Abstract
Deep infiltrating endometriosis (DIE) is characterized by chronic pain, hyperproliferation of endometriotic cells and fibrosis. Since cannabinoids are endowed with antiproliferative and antifibrotic properties, in addition to their psychogenic and analgesic effects, cannabinoid agonists have been evaluated in DIE both in vitro and in vivo. The in vitro effects of the cannabinoid agonist WIN 55212-2 were evaluated on primary endometriotic and endometrial stromal and epithelial cell lines extracted from patients with or without DIE. Cell proliferation was determined by thymidine incorporation and production of reactive oxygen species by spectrofluorometry. ERK and Akt pathways were studied by immunoblotting. Immunoblotting of α-smooth muscle actin was studied as evidence of myofibroblastic transformation. The in vivo effects of WIN 55212-2 were evaluated on Nude mice implanted with human deep infiltrating endometriotic nodules. The in vitro treatment of stromal endometriotic cells by WIN 55212-2 decreased cell proliferation, reactive oxygen species production, and α-smooth muscle actin expression. The decrease in cell proliferation induced by WIN 55212-2 was not associated with a decrease in ERK activation, but was associated with the inhibition of Akt activation. WIN 55212-2 abrogated the growth of endometriotic tissue implanted in Nude mice. Cannabinoid agonists exert anti-proliferative effects on stromal endometriotic cells linked to the inhibition of the Akt pathway. These beneficial effects of cannabinoid agonists on DIE have been confirmed in vivo.
Endometriosis, a common disease that affects about 6 to 10% of women of childbearing age,1 is characterized by the presence of endometrial tissue outside of the uterine cavity.1,2 Deep infiltrating endometriosis (DIE) is an aggressive form of the disease that involves muscularis regardless of location (bladder, intestine, ureter, etc.).3 DIE is responsible for chronic pelvic pain whose intensity is correlated with the depth of lesions,4,5 and induces disability6 and infertility.7 In DIE, the tissular lesion includes fibrosis in addition to the endometrial tissue (endometriotic gland, simple endometrial epithelium with stroma). The presence of fibrosis in DIE explains the low hormonal dependence of lesions and the difficulties in surgical resection.8 The medical treatments currently used are based on hormonal therapy that blocks ovarian function, but whose effects are transient. Therefore, surgical resection of the lesions is the only curative treatment. Surgery is often necessarily extensive and is associated with significant morbidity.9 It would be interesting to find an alternative medical treatment that targets the hyperproliferative phenotype of endometriotic cells which is associated with increased endogenous oxidative stress and activation of the ERK pathway.10
The cannabinoids are well known for their psychogenic effects and their role in inflammation and immunity.11 They are also endowed with properties that can be used in the control of three major aspects of DIE: hyperproliferation, fibrosis, and chronic pain. Because of their implication in proliferation, apoptosis, and angiogenesis, the cannabinoids control cell growth.12,13,14 Their antiproliferative effects result from the inhibition of growth factors15,16 and the deregulation of such signaling pathways as Ras-Raf-MKKK1-ERK1/2,17 PI3K-Akt/PKB-mTOR18 and c-Jun N-terminal kinase-MAPK.19 These mechanisms have suggested new targets in cancer treatment20 and also in endometriosis, since endometriotic cells have a hyperproliferative phenotype and pro-angiogenic properties.21 In addition, several experimental studies have reported an antifibrotic role of cannabinoid agonists.22,23,24,25,26,27,28 If such antifibrotic effect of cannabinoid agonists could be demonstrated in DIE it would allow a less extensive surgery. Finally, cannabinoids have analgesic properties and have been used for a long time in treating chronic pain.29,30,31
Therefore, we have evaluated the effects of cannabinoid agonists in vitro on cells extracted from biopsies of deep infiltrating endometriosis and in vivo on a mouse model of endometriosis. We conclude from our data that cannabinoid agonists represent a promising approach in the treatment of DIE.
Materials and Methods
Sample Collection
Biopsies of eutopic endometrium and deep infiltrating endometriotic nodules were obtained from 14 patients undergoing surgical treatment for DIE with rectal involvement. Low rectal endometriosis was defined preoperatively, based on the following clinical and endoscopic ultrasonographic criteria: a) rectal invasion of the infraperitoneal rectum located within 8 cm of the dentate line, reachable on rectal examination; and b) full-thickness invasion of the muscular layer greater than 15 mm on rectal endoscopic ultrasonography.9
DIE was confirmed in all cases by a pathologist experienced in endometriosis pathology (S.A.). All of the patients had been treated by luteinizing hormone releasing hormone agonists before surgery since for at least one month. Control endometrial specimens were obtained from 12 patients without macroscopic endometriosis undergoing laparoscopy for other reasons (tubal infertility, nonendometriotic ovarian cyst, myoma). Ethics approval for this study has been obtained from the ethics committee at Cochin Hospital (n°05-2006 “génomique et protéomique de l’endométriose”). Written informed consent was obtained from each patient and control. Specimens were collected under sterile conditions and immediately transported to our laboratory in Dulbecco’s modified Eagle’s medium (Gibco Invitrogen, Cergy Pontoise, France) with 10% fetal calf serum. No steroid hormones were added in cell culture. Estrogen (e2) and progesterone (p4) were undetectable in cell culture supernatants as determined by an immunodiagnostic system (Advia CentaurXP, Siemens, Health Care Diagnostics, France, Saint-Denis). The absence of steroid hormones in culture supernatants reproduces clinical conditions in patients submitted to a treatment by luteinizing hormone releasing hormone agonists. The period of time elapsed between the biopsy and the procedure of cell isolation never exceeded 1 hour. The same culture medium was used throughout the study.
Cell Isolation and Culture
Primary endometrial and deep endometriotic cell cultures were prepared from biopsies as described.32 Biopsy specimens were rinsed and minced into small pieces then digested with 5% dispase and collagenase (2 mg/ml, Gibco Invitrogen, Cergy Pontoise, France) for 1 hour at 37°C and separated using serial filtration. Red blood cells were removed by hypotonic lysis (0.15 mol/L NH4Cl, 1 mmol/L KHCO3, 0.1 mmol/L Na2EDTA). Debris was removed using 100-μm aperture sieves. Epithelial cells were retained on 40-μm aperture sieves while stromal cells remained in the filtrate. Both cells were plated onto Primaria flasks (Becton Dickinson Labware, Le Pont de Claix, France) and cultured in Dulbecco’s modified Eagle’s medium (Gibco Invitrogen, Cergy Pontoise, France) with 10% fetal calf serum. For each patient with DIE, four cell populations were obtained: eutopic endometrial stromal cells (Es), eutopic endometrial epithelial cells (Ee), deep infiltrating endometriotic stromal cells (Ds), and deep infiltrating endometriotic epithelial cells (De). For each control, we used two cell populations: control endometrial stromal cells (Cs) and control endometrial epithelial cells (Ce).
The purity of stromal and epithelial cell suspension was assessed by staining with 1:100 fluorescein isothiocyanate-labeled anti-cytokeratin and 1:100 Cy3-labeled anti-vimentin antibodies (Sigma Aldrich, St. Louis, MO). Fluorescence was analyzed using an Olympus fluorescent microscope (Hambourg, Germany) and images were captured using the Cell Imaging station (Olympus). Both populations were negative for CD3 (T cells), CD45 (leukocytes), and CD11b (monocytes and granulocytes) staining. All of the experiments were performed on primary cultures of each cell population and the various tests were performed in triplicates.
Cell Proliferation and Viability Assays
The cells that had reached confluence were harvested following trypsin treatment. Cells (104 per well) were seeded in 96-well plates (Nunc, Roskilde, Denmark) and incubated for 48 hours in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum at 37°C under 5% CO2 alone or with various concentrations of WIN 55212-2 (from 0.3 μmol/L to 40 μmol/L as described in figure legends). Cell proliferation was determined by pulsing the cells with [3H]-thymidine (1 μCi per well, Amersham, GE Health care) during the last 18 hours of culture33 and measuring the radioactivity incorporated by liquid scintillation counting. Results are expressed as counts per minutes (cpm).
Cell viability was analyzed by Hoechst 33342 staining (Molecular Probes). Cells were incubated in 100 μl PBS with 1 μg/ml Hoechst 33342 for 30 minutes. Fluorescence intensity was recorded at the end of the incubation with a fluorescence excitation/emission maxima of 350/461 nm.
Cellular Production of Reactive Oxygen Species
Cells (104 per well) were seeded in 96-well plates (Nunc, Roskilde, Denmark) and incubated for 18 hours alone or with various concentrations of WIN 55212-2 (from 0.3 μmol/L to 40 μmol/L as described in figure legends). Cellular levels of O2·−, H2O2 and nitric oxide (NO) were assessed by spectrofluorometry using the oxidation of 125 μmol/L dihydroethidium (DHE) (Interchim, Montluçon, France), 100 μmol/L 2′,7′-dichlorohydrofluorescein diacetate (H2DCFDA) (Molecular Probes, Eugene, OR) and 4′,5′-diaminofluorescein diacetate (DAF2DA) (Sigma Aldrich, Saint Quentin-Fallavier, France) respectively, during 5 hours. The levels of O2·−, H2O2, and NO were calculated in each sample as follows: reactive oxygen species rate (arbitrary units/min/104cells) = (fluorescence intensity [arbitrary unit] at T 300 minutes − fluorescence intensity [arbitrary unit] at T 0/300 minutes/number of cells.33
Immunoblotting of Cell Lysates
Untreated cells or cells treated by 20 μmol/L of WIN 55212-2 were lysed in ice-cold radioimmunoprecipitation assay buffer (10 mmol/L TrisHCl, pH 7.5, 5 mol/L NaCl, 1% Triton X-100, 0.1% SDS) supplemented with 25 mmol/L sodium fluoride, 0.5 mmol/L sodium orthovanadate, and 1% anti-protease. Equal amounts of proteins (30 μg) were loaded and separated by 10% SDS-polyacrylamide gel electrophoresis. Transfer and blocking experiments were performed. For CB1 and CB2 staining, polyvinylidene difluoride membrane was saturated with 5% skim milk for 1 hour, then incubated with rabbit anti-human CB1 IgG antibodies or with rabbit anti-human CB2 IgG antibodies (Interchim, Montluçon, France) overnight at 4°C. For ERK and pERK staining, polyvinylidene difluoride membrane was saturated with 5% skim milk 1 hour then incubated with rabbit anti-human ERK IgG antibodies and rabbit anti-human pERK IgG antibodies (Santa Cruz Biotechnology, Santa Cruz, California) overnight at 4°C. For α-smooth muscle actin (SMA) staining, polyvinylidene difluoride membrane was saturated with 5% skim milk 1 hour, and then incubated with mouse anti-human α-SMA IgG antibodies (Sigma Aldrich, St. Louis, MO) overnight at 4°C.
The binding of specific antibodies was revealed using horseradish peroxidase-conjugated goat anti-rabbit IgG antibodies for CB1, CB2, ERK, or pERK, or horseradish peroxidase-conjugated goat anti-mouse IgG antibodies for α-SMA, then visualized by an Enhanced ChemoLuminescence system (Pierce Perbio Sciences, Brebières, France).34
Mouse Model
Fourteen Nude mice were grafted with two fragments of a nodule from one patient with DIE. Female nude mice between 6 and 8 weeks of age weighing 20 to 22 g were used in this study. Animals received humane care in compliance with institutional guidelines. Mice were anesthetized with intraperitoneal injections of Avertine. An incision was made on the ventral midline and two fragments of human endometriotic nodules were sutured to the parietal peritoneum with 7/0 prolene. Each fragment was measured using a rule caliper. An additional fragment was fixed with 10% formol and set in paraffin for initial histological analysis. The abdominal wall was sutured with a 6/0 nylon thread.35 A subcutaneous injection of 0.5 μg β-estradiol was performed on day 1 and day 2 to facilitate the implantation of endometriotic nodules.36
On day 7 after implantation, mice were operated to confirm the viability of the implant. Treatment started on day 7: the treated group received intraperitoneal injections of WIN 55212-2 (3 mg/kg in 100 μl of PBS, 5 days a week for 2 weeks) and the control group received intraperitoneal injections of 100 μl PBS under the same conditions. After 2 weeks of treatment, animals were sacrificed by cervical dislocation. Implants were extracted, measured. The volumes of the implant were calculated as follows: TV (mm3) = (L × W2)/2, where L is the longest and W the shortest radius of the tumor in millimeters. Results were expressed as means of tumor volumes ± SE.
Statistical Analysis
All of the results are the means of independent triplicate experiments for each cellular population from each patient. Means were compared by the Student’s test. A level of P < 0.05 was accepted as significant.
Results
Biopsies
Fourteen patients were included in the study. Twelve biopsies of eutopic endometrium and 14 biopsies of deep endometriotic nodules were obtained from those patients. Twelve Ee cell lines and 12 Es cell lines were extracted from 12 samples of eutopic endometrium and 14 De cell lines and 14 Ds cell lines were extracted from 14 samples of deep infiltrating endometriotic nodules. Four cell lines were eliminated for failure of culture (n = 3) or bacterial contamination (n = 1). Finally, 10 Ee cell lines, 11 Es cell lines, 13 De cell lines, and 14 Ds cell lines could be studied.
Twelve control women were included in the study. Twelve biopsies of healthy endometrium were obtained. The 12 samples of endometrium provided 12 epithelial and stromal control endometrial cell lines. Three cell lines were eliminated for failure of culture. Finally, 11 Ce cell lines and 10 Cs cell lines have been used in this study.
Expression of Cannabinoid Receptors CB1 and CB2 by Endometriotic Cells
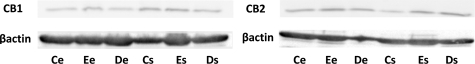
The CB1 and CB2 receptors were equally present in the epithelial and stromal cell lines derived from eutopic endometrium (Ee, Es) and deep infiltrating endometriotic nodules (De, Ds) (Figure 1).
Figure 1.
Expression of CB1 and CB2 on endometriotic cells. Expression of cannabinoid receptors CB1 and CB2 was determined by western blots on each cell line lysate: 10 eutopic endometrial epithelial cell lines (Ee), 11 eutopic endometrial stromal cell lines (Es), 13 deep infiltrating endometriotic epithelial cell lines (De) and 14 deep infiltrating endometriotic stromal cell lines (Ds), 11 control endometrial epithelial cell lines (Ce), and 10 control endometrial stromal cell lines (Cs). The figure represents one representative Western blot obtained with all of the cell lines extracted from one patient and from one control. CB1 and CB2 are equally present in Ce, Ee, De, Cs, Es, and Ds cells.
Effect of WIN 55212-2 on the Proliferation of Endometriotic Stromal Cells
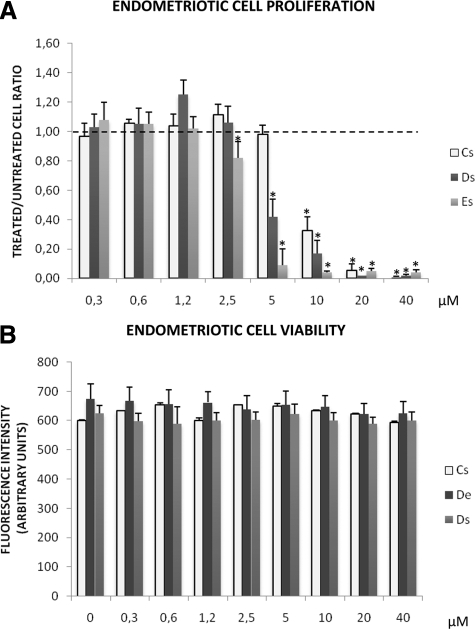
The Es, Ds, and Cs cells were treated with increasing concentrations of WIN 55212-2 (0.3 μmol/L to 40 μmol/L). At the highest concentration (40 μmol/L), the proliferative rate of Es, Ds, and Cs cells was reduced by 96%, 98%, and 99% respectively (P < 0.001 in all cases). The decrease was dose-dependent and not significant at concentrations below 5 μmol/L (Cs), 2.5 μmol/L (Ds), and 1.25 μmol/L (Es) (Figure 2A).
Figure 2.
Effect of WIN 55212-2 on cell proliferation and cell viability. A: Proliferative rate of each stromal endometriotic cell line (11 eutopic endometrial stromal cell lines, Es, and 14 deep infiltrating endometriotic stromal cell lines, Ds) and of three control endometrial stromal cell lines (Cs) treated in vitro with increasing concentrations (0.3–40 μmol/L) of WIN 55212-2. Black dotted line shows the basal level of proliferation of untreated endometriotic cells. Cell proliferation was determined by thymidine incorporation. Results are expressed as ratios of treated versus untreated cells: *P < 0.05. B: Cell viability of each stromal endometriotic cell line (11 eutopic endometrial stromal cell lines, Es, and 14 deep infiltrating endometriotic stromal cell lines, Ds) and of three control endometrial stromal cell lines (Cs) untreated or treated in vitro with increasing concentrations (0.3 to 40 μmol/L) of WIN 55212-2. Analysis by Hoechst 33342 staining. Fluorescence intensity was recorded with a fluorescence excitation/emission maxima of 350/461 nm.
At 5 μmol/L, the proliferative rate of Ds cells and Es cells were significantly decreased by 57% (P < 0.01) and 72% (P < 0.001) respectively compared to Cs (Figure 2A). At 1.25 μmol/L, the proliferative rate of Ds cells was significantly increased by 98% (P < 0.001) (Figure 2A).
Effect of WIN 55212-2 on the Viability of Endometriotic Stromal Cells
WIN 55212-2 was not cytotoxic to Es and Ds cells at the concentration tested as shown by Hoechst 33342 staining (Figure 2B).
Effect of WIN 55212-2 on the Endogenous Production of Reactive Oxygen Species by Endometriotic Stromal Cells
Production of Superoxide Anions (O2·−)
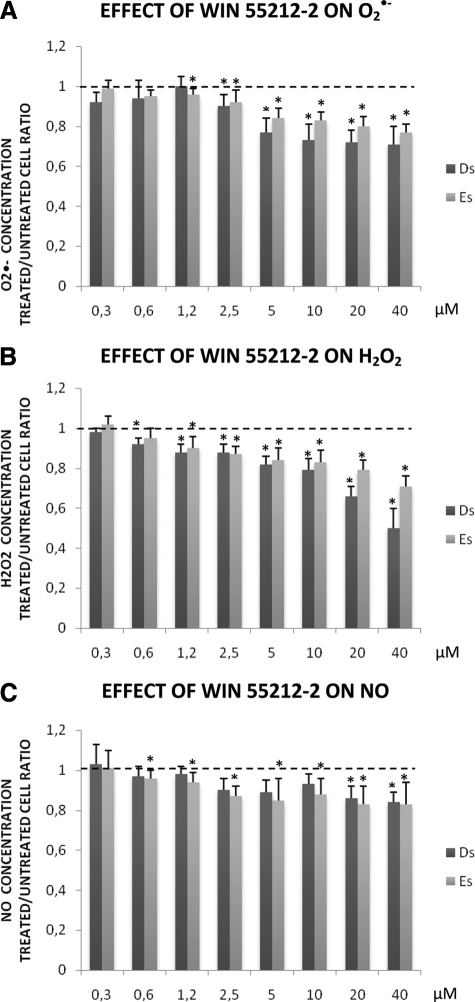
Using 40 μmol/L WIN 55212-2, the production of O2·− in Es and Ds cells was significantly reduced by 23% and 39%, respectively (Figure 3A, P < 0.001 in both cases). The production of O2·− was dose-dependently decreased and was not significant below 1.25 μmol/L.
Figure 3.
Effect of WIN 55212-2 on ROS production. Productions of O2·− (A), H2O2 (B), and NO (C) in each stromal endometriotic cell line (11 eutopic endometrial stromal cell lines, Es, and 14 deep infiltrating endometriotic stromal cell lines, Ds) treated in vitro with increasing concentrations (0.3 to 40 μmol/L) of WIN 55212-2 compared to untreated cells. Black dotted line shows the basal level of production in untreated cells. Production of ROS was assessed by spectrofluorometry using specific fluorochromes. Results are expressed as ratios of treated versus untreated cells: *P < 0.05.
Production of H2O2
Using 40 μmol/L WIN 55212-2, the production of H2O2 in Es and Ds cells was significantly reduced by 29% and 50%, respectively (Figure 3B, P < 0.001 in both cases). The production of H2O2 was dose-dependently decreased and was not significant below 0.6 μmol/L.
Production of NO
Using 40 μmol/L WIN 55212-2, the production of NO in Es and Ds cells was significantly reduced by 17% and 16%, respectively (Figure 3C, P < 0.05 in both cases). The production of NO was dose-dependently decreased and was not significant below 10 μmol/L (Ds) and 0.3 μmol/L (Es).
Effect of WIN 55212-2 on the ERK Pathway
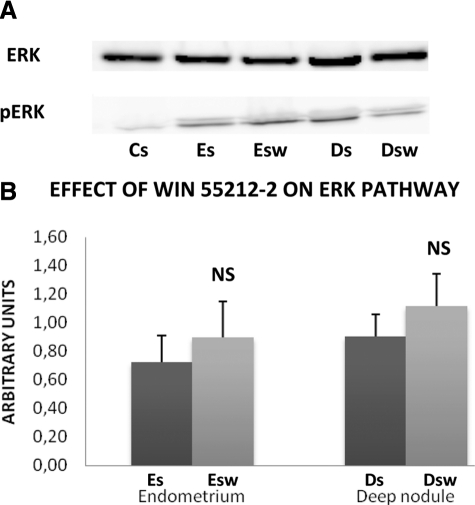
The ERK protein was equally present in the stromal cell lines derived from control endometrium (Cs), eutopic endometrium from untreated and treated patients (Es, Esw) and deep infiltrating endometriotic nodules from untreated and treated patients (Ds, Dsw). The phosphorylated activated form of ERK, pERK, was undetectable in Cs cells but equally present in Es, Esw, Ds, and Dsw cells (Figure 4A).
Figure 4.
Effect of WIN 55212-2 on ERK pathway. A: Representative Western blot of ERK and pERK. Western blotting of ERK and pERK were performed on lysates of each untreated stromal cell line (10 control endometrial stromal cell lines, Cs, 11 eutopic endometrial stromal cell lines, Es, and 14 deep infiltrating endometriotic stromal cell lines, Ds) and on lysates of each stromal cell line treated in vitro by 20 μmol/L of WIN 55212-2 (11 eutopic endometrial stromal cell lines, Esw, and 14 deep infiltrating endometriotic stromal cell lines, Dsw) using specific anti-ERK and anti-pERK antibodies. B: Quantitative analysis of ERK and pERK. Quantitative analysis of ERK and pERK was performed in each untreated stromal endometriotic cell line (11 eutopic endometrial stromal cell lines, Es, and 14 deep infiltrating endometriotic stromal cell lines, Ds) and in each stromal endometriotic cell line treated in vitro by 20 μmol/L WIN 55212-2 (11 eutopic endometrial stromal cell lines, Esw, and 14 deep infiltrating endometriotic stromal cell lines, Dsw). The mean optical density ratio pERK/ERK was calculated in each endometriotic cell type. NS: no significant.
The differences were significant neither between Esw cells and Es cells, nor between Dsw and Ds cells (Figure 4B).
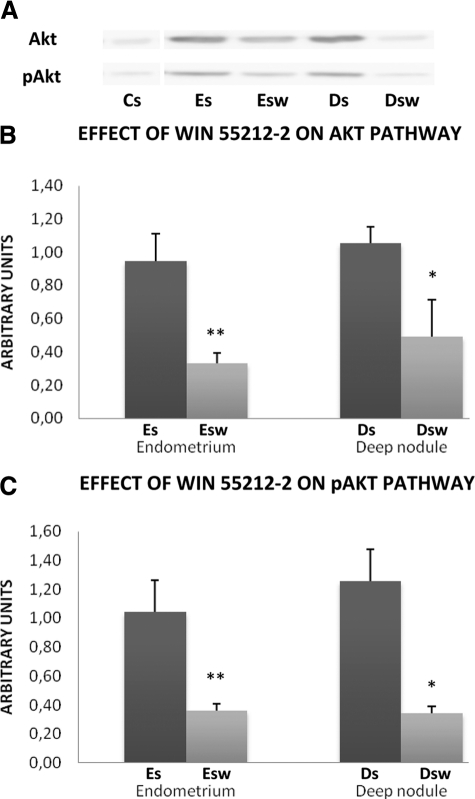
Effect of WIN 55212-2 on the Akt Pathway
The Akt protein and its phosphorylated activated form, pAkt, were undetectable in Cs cells. Akt and pAkt were present in cell lines derived from patients but at higher levels in Es cells and in Ds cells than in Esw cells and in Dsw cells (Figure 5A).
Figure 5.
Effect of WIN 55212-2 on Akt pathway. A: Representative Western blot of Akt and pAkt. Western blotting of Akt and pAkt were performed on lysates of each untreated stromal cell line (10 control endometrial stromal cell lines, Cs, 11 eutopic endometrial stromal cell lines, Es, and 14 deep infiltrating endometriotic stromal cell lines, Ds) and on lysates of each stromal cell line treated in vitro by 20 μmol/L of WIN 55212-2 (11 eutopic endometrial stromal cell lines, Esw, and 14 deep infiltrating endometriotic stromal cell lines, Dsw) using specific anti-Akt and anti-pAkt, antibodies. B: Quantitative analysis of Akt and (C) quantitative analysis of pAkt. Quantitative analysis of Akt and pAkt was performed in each untreated stromal endometriotic cell line (11 eutopic endometrial stromal cell lines, Es, and 14 deep infiltrating endometriotic stromal cell lines, Ds) and in each stromal endometriotic cell line treated in vitro by 20 μmol/L WIN 55212-2 (11 eutopic endometrial stromal cell lines, Esw, and 14 deep infiltrating endometriotic stromal cell lines, Dsw). The mean optical density of Akt and pAkt were calculated in each endometriotic cell type: *P < 0.05, **P < 0.01.
Akt level was reduced by 65% in Esw cells versus Es cells (P < 0.01) and by 50% in Dsw cells versus Ds cells (P < 0.05) (Figure 5B).
pAkt level was reduced by 65% in Esw cells versus Es cells (P < 0.01) and by 73% in Dsw cells versus Ds cells (P < 0.01) (Figure 5C).
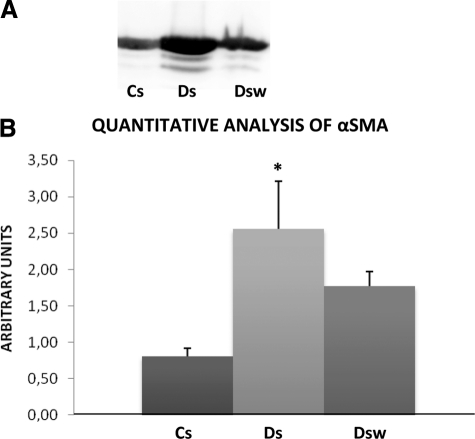
Expression of αSMA by Deep Infiltrating Endometriotic Stromal Cells
The αSMA protein was present in CS cells, Ds cells and Dsw cells, but in higher amounts in Ds cells (Figure 6A).
Figure 6.
Effect of WIN 55212-2 on expression of αSMA. A: Representative Western blot of αSMA. Western blotting of αSMA was performed on lysates of each untreated stromal cell line (10 control endometrial stromal cells, Cs, and 14 deep infiltrating endometriotic stromal cells, Ds) and in each 14 deep infiltrating endometriotic stromal treated in vitro by 20 μmol/L WIN 55212-2 (Dsw) using specific antibody anti-αSMA. B: Quantitative analysis of αSMA. Quantitative analysis of αSMA was performed in each untreated cell line (10 control endometrial stromal cells, Cs, and 14 untreated deep infiltrating endometriotic stromal cells, Ds) and in each 14 deep infiltrating endometriotic stromal cell line treated in vitro by 20 μmol/L WIN 55212-2 (Dsw). The mean optical density of αSMA was calculated in each endometriotic cell type: *P < 0.05.
αSMA level was threefold higher in Ds cells than in Cs cells (P < 0.05). Its decrease (30%) in Dsw cells compared to Ds cells did not reach significance Figure 6B).
Mouse Model
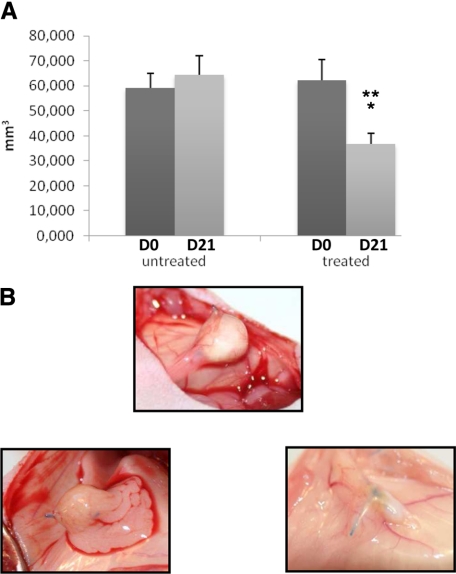
Fourteen nude mice were grafted with two fragments of nodules from one patient with DIE. Two mice died immediately after surgery and one mouse died on day 1 in the aftermath of an evisceration. Two fragments of nodules were removed, one on day 7 for lack of implantation and one on day 21 because of an abscess. The control group included six mice with 11 implants and the treated group included five mice with 9 implants.
In control mice, there was no difference between the volume of DIE implants on day 0 (59.3 mm3) and the volume of DIE implants on day 21 (64.5 mm3). In treated mice, the volume of DIE implants on day 21 was reduced by 41% compared to the volume of DIE implants on day 0 (36.7 mm3 versus 62.1 mm3, respectively, P < 0.01). On day 21, the volume of DIE implants in treated mice was reduced by 43% compared to the volume of DIE implants in control mice (36.7 mm3 versus 64.5 mm3 respectively, P < 0.01) (Figure 7A and B).
Figure 7.
Effect on WIN 55212-2 on deep infiltrating endometriotic tissue on mouse model. A: Volumes of implants were calculated on days 0 and 21 of untreated and treated mice. Volumes on day 21 versus volumes on day 0: *P < 0.01; volumes on day 21 in treated mice versus untreated mice: **P < 0.01. B: Macroscopic views of deep infiltrating implant in untreated mice (top) and in treated mice on days 0 and 21 (bottom).
Discussion
This report describes the effects of WIN 55212-2, a cannabinoid agonist, on deep infiltrating endometriotic cell proliferation, and also its on fibrosis which is another characteristic of DIE.
WIN 55212-2 was chosen because it is a nonselective agonist and because both types of cannabinoid receptors, CB1 and CB2, are expressed by stromal and epithelial cells in the eutopic endometrium, in the deep infiltrating endometriotic nodules and in the healthy endometrium as well. The antiproliferative effect of WIN 55212-2 is more powerful on eutopic endometrial stromal cells and deep infiltrating endometriotic stromal cells than on control endometrial stromal cells. This can be explained by the hyperproliferative phenotype of endometriotic cells compared to normal endometrial cells. An antiproliferative effect of cannabinoid agonists has already been reported in cancer, in particular breast cancer,15 prostate cancer,15,16 colorectal cancer,37 and melanoma.38 The antiproliferative effect of this molecule on endometriotic cells is not so surprising since cancer cells that have several metabolic characteristics in common with endometriotic cells.21 Reciprocally, we have already shown that 5-FU, an antimetabolite commonly used in the treatment of cancer was effective in DIE.39
To understand the mechanism of the antiproliferative properties of WIN 55212-2, we have studied the effects of the molecule on the endogenous oxidative stress, which is increased in endometriotic cells.10 At high concentrations of WIN 55212-2, the inhibition of O2·− and H2O2 productions was higher in the deep infiltrating endometriotic stromal cells than in the eutopic endometrial stromal cells. The regulation of ROS production by cannabinoid agonists has also been shown in macrophages40 and in cardiomyocytes.41
ROS have been shown to increase the proliferation of endometriotic cells through the activation of ERK1/2. Therefore, the decreased production of ROS by WIN could have led to a decrease in ERK activation. Despite the decrease in ROS production and the decreased proliferation of endometriotic cells by WIN, ERK1/2 activation remained stable in WIN-treated endometriotic cells. On the other hand, a direct activation of ERK by WIN has been shown.17,38 This agonistic effect could have been neutralized in endometriotic cells by the antioxidant effect of win on those cells. This result rules out the involvement of the ERK pathway as the one involved in the control of endometriotic cell proliferation by WIN.
On the other hand, the Akt pathway which is involved in the antiproliferative effect of cannabinoids,42,43 has never been explored in endometriosis. In our hands, WIN 55212-2 inactivates the Akt pathway. ROS having few interactions with the Akt pathway, this data confirms the absence of connection between the antioxidant effects of WIN and its antiproliferative effects that appear to be mediated by the Akt pathway.
These data could also explain the absence of cytotoxic effect of WIN 55212-2 on endometriotic cells although cannabinoid agonists have a cytotoxic effect on various cells including glial cells,42 human T-cell lymphoma,43 breast cancer,44 or prostate cancer.45 Actually, the apoptosis induced by cannabinoid agonists in glial cells of rats is initiated by the combined inhibitions of ERK and Akt pathways.42 In our model, the absence of cytotoxicity of WIN 55212-2 on endometriotic cells can be explained by the noninhibition of the ERK pathway.
The second benefit potentially expected from the use of cannabinoid agonists in patients with DIE is the inhibition of the fibrotic process. In our hand, WIN 55212-2 inhibits αSMA expression, a marker of myofibroblastic transformation. The antifibrotic property of those substances has been already demonstrated in the skin,22 in the heart,23 in the pancreas,26 and in the liver,25,28 where they exert a curative effect on fibrosis.27 In vitro, the synthesis of type I collagen by fibroblasts from skin biopsies of patients with diffuse systemic sclerosis24 is selectively inhibited by cannabinoid agonists. Limiting the fibrotic process, as shown in our experiments, would allow a less extensive surgery.
In the last part of this report we confirm the antiproliferative effect of WIN 55212-2 in an in vivo model of endometriosis in nude mice. In most studies, the lifetime of implants in nude mice does not exceed 4 weeks. Then a dedifferentiation appears and a B cell infiltrate occurs from the third week on.36 We have circumvented this difficulty by sacrificing the mice 15 days after implantation. Mortality was less than 25% and the implantation rate was above 90% which is similar to the data published.36 After 2 weeks, the treatment by WIN 55212-2 proved effective with a significant decrease in the volume of the implants.
In conclusion, WIN 55212-2 has in vitro antiproliferative and antifibrotic effects in deep infiltrating endometriotic cells. The antiproliferative effect is linked to the inactivation of the Akt pathway. The effectiveness of WIN 55212-2 in vitro, confirmed in vivo in a mouse model of DIE, suggests that the cannabinoid agonists represent a promising therapeutic approach in the treatment of DIE.
Acknowledgments
The authors are grateful to Ms. Agnes Colle for editing the manuscript.
Footnotes
Address reprint requests to Frédéric Batteux, M.D., Ph.D., Laboratoire d’immunologie, Hôpital Cochin, 75679 Paris cedex 14. E-mail: frederic.batteux@cch.aphp.fr.
Supported in part by funds from Paris Decartes University.
B.D. and F.B. contributed equally to the direction of this work.
References
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308:1587–1589. doi: 10.1126/science.1111445. [DOI] [PubMed] [Google Scholar]
- Chapron C, Bourret A, Chopin N, Dousset B, Leconte M, Amsellem-Ouazana D, de Ziegler D, Borghese B. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod. 25:884–889. doi: 10.1093/humrep/deq017. [DOI] [PubMed] [Google Scholar]
- Chapron C, Fauconnier A, Dubuisson JB, Barakat H, Vieira M, Breart G. Deep infiltrating endometriosis: relation between severity of dysmenorrhoea and extent of disease. Hum Reprod. 2003;18:760–766. doi: 10.1093/humrep/deg152. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril. 1991;55:759–765. doi: 10.1016/s0015-0282(16)54244-7. [DOI] [PubMed] [Google Scholar]
- Laursen BS, Bajaj P, Olesen AS, Delmar C, Arendt-Nielsen L. Health related quality of life and quantitative pain measurement in females with chronic non-malignant pain. Eur J Pain. 2005;9:267–275. doi: 10.1016/j.ejpain.2004.07.003. [DOI] [PubMed] [Google Scholar]
- De Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376:730–738. doi: 10.1016/S0140-6736(10)60490-4. [DOI] [PubMed] [Google Scholar]
- Bonte H, Chapron C, Vieira M, Fauconnier A, Barakat H, Fritel X, Vacher-Lavenu MC, Dubuisson JB. Histologic appearance of endometriosis infiltrating uterosacral ligaments in women with painful symptoms. J Am Assoc Gynecol Laparosc. 2002;9:519–524. doi: 10.1016/s1074-3804(05)60530-0. [DOI] [PubMed] [Google Scholar]
- Dousset B, Leconte M, Borghese B, Millischer AE, Roseau G, Arkwright S, Chapron C. Complete surgery for low rectal endometriosis: long-term results of a 100-case prospective study. Ann Surg. 251:887–895. doi: 10.1097/SLA.0b013e3181d9722d. [DOI] [PubMed] [Google Scholar]
- Ngo C, Chereau C, Nicco C, Weill B, Chapron C, Batteux F. Reactive oxygen species controls endometriosis progression. Am J Pathol. 2009;175:225–234. doi: 10.2353/ajpath.2009.080804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- Bifulco M, Di Marzo V. Targeting the endocannabinoid system in cancer therapy: a call for further research. Nat Med. 2002;8:547–550. doi: 10.1038/nm0602-547. [DOI] [PubMed] [Google Scholar]
- Guzman M, Sanchez C, Galve-Roperh I. Cannabinoids and cell fate. Pharmacol Ther. 2002;95:175–184. doi: 10.1016/s0163-7258(02)00256-5. [DOI] [PubMed] [Google Scholar]
- Pisanti S, Borselli C, Oliviero O, Laezza C, Gazzerro P, Bifulco M. Antiangiogenic activity of the endocannabinoid anandamide: correlation to its tumor-suppressor efficacy. J Cell Physiol. 2007;211:495–503. doi: 10.1002/jcp.20954. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Melck D, Palmisano A, Bisogno T, Laezza C, Bifulco M, Di Marzo V. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc Natl Acad Sci USA. 1998;95:8375–8380. doi: 10.1073/pnas.95.14.8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melck D, De Petrocellis L, Orlando P, Bisogno T, Laezza C, Bifulco M, Di Marzo V. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology. 2000;141:118–126. doi: 10.1210/endo.141.1.7239. [DOI] [PubMed] [Google Scholar]
- Gomez del Pulgar T, Velasco G, Sanchez C, Haro A, Guzman M. De novo-synthesized ceramide is involved in cannabinoid-induced apoptosis. Biochem J. 2002;363:183–188. doi: 10.1042/0264-6021:3630183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312 (Pt 2):637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda D, Galve-Roperh I, Haro A, Guzman M. The CB(1) cannabinoid receptor is coupled to the activation of c-Jun N-terminal kinase. Mol Pharmacol. 2000;58:814–820. doi: 10.1124/mol.58.4.814. [DOI] [PubMed] [Google Scholar]
- Fogli S, Breschi M. The molecular bases of cannabinoid action in cancer. Cancer Ther. 2008;6:103–116. [Google Scholar]
- Varma R, Rollason T, Gupta JK, Maher ER. Endometriosis and the neoplastic process. Reproduction. 2004;127:293–304. doi: 10.1530/rep.1.00020. [DOI] [PubMed] [Google Scholar]
- Akhmetshina A, Dees C, Busch N, Beer J, Sarter K, Zwerina J, Zimmer A, Distler O, Schett G, Distler JH. The cannabinoid receptor CB2 exerts antifibrotic effects in experimental dermal fibrosis. Arthritis Rheum. 2009;60:1129–1136. doi: 10.1002/art.24395. [DOI] [PubMed] [Google Scholar]
- Defer N, Wan J, Souktani R, Escoubet B, Perier M, Caramelle P, Manin S, Deveaux V, Bourin MC, Zimmer A, Lotersztajn S, Pecker F, Pavoine C. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy. FASEB J. 2009;23:2120–2130. doi: 10.1096/fj.09-129478. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalez E, Selvi E, Balistreri E, Lorenzini S, Maggio R, Natale MR, Capecchi PL, Lazzerini PE, Bardelli M, Laghi-Pasini F, Galeazzi M. Cannabinoids inhibit fibrogenesis in diffuse systemic sclerosis fibroblasts. Rheumatology (Oxford) 2009;48:1050–1056. doi: 10.1093/rheumatology/kep189. [DOI] [PubMed] [Google Scholar]
- Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, Zimmer A, Mallat A, Lotersztajn S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742–755. doi: 10.1053/j.gastro.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Michalski CW, Maier M, Erkan M, Sauliunaite D, Bergmann F, Pacher P, Batkai S, Giese NA, Giese T, Friess H, Kleeff J. Cannabinoids reduce markers of inflammation and fibrosis in pancreatic stellate cells. PLoS One. 2008;3:e1701. doi: 10.1371/journal.pone.0001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Luque J, Ros J, Fernandez-Varo G, Tugues S, Morales-Ruiz M, Alvarez CE, Friedman SL, Arroyo V, Jimenez W. Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J Pharmacol Exp Ther. 2008;324:475–483. doi: 10.1124/jpet.107.131896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, Serriere-Lanneau V, Ledent C, Mallat A, Lotersztajn S. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671–676. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153:319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Lowitz KA, Hohmann AG, Martin WJ, Hathaway CB, Bereiter DA, Walker JM. Suppression of noxious stimulus-evoked expression of Fos protein-like immunoreactivity in rat spinal cord by a selective cannabinoid agonist. Neuroscience. 1996;70:791–798. doi: 10.1016/s0306-4522(96)83015-6. [DOI] [PubMed] [Google Scholar]
- Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN. Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab. 1997;82:1621–1628. doi: 10.1210/jcem.82.5.3919. [DOI] [PubMed] [Google Scholar]
- Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, Levy E, Goldwasser F, Panis Y, Soubrane O, Weill B, Batteux F. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–956. [PubMed] [Google Scholar]
- Song JS, Kang CM, Yoo MB, Kim SJ, Yoon HK, Kim YK, Kim KH, Moon HS, Park SH. Nitric oxide induces MUC5AC mucin in respiratory epithelial cells through PKC and ERK dependent pathways. Respir Res. 2007;8:28. doi: 10.1186/1465-9921-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner-Tran KL, Webster-Clair D, Osteen KG. Experimental endometriosis: the nude mouse as a xenographic host. Ann NY Acad Sci. 2002;955:328–339. doi: 10.1111/j.1749-6632.2002.tb02793.x. discussion 340–322, 396–406. [DOI] [PubMed] [Google Scholar]
- Grummer R, Schwarzer F, Bainczyk K, Hess-Stumpp H, Regidor PA, Schindler AE, Winterhager E. Peritoneal endometriosis: validation of an in-vivo model. Hum Reprod. 2001;16:1736–1743. doi: 10.1093/humrep/16.8.1736. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Bisogno T, Matias I, De Petrocellis L, Cascio MG, Cosenza V, D'Argenio G, Scaglione G, Bifulco M, Sorrentini I, Di Marzo V. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology. 2003;125:677–687. doi: 10.1016/s0016-5085(03)00881-3. [DOI] [PubMed] [Google Scholar]
- Blazquez C, Carracedo A, Barrado L, Real PJ, Fernandez-Luna JL, Velasco G, Malumbres M, Guzman M. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006;20:2633–2635. doi: 10.1096/fj.06-6638fje. [DOI] [PubMed] [Google Scholar]
- Ngo C, Nicco C, Leconte M, Chereau C, Weill B, Batteux F, Chapron C. Antiproliferative effects of anastrozole, methotrexate, and 5-fluorouracil on endometriosis in vitro and in vivo. Fertil Steril. 2009;94:1632–1638. doi: 10.1016/j.fertnstert.2009.09.031. [DOI] [PubMed] [Google Scholar]
- Han KH, Lim S, Ryu J, Lee CW, Kim Y, Kang JH, Kang SS, Ahn YK, Park CS, Kim JJ. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc Res. 2009;84:378–386. doi: 10.1093/cvr/cvp240. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Batkai S, Patel V, Kashiwaya Y, Liaudet L, Evgenov OV, Mackie K, Hasko G, Pacher P. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 85:773–784. doi: 10.1093/cvr/cvp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellert-Miklaszewska A, Kaminska B, Konarska L. Cannabinoids down-regulate PI3K/Akt and Erk signalling pathways and activate proapoptotic function of Bad protein. Cell Signal. 2005;17:25–37. doi: 10.1016/j.cellsig.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Jia W, Hegde VL, Singh NP, Sisco D, Grant S, Nagarkatti M, Nagarkatti PS. Delta9-tetrahydrocannabinol-induced apoptosis in Jurkat leukemia T cells is regulated by translocation of Bad to mitochondria. Mol Cancer Res. 2006;4:549–562. doi: 10.1158/1541-7786.MCR-05-0193. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Nagarkatti M, Nagarkatti PS. Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol. 2005;174:3281–3289. doi: 10.4049/jimmunol.174.6.3281. [DOI] [PubMed] [Google Scholar]
- Sarfaraz S, Afaq F, Adhami VM, Malik A, Mukhtar H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. J Biol Chem. 2006;281:39480–39491. doi: 10.1074/jbc.M603495200. [DOI] [PubMed] [Google Scholar]