Abstract
Chronic injury to intrahepatic bile duct epithelial cells (BDECs) elicits expression of various mediators, including the αVβ6 integrin, promoting liver fibrosis. We tested the hypothesis that tissue factor (TF)-dependent thrombin generation and protease activated receptor-1 (PAR-1) activation contribute to liver fibrosis induced by cholestasis via induction of αVβ6 expression. To test this hypothesis, mice deficient in either TF or PAR-1 were fed a diet containing 0.025% α-naphthylisothiocyanate (ANIT), a BDEC-selective toxicant. In genetically modified mice with a 50% reduction in liver TF activity fed an ANIT diet, coagulation cascade activation and liver fibrosis were reduced. Similarly, liver fibrosis was significantly reduced in PAR-1−/− mice fed an ANIT diet. Hepatic integrin β6 mRNA induction, expression of αVβ6 protein by intrahepatic BDECs, and SMAD2 phosphorylation were reduced by TF deficiency and PAR-1 deficiency in mice fed the ANIT diet. Treatment with either an anti-αVβ6 blocking antibody or soluble transforming growth factor-β receptor type II reduced liver fibrosis in mice fed the ANIT diet. PAR-1 activation enhanced transforming growth factor-β1-induced integrin β6 mRNA expression in both transformed human BDECs and primary rat BDECs. Interestingly, TF and PAR-1 mRNA levels were increased in livers from patients with cholestatic liver disease. These results indicate that a TF-PAR-1 pathway contributes to liver fibrosis induced by chronic cholestasis by increasing expression of the αVβ6 integrin, an important regulator of transforming growth factor- β1 activation.
Chronic injury to intrahepatic bile duct epithelial cells (BDECs) causes cholestatic liver disease characterized by an increase in toxic bile constituents in the hepatic parenchyma. This elicits inflammation and a profibrogenic response characterized by excessive deposition of extracellular matrix (eg, collagens), which may lead to liver cirrhosis.1 The cellular and molecular mechanisms of liver fibrosis caused by intrahepatic BDEC injury are not completely understood. Recent animal studies have clearly demonstrated that αVβ6 is critical for the development of liver fibrosis in two distinct models of cholestasis, and that it promotes fibrosis by activating the profibrogenic growth factor, transforming growth factor (TGF)-β1.2,3 However, the mechanism of αVβ6 up-regulation on BDECs during cholestasis is not known.
Injury to intrahepatic BDECs can be modeled in rodents by administration of the xenobiotic α-naphthylisothiocyanate (ANIT).4 Repetitive canilicular transport of an unstable ANIT-glutathione conjugate from hepatocytes into the bile exposes BDECs to toxic concentrations of ANIT.5,6 In animals administered large doses of ANIT, hepatocellular necrosis manifests proximal to damaged bile ducts7 by mechanisms likely involving both high concentrations of hydrophobic bile acids released from damaged bile ducts8 and infiltration of inflammatory cells.9 In contrast, rodents fed a small dose of ANIT in their diet (eg, 0.25–1 Gram/kg diet) develop modest BDEC injury associated with BDEC proliferation, periportal inflammation, profibrogenic gene expression, and peribiliary collagen deposition.10,11,12,13 Consequently, ANIT-induced chronic BDEC injury provides an important model to evaluate the pathogenesis of liver fibrosis stemming from cytotoxic challenge to the intrahepatic BDECs.
Tissue factor (TF) is a transmembrane protein and the principal activator of the extrinsic blood coagulation cascade. It is involved in both physiological hemostasis and the pathogenesis of various diseases.14 In the liver, TF is expressed by various cells including BDECs.15 Previous studies have shown that in rodents the coagulation cascade is activated by obstructive cholestasis and after acute toxic insult to the biliary epithelium.15,16 Similarly, generation of the coagulation protease thrombin is evident in patients with primary biliary cirrhosis (PBC).17,18 Pharmacological anticoagulation and a protease activated receptor-1 (PAR-1) antagonist reduced hepatic collagen levels after bile duct ligation,16,19 suggesting that thrombin contributes to liver fibrosis during obstructive cholestasis. These experiments demonstrate a role for PAR-1 in fibrosis induced by cholestasis. However, the mechanism of coagulation cascade activation during cholestasis and downstream consequences of PAR-1 activation that promote liver fibrosis during cholestasis are not known.
In the present studies, we tested the hypothesis that a TF-dependent procoagulant response accompanies intrahepatic BDEC injury and promotes liver fibrosis by a mechanism requiring thrombin-dependent induction of the αVβ6 integrin by BDECs.
Materials and Methods
Mice
All studies were carried out with mice of 8 to 16 weeks of age. Wild-type C57Bl/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice heterozygous for murine TF (mTF+/−hTF+ mice; hereafter referred to as TF+/− mice) and control mice (mTF+/+hTF+ mice; hereafter referred to as TF+/+ mice), each expressing a low level (approximately 1%) of human TF (hTF), were kindly provided by Dr. Nigel Mackman (University of North Carolina-Chapel Hill). Age-matched TF+/− mice and TF+/+ littermates were used for these studies. PAR-1−/− mice and PAR-1+/+ mice on an identical genetic background (N8 C57Bl/6J) were maintained by homozygous breeding. Mice were maintained in an AAALAC-accredited facility at the University of Kansas Medical Center. Mice were housed at an ambient temperature of 22°C with alternating 12-hour light/dark cycles, and provided water and rodent chow ad libitum (Teklad 8604; Harlan, Indianapolis, IN) before feeding custom diets. All animal procedures were performed according to the guidelines of the American Association for Laboratory Animal Science and were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Diets and Sample Collection
Diets were prepared by Dyets, Inc. (Bethlehem, PA). The ANIT diet was an AIN-93M purified diet containing 0.025% ANIT (Sigma-Aldrich, St. Louis, MO). The control diet was AIN-93M, a purified diet formulated for maintenance of mature rodents. For antibody studies, wild type C57Bl/6J mice were administered once weekly intraperitoneal injections of isotype control antibody (clone 1E6, 10 mg/kg), recombinant murine TGF-β receptor II-Fc fusion protein (mTGFβRII-Fc, 5 mg/kg)2 or anti-αVβ6 antibody (clone 6.3G9, 1 or 10 mg/kg),2 each diluted in sterile, endotoxin-free phosphate-buffered saline. Mice were fed the diets for 2 weeks, subsequently anesthetized with isoflurane, and blood was collected from the caudal vena cava into sodium citrate (final, 0.38%) or an empty syringe for the collection of plasma and serum, respectively. The liver was removed, washed in saline, and the intact gall bladder removed. The left medial lobe of the liver was affixed to a cork with OCT and frozen for 3 minutes in liquid nitrogen-chilled isopentane. Sections of the left lateral lobe were fixed in neutral-buffered formalin for 48 hours before routine processing. The remaining liver was cut into 100-mg pieces and flash-frozen in liquid nitrogen.
Biomarkers of Injury and Coagulation and MCP-1 Determination
Serum ALT activity was determined using a commercial assay (ThermoFisher, Waltham, MA). Plasma thrombin-antithrombin levels were determined using a commercial enzyme-linked immunosorbent assay (ELISA) (Siemens Health care Diagnostics, Waltham, MA). Supernatant MCP-1 levels were determined using a commercial ELISA (eBioscience, San Diego, CA).
Cell Culture and Treatments
Transformed human bile duct epithelial cells (MMNK-1 cells)20 were kindly provided by Dr. Melissa Runge-Morris (Wayne State University, Detroit, MI), on behalf of Dr. Naoya Kobayashi (Okayama University, Okayama, Japan). The cells were maintained at 37°C at 5% CO2 in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 10 U/ml penicillin, and 10 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO), serum-starved for 60 minutes, and then stimulated with recombinant human TGF-β1 (5 ng/ml, PeproTech, Rocky Hill, NJ) or its vehicle (endotoxin-free PBS containing 2 mg/ml bovine serum albumin), and with human α-thrombin (10 U/ml, Enzyme Research Laboratories, South Bend, IN), 10 μmol/L TFLLRN, 100 μmol/L SFLLRN, 100 μmol/L FSLLRN (AnaSpec, Inc., San Jose, CA) or associated vehicle (endotoxin-free PBS, Sigma-Aldrich). Primary rat BDECs were kindly provided by Dr. Vasanthi Bhaskaran, Dr. Monica Otieno, and Dr. Lois Lehman-Mckeeman (Bristol-Myers Squibb, Princeton, NJ). Rat BDECs were maintained in LYD medium [DMEM/F12 supplemented with 1.2 mg/ml sodium bicarbonate, 5 mg/ml d-glucose, 3 μmol/L forskolin, 1 μmol/L dexamethasone, 5 nmol/L triiodothyronine (Sigma), 10% Nuserum IV, 20 ng/ml epidermal growth factor, 12.5 μg/ml bovine pituitary extract, 1% ITS premix (BD Biosciences, San Jose CA) and 50 μg/ml gentamicin (Invitrogen, Carlsbad CA) pH 7.35]. The rat BDECs were co-stimulated with TGF-β1 and thrombin as described above.
RNA Isolation, cDNA Synthesis, and Real-Time PCR
Total RNA was isolated from 100 mg of snap-frozen liver or adherent cells using TRI Reagent (Molecular Research Center, Cincinnati, OH), from which 1 μg of cDNA was synthesized using a high capacity cDNA synthesis kit (Applied Biosystems, Foster City, CA). Subsequent real-time PCR analysis was performed using a StepOnePlus instrument (Applied Biosystems) and 2X TaqMan Master Mix and TaqMan gene expression assays (Applied Biosystems). The levels of each gene were adjusted to the levels of 18S RNA for in vivo studies and GAPDH for in vitro studies and the relative levels of each gene evaluated using the ΔΔCt method.
Detection of Phosphorylated SMAD2 in Liver Homogenate
Approximately 100 mg of liver was homogenized in 1.5 ml of a buffer containing 10 mmol/L HEPES, 10 mmol/L KCl, 300 mmol/L sucrose, 1.5 mmol/L MgCl2, 0.5 mmol/L dithiothreitol , 1 mmol/L phenylmethylsulfonyl fluoride, and a protease/phosphatase inhibitor cocktail (Pierce), and subjected to centrifugation at 3600 × g for 10 minutes at 4°C. The resulting pellet was lysed in 0.2 ml of a buffer containing 20 mmol/L HEPES, 100 mmol/L KCl, 100 mmol/L NaCl, 20% glycerol, 1 mmol/L phenylmethylsulfonyl fluoride, and a protease/phosphatase inhibitor cocktail (Pierce), incubated on ice for 30 minutes, then subjected to centrifugation at 20,800 × g for 15 minutes at 4°C. The supernatant (ie, nuclear fraction) was collected and protein levels determined using a Bio-Rad Dc kit (Bio-Rad). 100 μg of this protein extract was used for the detection of phosphorylated SMAD2 (Ser465/467) using a commercially available PathScan ELISA (Cell Signaling Technology).
Immunofluorescent Staining and Confocal Microscopy
For all immunofluorescent staining, 5-μm frozen sections obtained from the isopentane-fixed livers were used. For each staining, the sections were fixed with 4% neutral buffered formalin for 10 minutes. For type 1 collagen staining, sections were blocked with 10% goat serum in PBS for 1 hour at room temperature and then incubated with primary antibody (rabbit anti-mouse type 1 collagen, AB765, Millipore, Temecula, CA) diluted to 0.5 μg/ml in block buffer overnight at 4°C. The sections were washed with PBS and then incubated with an anti-rabbit Alexa Fluor 594-conjugated secondary antibody (Invitrogen, Carlsbad, CA) for 2 hours at room temperature. For αVβ6 staining, sections were blocked with 10% goat serum in PBS for 1 hour at room temperature and then incubated with primary antibody (human/mouse chimeric anti-αVβ6, ch2A1)21 diluted 1:200 in block buffer overnight at 4°C. The sections were then washed and incubated with a rat anti-mouse cytokeratin 19 (CK19) antibody diluted 1:2000 in block buffer for 2 hours at room temperature (clone TROMA-3, Developmental Studies Hybridoma Bank, Iowa City, IA). The TROMA-3 antibody developed by R. Kemler was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA. The sections were then incubated with cross-adsorbed secondary antibodies: Alexa Fluor 488-conjugated goat anti-rat IgG and Alexa Fluor 594-conjugated goat anti-human IgG (Invitrogen, Carlsbad, CA), each diluted 1:500 in block buffer. Slides were then washed with PBS after secondary antibody addition and fluorescent staining in the livers sections was visualized using an Olympus BX41 microscope (Olympus, Lake Success, NY). Staining protocols for confocal microscopy were similar with substitution of goat anti-human IgG Alexa Fluor 546-conjugated antibody. Slides were visualized using a Nikon TE2000-U motorized inverted scope (Nikon Instruments Inc., Melville, NY) outfitted with a three-laser confocal system. Images were captured using Nikon EZC1 3.8 confocal imaging software.
Quantification of type 1 collagen staining in 10 randomly selected 100X fields per tissue was performed using Scion Image (Scion Corporation, Frederick, MD) as described previously.22 Quantification of αVβ6 staining was performed using Scion Image as follows. Ten randomly selected 400X fields containing periportal regions (positive for CK19 staining) were captured per tissue. For each identical field the red fluorescence (positive red αVβ6 staining) was also captured. For each image the area of CK19 staining (positive pixels) and accompanying αVβ6 staining intensity (integrated density) was determined and αVβ6 staining intensity expressed as a ratio of the CK19 area. For representative photomicrographs, red and green images were merged using the Olympus DP Manager software.
Trichrome Staining
Formalin-fixed sections were stained using a Chromaview Gomori Trichrome staining kit (Thermo-Fisher, Waltham, MA) as described by the manufacturer’s protocol.
Flow Cytometry
MMNK-1 cells were collected by trypsinization and pelleted by centrifugation at 700 × g for 2 minutes. The cells were then adjusted to a density of 1 × 106 cells/ml in block buffer (10% goat serum in PBS) and incubated for 20 minutes at 4°C. Cells were then incubated for 1 hour at 4°C with anti-mouse PAR-1 antibody (ATAP2, Santa Cruz Biotechnology, Santa Cruz, CA) or IgG2a isotype control (BioLegend, San Diego, CA) at a concentration of 1 μg/106 cells. Cells were pelleted by centrifugation at 700 × g and washed five times with 2% fetal bovine serum in PBS. Cells were then incubated with Alexa Fluor 647-conjugated goat anti-mouse secondary antibody at a concentration of 1 μg/106 cells for 1 hour at 4°C. Cells were washed five times with 2% fetal bovine serum in PBS and then fixed in 4% paraformaldehyde (BioLegend) for 20 minutes. Cells were then resuspended in 2% fetal bovine serum in PBS and 20,000 events were measured on a FACSCalibur (BD Biosciences, San Jose, CA).
Human Liver Samples
Research involving human livers was reviewed by the University of Kansas Medical Center Human Research Protection Program and the use of de-identified human liver samples was approved. The specimens used in this study were collected by Erik Schadde, M.D, Richard Gilroy, M.D., Bashar Abdulkarim, M.D., Ph.D., Jameson Forster, M.D., Mojtaba Olyaee, M.D., and Atta M. Nawabi, M.D., and provided by the KU Liver Center Tissue Bank. The project Determination of Gene Expression Related to Coagulation Signaling in Liver Disease is sponsored by the Liver Center at The University of Kansas Medical Center. Diseased liver tissue used was from patients with primary biliary cirrhosis (four females, ages 59, 56, 60, 61; one male, age 47) and patients with primary sclerosing cholangitis (three males, ages 44, 45, 57). Donor liver samples without histological evidence of cholestasis, fibrosis or severe inflammation were selected as control tissues (five females, ages 41, 48, 56, 52, 66; five males, ages 57, 45, 57, 58, 48).
Statistics
Comparison of two groups was performed using Student’s t-test. Comparison of 3 or more groups was performed using one- or two-way analysis of variance, as appropriate, and the Student-Newman-Keuls post hoc test. The criterion for statistical significance was P < 0.05.
Results
TF Deficiency Reduces Coagulation and Attenuates Liver Fibrosis in Mice Fed the ANIT Diet
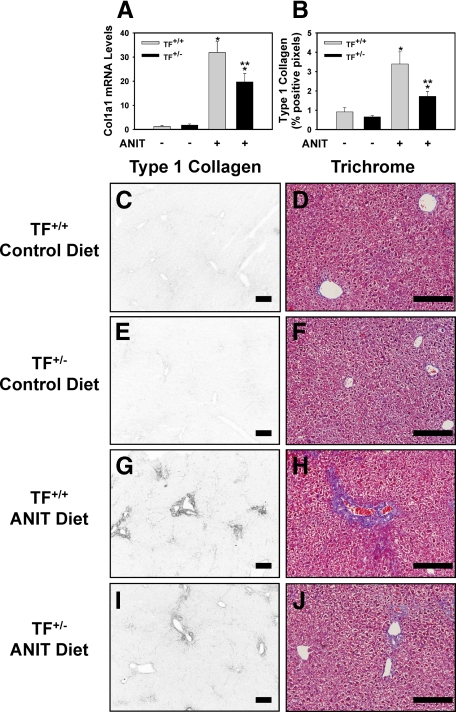
Heterozygous TF deficiency (TF+/−), which reduces liver TF activity by 50%,15 did not affect plasma thrombin-antithrombin levels in mice fed the control diet (Table 1). Plasma thrombin-antithrombin levels increased significantly in TF+/+ mice fed the ANIT diet compared to mice fed control diet (Table 1), and this increase was largely prevented in TF+/− mice (Table 1, P = 0.070 for the comparison of TF+/− mice fed ANIT diet with TF+/− mice fed control diet.). TF deficiency did not affect the modest increase in serum ALT activity in mice fed the ANIT diet (Table 1). The induction of α-1 type I collagen (Col1a1) mRNA in liver was significantly reduced in TF+/− mice fed the ANIT diet compared to TF+/+ mice fed the ANIT diet (Figure 1A). Increased periportal deposition of type 1 collagen was evident in the livers of TF+/+ mice fed the ANIT diet compared to mice fed the control diet (Figure 1, C–G). Similarly, trichrome staining revealed marked periportal collagen deposition in livers of TF+/+ mice fed the ANIT diet (Figure 1H). In agreement with the reduction in Col1a1 mRNA levels, collagen deposition was significantly reduced in the livers of TF+/− mice fed the ANIT diet (Figure 1, B and G–J).
Table 1.
Effect of TF Deficiency on Serum ALT Activity and Plasma TAT Levels in Mice Fed the ANIT Diet
| Diet | Genotype | ALT (U/L) | TAT (ng/ml) |
|---|---|---|---|
| Control diet | TF+/+ | 35.9 ± 6.9 | 7.2 ± 0.8 |
| Control diet | TF+/− | 28.4 ± 3.6 | 6.3 ± 0.5 |
| ANIT diet | TF+/+ | 148.9 ± 15.6* | 12.4 ± 2.6* |
| ANIT diet | TF+/− | 163.1 ± 22.8* | 8.4 ± 0.6† |
Mice wild type for TF (TF+/+) and mice heterozygous for TF (TF+/−) were fed either the control diet or an identical diet containing 0.025% ANIT for 14 days. n = 4–8 mice per group. Data are expressed as the mean ± SEM.
Significantly different from the same mice fed the control diet.
Significantly different from the TF+/+ mice fed the ANIT diet.
Figure 1.
Reduced liver fibrosis in livers of TF-deficient mice fed the ANIT diet. Mice wild type for TF (TF+/+) and mice heterozygous for TF (TF+/-) were fed either the control diet or an identical diet containing 0.025% ANIT for 14 days. A: Levels of Col1a1 mRNA were determined in liver. B: Quantification of type 1 collagen staining (see below) as described in Materials and Methods; n = 4–8 mice per group. Data are expressed as the mean ± SEM. *P < 0.05; significantly different from the same mice fed the control diet. **P < 0.05; significantly different from TF+/+ mice fed the ANIT diet. C–J: Representative photomicrographs showing immunofluorescent type 1 collagen staining and trichrome staining (Scale bar = 20 μm) of liver sections from TF+/+ mice (C and D, G and H) or TF+/− mice (E and F, I and J) fed control diet (C–F) or ANIT diet (G–J). For type 1 collagen staining, the images were converted to grayscale and inverted such that positive staining is dark.
Role of Protease Activated Receptor-1 in Liver Fibrosis in Mice Fed the ANIT Diet
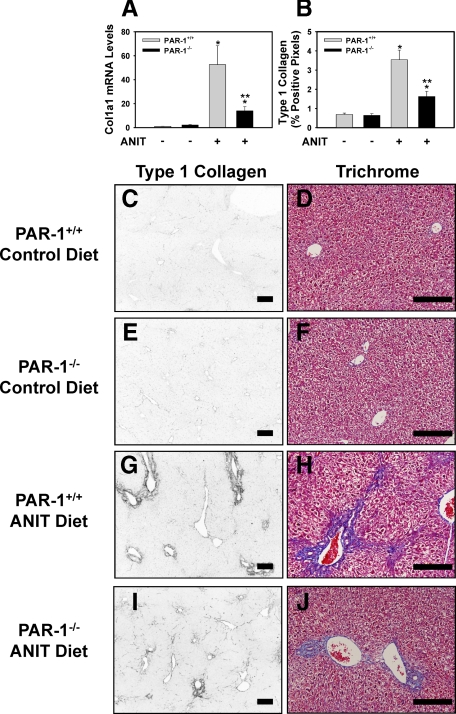
One mechanism whereby TF-dependent thrombin generation could contribute to liver fibrosis in mice fed the ANIT diet is by activation of PAR-1. To determine the role of PAR-1 in hepatic injury and fibrosis induced by the ANIT diet we used PAR-1−/− mice. Serum ALT activity increased similarly in PAR-1+/+ mice and PAR-1−/− mice fed the ANIT diet (data not shown). Reflecting the effect of TF deficiency on liver fibrosis in mice fed the ANIT diet, Col1a1 mRNA and collagen deposition were significantly reduced in the livers of PAR-1−/− mice compared to PAR-1+/+ mice fed the ANIT diet (Figure 2, A–J).
Figure 2.
Reduced liver fibrosis in livers of PAR-1−/− mice fed the ANIT diet. PAR-1+/+ mice and PAR-1−/− mice were fed either the control diet or an identical diet containing 0.025% ANIT for 14 days. A: Levels of Col1a1 mRNA were determined in liver. B: Quantification of type 1 collagen staining (see below) as described in the Methods; n = 4–12 mice per group. Data are expressed as the mean ± SEM *P < 0.05; significantly different from the same mice fed the control diet. **P < 0.05; significantly different from PAR-1+/+ mice fed the ANIT diet. C–J: Representative photomicrographs showing immunofluorescent type 1 collagen staining and trichrome staining (Scale bar = 20 μm) of liver sections from PAR-1+/+ mice (C and D, G and H) or PAR-1−/− mice (E and F, I and J) fed control diet (C–F) or ANIT diet (G–J). For type 1 collagen staining, the images were converted to grayscale and inverted such that positive staining is dark.
Effect of TF- or PAR-1 Deficiency on Induction of Fibrogenic Genes in the Livers of Mice Fed the ANIT Diet
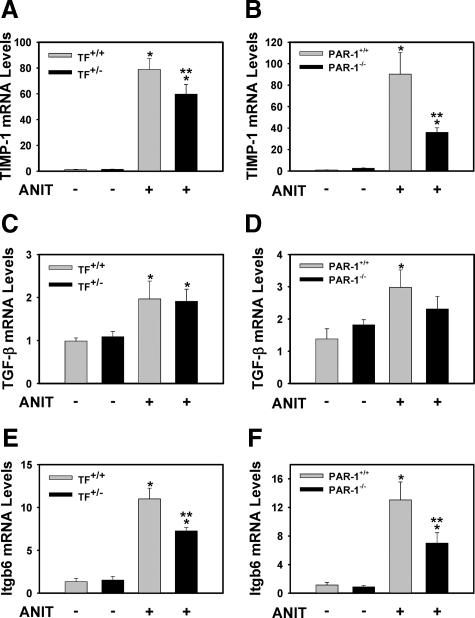
Insofar as both TF deficiency and PAR-1 deficiency reduced liver fibrosis in mice fed the ANIT diet, we examined similarities in the induction of profibrogenic genes in liver as a prelude to identify the mechanism(s) whereby coagulation protease signaling contributes to liver fibrosis. Both TF deficiency and PAR-1 deficiency attenuated induction of tissue inhibitor of metalloproteinase-1 (TIMP-1) mRNA in livers of mice fed the ANIT diet (Figure 3, A and B). Interestingly, levels of TGF-β1 mRNA in livers of mice fed the ANIT diet were not significantly affected by either TF- or PAR-1 deficiency (Figure 3, C and D). However, induction of integrin β6 mRNA was significantly reduced by TF deficiency and PAR-1 deficiency (Figure 3, E and F).
Figure 3.
Effect of TF deficiency and PAR-1 deficiency on the expression of fibrogenic genes in livers of mice fed the ANIT diet. Mice wild type for TF (TF+/+) and mice heterozygous for TF (TF+/−), and PAR-1+/+ mice and PAR-1−/− mice were fed either the control diet or an identical diet containing 0.025% ANIT for 14 days. Levels of TIMP-1 (A and B), TGF-β (C and D), and integrin β6 (Itgb6) mRNA (E and F) in liver were determined as described in Materials and Methods; n = 4–12 mice per group. Data are expressed as the mean ± SEM. *P < 0.05; significantly different from the same mice fed the control diet. **P < 0.05; significantly different from control mice (TF+/+ mice or PAR-1+/+ mice) fed the ANIT diet.
TF and PAR-1 Contribute to the αVβ6 Integrin Expression by BDECs and SMAD2 Phosphorylation in Livers of Mice Fed the ANIT Diet
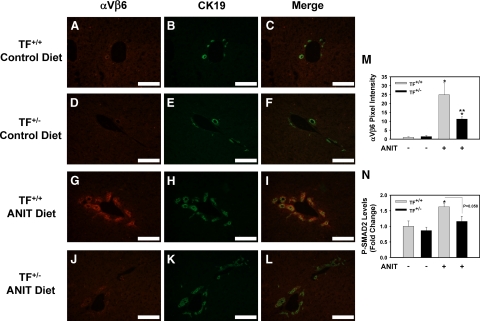
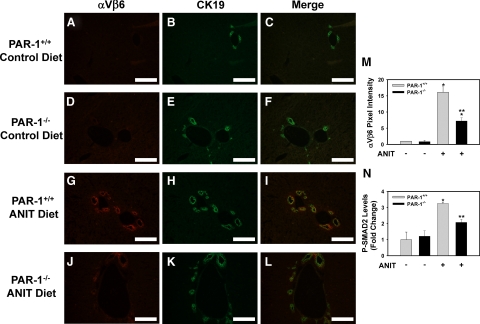
The αVβ6 integrin is highly expressed in BDECs in mice subjected to common bile duct ligation and in livers of patients with cholestatic liver disease.2 Indeed, we found that integrin β6 mRNA levels increased in the livers of patients with PBC and PSC compared to control livers (data not shown). Recent studies using pharmacological and genetic strategies indicated a critical role for this integrin in the activation of TGF-β and liver fibrosis induced by chronic cholestasis.2,3 Of importance, the mechanism whereby αVβ6 integrin expression increases during chronic cholestasis is not known. To determine whether the effect of TF- and PAR-1 deficiency on integrin β6 mRNA levels (Figure 3, E and F) was mirrored by changes in the biliary levels of the β6 protein, we used immunofluorescent staining. Liver sections from mice fed either control diet or ANIT diet were co-stained for the β6 integrin and CK19, the latter a biomarker of BDECs in the adult mouse liver. Minimal αVβ6 integrin staining was observed in livers of TF+/+ mice and TF+/− mice fed the control diet (Figure 4, A–F). An increase in BDEC number, as indicated by an increase in CK19-positive staining, was evident in the livers of TF+/+ mice and TF+/− mice fed the ANIT diet (Figure 4, H and K). αVβ6 integrin expression increased substantially in TF+/+ mice fed the ANIT diet and the staining colocalized with CK19, indicating αVβ6 expression by BDECs (Figure 4, G–I). Visualization of αVβ6/CK19 co-staining by confocal microscopy indicated colocalization of these proteins within the same plane of cells (Supplemental Figure S1 at http://ajp.amjpathol.org). Compared to TF+/+ mice fed the ANIT diet, the intensity of αVβ6 staining was significantly reduced in TF+/− mice fed the ANIT diet (Figure 4, J–M). Moreover, an analogous pattern of CK19 and αVβ6 staining was observed in liver sections from PAR-1+/+ mice and PAR-1−/− mice fed the ANIT diet (Figure 5, A–M).
Figure 4.
Effect of TF deficiency on αVβ6 integrin expression by BDECs in mice fed the ANIT diet. Mice wild type for TF (TF+/+) (A–C, G–I) and mice heterozygous for TF (TF+/−) (D–F, J–L) were fed either the control diet (A–F) or an identical diet containing 0.025% ANIT (G–L) for 14 days. Representative photomicrographs showing immunofluorescent staining for αVβ6 integrin (red), cytokeratin 19 (CK19, green), and the digital merge (yellow) were captured from liver sections. Scale bar = 10 μm. M: αVβ6 integrin staining intensity adjusted for CK19 area was determined as described in the Methods; n = 3–6 mice per group. N: Levels of phosphorylated (Ser465/467) SMAD2 were determined in liver homogenates by ELISA as described in Materials and Methods. Data are expressed as the mean ± SEM. *P < 0.05; significantly different from the same mice fed the control diet. **P < 0.05; significantly different from TF+/+ mice fed the ANIT diet.
Figure 5.
Effect of PAR-1 deficiency on αVβ6 integrin expression by BDECs in mice fed the ANIT diet. PAR-1+/+ mice (A–C, G–I) and PAR-1−/− mice (D–F, J–L) were fed either the control diet or an identical diet containing 0.025% ANIT for 14 days. Representative photomicrographs showing immunofluorescent staining for αVβ6 integrin (red), cytokeratin 19 (CK19, green), and the digital merge (yellow) were captured from liver sections. Scale bar = 10 μm. M: αVβ6 integrin staining intensity adjusted for CK19 area was determined as described in Materials and Methods; n = 3–7 mice per group. N: Levels of phosphorylated (Ser465/467) SMAD2 were determined in liver homogenates by ELISA as described in Materials and Methods. n = 4–7 mice per group. Data are expressed as the mean ± SEM. *P < 0.05; significantly different from the same mice fed the control diet. **P < 0.05; significantly different from PAR-1+/+ mice fed the ANIT diet.
To determine whether αVβ6 expression correlated with activation of TGF-β signaling, we evaluated levels of phosphorylated SMAD-2 in liver. TGF-β signaling culminates in SMAD2 phosphorylation, and this readout has been used previously as an indicator of TGF-β activation.2,23 Both TF deficiency (P = 0.058) and PAR-1 deficiency reduced the levels of phospho-SMAD2 in livers of mice fed the ANIT diet, consistent with the reduced αVβ6 protein expression in TF-deficient and PAR-1-deficient mice (Figures 4N and 5N).
The αVβ6 Integrin and TGF-β Contribute to Liver Fibrosis in Mice Fed the ANIT Diet
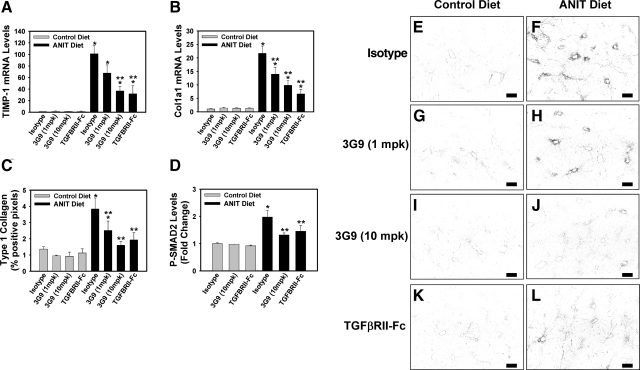
The role of αVβ6 and TGF-β1 in the liver fibrosis induced by the ANIT diet has not been described previously. To this end, we evaluated whether TF- and PAR-1-dependent up-regulation of αVβ6 could contribute to liver fibrosis in this model. Compared to wild type mice administered an isotype control antibody (1E6), wild type mice administered a function blocking anti-αVβ6 antibody (clone 6.3G9)24 and fed the ANIT diet demonstrated a dose-dependent inhibition of TIMP-1 and Col1a1 gene expression (Figure 6, A and B) and Type 1 collagen deposition (Figure 6, C, E–J) in the liver. Of importance, administration of the anti-αVβ6 antibody significantly reduced SMAD2 phosphorylation in the livers of mice fed the ANIT diet, indicating inhibition of TGF-β activation (Figure 6D). Since αVβ6 led to activation of the TGF-β cytokine,25 we evaluated the contribution of TGF-β to liver fibrosis in this model using a recombinant murine TGF-β receptor type II-Fc fusion protein (mTGFβRII-Fc).2 Administration of mTGFβRII-Fc blocked SMAD2 phosphorylation in liver (Figure 6D) and also significantly reduced TIMP-1 and Col1a1 gene expression (Figure 6, A and B) and Type 1 collagen deposition (Figure 6, C, E, F, K, L) in the livers of wild type mice fed the ANIT diet. These results indicate that αVβ6 contributes to TGF-β activation and liver fibrosis in mice fed the ANIT diet, and are consistent with the hypothesis that a TF-PAR-1-dependent pathway contributes to liver fibrosis by up-regulating αVβ6 expression.
Figure 6.
Effect of inhibitory anti-αVβ6 integrin antibody and soluble TGF-β receptor treatment on liver fibrosis in mice fed the ANIT diet. Wild-type C57Bl/6J mice were fed either the control diet or an identical diet containing 0.025% ANIT for 14 days. Mice were given intraperitoneal injections of isotype control antibody (1E6, 10 mg/kg), recombinant TGF-β receptor type II-Fc fusion protein (TGFBRII-Fc, 5 mg/kg), or inhibitory anti-αVβ6 antibody (6.3G9, 1 or 10 mg/kg) on experiment days 1 and 7. Levels of TIMP-1 (A) and Col1a1 mRNA (B) in liver were determined as described in Materials and Methods. n = 10 mice per group. C: Quantification of type 1 collagen staining (E–L) as described in Materials and Methods; n = 5 mice per group. D: Levels of phosphorylated (Ser465/467) SMAD2 were determined in liver homogenated by ELISA as described in Materials and Methods. n = 10 mice per group. Data are expressed as the mean ± SEM. *P < 0.05; significantly different from the same mice fed the control diet. **P < 0.05; significantly different from isotype control-treated mice fed the ANIT diet. E–L: Representative photomicrographs showing immunofluorescent type 1 collagen staining of liver sections (scale bar = 20 μm). The images were converted to grayscale and inverted such that positive staining is dark.
Expression of PAR-1 by Transformed Human Bile Duct Epithelial Cells
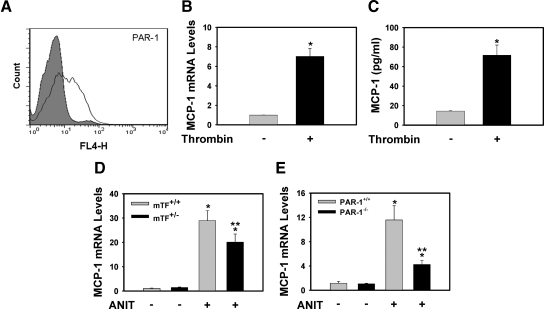
One possible mechanism whereby PAR-1 could contribute to expression of integrin β6 mRNA is by direct stimulation of BDECs. Although other epithelial cells have been shown to express PAR-1,26,27 the expression of PAR-1 by BDECs has not been described. To address this question we selected MMNK-1 cells, a transformed human bile duct epithelial cell line shown to express low levels of integrin β6 mRNA,3 analogous to normal BDECs in vivo. Of importance, MMNK-1 cells expressed PAR-1 mRNA (data not shown) and PAR-1 protein on the cell surface (Figure 7A). Stimulation of MMNK-1 cells with human α-thrombin or the specific PAR-1 agonist peptide TFLLRN28 induced MCP-1 mRNA expression (Figure 7B and data not shown), a positive control for PAR-1 activation. Thrombin stimulation also increased MCP-1 levels in the culture supernatant (Figure 7C). Interestingly, hepatic MCP-1 mRNA induction was reduced by TF deficiency and PAR-1 deficiency in mice fed the ANIT diet (Figure 7, D and E). The results suggest a PAR-1-dependent pathway of MCP-1 expression during chronic cholestatic liver injury. Furthermore, these data demonstrate that activation of PAR-1 on BDECs activates signaling pathways that lead to gene expression changes.
Figure 7.
Thrombin stimulation of transformed human BDECs. A: Flow cytometric detection of PAR-1 expression on the surface of MMNK-1 cells (isotype control, gray; white, anti-PAR-1). For B and C, MMNK-1 cells were stimulated with human α-thrombin (10 U/ml) for 4 hours, and MCP-1 mRNA (B) and supernatant MCP-1 (C) levels were determined. Data are expressed as mean ± SEM from at least three independent experiments. *Significantly different from vehicle-treated cells. For D and E, mice wild type for TF (TF+/+) and mice heterozygous for TF (TF+/−), and PAR-1+/+ mice and PAR-1−/− mice were fed either the control diet or an identical diet containing 0.025% ANIT for 14 days. Levels of MCP-1 mRNA in liver were determined as described in Materials and Methods; n = 4–12 mice per group. Data are expressed as the mean ± SEM. *P < 0.05; significantly different from the same mice fed the control diet. **P < 0.05; significantly different from control mice (TF+/+ mice or PAR-1+/+ mice) fed the ANIT diet.
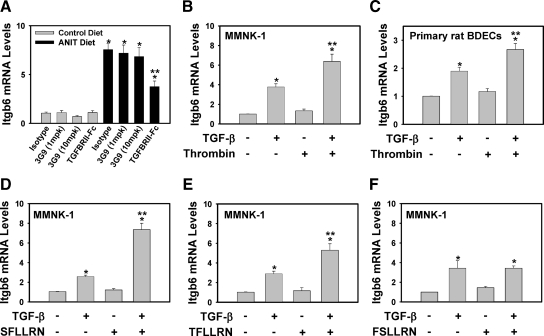
PAR-1 Activation Enhances TGF-β1 Induction of Integrin β6 mRNA Expression
In contrast to thrombin stimulation of MMNK-1 cells in vitro, thrombin generated during cholestasis likely promotes fibrosis in synergy with other mediators. Accordingly, thrombin could modify the response of BDECs to other profibrogenic mediators that induce integrin β6 mRNA expression. Interestingly, we found that mice fed the ANIT diet and treated with mTGFβRII-Fc showed a significant inhibition of hepatic integrin β6 expression as compared to isotype control-treated mice (Figure 8A). Consistent with this result, stimulation of MMNK-1 cells and primary rat BDECs with TGF-β1 increased integrin β6 mRNA levels (Figure 8, B and C). Stimulation of MMNK-1 cells and primary rat BDECs with thrombin alone did not affect integrin β6 mRNA levels. In contrast, thrombin enhanced TGF-β1-mediated induction of integrin β6 mRNA expression in MMNK-1 cells and primary rat BDECs (Figure 8, B and C). We also evaluated the role of PAR-1 in this cell system by using pharmacological agonists of this receptor. The PAR-1 agonist peptides SFLLRN and TFLLRN, which are based on the N-terminal tethered-ligand sequence of PAR-1,28 also enhanced TGF-β1-induced integrin β6 mRNA expression in MMNK-1 cells (Figure 8, D and E). In contrast, the peptide FSLLRN, which does not activate PAR-1, did not enhance TGF-β1-induced integrin β6 mRNA expression in MMNK-1 cells (Figure 8F).The data indicate that activation of PAR-1 on BDECs enhances TGF-β1 induction of integrin β6 mRNA expression.
Figure 8.
PAR-1 activation enhances TGF-β-induced integrin β6 mRNA expression. A: Wild-type C57Bl/6J mice were fed either the control diet or an identical diet containing 0.025% ANIT for 14 days. Mice were given intraperitoneal injections of isotype control antibody (1E6, 10 mg/kg), recombinant TGF-β receptor type II-Fc fusion protein (TGFBRII-Fc, 5 mg/kg), or inhibitory anti-αVβ6 antibody (3G9, 1 or 10 mg/kg) on experiment days 1 and 7. Levels of integrin β6 (Itgb6) mRNA in liver were determined as described in Materials and Methods; n = 10 mice per group. Data are expressed as the mean ± SEM. *Significantly different from the same mice fed the control diet. #Significantly different from isotype control-treated mice fed the ANIT diet. MMNK-1 cells (B) or primary rat BDECs (C) were stimulated with TGF-β1 (5 ng/ml) or vehicle in the presence or absence of human α-thrombin (10 U/ml) for 4 hours, and Itgb6 mRNA levels determined. For D–F, MMNK-1 cells were stimulated with TGF-β1 (5 ng/ml) or vehicle in the presence or absence of 100 μmol/L SFLLRN (D), 10 μΜ TFLLRN (E), or 100 μmol/L FSLLRN (F). Data are expressed as the mean ± SEM from at least four independent experiments. *P < 0.05; significantly different from the respective treatment group in the absence of TGF-β1. **P < 0.05; significantly different from cells treated with TGF-β1 alone.
TF and PAR-1 mRNAs Are Increased in Livers of Mice Fed the ANIT Diet and in Patients with Primary Biliary Cirrhosis and Sclerosing Cholangitis
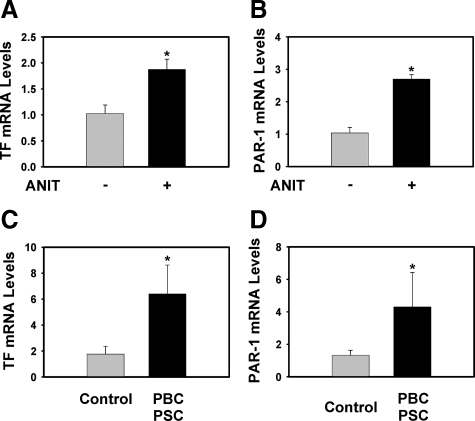
It is unlikely that patients with cholestatic liver disease are uniformly procoagulant to an extent considered pathological (ie, thrombosis), especially insofar as prothrombin time can be prolonged in these patients. However, a few studies have shown modest thrombin generation and increased circulating tissue factor levels in patients with PBC.17,18 Although such changes may be insufficient to dramatically affect systemic hemostasis, low levels of hepatic thrombin generation could be sufficient to trigger intracellular signaling in the liver. Of importance, we found that similar to mice fed the ANIT diet (Figure 9, A and B), levels of both TF mRNA and PAR-1 mRNA were increased in liver samples from patients with fibrosis associated with chronic cholestatic liver disease (PBC [n = 5] and PSC [n = 3]) compared to control liver samples without evidence of inflammation or fibrosis (Figure 9, C and D).
Figure 9.
Increased expression of TF and PAR-1 mRNA in mice fed the ANIT diet and in patients with cholestatic liver disease. A and B: TF and PAR-1 mRNA levels were determined in livers from wild-type C57Bl/6J mice fed either control diet or identical diet containing 0.025% ANIT for 14 days. Data are expressed as the mean ± SEM; n = 5–13 mice per group. *P < 0.05; significantly different from mice fed the control diet. C and D: TF and PAR-1 mRNA levels were determined in livers from patients with PBC (n = 5) and PSC (n = 3) and from control livers (n = 10) as described in Materials and Methods. Data are expressed as the mean ± SEM. *Significantly different from the control livers.
Discussion
It is increasingly clear that BDECs are not simply passive targets of injury in models of cholestatic liver disease. Rather, these cells actively participate in liver fibrosis by producing a wide array of mediators.29 One of these is the heterodimeric integrin αVβ6, which has diverse functions including activation of latent-TGF-β to its mature, active form.25 Elegant studies using pharmacological and genetic interventions demonstrated a critical role for the αVβ6 integrin in liver fibrosis induced by MDR2 deficiency or bile duct ligation in rodents.2,3 We found that administration of a function blocking anti-αVβ6 integrin antibody also reduced liver fibrosis in the ANIT diet model of cholestasis, indicating the effectiveness of αVβ6 blockade across multiple models of cholestasis, and further supporting the case for taking therapeutics forward. Moreover, administration of a murine recombinant TGF-β receptor type II-Fc fusion protein significantly reduced liver fibrosis in mice fed the ANIT diet. Taken together, the results implicate the αVβ6 integrin and TGF-β in the pathogenesis of liver fibrosis induced by the ANIT diet.
The TGF-β isoforms are expressed as part of an inactive complex by both normal and diseased tissues.30 In liver, TGF-β is expressed by several cell types including myofibroblasts, hepatocytes, and Kupffer cells.31 Our findings in the ANIT diet model and previous studies2,3 indicate that the αVβ6 integrin is required for activation of TGF-β to its profibrogenic form during cholestasis, and that the αVβ6 integrin expression is primarily localized to the BDEC. One potential cellular source of latent TGF-β complex relevant to cholestatic liver disease is the periportal myofibroblast.32 Liver myofibroblasts have been shown to express the latent TGF-β complex,33 and the proximity of these cells to BDECs may deliver latent TGF-β to αVβ6 integrin on BDECs for activation. Another intriguing possibility is that BDECs themselves express the requisite latent TGF-β. Increased expression of TGF-β1 by BDECs after bile duct ligation has been described previously, although BDEC expression levels were low compared to the adjacent fibroblasts.34 Of importance, the expression of αVβ6 integrin appears to be the critical, rate-limiting step for the activation of latent TGF-β during cholestasis.
Expression of the αVβ6 integrin is negligible in normal liver.2 However, after biliary injury, hepatic integrin β6 mRNA levels increase and expression of the αVβ6 integrin increases on BDECs.2,3 Similar to extrahepatic, obstructive cholestasis (eg, bile duct ligation), we found that chronic xenobiotic-induced intrahepatic BDEC injury markedly increased integrin β6 mRNA levels in liver and expression of the integrin β6 protein selectively on BDECs. Of importance, the mechanism whereby αVβ6 integrin expression increases during cholestatic liver injury has not been identified. Potential inducers of αVβ6 include direct cellular injury and a number of profibrogenic mediators produced in response to chronic cholestatic liver injury. Analogous to previous studies in other extrahepatic cell types,35,36 we found that TGF-β1 stimulation of BDECs induced integrin β6 mRNA expression, suggesting the presence of a feed-forward pathway whereby TGF-β1 amplifies activation of additional latent-TGF-β via induction of integrin β6 mRNA (Figure 10). In agreement with our findings in vitro, administration of a soluble TGF-β receptor type II attenuated the induction of integrin β6 mRNA expression in livers of mice fed the ANIT diet. Interestingly, administration of the anti-αVβ6 integrin antibody did not impact induction of integrin β6 mRNA in mice fed the ANIT diet, suggesting that the αVβ6 integrin is not the only activator of TGF-β in this model, or that low levels of αVβ6 are sufficient for localized activation of TGF-β and subsequent integrin β6 mRNA induction. Overall, the data indicate that TGF-β contributes to integrin β6 mRNA induction during cholestasis. However, TGF-β stimulates various cell types concurrently with other pathways activated by liver damage and interaction between these pathways may increase profibrogenic stimuli.
Figure 10.
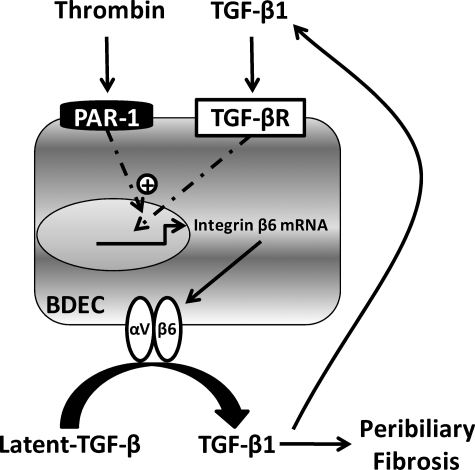
Proposed mechanism of amplified β6 integrin expression by thrombin and TGF-β1 in bile duct epithelial cells (BDECs). TGF-β1 generated during chronic cholestasis induces β6 integrin expression in BDECs, which is expressed on the cell surface as an αVβ6 integrin heterodimer. The αVβ6 integrin contributes to activation of additional TGF-β1 that stimulates peribiliary fibroblasts to produce collagen, leading to peribiliary fibrosis. In a feed-forward amplification loop, TGF-β1 also stimulates additional β6 integrin expression by BDECs. Chronic cellular injury elicits tissue factor-dependent coagulation cascade activation and thrombin generation. Thrombin activation of PAR-1 on BDECs contributes to peribiliary fibrosis by enhancing TGF-β1-induced β6 integrin expression.
One pathway activated by chronic liver damage is the coagulation cascade. Pharmacological inhibition of thrombin or its receptor PAR-1 reduced hepatic collagen deposition after bile duct ligation.16,19 Despite this and other evidence supporting a role for thrombin and PAR-1 in liver fibrosis, the mechanism of coagulation activation and the distal mechanism whereby PAR-1 activation contributes to liver fibrosis has not been identified. Our results indicate that pathological coagulation cascade activation during chronic cholestasis in mice fed the ANIT diet is TF-dependent, and that the thrombin generated contributes to liver fibrosis by activating PAR-1. Of interest, TF deficiency and PAR-1 deficiency attenuated αVβ6 integrin expression and TGF-β activation in livers of mice fed the ANIT diet. These results reveal a novel mechanism whereby coagulation protease signaling contributes to liver fibrosis during cholestasis (Figure 10). Of importance, the mechanism whereby thrombin contributes to liver fibrosis may be model-dependent.
Previous studies suggested that the expression of PAR-1 in the liver is restricted to non-parenchymal cells.37 We found that PAR-1 is expressed on the surface of transformed human BDECs (ie, MMNK-1 cells) and that these cells respond to thrombin and PAR-1 agonist peptide stimulation by producing MCP-1. Of importance, PAR-1 deficiency also reduced MCP-1 expression in mice fed the ANIT diet. Insofar as MCP-1 contributes to liver fibrosis after bile duct ligation,38 this suggests another mechanism whereby PAR-1 could contribute to liver fibrosis in this model. Although treatment of MMNK-1 cells or primary rat BDECs with thrombin had no effect on integrin β6 mRNA levels in vitro, thrombin enhanced TGF-β1-induced integrin β6 mRNA expression in both cell types. Similar experiments using agonist peptides mimicking the PAR-1 N-terminal tethered-ligand support the hypothesis that this effect is PAR-1-dependent. These experiments reveal a novel mechanism whereby thrombin and TGF-β synergistically promote profibrogenic gene expression and provide an important in vitro correlate for our observation that both PAR-1 and TGF-β contribute to αVβ6 expression in vivo. Interestingly, another study showed that stimulation of pulmonary epithelial cells with thrombin or PAR-1 agonist peptide increased αVβ6 integrin-dependent activation of latent TGF-β.39 In the latter study, de novo synthesis of integrin β6 mRNA was not required. Taken together, the results suggest that the coagulation cascade fine tunes, via PAR-1, the TGF-β-dependent induction of integrin β6 expression and the fibrogenic activity of αVβ6 in models of fibrosis (Figure 10).
Despite prolonged prothrombin time in patients with liver disease,17 moderate thrombin generation occurs, as indicated by elevated plasma thrombin-antithrombin levels.17,18 This low level of thrombin generation could be sufficient to elicit signaling through PAR-1 that contributes to disease progression. We found that TF and PAR-1 mRNA levels, as well as integrin β6 mRNA levels (not shown) were increased in patients with cholestatic liver disease. Another study also found elevated PAR-1 expression in patients with PBC.40 Moreover, we have shown previously that normal murine BDECs express TF in vitro and in vivo.15 The intricate association and apparent cellular co-localization of TF, PAR-1 and αVβ6 in liver is intriguing, as it suggests a paradigm where critical profibrogenic processes can respond rapidly to BDEC injury. That is, BDEC injury could result in membrane disruption that exposes TF or TF-positive microparticles to the blood, generating locally high thrombin concentrations. Subsequent activation of PAR-1 on BDECs could promote αVβ6 expression and peribiliary fibrosis.
In summary, the results indicate that TF and PAR-1 contribute to liver fibrosis in a model of chronic xenobiotic-induced cholestatic liver injury by enhancing TGF-β-dependent αVβ6 expression by BDECs. Continued investigation of the mechanism whereby coagulation protease signaling contributes to liver fibrosis may lead to novel approaches to limit TGF-β activation and fibrosis in patients.
Acknowledgments
We wish to recognize the outstanding technical assistance provided by Ruipeng (Frank) Wang and Huina Cai, and to thank Stanton Fernald in the Interdisciplinary Center for Male Contraceptive Research & Drug Development Imaging Core of the University of Kansas Medical Center for assistance with confocal microscopy.
Footnotes
Address reprint requests to James P. Luyendyk, Ph.D., Department of Pharmacology, Toxicology and Therapeutics, The University of Kansas Medical Center, 3901 Rainbow Blvd., MS-1018, Kansas City, KS 66160. E-mail: jluyendyk@kumc.edu.
Supported by National Institutes of Health grant R01 ES017537 (J.P.L.) and Center of Biomedical Research Excellence grant P20 RR021940 as well as the Molecular Biology Core and the Histology Core supported by the Center of Biomedical Research Excellence grant. B.P.S. was supported in part by National Institutes of Health training grant T32ES007079.
S.M.V. is an employee of Stromedix, Inc. and is developing anti-αvβ6 monoclonal antibodies for therapeutic use. P.H.W. is an employee of Biogen Idec, Inc. and was previously developing anti-αvβ6 monoclonal antibodies for therapeutic use.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46:1404–1412. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D. Inhibition of integrin alphavbeta6 on cholangiocytes blocks transforming growth factor-beta activation and retards biliary fibrosis progression. Gastroenterology. 2008;135:660–670. doi: 10.1053/j.gastro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaa GL, Priestly BG. Intrahepatic cholestasis induced by drugs and chemicals. Pharmacol Rev. 1976;28:207–273. [PubMed] [Google Scholar]
- Roth RA, Dahm LJ. Neutrophil- and glutathione-mediated hepatotoxicity of alpha-naphthylisothiocyanate. Drug Metab Rev. 1997;29:153–165. doi: 10.3109/03602539709037578. [DOI] [PubMed] [Google Scholar]
- Dietrich CG, Ottenhoff R, de Waart DR, Oude Elferink RP. Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology. 2001;167:73–81. doi: 10.1016/s0300-483x(01)00459-0. [DOI] [PubMed] [Google Scholar]
- Becker BA, Plaa GL. The nature of alpha-naphthylisothiocyanate-induced cholestasis. Toxicol Appl Pharmacol. 1965;7:680–685. doi: 10.1016/0041-008x(65)90125-0. [DOI] [PubMed] [Google Scholar]
- Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali P, Wu P, Lahiji PA, Brown EJ, Maher JJ. ANIT toxicity toward mouse hepatocytes in vivo is mediated primarily by neutrophils via CD18. Am J Physiol Gastrointest Liver Physiol. 2006;291:G355–G363. doi: 10.1152/ajpgi.00458.2005. [DOI] [PubMed] [Google Scholar]
- Moritoki Y, Ueno Y, Kanno N, Yamagiwa Y, Fukushima K, Gershwin ME, Shimosegawa T. Lack of evidence that bone marrow cells contribute to cholangiocyte repopulation during experimental cholestatic ductal hyperplasia. Liver Int. 2006;26:457–466. doi: 10.1111/j.1478-3231.2006.01250.x. [DOI] [PubMed] [Google Scholar]
- Lesage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, Phinizy JL, Francis H, Alpini G. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G182–G190. doi: 10.1152/ajpgi.2001.281.1.G182. [DOI] [PubMed] [Google Scholar]
- Tjandra K, Sharkey KA, Swain MG. Progressive development of a Th1-type hepatic cytokine profile in rats with experimental cholangitis. Hepatology. 2000;31:280–290. doi: 10.1002/hep.510310204. [DOI] [PubMed] [Google Scholar]
- Xu J, Lee G, Wang H, Vierling JM, Maher JJ. Limited role for CXC chemokines in the pathogenesis of alpha-naphthylisothiocyanate-induced liver injury. Am J Physiol Gastrointest Liver Physiol. 2004;287:G734–G741. doi: 10.1152/ajpgi.00300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N. The many faces of tissue factor. J Thromb Haemost. 2009;7 Suppl 1:136–9:136–139. doi: 10.1111/j.1538-7836.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyendyk JP, Cantor GH, Kirchhofer D, Mackman N, Copple BL, Wang R. Tissue factor-dependent coagulation contributes to alpha-naphthylisothiocyanate-induced cholestatic liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G840–G849. doi: 10.1152/ajpgi.90639.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Salam OM, Baiuomy AR, Ameen A, Hassan NS. A study of unfractionated and low molecular weight heparins in a model of cholestatic liver injury in the rat. Pharmacol Res. 2005;51:59–67. doi: 10.1016/j.phrs.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Segal H, Cottam S, Potter D, Hunt BJ. Coagulation and fibrinolysis in primary biliary cirrhosis compared with other liver disease and during orthotopic liver transplantation. Hepatology. 1997;25:683–688. doi: 10.1002/hep.510250332. [DOI] [PubMed] [Google Scholar]
- Biagini MR, Tozzi A, Marcucci R, Paniccia R, Fedi S, Milani S, Galli A, Ceni E, Capanni M, Manta R, Abbate R, Surrenti C. Hyperhomocysteinemia and hypercoagulability in primary biliary cirrhosis. World J Gastroenterol. 2006;12:1607–1612. doi: 10.3748/wjg.v12.i10.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Distrutti E, Severino B, Fiorentina R, Baldoni M, Caliendo G, Santagada V, Morelli A, Cirino G. PAR1 antagonism protects against experimental liver fibrosis. Role of proteinase receptors in stellate cell activation Hepatology. 2004;39:365–375. doi: 10.1002/hep.20054. [DOI] [PubMed] [Google Scholar]
- Maruyama M, Kobayashi N, Westerman KA, Sakaguchi M, Allain JE, Totsugawa T, Okitsu T, Fukazawa T, Weber A, Stolz DB, Leboulch P, Tanaka N. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation. 2004;77:446–451. doi: 10.1097/01.TP.0000110292.73873.25. [DOI] [PubMed] [Google Scholar]
- Van Aarsen LA, Leone DR, Ho S, Dolinski BM, McCoon PE, LePage DJ, Kelly R, Heaney G, Rayhorn P, Reid C, Simon KJ, Horan GS, Tao N, Gardner HA, Skelly MM, Gown AM, Thomas GJ, Weinreb PH, Fawell SE, Violette SM. Antibody-mediated blockade of integrin alpha v beta 6 inhibits tumor progression in vivo by a transforming growth factor-beta-regulated mechanism. Cancer Res. 2008;68:561–570. doi: 10.1158/0008-5472.CAN-07-2307. [DOI] [PubMed] [Google Scholar]
- Kim ND, Moon JO, Slitt AL, Copple BL. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006;90:586–595. doi: 10.1093/toxsci/kfj111. [DOI] [PubMed] [Google Scholar]
- Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, Goyal J, Feghali-Bostwick CA, Matteson EL, O'Hara C, Lafyatis R, Davis GS, Huang X, Sheppard D, Violette SM. Partial inhibition of integrin alpha (v) beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- Weinreb PH, Simon KJ, Rayhorn P, Yang WJ, Leone DR, Dolinski BM, Pearse BR, Yokota Y, Kawakatsu H, Atakilit A, Sheppard D, Violette SM. Function-blocking integrin alphavbeta6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J Biol Chem. 2004;279:17875–17887. doi: 10.1074/jbc.M312103200. [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Rondeau E, Vigneau C, Berrou J. Role of thrombin receptors in the kidney: lessons from PAR1 knock-out mice. Nephrol Dial Transplant. 2001;16:1529–1531. doi: 10.1093/ndt/16.8.1529. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Gabazza EC, Hayashi T, Ido M, Adachi Y, Suzuki K. Thrombin stimulates the expression of PDGF in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L503–L510. doi: 10.1152/ajplung.2000.279.3.L503. [DOI] [PubMed] [Google Scholar]
- Blackhart BD, Emilsson K, Nguyen D, Teng W, Martelli AJ, Nystedt S, Sundelin J, Scarborough RM. Ligand cross-reactivity within the protease-activated receptor family. J Biol Chem. 1996;271:16466–16471. doi: 10.1074/jbc.271.28.16466. [DOI] [PubMed] [Google Scholar]
- Glaser SS, Gaudio E, Miller T, Alvaro D, Alpini G. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med. 2009;11:e7. doi: 10.1017/S1462399409000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura SL. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol. 2009;175:1362–1370. doi: 10.2353/ajpath.2009.090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff JA, Wells RG. Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–1444. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangasser-Stephan K, Gartung C, Lahme B, Gressner AM. Expression of isoforms and splice variants of the latent transforming growth factor beta binding protein (LTBP) in cultured human liver myofibroblasts. Liver. 2001;21:105–113. doi: 10.1034/j.1600-0676.2001.021002105.x. [DOI] [PubMed] [Google Scholar]
- Sedlaczek N, Jia JD, Bauer M, Herbst H, Ruehl M, Hahn EG, Schuppan D. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol. 2001;158:1239–1244. doi: 10.1016/S0002-9440(10)64074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L, Hill CS. Smad4 dependency defines two classes of transforming growth factor {beta} (TGF-{beta}) target genes and distinguishes TGF-{beta}-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol. 2005;25:8108–8125. doi: 10.1128/MCB.25.18.8108-8125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannad F, Nica D, Fulle MI, Grenier D, Putnins EE, Johnston S, Eslami A, Koivisto L, Jiang G, McKee MD, Hakkinen L, Larjava H. Absence of alphavbeta6 integrin is linked to initiation and progression of periodontal disease. Am J Pathol. 2008;172:1271–1286. doi: 10.2353/ajpath.2008.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copple BL, Moulin F, Hanumegowda UM, Ganey PE, Roth RA. Thrombin and protease-activated receptor-1 agonists promote lipopolysaccharide-induced hepatocellular injury in perfused livers. J Pharmacol Exp Ther. 2003;305:417–425. doi: 10.1124/jpet.102.046391. [DOI] [PubMed] [Google Scholar]
- Seki E, De MS, Inokuchi S, Taura K, Miyai K, van RN, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. Ligation of protease-activated receptor 1 enhances alpha (v) beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest. 2006;116:1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW. Identification of novel molecules and pathogenic pathways in primary biliary cirrhosis: cDNA array analysis of intrahepatic differential gene expression. Gut. 2001;49:565–576. doi: 10.1136/gut.49.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]