Abstract
The polyphenol epigallocatechin-3-gallate (EGCG) in combination with doxorubicin (Dox) exhibits a synergistic activity in blocking the growth and colony-forming ability of human prostate cell lines in vitro. EGCG has been found to disrupt the mitochondrial membrane potential, induce vesiculation of mitochondria, and induce elevated poly (ADP-ribose) polymerase (PARP) cleavage and apoptosis. EGCG in combination with low levels of Dox had a synergistic effect in blocking tumor cell growth. In vivo tumor modeling studies with a highly metastatic tumor line, PC-3ML cells, revealed that EGCG (228 mg/kg or 200 μmol/L) appeared to sensitize tumors to Dox. EGCG combined with low levels of Dox (0.14 mg/kg or 2 μmol/L) blocked tumor growth by PC-3ML cells injected intraperitoneally (ie, in CB17 severe combined immunodeficiencies) and significantly increased mouse survival rates. Similarly, relatively low levels of EGCG (57 mg/kg or 50 μmol/L) plus Dox (0.07 mg/kg or 1 μmol/L) eradicated established tumors (ie, in nonobese diabetic–severe combined immunodeficiencies) that were derived from CD44hi tumor-initiating cells isolated from PCa-20a cells. Flow cytometry results showed that EGCG appeared to enhance retention of Dox by tumor cells to synergistically inhibit tumor growth and eradicate tumors. These data suggest that localized delivery of high dosages of EGCG combined with low levels of Dox may have significant clinical application in the treatment of metastatic prostate and/or eradication of primary tumors derived from tumor-initiating cells.
Epidemiological studies have suggested a protective effect of tea consumption against human cancers of the breast, cervix, colon and rectum, gallbladder, liver, lung, nasopharynx, pancreas, prostate, stomach, ovary, and uterus.1,2,3,4 Recent epidemiological studies have demonstrated the cancer preventive properties of green tea polyphenols in prostate cancer.2,5,6
Animal tumor modeling studies using green tea, green tea leaves, green tea extracts, polyphenol mixtures, green tea catechin mixtures, and the individual catechins have demonstrated chemopreventive efficacy in several cancers. Using the Transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse model for prostate cancer, Gupta et al7 showed that a polyphenol extract delivered by gavage (500 mg/kg/day) partially delayed the onset of prostate cancer and inhibited prostate cancer growth to increase mouse survival rates. Polyphenol consumption caused significant apoptosis of the tumor cells, which possibly resulted in reduced dissemination of cancer cells, thereby causing inhibition of tumor development, progression, and metastasis. However, a similar animal model study with epigallocatechin gallate (EGCG; ie, purified from polyphenols) added to the drinking water only slightly reduced the incidence of prostate cancer and tumor progression.8 In addition, a recent study by Kinney and colleagues9 revealed that oral administration of polyphenols did not inhibit tumor progression in TRAMP mice. It was suggested that the limited activity of polypenols may be due to a relatively short half life plus low oral bioavailability of polyphenols (ie, slow absorption combined with high metabolic clearance by the liver).6 Further studies are required to improve delivery and assess whether polyphenols have significant anti-tumorigenic activity against prostate cancer.
The variability in results reported on polyphenol activities prompted us to evaluate whether localized delivery of drug might significantly improve the therapeutic activity of EGCG. Secondly, we reasoned that EGCG delivered locally in combination with low levels of a known therapeutic agent (ie, Doxorubicin [Dox]) used at subtherapeutic dosages may have a synergistic activity in eradicating tumors while having minimal side effects. In this regard, several reports have previously shown that green tea components (ie, theanine and caffeine) in combination with Dox can block ovarian cancer growth in mice,9,10 but the studies were poorly controlled and it was difficult to determine whether the two agents exhibited synergistic effects in vivo. Moreover, optimal delivery of the drug to maximize anti-tumor activities was not explored in these studies.
In this article, we attempted to show that EGCG and Dox exhibit synergistic effects to block tumor cell growth and colony forming ability in vitro and tumor growth in vivo. The data showed that EGCG independently reduced mitochondrial membrane competence to induce apoptosis. EGCG also increased Dox retention by the tumor cells in vitro and metastatic tumors in vivo. Thus, EGCG appears to sensitize tumors to relatively low levels of Dox to significantly increase their combined anti-tumor activity. In sum, the data showed, for the first time, that localized delivery of EGCG plus Dox exerted a synergistic activity in blocking tumor growth i.p. by a highly metastatic tumor line to improve significantly overall mouse survival rates.
Materials and Methods
Cell Cultures
The IBC-10a and PCa-20a cell strains were isolated by our lab from the right peripheral zone of a prostate gland with Gleason score (GS) 6 and 7 tumors, respectively. Cells were immortalized by transfections with LXSN-hTERT retroviral vector (courtesy of Johng Rhim, Center for Prostate Disease Research, Uniformed Services University of the Health Sciences (USUHS), Bethesda, MD) using methods previously described.11 IBC-10a12,13 and PCa-20a cells were found to be CK5, CK18, p63, and PTEN positive, and have been classified as intermediate basal cells. Cells were grown in serum free complete Keratinocyte media (CKM) containing epidermal growth factor and pituitary extract plus 1% penicillin/streptomycin (Invitrogen, Inc., Carlsbad, CA). PC-3ML cells were subcloned by our lab12 from the parent PC-3 cells ATTC (American Tissue Culture Consortium, Bethesda, MD) based on their invasive properties in vitro and ability to metastasize to the bone marrow in CB17-severe combined immunodeficiencies (SCIDs). PC-3ML cells were maintain Dulbecco’s modified Eagle’s medium plus 10% fetal bovine serum (FBS) according to previously described protocols of the American Tissue Culture Consortium.
Primary Organ Cultures
Primary organ cultures were established from freshly minced pieces of prostate tissue (<1 mm diameter [dia]) and the pathology carried out by Dr. Garcia on adjacent pieces according to methods developed by our lab to establish IBC-10a cultures.12,13 Pieces of tumor were plated on tissue culture dishes in ∼two drops of CKM for ∼4 hours in the CO2 incubator to allow tissue adherence to the dish. Then, additional media was added and epithelial cells were induced to grow out on the dish in the presence of CKM containing 1 nm dihydrotestosterone and 1 ng/ml hepatocyte growth factor (Sigma-Aldrich, Inc., St. Louis, MO) over a 6 to 10 day period. Immunolabeling with CK18 and CK5 antibodies indicated the cells were of epithelial origin. Alternatively, stromal fibroblasts were induced to grow out from pieces of prostate tissue incubated in Dulbecco’s modified Eagle’s medium containing 1 nm dihydrotestosterone (DHT) and 10% FBS. After the outgrowth of cells from tumor pieces, we examined the dosage-dependent response of these cells to EGCG and Dox. H&E sections were prepared from the organ tissue pieces for histological examination and diagnosis of GS. Dr. Fernando Garcia also carried out the diagnosis of the tumor GS by using adjacent tumor pieces. The Gleason score of the tumor pieces used in this study ranged from GS6 to GS8. Isolation of prostate organ cell cultures was done with the approval from the Drexel University College of Medicine Institutional Review Board.
Cell Growth and Cell Cycle Analysis
Cell growth assays were carried out by using the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) assay.12,13,14 Cells were labeled with 1 nm propidium iodide for flow cytometry analysis of the changes in cell cycle and DNA content of the cells. The DNA histograms were analyzed by using ModiFitLT software (Verily Software House, Topsham, ME).
Colony Forming Assays
Cells suspended in CKM plus 10% FBS were seeded in 0.5% soft agar as single cell suspensions according to Sambrook et al15 in 12-well dishes (Corning Inc., Corning, NY) at 1 × 103 cells/dish. We counted the numbers of colonies formed after 30 to 40 days, which were >100 μm dia.
Apoptosis Assays
Apoptosis was measured by flow cytometry by using Annexin V antibodies and a kit from Guava, Inc. (Redwood, CA) according to published methods by our group.15
Di06(3) Staining
A stock solution of DiOC6(3) (Invitrogen, Eugene, OR) was prepared by diluting DiOC6(3) to 400 nm in PBS, pH 7.4. DiOC6(3) was added to cells for ∼1 minute and then cells were washed with fresh medium and photographed by using a Zeiss Confocal Microscope (Zeiss Microscopes, Thornwood, NY).
Flow Cytometry: Measurements of Mitochondrial Membrane Potential
A stock solution of JC-1 (Invitrogen) at 20 μm (2 μl stock solution plus 1 ml PBS) was prepared. Cells in suspension at 37°C were incubated with 1 μm JC-1 for 30 minutes then placed on ice until reading in the flow cytometer. As a negative control, aliquots of cells were labeled in the presence of the protonophore CCCP (100 μm). JC-1 accumulates as J-aggregates (A590 nm, red) in metabolically active mitochondria only, while the depolarization of the mitochondrial membranes leads to JC-1 monomer formation (A 527 nm, green). Autofluorescence and JC-1 dependent fluorescence changes were recorded by using a Guava flow cytometer (Guava, Inc.) using 488 nm excitation with 530/30 nm (FL1, green) and 585/42 nm (FL2, orange) band pass emission filters. The sample flow rate was adjusted to about 1000 cells/min. The respective gates were defined by using the distinctive forward-scatter and side-scatter properties of the individual cell populations. The data from 2000 cells/assay were analyzed by using Guava Express software.
Reactive Oxygen Species Assays
To determine the involvement of reactive oxygen species (ROS) in apoptosis, the ROS inhibitor N-acetylcysteine was added to cells either alone or in combination with EGCG. All reagents were purchased from Sigma-Aldrich unless otherwise stated. Intracellular ROS generation in the control and EGCG-treated cells was monitored by using H2O2- sensitive probe 5 (and 6)-chloromethyl-2′,7′-dichlorodi-hydrofluorescein diacetate (CM-H2DCFDA), as previously described.16 Briefly, cells were incubated with H2DCFDA (5 μm) for 30 minutes at 37°C and washed twice with PBS. Then, the fluorescent intensity of 5 × 105 cells was measured by using a spectrofluorometer (excitation A500 nm; emission A530 nm).
Measurements of Dox Retention
Flow cytometry was used to quantify the intracellular accumulation and retention of Dox according to published methods.17 Cells were washed twice in PBS, harvested after Trypsin-EDTA treatment, and washed twice in PBS. The Dox fluorescence was measured by using a Guava, Inc., flow cytometer. Using the excitation with an argon laser at 488 nm, emission of 10,000 events per sample was detected on FL2 (A550 nm).
Gel Electrophoresis and Western Blots
Following the different treatments, cells were lysed with modified radioimmunoprecipitation assay buffer.15 Western blots were carried out according to standard procedures15 by using Super Signal West Pico chemiluminescent substrate (Pierce Biotechnology, Inc., Rockford, IL) for antibody detection. The reaction was visualized by using the ChemiDoc XRS Gel Documentation system (Bio-Rad Laboratories, Inc., Hercules, CA). Protein measurements were carried out according to Sambrook et al.15 anti-poly (ADP-ribose) polymerase (PARP) monoclonal antibodies were from Pharmingen (San Diego, CA); matrix metalloproteinase-2 (MMP-2), tubulin, and glyceraldehyde-3-phosphate dehydrogenase antibodies were from Sigma-Aldrich.
Drug Studies
EGCG (Sigma-Aldrich) was prepared fresh by resuspension of EGCG in PBS, pH.5, containing 3% ascorbic acid and 10 mm EDTA (the solvent). The EGCG solution was filter sterilized and added to cells or injected in mice. In control experiments, cells or mice were treated with the solvent alone. After treatment, the conditioned media or crude cell extracts prepared in radioimmunoprecipitation assay buffer were collected and stored at −80°C. Dox (Sigma-Aldrich) was solubilized in sterile PBS at 1 mg/ml, and aliquots were added to cell cultures or injected in mice.
Isolation of CD44hi PCa-20a Cells
CD44hi PCa-20a subpopulations were isolated from passage 5 PCa-20a cells by using magnetic beads coated with CD44hi antibodies (1:50 dilution; ab41478, Abcam Inc., San Francisco, CA). In brief, a kit and procedures from Miltenyl Biotech (Auburn, CA) was used according to methods developed in our lab.12 Cells were suspended in CKM counted with a Hemacytometer and injected i.p. in 6-week-old male nonobese diabetic (NOD)-SCIDs.
Tumor Xenografts
Single-cell suspensions of PC-3ML cells (1 × 106 cells) at <passage 10 were injected i.p. in 5- to 6-week-old male CB17-SCID mice (Taconic Labs, Pottstown, NY). When tumors reached a minimal volume of 0.15 to 0.18 mm3 (after ∼1 week), the drug was injected i.p. in a volume of 0.50 ml per mouse. CD44hi cells freshly isolated from PCa-20a cells were injected i.p. at 100 and 1000 cells/animal in a volume of 0.5 ml CKM in 6-week-old male NOD-SCID mice (Taconic Labs). CD44hi cells were allowed to form tumors for 1 month before treatment of mice with EGCG and Dox. Each group had 5 or 10 mice, and tumor volumes were determined according to the formula (L × l2)/2 by measurement of tumor length (L) and width (l) with a caliper. Mice were kept in a double barrier facility and handled according to an approved Institutional Animal Care Use Committee (IACUC) protocol in accordance with our institution’s policy.
Statistical Analysis
The results are presented as the mean ± 1 SD. Significant changes were assessed by Student’s t-test. A value of P < 0.05 was accepted as the level of significance.
Results
Synergistic Effects of EGCG and Dox on Cell Growth of Malignant PC-3ML Cells and Primary Prostate IBC-10a and PCa-20a Cell Lines
We postulated that EGCG may increase the sensitivity of prostate tumor lines to Dox. We found that EGCG at dosages >30 μm independently inhibited growth of malignant PC-3ML human prostate cells after 3 and 6 days (Figure 1A; (half maximal inhibitory concentration) IC50 = 20 μm). EGCG (ie, at 20 μm) combined with Dox (ie, 2 nm) inhibited growth in a synergistic manner after 3 and 6 days, respectively, P < 0.05 (Figure 1B). Growth was almost completely inhibited at concentrations of EGCG greater than 30 μm in the presence of Dox (2 nm). Low levels of Dox (1 and 2 nm) failed to inhibit growth of PC-3ML cells after 3, 6, and 9 days (data not shown).
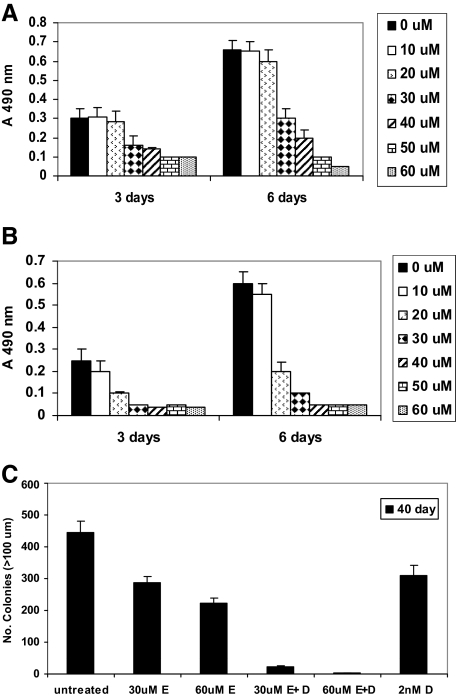
Figure 1.
MTS assays showing the effects of increased dosages of EGCG (0 to 60 μmol/L) on growth of PC-3ML cells in the absence (ie, solvent buffer) (A) and presence (B) of 2 nmol/L Dox. Differences in growth rates were significant (P < 0.05). Cells were plated at 5000 cells/well, and growth was measured after 3 and 6 days, respectively. C: Colony-forming assays with PC-3ML cells seeded for 40 days. The number of colonies (>100 μm dia) by untreated cells and cells exposed to 30 μmol/L EGCG (30 μmol/L E), 60 μmol/L EGCG (60 μmol/L E), 30 μmol/L EGCG plus 2 nmol/L Dox (30 μmol/L E+D), 60 μmol/L EGCG plus 2 nmol/L Dox (60 μmol/L E+D), and 2 nmol/L Dox alone (2 μmol/L D) is shown. Differences in colony forming assays were significant (P < 0.05). Cells were seeded at 5 × 103 cells/well in 12-well dishes for 10 days in complete Dulbecco’s modified Eagle’s medium plus 10% FBS, and cells were exposed to drug with a change of media every 5 days for an additional 30 days. Control wells were treated with solvent buffer alone.
We then examined if similar or lower dosages of drug were effective in blocking growth of primary prostate cell lines. We found that EGCG also inhibited growth of two different primary prostate cell lines, IBC-10a and PCa-20a cells, derived from human prostate Gleason score 6 and 7 tumors, respectively. For example, Figure S1A (see Supplemental Figure S1, A and B, at http://ajp.amjpathol.org) shows the effects of increased dosages of EGCG on growth of IBC-10a cells. The IC50 = 30 μm and growth was almost completely inhibited at >40 μm EGCG (P < 0.05; Figure S1A). For Dox, the IC50 = 5 nm (data not shown). Although EGCG and Dox had a minimal effect on growth at concentrations <20 μm EGCG or <2 nm Dox (data not shown), we found that 20 μm EGCG combined with 2 nm Dox almost completely inhibited growth after 1 to 7 days (P < 0.05; Figure S1B). In contrast, increased dosages of EGCG (0 to 60 μm for 9 days) failed to inhibit the growth of WI38 human fibroblasts (Figure S1B). Likewise 60 μm EGCG combined with 2 nm Dox did not reduce the growth rates of human WI38 fibroblasts derived from lung tissue.
Colony Forming Assays
Colony forming assays were carried out with PC-3ML cells. The data revealed that 30 and 60 μm EGCG reduced the colony forming ability of PC-3ML cells by ∼36% and ∼50%, respectively. In comparison, 30 and 60 μm EGCG in combination with 2 nm Dox completely inhibited colony formation (ie, by 30 to 40 days). Dox alone (ie, at 2 nm) partially reduced colony forming ability by ∼30% (Figure 1C). Likewise assays of colonies (ie, >50 μm dia) formed after 21 days revealed results similar to that observed at 40 days. Since IBC-10a and PCa-20a cells are nonmalignant and do not readily form colonies, colony forming assays were not performed on these cells. Taken together, the data indicate that EGCG combined with Dox inhibited tumor cell growth in vitro in a synergistic manner.
EGCG Reduces Mitochondrial Membrane Potential
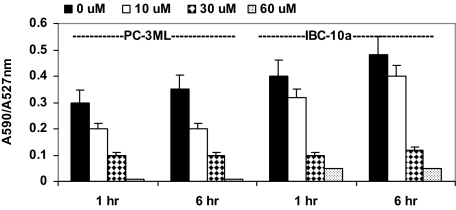
JC1 is a fluorescent dye that accumulates as a monomer in the cytoplasm (emission 527 nm normally visualized in the “green” channel) and as an aggregate in intact mitochondria (590 mm emission normally visualized in the “red” channel). With disruption of the mitochondrial membrane integrity, JC1 is released from the mitochondria and there is a net loss of red fluorescence resulting in a shift of the 590/527 ratio. Flow cytometry showed that when living PC-3ML cells were exposed to JC1 (ie, 10 μm for 30 minutes), the ratio of red/green fluorescent signal (ie, A590/A527 nm) was normally between 0.3 and 0.35 after 1 and 6 hours incubation, respectively. In PC-3ML cells treated with 10, 30, and 60 μm EGCG, there was a significant decrease in the A590/A527 ratios to ∼0.2, 0.1, and 0.05, respectively, after 1 or 6 hours treatment (Figure 2). Similar types of studies were then carried out in the primary cell lines. In IBC-10a cells treated with 10, 30, and 60 μm EGCG, the A590/A527 ratio decreased from ∼0.4–0.48 to ∼0.3–0.38, 0.1 and 0.05, respectively, after 1 and 6 hours (Figure 2). Similar decreases in the A590/A525 ratio were observed in cells exposed to increased dosages of EGCG (ie, 0 to 60 μm) plus 2 nm Dox for 1 hour. However, treatment of either cell line with Dox alone (1 and 2 nm for 6 hours) had no effect on A590/A527 ratio (data not shown), indicating Dox does not affect mitochondria membrane potential. Surprisingly, EGCG did not induce increases in ROS. That is, flow cytometry showed that 0 to 60 μm EGCG for 1 to 6 hours (plus or minus 2 nm Dox) did not induce expression of ROS (data not shown).
Figure 2.
Flow cytometry analysis of JC1 staining of live PC-3ML and IBC-10a cells. The ratio of A590/A527 nm fluorescence in cells labeled with 10 μmol/L JC1 is shown. Cells were treated with increased dosages of EGCG (0, 10, 30, and 60 μmol/L) for the 1-hour and 6-hour intervals, respectively. Controls were treated with solvent alone.
DiOC6(3) Staining
In comparative studies of PC-3ML and fibroblasts, fluorescent imaging of live cells incubated with DiOC6(3) (<0.1 μg/ml) revealed that the dye stained the mitochondrial networks (green) throughout the cytoplasm in PC-3ML cells and human WI38 fibroblasts (see Supplemental Figure S2, A–E, at http://ajp.amjpathol.org). Following treatment of PC-3ML cells with 60 μm EGCG (Figure S2B) or 60 μm EGCG plus 2 nm Dox (Figure S2C) for 1 hour, the mitochondria networks appeared vesiculated, indicating EGCG has a dramatic effect on mitochondrial integrity. Dox alone (ie, 2 nm Dox for 1 to 6 hours), also had some affect on the mitochondria networks, although they largely remained intact (Figure S2D). In comparison, EGCG and Dox (ie, 60 μm EGCG plus 2 nm Dox for 6 hours) had zero effect on the mitochondrial in human WI38 fibroblasts (Figure S2E).
Human Prostate Organ Culture Studies
We have extended the studies on cell lines to examine the effects of EGCG and Dox on growth of human prostate organ cultures established from tumors of increased Gleason score (GS6 to GS8). We have found that we can routinely establish epithelial (or fibroblast) cultures from minced pieces of human prostate tissue by ∼6 to 10 days. We found that growth of epithelial cultures growing out from GS6 to GS8 tumors (n = 5/GS) was completely inhibited by >30 μm EGCG and that the epithelial cells lifted off the culture dish after 2 to 3 days treatment. In comparison, fibroblast cultures growing out from the tumors were not affected by 30 to 60 μm EGCG in the absence or presence of 2 nm Dox for 3 days. Moreover, after treatment for 3 days and removal of the drugs, the fibroblasts continued to proliferate. DiO6(3) labeling revealed that treatment of the epithelial cultures with 20 to 30 μm EGCG for 1 hour induced vesiculation of the mitochondria in all of the cells (Figure S3A; see Supplemental Figure S3, A–D, at http://ajp.amjpathol.org). EGCG (30 μm for 24 hours) eventually caused the epithelial cells to round up and lift off the surface of the culture dishes. In contrast, EGCG (30 μm) alone or ECGC (30 μm) in combination with 2 nm Dox for 6 hours had no effect on the mitochondria in fibroblast cultures grown out from the tumor pieces (Figure S3B). Histological sections of the tumor pieces revealed that 50 μm EGCG for 24 hours eliminated the epithelial cells from glands of the tumor, but did not affect the basal cells or the stromal cells (compare Figure S3, C and D).
Apoptosis Assays on Primary and Malignant PC-3ML Cells
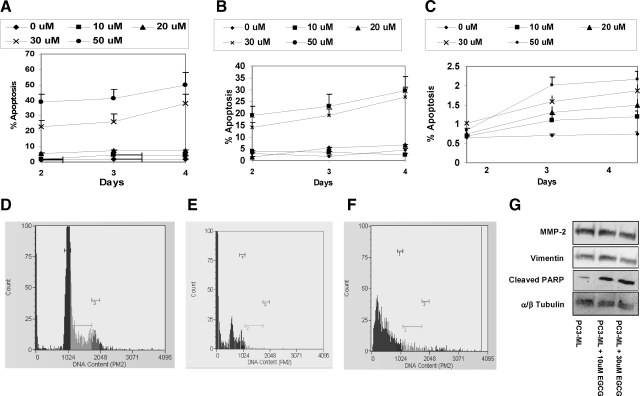
Flow cytometry assays using Annexin V antibodies showed that EGCG (0 to 50 μm) induced significant apoptosis in IBC-10a and PC-3ML cells (Figure 3, A and B). Low levels of EGCG (ie, <20 μm) induced low levels of apoptosis (ie, <5%) in both cell types after 2, 3, and 4 days treatment (Figure 3, A and B). However, EGCG at 30 and 50 μm levels induced significant apoptosis (ie, >15% to 50%) in both IBC-10a and PC-3ML cells, respectively, by 2, 3, and 4 days (Figure 3, A and B). Studies with WI38 fibroblasts showed that both untreated and EGCG treated cells exhibited low levels of apoptosis (<2%) after 2, 3, and 4 days (Figure 3C). Surprisingly, treatment of these three cell lines with increased dosages of EGCG (0 to 50 μm) in the presence of 2 nm Dox did not significantly increase the percent apoptosis after 2, 3, and 4 days, respectively (data not shown). Also, cells treated with Dox alone (at 2 nm for 2, 3, and 4 days) failed to exhibit a significant degree of apoptosis (ie, >2% to 5%).
Figure 3.
A–C: Percent apoptosis in IBC-10a (A), PC-3ML (B), and WI38 fibroblasts (C) treated with increased dosages of EGCG (0 to 50 μmol/L) for 2, 3, and 4 days, respectively. Annexin V (Guava) labeling. D–F: Flow cytometry histograms of propidium iodide-labeled IBC-10a cells that were treated for 24 hours with 2 nmol/L Dox (D); 30 μmol/L EGCG (E); and 30 μmol/L EGCG plus 2 nmol/L Dox (F). Control cells in A–F were treated with solvent buffer. G: Western blots showing the levels of MMP-2, vimentin, α/β tubulin, and PARP cleavage in PC-3ML cells treated with 0, 10, and 30 μmol/L EGCG for 24 hours.
Cell Cycle Analysis on Primary and Malignant PC-3ML Cells
Flow cytometry of propidium iodide labeled IBC-10a and PC-3ML cells showed that cells exposed to 30 μm EGCG and 30 μm EGCG plus 2 nm Dox for 24 hours exhibited a ∼100% decrease in cells in G2/M (ie, M3 <1%). Dox alone (2 nm for 24 hours) failed to reduce the percent cells in G2/M from that observed in untreated controls, which were treated with solvent (ie, M1: ∼29% to 35%; M2: ∼9% to 15%; and M3: ∼5% to 8% of the cells). Figure 3, D–F, shows, for example, histograms from IBC-10a cells (at ∼80% confluence), which were exposed for 24 hours to either 2 nm Dox, 30 μmol/L EGCG, or 30 μm EGCG plus 2 nm Dox (Figure 3, D–F, respectively).
PARP Cleavage Studies on Primary and Malignant PC-3ML Cells
Western blots with an antibody that specifically recognized the cleaved PARP fragment showed that 10 and 30 μm EGCG for 24 hours induced a significant increase (ie, ∼10 and 20-fold, respectively) in PARP cleavage compared with untreated cells (Figure 3D). Control blots with antibodies specific for MMP-2, vimentin and tubulin indicated there was no change in the levels of these proteins in the crude cell extracts (Figure 3G).
Taken together, the studies indicate that EGCG and not Dox is primarily responsible for the induction of apoptosis in primary prostate cells and malignant PC-3ML cells. Thus, the synergistic effects of EGCG and Dox on cell growth appear to arise from independent activities of the two drugs.
EGCG Increases Dox Retention by PC-3ML Cells
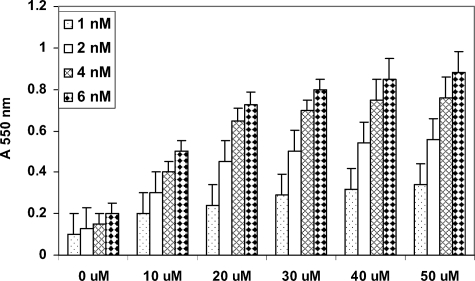
We postulate that EGCG might increase cellular retention of Dox to enhance the therapeutic efficacy of Dox. Flow cytometry measurements (absorbance 550 nm) of Dox retention by PC-3ML cells revealed that following treatment with increased dosages of EGCG (0 to 50 μm for 24 hours), there was a significant increase in Dox retention (ie, at EGCG concentrations >20 μm; Figure 4). In comparison, untreated PC-3ML cells retained relatively little Dox (Figure 4). Similarly, in studies of PC-3ML tumors in SCID mice, we found that EGCG promoted Dox retention by the tumors. That is, PC-3ML cells isolated from tumors exposed to EGCG plus Dox (ie, at 100 μm and 2 nm levels, respectively, for 45 days) retained Dox (A 550 nm = ∼0.65 ± 0.10), whereas cells from tumors treated with Dox alone (ie, 2 nm levels for 45 days) retained relatively little Dox (A 550 nm = ∼0.15 ± 0.05).
Figure 4.
The effect of increased concentrations of EGCG (0 to 50 μmol/L) pretreatment for 24 hours on the retention of increased dosages of Dox (1 to 6 μmol/L) retention (A550) by PC-3ML cells. Cells were plated at 5 × 105 cells/well for 24 hours. Then cells were treated with EGCG for 24 hours, exposed to Dox for 30 minutes, washed, and the amount of drug retention measured at A550. Measurements represent the mean ± 1 SD from two experiments using four wells per assay. The differences between treatments were significant (P < 0.005). Dox was prepared in the presence of 20 μl lipofectamine 2000. Control cells were treated with solvent buffer plus lipofectamine.
In similar types of experiments, we found that EGCG also increased Hoechst dye retention by PC-3ML cells in culture. Flow cytometry (A360 nm) showed that following treatment of PC-3ML cells with EGCG (ie, >40 μm for 7 hours) there was a >twofold increase in Hoechst 33342 dye retention compared with untreated cells (Figure S4; see Supplemental Figure S4 at http://ajp.amjpathol.org).
Mouse Tumor Modeling Studies with PC-3ML Cells
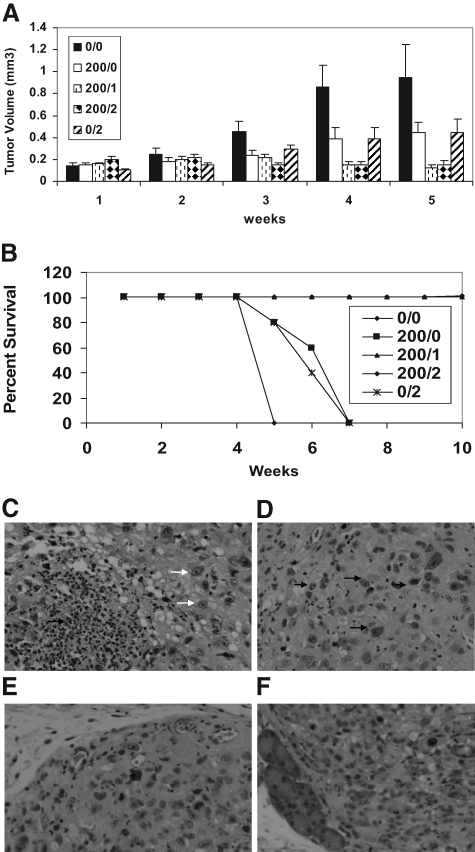
The goal was to assess whether EGCG alone or in combination with Dox could block the metastatic growth of PC-3ML tumor cells injected i.p. Figure 5A shows that mice injected with PC-3ML cells i.p. formed numerous tiny nodules of 0.18 to 0.20 mm3 volume after 1 week. The numbers of nodules and distribution were highly variable, but usually we found nodules on the liver, colon, kidney, and mesenteric tissues. In mice treated with vehicle, the tumors grew to >0.9 mm3 after 5 weeks and the mice died. Treatment of mice with EGCG alone (ie, 200 μm/0 μm) or Dox alone (ie, 0 μm/2 μm) reduced tumor size significantly to <0.6 mm3 after ∼3 to 5 weeks, and the mice survival rates were 100%. The treatment of mice with micromolar levels of EGCG and Dox combined (ie, at micromolar ratios of EGCG/Dox of 200/2 and 200/1) blocked tumor growth and reduced the size of the existing tumors (ie, to <0.1 mm3) after 5 weeks (n = 5 mice/treatment). In addition, the combination drug treatments with EGCG and Dox reduced the number of lesions from ∼30 nodules/mouse to ∼two to three nodules per mouse (Figure 5A).
Figure 5.
Effects of EGCG and Dox on tumor growth in CB17-SCIDs after 1 to 5 weeks (A). PC-3ML cells were injected i.p. at 1 × 105 cells. After 1 week, mice were treated with EGCG and Dox at micromolar concentrations of EGCG/Dox of 0/0, 200/0, 200/1, 200/2, and 0/2, respectively; P < 0.05. Mice were treated every 2 days by injection of drug i.p. in 1 ml PBS (n = 5 mice/treatment). B: Effects of EGCG and Dox on mouse survival rates after 10 weeks as described in A. C–F: H&E-stained thin sections of tumors from mice treated for 45 days with EGCG 200 μmol/L (C); EGCG/Dox (200 μmol/L/2 μmol/L) (D); Dox-2 μmol/L (E); and untreated (ie, solvent buffer) (F) PC-3ML tumors. Note: The EGCG and EGCG/Dox-treated tumor cells were more amorphous in appearance and contained numerous irregular shaped nuclei, transparent nuclei, and pycnotic nuclei (white and black arrows). Original magnification, ×200.
In a separate set of experiments, we monitored mouse survival rates over prolonged intervals of ∼10 weeks. All of the untreated mice died after 5 weeks (Figure 5B). Mice treated with EGCG alone or Dox alone died by ∼5 to 8 weeks. However, the survival rates were increased to ∼100% in mice treated with a combination of EGCG/Dox of 200/1 and 200/2 for 9 weeks (Figure 5B). We also examined tumor recurrence rates in mice treated with EGCG/Dox (200/1) for 9 weeks (n = 10 mice), followed by discontinuation of the treatment for an additional 12 weeks. Although all of the mice survived in the latter experiments, we found tumor recurrence in 30% of the mice (n = 3/10). Tiny tumor nodules (∼0.5 mm dia) were found on the colon and mesenchymal tissues in these mice, and we expect these mice would eventually succumb to cancer.
Histology of H&E stained sections revealed that the EGCG (200 μm) and EGCG/Dox (200 μm/2 μm) treated tumors were highly necrotic (Figure 5, C and D) compared with the Dox (2 μm) treated tumors (Figure 5E) or control tumors (Figure 5F), which were uniform solid masses of cells. No differences in the overall vascularization or numbers of blood vessels were apparent in the EGCG or EGCG/Dox treated tumors, albeit the tumors tended to be bloodied, suggesting vascular leakage had occurred. Interestingly, the nuclei of EGCG and EGCG/Dox treated tumors were highly irregular in appearance ranging from pycnotic to vacuous looking (Figure 5, C and D, arrows). In comparison, the nuclei of untreated and Dox treated tumors were uniform in appearance and density (Figure 5, E and F).
Tumor Modeling Studies with CD44hi Tumor Initiating Cells Isolated from PCa-20a Cells
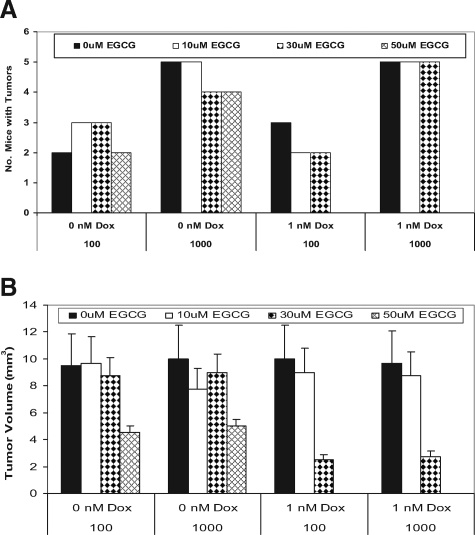
Several groups have shown that CD44hi cells may constitute a tumor initiating cell with higher malignant potential than the CD44lo subpopulations.18,19 We have found that the PCa-20a parent cells injected s.c. (1 × 106 cells/animal), and CD44hi subpopulations of PCa-20a cells (ie, 1000 cells injected s.c.) can form tumors in NOD-SCIDs after ∼3 months (data not shown). To test whether EGCG in combination with Dox can block growth of CD44hi cells, we have isolated CD44hi subpopulations from PCa-20a cells for tumor modeling studies in NOD-SCIDs. We found that following injection i.p. at 100 and 1000 cells/animal, one to two tumors per mouse formed (ie, in 2/5 and 5/5, respectively, of the mice) after ∼2 months (data not shown). To test if EGCG plus Dox could eradicate these tumors, mice inoculated with CD44hi cells for 2 months were subsequently treated with increased EGCG concentrations of 10, 30, and 50 μm biweekly for 2 months before sacrifice. The data showed that EGCG alone (ie, even at high concentrations of 50 μm or 57 mg/kg) had zero effect on the total number of tumors (Figure 6A). Likewise, 1 μm Dox plus 10 to 30 μm EGCG combined with 1 μm Dox did not reduce the number of tumors per animal. However, 50 μm EGCG in combination with 1 μm Dox eliminated tumors in mice injected with either 100 or 1000 cells per animal (Figure 6A). In follow up experiments, mice (n = 5) were inoculated with 1000 cells per animal and treated with 50 μm EGCG plus 1 μm Dox for 2 months. We then discontinued treatment for 3 months and found that these mice remained healthy and free of tumor burden.
Figure 6.
Effects of EGCG and Dox on tumor growth in NOD-SCIDs. CD44hi subpopulations freshly isolated from PCa-20a cells were injected i.p. at increased numbers of 100 and 1000 cells/animal. After 2 months, mice were treated with EGCG, Dox, and EGCG plus Dox. EGCG was used at 10, 30, and 50 μmol/L EGCG. Dox was used at 1 μmol/L. Mice were dosed biweekly by i.p. injection of drug in 0.5 ml PBS (n = 5 mice/treatment) for 2 months. A: Number of mice with tumors. B: Measurements of tumor volume (mm3).
In the experiments described in Figure 6A, tumors were measured with calipers after sacrifice of the animals. We found that the tumors averaged ∼10 mm3 in untreated mice and mice treated with EGCG at 10 and 30 μm, and the size was independent of whether mice were injected with 100 or 1000 cells/animal (Figure 6B). In mice treated with 50 μm EGCG alone, the tumors were reduced in size to ∼4.5 mm3. In mice treated with 30 μm EGCG plus 1 μm Dox, the tumors were also reduced in size to ∼2.5 mm3. Histology revealed that large areas of necrosis arose in response to the drug. Finally, in mice treated 50 μm EGCG plus 1 μm Dox, tumors were completely eliminated. In additional experiments, we treated the tumor bearing mice (ie, inoculated with 1000 CD44hi for 2 months) with 50 μm EGCG plus 1 μm Dox for 2 months and then discontinued treatment for 4 months. Tumors did not reappear in any of these mice (n = 10). The data suggest that EGCG in combination with Dox can eradicate established tumors derived from CD44hi tumor initiating cells.
Discussion
For the first time, we have shown that localized delivery of relatively high dosages of EGCG (228 mg/kg or 200 μmol/L) in combination with very low dosages of Dox (<0.14 mg/kg or 2 μm) can either eradicate or significantly reduce tumor size of highly aggressive PC-3ML tumors in CB17-SCID mice. More importantly, EGCG in combination with Dox increased mouse survival rates from 0% to 100% with a significantly reduced incidence of tumor recurrence in long-term survival studies. We believe that the drug therapy was successful due to the fact that EGCG promoted Dox retention by the tumor cells. In addition, the use of low levels of Dox allowed frequent localized delivery of the drugs, which, thereby, increased drug bioavailability and uptake by existing tumor cells and newly arising tumor cells. Since the tumors were relatively small (<0.2 mm3) at the time the treatments were initiated, it was possible to ensure tumor cell uptake of the drugs and eradicate the tumors. Mechanistically, EGCG appeared to induce apoptosis by disrupting mitochondrial membrane integrity. In addition, EGCG enabled increased Dox retention by the PC-3ML tumor cells to increase the therapeutic efficacy of Dox. In the experiments where the PC-3ML tumors were allowed to grow to ∼1 cm dia before onset of treatment, EGCG alone or in combination with low dosages of Dox blocked tumor growth, induced extensive tumor necrosis, and prevented metastases. However, under these conditions the tumors did not reduce in size, perhaps due to an inability of the drugs to penetrate the tumor mass. It may turn out that localized delivery of high dosages of EGCG in combination with much higher dosages of Dox (ie, 10 μm levels) are required to eradicate the larger sized tumors. Similar types of studies with CD44hi tumor initiating cells isolated from PCa-20a cells further revealed that relatively low dosages of EGCG (ie, 50 μm or 57 mg/kg) in combination with subtherapeutic dosages of Dox (1 μm) blocked growth and led to eradication of established tumors (ie, tumors′ 10 mm dia). Overall, it appeared that these tumors were more sensitive to the combination drug therapy than that observed with PC-3ML tumors.
The in vitro studies provided novel insights as to the possible mechanism of action of EGCG. The DiO6(3) staining studies clearly indicated that EGCG targets the mitochondria. That is, EGCG at dosages >30 μm rapidly penetrated the epithelial cells within ∼60 minutes to induce vesiculation of the mitochondria. Flow cytometry studies with the lipophilic cationic dye 5,5,6,6-tetrachloro-1,1,tetraethylbenzimidazolocarbo-cyanine iodide (JC-1) provided quantitative evidence that EGCG disrupted the mitochondrial membrane potential. In response to EGCG (30 μm for 60 minutes), there was a loss of orange fluorescence staining of the mitochondria as a result of a decrease in mitochondrial membrane potential and the JC-1 dye leaking into the cytoplasm to form a monomer that fluoresces green. Loss of mitochondrial ΔΨ was associated with the subsequent onset of apoptosis. Under these conditions EGCG induced apoptosis. We found that PARP cleavage and Annexin V expression both increased significantly in response to EGCG. Furthermore, flow cytometry analysis of propidium iodide stained cells showed that there was a dramatic reduction in cells in G2/M. The dramatic rise in apoptosis rates corresponded with a significant reduction in growth rates and colony forming abilities of the cells. The growth assays showed that >30 μm EGCG reduced both PC-3ML and IBC-10a growth >70% after 3 and 6 days. Taken together, the data strongly suggest that EGCG is an effective inhibitor of prostate cell survival and growth.
More importantly, we found that EGCG combined with low levels of Dox had a synergistic effect in blocking growth and colony forming ability of both premalignant and highly malignant tumor cells in vitro. EGCG appeared to block the export of Dox from PC-3ML and IBC-10a cells to effectively increase Dox retention. Thus, EGCG appeared to thereby increase the therapeutic activity of relatively nontoxic levels of Dox. This effect combined with EGCG induced apoptosis appeared to significantly compromise tumor cell growth and survival. It would appear that EGCG is not taken up by fibroblasts since EGCG treated IBC-10a or PC-3ML cell pellets were brown, whereas the fibroblast cell pellets were translucent (data not shown).
Green tea has previously been reported to have useful antioxidative effects and to inhibit carcinogenesis.1,2,20,21,22,23,24,25,26 These effects of green tea are due to components of green tea such as the catechin group, the vitamin group, caffeine, and theanine.1,2,20,21,22,23,24,25,26 Interestingly, there have also been several reports showing green tea components in combination with Dox can block tumor growth. Sadzuka et al27,28 found that theanine and caffeine inhibited Dox efflux from Ehrlich ascites tumor cells and that theanine rendered Dox resistant Ehrlich ascites tumors sensitive to Dox and increased Dox suppression of metastasis. They suggested that flavonoids enhanced the Dox induced antitumor activity and increase the Dox concentration in tumors through the inhibition of Dox efflux.27,28 For example, green tea in combination with adriamycin or Dox inhibited growth of M5076 ovarian sarcoma cells, whereas adriamycin and Dox alone failed to inhibit tumor growth. Green tea extracts in combination with Dox also have been found to enhance the inhibitory effect of Dox on Ehrlich ascites tumors in mice.28 Likewise, EGCG was found enhance the apoptotic effect of sulindac and tamoxifen in tumor cells.29 Our results support these studies and clearly indicate that EGCG can enhance Dox retention by prostate tumor cells and increase the anti-tumorigenic and anti-metastatic activities of Dox in vivo. Our data further show that using high concentrations of EGCG with low dosages of Dox is an ideal therapeutic strategy that allows for frequent treatments and more efficient eradication of tumors. Note that similar anti-tumorigenic results were observed by using EGCG (100 μM) in combination with low levels of mitoxantrone (ie, 1 μm) to treat metastatic PC-3ML tumors injected i.p. in SCID mice (unpublished data).
Unfortunately, the short-half life of EGCG is a limitation and it would be difficult to attain such high dosages of EGCG in patients. However, recent studies with the prodrug Peracetate-EGCG have shown it is very stable29 and is readily taken up by tumor cells and converted to EGCG.30 It may, therefore, be feasible to achieve therapeutic dosages at relatively low dosages of Peracetate-EGCG, which could be achievable in clinical studies.
EGCG plus Dox eradicate tumors derived from CD44hi tumor initiating cells. In the past 10 years, there has been a concerted effort to identify adult prostate stem cells by using putative prostate stem cell markers, including ABCG2, α2β1 integrin, CD117 (c-Kit), CD44, CD133, Oct-3/4, cytokeratin 6a, p63, and Sca-1.31,32,33,34,35,36,37,38,39 Using the above markers, various groups have reported efficient isolation of putative adult stem cells from the prostate; however, only a few groups have demonstrated true stem cell properties by using in vivo transplantation assays to generate structures that recapitulate the glandular architecture of the prostate. Isolated CD44hi cells from human DU145 cells have been found to display increased tumorigenic and invasive potential (as compared with the CD44lo counterparts), and these cells display higher levels of Shh and Gli-2.18 Wei et al40 further demonstrated that a CD133+/CD44+/α2β1hi subpopulation of DU145 prostate cancer cells exhibited greater tumorigenic capacity both in vitro and in vivo than their negative counterparts. Our data showed CD44hi subpopulations formed tumors in NOD-SCIDs, and we have termed these cells tumor initiating cells since there is little evidence they are stem cells per se. Like other labs we found that CD44lo cells failed to form tumors unless injected at very high titers of >10,000 cells/animal (data not shown). In experiments with the tumors established from CD44hi cells, we found that EGCG (50 μm or 57 mg/kg) could significantly reduce tumor volume, but lower titers of EGCG had little or no effect on the tumor cells or the tumor size. More importantly, 30 μm EGCG plus 1 μm Dox reduced tumor volume significantly, and 50 μm EGCG in combination with 1 μm Dox completely eliminated established tumors. In the latter study, we found that after discontinuation of therapy, mice survived disease-free for 3 months when they were sacrificed. The data suggest that EGCG plus Dox might prevent tumor progression in early stage tumors and eradicate CD44hi tumor initiating cells.
Overall, the data show that EGCG is an ideal therapeutic agent with which to sensitize tumors to toxic drugs like Dox or mitoxantrone. EGCG has minimal toxic side effects as it does not appear to accumulate in normal cells and tissue or to hamper their survival. For example, the drug did not reduce fibroblast growth or induce apoptosis in fibroblasts or basal cells in organ culture. Neither did the high dosages given to mice appear to have any toxic side effects (ie, hair loss, neuropathy, weight loss, and tissue damage). More importantly, EGCG readily accumulated in tumor cells (and tumors) and functioned to not only reduce tumor cell viability, but also to block expression of genes essential for invasion and metastases (ie, MMP-2/9; data not shown). Basically, EGCG appeared to interfere with membrane transport processes, thereby blocking the export of Dox. With prolonged treatment there was a high rate of apoptosis (ie, >40%), possibly due to EGCG induced impairment of mitochondrial integrity and function.
In conclusion, based on the preclinical mouse tumor modeling studies presented here, we believe that EGCG might be effectively used at high dosages delivered locally to increase the efficacy of Dox in eradicating highly aggressive, metastatic tumors or primary tumors arising from tumor initiating cells. One primary benefit is that the drugs can be given frequently and Dox can be used at nontoxic levels 10-fold below what is normally required to block tumor growth or tumor metastases.
Acknowledgments
We thank Dr. Fernando U. Garcia for his help in obtaining tumor tissue for the organ cultures.
Footnotes
Address reprint requests to Mark E. Stearns, Drexel University College of Medicine, Dept. of Pathology, MS 435, 245 N. Broad St., Philadelphia, PA, 19102. E-mail: stearnsm1@aol.com.
Supported by NIH-NCI-CA076639-7 to M.E.S.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Kelloff GJ, Crowell JA, Hawk ET, Steele VE, Lubet RA, Boone CW, Covey JM, Doody LA, Omenn GS, Greenwald P, Hong WK, Parkinson DR, Bagheri D, Baxter GT, Blunden M, Doeltz MK, Eisenhauer KM, Johnson K, Knapp GG, Longfellow DG, Malone WF, Nayfield SG, Seifried HE, Swall LM, Sigman CC. Clinical development plan: tea extracts, green tea polyphenols, and epigallocatechin gallate. J Cell Biochem Suppl. 1996;26:236–257. [PubMed] [Google Scholar]
- Bushman JL. Green tea and cancer in humans: a review of the literature. Nutr Cancer. 1998;31:151–159. doi: 10.1080/01635589809514697. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Ahn WS, Yoo J, Huh SW, Kim CK, Lee JM, Namkoong SE, Bae SM, Lee IP. Protective effects of green tea extracts (Polyphenon E and EGCG) on human cervical lesions. Eur J Cancer Prev. 2003;12:383–390. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]
- Nelson WG. Agents in development for prostate cancer prevention. Expert Opin Invest Drugs. 2004;13:1541–1554. doi: 10.1517/13543784.13.12.1541. [DOI] [PubMed] [Google Scholar]
- Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int J Cancer. 2004;108:130–135. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA. 2001;98:10350–10355. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttie A, Nyska A, Haseman JK, Moser GJ, Hackett TR, Goldsworthy TL. A grading scheme for the assessment of proliferative lesions of the mouse prostate in the TRAMP model. Toxicol Pathol. 2003;31:31–38. doi: 10.1080/01926230390173842. [DOI] [PubMed] [Google Scholar]
- Shannon R, Kinney M, Zhang W, Pascual M, Greally JM, Gillard BM, Karasik E, Foster BA, Karpf AR. Lack of evidence for green tea polyphenols as DNA methylation inhibitors in murine prostate. Cancer Prevention Research. 2009;2:1065–1072. doi: 10.1158/1940-6207.CAPR-09-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K, Miyazawa T. Chemiluminescence–high-performance liquid chromatographic determination of tea catechin, (-)-epigallocatechin 3-gallate, at picomole levels in rat and human plasma. Anal Biochem. 1997;248:41–49. doi: 10.1006/abio.1997.2098. [DOI] [PubMed] [Google Scholar]
- Miki J, Furusato B, Li H, Gu Y, Takahashi H, Egawa S, Sesterhenn IA, McLeod DG, Srivastava S, Rhim JS. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007;67:3153–3161. doi: 10.1158/0008-5472.CAN-06-4429. [DOI] [PubMed] [Google Scholar]
- Goodyear SM, Amatangelo MD, Stearns ME. Dysplasia of human prostate CD133hi SPs in NOD-SCIDs is blocked by c-myc anti-sense. The Prostate. 2009;69:689–698. doi: 10.1002/pros.20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear SM, Amatangelo MD, Stearns ME. Role of tumor initiating cells in prostate cancer. Trends in Cancer Res. 2009;5:71–88. [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Woodbury, New York: Cold Spring Harbor Laboratory Press,; Molecular CloningA Laboratory Manual, (3rd ed.) 2003:1:2.82–2.108. [Google Scholar]
- Stearns ME, Jason B, Zhang H, Francis MK. Sell, C Insulin-like growth factor 1 (IGF-1) induction of vascular endothelial growth factor (VEGF) is dependent upon activated. Ras Cancer Res. 2005;65:2085–2088. doi: 10.1158/0008-5472.CAN-04-4100. [DOI] [PubMed] [Google Scholar]
- Sharma V, Joseph C, Ghosh S, Agarwal A, Mishra MK, Sen E. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol Cancer Ther. 2007;6:2544–2553. doi: 10.1158/1535-7163.MCT-06-0788. [DOI] [PubMed] [Google Scholar]
- Klarmann GJ, Hurt EM, Mathews LA, Zhang XH, Duhagon MA, Mistree T, Thomas SB, Farrar WL. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin Expl Metastsis. 2009;26:433–446. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear SM, Kheyfets SB, Garcia FU, Stearns ME. Role of the VEGFR3/VEGFD receptor axis in TGFβ1 activation of primary prostate cell lines. The Prostate. 2009;69:982–990. doi: 10.1002/pros.20945. [DOI] [PubMed] [Google Scholar]
- Mukhtar T, Wang Z, Katiyer SK, Agarwal R. Tea components: antimutagenic and anticarcinogenic effects. Prev Med. 1992;21:351–360. doi: 10.1016/0091-7435(92)90042-g. [DOI] [PubMed] [Google Scholar]
- Stearns ME, Wang M, Stearns M. IL-10 blocks collagen IV invasion by “invasion stimulating factor” activated PC-3 ML cells: upregulation of TIMP-1 expression. Oncol Res. 1995;7:157–163. [PubMed] [Google Scholar]
- Katiyar SK, Agarwal R, Mukhtar H. Inhibition of spontaneous and photo-enhanced lipid peroxidation in mouse epidermal microsomes by epicatechin derivatives from green tea. Cancer Lett. 1994;79:61–66. doi: 10.1016/0304-3835(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Nishida H, Omori M, Fukutomi Y, Ninomiya M, Nishiwaki S, Suganuma M, Moriwaki H, Muto Y. Inhibitory effects of (-)-epigallocatechin gallate on spontaneous hepatoma in C3H/HeNCrj mice and human hepatoma-derived PLC/PRF/5 cells. Jpn J Cancer Res. 1994;85:221–225. doi: 10.1111/j.1349-7006.1994.tb02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagesaka-Mitane Y, Sugiura T, Miwa Y, Yamaguchi K, Kyuki K. Effect of tea-leaf saponin on blood pressure of spontaneously hypertensive rats. Yakugaku Zasshi. 1996;116:388–395. doi: 10.1248/yakushi1947.116.5_388. [DOI] [PubMed] [Google Scholar]
- Sano M, Takahashi Y, Yoshino K, Shimoi K, Nakamura Y, Tomita I, Oguni I, Konomoto H. Effect of tea (Camellia sinensis L.) on lipid peroxidation in rat liver and kidney: a comparison of green and black tea feeding. Biol Pharm Bull. 1995;18:1006–1008. doi: 10.1248/bpb.18.1006. [DOI] [PubMed] [Google Scholar]
- Nagata T. Studies on useful components of tea in leaves of the Genus Camellia. Bull Nat Res Inst Tea. 1986;21:60–120. [Google Scholar]
- Sadzuka Y, Sugiyama T, Sonobe T. Efficacies of tea components on Dox induced antitumor activity and reversal of multidrug resistance. Toxicology Letters. 2000;114:155–162. doi: 10.1016/s0378-4274(99)00290-8. [DOI] [PubMed] [Google Scholar]
- Sadzuka Y, Sugiyama T, Hirota S. Modulation of cancer chemotherapy by green tea. Clin Cancer Res. 1998;4:153–156. [PubMed] [Google Scholar]
- Suganuma M, Okabe S, Kai Y, Sueoka N, Sueoka E, Fujiki H. Synergistic effects of (-)-epigallocatechin gallate with (-)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Res. 1999;59:44–47. [PubMed] [Google Scholar]
- Landis-Piwowar KR, Huo C, Chen D, Milacic V, Shi G, Chan TH, Dou QP. A novel PEGCG of the green tea polyphenol (-)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007;67:4303–4310. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- Collins AT, Habib FK, Maitland NJ, Neal DE. Identification and isolation of human prostate epithelial stem cells based on α2β1-integrin expression. J Cell Sci. 2001;114:3865–3872. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Moll R, Hesse U, Prasad AR, Gandolfi JA, Hasan SR, Bartholdi M, Cress AE. Identification of a stem cell candidate in the normal human prostate gland. Eur J Cell Biol. 2005;84:341–354. doi: 10.1016/j.ejcb.2004.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon F. p63 and the epithelial stem cell: more than status quo? Genes Development. 2004;18:465–469. doi: 10.1101/gad.1190504. [DOI] [PubMed] [Google Scholar]
- Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- Sotomayor P, Godoy A, Smith GJ, Huss WJ. Oct4A is expressed by a subpopulation of prostate neuroendocrine cells. Prostate. 2009;69:401–410. doi: 10.1002/pros.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Griend V, Karthaus DJ, Dalrymple WLS, Meeker A, DeMarzo AM, Isaacs JT. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008;68:9703–9711. doi: 10.1158/0008-5472.CAN-08-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci USA. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Sadzuka Y. Enhancing effects of green tea components on the antitumor activity of adriamycin against M5076 ovarian sarcoma. Cancer Lett. 1998;133:19–26. doi: 10.1016/s0304-3835(98)00185-2. [DOI] [PubMed] [Google Scholar]
- Wei C, Guomin W, Yujun L, Ruizhe Q. Cancer stem-like cells in human prostate carcinoma cells DU145: the seeds of the cell line? Cancer Biol Ther. 2007;6:763–768. doi: 10.4161/cbt.6.5.3996. [DOI] [PubMed] [Google Scholar]