in the OIR mouse model.8 Our results revealed a parabolic correlation between body weight and extent of NV at P17 (r2 = 0.83; Figure 1A). Mice with body weights between 5 and 7.5 g at P17 displayed the highest amount of NV, while mice with either less than 5 g or more than 7.5 g body weight at P17 showed significantly lower severity of NV.
Figure 1.
Postnatal weight gain modifies course and severity of OIR. A: Neovascularization (NV) in mice with oxygen-induced retinopathy shows a parabolic correlation with body weight at P17 (r2 = 0.83). Examplary lectin-stained retinal flat-mounts illustrate the highest amount of NV (black areas) in medium weight pups (middle) compared with high weight pups (top) and low weight pups (bottom); n = 15 litters. B: Longitudinal development of postnatal weight measured in 226 C57BL/6 wild-type OIR pups. Mice were exposed to hyperoxia (75% O2) from P7–P12, followed by relative hypoxia at room air (21% O2) from P12 onwards. Three groups were defined based on weights at P17: PWG (P17 weight < 5 g), MWG (P17 weight 5–7.5 g), and EWG (P17 weight >7.5g). The dashed line represents normoxic control mice. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001 for significant differences from MWG group (ANOVA with Tukey-Kramer honestly significant difference); n = 10–98 mice/group and time point. C and D: Retinal NV and VO from all three weight groups. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; *****P < 10−5 for difference from MWG group (ANOVA with Tukey-Kramer honestly significant difference). n = 3–42 retinas/group and time point. E: P17 weight data for three independent litters that were split at P1. One-half of each litter was raised with additional surrogate mother (black bar), while the other half of each litter was raised without additional surrogate (w/o surrogate; gray bar). Weights from surrogated pups resemble EWG values at P17, while littermates without surrogate resemble MWG values at P17. F and G: Retinal NV is lower in surrogated pups at P17 compared with nonsurrogated littermates. NV values in surrogated pups resemble EWG values. NV values in nonsurrogated pups resemble MWG values. VO does not differ between the two groups; **P < 0.01.
We next asked if the difference in body weight at P17 was due to different birth weights or differences in postnatal weight gain. On the basis of the three different weight clusters observed in Figure 1A, three distinct groups were defined and followed from P1 to P21: P17 body weight below 5 g (group 1), P17 body weight between 5 and 7.5 g (group 2), and P17 body weight above 7.5 g (group 3). Longitudinal weight data collected from a total of 226 C57BL/6 wild-type mice revealed that these three groups do not differ in weights at P1 (Figure 1B). It was thus postnatal weight gain that results in the observed differences at P17. On the basis of this result, group 1 was labeled PWG (<5 g at P17), group 2 MWG (5–7.5 g at P17), and group 3 EWG (>7.5 g at P17). Starting from identical weights at P1, EWG pups are characterized by significantly higher weights compared with MWG, PWG, and the normoxic control group by P7. The MWG group closely follows the normoxic growth curve up to and throughout the hyperoxic incubation period from P7–P12. However, after return to room air at P12, MWG pups display a marked slow-down in weight gain compared with normoxic controls that is only corrected by P19. In a strikingly different pattern, PWG pups show slower weight gain during hyperoxic incubation (P7–P12) followed by a 14% weight loss during the early hypoxic phase and significantly slower growth rates throughout the whole observation period.
To investigate how these different postnatal weight gain patterns correlate with retinopathy, we analyzed NV and VO on a total of 216 retinas from P15 to P35 (Figure 1, C and D). Both EWG and MWG pups exhibit a similar time course in the evolution and regression of NV, with maximal severity at P17 and relatively rapid regression thereafter (Figure 1C). However, peak severity of NV is significantly lower in EWG mice compared with MWG (5.6 ± 0.9 versus 8.5 ± 0.3%NV at P17; P < 0.0001), and resolution of NV is correspondingly found earlier in EWG mice (P21 versus P25). Different from both EWG and MWG mice, PWG pups show a delayed onset of NV with lower magnitude at P17 (3.9 ± 0.2%NV; P < 10−5). However, in contrast to EWG and MWG pups, NV severity in PWG pups keeps rising beyond P17, resulting in a prolonged NV peak from P19–P21 and only slow resolution thereafter. Complete NV resolution in PWG pups is only reached by P35. Concordant with the findings for NV, the time course and severity of VO also differs significantly between groups (Figure 1D). EWG mice show the quickest VO repair followed closely by MWG mice (13.8 ± 1.2 versus 16.9 ± 0.8% VO at P17; P > 0.05). PWG mice, in contrast, exhibit a markedly delayed VO repair with 25.2 ± 0.8%VO remaining at P17 (P < 0.0001) and 5% of the retina still being avascular by P35.
To investigate whether postnatal weight gain is causally linked to the observed differences in OIR patterns, we separated each of three C57BL/6 litters into two groups at P1. One-half of each litter (three pups) remained with their nursing mother. The other half of each litter was transferred to a new cage with two surrogate dams. Importantly, at P1 when the litters were separated, weights are evenly distributed across groups (Figure 1B). This experimental setup allowed us to investigate i) whether differences in postnatal nutritional supply determine in which weight group the litter-matched pups develop and ii) whether pups from the same litter develop different degrees of retinopathy based on their nutritional supply. Our results demonstrate that in all three litters, the pups with an additional surrogate dam develop into EWG pups (8.85 ± 0.5 g at P17), while their littermates without surrogate follow the MWG growth curve (6.67 ± 0.5 g at P17; Figure 1E). OIR pups with an extra surrogate dam exhibit better weight gain and lower NV at P17 compared with pups reared by only their mother (3.7 ± 0.8 versus 8.0 ± 0.4%NV; P < 0.01; Figure 1F). VO at P17 does not differ with the addition of a surrogate (16.0 ± 3.0 versus 19.0 ± 1.6%VO; P > 0.05; Figure 1G). These results parallel the values obtained for EWG and MWG mice in the larger cohort (Figure 1, C and D) and thus provide evidence that alteration of nutritional supply in littermate-controlled mice (presenting identical genetic backgrounds) alters postnatal weight gain and the severity of retinopathy. PWG pups do not arise in these littermate-controlled experiments because due to the litter split at P1, the two groups contain only three to four pups each, and even with only one dam, adequate nutritional supply for three to four pups is provided.
Prolonged Up-Regulation of Retinal VEGF in PWG Pups
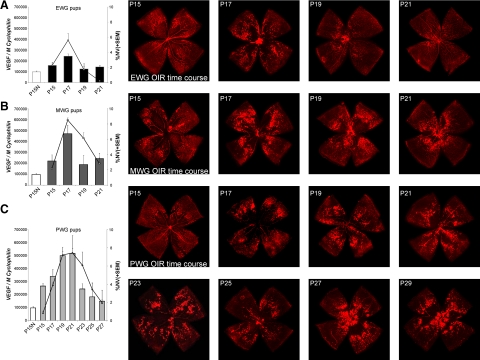
After establishing a correlation between nutritional supply, postnatal weight gain, and pathological retinal angiogenesis, we aimed to investigate potential differences in retinal VEGF expression between groups. VEGF was significantly up-regulated during the hypoxic phase of OIR in all three groups (P15–P21; Figure 2A–C). Notably, in each weight group, the time course of VEGF expression (bars) parallels the time course of NV (lines). EWG mice show the least VEGF up-regulation, peaking at P17 together with the peak of NV formation. MWG mice display a similar time course of VEGF induction, however, with significantly higher VEGF up-regulation at P17 that matches the more pronounced NV spike at P17. PWG mice, in contrast, exhibit a prolonged VEGF up-regulation with a broad peak from P19–P21 that parallels the protracted NV formation in these pups. Lectin-stained representative retinal flat-mounts illustrate the described differences in NV and VO time courses (Figure 2, right panel). Protein measurements for VEGF show a tight correlation with the mRNA data and confirm the limited VEGF up-regulation in EWG pups at P17 compared with PWG or MWG pups (Supplemental Figure 1A, see http://ajp.amjpathol.org). These results establish a correlation between postnatal weight gain, retinal VEGF overexpression, and the course of proliferative retinopathy.
Figure 2.
Retinal VEGF mRNA up-regulation mirrors duration and severity of proliferative retinopathy. A: In EWG pups, retinal up-regulation of VEGF mRNA (bars) peaks at P17, coinciding with the spike in retinal neovascularization (NV; lines). Representative lectin-stained retinal flat-mounts illustrate relatively mild proliferative retinopathy with almost complete resolution by P21. B: MWG pups show a similar pattern of VEGF up-regulation and course of retinopathy. Peak VEGF expression as well as maximal disease severity, however, are higher compared with EWG pups. Representative images on the right illustrate the more severe course of retinopathy. C: PWG pups exhibit a strikingly different pattern of VEGF up-regulation and retinopathy with peak VEGF expression between P19 and P21, coinciding with prolonged maximal NV formation. Representative retinal flat-mounts illustrate the extended course of retinopathy in PWG pups with active NV and remaining avascular retinal areas until P29.
Reduced Serum Markers of Postnatal Metabolic Supply in PWG Pups
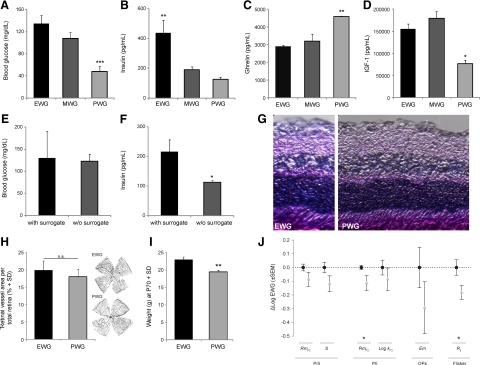
In addition to analyzing local VEGF up-regulation in the retina of OIR mice, we aimed to indentify serum markers that were differentially regulated between the three postnatal weight gain groups. Because fasting measurements are not feasible in nursing pups we tested glucose, IGF-1, and insulin under non-fasting conditions (at the same hour of the day to control for circadian fluctuations). Both non-fasting glucose and insulin levels have been shown to correlate with food intake.13 In our study, non-fasting glucose and insulin were highest in EWG pups and lowest in PWG pups at P17, indicating enhanced nutrient intake in EWG pups and deficiencies in PWG pups (Figure 3, A and B). This notion is supported by increased ghrelin levels in PWG pups at P17 (Figure 3C). Ghrelin is a peptide hormone that is released from gastric endocrine cells during fasting.21 Increased serum ghrelin levels in PWG pups therefore indicate a fasting state in PWG pups compared with EWG or MWG pups at P17. Importantly, increased ghrelin, reduced blood glucose and low serum insulin levels at P17 coincide with the weight loss phase in PWG pups (P12–P17; Figure 1B). In line with data from clinical studies showing a correlation between low IGF-1 and poor postnatal weight gain,6,7 our data also demonstrate significantly lower IGF-1 levels in PWG pups compared with EWG or MWG pups at P17 (Figure 3D). At P19, however, when NV formation starts to peak in PWG pups, IGF-1 levels have risen to normal values (Supplemental Figure 1B, see http://ajp.amjpathol.org). Importantly, equal levels of nonfasting blood glucose, but increased levels of serum insulin are found in surrogated versus nonsurrogated groups at P17 (Figure 3, E and F). This pattern resembles the difference observed between EWG versus MWG pups in the larger cohort at P17 and is indicative of more frequent feeding and better metabolic supply in surrogated pups. Combined, these results demonstrate that the different weight gain groups have distinct levels of serum markers associated with metabolic supply and energy sensing that can be modified by altering nutritional supply.
Figure 3.
Postnatal weight gain groups differ in serum markers of metabolic supply and long-term functional outcome. A–D: Non-fasting serum levels for glucose (A), insulin (B), ghrelin (C), and IGF-1 (D) differ between the three weight groups. Significantly lower levels for nonfasting glucose and IGF-1 combined with higher serum ghrelin levels indicate low nutritional supply in PWG pups. *P < 0.05; **P < 0.01; ***P < 0.001 (ANOVA with Tukey-Kramer honestly significant difference); n = 3–10 retinas/group. E and F: Surrogated pups from the litter-matched experiment show similar nonfasting blood glucose levels but higher insulin serum levels compared with their nonsurrogated littermates; *P < 0.05 (Student’s t-test). G: H&E-stained retinal cross-sections from adult PWG mice (right) show no morphological difference to EWG mice (left). H: Vascularization of the inner retina shows no difference between adult EWG and PWG mice. Exemplary flat-mounts from P70 EWG (top) and PWG (bottom) mice are shown on the right. I: At P70, PWG pups retain a significant difference in body weight compared to EWG pups (19.46 ± 0.5 versus 23.05 ± 0.75 g). J: Functional retinal analysis using ERG shows significant deficits in PWG mice at P70 compared with EWG pups. PIII = photoreceptor function with RmP3 being the saturating amplitude of the rod photoreceptor response and S the sensitivity of the rod photoreceptor response. PII = bipolar cell function with RmP2 being the saturating amplitude of the bipolar cell component and LogkP2 the sensitivity of the bipolar cell response. OPs = oscillatory potentials with Em being the maximal energy in the oscillatory potential. Flicker = retinal response to intermittent light stimulus with R8 being the response amplitude at 8 Hz.
Persistent Deficits in Adult Retinal Function in PWG Pups
To evaluate whether the observed perinatal differences in metabolic supply, retinal VEGF expression, and OIR severity translate into persistent phenotypic changes in adult mice, we analyzed retinas from both EWG and PWG mice at P70 (Figure 3, G and H). Although EWG pups represent mice with the most benign course of retinopathy while PWG pups represent mice with significantly prolonged vascular injury, we observed no obvious morphological differences between EWG and PWG mice on H&E-stained retinal cross-sections at P70 (Figure 3G). Similarly, both groups do not differ in retinal vascularization at P70, indicating that repair of the OIR-induced vascular damage as assessed in retinal cross section is complete in both groups by this age (Figure 3H). Notably, adult EWG and PWG mice do display a significant body weight difference at P70 (23.05 ± 0.75 versus 19.4 6 ± 0.5 g; Figure 3I), suggesting that poor postnatal weight gain during early development is not fully compensated in later life. This is in line with clinical data that found persistent growth deficits in small for gestational age infants with low early weight gain.22 As retinal function has been found to be abnormal despite no apparent histological changes in other eye disease models,23 a comparison of retinal function in adult EWG and PWG mice by ERG was performed (Figure 3J). We found attenuated ERG responses in PWG mice for all six parameters tested, with significantly reduced amplitudes in RmP2 (bipolar cell function) and 8 Hz Flicker amplitude (R8; inner retinal function).
Discussion
Clinical studies have recently identified postnatal weight gain as a reliable predictor of ROP severity independent from weight at birth.3,4,5,6,7 In clinical studies, however, weight gain can depend on several factors, including genetic background, neonatal care, and nutritional supply. In the current study, we used the OIR mouse model to isolate the effect of postnatal nutritional supply on weight gain and proliferative retinopathy. Although the OIR mouse model does not entail fluctuating oxygen levels common in ROP babies and induces central vaso-obliteration as opposed to peripheral avascular areas as seen in human ROP or the 50/10 rat OIR model,24,25,26 it does represent one of the most widely used models for human ROP. Investigating three OIR groups of wild-type mice distinguished solely by the nutritional supply provided by their nursing mother, we found a severely protracted course of retinopathy in mice with poor postnatal weight gain that had serum markers indicative of nutritional deficiencies (Figures 1, C and D, and 3A–C). Paralleling the prolonged course of retinopathy in these mice, retinal VEGF overexpression is protracted (Figure 2), and IGF-1 levels, which are associated with nutrient intake and postnatal growth,27 are reduced during the early post-OIR phase (Figure 3D). Notably, IGF-1 (together with its binding protein IGF binding protein-3) is a permissive factor for retinal angiogenesis both in normal development and during vessel regrowth after hyperoxic damage.28,29,30,31 The lower IGF-1 levels in PWG pups in the immediate post-OIR phase could therefore negatively affect VO repair.29,30 Persistent avascular areas, in turn, could act as triggers for prolonged overexpression of hypoxia-regulated VEGF and stimulate a protracted course of NV. Later during disease progression, when PWG pups reach their peak NV formation at P19, IGF-1 levels have returned to normal and enable VEGF-driven pathological NV formation (Supplemental Figure 1B, see http://ajp.amjpathol.org).
Importantly, the lack of IGF-1 and possibly other growth factors in the immediate post-OIR phase may also resolve the somewhat unexpected finding of a larger VO area being associated with lower NV formation in PWG pups at P17. PWG pups show VEGF up-regulation as early as P15 (Figure 2C). This indicates that the VO area does trigger a hypoxic response in the retinas of these pups. However, the retinal vessels in metabolically undersupplied and IGF-1-deficient PWG pups are unable to fully respond to the VEGF stimulus at P17. The paradox of large VO with little NV in PWG pups may thus be interpreted as an inability of PWG pups to respond adequately to hypoxic retinal tissue damage in the early post-OIR phase due to a lack of metabolic supply and/or IGF-1.
Interestingly, MWG pups show the highest peak percentage of retinal NV, but this is limited to a very short time span (P17). When PWG pups reach their NV peak (P19–P21), VEGF levels are comparable to MWG pups (Figure 2), but the extent of NV does not reach the maximal levels seen in MWG pups (8.5 versus 7.4%NV, P = 0.01). This indicates that VEGF up-regulation is not the sole factor determining NV formation. Rather, other growth factors synergizing with VEGF and/or influencing its signaling seem to play critical roles in this process. IGF-1 and its permissive role for VEGF signaling could to be an important cofactor in this respect.32
These observations have important implications with regard to an ongoing clinical trial that investigates the prophylactic effect of systemic injections of equimolar IGF-1/IGF binding protein-3 in infants at high risk for ROP.33 The pathophysiological rationale behind this trial lies in IGF-1 levels being high during the third trimester of pregnancy34 but low in prematurely born infants due to the loss of factors normally transmitted to the fetus from the mother and an inability of the prematurely born infant’s immature liver to produce sufficient amounts of endogenous IGF-1.27 Animal studies have shown that IGF-1 supplementation can reduce retinopathy.20 The results from our study support the notion that poor postnatal nutrition is associated with low IGF-1 levels and a more prolonged duration of vaso-obliteration and proliferative retinopathy. Thus, good postnatal nutrition (along with additional IGF-1 supplementation in those infants unable to produce sufficient IGF-1) might be beneficial to improve functional retinal vascularization and subsequently reduce the duration of hypoxia-triggered VEGF overexpression and neovascularization in infants at risk of ROP.
Other ongoing clinical trials investigate the therapeutic potential of intravitreal VEGF-inhibition in ROP infants (http://www.clinicaltrials.gov search terms “ROP and VEGF,” last accessed April 14, 2010). Our results suggest that the course of retinal VEGF overexpression is closely linked to postnatal nutritional supply and weight gain. Possibly secondary to an inability to repair the VO area with functional vessels, PWG pups show a prolonged overexpression of retinal VEGF. This indicates that infants with poor postnatal weight gain might also have the most pronounced VEGF overproduction.
Finally, the least invasive initial approach to alleviate the severity of ROP could lie in adjusting the infant’s nutritional intake to meet all demands of development, including those of the immature retina. The magnitude of the effect of adequate metabolic supply on OIR severity in our study was striking: Otherwise identical C57BL/6 pups showed duration of proliferative retinal disease between 6 days in EWG pups (P15–P21) and 20 days in PWG pups (P15–P35) (Figure 1, C and D). Importantly, pups with adequate nutritional supply during early postnatal development also had superior retinal function in adulthood compared with PWG pups (Figure 3D). These ERG changes might be the consequence of more severe retinopathy or other developmental problems associated with poor weight gain.35 It is paramount to note that not only total caloric intake but also the composition of available nutrients significantly alters the severity of proliferative retinopathy.36,37 The importance of finely balancing nutritional intake during infancy is further illustrated by findings that overnutrition during early development can increase cardiovascular risk factors later in life.38
Beyond these important clinical implications, our data suggests that researchers using the OIR mouse model must take postnatal weight gain into account when interpreting their results. Differences in weight gain (eg, between treatment and control group or between transgenic animals) can be extremely potent confounders of any data obtained in the OIR mouse model. At P17 (the standard date for OIR quantification), PWG pups have not yet reached their maximal disease severity and exhibit a twofold lower NV severity compared with MWG pups. Four days later, however, this pattern is reversed with almost threefold higher NV severity in PWG pups compared with MWG pups. It is important to emphasize that while PWG and EWG pups have both lower NV compared with MWG pups at P17, the time course illustrates that NV in PWG pups at P17 is still rising (and reaching peak levels at P19–P21), while in EWG pups, the low P17 NV values represent the maximal NV response reached over the whole disease period. Thus, when considering the whole time course, the NV response in EWG pups is relatively mild and limited in duration, while the NV response in PWG pups is significantly protracted compared with both EWG and MWG pups. Group differences in postnatal weight gain might therefore lead to erroneous conclusions from OIR studies. As a consequence, weight data should always be monitored in studies using the OIR model, and mice with consistent weights must be used for comparison.
In summary, the current study provides evidence for a strikingly profound effect of nutritional supply and postnatal weight gain on OIR. Our data establish, for the first time, a direct correlation between postnatal metabolic supply, weight gain, retinal VEGF expression, and the severity and long-term outcome of proliferative retinopathy. The implications relate to both fundamental researchers working with the OIR model as well as to clinicians treating premature infants at risk of ROP.
Footnotes
Address reprint requests to Lois E.H. Smith, M.D., Ph.D., Harvard Medical School, Children’s Hospital Boston, 300 Longwood Avenue, Boston, MA 02115. E-mail: lois.smith@childrens.harvard.edu.
Supported by Deutsche Forschungsgemeinschaft (A.S.), Canadian Institutes of Health Research, Canadian National Institute for the Blind (P.S.), and Juvenile Diabetes Research Foundation International (J.C.). National Institutes of Health grant EY020308 (J.D.A.), National Institutes of Health grants EY017017 and EY017017-S1, V. Kann Rasmussen Foundation, Roche Foundation for Anemia Research, Children’s Hospital Boston Mental Retardation and Developmental Disabilities Research Center, Research to Prevent Blindness Senior Investigator Award, Alcon Research Institute Award, and MacTel Foundation (L.E.H.S.).
J.C. and P.S. contributed equally.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Saugstad OD. Oxygen and retinopathy of prematurity. J Perinatol. 2006;26(Suppl 1):S46–S50. doi: 10.1038/sj.jp.7211475. discussion S63–S44. [DOI] [PubMed] [Google Scholar]
- Sears JE, Pietz J, Sonnie C, Dolcini D, Hoppe G. A change in oxygen supplementation can decrease the incidence of retinopathy of prematurity. Ophthalmology. 2009;116:513–518. doi: 10.1016/j.ophtha.2008.09.051. [DOI] [PubMed] [Google Scholar]
- Hellstrom A, Hard AL, Engstrom E, Niklasson A, Andersson E, Smith L, Lofqvist C. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009;123:e638–e645. doi: 10.1542/peds.2008-2697. [DOI] [PubMed] [Google Scholar]
- Fortes Filho JB, Bonomo PP, Maia M, Procianoy RS. Weight gain measured at 6 weeks after birth as a predictor for severe retinopathy of prematurity: study with 317 very low birth weight preterm babies. Graefes Arch Clin Exp Ophthalmol. 2009;247:831–836. doi: 10.1007/s00417-008-1012-3. [DOI] [PubMed] [Google Scholar]
- Hellstrom A, Ley D, Hansen-Pupp I, Niklasson A, Smith L, Lofqvist C, Hard AL. New insights into the development of retinopathy of prematurity: importance of early weight gain. Acta Paediatr. 2010;99:502–508. doi: 10.1111/j.1651-2227.2009.01568.x. [DOI] [PubMed] [Google Scholar]
- Lofqvist C, Andersson E, Sigurdsson J, Engstrom E, Hard AL, Niklasson A, Smith LE, Hellstrom A. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol. 2006;124:1711–1718. doi: 10.1001/archopht.124.12.1711. [DOI] [PubMed] [Google Scholar]
- Lofqvist C, Hansen-Pupp I, Andersson E, Holm K, Smith LE, Ley D, Hellstrom A. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulinlike growth factor I. Arch Ophthalmol. 2009;127:622–627. doi: 10.1001/archophthalmol.2009.69. [DOI] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Lofqvist C, Hellstrom A, Smith LE. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D, Muhlfeld C. Perinatal adaptation in mammals: the impact of metabolic rate. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:780–784. doi: 10.1016/j.cbpa.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Connor KM, Sapieha P, Willett KL, Krah NM, Dennison RJ, Chen J, Guerin KI, Smith LE. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009;12:297–301. doi: 10.1007/s10456-009-9155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson JR, Whincup PH, Walker M, Thomas M, Alberti KG. Biochemical measures in a population-based study: effect of fasting duration and time of day. Ann Clin Biochem. 2002;39:493–501. doi: 10.1258/000456302320314511. [DOI] [PubMed] [Google Scholar]
- Akula JD, Mocko JA, Benador IY, Hansen RM, Favazza TL, Vyhovsky TC, Fulton AB. The neurovascular relation in oxygen-induced retinopathy. Mol Vis. 2008;14:2499–2508. [PMC free article] [PubMed] [Google Scholar]
- Hood DC, Birch DG. A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis Neurosci. 1992;8:107–126. doi: 10.1017/s0952523800009275. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh EN, Jr, Lamb TD. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993;1141:111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Akula JD, Mocko JA, Moskowitz A, Hansen RM, Fulton AB. The oscillatory potentials of the dark-adapted electroretinogram in retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2007;48:5788–5797. doi: 10.1167/iovs.07-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JM, Duffner LA. The effect of postnatal growth retardation on abnormal neovascularization in the oxygen exposed neonatal rat. Curr Eye Res. 1996;15:403–409. doi: 10.3109/02713689608995831. [DOI] [PubMed] [Google Scholar]
- Vanhaesebrouck S, Daniels H, Moons L, Vanhole C, Carmeliet P, De Zegher F. Oxygen-induced retinopathy in mice: amplification by neonatal IGF-I deficit and attenuation by IGF-I administration. Pediatr Res. 2009;65:307–310. doi: 10.1203/PDR.0b013e3181973dc8. [DOI] [PubMed] [Google Scholar]
- Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- Knops NB, Sneeuw KC, Brand R, Hille ET, den Ouden AL, Wit JM, Verloove-Vanhorick SP. Catch-up growth up to ten years of age in children born very preterm or with very low birth weight. BMC Pediatr. 2005;5:26. doi: 10.1186/1471-2431-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie JM, Ohlemiller KK, Shah GN, Ulmasov B, Becker TA, Waheed A, Hennig AK, Lukasiewicz PD, Sly WS. Carbonic anhydrase XIV deficiency produces a functional defect in the retinal light response. Proc Natl Acad Sci USA. 2007;104:8514–8519. doi: 10.1073/pnas.0702899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–731. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- Hartnett ME. The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: knowledge from the 50/10 OIR model. Doc Ophthalmol. 2010;120:25–39. doi: 10.1007/s10633-009-9181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JM, Yanni SE, Penn JS. The development of the rat model of retinopathy of prematurity. Doc Ophthalmol. 2010;120:3–12. doi: 10.1007/s10633-009-9180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom E, Niklasson A, Wikland KA, Ewald U, Hellstrom A. The role of maternal factors, postnatal nutrition, weight gain, and gender in regulation of serum IGF-I among preterm infants. Pediatr Res. 2005;57:605–610. doi: 10.1203/01.PDR.0000155950.67503.BC. [DOI] [PubMed] [Google Scholar]
- Hellstrom A, Carlsson B, Niklasson A, Segnestam K, Boguszewski M, de Lacerda L, Savage M, Svensson E, Smith L, Weinberger D, Albertsson Wikland K, Laron Z. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab. 2002;87:3413–3416. doi: 10.1210/jcem.87.7.8629. [DOI] [PubMed] [Google Scholar]
- Chang KH, Chan-Ling T, McFarland EL, Afzal A, Pan H, Baxter LC, Shaw LC, Caballero S, Sengupta N, Li Calzi S, Sullivan SM, Grant MB. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development, Proc Natl Acad Sci USA. 2007;104:10595–10600. doi: 10.1073/pnas.0702072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL, Albertsson-Wikland K, Carlsson B, Niklasson A, Sjodell L, LeRoith D, Senger DR, Smith LE. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci USA. 2001;98:5804–5808. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofqvist C, Chen J, Connor KM, Smith AC, Aderman CM, Liu N, Pintar JE, Ludwig T, Hellstrom A, Smith LE. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci USA. 2007;104:10589–10594. doi: 10.1073/pnas.0702031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, Robinson G, Driver S, Bischoff J, Zhang B, Schaeffer JM, Senger DR. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5:1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- Lofqvist C, Niklasson A, Engstrom E, Friberg LE, Camacho-Hubner C, Ley D, Borg J, Smith LE, Hellstrom A. A pharmacokinetic and dosing study of intravenous insulin-like growth factor-I and IGF-binding protein-3 complex to preterm infants. Pediatr Res. 2009;65:574–579. doi: 10.1203/PDR.0b013e31819d9e8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caufriez A, Frankenne F, Englert Y, Golstein J, Cantraine F, Hennen G, Copinschi G. Placental growth hormone as a potential regulator of maternal IGF-I during human pregnancy. Am J Physiol. 1990;258:E1014–E1019. doi: 10.1152/ajpendo.1990.258.6.E1014. [DOI] [PubMed] [Google Scholar]
- Kjellmer I, Liedholm M, Sultan B, Wennergren M, Wallin Gotborg C, Thordstein M. Long-term effects of intrauterine growth retardation. Acta Paediatr Suppl. 1997;422:83–84. doi: 10.1111/j.1651-2227.1997.tb18352.x. [DOI] [PubMed] [Google Scholar]
- Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr, Serhan CN, Smith LE. Increased dietary intake of ω-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Sapieha P, Connor KM, Sangiovanni JP, Chen J, Aderman CM, Willett KL, Krah NM, Dennison RJ, Seaward MR, Guerin KI, Hua J, Smith LE. PPARγ mediates a direct antiangiogenic effect of ω3-PUFAs in proliferative retinopathy. Circ Res. 2010;107:495–500. doi: 10.1161/CIRCRESAHA.110.221317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal A, Cole TJ, Fewtrell M, Kennedy K, Stephenson T, Elias-Jones A, Lucas A. Promotion of faster weight gain in infants born small for gestational age: is there an adverse effect on later blood pressure? Circulation. 2007;115:213–220. doi: 10.1161/CIRCULATIONAHA.106.617811. [DOI] [PubMed] [Google Scholar]