Abstract
Few rodent models of human immunodeficiency virus type one (HIV-1) infection can reflect the course of viral infection in humans. To this end, we investigated the relationships between progressive HIV-1 infection, immune compromise, and neuroinflammatory responses in NOD/scid-IL-2Rγcnull mice reconstituted with human hematopoietic CD34+ stem cells. Human blood-borne macrophages repopulated the meninges and perivascular spaces of chimeric animals. Viral infection in lymphoid tissue led to the accelerated entry of human cells into the brain, marked neuroinflammation, and HIV-1 replication in human mononuclear phagocytes. A meningitis and less commonly an encephalitis followed cM-T807 antibody-mediated CD8+ cell depletion. We conclude that HIV-1–infected NOD/scid-IL-2Rγcnull humanized mice can, at least in part, recapitulate lentiviral neuropathobiology. This model of neuroAIDS reflects the virological, immunological, and early disease-associated neuropathological components of human disease.
Reconstitution of mice with human cells susceptible to HIV-1 is an attractive approach to address the basic pathobiological mechanisms of viral infection and for the screening of therapeutic modalities. Application of mice injected intracranially with HIV-1 infected human monocyte-derived macrophages (MDM) alone or in combination with peripheral blood lymphocytes (PBL) reconstitution for the study of neuroAIDS is well established.1,2,3,4,5,6 However, traumatic injury to the brain from injection, PBL activation by the mouse environment and developed graft-versus host reactivity are significant disadvantages of these models.
Long-term functional engraftment of a human immune system was achieved in immune deficient mice reconstituted with human hematopoietic stem cells (HSC) in divergent genetic backgrounds: NOD/scid-IL-2Rγcnull (NSG),7,8,9,10 BALB/c-Rag2−/−γc−/− (BRG),11,12,13,14,15 and NOD/scid mice reconstituted with fetal thymus/liver and HSC (BLT).16,17 Despite rapid research advances made in these rodent animal models on HIV-1 disease pathobiology, their importance for studies of HIV-1–related complications, including neuroAIDS, have not been explored.
Central nervous system (CNS) inflammatory responses are driven by chronic low-level infection affecting activation of brain mononuclear phagocytes (MP; blood borne macrophage and microglia).18,19,20,21,22 HIV-1–associated neurocognitive disorders (HAND), in particular, are a common cause of morbidity for virus-infected people.23 However, human disease can be paralleled only in studies of simian immunodeficiency virus (SIV)-infected rhesus macaques.24,25 Moreover, only the later stages of disease have been studied in detail where encephalitis is seen as a result of virus-infected MP and MP-derived multinucleated giant cells (MGC), astrogliosis, myelin pallor, and neuronal dropout predominate.26,27 Little is known about the early stages of disease after acute infection. Here the pathobiological findings include aseptic meningitis. This can consist of inflammatory T-cell reaction with overt vasculitis and leptomeningitis. Immune activation of brain parenchyma with increased number of microglial cells, up-regulation of major histocompatibility complex class II antigens, and local production of cytokines was described.28,29 In both acute and chronic infection phases, CD8+ cytotoxic T lymphocytes (CTL) play a dual role: control viral replication and modulate neurological disease.30,31,32,33,34,35,36,37,38 In HIV-1–infected patients and SIV-infected monkeys, virus-induced innate and adaptive immune responses trigger activation of oxidative stress and lead to neuronal injury. These include secretion of viral proteins, proinflammatory cytokines, platelet-activating factor, arachidonic acid metabolites, quinolinic acid, and activation of inducible nitric oxide synthase (iNOS).2 iNOS produced in an oxidative environment with excessive production of NO leads to peroxynitrite formation and cell toxicity.39,40,41,42,43 However, the pathobiology of the early stage HAND remains unclear because investigations are not readily done in infected patients.
Here, we investigated whether CNS pathologies could be seen in HIV-1–infected humanized mice. Several notable observations were made to human cells repopulation, progressive HIV-1 infection, and CD8+ cell depletion. First, brains were repopulated with human CD163+, CD14+ macrophages, predominantly located in meninges and perivascular spaces. Second, productive infection accelerated the entry of human cells into the brain. This was seen by immunostaining for human CD163 and HLA-DR+ cells and the expression of human HLA-DQ by real-time PCR. HIV-1 p24+ cells with macrophage and lymphocyte morphology were seen in the meninges and perivascular spaces. Third, CD8+ T cell depletion initiated by cM-T807 antibodies resulted in increased HIV-1gag RNA and iNOS expression in the brain. Fourth, the development of a meningitis and rarely a meningoencephalitis were observed. These findings, taken together, demonstrate a natural progression of HIV-1 CNS disease in rodents in a fashion qualitatively similar to SIV-infected macaques. This is the first study, to our knowledge, showing relationships between ongoing viral replication and CNS HIV-1 pathobiology in mice permanently reconstituted with a human immune system.
Materials and Methods
Animals
NOD/scid-IL-2Rγcnull mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and were bred under specific pathogen-free conditions in accordance with ethical guidelines for care of laboratory animals at the University of Nebraska Medical Center (UNMC) as set forth by the National Institutes of Health.
CD34+ Cell Isolation and Transplantation
Human umbilical cord blood was obtained with parental written informed consent from healthy full-term newborns (Department of Gynecology and Obstetrics, UNMC). After density gradient centrifugation, CD34+ cells were enriched using immunomagnetic beads according to the manufacturer’s instructions (CD34+ selection kit; Miltenyi Biotec Inc., Auburn, CA). Purity of CD34+ cells was evaluated by flowcytometry and was >90%. Cells were either frozen or immediately transplanted into newborn mice irradiated at 1 Gy using a C9 cobalt 60 source (Picker Corporation, Cleveland, OH). The number of animal deaths was as follows: i) acute death during first 7 days after sublethal irradiation of newborn pups by 1 Gy <1%; ii) death during 1–2 months postirradiation <1%. CD34+ cells were injected intrahepatically (i.h.) at 105 cells per mouse in 20 μl phosphate-buffered saline (PBS) using a 30-gauge needle. Newborns received cells from single donors. Two to seven littermates were reconstituted with cells isolated from cord blood sample derived from one donor. The number of animals reconstituted was dependent on the number of CD34+ cells isolated from cord blood. Mice were weaned at 3 weeks of age. Mice were then evenly distributed between different experimental groups (Table 1). Animals that developed sign of chronic graft-versus host disease from 5 to 6 months of age (such as hair loss, loss of weight was observed in ∼5%) were sacrificed and were not included in the analysis.
Table 1.
Profiles of HIV-1–Infected and CD8+ Cell–Depleted Animals Used in This Study*
| Experimental Groups | Number of Animals | Age, Weeks | Human CD45+ Cells, % | Viral Load, log10 RNA Copies/ml |
|---|---|---|---|---|
| Control† | 18 | 25 (21–32) | 25.8 (3.8–50.5) | NA |
| HIV-1–infected‡ | 22 | 29 (26–36) | 28.7 (6.0–50.2) | 5.06 (4.12–6.18) |
| HIV-1–infected/CD8+ cell depleted§ | 12 | 29 (26–32) | 20.2 (3.1–52.6) | 5.58 (4.39–7.54) |
| CD8+ cell–depleted | 7 | 31 (26–35) | 16.5 (3.1–80.6) | NA |
All rodent brains were evaluated by immunohistochemistry.
Control, uninfected reconstituted mice.
Productive viral infection was assessed by the presence of viral RNA in peripheral blood, HIV-1p24+ cells in lymphoid tissues or HIV-1gag mRNA in brain tissue.
Animals were CD8+ cell depleted at 2 weeks (n = 5) or 5–7 weeks after infection. Median and the range are shown in parentheses.
Viral Stocks
The CCR5 coreceptor-using HIV-1ADA strain was propagated in human MDM. Monocytes were isolated from leukopaks and generating MDM.44 Viral preparations were screened and found to be negative for endotoxin (<10 pg/ml) (Associates of Cape Cod, Woods Hole, MA) and mycoplasma (Gen-Probe II; Gen-Probe, San Diego, CA). The viral titers were assayed on MDM and 105 tissue culture infectious dose50 (TCID50)/ml.
HIV-1 Infection
HIV-1ADA was injected intraperitoneally (i.p.) at 104 TCID50. The levels of viral RNA copies/ml were analyzed by automated COBAS Amplicor System (Roche Molecular Diagnostics, Basel, Switzerland). For assay use, mouse plasma samples (20 μl each) were diluted to 700 μl with normal human serum which increased the detection limit to 1750 viral RNA copies/ml. HIV-1 infection was confirmed by virologic and histological examination in 26 animals (Table 1). Eighteen reconstituted animals not exposed to HIV-1 served as controls. No mortalities were induced by HIV-1 infection.
CD8+ T Cell Depletion
The cM-T807 mAb was obtained from the National Institutes of Health/National Center for Research Resources. Each mouse received 10 mg/kg of cM-T807 subcutaneously (s.c.) and 5 mg/kg i.p. at 3 day intervals.
Flow Cytometry
Peripheral blood samples were collected from the submandibular vein in EDTA-coated tubes by using lancets (MEDIpoint, Inc., Mineola, NY) or by cardiocentesis at the end of observation. Blood leukocytes and cells suspensions from half of spleen were tested for human pan-CD45, CD3, CD4, CD8, CD11c, CD14, CD19, and HLA-DR markers as seven-color combinations. Antibodies and isotype controls were obtained from BD PharMingen (San Diego, CA), and staining was analyzed with a FACSDiva (BD Immunocytometry Systems, Mountain View, CA). Presence of CD8+ cells before and after depletion was assessed by using anti–CD8-PE clone DK25 (Dako, Carpinteria, CA). Results were expressed as percentages of total number of gated lymphocytes. The gating strategy was human CD45→CD3→CD4/CD8, CD45→CD19, CD45→CD14.
Immunohistochemistry
Brains were removed immediately after euthanasia and processed. Tissue was divided by hemispheres (for sagittal section histology and real time PCR) or left as a whole for horizontal sectioning. Tissue samples (brain and spleen half) were fixed with 4% paraformaldehyde overnight and embedded in paraffin. Five-micron-thick sections were stained with mouse monoclonal antibodies for CD163 (clone 10D6, 1:50, Vector Laboratories, Burlingame, CA), HLA-DQ/DP/DR (clone CR3/43, 1:100), CD8 (clone 144, 1:50), CD68 (clone KP-1, 1:50), HIV-1 p24 (clone Kal-1, 1:10), CD3 (1:100, rabbit polyclonal), and glial fibrillary acidic protein (GFAP; 1:1000 rabbit polyclonal). Except for CD163 antibody, all other antibodies were obtained from Dako. Mouse monoclonal antibodies to human CD14 (clone 7, 1:50) and CD4 (clone 1F6, 1:40) were purchased from Biocare Medical, LLC (Concord, CA) and Novocastra (Norwell, MA), respectively. Ionized calcium-binding adaptor molecule 1 for mouse/human microglial/macrophages cells (Iba-1, 1:500; rabbit polyclonal) were purchased from Wako Chemicals USA, Inc. (Richmond, VA). The polymer-based HRP- and AP-conjugated anti-mouse and anti-rabbit Dako EnVision systems were used as secondary detection reagents, and 3,3′-diaminobenzidine (DAB) and Permanent Red (Dako) were used as chromogens. All paraffin-embedded sections were counterstained with Mayer’s hematoxylin. Deletion of primary Ab or mouse IgG served as controls. Images were obtained by Optronics digital camera fixed to a Nikon Eclipse E800 (Nikon Instruments, Melville, NY) using MagnaFire 2.0 software. Tissue sections (2–4 sagittal, average total area of 78.8 mm2) were examined for human HLA-DR, CD163 and CD8 immunopositive cells using a ×40 objective. Number of cells in meninges, perivascular spaces, and parenchyma/section were calculated for each mouse. The assessments of cellular infiltrates in meninges, perivascular spaces, and parenchyma were done in blinded manner by four independent investigators. Intensity of astrocytes staining (GFAP), microglia and blood-borne macrophages (Iba-1) were scored by two investigators using ×10 and ×20 objectives. Findings were compared to animals that were not manipulated (score of 0). A score of 1 represented few activated astrocytes or activated microglial cells. A score of 2 is consistent with moderate activation of astrocytes and microglia. A score of 3 showed hypertrophic astrocytes with concomitant processes and microglial nodules.
Real-Time RT-PCR
Total RNA from cortex, midbrain and cerebellum/brain stem sections were extracted with TRIzol (Invitrogen, Carlsbad, CA) and reverse transcribed to cDNA with random hexamers and Moloney murine leukemia virus reverse transcriptase (Invitrogen). Real-time quantitative PCR was performed with cDNA using an ABI PRISM 7000 sequence detector (Applied Biosystems, Foster City, CA). Human HLA-DQ,45 mouse TNF-α, Mac-1, GAPDH expression were analyzed using TaqMan gene expression assays, and for HIV-1gag the primers and probe used were: forward, 5′-ACATCA AGCCATGCAAAT-3′; reverse, 5′-ATCTGGCCTGGT GCAATAGG-3′; and probe (FAM), 5′-CATCAATGAGGA AGCTGCAGAATGGGATAG A-3′ (TAMRA).46 Expression of mouse GFAP and iNOS were determined using SYBR-green method and the primers were: GFAP, forward 5′-ACTGGGTACCATGCCACGTT-3′; reverse 5′-GGGAGTGGAGGAGTCATTCG-3′, and iNOS forward 5′-GGCAGCCTGTGAGACCTTTG-3′; reverse 5′-GAAGCGTTTCGGGATCTGAA-3′. Quantification was done using standard curve method, as described in User Bulletin 2 obtained with ABI PRISM 7000 sequence detector. Gene expression including HIV-1 gag was expressed relative to GAPDH and used as an endogenous control. All PCR reagents were obtained from Applied Biosystems.
Statistical Analysis
Data were analyzed using GraphPad Prizm and Excel softwares; statistical tests used were nonparametric t-test (Mann–Whitney U-test), non-parametric Spearman correlation test, chi test (χ2), and one-way analysis of variance for comparisons of multiple groups. A P value of <0.05 was considered statistically significant.
Results
HIV Infection in Humanized Mice
We reconstituted newborn NSG mice with CD34+ human HSC isolated from umbilical cord blood. Details of different experimental groups of animals are summarized in Table 1. Human lymphoid tissue development was evaluated by flow cytometric analysis of human cells in peripheral blood (CD45, CD3, CD4, CD8, CD19, and CD14) and in spleens at the end of observation. This was performed to determine the relative abundance of immune cell groups. We observed that HIV-1–infected animals showed peak viremia 5–6 weeks after viral challenge with HIV-1ADA at 104 TCID50. All infected animals by this time had detectable viral load at the range 3.76–6.50 Log10 copies/ml. CD8+ cell depletion was used to accelerate viral dynamics with two sequential injections of cM-T807 antibodies (s.c. and i.p. within 3 days interval), either at 2 weeks or 5–7 weeks after infection. After treatment, human CD8+ cells were absent in blood but reappeared in circulation 2–3 weeks postdepletion. CD8+ cell depletion at earlier stage of infection (2w postinfection) resulted in accelerated increase in viral load and Δ log10 was 2.02 (P = 0.04). In mice with established infection for 5–7 weeks, CD8+ cell depletion resulted in a Δ log10 of 0.87 (P = 0.013). In the same stage of disease, non-depleted animals did show decline of viral load over the same time interval.
Transient antibody-mediated depletion of CD8+ cells increased the numbers and level of CD3+CD4+ cell proliferation. CD8+ cell depletion affected total number of human cells in lymphoid tissues. The effect of CD8+ cell depletion on viral load dynamics, total CD4+ T cells, other immune parameters, and pathologies were previously discussed.7
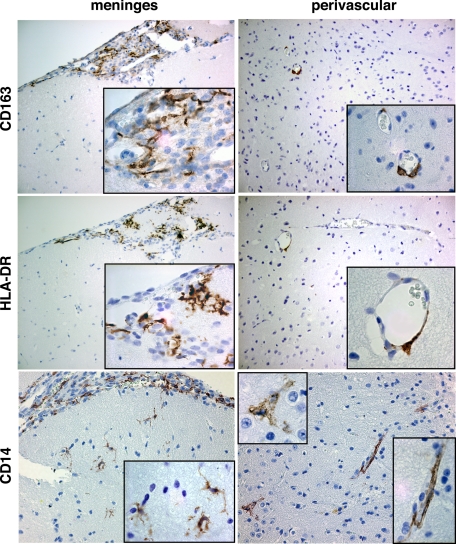
Human Macrophage Ingress into Rodent Brain
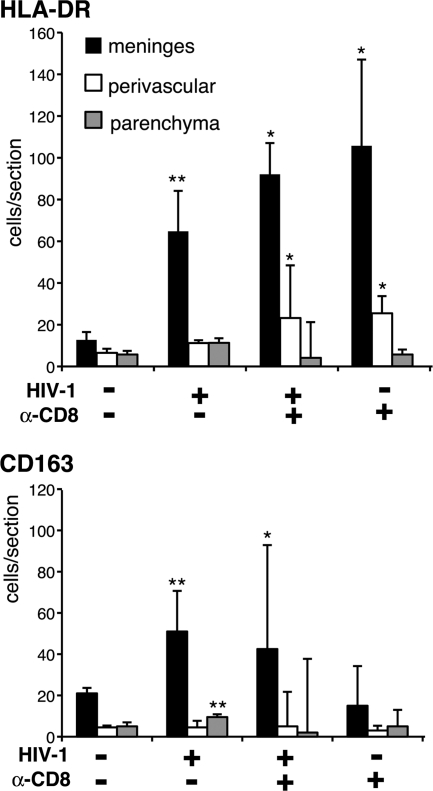
Repopulation of mouse brain with human cells was assessed by immunohistochemistry. Figure 1 represents brain sections from an uninfected mouse stained for different human cell markers. Human specific antibodies to CD163, CD14, HLA-DR, and CD68 detected MP in meninges. Twenty to 50% of CD163+ staining colocalized with human HLA-DR. Few human CD163+/HLA-DR+ cells had dendritic cell morphology. Among the reconstituted mice, a marked difference in the number of human cells infiltrated into the brain was noticed. However, consistent regional and cellular patterns were observed. The presence of human cells was readily seen in the cerebellum and surrounding meninges. Human cells were observed less frequently (from most to least abundant) in the cortical meninges, midbrain perivascular spaces, hippocampus, brain stem, cortex, and choroids plexus. The majority of human macrophages in the meninges and perivascular spaces had an elongated shape. Rarely found in the parenchymal perivascular spaces were human CD14+ and CD163+ cells showing ramified microglial morphology (∼5% of animals). CD3+ cells were infrequent in meninges and white matter tracks (data not shown). The frequency of human cells stained with monoclonal antibodies HLA-DR and CD163 on 5-μm-thick paraffin-embedded sections (2–5 per mouse collected at different levels of brain) was manually evaluated (Figure 2). The four groups of animals from which the HLA-DR+ and CD163+ cells were counted include uninfected, HIV-1 infected, HIV-1 infected/CD8+ cell depleted, and uninfected/CD8+ cell depleted. The number of positive cells was counted from different regions of the brain: meninges, perivascular spaces and parenchyma. HIV-1 infection increased the ingress of HLA-DR+ cells into the meninges. Depletion of CD8+ cells augmented the influx of HLA-DR+ cells in meninges and perivascular spaces, but it was not statistically different from non-depleted animals. This increase in the number of HLA-DR+ cells was also seen in uninfected animals with CD8+ cell depletion. However, when we analyzed the number of CD163+ MPs in HIV-1–infected and infected CD8 cell–depleted animals, a significant increase in CD163+ MPs (P < 0.01 and P < 0.05, compared to uninfected) was observed. This suggested that the increased number of HLA-DR+ cells in the brains of uninfected/CD8+ cell depleted animals were activated lymphocytes.
Figure 1.
Human cells in meninges and brain perivascular spaces. Representative paraffin-embedded 5-μm brain sections from uninfected control animal stained for human CD163, HLA-DR, and CD14 markers show cells with macrophage, microglia, and dendritic cell morphology (brown). Sections were counterstained with hematoxylin. Original magnification ×200; Insets magnification ×1000.
Figure 2.
Human cells in mouse brain. The majority of human cells were in the meninges. HIV-1 infection and CD8+ cell depletion increased human cell infiltration into the meninges as assessed by human HLA-DR staining of macrophages and lymphocytes. Representative tissue sections (2–4 sagittal with average total area of 78.8 mm2) were examined for human HLA-DR and CD163 using a ×40 objective. Average numbers of cells in meninges, perivascular spaces, and parenchyma/section were calculated for each mouse. Mean ± SEM cells per section are shown. P values were determined by analysis of variance and animal groups were compared by non-parametric Mann–Whitney U tests: *P < 0.05, **P < 0.01 compared to control. Numbers of histologically analyzed animals per groups are placed in parentheses.
CD8+ Cell Depletion Accelerated Neuropathology and CNS Viral Load
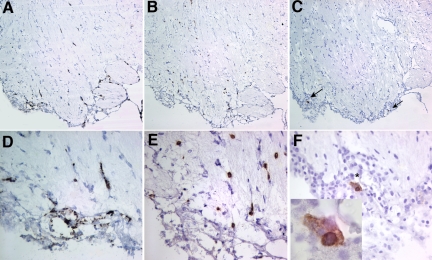
Continuous HIV-1 infection for 5–13 weeks induced neuropathology. This was evident by the presence of increased infiltration of meninges with human HLA-DR/CD163+ cells (Figure 2). Few HIV-1p24–positive human cells in the brain were observed, predominantly in the meninges. Figure 3, A–F shows the brain sections of an HIV-1–infected mouse sacrificed at 5 weeks postinfection, where HIV-1+ cells are seen in meninges and several scattering infected cells were also present in olfactory bulb and striatum, accompanied by CD8+ cell infiltration. Increased infiltration of CD8+ cells was observed in the brains of infected animals, which is rarely seen in uninfected brains. Meningitis, characterized by the presence of cell clusters in the meninges in addition to increased infiltration of HLA-DR/CD163+ cells, was seen in 2/10 animals sacrificed at 5 weeks and 2/12 animals sacrificed at 8–13 weeks after infection. Minimal astrocyte and microglial activation were also observed in cerebral cortex and microglial nodules formation in brain stem.
Figure 3.
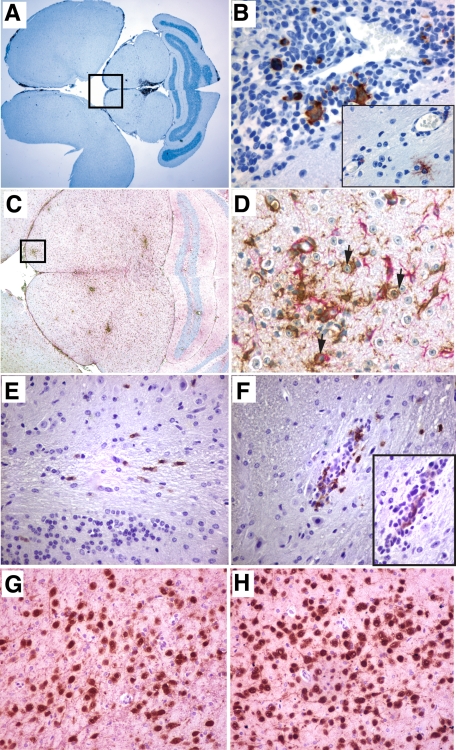
Influx of CD8+ cells in the brain of HIV-1–infected mice. Sagittal brain section of 36-week-old mouse #596 infected for 5 weeks is shown (olfactory bulb). Sections were stained for (A and D) human CD163, (B and E) human CD8, and (C and F) HIV-1p24 antigen. A–C: Distribution of human macrophages in meninges and along the vessels, scattering through parenchyma are CD8+ cells and rare HIV-1p24-positive cells (arrows, C). Inset in F shows magnified view of a group of infected cells in meninges. Sections were counterstained with hematoxylin. Magnifications: A–C ×100, D–F ×400 and inset ×1000.
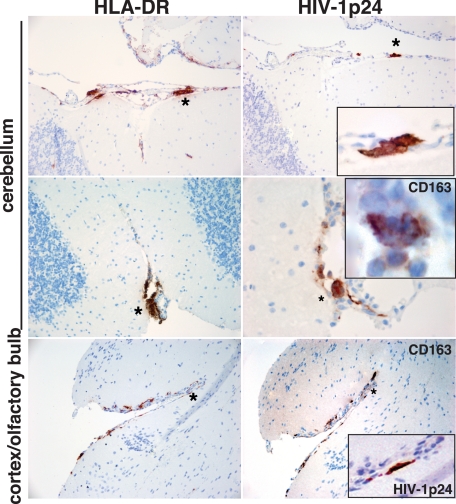
HIV-1–infected and CD8+ cell–depleted mice had more prominent pathology. Eight of 12 showed neuropathology. Six developed meningitis with clusters of human macrophages and lymphocytes (HLA-DR+ and CD163+ cells) in meninges (Figure 4). More importantly, two animals showed significant meningoencephalitis with robust human HLA-DR cell infiltration of HLA-DR+ cells into the meninges. These cells showed lymphocyte morphology. Figure 5 shows the brain sections from a meningoencephalitic mouse stained for human immune cell markers. Perivascular cuffing with HLA-DR+, CD8+ cells, as well as readily identified human cells were scattered throughout the neuropil. The meningoencephalitis was seen at 3 weeks after depletion with a total infection time of 8 weeks. In these animals with meningoencephalitis, CD8+ cells restored in larger numbers than usual in periphery. HIV-1p24+ cells were found frequently in the meninges and perivascular spaces in both meningitis and meningoencephalitic brains (Figures 4 and 5, A–H). Perivascular cuffs in encephalitic mice also contained HIV-1p24+ cells. Infected cells in the meninges and parenchyma were surrounded by locally activated mouse astrocytes and microglia, shown in sections stained with antibodies to mouse GFAP (activated astrocytes) and Iba-1 (activated microglia).
Figure 4.
Meningitis in HIV-1–infected and CD8+ cell–depleted mice. Horizontal brain sections of a 25-week-old mouse #209 infected for 4 weeks and CD8+ cell depleted for 2 weeks were stained for human HLA-DR (left column), HIV-1p24 antigen, and human CD163 (right column). Panels show the accumulation of human cells in cerebellar and olfactory bulb meninges. Insets represent magnified views of selected area (Asterisk) on adjacent section stained for human macrophage marker CD163 (MGC, middle row) and viral protein (top and bottom rows). Sections were counterstained with hematoxylin. Magnification ×10, inset magnification is ×100.
Figure 5.
Meningoencephalitis in HIV-1–infected and CD8+ cell–depleted mice. Horizontal brain sections of a 29-week-old mouse #305 infected for 8 weeks and CD8+ cell–depleted for 3 weeks are shown. Sections were stained for (A) human HLA-DR, (B) HIV-1p24 antigen, and (C and D) mouse astrocytes (GFAP, Permanent Red) and microglia (Iba-1, DAB), (E and F) human CD8+ cells, and the inset is the adjacent section stained for HIV-1p24, and (G and H) neuronal nuclear protein (NeuN, DAB) and MAP-2 (Permanent Red). A: Accumulation of human cells in meninges and periaqueductal structures. B: Magnified views of selected area on the adjacent section stained for viral protein showing viral cytopathic effects, perivascular-infected cells, and infected cells with microglial morphology. C: Diffused activation of astrocytes and microglia, perivascular cuffs. D: Magnified view of selected region showing activated phagocytizing microglial cells (arrows). E and F: Scattering CD8+ cells in cerebellar white matter tracts (E) and perivascular infiltration of CD8+ cells near HIV-1 p24 infected cell (F and inset). G and H: Neuronal density in periaqueductal gray matter in the same mouse and in control reconstituted animal of the same age, respectively. Sections were counterstained with hematoxylin. Magnifications: A, ×1; B, ×20; inset, × 400; C, ×4; D, ×400; E and F and inset, × 400; G and H, ×20.
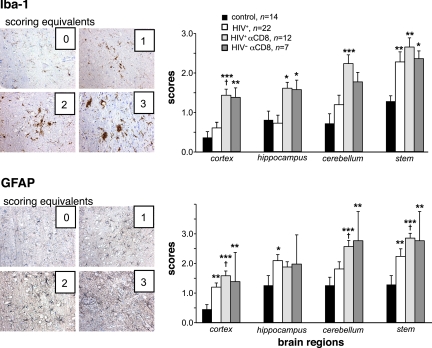
Murine glial responses were assessed by GFAP and Iba-1 staining of brain sections obtained from all four experimental groups and scored by two investigators from four different regions of the brain (Figure 6). Prominent glial activation was seen in the majority of CD8+ cell–depleted animals regardless of levels of viral infection. In reconstituted control animals, murine cells did not demonstrate morphologies reflective of reactive astrocytes and microglia in cortex. Minimal activation was found in hippocampus and cerebellum. Few microglial nodules (2–3) and gliotic changes were in the brain stem of aged NSG mice (data not shown). Significant increase in glial activation in HIV-1 infected and HIV-1 infected/CD8+ cell depleted animals was observed compared to uninfected controls. A summary of the morphological findings is listed in Table 2. Meningitis was present in HIV-1–infected compared to HIV-1–infected and CD8+ cell depleted animals (18.4% vs 50%, χ2 = 28.4, P < 0.00001).
Figure 6.
Glial reactions in HIV-1–infected and CD8+ cell–depleted mice. HIV-1 infection induced a diffuse astrogliosis that was increased by CD8+ cell depletion as seen by GFAP staining. This paralleled microglial activation responses measured by Iba-1 staining. Histopathological examination was performed on a minimum of four brain regions derived from paraffin-embedded brain tissue and recorded by two independent investigators. Findings were compared to animals that were not manipulated in any manner (score of 0). A score of 1 represents few activated astrocytes or activated microglial cells. A score of 2 is coincident with moderate activation of astrocytes and microglia. A score of 3 shows hypertrophic astrocytes and concomitant processes and microglial nodules. P values were determined by analysis of variance and animal groups were compared by nonparametric Mann–Whitney U tests. Mean ± SEM score per region are illustrated. *P < 0.05, **P < 0.01, ***P < P < 0.001 statistically different compared to uninfected controls, †P < 0.05 compared to HIV-1–infected.
Table 2.
Neuropathological Features of HIV-1–Infected Humanized Mice
| Experimental Groups and No. of Evaluated Brains | Pathologic Findings
|
||
|---|---|---|---|
| Glial Activation* | Meningitis† | Encephalitis‡ | |
| Control, n = 18 | 4 | 0 | 0 |
| HIV-1–infected, n = 22 | 20 | 4 | 0 |
| HIV-1–infected/CD8-depleted, n = 12 | 12 | 6 | 2 |
| CD8-depleted, n = 7 | 7 | 0 | 0 |
Control, uninfected reconstituted mice.
Focal mild glial activation in cortex and midbrain and more severe activation in cerebellum and brain stem were observed. Scoring for glial activation took into account the increased number of astrocyte processes and hypertrophy as well as microglial morphology. The latter included ramified and amoeboid morphology, increased cytoplasmic and processes proportion, and nodules (Figure 6).
Prominently human and mouse macrophages with limited number of lymphocytes were present.
In encephalitic animals, diffuse activation of astrocytes and microglia was seen with significant meningeal, parenchymal, and perivascular infiltration of human lymphocytes. HIV-1–infected human MGC were found in meninges but not in the parenchyma.
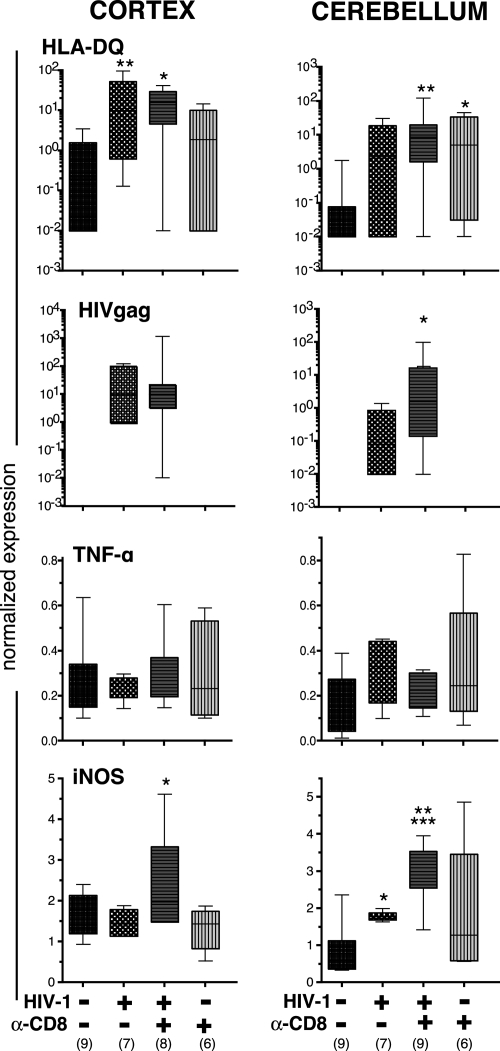
To assess the total number of human cells and HIV-1 viral load in the brain, we dissected the cerebellum, midbrain and cortex, extracted RNA, and amplified human HLA-DQ RNA and viral HIV-1gag RNA. Both parameters were analyzed by real-time RT-PCR, and the results confirmed immunohistological findings (Figure 7). As shown in Figure 7, there was significant variability in the presence of human cells in mice. In approximately half of uninfected animals, HLA-DQ expression was not observed, showing a limited number of activated human cells in the brains of these animals. HIV-1 infection per se stimulated the influx of human cells (Figure 2) and HLA-DQ expression was found in all cortical and 80% of cerebellar tissues of animals infected for 8–13 weeks. CD8+ cell depletion in HIV-1–infected mice did not increase HLA-DQ expression significantly in cerebellum and cortex, when compared to infected/nondepleted animals. In infected mice, 40% (cerebellum) to 70% (cortex) of samples had detectable HIV-1gag expression. CD8+ cell depletion in infected mice increased the expression of HIV-1gag that reached statistical significance in cerebellum (P < 0.05) (Figure 7). Comparison of viral load in peripheral blood and the expression of HIV-1gag in cortex and cerebellum of each animal showed that some animals with low peripheral viral load do have detectable levels of HIV-1gag expression in the brain7 (Table 3). Statistical analyses by nonparametric Spearman r test showed positive correlation between viral load in peripheral blood and HIV-1gag expression in both cortex (r = 0.572, P < 0.02, n = 17) and cerebellar samples (r = 0.732, P < 0.01, n = 13). We did not find statistically significant positive correlation between expression of HIVgag and HLA-DQ either in cortex (r = 0.383, P = 0.117, n = 18) or cerebellar samples (r = 0.332, P = 0.246, n = 14).
Figure 7.
HIV-1 infection and CD8+ cell depletion affect immune and viral brain biomarkers. RNA was extracted from cortex and cerebellar tissues, and RT-PCR was performed to assess the expression of HLA-DQ (to estimate human cells), HIV-1gag, TNF-α, and iNOS. Animals were injected with α-CD8 antibody after (post) infection with HIV-1. Uninfected animals were injected with the antibody at similar age. Expression of all genes was normalized to GAPDH used as an endogenous control. Median lines, the range and interquartile range, and number of analyzed animals (in parenthesis) are shown. Statistical analyses were determined by analysis of variance and comparison by nonparametric Mann–Whitney U test between groups. *P < 0.05, **P < 0.01 compared to uninfected controls, ***P < 0.01 to HIV-1–infected.
Table 3.
HIV-1 Viral Load in Peripheral Blood and HIV-1gag RNA in Brain*
| Mouse # | Age, Weeks | HIV-1 Infection Weeks† | CD8+cell Depletion, Weeks‡ | HIV-1 RNA Copies/ml§ | HIV-1gag RNA | |
|---|---|---|---|---|---|---|
| Cortex | Cerebellum | |||||
| HIV-1–infected | ||||||
| 338 | 26 | 13 | 1,526,000 | 120.258 | ND | |
| 329 | 27 | 13 | 112,000 | 0.968 | 0.331 | |
| 348 | 29 | 8 | 75,250 | 77.572 | ND | |
| 334 | 29 | 11 | 23,730 | 10.253 | ND | |
| 315 | 31 | 11 | 13,335 | 0.875 | 0.154 | |
| 352 | 32 | 11 | 153,300 | 8.968 | 1.416 | |
| 311 | 33 | 6 | <1750 | 0.189 | ND | |
| HIV-1–infected/CD8+ cell–depleted | ||||||
| 422 | 33 | 7 | 1 | 35,000,000 | 8.980 | 12.533 |
| 458 | 33 | 7 | 1 | 47,250 | 0.181 | 0.131 |
| 429 | 33 | 7 | 1 | 451,500 | ND | 20.697 |
| 202 | 26 | 4 | 2 | 24,745 | 6.268 | 0.155 |
| 209 | 26 | 4 | 2 | 89,600 | 32.304 | 1.610 |
| 212 | 26 | 4 | 2 | 35,000,000 | 1163.023 | 101.180 |
| 341 | 31 | 4 | 2 | 313,600 | 10.247 | 0.498 |
| 343 | 29 | 4 | 2 | 184,800 | 10.738 | ND |
End point data are shown for animals with determined peripheral viral load. Study subjects without this information were not included. Complete information of the peripheral reconstitution of presented animals was published in Gorantla et al., 2010.7
Weeks after HIV-1 infection.
Weeks after CD8+ cell depletion.
Detection limit for peripheral blood was 1750 copies/ml.
ND, not detected.
RT-PCR Analysis of Neuroinflammatory Markers and Cytokines
To analyze the effects of human cell–derived activation factors on murine glial cells, RT PCR analyses for mouse GFAP, Mac1, TNF-α, and iNOS were performed in the cortex, midbrain and cerebellum. Expression of these molecules was not associated with the number of human cells in circulation (total number of CD45+ cells) or HLA-DQ expression in brain tissues. Significant changes in the expression of GFAP (astrocyte activation) were not observed either between different experimental groups or brain regions. TNF-α was not statistically significantly increased (by analysis of variance) compared to controls (P = 0.137, Figure 7). However, increased Mac1 expression by 10–50% (an indicator of microgliosis) in the cerebellum of all experimental groups compared to controls was observed (P < 0.0001 by analysis of variance). In addition, iNOS expression was significantly up-regulated in HIV-1–infected/CD8+ cell–depleted animals compared to all other experimental groups by ∼30% in cortex (P < 0.05). In cerebellum of HIV-1–infected/CD8+ cell–depleted animals iNOS expression was up-regulated by six and two folds compared to control and HIV-1–infected mice (P < 0.01 and P < 0.05, respectively, Figure 7).
Discussion
This study is the first, to our knowledge, to demonstrate the relationships between chronic progressive HIV-1 infection, blood-borne macrophage migration to brain, and CNS disease in humanized mice. The new model circumvents limitations seen previously in other systems that include: traumatic injury induced by injection of MDM, severe inflammation associated with graft-versus-host reaction, inability of long-term observation, and the absence of adaptive immune responses. NOD/scid-IL2Rγcnull genotype support human cell survival47,48,49 and provide a valuable new platform to evaluate changes in brain morphology associated with continuous HIV-1 infection. Moreover, due to the engrafted humanized lymphoid tissue, abnormalities associated with HIV-1 infection alone or in combination with CD8 cell depletion and the consequences of viral infection on brain pathology can be elucidated in a murine environment.
Several notable observations were made reflective of early neuroAIDS. Human cells with MP morphology were readily seen in the meninges and parenchymal perivascular spaces. This finding is in concordance with known abilities of mouse and human myeloid bone-marrow derived cells to enter the brain, and not only populate perivascular spaces, but also acquire microglial cell phenotypes.50 This was seen without inciting brain damage by direct injection of either HIV-1–infected human peripheral mononuclear cells or MDM into the brain to induce neuropathology.1,5 Peripheral activation of the human immune system by HIV-1 infection increased the influx of human cells into the brain. However, this was not a lymphocyte-mediated graft-versus-host–like reaction and did not induce significant histological changes in HIV-1–infected animals without CD8+ cell depletion.
CD8+ cell depletion in humanized mice was used to accelerate brain disease as of previously published data for SIV infected nonhuman primate models.32,33,34,35,37,51,52 Depletion of CD8+ cells after productive infection significantly increased peripheral and brain viral load, migration of human cells into the CNS, and formation of MGC in meninges as well as diffusing glial reactions. In two cases, as a result of CD8+ cell depletion and CD4+ cell activation, mice developed severe meningoencephalitis, acute glial activation, and phagocytosis of neurons by microglial cells. We observed that the presence of infected cells in meninges itself induced mild local astrocyte or microglial reaction. Microglial activation was associated with the formation of nodules in the brain of the infected mice (5–10) and significantly exceeded microglial nodules in age-matched NSG control mice (2–3). The number of microglial nodules further increased with CD8+ cell depletion in HIV-1–infected mice. Such microglial responses began in the basal pons and expanded along white matter tracks. Importantly, such observations paralleled those seen in rhesus macaques infected with SIVmac239 or SIVmac239/17E in the absence of perivascular cuffing.53 However, increased expression of MHC-II by microglial cells were found concentrated in white matter of the basal pons and followed broad distributions regardless of viral tropisms.53 In HIV-1 infection of humans, microglial nodules are associated with the presence of viral gene products or opportunistic pathogens.54 However, in humanized mice the microglial nodules were negative for HIV-1p24 antigen.
We do not have direct evidence that all analyzed chimeric mice had cellular adaptive response against HIV-1. However, the contribution of CTL in the control of viral replication is possible as shown previously7 and CD8+ cells were present in brain parenchyma of some animals at 5 weeks after infection. We did not find severe encephalitis in all infected and CD8+ cell–depleted animals. Severe encephalitis may not be expected at 2–3 weeks postdepletion because in these animals the number of human brain resident macrophages is limited and HIV-1 infection of mouse macrophages/microglia is restricted due to viral receptor specificity. We also cannot exclude donor-dependent effects. The development of encephalitis after CD8+ cell depletion in this model could be linked, in part, to viral infection and human cell ingress. Mouse brain inflammation was seen in association with ongoing peripheral and CNS viral replication, particularly with increased expression of murine iNOS.
Changes in iNOS expression denote important pathological disturbances in oxidative stress-mediated damage in the human brain during HIV-1 infection41,55,56,57 and other neurodegenerative diseases.58 Preferential increase in cerebellar Mac-1 expression in HIV-1 infected and CD8+ cell depleted animals may be attributed to mouse brain anatomy and specificity of cell migration during inflammation.59,60
In SIV monkey models, depletion of CD8+ cells induced profound neuropathology and encephalitis attributed to the loss of CTL control of viral replication.33,34 Similarities and differences between the neuropathological findings seen in HIV-1–infected humanized mice, human HIV-1 infection, and SIV-infected nonhuman primate models are listed in Table 4.28,31,61,62,63,64,65,66,67,68,69 It is not surprising that all HIV-1–infected animals did not develop acute inflammatory responses in the brain because such aseptic meningitis was seen only in 20% of HIV-1 infected humans.28,61,70,71,72 The encephalitis observed after CD8 cell depletion in these mice were distinct from that reported in HIV-1 and SIV encephalitis. Differences are notable for the presence and distribution of MGC. In the humanized mice MGC are in the meninges, but not in parenchyma, where limited numbers of human microglial cells were seen.
Table 4.
Comparative Analyses of the Neuropathological Findings Seen in HIV-1–Infected Humanized Mice, HIV-1–Infected Humans, and SIV-Infected Nonhuman Primate Models*
| Pathologic Findings | Humanized Mice HIV-1 | Human HIV-1 | Nonhuman Primates SIV |
|---|---|---|---|
| Meningeal and perivascular macrophage infection | Yes | Yes | Yes |
| Meningitis | Yes | Yes | Yes |
| Encephalitis | No | Yes | Yes |
| Parenchymal microglial infection | Limited | Yes | Yes |
| Microglial activation | Yes | Yes | Yes |
| Lymphocyte brain infiltration | Yes | Yes | Yes |
| Astrogliosis | Yes | Yes | Yes |
| Induction of encephalitis by CD8+cell depletion | Yes | NA | Yes |
To further validate the mouse model, we investigated the development of cognitive and motor dysfunctions, as they are the common features of HAND. To confirm the development of cognitive and motor dysfunction as the common complications in HIV-1–infected patients, we evaluated adult NSG mice to achieve spatial memory formation.73,74 This mouse strain shows indices of spatial memory, but irradiation at birth dramatically reduced the formation of spatial memory (data not shown). Irradiation induced damage can be avoided using myeloablation by chemicals75 or antibodies.76,77 NOG mice could be engrafted with human cells without myeloablation as was reported recently.9 We are currently exploring such methods to generate humanized mice, which could be used for behavioral studies. We also found age-dependent neurodegeneration in NSG mice, especially in pons/brain stem with glial nodule formation, which was not seen in age-matched immune competent mice. This should be considered as a baseline, and we suggest caution to avoid over interpreting HIV-1–mediated neuropathology. We also assume that housing of humanized mice in a specific pathogen-free environment eliminates the exposure to LPS that is known to affect pathogenesis in HIV-1 infection.78,79,80
Altogether, while SIV-infected nonhuman primates are well acknowledged models for HIV-1 pathogenesis studies, species specificity and expense preclude their widespread use. Thus, relevant small animal models like the model developed in the current report are greatly needed. A comparative description of previous mouse models of NeuroAIDS is presented in the Supplemental table, S1 (available at http://ajp.amjpathol.org).1,2,3,4,6,7,62,63,64,65,66,67,68,69,81,82,83,84,85,86,87,88,89,90 What is reported now can permit future studies of viral neuropathogenesis studies in rodents and for investigations of novel antiretroviral and adjunctive therapies.
Acknowledgments
We thank Lisa Kosloski, Mohammed Ali, Alexander Smith, and Jillian Ann Braun-Jankovich for assistance with immunohistochemical staining and cell counting. We thank Robin Taylor for critical reading of the manuscript and administrative assistance.
Footnotes
Address reprint requests to Larisa Poluektova, M.D., Ph.D., Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, 985880 Nebraska Medical Center, Omaha, NE 68198-5880. E-mail: lpoluekt@unmc.edu.
Supported by National Institutes of Health grants R21NS060642-01 (L.P.); P20 RR015635, 1 P01 NS043985-01, and 1P01DA028555-01 (L.P. and H.E.G.); and P20DA026146, 5R01 NS36126, PO1 NS31492, 2R01 NS034239, P30 AI42845, P01MH64570, and 1 R01MH083516 (H.E.G).
Author J.F.-D. is deceased.
Supplemental Material for this article can be found on http://ajp.amjpathol.org.
Current address for A.H.: School of Biological Sciences, University of Nebraska-Lincoln, Lincoln, NE.
References
- Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, Patil A, Nottet HSLM, Epstein L, Gelbard H, Flanagan E, Reinhard J, Pirruccello SJ, Gendelman HE. Human immunodeficiency virus encephalitis in SCID mice. Am J Pathol. 1996;149:1027–1053. [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman HE. An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis. J Neurovirol. 1997;3:401–416. doi: 10.3109/13550289709031186. [DOI] [PubMed] [Google Scholar]
- Poluektova LY, Munn DH, Persidsky Y, Gendelman HE. Generation of cytotoxic T cells against virus-infected human brain macrophages in a murine model of HIV-1 encephalitis. J Immunol. 2002;168:3941–3949. doi: 10.4049/jimmunol.168.8.3941. [DOI] [PubMed] [Google Scholar]
- Poluektova L, Gorantla S, Faraci J, Birusingh K, Dou H, Gendelman HE. Neuroregulatory events follow adaptive immune-mediated elimination of HIV-1-infected macrophages: studies in a murine model of viral encephalitis. J Immunol. 2004;172:7610–7617. doi: 10.4049/jimmunol.172.12.7610. [DOI] [PubMed] [Google Scholar]
- Tyor WR, Power C, Gendelman HE, Markham RB. A model of human immunodeficiency virus encephalitis in scid mice. Proc Natl Acad Sci USA. 1993;90:8658–8662. doi: 10.1073/pnas.90.18.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poluektova L, Meyer V, Walters L, Paez X, Gendelman HE. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 2005;52:344–353. doi: 10.1002/glia.20253. [DOI] [PubMed] [Google Scholar]
- Gorantla S, Makarov E, Finke-Dwyer J, Gebhart CL, Domm W, Dewhurst S, Gendelman HE, Poluektova LY. CD8+ cell depletion accelerates HIV-1 immunopathology in humanized mice. J Immunol. 2010;184:7082–7091. doi: 10.4049/jimmunol.1000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Terashima K, Ohta S, Horibata S, Yajima M, Shiozawa Y, Dewan MZ, Yu Z, Ito M, Morio T, Shimizu N, Honda M, Yamamoto N. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood. 2007;109:212–218. doi: 10.1182/blood-2006-04-017681. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Ohta S, Yajima M, Terashima K, Ito M, Mugishima H, Fujiwara S, Shimizu K, Honda M, Shimizu N, Yamamoto N. Humanized NOD/SCID/IL2R{gamma}null mice transplanted with hematopoietic stem cells under nonmyeloablative conditions show prolonged life spans and allow detailed analysis of human immunodeficiency virus type 1 pathogenesis. J Virol. 2007;81:13259–13264. doi: 10.1128/JVI.01353-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- Baenziger S, Tussiwand R, Schlaepfer E, Mazzucchelli L, Heikenwalder M, Kurrer MO, Behnke S, Frey J, Oxenius A, Joller H, Aguzzi A, Manz MG, Speck RF. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2-/-gamma c-/- mice. Proc Natl Acad Sci USA. 2006;103:15951–15956. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla S, Sneller H, Walters L, Sharp JG, Pirruccello SJ, West JT, Wood C, Dewhurst S, Gendelman HE, Poluektova L. Human immunodeficiency virus type 1 pathobiology studied in humanized BALB/c-Rag2-/-gammac-/- mice. J Virol. 2007;81:2700–2712. doi: 10.1128/JVI.02010-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berges B, Akkina S, Folkvord J, Connick E, Akkina R. Mucosal transmission of R5 and X4 tropic HIV-1 via vaginal and rectal routes in humanized Rag2-/- gammac -/- (RAG-hu) mice. Virology. 2008;373:342–351. doi: 10.1016/j.virol.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An DS, Poon B, Fang RH, Weijer K, Blom B, Spits H, Chen IS, Uittenbogaart CH. Use of a novel chimeric mouse model with a functionally active human immune system to study human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2007;14:391–396. doi: 10.1128/CVI.00403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kovalev GI, Su L. HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood. 2007;109:2978–2981. doi: 10.1182/blood-2006-07-033159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Denton P, Estes J, Othieno F, Wei B, Wege A, Melkus M, Padgett-Thomas A, Zupancic M, Haase A, Garcia J. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007;204:705–714. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL, Laouar A, Yao J, Haridas V, Habiro K, Yang YG, Jeong JH, Lee KY, Kim YH, Kim SW, Peipp M, Fey GH, Manjunath N, Shultz LD, Lee SK, Shankar P. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196:1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- Clifford DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med. 2008;16:94–98. [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Letendre S, Ances B, Gibson S, Ellis RJ. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2007;15:32–39. [PubMed] [Google Scholar]
- Jones G, Power C. Regulation of neural cell survival by HIV-1 infection. Neurobiol Dis. 2006;21:1–17. doi: 10.1016/j.nbd.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O, Joag SV, Stephens EB. Selected models of HIV-induced neurological disease. Curr Top Microbiol Immunol. 1995;202:151–166. doi: 10.1007/978-3-642-79657-9_11. [DOI] [PubMed] [Google Scholar]
- Lackner AA, Smith MO, Munn RJ, Martfeld DJ, Gardner MB, Marx PA, Dandekar S. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991;139:609–621. [PMC free article] [PubMed] [Google Scholar]
- Budka H, Wiley CA, Kleihues P, Artigas J, Asbury AK, Cho ES, Cornblath DR, Dal Canto MC, DeGirolami U, Dickson D. HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1991;1:143–152. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Kraft-Terry SD, Buch SJ, Fox HS, Gendelman HE. A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron. 2009;64:133–145. doi: 10.1016/j.neuron.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray F, Scaravilli F, Everall I, Chretien F, An S, Boche D, Adle-Biassette H, Wingertsmann L, Durigon M, Hurtrel B, Chiodi F, Bell J, Lantos P. Neuropathology of early HIV-1 infection. Brain Pathol. 1996;6:1–15. doi: 10.1111/j.1750-3639.1996.tb00775.x. [DOI] [PubMed] [Google Scholar]
- An SF, Giometto B, Scaravilli F. HIV-1 DNA in brains in AIDS and pre-AIDS: correlation with the stage of disease. Ann Neurol. 1996;40:611–617. doi: 10.1002/ana.410400411. [DOI] [PubMed] [Google Scholar]
- Marcondes MC, Burudi EM, Huitron-Resendiz S, Sanchez-Alavez M, Watry D, Zandonatti M, Henriksen SJ, Fox HS. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol. 2001;167:5429–5438. doi: 10.4049/jimmunol.167.9.5429. [DOI] [PubMed] [Google Scholar]
- McCrossan M, Marsden M, Carnie FW, Minnis S, Hansoti B, Anthony IC, Brettle RP, Bell JE, Simmonds P. An immune control model for viral replication in the CNS during presymptomatic HIV infection. Brain. 2006;129:503–516. doi: 10.1093/brain/awh695. [DOI] [PubMed] [Google Scholar]
- Sopper S, Sauer U, Hemm S, Demuth M, Muller J, Stahl-Hennig C, Hunsmann G, ter Meulen V, Dorries R. Protective role of the virus-specific immune response for development of severe neurologic signs in simian immunodeficiency virus-infected macaques. J Virol. 1998;72:9940–9947. doi: 10.1128/jvi.72.12.9940-9947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissel SJ, Wang G, Trichel AM, Murphey-Corb M, Wiley CA. Longitudinal analysis of monocyte/macrophage infection in simian immunodeficiency virus-infected. CD8+ T-cell-depleted macaques that develop lentiviral encephalitis. Am J Pathol. 2006;168:1553–1569. doi: 10.2353/ajpath.2006.050240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Simon MA, Kuroda MJ, Lifton MA, Ollert MW, Vogel CW, Racz P, Tenner-Racz K, Scallon BJ, Dalesandro M, Ghrayeb J, Rieber EP, Sasseville VG, Reimann KA. A nonhuman primate model for the selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am J Pathol. 1999;154:1923–1932. doi: 10.1016/S0002-9440(10)65450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matano T, Shibata R, Siemon C, Connors M, Lane HC, Martin MA. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagopal S, Lorey SL, Barnett L, Basham R, Lebo L, Erdem H, Haman K, Avison M, Waddell K, Haas DW, Kalams SA. Enhancement of human immunodeficiency virus (HIV)-specific CD8+ T cells in cerebrospinal fluid compared to those in blood among antiretroviral therapy-naive HIV-positive subjects. J Virol. 2008;82:10418–10428. doi: 10.1128/JVI.01190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H, Zheng YM, Heber-Katz E, Fraser N, Rorke L, Fu ZF, Hanlon C, Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases [published erratum appears in Proc Natl Acad Sci USA: 1993 Jun 1;90(11):5378]. Proc Natl Acad Sci USA. 1993;90:3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson DC, McArthur JC, Dawson TM, Dawson VL. Rate and severity of HIV-associated dementia (HAD): correlations with Gp41 and iNOS. Mol Med. 1999;5:98–109. [PMC free article] [PubMed] [Google Scholar]
- Lane TE, Buchmeier MJ, Watry DD, Fox HS. Expression of inflammatory cytokines and inducible nitric oxide synthase in brains of SIV-infected rhesus monkeys: applications to HIV-induced central nervous system disease. Mol Med. 1996;2:27–37. [PMC free article] [PubMed] [Google Scholar]
- Nuovo G, Alfieri M. AIDS dementia is associated with massive, activated HIV-1 infection and concomitant expression of several cytokines. Mol Med. 1996;2:358–366. [PMC free article] [PubMed] [Google Scholar]
- Hori K, Burd PR, Furuke K, Kutza J, Weih KA, Clouse KA. Human immunodeficiency virus-1-infected macrophages induce inducible nitric oxide synthase and nitric oxide (NO) production in astrocytes: astrocytic NO as a possible mediator of neural damage in acquired immunodeficiency syndrome. Blood. 1999;93:1843–1850. [PubMed] [Google Scholar]
- Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locardi C, Puddu P, Ferrantini M, Parlanti E, Sestili P, Varano F, Belardelli F. Persistent infection of normal mice with human immunodeficiency virus. J Virol. 1992;66:1649–1654. doi: 10.1128/jvi.66.3.1649-1654.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota M, Kleinschmidt A, Ceccherini-Silberstein F, Aloisi F, Mengozzi M, Mantovani A, Brack-Werner R, Poli G. Upregulated expression of interleukin-8. RANTES and chemokine receptors in human astrocytic cells infected with HIV-1. J Neurovirol. 2000;6:75–83. doi: 10.3109/13550280009006384. [DOI] [PubMed] [Google Scholar]
- Cao X, Shores E, Hu-Li J, Anver M, Kelsall B, Russell S, Drago J, Noguchi M, Grinberg A, Bloom E. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- Asheuer M, Pflumio F, Benhamida S, Dubart-Kupperschmitt A, Fouquet F, Imai Y, Aubourg P, Cartier N. Human CD34+ cells differentiate into microglia and express recombinant therapeutic protein. Proc Natl Acad Sci USA. 2004;101:3557–3562. doi: 10.1073/pnas.0306431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopper S, Koutsilieri E, Scheller C, Czub S, Riederer P, ter Meulen V. Macaque animal model for HIV-induced neurological disease. J Neural Transm. 2002;109:747–766. doi: 10.1007/s007020200062. [DOI] [PubMed] [Google Scholar]
- Veazey RS, Acierno PM, McEvers KJ, Baumeister SH, Foster GJ, Rett MD, Newberg MH, Kuroda MJ, Williams K, Kim EY, Wolinsky SM, Rieber EP, Piatak M, Jr, Lifson JD, Montefiori DC, Brown CR, Hirsch VM, Schmitz JE. Increased loss of CCR5+ CD45RA- CD4+ T cells in CD8+ lymphocyte-depleted Simian immunodeficiency virus-infected rhesus monkeys. J Virol. 2008;82:5618–5630. doi: 10.1128/JVI.02748-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman NE, Sheffield LG, Purcell J, Joag SV, Narayan O, Cheney P. Gradient of microglial activation in the brain of SIV infected macaques. J NeuroAIDS. 1998;2:43–54. doi: 10.1300/J128v02n01_03. [DOI] [PubMed] [Google Scholar]
- Nebuloni M, Pellegrinelli A, Ferri A, Tosoni A, Bonetto S, Zerbi P, Boldorini R, Vago L, Costanzi G. Etiology of microglial nodules in brains of patients with acquired immunodeficiency syndrome. J NeuroVirol. 2000;6:46–50. doi: 10.3109/13550280009006381. [DOI] [PubMed] [Google Scholar]
- Lisak RP, Benjamins JA, Bealmear B, Nedelkoska L, Studzinski D, Retland E, Yao B, Land S. Differential effects of Th1, monocyte/macrophage and Th2 cytokine mixtures on early gene expression for molecules associated with metabolism, signaling and regulation in central nervous system mixed glial cell cultures. J Neuroinflammation. 2009;6:4. doi: 10.1186/1742-2094-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky MI, Nottet HS, Schmidtmayerova H, Dubrovsky L, Flanagan CR, Mullins ME, Lipton SA, Gendelman HE. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV- associated neurological disease. J Exp Med. 1995;181:735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson DC, Wildemann B, Sasaki M, Glass JD, McArthur JC, Christov VI, Dawson TM, Dawson VL. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science. 1996;274:1917–1921. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans. 2007;35:1119–1121. doi: 10.1042/BST0351119. [DOI] [PubMed] [Google Scholar]
- Fabis MJ, Phares TW, Kean RB, Koprowski H, Hooper DC. Blood-brain barrier changes and cell invasion differ between therapeutic immune clearance of neurotrophic virus and CNS autoimmunity. Proc Natl Acad Sci USA. 2008;105:15511–15516. doi: 10.1073/pnas.0807656105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional Differences in Blood-Brain Barrier Permeability Changes and Inflammation in the Apathogenic Clearance of Virus from the Central Nervous System. J Immunol. 2006;176:7666–7675. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- An SF, Scaravilli F. Early HIV-1 infection of the central nervous system. Arch Anat Cytol Pathol. 1997;45:94–105. [PubMed] [Google Scholar]
- Avgeropoulos N, Kelley B, Middaugh L, Arrigo S, Persidsky Y, Gendelman HE, Tyor WR. SCID mice with HIV encephalitis develop behavioral abnormalities. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:13–20. doi: 10.1097/00042560-199805010-00003. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L, Gendelman HE. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1599–1611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelivyanskaya ML, Nelson JA, Poluektova L, Uberti M, Mellon M, Gendelman HE, Boska MD. Tracking superparamagnetic iron oxide labeled monocytes in brain by high-field magnetic resonance imaging. J Neurosci Res. 2003;73:284–295. doi: 10.1002/jnr.10693. [DOI] [PubMed] [Google Scholar]
- Nukuna A, Gendelman HE, Limoges J, Rasmussen J, Poluektova L, Ghorpade A, Persidsky Y. Levels of human immunodeficiency virus type 1 (HIV-1) replication in macrophages determines the severity of murine HIV-1 encephalitis. J Neurovirol. 2004;10 Suppl 1:82–90. doi: 10.1080/753312757. [DOI] [PubMed] [Google Scholar]
- Peng H, Erdmann N, Whitney N, Dou H, Gorantla S, Gendelman HE, Ghorpade A, Zheng J. HIV-1-infected and/or immune activated macophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006;54:619–629. doi: 10.1002/glia.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sas AR, Bimonte-Nelson H, Smothers CT, Woodward J, Tyor WR. Interferon-{alpha} causes neuronal dysfunction in encephalitis. J Neurosci. 2009;29:3948–3955. doi: 10.1523/JNEUROSCI.5595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoges J, Persidsky Y, Bock P, Gendelman HE. Dexamethasone therapy worsens the neuropathology of human immunodeficiency virus type 1 encephalitis in SCID mice. J Infect Dis. 1997;175:1368–1381. doi: 10.1086/516469. [DOI] [PubMed] [Google Scholar]
- Limoges J, Persidsky Y, Poluektova L, Rasmussen J, Ratanasuwan W, Zelivyanskaya M, McClernon DR, Lanier ER, Gendelman HE. Evaluation of antiretroviral drug efficacy for HIV-1 encephalitis in SCID mice. Neurology. 2000;54:379–389. doi: 10.1212/wnl.54.2.379. [DOI] [PubMed] [Google Scholar]
- Masliah E, DeTeresa RM, Mallory ME, Hansen LA. Changes in pathological findings at autopsy in AIDS cases for the last 15 years. AIDS. 2000;14:69–74. doi: 10.1097/00002030-200001070-00008. [DOI] [PubMed] [Google Scholar]
- Michaels J, Sharer LR, Epstein LG. Human immunodeficiency virus type 1 (HIV-1) infection of the nervous system: a review. Immunodefic Rev. 1988;1:71–104. [PubMed] [Google Scholar]
- Li CM, Lee YY, Ho YR. Acute meningoencephalitis as initial presentation of human immunodeficiency virus infection: report of two cases. J Microbiol Immunol Infect. 2002;35:195–198. [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Stone WS, Ingram DK, Reynolds J, Gold PE, Conti LH, Pontecorvo MJ, Wenk GL, Olton DS. Individual differences in aging: behavioral and neurobiological correlates. Neurobiol Aging. 1989;10:31–43. doi: 10.1016/s0197-4580(89)80008-9. [DOI] [PubMed] [Google Scholar]
- Sjoo F, Hassan Z, Abedi-Valugerdi M, Griskevicius L, Nilsson C, Remberger M, Aschan J, Concha H, Gaughan U, Hassan M. Myeloablative and immunosuppressive properties of treosulfan in mice. Exp Hematol. 2006;34:115–121. doi: 10.1016/j.exphem.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Livingston AG, Wingard JR, Nishikawa S, Ogawa M. An assay for long-term engrafting human hematopoietic cells based on newborn NOD/SCID/beta2-microglobulin(null) mice. Exp Hematol. 2002;30:488–494. doi: 10.1016/s0301-472x(02)00784-1. [DOI] [PubMed] [Google Scholar]
- Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien E, Aukrust P, Sundan A, Muller F, Froland SS, Espevik T. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: correlation to disease progression and clinical events. Blood. 1998;92:2084–2092. [PubMed] [Google Scholar]
- Pandrea I, Gaufin T, Brenchley JM, Gautam R, Monjure C, Gautam A, Coleman C, Lackner AA, Ribeiro RM, Douek DC, Apetrei C. Cutting edge: experimentally induced immune activation in natural hosts of simian immunodeficiency virus induces significant increases in viral replication and CD4+ T cell depletion. J Immunol. 2008;181:6687–6691. doi: 10.4049/jimmunol.181.10.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, Zaman T, Stone D, Mefford M, Morgello S, Singer EJ, Wolinsky SM, Gabuzda D. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoges J, Poluektova L, Ratanasuwan W, Rasmussen J, Zelivyanskaya M, McClernon DR, Lanier ER, Gendelman HE, Persidsky Y. The efficacy of potent anti-retroviral drug combinations tested in a murine model of HIV-1 encephalitis. Virology. 2001;281:21–34. doi: 10.1006/viro.2000.0758. [DOI] [PubMed] [Google Scholar]
- Dou H, Birusingh K, Faraci J, Gorantla S, Poluektova LY, Maggirwar SB, Dewhurst S, Gelbard HA, Gendelman HE. Neuroprotective activities of sodium valproate in a murine model of human immunodeficiency virus-1 encephalitis. J Neurosci. 2003;23:9162–9170. doi: 10.1523/JNEUROSCI.23-27-09162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Ellison B, Bradley J, Kasiyanov A, Poluektova LY, Xiong H, Maggirwar S, Dewhurst S, Gelbard HA, Gendelman HE. Neuroprotective mechanisms of lithium in murine human immunodeficiency virus-1 encephalitis. J Neurosci. 2005;25:8375–8385. doi: 10.1523/JNEUROSCI.2164-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla S, Liu J, Wang T, Holguin A, Sneller HM, Dou H, Kipnis J, Poluektova L, Gendelman HE. Modulation of innate immunity by copolymer-1 leads to neuroprotection in murine HIV-1 encephalitis. Glia. 2008;56:223–232. doi: 10.1002/glia.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert D, Dash PK, Serradji N, Dong CZ, Clayette P, Heymans F, Dou H, Gorantla S, Gelbard HA, Poluektova L, Gendelman HE. Development of a platelet-activating factor antagonist for HIV-1 associated neurocognitive disorders. J Neuroimmunol. 2009;213:47–59. doi: 10.1016/j.jneuroim.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert D, Dash PK, Gorantla S, Dou H, Schifitto G, Maggirwar SB, Dewhurst S, Poluektova L, Gelbard HA, Gendelman HE. Neuroprotective activities of CEP-1347 in models of neuroAIDS. J Immunol. 2010;184:746–756. doi: 10.4049/jimmunol.0902962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla S, Liu J, Sneller H, Dou H, Holguin A, Smith L, Ikezu T, Volsky DJ, Poluektova L, Gendelman HE. Copolymer-1 induces adaptive immune anti-inflammatory glial and neuroprotective responses in a murine model of HIV-1 encephalitis. J Immunol. 2007;179:4345–4356. doi: 10.4049/jimmunol.179.7.4345. [DOI] [PubMed] [Google Scholar]
- Potula R, Haorah J, Knipe B, Leibhart J, Chrastil J, Heilman D, Dou H, Reddy R, Ghorpade A, Persidsky Y. Alcohol Abuse Enhances Neuroinflammation and Impairs Immune Responses in an Animal Model of Human Immunodeficiency Virus-1 Encephalitis. Am J Pathol. 2006;168:1335–1344. doi: 10.2353/ajpath.2006.051181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potula R, Poluektova L, Knipe B, Chrastil J, Heilman D, Dou H, Takikawa O, Munn DH, Gendelman HE, Persidsky Y. Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in an animal model of HIV-1 encephalitis. Blood. 2005;106:2382–2390. doi: 10.1182/blood-2005-04-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla S, Markarov E, Roy D, Finke-Dwyer J, Murrin LC, Gendelman HE, Poluektova L. Immunoregulation of a CB2 Receptor Agonist in a Murine Model of NeuroAIDS. J Neuroimmune Pharmacol. 2010;5:456–468. doi: 10.1007/s11481-010-9225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]