Abstract
Understanding the complex interactions between Leishmania and dendritic cells (DCs) is central to the modulation of the outcome of this infection, given that an effective immune response against Leishmania is dependent on the successful activation and maturation of DCs. We report here that Leishmania infantum promastigotes successfully infect mouse bone marrow-derived DCs without triggering maturation, as shown by a failure in the up-regulation of CD40 and CD86 expression, and that parasites strongly counteract the lipopolysaccharide-triggered maturation of DCs. A small increase in interleukin (IL)-12 and IL-10 transcription and secretion and a decrease in IL-6 were observed in infected cells. This arrested DC maturation state is actively promoted by parasites because heat-killed or fixed parasites increased cytokine and costimulatory molecule expression. At a molecular level, L. infantum rapidly induced activation of phosphatidylinositol 3-kinase/Akt and extracellular signal-regulated kinase 1/2, whereas no effect was observed in the c-Jun N-terminal kinase and p38 mitogen-activated protein kinase proinflammatory pathways. Moreover, parasites actively promoted cleavage of the nuclear factor-κB p65RelA subunit, causing its impairment. The blockade of phosphatidylinositol 3-kinase/Akt by either treatment of bone marrow-derived DCs with wortmannin or transfection with an Akt dominant-negative mutant resulted in a strong decrease in infection rates, revealing for the first time a crucial role of this pathway on Leishmania engulfment by DCs. Overall, our data indicate that activation of Akt and impairment of nuclear factor-κB are responsible for immunogenicity subversion of L. infantum-infected DCs.
Visceral leishmaniasis is a vector-borne parasite infection caused by species of the genus Leishmania (Leishmania donovani and Leishmania infantum/Leishmania chagasi) that disseminate hematogenously, infecting mononuclear phagocytic cells in the spleen, liver, lymph nodes, and bone marrow.1 A protective response against all forms of leishmaniasis is dependent on interleukin (IL)-12 production by antigen-presenting cells, which leads to the differentiation and proliferation of CD4+ Th1 cells with interferon-γ and tumor necrosis factor (TNF)-α production.2,3,4 Although Leishmania-infected neutrophils are believed to constitute one of the earliest sources of IL-12 in resistant C57BL/6 mice,5 it is currently believed that dendritic cells (DCs) are the critical source of early IL-12 production after Leishmania infection.6,7
DCs are specialized antigen-presenting cells that play a crucial role in driving adaptive immune responses.8 Depending on their maturation/activation state, DCs have the ability to polarize distinct T-cell subsets (T-helper cells [Th1, Th2, and Th17], regulatory T cells, and cytotoxic T cells), controlling the outcome of an infection. Recently, research focused on the role played by DCs during leishmaniasis and DC-based vaccination for the control of this infection has gained special attention. Although there is consensus that DCs play a critical role in the progression or resolution of leishmaniasis (reviewed in 9, 10), the data obtained in these studies have often generated conflicting results. Early in vitro experiments demonstrated that on phagocytosis of Leishmania major promastigote or amastigote, DCs became activated as determined by increased expression levels of costimulatory surface markers, IL-12p40 secretion, and their potential for priming primary CD4+-T lymphocytes.11 Nevertheless, studies performed with New World cutaneous and mucocutaneous Leishmania species are more controversial, because although Leishmania braziliensis uptake was shown to ultimately induce DC maturation,12,13 infection with Leishmania amazonensis or Leishmania mexicana parasites dramatically impaired the differentiation and function of DCs, irrespective of the parasite form used.14,15,16,17 Furthermore, the role played by DCs during visceral leishmaniasis is still poorly understood and needs further elucidation. A few studies have demonstrated that DC infection with visceral Leishmania species triggers, within minutes, the release of preformed membrane-associated IL-12p70.18 Nevertheless, the production of IL-12p40 was shown to be weak and transient6 and occurs in the absence of DC maturation.19,20,21
To date, the early molecular mechanisms by which Leishmania parasites control the DC activation/maturation state and thus their immunostimulatory abilities remain unclear. The DC maturation process is a well coordinated series of events tightly controlled by the balance of particular intracellular signaling pathways. Among these pathways, the nuclear factor-κB (NF-κB) signaling system is considered the master regulator of innate immunity and inflammatory responses and phosphoinositide 3-kinase (PI3K) has been regarded as an internal safety mechanism to control extensive inflammation, by limiting IL-12 production and enhancing the synthesis of the anti-inflammatory IL-10. Likewise the three primary kinases members of the mitogen-activated protein kinase (MAPK) family, p38, c-Jun N-terminal kinase (JNK), and extracellular signal regulated kinase (ERK), have been implicated in the regulation of several aspects of phenotypic and functional maturation of DCs, as well as cytokine production.22
Therefore, in the present study, we examined the ability of mouse bone marrow-derived DCs to phagocyte L. infantum promastigotes and assessed the effects of infection on the three major MAPKs pathways and the PI3K/Akt and the NF-κB signaling cascades. The impact of early infection on the DC phenotype and cytokine release profile was also analyzed. In addition, we investigated the ability of the parasite to subvert the lipopolysaccharide (LPS)-triggered DC maturation/activation process. Finally, we used specific inhibitors to understand the relevance of each pathway in the L. infantum promastigote-induced events in DCs. Our results clearly demonstrate that the uptake of visceral L. infantum promastigotes actively arrests the activation/maturation of bone-marrow DCs, thus promoting a silent infection through the biased modulation of PI3K/Akt and NF-κB pathways.
Materials and Methods
Materials
LPS from Escherichia coli (serotype 026:B6) was obtained from Sigma Chemical Co. (St. Louis, MO). SB203580 and SP600125 were from Calbiochem (San Diego, CA), PD098059 was obtained from RBI (Natick, MA) and wortmannin was from Sigma Chemical Co.
Animals and Parasites
Ten- to 12-week-old female BALB/c mice were obtained from Instituto de Biologia Molecular e Celular (Porto, Portugal) animal facilities. Animal care was in accordance with institutional guidelines. A cloned line of virulent L. infantum (MHOM/MA/67/ITMAP-263) was maintained by weekly subpassages (less than 10) in complete RPMI 1640 (RPMIc) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mmol/L HEPES (BioWhittaker, Walkersville, MD). In some experiments, the cloned line of virulent L. infantum promastigotes expressing monomeric green fluorescent protein (GFP) was used. L. infantum recombinant parasites overexpressing GFP genes and the neomycin phosphotransferase gene (neo) as a dominant positive selection marker conferring resistance to G418, were generated by transfection of the expression vector pSPαNEOαGFP. Expression of the GFP and neo genes in vector pSPαNEOαGFP is driven by the α-tubulin intergenic region of Leishmania enriettii.23 Transfection experiments were done as described elsewhere,24 and transfectants were selected and grown in the presence of G418 (100 μg/ml).
Bone Marrow-Derived Dendritic Cells
Bone marrow-dendritic cells (BMDCs) were derived as described previously.25 In brief, bone marrow from femurs and tibiae of 10- to 12-week-old BALB/c mice were flushed with RPMI 1640, using syringes and 25-gauge needles. The tissue was resuspended, and BMDCs were obtained by seeding 5 × 106 bone marrow cells in 25 ml of RPMIc supplemented with 50 μmol/L 2-mercaptoethanol (Sigma Chemical Co.) and 200 U/ml of granulocyte macrophage–colony-stimulating factor (GM-CSF) (PeproTech, Rocky Hill, NJ) (DC medium). Cells were cultured at 37°C and 5% CO2 for 3 days, after which the same amount of DC medium was added to each flask. At days 6 and 8, half of the culture supernatant was collected and centrifuged, and the cell pellet was resuspended in the same amount of fresh DC medium and put back into the original flasks. At day 10, the same procedure was performed but with use of only 100 U/ml of GM-CSF. BMDCs obtained after 12 days of culture displayed a phenotype highly enriched in CD11c+ cells (∼95%).
In Vitro Stimulation and Infection of Dendritic Cells
For in vitro infection, 12-day nonadherent BMDCs were seeded at 1 × 106 cells/ml of RPMIc containing 3 U/ml of GM-CSF in 24-well plates (for flow cytometry and enzyme-linked immunosorbent assay [ELISA] assays), at 2 × 105/200 μl in chamber slides (for infection quantification) or at 2 × 106cells/ml in 6-well plates (for Western blots and quantitative PCR assays). After an overnight incubation period, stationary-phase L. infantum promastigotes were added to the culture at an infection ratio of 10:1 (parasites/cell). Parallel experiments were also performed using polystyrene beads (FACS AccuDrop beads, 6 μm in diameter) and heat-killed (30 minutes at 56°C) or fixed parasites (10 minutes in glutaraldehyde). For Western blot assays, the cells were exposed to parasites for 10, 30, or 60 minutes and then immediately lysed in radioimmunoprecipitation buffer. For further experiments noninternalized parasites were removed by gently washing after 4 hours of infection, and fresh RPMIc supplemented with 3 U/ml of GM-CSF was added to the wells. Cells were immediately recovered or maintained for 24 hours (for flow cytometry and ELISA assays). When specific inhibitors of the different signaling pathways were used (500 nmol/L wortmannin, 20 μmol/L SB203580, 20 μmol/L PD098059, 2 μmol/L SP600125, or 10 μmol/L BAY 11-7082), cells were pretreated with the respective drugs for 1 hour and then submitted to infection as described above. BMDCs stimulated with LPS (1 μg/ml) were used as a positive control for DC activation/maturation. When indicated as LPS + infection, the cells were simultaneously exposed to LPS and parasites for 30 minutes (for Western blot assays) or pretreated with LPS for 1 hour and then infected (LPS → infection) for the indicated times (for flow cytometry and ELISA assays).
Determination of Percentage of Infected Cells
BMDCs cultured in chamber slides (Lab-Tek Chamber Slides, Nunc GmbH&Co. KG, Wiesbaden, Germany) were infected for a 4-hour period at a 10:1 (parasites/cell) ratio. After three washing steps, cells were immediately prepared or maintained in culture for additional 12, 24, 48, or 72 hours. Afterward, BMDCs were washed with PBS, fixed with 2% paraformaldehyde for 30 minutes and finally permeabilized with 0.1% (v/v) Triton X-100 in PBS for 10 minutes at 4°C. Cells were then mounted in Vectashield with the nuclear label 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA) and analyzed with a fluorescent microscope (Axioskop-Carl Zeiss, Jena, Germany) at ×1000 magnification. Images were captured with a digital camera (Spot 2, Diagnostic Instruments, Sterling Heights, MI) and the software Spot 3.1 (Diagnostic Instruments). In some experiments, the percentage of infected BMDCs was determined by flow cytometry evaluation of GFP-positive cells on infection with GFP-recombinant parasites. In brief, BMDCs were infected for a 4-hour period at a 10:1 (parasites/cell) ratio and after washing steps were analyzed in a FACScan cytometer equipped with FlowJo software (TreeStar Inc., Ashland, OR) to evaluate BMDCs presenting a GFP-positive signal.
Cell Lysate Preparation
Cells were washed in ice-cold PBS and harvested in radioimmunoprecipitation lysis buffer (50 mmol/L Tris-HCl, pH 8.0, 1% Nonidet P-40, 150 mmol/L NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 2 mmol/L EDTA, and 1 mmol/L dithiothreitol) freshly supplemented with protease and phosphatase inhibitor cocktails (Roche, Mannheim, Germany). The nuclei and the insoluble cell debris were removed by centrifugation at 12,000 × g for 10 minutes at 4°C. The extracts were collected and used as total cell lysates. Nuclear and cytosolic fractions were prepared using the Nuclear Extract Kit (Active Motif Inc., Carlsbad, CA) according to the manufacturer’s instructions. The protein concentration was determined using the bicinchoninic acid method, and the cell lysates were denatured at 95°C for 5 minutes in sample buffer (0.125 mmol/L Tris, pH 6.8, 2% w/v SDS, 100 mmol/L dithiothreitol, 10% glycerol, and bromphenol blue) for further use in Western blot analysis.
Western Blot Analysis
Western blots were performed to evaluate the levels of phospho-IκB-α, IκB-α, p65RelA, phospho-ERK1/ERK2, phospho-p38 MAPK, phospho-stress activated protein kinase (SAPK)/JNK, and phospho-Akt. In brief, 50 μg of protein was electrophoretically separated on a 12% (v/v) SDS-polyacrylamide gel, and transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Bedford, MA). The membranes were blocked with 5% (w/v) fat-free dry milk in Tris-buffered saline containing 0.1% (v/v) Tween 20 (TBS-T) for 1 hour at room temperature. Blots were then incubated overnight at 4°C with the primary antibodies against the different proteins to be studied: phospho-IκB-α (1:1000), IκB-α (1:1000), p65RelA (1:1000), phospho-ERK1/ERK2 (1:1000), phospho-p38 MAPK (1:1000), phospho-JNK (1:1000), and phospho-Akt (1:500). The membranes were then washed for 25 minutes with TBS-T and incubated for 1 hour at room temperature with alkaline phosphatase-conjugated anti-rabbit or anti-mouse antibodies (1:20,000) (GE Healthcare, Chalfont St. Giles, UK). The immune complexes were detected by membrane exposure to the ECF reagent for 5 minutes, followed by scanning for blue excited fluorescence on the Storm 860 imager (GE Healthcare). The generated signals were analyzed using ImageQuant TL software. To test whether similar amounts of protein for each sample were loaded, the membranes were stripped and reprobed with antibodies to total ERK1/2, SAPK/JNK, p38 MAPK, and Akt or with an anti-actin antibody, and blots were developed with alkaline phosphatase-conjugated secondary antibodies and visualized by enhanced chemifluorescence. Antibodies against phospho-IκB-α, IκB-α, p65RelA, phospho-p44/p42 MAPK (ERK1/ERK2), phospho-p38 MAPK, phospho-SAPK/JNK, and phospho-Akt (Ser473) were from Cell Signaling Technology (Danvers, MA). The pan anti-JNK antibody was from R&D Systems (Minneapolis, MN), and pan anti-ERK, p38 and Akt were from Cell Signaling Technology. The anti-actin antibody was purchased from Millipore Corporation.
Flow Cytometry Determination
For the analysis of surface costimulatory markers, 2 × 105 BMDCs were incubated for 20 minutes with saturating concentrations of fluorescein isothiocyanate-conjugated monoclonal antibodies to either CD40 (clone 3/23), CD86 (clone GL1), or anti CD11c-PE (clone HL3). Mouse isotype controls were used when necessary. All of the antibodies were obtained from BioLegend (San Diego, CA), except for anti-CD11c-PE antibody, which was obtained from BD Pharmingen (San Diego, CA). After two washing steps with PBS/2% fetal bovine serum, the cells were analyzed by flow cytometry in a FACScan cytometer equipped with FlowJo software. Cells were selected on the basis of forward scatter/side scatter values; BMDCs were gated on CD11c+ and dead cells were excluded from all samples by propidium iodide labeling.
Determination of Cytokines by ELISA
The levels of IL-12p40, TNF-α, IL-6, and IL-10 were measured in the culture supernatants by ELISA. All cytokine quantification was done according to the manufacturer’s recommendations (BD Pharmingen for IL-10 and BioLegend for IL12p40, TNF-α, and IL-6). Samples were assayed in triplicate, and the data are expressed as the average and SD of each cytokine assayed.
RNA Extraction and Real-Time RT-PCR
Total RNA was isolated from cells with TRIzol reagent (Invitrogen, Barcelona, Spain), according to the manufacturer’s instructions. The RNA concentration was determined by OD260 measurement using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE), and quality was inspected for the absence of degradation or genomic DNA contamination, using Experion RNA StdSens Chips in the Experion automated microfluidic electrophoresis system (Bio-Rad, Hercules, CA). RNA was stored in RNA Storage Solution (Ambion, Foster City, CA) at −80°C until use. Real-time RT-PCR reactions were run in duplicate for each sample on a Bio-Rad MyCycler iQ5. Primer sequences (Table 1) were designed using Beacon Designer software (version 7.2, PREMIER Biosoft International, Palo Alto, CA) and thoroughly tested. In brief, 1 μg of total RNA was reverse-transcribed using the iScript Select cDNA Synthesis Kit (Bio-Rad). Real-time PCR was performed as described previously.26 After amplification, a threshold was set for each gene and Ct values were calculated for all samples. Gene expression changes were analyzed using the built-in iQ5 Optical system software (version 2). The results were normalized using a reference gene, HPRT-1, determined with Genex software (MultiD Analyses AB, Göteberg, Sweden) as the most stable for the treatment conditions used.
Table 1.
Primer Sequences for Targeted cDNAs
| Targeted cDNA | Primer sequence | Reference sequence identification |
|---|---|---|
| HPRT1 | ||
| Forward | 5′-GTTGAAGATATAATTGACACTG-3′ | |
| Reverse | 5′-GGCATATCCAACAACAAAC-3′ | NM_013556 |
| CD40 | ||
| Forward | 5′-GCCACTGAGACCACTGATAC-3′ | |
| Reverse | 5′-TCTGACTCGTTCCTTTCTGTAG-3′ | NM_011611 |
| CD86 | ||
| Forward | 5′-TATCTCCAACAGCCTCTC-3′ | |
| Reverse | 5′-TGTAATCTCCTTCCAATACG-3′ | NM_019388 |
| IL-12p40 | ||
| Forward | 5′-CTCAGGATGGAAGAGTCC-3′ | |
| Reverse | 5′-CAAGTGGAATGCTAGAATATC-3′ | NM_008352 |
| TNF-α | ||
| Forward | 5′-CAAGGGACTAGCCAGGAG-3′ | |
| Reverse | 5′-TGCCTCTTCTGCCAGTTC-3′ | NM_013693 |
| IL-6 | ||
| Forward | 5′-TTCCATCCAGTTGCCTTC-3′ | |
| Reverse | 5′-TTCTCATTTCCACGATTTCC-3′ | NM_031168 |
| IL-10 | ||
| Forward | 5′-CCCTTTGCTATGGTGTCCTTTC-3′ | |
| Reverse | 5′-ATCTCCCTGGTTTCTCTTCCC-3′ | NM_010548 |
Calculation of Real-Time RT-PCR Results
Because the real-time RT-PCR results are presented as ratios of treated samples over untreated (control) or LPS-treated cells, the data do not follow a normal distribution. A two-base logarithmic transformation was therefore used to make observations symmetric and closer to a normal distribution. If x represents the fold change of a gene in one sample, then the two-base logarithmic transformation is log2(x) = ln(x1)/ln(2). This way, fold changes of 2 and 0.5 correspond to mean log2 values of 1 and −1, respectively.
Plasmid Preparation
Plasmids coding for a dominant-negative form of Akt, HA-Akt DN (K179M)27 or for a constitutively active form, HA-Akt CA (myr-HA-AKT), were obtained from Addgene (Cambridge, MA, plasmid numbers 16243 and 16244, respectively). An endotoxin-free Plasmid Purification Kit (Qiagen, Hilden, Germany) was used according to the manufacturer’s instructions to purify HA-Akt DN and HA-Akt CA. The DNA concentration was determined by OD260 measurement using a NanoDrop spectrophotometer.
Cell Transfection
Nucleofection of BMDCs was performed in the Amaxa Nucleofector according to the manufacturer’s instructions. In brief, the BMDCs were collected and washed twice in PBS and subsequently 2 × 105 cells were resuspended in 100 μl of Nucleofector solution. Plasmid DNA coding for pmaxGFP (2 μg), HA-Akt DN (2 μg), or HA-Akt CA (2 μg) was added to 2 × 105 cells, and the samples were transferred into certified cuvettes and nucleofected by using program Y-001. Immediately after transfection, 400 μl of medium previously warmed to 37°C, was added to each cuvette. The DCs were collected, dispensed in the wells of Lab-Tek Chamber Slides, and incubated at 37°C and 5% CO2 for 48 hours. Cells were then infected, and the rates of infection were determined was described above. For image acquisition, the actin network of cells was stained with Alexa Fluor 555 phalloidin (Invitrogen). Images were acquired in a confocal laser scanning microscope (Zeiss LSM 510 Meta). The filter set used included an excitation filter of 560 nm and an emission filter of 575 nm. The settings for contrast, brightness, pinhole, acquisition mode, and scanning time were maintained throughout the work.
Statistical Analysis
The results are presented as means ± SD, and the statistical difference between two groups was determined by the two-sided unpaired Student’s t-test. For multiple group comparisons, the one-way analysis of variance test, with a Bonferroni multiple comparison posttest was used. The tests were performed using GraphPad Prism (version 5.02; GraphPad Software, San Diego, CA). Statistically significant values are as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Visceral L. infantum Promastigotes Successfully Infect Murine BMDCs
Leishmania manipulates host immune responses to protect itself from elimination. Here, in an attempt to unravel the molecular mechanisms involved in DCs manipulation by visceral Leishmania parasites, primary murine BMDCs from susceptible BALB/c mice were infected in vitro with L. infantum promastigotes. Because Leishmania exhibits a strong tropism for macrophage, it was of major importance to ensure the high purity of BMDCs population submitted to infection. Flow cytometry analyses revealed a small population expressing the macrophage marker F4/80, which was excluded from all subsequent analyses. In addition, we did not observe any contamination with T-cell (CD3) or B-cell (B220) populations (data not shown). After a differentiation period of 12 days in the presence of GM- CSF, BMDCs were phenotypically characterized as CD4−CD8−CD11b+CD11c+DEC205− DCs.
We first examined whether L. infantum promastigotes could infect and survive within murine BMDCs. Cells were incubated with L. infantum promastigotes at a parasite/cell ratio of 10:1 for 4 hours, after which the parasite internalization was determined. As shown in Figure 1A, after 4 hours of parasite-cell incubation, between 50 and 60% of immature BMDCs were successfully infected by L. infantum promastigotes with an average of 3 parasites/cell (3.2 ± 1.1). The infection rate was confirmed by flow cytometry evaluation of GFP-L. infantum promastigotes (Figure 1B). After removal of nonphagocytosed parasites, infected BMDCs were maintained in culture for 12, 24, 48, or 72 hours to evaluate parasite development. The number of cells presenting intracellular parasites dropped significantly from 4 to 12 hours (P = 0.044), and the mean number of parasites per infected cell also presented a slight yet not significant decrease (Figure 1A). The infection rate seemed to stabilize after 24 hours, with no significant differences between 24 and 48 hours or 24 and 72 hours (Figure 1A). After the slight decrease observed at 12 hours, the number of parasites per cell showed an increasing tendency in posterior time points, being significant at 48 and 72 hours (P = 0.012 and 0.015, respectively). These results clearly suggest that the first 12 hours led to the resolution of a large part of the infection, which nevertheless did not induce the complete elimination of the parasite. Indeed, after this critical period, the infection remained stable for at least 72 hours, and parasites subsisted within cells thus showing that infections are not abortive.
Figure 1.
Kinetic analyses of visceral L. infantum infection. A: Cells were infected with promastigotes at a 10:1 (parasites/cell) ratio for 4 hours. BMDCs were immediately recovered or left in culture for an additional 12, 24, 48, or 72 hours. A fluorescent nuclear label, 4′,6-diamidino-2-phenylindole, was added to the cells, and the number of infected cells as well as the number of parasite per cell was counted with a fluorescence microscope at ×1000. Results are representative of three independent experiments. B: BMDCs infected with GFP-L. infantum promastigotes at a 10:1 (parasites/cell) ratio were recovered after 4 hours of incubation or left in culture for an additional 24 hours after the end of the infection. The cells were analyzed by flow cytometry, and the percentage of GFP-positive BMDCs was quantified. Each value represents the mean ± SD from three independent experiments. NS, not significant; *P < 0.05.
L. infantum Promastigotes Silently Infect Murine BMDCs and Counteract LPS-Triggered BMDC Activation/Maturation Process
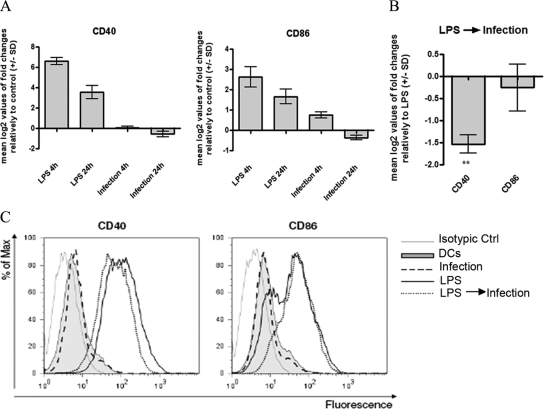
It was previously demonstrated that several Leishmania species suppress the up-regulation of costimulatory molecules in infected macrophages.28,29 However, the data generated using DCs as main infection targets for Leishmania are often incoherent and conflicting, depending on the type of DCs, species, and developmental stage of the parasite used. Hence, we investigated the activation status of murine BMDCs that were infected with L. infantum promastigotes by analyzing the transcription and surface expression of the DC maturation markers CD40 and CD86 by quantitative PCR (qPCR) and flow cytometry, respectively. As shown in Figure 2, A and C, and Table 2, L. infantum promastigotes failed to up-regulate the transcription and surface expression of CD40 and CD86 costimulatory molecules in BMDCs. Given that a significant variation in the percentage of infected BMDCs was observed during the time course of the experiment (Figure 1A), to avoid bias due to different parasite load, we evaluated the expression pattern of costimulatory molecules through the duration of the experiment. Nevertheless, the expression of the surface markers after infection was always similar to that of noninfected BMDCs (data not shown). To access the active involvement of parasites in the arrested maturation of infected BMDCs and to discard an unspecific phagocytic process, parallel experiments were performed with polystyrene beads and heat-killed or glutaraldehyde-fixed parasites. Surprisingly and in contrast to viable parasites, the exposure of BMDCs to killed L. infantum promastigotes or to the beads significantly increased the transcription (Supplemental Figure S1, see http://ajp.amjpathol.org) and expression of CD40 and CD86 (Table 2). These results show that the arresting of BMDCs maturation after engulfment of L. infantum promastigotes is a parasite-specific process that requires the active involvement of viable parasites.
Figure 2.
Effects of L. infantum infection on BMDC CD40 and CD86 costimulatory molecule expression. A: BMDCs plated at 2 × 106 cells/well were infected with L. infantum promastigotes in a 10:1 (parasites/cell) ratio for 4 hours. Cells were immediately recovered or left in culture for an additional 24 hours after the end of the infection. As a control, BDMCs were stimulated with 1 μg/ml LPS for 4 and 24 hours. The mRNA levels were assessed by qPCR for CD40 and CD86. Gene expression is indicated as mean log2 values of fold changes relative to untreated cells. B: BMDCs were treated with 1 μg/ml LPS or pretreated for 1 hour with LPS and then infected with L. infantum promastigotes in a 10:1 (parasites/cell) ratio, (LPS → Infection). After 4 hours of culture, cells were harvested, and the CD40 and CD86 mRNA levels were assessed by qPCR. Gene expression is indicated as mean log2 values of fold changes relative to LPS-treated cells. C: Cells were infected, treated with 1 μg/ml LPS or pretreated during 1 hour with LPS and then infected (LPS → Infection). After 24 hours, the surface expression of CD40 and CD86 was measured on gated CD11c+ cells by flow cytometry. As negative control, cells were incubated in RPMIc (DCs) for a similar time. Each value represents the mean ± SD from three independent experiments. **P < 0.01.
Table 2.
Effect of Viable and Killed L. infantum Promastigotes on BMDC CD40 and CD86 Surface Expression
| Cell Treatment | CD40 | CD86 |
|---|---|---|
| Cells | 14.73 ± 4.39 | 34.30 ± 2.63 |
| L. infantum | 12.32 ± 1.78 (NS) | 31.25 ± 2.44 (NS) |
| Heat-killed L. infantum | 28.64 ± 1.20** | 44.41 ± 8.68 (NS) |
| Fixed L. infantum | 37.57 ± 1.67*** | 89.46 ± 19.18 (NS) |
| Beads | 36.96 ± 5.02*** | 281.50 ± 74.54*** |
BMDCs were infected with L. infantun promastigotes at an infection ratio of 10:1 (parasites/cell). In parallel, cells were exposed to polystyrene beads and heat-killed or glutaraldehyde-fixed parasites in the same ratio as that used for infection. After 24 hours, the surface expression of CD40 and CD86 was measured on gated CD11c+ cells by flow cytometry. Each value represents the geometric mean ± SD from three independent experiments: NS, not significant;
P < 0.01;
P < 0.001.
We next tested the ability of parasites to manipulate the LPS-triggered maturation status of dendritic cells. Lipopolysaccharide is a TLR4 agonist that has long been used as a potent inducer of DC maturation.30 As expected, LPS stimulation induced the maturation of BMDCs, as judged by the increased transcription and expression of CD40 and CD86 (Figure 2, A and C). However, in LPS-stimulated BMDCs that were subsequently submitted to L. infantum infection, mRNA levels of CD40 were significantly decreased (Figure 2B). These data were corroborated by flow cytometry experiments that showed a decrease on the surface expression of this molecule (Figure 2C). Taken together, these observations confirm silent infection of murine BMDCs by visceral L. infantum promastigotes and show the ability of parasites to counteract the maturation/activation process triggered by other inflammatory stimuli.
L. infantum Promastigotes Induce Marginal Production of IL-12p40 and IL-10 and Manipulate the LPS-Induced Cytokine Profile in BMDCs
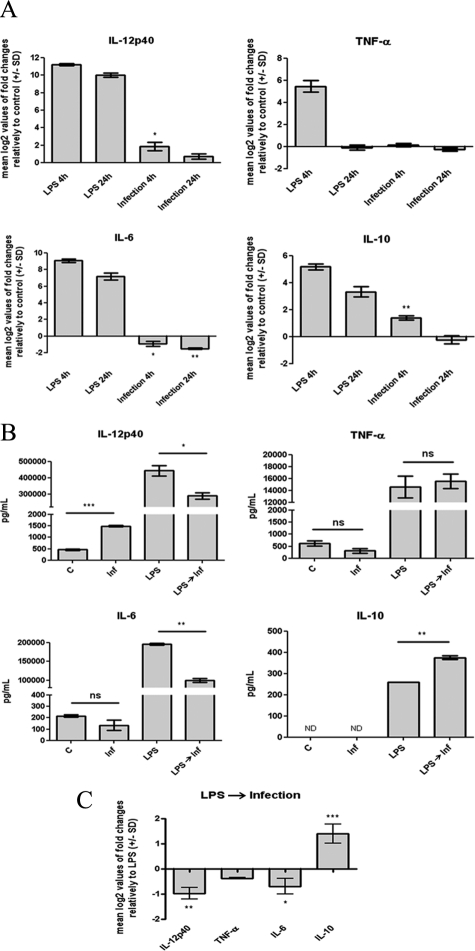
In response to intracellular pathogens, DCs secrete cytokines that will dictate the nature of the T-cell response. The quality of this immune response is influenced by the balance between the secretion of pro- and anti-inflammatory cytokines, in particular IL-12 and IL-10, respectively. Consequently, complementary experiments were performed to examine the effect of L. infantum promastigotes on BMDC cytokine production and on the ability of parasites to manipulate the LPS-induced cytokine profile. The transcription and secretion of proinflammatory (IL-6, IL12, and TNF-α) and anti-inflammatory (IL-10) cytokines were assessed by qPCR and ELISA, respectively. As shown in Figure 3A, the internalization of L. infantum promastigotes slightly induced transcription of IL-12p40 and IL-10, with mean log2 values of 1.9 ± 0.8 (P < 0.05) and 1.4 ± 0.3 (P < 0.01), respectively. Nevertheless, this effect was seen to be transient, because 24 hours after infection the mRNA levels of these cytokines in infected cells were not significantly different from those of noninfected BMDCs. In contrast, the transcription of the IL-6 gene was found to be significantly decreased in a time-sustained manner (Figure 3A). No significant differences were found for the TNF-α mRNA levels after infection. The consequences of these transcriptional changes were then evaluated at the protein level by ELISA assays performed in the culture supernatants obtained 24 hours postinfection. In accordance with qPCR results, L. infantum significantly increased the secretion of IL-12p40 (P < 0.001) and caused a slight 1.6-fold decrease in the IL-6 protein level (not statistically significant) (Figure 3B). Although there was an infection-induced increase in IL-10 gene transcription, no increase in IL-10 secretion was observed. This discrepancy might be explained by the low gene induction and by the lower sensitivity of the ELISA assay compared with the qPCR technique. This pattern of cytokine expression is specific and actively modulated by viable parasites, given that exposure to killed parasites or beads caused a different cytokine profile (Supplemental Figure S2, see http://ajp.amjpathol.org). Of particular interest is the difference in the amounts of IL-12 produced. Cells exposed to killed parasites showed a significantly higher transcription of IL-12 than cells exposed to viable promastigotes. This finding indicates that although some IL-12 was produced during DC infection, parasites actively limit the production of this cytokine, avoiding the triggering of a strong proinflammatory response.
Figure 3.
Effects of L. infantum infection on BMDC cytokine production. A: Cells plated at 2 × 106 cells/well were infected with L. infantum promastigotes in a 10:1 (parasites/cell) ratio or stimulated with 1 μg/ml LPS during the indicated times at 37°C with 5% CO2. The mRNA levels were assessed by real-time qPCR for IL-12p40, TNF-α, IL-6, and IL-10. Gene expression is indicated as mean log2 values of fold changes relative to untreated cells. B: The levels of IL-12p40, TNF-α, IL-6, and IL-10 were quantified by ELISA on 24-hour culture supernatants of 1 × 106 BMDCs that were either infected with L. infantum promastigotes (Inf), stimulated with 1 μg/ml LPS, or pretreated for 1 hour with LPS and then infected (LPS→ Inf). C, control. C: BMDCs (2 × 106/well) were stimulated with 1 μg/ml LPS or infected for 1 hour after LPS stimulation. Four hours after infection, the mRNA levels of IL-12p40, TNF-α, IL-6, and IL-10 were assessed by real-time qPCR. Gene expression is indicated as mean log2 values of fold changes relative to LPS-treated cells. Each value represents the mean ± SD from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
We also assessed the ability of parasites to manipulate the LPS-induced cytokine profile. LPS potently induced the transcription and secretion of IL-12p40, IL-6, TNF-α, and IL-10; however, this secretion profile was substantially altered by infection with L. infantum promastigotes. The infection caused a significant decrease in the transcription and secretion of the LPS-induced proinflammatory cytokines IL-12p40 (qPCR −0.76 ± 0.3, P < 0.05; ELISA P < 0.05) and IL-6 (qPCR −0.76 ± 0.3, P < 0.05; ELISA P < 0.01), whereas no significant effect was found for TNF-α (Figure 3, B and C). In contrast, the mRNA and protein levels of the anti-inflammatory cytokine IL-10 were significantly increased in LPS-treated cells exposed to parasites (qPCR 1.41 ± 0.4, P < 0.001; ELISA P < 0.01). Overall these results show that in BMDCs L. infantum promastigotes slightly induce the transcription and secretion of IL-12p40 and IL-10 while reducing IL-6 levels. In addition, our data indicate that parasites are able to shift the levels of cytokines induced by LPS to a more parasite-convenient anti-inflammatory status.
L. infantum Promastigotes Induce a Rapid Phosphorylation of ERK1/2 and Akt While They Actively Promote a Cleavage on NF-κB p65RelA Subunit
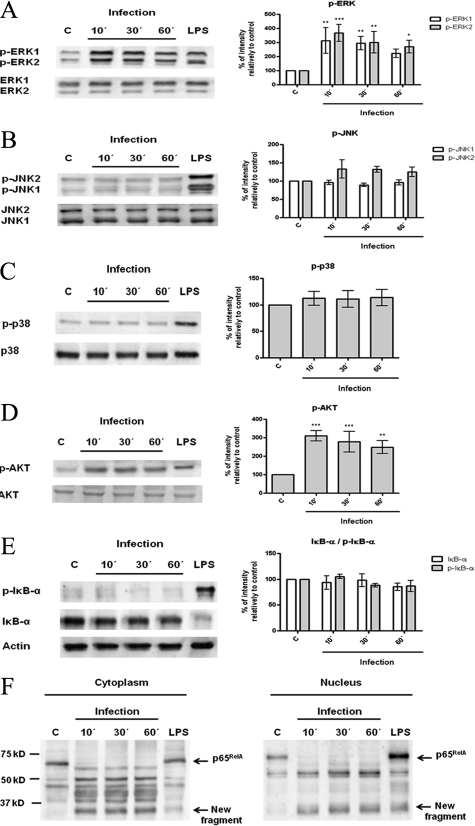
The initial interaction between host mononuclear phagocytes and Leishmania parasites results in the induction of intracellular signaling pathways that connect receptor-mediated events to nuclear transcription responses. To investigate these molecular mechanisms, we examined the effect of infection on the phosphorylated forms of MAPKs (ERK1/ERK2, SAPK/JNK, and p38 MAPK) and of Akt, which correspond to the active forms of the enzymes. The involvement of the transcription factor NF-κB was also evaluated by measuring the levels of its inhibitory protein, IκB-α, in both phosphorylated and nonphosphorylated forms and by assessing the nuclear translocation of the p65RelA subunit. As shown in Figure 4, A and D, infection of BMDCs with L. infantum promastigotes caused a rapid activation of ERK1/ERK2 and Akt. In contrast, SAPK/JNK and p38 pathways were not modulated by parasites (Figure 4, B and C). The NF-κB transcription factor cascade was not activated, at least by its canonical way, as evaluated by the absence of phosphorylation and subsequent degradation of the inhibitory protein IκB-α (Figure 4E). Interestingly, and not reported until now in DCs, parasites induced a rapid cleavage of the NF-κB p65RelA subunit, resulting a major new fragment of approximately 35 kDa (Figure 4F). The presence of this fragment on cytoplasmic and nuclear extracts of parasite-exposed cells indicates that cleavage may occur in cytoplasm and is followed by immediate translocation into the nucleus. Moreover, this cleavage was not observed with heat-killed or fixed promastigotes, pointing to a specific process that requires the activity of protein structures and viability of the parasite (Supplemental Figure S3F, see http://ajp.amjpathol.org). Similar results for all pathways assayed were obtained in another dendritic cell model, a fetal skin-derived dendritic cell line (data not shown).
Figure 4.
Interaction of L. infantum promastigotes with PI3K/Akt, MAPKs, and NF-κB signaling pathways. BMDCs were infected with L. infantum promastigotes in a cell/parasite ratio of 1:10, and cell lysates were prepared after 10, 30, or 60 minutes of infection. As a positive control, BMDCs were stimulated with 1 μg/ml of LPS for 30 minutes. The activation of specific intracellular signaling pathways was examined by Western blot analysis using specific antibodies to the phosphorylated (p-) forms of ERK1/2 (A), SAPK/JNK (B), p38 MAPK (C), Akt (D), and IκB-α (E). NF-κB activation was also evaluated by determination of the levels of its inhibitory protein IκB-α and by assessment of nuclear translocation of the NF-κB p65RelA subunit (F). Equal protein loading was assessed using antibodies to total ERK1/2, SAPK/JNK, p38 MAPK, and Akt or with an anti-actin antibody. The optical densities of the bands were obtained by scanning the membranes in a fluorescence scanner and then were analyzed with ImageQuant TL Software. The results are expressed as % intensity relative to control (C). Each value represents the mean ± SD from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
L. infantum Promastigotes Repress LPS-Induced NF-κB Activation and Exert a Synergistic Effect on ERK1/2 and Akt Phosphorylation
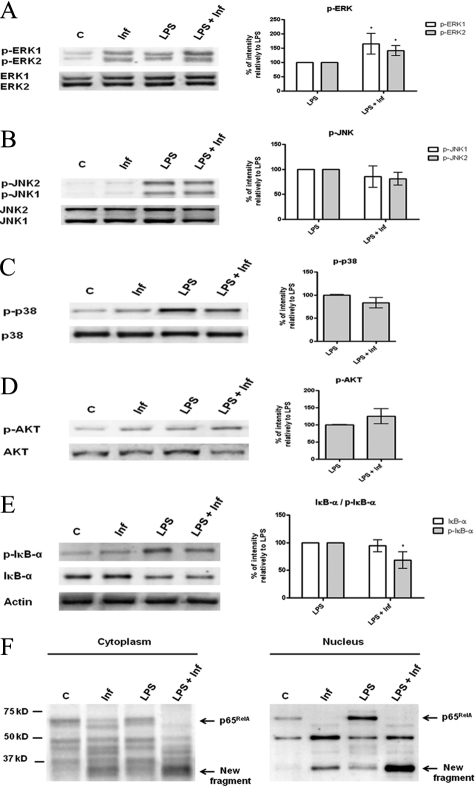
Further experiments were conducted to evaluate whether parasites were able to subvert/counteract, at signaling pathway levels, the effects of the effective maturation stimulus LPS. We exposed the cells simultaneously to LPS and L. infantum promastigotes for 30 minutes and analyzed the effects on NF-κB, MAPKs, and PI3K/Akt pathways (Figure 5, A–D). On LPS stimulation all of the pathways studied were activated and when BMDCs were coincubated with LPS and parasites, the phosphorylated forms of ERK and Akt were increased over the LPS-induced levels, revealing a synergistic effect (Figure 5, A and D). Nevertheless, the most striking effect of the coincubation of BMDCs with LPS and parasites was observed in the NF-κB transcription factor cascade. The phosphorylation of IκB-α was just slightly decreased, whereas the levels of total IκB-α remained approximately the same (Figure 5E); however, the NF-κB p65RelA subunit was dramatically affected. As reported above, the parasites seemed to actively promote the cleavage of the p65RelA subunit and in cells simultaneously exposed to parasites and LPS, this effect is clearly exacerbated (Figure 5F). This result suggests that LPS-triggered activation of NF-κB may somehow facilitate the cleavage promoted by the parasites. These events are currently under investigation in our laboratory.
Figure 5.
Effects of L. infantum infection on LPS-triggered signaling pathways. BMDCs were infected with L. infantum promastigotes for 30 minutes. infantum). In some experiments, infected BMDCs were simultaneously stimulated with 1 μg/ml LPS (LPS + Inf). Nonstimulated cells (C) or LPS-stimulated cells (LPS) were used as negative and positive controls, respectively. Cell extracts were analyzed by Western blot using specific antibodies to the phosphorylated (p-) forms of ERK1/2 (A), SAPK/JNK (B), p38 MAPK (C), Akt (D), and IκB-α (E) and for total IκB-α and NF-κB p65RelA (F). Equal protein loading was evaluated with antibodies to total ERK1/2, SAPK/JNK, p38 MAPK, and Akt or with an anti-actin antibody. The optical densities of the bands were obtained by scanning the membranes in a fluorescence scanner and then were analyzed with ImageQuant TL software. The results are expressed as % intensity relative to control. Each value represents the mean ± SD from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Taken together, these data show that L. infantum promastigotes are able to manipulate the LPS-triggered signaling cascades at different levels.
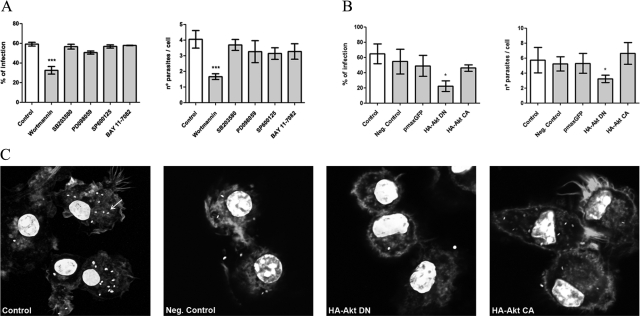
Inhibition of PI3K/Akt Blocks L. infantum Infection in BMDCs
The way Leishmania parasites signal to gain entry and survive in their host is not completely understood. In this study we provided clear evidence that L. infantum promastigotes interact and modulate different signaling systems in BMDCs. Thus, we decided to analyze the relevance of PI3K/Akt, MAPKs, and NF-κB pathways for L. infantum promastigotes uptake by cells. BMDCs were preincubated for 1 hour with wortmannin (PI3K/Akt inhibitor), SB203580 (p38MAPK inhibitor), PD098059 (ERK1/ERK2 inhibitor), or SP600125 (SAPK/JNK inhibitor) and then were exposed to parasites for 4 hours. To evaluate the role of NF-κB, cells were similarly treated with BAY 11-7082, an irreversible inhibitor of IκB-α phosphorylation.31 Preliminary experiments were conducted to evaluate and select the nontoxic concentrations of each inhibitor that efficiently inhibited the phosphorylation of the respective kinase mediator (data not shown). The effects of the different inhibitors on infection rates were checked by assessing the number of infected cells and number of parasites per cell.
As shown in Figure 6A, the engulfment of L. infantum promastigotes was significantly inhibited in the presence of wortmannin (32.53 ± 1.9%; P < 0.001), demonstrating for the first time that PI3K/Akt activation is required for the uptake of L. infantum parasites. This mechanism seemed to be specific for PI3K, because none of the other tested inhibitors altered parasite uptake so drastically. Because of this crucial role of the PI3K/Akt pathway on the entry of parasites, we decided to confirm these data by using a molecular approach that consisted of transient transfection of BMDCs with a dominant-negative mutant of Akt, HA-Akt DN (K179M). The results obtained supported the data from the pharmacological approach. The cells transfected with the negative mutant (HA-Akt DN) showed a 2.9 decrease in the parasite uptake and a 1.8 decrease in the number of parasites per cell compared with mock-transfected cells (negative control). In contrast, cells transfected with pmaxGFP or with a constitutively activated form of Akt (HA-Akt CA) did not showed significant differences in infection rates (Figure 6, B and C).
Figure 6.
Relevance of PI3K/Akt, MAPKs, and NF-κB signaling pathways on the engulfment of parasites by BMDCs. A: BMDCs previously treated with specific inhibitors for Akt (wortmannin), p38 MAPK (SB203580), ERK1/2 (PD098059), SAPK/JNK (SP600125), or NF-κB (Bay 11-7082) were incubated with L. infantum parasites for 4 hours. B: BMDCs were submitted to electroporation without any plasmid DNA (negative control) or transfected with pmaxGFP, a dominant-negative mutant of Akt (HA-Akt DN), or with a constitutively activated form of Akt (HA-Akt CA). After 48 hours, BMDCs were infected with L. infantum parasites. Four hours after infection, cells were stained with 4′,6-diamidino-2-phenylindole, and the numbers of infected cells as well as the numbers of parasites per cells were counted with a fluorescence microscope at ×1000. C: Images representative of fields of the different transfection experiments (original magnification: ×630) were acquired with a confocal laser scanning microscope (Zeiss LSM 510 Meta). Parasites were distributed in cytoplasm, and one of them is indicated by an arrow. Each value represents the mean ± SD from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
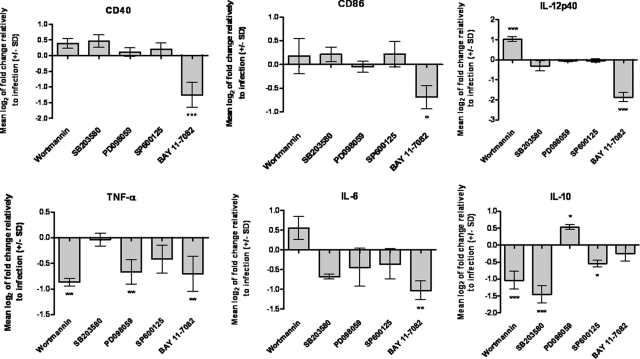
Activation of PI3K/Akt and Impairment of NF-κB by L. infantum Promastigotes Limit BMDC Maturation Status and the Release of Proinflammatory Cytokines
To assess the relevance of PI3K/Akt, MAPKs, and NF-κB pathways in the L. infantum modulation of BMDC functions, we used specific inhibitors of each pathway and checked their effects on the transcription of costimulatory molecules CD40 and CD86 and of the cytokines IL-6, IL-12p40, TNF-α, and IL-10. As shown in Figure 7, we found that the inhibition of PI3K/Akt by wortmannin before BMDC infection caused a slight increase in the transcription of costimulatory molecules CD40 and CD86 and proinflammatory cytokines IL-12p40 and IL-6. Remarkably, the opposite effect was observed for anti-inflammatory IL-10, which was found to be significantly repressed (−1.04 ± 0.3, P < 0.001). In contrast, pretreatment with PD098059 (inhibitor of ERK) marginally increased IL-10 transcription (0.53 ± 0.1, P < 0.05), suggesting a minor role for ERK in controlling the expression of this cytokine.
Figure 7.
Relevance of PI3K/Akt, MAPKs, and NF-κB signaling pathways on the expression of costimulatory molecules and cytokines by infected BMDCs. L. infantum parasites were incubated with BMDCs previously treated with specific inhibitors for Akt (wortmannin), p38 MAPK (SB203580), ERK1/2 (PD098059), SAPK/JNK (SP600125), or NF-κB (Bay 11-7082). The CD40, CD86, IL-12p40, TNF-α, IL-6, and IL-10 mRNA levels were assessed by qPCR 4 hours postinfection. Gene expression is indicated as mean log2 values of fold changes relative to L. infantum-infected cells. Each value represents the mean ± SD from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Transcription of the costimulatory molecules CD40 and CD86 as well as the proinflammatory cytokines IL-12p40, TNF-α, and IL-6 was significantly reduced by the NF-κB inhibitor BAY 11-7082. Because we showed that parasites do not activate this transcription factor and may even cause its impairment, these results have to be interpreted as the effects of inhibition over the basal activation state of the pathway, being evident the involvement of the NF-κB cascade in the transcriptional control of the above molecules. Therefore, we can postulate that the nonactivation/impairment of this pathway by L. infantum promastigotes justifies the lack of or weak expression of costimulatory and proinflammatory molecules by infected BMDCs. Overall, these data suggest that PI3K/Akt activation and nonactivation/impairment of NF-κB transcription factor are dominant mechanisms by which the parasite orchestrates and manipulates the inflammatory/maturation status of DCs.
Discussion
The molecular mechanisms by which Leishmania parasites evade/impair DC biological functions remain incompletely understood. These facts prompted us to study the interaction between visceral L. infantum promastigotes and DCs at multiple levels. Our data showed that murine BMDCs rapidly internalize L. infantum promastigotes and that a large part of the infection is cleared in the first 12 hours postinfection, a period after which the number of parasites per cell starts to increase. Concerning the ability of parasites to activate DCs, discrepant observations are found in literature, with some studies showing DC activation32,33,34,35 and others showing a silent infection.14,36 These discrepant results might be due to the use of different parasite species and life-cycle stages as well as different sources of DCs. In this study, we showed that L. infantum promastigotes were able to infect DCs, avoiding the induction of CD40 and CD86 expression in a specific process that was dependent viability, because killed parasites strongly induced these maturation markers.
In agreement with previous studies performed with L. major,11,37 L. amazonensis,17 L. donovani,6 and L. infantum promastigotes,7 we observed in our model a transient induction of the transcription of IL-12p40 and IL-10 that dropped dramatically at 24 hours postinfection. In contrast with recent reports that described the production of high levels of TNF-α by L. braziliensis-infected mouse DCs12 or L. infantum-infected human DCs,38 in our DC model L. infantum promastigotes were unable to up-regulate TNF-α and induced a time-sustained impairment of the transcription and secretion of the IL-6 cytokine. As observed for the expression of surface molecules, this cytokine profile of infected cells is specific for viable parasites. Of particular relevance was the fact that killed parasites induced a significantly higher transcription and secretion of IL-12 than cells exposed to viable parasites, indicating that L. infantum promastigotes actively limit the production of this proinflammatory cytokine. Given that in our model not all of the DCs are infected, the slight increase in IL-12 that we observed in cells exposed to viable promastigotes may result from the activity of noninfected bystander DCs, in a process similar to the one described by Carvalho et al.12 In fact, experiments performed in our laboratory by Ricardo Silvestre, Mariana Resende, Bruno Neves, Ali Ouaissi, Maria Teresa Cruz and Anabela Cordeiro da Silva with isolated populations of infected and bystander DCs showed that IL-12 is exclusively produced by the bystander DC population (manuscript in preparation).
As previously observed in macrophages,39,40,41,42 our data provide evidences that L. infantum promastigotes actively counteract the LPS-induced maturation process in dendritic cells. We observed that parasites significantly decrease the LPS-induced CD40 surface expression in BMDCs and that the levels of LPS-induced cytokines were also considerably affected after infection. The expression of the proinflammatory cytokines IL-6 and IL-12 was significantly reduced in LPS-treated cells infected with L. infantum promastigotes and in contrast, the LPS-induced expression of the anti-inflammatory cytokine IL-10 was synergistically enhanced by exposure to parasites. These results further support the evidence that L. infantum is able to bias the cytokine release profile toward a more parasite-convenient immunosuppressive profile.
At molecular level we showed that L. infantum promastigotes rapidly induced a time-sustained activation of PI3K/Akt and ERK1/2 signaling cascades, whereas the NF-κB transcription factor seems to be impaired. The available data regarding the involvement of the NF-κB signaling system in Leishmania infection of DCs are limited, although recent studies showed that L. mexicana lipophosphoglycan43 and L. major infection44 activate this family of transcription factors in human monocyte-derived DCs. The data obtained in this study demonstrated that L. infantum promastigotes are unable to “canonically” activate the NF-κB transcription factor, as shown by the inability of parasites to promote phosphorylation and posterior degradation of the NF-κB inhibitory protein, IκB-α. Surprisingly, parasites induced a cleavage on the NF-κB p65RelA subunit, resulting in a major new fragment of approximately 35 kDa. This cleavage was not observed in cells exposed to heat-killed or fixed parasites, indicating that protein structures of the parasite are directly involved in this process. Given the role of NF-κB on the activation/maturation process of DCs,45 its impairment by viable parasites provides an explanation for the maintenance of infected cells in an incomplete mature state. A similar observation was reported in a recent study performed in macrophages, showing that infection with Leishmania promastigotes induced a specific cleavage of the NF-κB p65RelA subunit.46 The authors postulated that the resulting p35RelA fragment may represent an important mediator by which Leishmania promastigotes induce several chemokines without inducing other NF-κB-regulated genes, such as iNOS and IL-12 that are detrimental for parasite survival. Moreover, we showed that Leishmania actively counteract the LPS-triggered activation of NF-κB cascade by reducing IκB-α phosphorylation and extensively cleaving the NF-κB p65RelA subunit. Because parasite-promoted cleavage seems to be somehow facilitated by LPS, we hypothesize that the cleavage still occurs in NF-κB-IκB-α complexes but is facilitated in LPS-treated cells because of the release of NF-κB from IκB-α. Supporting this hypothesis, Gregory et al46 have shown that the inhibition of IκB-α proteasomal degradation decreases the appearance of the p35RelA fragment in infected nuclei.
Regarding the other signaling pathways analyzed in this study, we observed that among the members of the MAPK family, only ERK was clearly activated by L. infantum promastigotes, whereas JNK and p38 were not affected. The MAPKs transduction pathways are understood to play positive as well as negative regulatory roles in inflammatory cytokine production22 and several reports indicate that activation of ERK can prevent proper DC maturation and cause a shift through a Th2 DC-polarizing cytokine/chemokine profile.26,47,48 However, our results indicated that the ERK pathway plays a minor role in L. infantum-induced DC costimulatory molecules and cytokine release profile. Treatment of cells with PD098059, a specific inhibitor of ERK1/2, minimally affected transcription of genes encoding CD40, CD86, or the studied cytokines. Interestingly we found that, in contrast to the observed effect for NF-κB, p38, and JNK, Leishmania synergizes with LPS to induce ERK activation.
For the first time, we provide evidence that infection of murine DCs by L. infantum promastigotes led to the activation of PI3K/Akt pathway. We verified that the activation of this signaling cascade by Leishmania parasites plays a major role in their ability to impair the transcription of proinflammatory cytokines. Preincubation of cells with wortmannin, a specific inhibitor of PI3K, followed by infection, caused an increase in the transcription of CD40, IL-12p40, and IL-6 encoding genes. In contrast, the parasite-induced IL-10 expression was strongly repressed. Further, a recent report indicated that inhibition of Akt in L. amazonensis promastigote-infected macrophages increased IL-12p40 production at the transcriptional level.49 As observed for ERK, the PI3K/Akt pathway was synergistically activated in BMDCs coincubated with LPS and L. infantum promastigotes. This synergism on Akt phosphorylation might explain at a molecular level the observed decrease in IL-12p40 and the increase in IL-10 expression in cells treated with LPS before infection.
We also showed that the integrity of the PI3K/Akt pathway is essential for the capacity of L. infantum promastigotes to infect cells. Treatment of BMDCs with wortmannin or transfection with a dominant-negative mutant of Akt profoundly impairs the infection rates, reducing the percentage of infected cells and the number of parasites per cell. Modulation of host cell PI3K-dependent signaling by microbial pathogens is becoming recognized as an important strategy for establishment of infection and intracellular survival of microorganisms.50,51,52 Interestingly, similar to our observations for Leishmania, Trypanosoma cruzi, an intracellular protozoan parasite of the same family as Leishmania, activates the PI3K/Akt pathway in host cells, this activation being crucial for the entry of parasites.53,54,55
In summary, this study demonstrated for the first time that a visceral Leishmania species can differentially target PI3K/Akt, MAPKs, and NF-κB to modulate the maturation, activation, and immunostimulatory abilities of DCs. Overall, our results show that the activation of the PI3k/Akt pathway and the impairment of NF-κB transcription factor are crucial strategies by which Leishmania parasites subvert DC immunostimulatory abilities. Knowledge of the intracellular signaling profile triggered by Leishmania parasites in host immune cells sheds light on the mechanisms leading to immune evasion. This awareness will surely reveal new potential targets for the development of therapeutic strategies against visceral leishmaniasis.
Acknowledgments
We thank Marc Ouellette for providing the pSP expression vector.
Footnotes
Address reprint requests to Bruno Neves, Ph.D., Centro de Neurociências e Biologia Celular, Universidade de Coimbra, Azinhaga de Santa Comba, Celas 3000-548 Coimbra, Portugal. E-mail: neves_bruno@sapo.pt.
Supported by Fundação para a Ciência e a Tecnologia (FCT) and Fundo Europeu de Desenvolvimento Regional (FEDER) Ciência 2010 (project numbers PTDC/SAU-FCF/67351/2006 and PTDC/SAU-FCF/101017/2008 and FCT grants SFRH/BD/30563/2006, SFRH/BD/48626/2008, SFRH/BDP/48340/2008, SFRH/BD/64528/2009, SFRH/BD/37352/2007, and SFRH/BD/28316/2006).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Kaye PM, Svensson M, Ato M, Maroof A, Polley R, Stager S, Zubairi S, Engwerda CR. The immunopathology of experimental visceral leishmaniasis. Immunol Rev. 2004;201:239–253. doi: 10.1111/j.0105-2896.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- Ghalib HW, Whittle JA, Kubin M, Hashim FA, el-Hassan AM, Grabstein KH, Trinchieri G, Reed SG. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J Immunol. 1995;154:4623–4629. [PubMed] [Google Scholar]
- Liew FY, Parkinson C, Millott S, Severn A, Carrier M. Tumour necrosis factor (TNFα) in leishmaniasis. I. TNFα mediates host protection against cutaneous leishmaniasis. Immunology. 1990;69:570–573. [PMC free article] [PubMed] [Google Scholar]
- Murray HW, Hariprashad J. Interleukin 12 is effective treatment for an established systemic intracellular infection: experimental visceral leishmaniasis. J Exp Med. 1995;181:387–391. doi: 10.1084/jem.181.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmoy M, Megnekou R, Allenbach C, Zweifel C, Perez C, Monnat K, Breton M, Ronet C, Launois P, Tacchini-Cottier F. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J Leukoc Biol. 2007;82:288–299. doi: 10.1189/jlb.0706440. [DOI] [PubMed] [Google Scholar]
- Gorak PM, Engwerda CR, Kaye PM. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur J Immunol. 1998;28:687–695. doi: 10.1002/(SICI)1521-4141(199802)28:02<687::AID-IMMU687>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Schleicher U, Liese J, Knippertz I, Kurzmann C, Hesse A, Heit A, Fischer JA, Weiss S, Kalinke U, Kunz S, Bogdan C. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J Exp Med. 2007;204:893–906. doi: 10.1084/jem.20061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- Antoine JC, Prina E, Courret N, Lang T. Leishmania spp.: on the interactions they establish with antigen-presenting cells of their mammalian hosts. Adv Parasitol. 2004;58:1–68. doi: 10.1016/S0065-308X(04)58001-6. [DOI] [PubMed] [Google Scholar]
- Soong L. Modulation of dendritic cell function by Leishmania parasites. J Immunol. 2008;180:4355–4360. doi: 10.4049/jimmunol.180.7.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecny P, Stagg AJ, Jebbari H, English N, Davidson RN, Knight SC. Murine dendritic cells internalize Leishmania major promastigotes, produce IL-12 p40 and stimulate primary T cell proliferation in vitro. Eur J Immunol. 1999;29:1803–1811. doi: 10.1002/(SICI)1521-4141(199906)29:06<1803::AID-IMMU1803>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Carvalho LP, Pearce EJ, Scott P. Functional dichotomy of dendritic cells following interaction with Leishmania braziliensis: infected cells produce high levels of TNF-α, whereas bystander dendritic cells are activated to promote T cell responses. J Immunol. 2008;181:6473–6480. doi: 10.4049/jimmunol.181.9.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Inchaustegui DA, Xin L, Soong L. Leishmania braziliensis infection induces dendritic cell activation. ISG15 transcription, and the generation of protective immune responses. J Immunol. 2008;180:7537–7545. doi: 10.4049/jimmunol.180.11.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CL, Misslitz A, Colledge L, Aebischer T, Blackburn CC. Silent infection of bone marrow-derived dendritic cells by Leishmania mexicana amastigotes. Eur J Immunol. 2001;31:876–883. doi: 10.1002/1521-4141(200103)31:3<876::aid-immu876>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Boggiatto PM, Jie F, Ghosh M, Gibson-Corley KN, Ramer-Tait AE, Jones DE, Petersen CA. Altered dendritic cell phenotype in response to Leishmania amazonensis amastigote infection is mediated by MAP kinase, ERK. Am J Pathol. 2009;174:1818–1826. doi: 10.2353/ajpath.2009.080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favali C, Tavares N, Clarencio J, Barral A, Barral-Netto M, Brodskyn C. Leishmania amazonensis infection impairs differentiation and function of human dendritic cells. J Leukoc Biol. 2007;82:1401–1406. doi: 10.1189/jlb.0307187. [DOI] [PubMed] [Google Scholar]
- Xin L, Li K, Soong L. Down-regulation of dendritic cell signaling pathways by Leishmania amazonensis amastigotes. Mol Immunol. 2008;45:3371–3382. doi: 10.1016/j.molimm.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones M, Ahuja SK, Melby PC, Pate L, Reddick RL, Ahuja SS. Preformed membrane-associated stores of interleukin (IL)-12 are a previously unrecognized source of bioactive IL-12 that is mobilized within minutes of contact with an intracellular parasite. J Exp Med. 2000;192:507–516. doi: 10.1084/jem.192.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Martín Y, Colmenares M, Gozalbo-Lopez B, Lopez-Nunez M, Savage PB, Martinez-Naves E. Immature human dendritic cells infected with Leishmania infantum are resistant to NK-mediated cytolysis but are efficiently recognized by NKT cells. J Immunol. 2006;176:6172–6179. doi: 10.4049/jimmunol.176.10.6172. [DOI] [PubMed] [Google Scholar]
- Caparrós E, Serrano D, Puig-Kroger A, Riol L, Lasala F, Martinez I, Vidal-Vanaclocha F, Delgado R, Rodriguez-Fernandez JL, Rivas L, Corbi AL, Colmenares M. Role of the C-type lectins DC-SIGN and L-SIGN in Leishmania interaction with host phagocytes. Immunobiology. 2005;210:185–193. doi: 10.1016/j.imbio.2005.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejle K, Lindroth M, Magnusson KE, Rasmusson B. Wild-type Leishmania donovani promastigotes block maturation, increase integrin expression and inhibit detachment of human monocyte-derived dendritic cells—the influence of phosphoglycans. FEMS Microbiol Lett. 2008;279:92–102. doi: 10.1111/j.1574-6968.2007.01013.x. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Moroi Y, Uchi H, Furue M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J Dermatol Sci. 2006;42:1–11. doi: 10.1016/j.jdermsci.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Laban A, Tobin JF, Curotto de Lafaille MA, Wirth DF. Stable expression of the bacterial neor gene in Leishmania enriettii. Nature. 1990;343:572–574. doi: 10.1038/343572a0. [DOI] [PubMed] [Google Scholar]
- Papadopoulou B, Roy G, Ouellette M. A novel antifolate resistance gene on the amplified H circle of Leishmania. EMBO J. 1992;11:3601–3608. doi: 10.1002/j.1460-2075.1992.tb05444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Neves BM, Cruz MT, Francisco V, Garcia-Rodriguez C, Silvestre R, Cordeiro-da-Silva A, Dinis AM, Batista MT, Duarte CB, Lopes MC. Differential roles of PI3-Kinase. MAPKs and NF-κB on the manipulation of dendritic cell Th1/Th2 cytokine/chemokine polarizing profile. Mol Immunol. 2009;46:2481–2492. doi: 10.1016/j.molimm.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Hu MC, Miller SA, Yu Z, Xia W, Lin SY, Hung MC. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-κB pathway. J Biol Chem. 2000;275:8027–8031. doi: 10.1074/jbc.275.11.8027. [DOI] [PubMed] [Google Scholar]
- Reiner NE, Ng W, McMaster WR. Parasite-accessory cell interactions in murine leishmaniasis. II. Leishmania donovani suppresses macrophage expression of class I and class II major histocompatibility complex gene products. J Immunol. 1987;138:1926–1932. [PubMed] [Google Scholar]
- Saha B, Das G, Vohra H, Ganguly NK, Mishra GC. Macrophage-T cell interaction in experimental visceral leishmaniasis: failure to express costimulatory molecules on Leishmania-infected macrophages and its implication in the suppression of cell-mediated immunity. Eur J Immunol. 1995;25:2492–2498. doi: 10.1002/eji.1830250913. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- Flohé SB, Bauer C, Flohé S, Moll H. Antigen-pulsed epidermal Langerhans cells protect susceptible mice from infection with the intracellular parasite Leishmania major. Eur J Immunol. 1998;28:3800–3811. doi: 10.1002/(SICI)1521-4141(199811)28:11<3800::AID-IMMU3800>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Henri S, Curtis J, Hochrein H, Vremec D, Shortman K, Handman E. Hierarchy of susceptibility of dendritic cell subsets to infection by Leishmania major: inverse relationship to interleukin-12 production. Infect Immun. 2002;70:3874–3880. doi: 10.1128/IAI.70.7.3874-3880.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell MA, Marovich M, Lira R, Braun M, Sacks D. Leishmania priming of human dendritic cells for CD40 ligand-induced interleukin-12p70 secretion is strain and species dependent. Infect Immun. 2002;70:3994–4001. doi: 10.1128/IAI.70.8.3994-4001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stebut E, Belkaid Y, Nguyen BV, Cushing M, Sacks DL, Udey MC. Leishmania major-infected murine Langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous Leishmaniasis. Eur J Immunol. 2000;30:3498–3506. doi: 10.1002/1521-4141(2000012)30:12<3498::AID-IMMU3498>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Prina E, Abdi SZ, Lebastard M, Perret E, Winter N, Antoine JC. Dendritic cells as host cells for the promastigote and amastigote stages of Leishmania amazonensis: the role of opsonins in parasite uptake and dendritic cell maturation. J Cell Sci. 2004;117:315–325. doi: 10.1242/jcs.00860. [DOI] [PubMed] [Google Scholar]
- Xin L, Li Y, Soong L. Role of interleukin-1β in activating the CD11c(high) CD45RB-dendritic cell subset and priming Leishmania amazonensis-specific CD4+ T cells in vitro and in vivo. Infect Immun. 2007;75:5018–5026. doi: 10.1128/IAI.00499-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R, Barat C, Ouellet M, Lodge R, Tremblay MJ. Leishmania infantum amastigotes enhance HIV-1 production in cocultures of human dendritic cells and CD4 T cells by inducing secretion of IL-6 and TNF-α. PLoS Negl Trop Dis. 2009;3:e441. doi: 10.1371/journal.pntd.0000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P, McGachy A, Anderson M, Paul A, Coombs GH, Mottram JC, Alexander J, Plevin R. Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: the role of cysteine peptidases and the NF-κB signaling pathway. J Immunol. 2004;173:3297–3304. doi: 10.4049/jimmunol.173.5.3297. [DOI] [PubMed] [Google Scholar]
- Chandra D, Naik S. Leishmania donovani infection down-regulates TLR2-stimulated IL-12p40 and activates IL-10 in cells of macrophage/monocytic lineage by modulating MAPK pathways through a contact-dependent mechanism. Clin Exp Immunol. 2008;154:224–234. doi: 10.1111/j.1365-2249.2008.03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye PM, Rogers NJ, Curry AJ, Scott JC. Deficient expression of co-stimulatory molecules on Leishmania-infected macrophages. Eur J Immunol. 1994;24:2850–2854. doi: 10.1002/eji.1830241140. [DOI] [PubMed] [Google Scholar]
- Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18:293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueta-Donohué J, Carrillo N, Valdes-Reyes L, Zentella A, Aguirre-Garcia M, Becker I, Gutierrez-Kobeh L. Leishmania mexicana: participation of NF-κB in the differential production of IL-12 in dendritic cells and monocytes induced by lipophosphoglycan (LPG). Exp Parasitol. 2008;120:1–9. doi: 10.1016/j.exppara.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Jayakumar A, Donovan MJ, Tripathi V, Ramalho-Ortigao M, McDowell MA. Leishmania major infection activates NF-κB and interferon regulatory factors 1 and 8 in human dendritic cells. Infect Immun. 2008;76:2138–2148. doi: 10.1128/IAI.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-κB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–1046. [PubMed] [Google Scholar]
- Gregory DJ, Godbout M, Contreras I, Forget G, Olivier M. A novel form of NF-κB is induced by Leishmania infection: involvement in macrophage gene expression. Eur J Immunol. 2008;38:1071–1081. doi: 10.1002/eji.200737586. [DOI] [PubMed] [Google Scholar]
- Puig-Kröger A, Relloso M, Fernandez-Capetillo O, Zubiaga A, Silva A, Bernabeu C, Corbi AL. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood. 2001;98:2175–2182. doi: 10.1182/blood.v98.7.2175. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhland A, Kima PE. Activation of PI3K/Akt signaling has a dominant negative effect on IL-12 production by macrophages infected with Leishmania amazonensis promastigotes. Exp Parasitol. 2009;122:28–36. doi: 10.1016/j.exppara.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos S, Desjardins M. Subversion of a young phagosome: the survival strategies of intracellular pathogens. Cell Microbiol. 2000;2:365–377. doi: 10.1046/j.1462-5822.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol. 2001;154:631–644. doi: 10.1083/jcb.200106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes BK, Mahony JB. Identification of MEK- and phosphoinositide 3-kinase-dependent signalling as essential events during Chlamydia pneumoniae invasion of HEp2 cells. Cell Microbiol. 2002;4:447–460. doi: 10.1046/j.1462-5822.2002.00203.x. [DOI] [PubMed] [Google Scholar]
- Woolsey AM, Sunwoo L, Petersen CA, Brachmann SM, Cantley LC, Burleigh BA. Novel PI 3-kinase-dependent mechanisms of trypanosome invasion and vacuole maturation. J Cell Sci. 2003;116:3611–3622. doi: 10.1242/jcs.00666. [DOI] [PubMed] [Google Scholar]
- Todorov AG, Einicker-Lamas M, de Castro SL, Oliveira MM, Guilherme A. Activation of host cell phosphatidylinositol 3-kinases by Trypanosoma cruzi infection. J Biol Chem. 2000;275:32182–32186. doi: 10.1074/jbc.M909440199. [DOI] [PubMed] [Google Scholar]
- Wilkowsky SE, Barbieri MA, Stahl P, Isola EL. Trypanosoma cruzi: phosphatidylinositol 3-kinase and protein kinase B activation is associated with parasite invasion. Exp Cell Res. 2001;264:211–218. doi: 10.1006/excr.2000.5123. [DOI] [PubMed] [Google Scholar]