Abstract
Complement is implicated in the inflammatory response and the secondary neuronal damage that occurs after traumatic spinal cord injury (SCI). Complement can be activated by the classical, lectin, or alternative pathways, all of which share a common terminal pathway that culminates in formation of the cytolytic membrane attack complex (MAC). Here, we investigated the role of the alternative and terminal complement pathways in SCI. Mice deficient in the alternative pathway protein factor B (fB) were protected from traumatic SCI in terms of reduced tissue damage and demyelination, reduced inflammatory cell infiltrate, and improved functional recovery. In a clinically relevant paradigm, treatment of mice with an anti-fB mAb resulted in similarly improved outcomes. These improvements were associated with decreased C3 and fB deposition. On the other hand, deficiency of CD59, an inhibitor of the membrane attack complex, resulted in significantly increased injury and impaired functional recovery compared to wild-type mice. Increased injury in CD59-deficient mice was associated with increased MAC deposition, while levels of C3 and fB were unaffected. These data indicate key roles for the alternative and terminal complement pathways in the pathophysiology of SCI. Considering a previous study demonstrating an important role for the classical pathway in promoting SCI, it is likely that the alternative pathway plays a critical role in amplifying classical pathway initiated complement activation.
Post-traumatic inflammation after spinal cord injury (SCI) is thought to play an important role in secondary neuronal injury and impaired functional recovery, and while it is difficult to protect against the initial trauma, the subsequent inflammatory response represents a therapeutic target. Currently, the only clinically approved treatment for SCI is high-dose methylprednisolone, an antiinflammatory reagent that results in mild improvement for some patients.1,2,3
Complement activation is a key component of the inflammatory cascade, and although available data indicate that it plays an important role in SCI, details of its activation and pathogenic mechanisms are limited. Early studies showed that patients with spinal cord injury have elevated complement levels in their sera,4 and more recent studies have used rodent models to demonstrate a role for complement in SCI. These studies include analysis of complement activation and deposition after SCI5,6,7 and the demonstration that complement inhibition7,8,9,10 or complement deficiency7,11 ameliorates injury and improves functional recovery after traumatic injury.
Complement can be activated by one of three pathways: the classical, lectin, or alternative. Classical pathway activation is usually antibody-dependent and is initiated when C1q binds to an immune complex. The lectin pathway is activated when mannose binding protein (MBL) or ficolins bind to conserved carbohydrate structures. The alternative pathway is activated by spontaneous hydrolysis of C3 to a cleavage product (C3b analog) that binds factor B (fB), leading to formation of the alternative pathway C3 convertase. The alternative pathway also provides an amplification loop for the classical and lectin pathways. All pathways converge at C3 activation with the subsequent cleavage of C5. During this process, the anaphylatoxins C3a and C5a are generated, and C5 cleavage initiates the terminal complement pathway that culminates in the formation of the membrane attack complex (MAC). The MAC can be directly cytolytic and can stimulate the production of proinflammatory molecules when deposited in cell membranes at sublytic concentrations (for a review of the complement system, see Ref. 12). Complement activation on host tissue is controlled by various complement inhibitory proteins. Decay accelerating factor (DAF), membrane cofactor protein (MCP), and, in rodents, Crry, are membrane-bound inhibitors that all function to prevent C3 activation (by any pathway). Complement receptor 1 (CR1) also inhibits C3 activation, at least in soluble form. CD59 is a membrane-bound inhibitor of the terminal pathway that prevents the formation and membrane insertion of the MAC by binding the terminal complement proteins (C8 and C9) as they unfold. The plasma proteins factor H (fH) and C4-binding protein inhibit the alternative and classical complement pathways, respectively, both in the fluid phase and on cell surfaces after their attachment.
In the previous studies using rodent models alluded to above, SCI was shown to activate the classical, alternative, and terminal pathways of complement by the demonstration of C1q, C4, fB, and MAC deposition in spinal cords.5 In other studies, complement inhibition at the C3 activation step with vaccinia virus complement control protein (VCP),8 soluble CR1,10 and a targeted form of Crry7 were all protective against SCI and improved functional recovery. More recent studies have shown that C1q deficiency and C1-inhibitor are protective in models of SCI,9,11 indicating an important role for the classical pathway. Further to these findings, pathogenic antibodies produced by infiltrating B cells have been shown to contribute to SCI in a mouse model, and these antibodies associate with deposits of C1q.13
Of note, a number of rodent models of SCI have been developed over the years in attempts to mimic human SCI. These include weight-drop, contusion, compression, laceration, and chemically-mediated SCI. All these models have their advantages and disadvantages. The weight-drop method originally described in 1911, has evolved into a standard protocol developed by New York University (NYU) that allows for a controlled injury and minimizes the risk of multiple injuries and can be monitored for impact velocity and tissue displacement.14,15 Compression models use aneurysm clips or forceps that can allow for specific force application that correlate with injury and neurological outcomes.16,17 Various contusion (controlled pneumatic compression) models have been developed with the Ohio State University (OSU) device and the Infinite Horizon (IH) device that provides correlative injury and behavioral outcomes and allow for control of velocity, force, and tissue displacement, which eliminates the need for exclusion of animals that can occur with other models.18,19 The final models, laceration and chemically-induced SCI, are often used in research and present with injuries that allow for mechanistic studies but are not frequently seen in patients with SCI.20,21 Therefore, justification of the use of a particular model will depend on the types of questions being asked, the direct application to clinical relevant disease and applicability to other model systems. In the present study we used the weight-drop method that provides a consistent and standardized approach to study the role of factors in the pathogenesis of SCI. We used fB-deficient mice to investigate the role of the alternative complement pathway in SCI and further investigated the therapeutic potential of alternative pathway inhibition. We additionally investigated the role of the terminal complement pathway in SCI by using mice deficient in CD59 and thus less able to effectively control MAC formation.
Materials and Methods
Animals and Anti-fB Antibody
Female C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Breeding pairs of fB-deficient mice on C57BL/6 background were generated as described22 and provided by Dr. J. Thurman (University of Colorado Health Sciences Center, Denver, CO) and a breeding colony established. In mice, there are two genes encoding CD59; CD59a is widely expressed and is the primary regulator of the MAC in mice, whereas CD59b expression is limited to the testis and, at very low levels, bone marrow.23 In this study, CD59a-deficient mice on C57BL/6 background were used and were generated as described.24 fB and CD59 deficiency was confirmed by genotyping. Mice weighing between 18–22 g (6–8 weeks old) were used in experiments. All mice were fed on standard laboratory food and given tap water ad libitum with a light–dark cycle of 12 hours. The isolation and characterization of anti-fB mAb 1379 used in these studies was described previously, and the mAb effectively inhibits the mouse alternative pathway.25 The mAb was generously provided by Drs. V. M. Holers, J. M. Thurman (University of Colorado Health Sciences Center, Denver, CO), and G. S. Gilkeson (Medical University of South Carolina).
Injury Model and Antibody Treatment
Wild-type (wt) mice were randomized into sham, vehicle control (PBS), and anti-fB mAb treatment groups. Other groups consisted of fB-deficient (fB−/−) and CD59a-deficient (CD59−/−) mice. For anti-fB mAb treatment, wt mice were randomized into four groups, with mice in each group receiving an intravenous (tail vein) injection of 100 μl PBS vehicle control or 2 mg anti-fB mAb in 100 μl PBS at 1 and 12 hours after surgery, 12 and 24 hours after surgery, or 24 and 36 hours after surgery. The 2-mg dose used was based on previous studies characterizing the therapeutic effect of this Ab in mouse models of inflammation.25,26,27 For the surgical procedure, mice were anesthetized with ketamine/xylazine anesthesia (80 mg/kg/10 mg/kg, i.p.) and subjected to laminectomy at the level of the 12th thoracic vertebra (T12), followed by contusion-induced SCI using the NYU weight drop impactor as described previously.7 After injury, the muscles and the subcutaneous tissue were closed in layers, the skin was closed with metal wound clips, and mice were given 1 ml of saline per day for 3 days to compensate for loss of blood and dehydration. Manual bladder expression was performed twice daily until full bladder function was observed. Sham mice received a dorsal laminectomy without impact injury. There was less than 5% surgical mortality, and zero mortality of mice that recovered from surgery. Groups of mice were sacrificed at 1, 3, and 21 days after injury, and spinal cords were isolated for analysis. The care of mice and surgical procedures were approved by the Medical University of South Carolina’s committee for animal research, in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Locomotor Function Analysis
Open-field observations of locomotor function recovery were independently scored by two observers blinded to experimental groups using the Basso Mouse Scale (BMS).28 The ten point BMS scale (0–9) is based on a systematic in-depth analysis of locomotor function after SCI in mice and is sufficiently sensitive to determine gross locomotor recovery. Bladders were expressed before testing, and each mouse was evaluated for 4–5 minutes on the day before surgery, immediately after surgery, and then twice daily postoperatively until the end of the study (21 days).
Histopathological Analysis
Spinal cords were removed at 1, 3, and 21 days postinjury for histological analysis. Immediately after sacrifice, mice were perfused transcardially with PBS followed by 4% paraformaldehyde in PBS. Spinal cords were then removed, placed into 4% paraformaldehyde/PBS, and either cryoprotected in 30% sucrose for 48 hours before storing at −80°C or placed in formalin and processed to paraffin for histological analysis.
For histological assessment, sections of spinal cord were stained with hematoxylin and eosin (H&E) or luxol fast blue (LFB) as previously described.29 Histopathological damage was assessed quantitatively by an independent reviewer blinded to the experimental groups. H&E and LFB sections were scored from 0 to 3 for the presence and intensity of inflammatory cell infiltration, neuronal vacuolation, and hemorrhage (where 0, is no evidence and 3 severe). Scores were then expressed as a cumulative score of 0–9. When assessing recovery at 21 days after injury, the extent of demyelination, graded between 0–3 (with 0 being no evidence of demyelination) was added for a cumulative score of 0–12. To further quantify spinal cord injury, morphometric analyses was conducted to determine the degree of tissue sparing after injury. Transverse sections of spinal cord were stained with H&E, and the cross-sectioned area of spinal cord was measured at 150-μm increments extending 2 mm either side of the injury epicenter using Zeiss Axiovision image analysis software (Carl Zeiss, Oberkochen, Germany). Measurements were averaged for animals in each group at each time point as previously described.30 All assessments were performed in a blinded fashion.
Neutrophil and Macrophage Infiltration
The presence of infiltrating neutrophils and macrophages was assessed using immunohistochemistry on frozen spinal cord sections. Standard immunohistochemical methods were used as previously described.31 Neutrophils and macrophages were identified by anti-mouse Gr-1 and Mac-3 (BD Biosciences), respectively. Neutrophils and macrophages were quantified at the spinal cord injury epicenter, defined as the section exhibiting maximal tissue damage. The total number of neutrophils and macrophages were quantified using computerized image analysis methods, as previously described.32,33 Results are expressed as the number of neutrophils/macrophages per mm2. Specificity of immunostaining was confirmed by both the use of isotype control antibody and by the omission of primary antibody.
Complement Deposition
Spinal cord cryosections were fixed in cold acetone for 5 minutes and then washed in running water followed by PBS. Sections were then incubated for 1 hour at room temperature with either anti-mouse C3 fluorescein isothiocyanate (FITC) (Dako, Ely, UK), mouse anti-mouse fB mAb 1379 (see above), rabbit anti rat C9 that is cross reactive with mouse C9,34 or rat anti-mouse CD59 mAb 7A6.35 For C9 and CD59 visualization, donkey anti-rabbit FITC and donkey anti-rat FITC antibodies were used. For fB visualization, we used a mouse on mouse staining kit and protocol from Vector laboratories (Burlingame, CA). Sections were then counterstained with DAPI (Thermo Scientific, Rockford, IL) for nuclear detail. Sections were then coverslipped and analyzed for fluorescence intensity using a Zeiss LSM5 Confocal microscope. Fluorescence intensity for each complement component was scored on a scale of 0–3, where 0 is no staining, 1, mild, 2, moderate, and 3, intense. All observations were made by an observer blinded to group identities.
Statistical Analysis
All data are presented as Mean ± SEM or mean ± SD as indicated. Analyses were performed using SPSS 13.0 for Windows. Statistical significance between groups was determined by two-way analysis of variance with Bonferroni/Dunn’s corrected post hoc t-tests. For Locomotor functional analysis, repeated measures of analysis of variance was used to determine differences between the groups. P < 0.05 was considered statistically significant.
Results
Recovery of Locomotor Function Post-Traumatic Injury
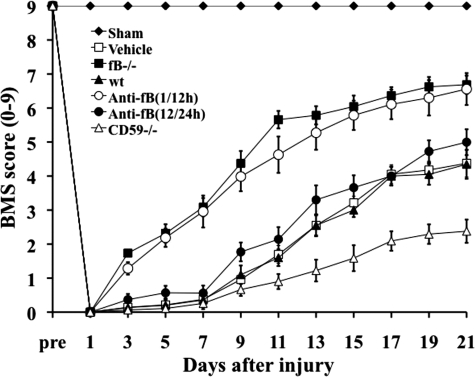
Contusion injury to the spinal cord was induced in mice deficient in fB or CD59, in wt untreated mice, and in wt mice subsequently treated with anti-fB mAb or PBS (vehicle). Locomotor function was assessed by the BMS scale, and all mice subjected to contusion injury exhibited a BMS score of 0 immediately after injury. Over the course of 21 days, locomotor function significantly improved in fB−/− mice and wt mice treated with anti-fB mAb at 1 and 12 hours after SCI compared to wt and PBS treated controls (Figure 1). There were no significant differences in BMS scores between fB−/− mice and anti-fB–treated (1/12 hours) mice over the course of the experiment. When anti-fB mAb was administered at 12 and 24 hours after SCI, there was a trend toward improved locomotor function when compared to controls, but the difference did not reach significance (Figure 1). We also administered anti-fB mAb at 24 and 36 hours post-SCI, but there was no difference in functional recovery compared to PBS treated mice (data not shown). Unless otherwise stated, all data shown below for anti-fB treated mice was obtained using a 1 and 12 hour post-SCI treatment schedule. In contrast to the improvement seen in fB−/− mice and anti-fB (1/12 hours) treated mice, recovery of locomotor function was significantly impaired in CD59−/− mice compared to wt and vehicle treated controls. There was no difference in functional recovery between PBS (vehicle) treated mice and untreated wt mice after SCI.
Figure 1.
Locomotor recovery after SCI in complement deficient and inhibited mice. Open-field 10-point BMS scores were recorded for 21 days after contusion-induced SCI in the indicated groups of mice. Anti-fB mAb treatment group received an i.v. injection of 2 mg at 1 and 12 or 12 and 24 hours after injury. BMS scores are significantly higher for fB−/− mice and anti-fB mAb (1/12 hours)-treated mice compared to wt or vehicle (PBS)-treated mice from day 3 postinjury. No significant difference between vehicle controls and anti-fB mAb (12/24 hours) treated mice. BMS scores are significantly lower in CD59−/− mice compared to wt and vehicle control mice from day 11 after injury (P < 0. 01). Mean ± SE, n = 8–10.
Effect of Complement Deficiency and Complement Inhibition on Tissue Damage and Demyelination after SCI
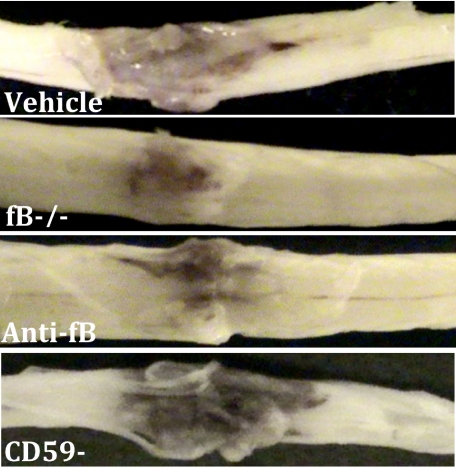
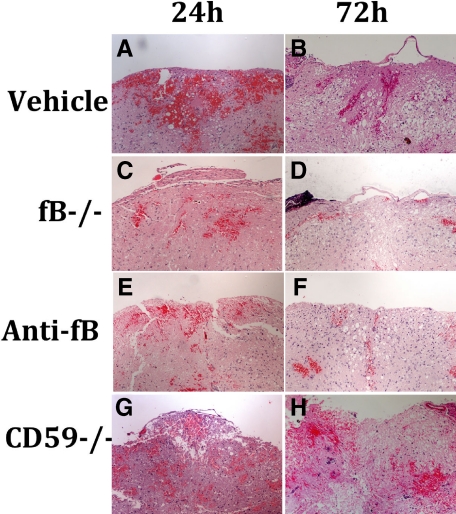
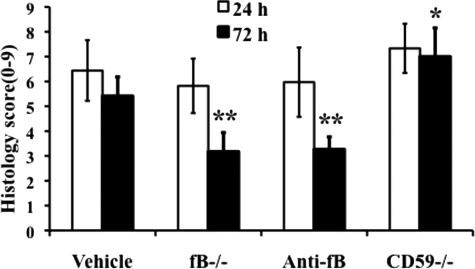
Spinal cord contusion results in a primary hemorrhage, inflammation, and loss/damage of neurons. We determined the effect of fB deficiency, fB inhibition (1/12 hours), or CD59 deficiency on spinal cord tissue injury by macroscopic examination of spinal cords and by assessment of histological changes within the spinal cords postinjury. At 72 hours after injury, macroscopic examination of control spinal cords demonstrated marked indentation at the injury site with evidence of hemorrhage. These features were markedly reduced in fB−/− and anti-fB mAb treated animals, but appeared exacerbated in spinal cords from CD59−/− mice (Figure 2). We next analyzed spinal cords microscopically at the injury epicenter using H&E stain to assess the extent of inflammatory cell infiltrate, neuronal vacuolation, and hemorrhage. Sections were prepared from cords isolated at 24 and 72 hours postinjury, and the sections were graded for a total cumulative score of 0–9 (see Materials and Methods). Injury to spinal cords was evident in all groups at both 24 and 72 hours (Figure 3). No significant differences were noted at 24 hours postinjury, but at 72 hours there was significantly less damage seen in fB−/− mice and anti-fB mAb–treated mice compared to control mice. In contrast, damage was significantly exacerbated in CD59−/− mice compared to controls 72 hours after injury. Representative images of spinal cord sections postinjury are shown in Figure 3, with quantification of data shown in Figure 4. Histologically, control and CD59−/− spinal cord sections demonstrated evidence of hemorrhage, pronounced inflammation, neuronal cell vacuolation, and demyelination, and while these features existed in fB−/− mice and anti-fB mAb–treated mice, they were markedly reduced.
Figure 2.
Macroscopic images of spinal cords isolated at 72 hours postinjury. Mice received anti-fB mAb or PBS (vehicle) at 1 and 12 hours after injury. Representative images shown, n = 6–8 per group.
Figure 3.
Histopathology of spinal cord sections after SCI. A–H: Transverse sections from the epicenter of injury were prepared from spinal cords isolated at 24 and 72 hours after injury and stained with H&E. Mice received anti-fB mAb or PBS (vehicle) at 1 and 12 hours after injury. Representative images shown, n = 6 per group.
Figure 4.
Quantitative assessment of histopathological inflammation and injury. H&E-stained sections from the epicenter of injury were scored from 0 to 3 for the presence and intensity of inflammatory cell infiltration, neuronal vacuolation, and hemorrhage (0 = no evidence, 3 = severe). Scores were then expressed as a cumulative score of 0–9. Assessments were made from spinal cords isolated at 24 and 72 hours postinjury. Mice received anti-fB mAb or PBS (vehicle) at 1 and 12 hours after injury. *P < 0.01, **P < 0.001 compared to vehicle control. Mean ± SD, n = 6 per group.
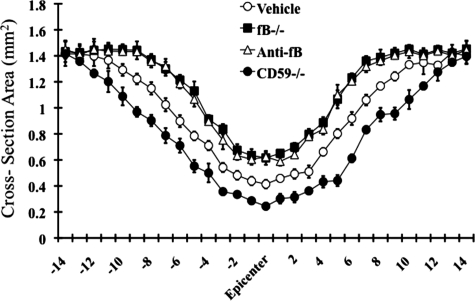
To further quantify the impact of complement deficiency or inhibition on SCI, we analyzed tissue destruction by determining the cross-sectional area of spinal cords at 100-μm increments extending 2 mm either side of the initial injury impact site. In accord with our subjective histological assessments, there was significantly increased tissue sparing in fB-deficient and fB-inhibited mice, and significantly less tissue sparing in CD59−/− mice, when compared to control mice (Figure 5).
Figure 5.
Morphometric analysis of tissue sparing 3 days after injury. The cross-sectional area of H&E stained spinal cord sections was measured at 150 μm increments extending 2 mm either side of the injury site. There was a significant level of tissue sparing in fB−/− mice and anti-fB–treated mice compared with vehicle control (PBS)-treated mice, and a significant decrease in tissue sparing in CD59−/− mice compared with wt (at injury site and out to 1.2 mm each side of the injury site, P < 0.01). Mice received anti-fB mAb or PBS at 1 and 12 hours after injury. Mean ± SD n = 6 per group.
Expression of Neutrophils and Macrophage Infiltration after Spinal Cord Injury
After SCI, the infiltration and activation of neutrophils and macrophages is considered to play an important role in the propagation of inflammation and tissue damage. Neutrophils are typically the first leukocytes to arrive at the injury site, while macrophages/microglia appear later but, unlike neutrophils, persist in the spinal cord.37 Complement activation products provide chemotactic and activating signals for neutrophils and macrophages, as well as induce the expression of inflammatory cytokines/chemokines and adhesion molecules. Using complement deficient and inhibited mice, we investigated the influence of the alternative and terminal complement pathways in neutrophil and macrophage infiltration after SCI. Inflammatory cell influx was evaluated by immunohistochemical analysis using Gr-1, a marker antibody for neutrophils, and Mac-3, a marker antibody for microglia/macrophages. Neutrophils and macrophages were quantified at the site of injury in mice from all groups at 24 and 72 hours post-SCI.
Neutrophil infiltration was pronounced at 24 hours after injury in all groups, and as expected, numbers declined by 72 hours post-SCI. However, at both 24 and 72 hours post-SCI, neutrophil numbers were significantly reduced in spinal cords from fB-deficient and fB-inhibited mice compared to control mice (Figure 6A). At 24 hours post-SCI, there was no significant difference in neutrophil numbers between CD59−/− and control mice, but neutrophil numbers were significantly higher in CD59−/− mice at 72 hours post-SCI. Not surprisingly, there was no difference in the number of macrophages between any of the groups at 24 hours post-SCI, in accord with previous data on the dynamics of macrophage infiltration following SCI. At 72 hours post-SCI, macrophage numbers were increased in all groups, but numbers were significantly lower in fB-deficient and fB-inhibited mice compared to control mice (Figure 6B). In CD59−/− mice at 72 hours post-SCI, macrophage numbers were the same as in control mice, and this is in contrast to the increased neutrophil infiltration seen in CD59−/− mice. These data indicate an important role for the alternative pathway in neutrophil and macrophage infiltration after SCI, and together with the above data demonstrate that the infiltration of these leukocytes is associated with tissue injury.
Figure 6.
Neutrophil (A) and macrophage (B) infiltration after SCI. Sections from the epicenter of injury were prepared from spinal cords isolated at 24 and 72 hours after injury. Mice received anti-fB mAb or PBS (vehicle) at 1 and 12 hours after injury. Neutrophils and macrophages were identified and counted by immunohistochemistry using anti-mouse Gr-1 and Mac-3 antibodies. Results are expressed as the number of neutrophils/macrophages per mm2. Mean ± SD n = 6 per group. *P < 0.01 vs. vehicle control.
In this study, the total number of neutrophils and macrophages present on each section was counted. No distinction was made between white and gray matter, due to the extensive damage noted at 24 and 72 hours postinjury in some groups and because sections were analyzed using immunohistochemistry, which did not permit morphological evaluation.
Deposition of Complement Activation
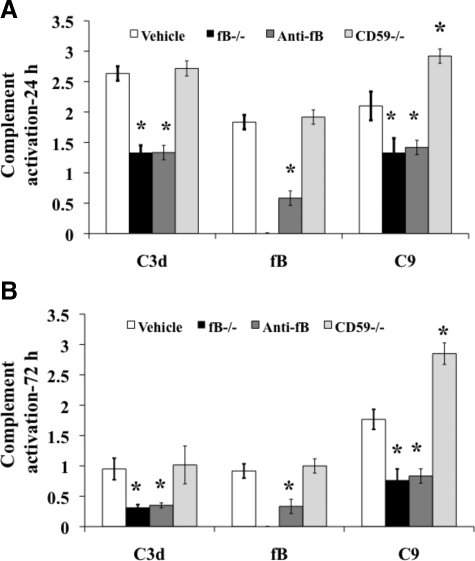
To correlate post-traumatic inflammation and injury with complement activation, we examined spinal cord sections for deposited C3, fB, and C9 (MAC) by immunofluorescence microscopy. In agreement with previous data obtained using mice7 (for C3 deposition) and rats5 (for fB and C9 deposition), there was pronounced deposition of all three complement proteins in the white and gray matter of control mice at 24 hours postinjury. C3 and C9 deposition was significantly lower in fB-deficient and fB-inhibited mice compared to control mice. As expected, fB was undetectable in fB-deficient mice and was detected at only very low levels in fB-inhibited mice. Deficiency of CD59 resulted in significantly increased levels of C9 deposition compared to all groups (including controls) but did not effect levels of C3 or fB deposition (Figure 7A). Similar relative profiles for C3, fB, and C9 deposition were seen at 72 hours post-SCI in all groups, although C3 and fB levels were lower than at 24 hours after SCI (Figure 7B). C9 levels were similar at 24 and 72 hours after SCI (Figure 7). Representative immunofluorescence images showing complement deposition, as well as CD59 expression, at 24 hours after injury are shown in supplemental Figure 1 (at http://ajp.amjpathol.org).
Figure 7.
Quantitative assessment of complement deposition post-SCI. The deposition of C3 (C3 days), fB, and C9 (MAC) was analyzed at 24 hours (A) and 72 hours (B) after injury in spinal cord sections from the epicenter of injury. Mice received anti-fB mAb or PBS (vehicle) at 1 and 12 hours after injury. Analysis was performed using immunofluorescence microscopy, and sections scored for fluorescence intensity on a 4 point scale (see Materials and Methods). Mean ± SD, n = 6. *P < 0.01.
There was no evidence of C3, fB, or C9 staining in spinal cords from sham operated mice, and expression of CD59 was similar in wt control treated and fB−/− mice and absent in CD59−/− mice (supplemental Figure 1 at http://ajp.amjpathol.org). In this study, we detected C3 deposition at 72 hours post-SCI, although at low levels. This is in contrast to our previous report that C3 was undetectable in mouse spinal cords 72 hours after injury, and we attribute this difference to the use of a different detection antibody (see Materials and Methods).
Histological Analysis of Recovery
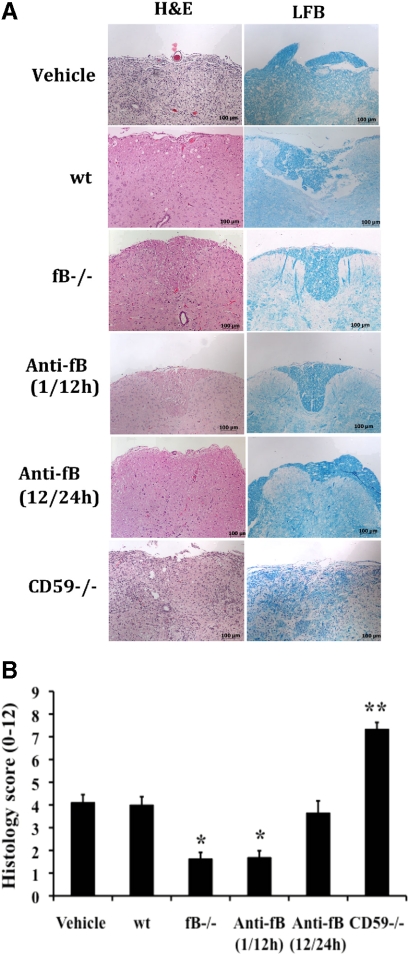
Data presented above show that after SCI, fB-deficient mice and fB-inhibited mice (when treated at 1 and 12 hours after SCI) have a significantly improved outcome compared with controls in terms of inflammation, tissue injury, and functional locomotor recovery. To determine the extent of histological recovery, we analyzed spinal cords 21 days after the initial impact injury. H&E and LFB staining of cord sections from wt and PBS treated controls revealed demylineation in the central core of the white matter beneath the impact site and evidence of vacuolation with some necrosis with inflammatory cells still present (Figure 8). By comparison, at 21 days after SCI, there was markedly less demyelination and inflammation and no evidence of necrosis in cords from fB-deficient mice and mice treated with anti-fB mAb at 1 and 12 hours after SCI. There was no apparent difference in the extent of demyelination between the fB-deficient and fB-inhibited (1/12 hours) groups. In contrast, CD59−/− mice exhibited a serious lack of structural organization, abundant inflammatory cell infiltration, scaring, vacuolation, and no obvious myelin structure. Also, in agreement with locomotor recovery data, when anti-fB mAb was administered 12 and 24 hours after SCI (or 24 and 36 hours, not shown), there was no significant improvement in histological evidence of recovery when compared to wt and PBS treated controls.
Figure 8.
Histopathology of spinal cord sections 21 days after injury. A: Images of transverse sections from spinal cords at the epicenter of injury. Sections stained with either H&E or LFB. Representative images, n = 6 per group. B: Quantitative assessment of histopathological inflammation, injury, and demyelination. H&E-stained sections were scored on a 0–3 scale for the presence and intensity of inflammatory cell infiltration, neuronal vacuolation, and hemorrhage. LFB-stained sections scored for demyelination (0 = no evidence, 3 = severe). Anti-fB mAb treatment groups received injections at 1 and 12, or 12 and 24 hours after injury. Vehicle control group (PBS) received injections at 1 and 12 hours after injury. Scores were then expressed as a cumulative score of 0–12. Mean ± SD, n = 6 per group. *P < 0.001 and **P < 0.0001 vs. vehicle control and wt.
Discussion
Post-traumatic inflammation and secondary tissue damage after SCI is characterized by, among other things, complement activation, expression of inflammatory cytokines and chemokines, the up-regulation of adhesion molecules, and the infiltration of neutrophils and macrophages. Complement activation products play known roles in promoting the expression of these inflammatory molecules and the recruitment and activation of leukocytes. A key role for complement in SCI has been shown by the demonstration that deficiency of C3 or inhibition of C3 activation, the point of convergence for all three complement activation pathways, protects against inflammation and injury.7,8,10 The classical, alternative, and terminal complement pathways are activated in rodent SCI, as demonstrated by the deposition of C1q and C4 (classical), fB (alternative) and C9 (terminal) on neurons, oligodendrocytes, and injured axons.5 However, C1q deficiency improves histological and functional locomotor outcome after SCI, indicating that the classical pathway play a key role in tissue damage.11 There is strong evidence that activation of the classical pathway is mediated by antibodies that are produced after SCI. After contusion injury, there is an accumulation of intraspinal antibody-secreting B cells, and antibody and C1q colocalize at the site of injury and demyelination, while B cell deficiency confers neuroprotection and there is only minimal C1q deposition in the spinal cords of B cell–deficient mice. Further linking antibody-mediated complement activation to SCI pathology, it has been shown that the injection of isolated SCI antibodies into intact spinal cords causes complete transient paralysis in wt mice, while C3 deficient mice exhibit significantly reduced hind limb deficits and pathology.13,38
In the present study, we show that fB-deficient mice have an improved outcome after SCI. The level of protection afforded to fB-deficient mice was similar to that seen in our previous study using C3-deficient mice.7 These data indicate a dependence on the alternative pathway for complement-mediated SCI. Together with the previous findings described above using B cell and C1q-deficient mice, it is likely that the alternative pathway plays a critical role in amplifying antibody-mediated classical pathway initiated complement activation. In this context, reduced pathology and improved locomotor recovery in fB-deficient mice was associated with significantly reduced C3 and MAC deposition compared to wt control mice, even though the classical (and lectin) pathway is intact in fB-deficient mice. A similar dependence on both antibody-mediated lectin pathway activation and alternative pathway activation has been described in models of intestine ischemia and reperfusion injury.39,40,41
In a more clinically relevant paradigm, we demonstrated that specific inhibition of the alternative pathway with anti-fB mAb was also protective against SCI, and to a similar level as fB deficiency. Selective inhibition of the alternative pathway while leaving the classical and lectin pathways intact has potential benefits over inhibiting all pathways at the C3 activation step. Complement is an important component of host defense, and the classical and lectin pathways provide important contributions to defense against some pathogens. Thus, compared with C3 inhibition, alternative pathway inhibition would be less generally immunosuppressive and may have less potential for increasing the incidence of sepsis or infection. Complement is also implicated in neuroprotective and regenerative mechanisms. Activated microglia and macrophages have been shown to be important for neuron protection and axon growth in animal models,42 and complement activation products, in particular C5a, can recruit and activate these cells. A recent study demonstrated that the treatment of rats 14 days post-SCI with a C5a receptor antagonist reduced locomotor recovery and myelination.43 Complement also participates in phagocytic clearance of apoptotic cells and cellular debris via their opsonization with C1q and C3 activation products, and interfering with this clearance pathway can impair axon growth (for reviews, see Refs. 42, 44). Thus, compared with total complement blockade, allowing activation of the classical (or lectin) pathway while inhibiting alternative pathway amplification could feasibly improve the balance between the neurodegenerative and neuroprotective effects of complement inhibition.
The exacerbated injury and impaired recovery of locomotor function in CD59a-deficient mice, together with increased deposits of the MAC (C9) in these mice compared to wt control treated mice, indicate an important contribution of the terminal pathway and the MAC to secondary neuronal injury after trauma. Furthermore, significant functional deficit persisted in CD59-deficient mice at 21 days after injury compared with controls. It is possible that neoepitope expression and cell lysis resulting from enhanced MAC formation in CD59-deficient mice could result in enhanced complement activation. However, while there were significantly increased deposits of C9 in CD59-deficient mice at both 24 and 72 hours post-SCI compared with wt treated control mice, CD59 deficiency had little effect on C3 or fB deposition. Previous studies using CD59a-deficient mice have similarly implicated the MAC in spinal cord demyelination and axonal injury in murine acute experimental allergic encephalomyelitis,45 as well as in axonal damage during Wallerian degeneration (peripheral nerve injury).46
The alternative and terminal complement pathways have also been implicated in causing neuropathology in a different model of traumatic neuroinflammation, traumatic brain injury. In these previous studies, CD59a deficiency impaired neurological outcome and increased neuronal cell death and brain tissue destruction compared to wt mice,47 whereas fB deficiency was protective.48 The anti-fB mAb used in the current study has also previously been shown to be protective in a model of traumatic brain injury.36 Administration of anti-fB mAb at 1 and 24 hours after traumatic brain injury resulted in significantly reduced neuronal cell death and tissue injury compared to controls for up to 7 days after injury (most distal time point analyzed). Of note, it was shown in this previous study that anti-fB mAb (1379) significantly reduced serum alternative pathway activity 24 hours after injury, but that alternative pathway activity returned to normal by 7 days, even with repeated mAb injection. In agreement with this previous data, we also found that anti-fB mAb significantly reduced serum alternative pathway activity 24 hours after administration (by 70%, see supplemental Figure 2 at http://ajp.amjpathol.org). Together with our data showing that anti-fB mAb administered 12 or more hours after SCI is not protective, these data together indicate that short term inhibition of the alternative pathway early after SCI is key to reducing injury and improving functional recovery and that continued complement inhibition is unlikely to have any further beneficial effect.
Neutrophils and macrophages are recruited to the spinal cord after SCI, and their infiltration and activation is strongly linked with neuronal and glial cell death.42 We previously demonstrated that C3 deficiency reduced neutrophil numbers in the spinal cord at 24 and 72 hours postinjury.7 C1q deficiency, however, did not effect neutrophil infiltration after SCI (at either 24 or 72 hours).11 In the current study, we show that compared with control-treated mice, there are significantly reduced neutrophil numbers in fB-deficient and fB-inhibited mice at both time points post-SCI. We similarly showed reduced macrophage/microglia numbers in fB-deficient or fB-inhibited mice compared with controls at 72 hours postinjury, whereas it was previously shown that C1q deficiency was (interestingly) associated with increased macrophage infiltration.11 Together, these data indicate a key role for the alternative pathway in neutrophil and macrophage infiltration after SCI. It is interesting that unlike fB deficiency, CD59 deficiency did not significantly effect the number of macrophages at 72 hours postinjury. Neutrophils, on the other hand, were present in significantly higher numbers in CD59-deficient mice compared to wt treated controls at 72 hours after injury. The MAC can influence cellular infiltration when deposited in sublethal quantities in cell membranes by inducing the expression of adhesion molecules, cytokines, and chemokines, including IL-8.49,50,51,52,53,54 Why there is an apparent difference in CD59-deficient mice on neutrophil and macrophage infiltration at 72 hours postinjury (compared to controls) is not clear, but a consequence could be the protraction of neutrophil mediated inflammatory events and a reduction in macrophage-mediated protective events.
In addition to showing that the complement inhibitor CD59 plays an important role in providing protection against SCI, the data have potential clinical implications. As discussed above, for reasons relating to the protective roles of complement that are relevant for SCI patients, there are potential benefits of selectively inhibiting the alternative pathway of complement to reduce secondary damage and improve functional recovery after SCI. However, the MAC plays a very limited known role in host defense and immune homeostatic/repair mechanisms, and because our data indicate that the MAC is a significant contributor to pathology in SCI, selective inhibition of the terminal pathway could potentially have even greater benefit over blocking complement at an early step in any pathway.
Footnotes
Address reprint requests to Dr. Stephen Tomlinson, Department of Microbiology and Immunology, Children’s Research Institute, Medical University of South Carolina, Charleston, SC 29425; or Dr. Hongbin Song, Institute of Disease Control and Prevention, Academy of Military Medical Science, Beijing 100071, China. E-mail: tomlinss@musc.edu and Hongbinsong@263.net.
See related Commentary on 2685
Supported by a grant from Christopher and Dana Reeve Foundation (S.T.), the Veterans Administration and National Institutes of Health (National Institute of Neurological Diseases and Stroke, NS050452 to M.S.K.), Science and Technology Research of China (2008ZXJ09004-050), 863 Project (2007AA02Z144), and National Natural Science Foundation of China (30801004 to H.S.).
CME Disclosure: None of the authors disclosed any relevant financial relationships.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Fiore C, Inman DM, Hirose S, Noble LJ, Igarashi T, Compagnone NA. Treatment with the neurosteroid dehydroepiandrosterone promotes recovery of motor behavior after moderate contusive spinal cord injury in the mouse. J Neurosci Res. 2004;75:391–400. doi: 10.1002/jnr.10821. [DOI] [PubMed] [Google Scholar]
- Tsai EC, Tator CH. Neuroprotection and regeneration strategies for spinal cord repair. Curr Pharm Des. 2005;11:1211–1222. doi: 10.2174/1381612053507404. [DOI] [PubMed] [Google Scholar]
- Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebhun J, Madorsky JG, Glovsky MM. Proteins of the complement system and acute phase reactants in sera of patients with spinal cord injury. Ann Allergy. 1991;66:335–338. [PubMed] [Google Scholar]
- Anderson AJ, Robert S, Huang W, Young W, Cotman CW. Activation of complement pathways after contusion-induced spinal cord injury. J Neurotrauma. 2004;21:1831–1846. doi: 10.1089/neu.2004.21.1831. [DOI] [PubMed] [Google Scholar]
- Nguyen HX, Galvan MD, Anderson AJ. Characterization of early and terminal complement proteins associated with polymorphonuclear leukocytes in vitro and in vivo after spinal cord injury. J Neuroinflammation. 2008;5:26. doi: 10.1186/1742-2094-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao F, Atkinson C, Song H, Pannu R, Singh I, Tomlinson S. Complement plays an important role in spinal cord injury and represents a therapeutic target for improving recovery following trauma. Am J Pathol. 2006;169:1039–1047. doi: 10.2353/ajpath.2006.060248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DN, Smith SA, Zhang YP, Mengsheng Q, Lahiri DK, Morassutti DJ, Shields CB, Kotwal GJ. Vaccinia virus complement control protein reduces inflammation and improves spinal cord integrity following spinal cord injury. Ann NY Acad Sci. 2004;1035:165–178. doi: 10.1196/annals.1332.011. [DOI] [PubMed] [Google Scholar]
- Tei R, Kaido T, Nakase H, Sakaki T. Protective effect of C1 esterase inhibitor on acute traumatic spinal cord injury in the rat. Neurol Res. 2008;30:761–767. doi: 10.1179/174313208X284241. [DOI] [PubMed] [Google Scholar]
- Li LM, Li JB, Zhu Y, Fan GY. Soluble complement receptor type 1 inhibits complement system activation and improves motor function in acute spinal cord injury. Spinal Cord. 2010;48:105–111. doi: 10.1038/sc.2009.104. [DOI] [PubMed] [Google Scholar]
- Galvan MD, Luchetti S, Burgos AM, Nguyen HX, Hooshmand MJ, Hamers FP, Anderson AJ. Deficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury. J Neurosci. 2008;28:13876–13888. doi: 10.1523/JNEUROSCI.2823-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- Ankeny DP, Guan Z, Popovich PG. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A.R. A. Surgery of experimental lesions of spinal cord equivalent to crush injury of fracture dislocation of spinal column: a preliminary report. J Am Med Assoc. 1911;57:878–880. [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. discussion 126–128. [DOI] [PubMed] [Google Scholar]
- Rivlin AS, Tator CH. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol. 1978;10:38–43. [PubMed] [Google Scholar]
- Blight AR. Morphometric analysis of a model of spinal cord injury in guinea pigs, with behavioral evidence of delayed secondary pathology. J Neurol Sci. 1991;103:156–171. doi: 10.1016/0022-510x(91)90159-5. [DOI] [PubMed] [Google Scholar]
- Noyes DH. Electromechanical impactor for producing experimental spinal cord injury in animals. Med Biol Eng Comput. 1987;25:335–340. doi: 10.1007/BF02447434. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Schrimsher GW, Reier PJ. Forelimb motor performance following dorsal column, dorsolateral funiculi, or ventrolateral funiculi lesions of the cervical spinal cord in the rat. Exp Neurol. 1993;120:264–276. doi: 10.1006/exnr.1993.1060. [DOI] [PubMed] [Google Scholar]
- Watson BD, Prado R, Dietrich WD, Ginsberg MD, Green BA. Photochemically induced spinal cord injury in the rat. Brain Res. 1986;367:296–300. doi: 10.1016/0006-8993(86)91606-9. [DOI] [PubMed] [Google Scholar]
- Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol. 2003;170:1517–1523. doi: 10.4049/jimmunol.170.3.1517. [DOI] [PubMed] [Google Scholar]
- Baalasubramanian S, Harris CL, Donev RM, Mizuno M, Omidvar N, Song WC, Morgan BP. CD59a is the primary regulator of membrane attack complex assembly in the mouse. J Immunol. 2004;173:3684–3692. doi: 10.4049/jimmunol.173.6.3684. [DOI] [PubMed] [Google Scholar]
- Holt DS, Botto M, Bygrave AE, Hanna SM, Walport MJ, Morgan BP. Targeted deletion of the CD59 gene causes spontaneous intravascular hemolysis and hemoglobinuria. Blood. 2001;98:442–449. doi: 10.1182/blood.v98.2.442. [DOI] [PubMed] [Google Scholar]
- Thurman JM, Kraus DM, Girardi G, Hourcade D, Kang HJ, Royer PA, Mitchell LM, Giclas PC, Salmon J, Gilkeson G, Holers VM. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol. 2005;42:87–97. doi: 10.1016/j.molimm.2004.07.043. [DOI] [PubMed] [Google Scholar]
- Taube C, Thurman JM, Takeda K, Joetham A, Miyahara N, Carroll MC, Dakhama A, Giclas PC, Holers VM, Gelfand EW. Factor B of the alternative complement pathway regulates development of airway hyperresponsiveness and inflammation. Proc Natl Acad Sci USA. 2006;103:8084–8089. doi: 10.1073/pnas.0602357103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier AH, Falk LA. Isolation of murine macrophages. Curr Protoc Immunol. 2001;Chapter 14:Unit 14:11. doi: 10.1002/0471142735.im1401s11. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Clark G. Staining Procedures. Baltimore, MD: Williams and Wilkins,; 1981:pp 111–129. [Google Scholar]
- Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol. 2003;184:456–463. doi: 10.1016/s0014-4886(03)00257-7. [DOI] [PubMed] [Google Scholar]
- Atkinson C, Song H, Lu B, Qiao F, Burns TA, Holers VM, Tsokos GC, Tomlinson S. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J Clin Invest. 2005;115:2444–2453. doi: 10.1172/JCI25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T, Esposito E, Mazzon E, Di Paola R, Caminiti R, Bramanti P, Cappelani A, Cuzzocrea S. Absence of endogenous interleukin-10 enhances secondary inflammatory process after spinal cord compression injury in mice. J Neurochem. 2009;108:1360–1372. doi: 10.1111/j.1471-4159.2009.05899.x. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Di Paola R, Cannavo G, Muia C, Bramanti P, Cuzzocrea S. Role of endogenous ligands for the peroxisome proliferators activated receptors alpha in the secondary damage in experimental spinal cord trauma. Exp Neurol. 2005;194:267–278. doi: 10.1016/j.expneurol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Matsuo S, Nishikage H, Yoshida F, Nomura A, Piddlesden SJ, Morgan BP. Role of CD59 in experimental glomerulonephritis in rats. Kidney Int. 1994;46:191–200. doi: 10.1038/ki.1994.259. [DOI] [PubMed] [Google Scholar]
- Harris CL, Hanna SM, Mizuno M, Holt DS, Marchbank KJ, Morgan BP. Characterization of the mouse analogues of CD59 using novel monoclonal antibodies: tissue distribution and functional comparison. Immunology. 2003;109:117–126. doi: 10.1046/j.1365-2567.2003.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinhase I, Rozanski M, Harhausen D, Thurman JM, Schmidt OI, Hossini AM, Taha ME, Rittirsch D, Ward PA, Holers VM, Ertel W, Stahel PF. Inhibition of the alternative complement activation pathway in traumatic brain injury by a monoclonal anti-factor B antibody: a randomized placebo-controlled study in mice. J Neuroinflammation. 2007;4:13. doi: 10.1186/1742-2094-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15:415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- Stahl GL, Xu Y, Hao L, Miller M, Buras JA, Fung M, Zhao H. Role for the alternative complement pathway in ischemia/reperfusion injury. Am J Pathol. 2003;162:449–455. doi: 10.1016/S0002-9440(10)63839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, Jensenius JC, Ezekowitz RA, Moore FD, Carroll MC. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol. 2006;177:4727–4734. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- Huang Y, Qiao F, Atkinson C, Holers VM, Tomlinson S. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J Immunol. 2008;181:8068–8076. doi: 10.4049/jimmunol.181.11.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JK, Popovich PG. Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JJ, Anderson AJ, Barnum SR, Stevens B, Tenner AJ. The complement cascade: yin-Yang in neuroinflammation–neuro-protection and -degeneration. J Neurochem. 2008;107:1169–1187. doi: 10.1111/j.1471-4159.2008.05668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead RJ, Neal JW, Griffiths MR, Linington C, Botto M, Lassmann H, Morgan BP. Deficiency of the complement regulator CD59a enhances disease severity, demyelination and axonal injury in murine acute experimental allergic encephalomyelitis. Lab Invest. 2004;84:21–28. doi: 10.1038/labinvest.3700015. [DOI] [PubMed] [Google Scholar]
- Ramaglia V, King RH, Morgan BP, Baas F. Deficiency of the complement regulator CD59a exacerbates Wallerian degeneration. Mol Immunol. 2009;46:1892–1896. doi: 10.1016/j.molimm.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Stahel PF, Flierl MA, Morgan BP, Persigehl I, Stoll C, Conrad C, Touban BM, Smith WR, Beauchamp K, Schmidt OI, Ertel W, Leinhase I. Absence of the complement regulatory molecule CD59a leads to exacerbated neuropathology after traumatic brain injury in mice. J Neuroinflammation. 2009;6:2. doi: 10.1186/1742-2094-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinhase I, Holers VM, Thurman JM, Harhausen D, Schmidt OI, Pietzcker M, Taha ME, Rittirsch D, Huber-Lang M, Smith WR, Ward PA, Stahel PF. Reduced neuronal cell death after experimental brain injury in mice lacking a functional alternative pathway of complement activation. BMC Neurosci. 2006;7:55. doi: 10.1186/1471-2202-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman KE, Vaporciyan AA, Bonish BK, Jones ML, Johnson KJ, Glovsky MM, Eddy SM, Ward PA. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94:1147–1155. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonermark M, Deppisch R, Riedasch G, Rother K, Hansch GM. Induction of mediator release from human glomerular mesangial cells by the terminal complement components C5b-9. Int Arch Allergy Appl Immunol. 1991;96:331–337. doi: 10.1159/000235517. [DOI] [PubMed] [Google Scholar]
- Kilgore KS, Shen JP, Miller BF, Ward PA, Warren JS. Enhancement by the complement membrane attack complex of tumor necrosis factor alpha-induced endothelial cell expression of E-selectin and ICAM-1. J Immunol. 1995;155:1434–1441. [PubMed] [Google Scholar]
- Kilgore KS, Flory CM, Miller BF, Evans VM, Warren JS. The membrane attack complex of complement induces interleukin-8 and monocyte chemoattractant protein-1 secretion from human umbilical vein endothelial cells. Am J Pathol. 1996;149:953–961. [PMC free article] [PubMed] [Google Scholar]
- Saadi S, Holzknecht RA, Patte CP, Platt JL. Endothelial cell activation by pore-forming structures: pivotal role for interleukin-1alpha. Circulation. 2000;101:1867–1873. doi: 10.1161/01.cir.101.15.1867. [DOI] [PubMed] [Google Scholar]
- Dobrina A, Pausa M, Fischetti F, Bulla R, Vecile E, Ferrero E, Mantovani A, Tedesco F. Cytolytically inactive terminal complement complex causes transendothelial migration of polymorphonuclear leukocytes in vitro and in vivo. Blood. 2002;99:185–192. doi: 10.1182/blood.v99.1.185. [DOI] [PubMed] [Google Scholar]