Abstract
Adipose tissue secretes adipocytokines for energy homeostasis, but recent evidence indicates that some adipocytokines also have a profound local impact on wound healing. Upon skin injury, keratinocytes use various signaling molecules to promote reepithelialization for efficient wound closure. In this study, we identify a novel function of adipocytokine angiopoietin-like 4 (ANGPTL4) in keratinocytes during wound healing through the control of both integrin-mediated signaling and internalization. Using two different in vivo models based on topical immuno-neutralization of ANGPTL4 as well as ablation of the ANGPTL4 gene, we show that ANGPTL4-deficient mice exhibit delayed wound reepithelialization with impaired keratinocyte migration. Human keratinocytes in which endogenous ANGPTL4 expression was suppressed by either siRNA or a neutralizing antibody show impaired migration associated with diminished integrin-mediated signaling. Importantly, we identify integrins β1 and β5, but not β3, as novel binding partners of ANGPTL4. ANGPTL4-bound integrin β1 activated the FAK-Src-PAK1 signaling pathway, which is important for cell migration. The findings presented herein reveal an unpredicted role of ANGPTL4 during wound healing and demonstrate how ANGPTL4 stimulates intracellular signaling mechanisms to coordinate cellular behavior. Our findings provide insight into a novel cell migration control mechanism and underscore the physiological importance of the modulation of integrin activity in cancer metastasis.
Wound healing consists of a finely tuned pattern of integrated biological events aimed at reestablishing a new epithelial barrier. This process includes inflammation, cell migration, proliferation, and extracellular matrix (ECM) remodeling. Integrins are crucial mediators of cell migration that are essential throughout the wound healing process.1 The binding of integrins to their cognate matrix proteins induces a conformational change that is propagated to the cytoplasmic domain and activates both focal adhesion kinase (FAK)-dependent and FAK-independent signaling pathways.2 FAK is a nonreceptor protein tyrosine kinase that is involved in signal transduction from integrin-enriched focal adhesion sites that mediate cell contact with the matrix proteins. The multiple protein–protein interaction sites allow FAK to associate with adaptor and structural proteins to modulate the activities of mitogen-activated protein kinases, stress-activated protein kinases, and small GTPases.2 Integrins can also cooperate with specific growth factor receptors to activate non–FAK-dependent pathways such as the phosphatidylinositol 3-kinase, mitogen-activated protein kinase, 14-3-3, and protein kinase C (PKC)-mediated pathways.3,4,5,6,7 Although the importance of the cell-matrix interactions in wound healing is well-recognized, the mechanism underlying these events needs further study.
During the wound repair process, changes in ECM composition have a direct effect on cell–matrix communication and, consequently, the behavior of the epithelial cells. The ECM is composed of matrix structural proteins and matricellular proteins, among others. Matricellular proteins, such as secreted protein acidic and rich in cysteine (SPARC), thrombospondin, tenascin, and osteopontin, belong to a group of extracellular factors that modulate cell–matrix communication but do not serve primary structural roles.8 They are expressed when tissues undergo events that require tissue renewal, tissue remodeling, or embryonic development. Despite the importance of matricellular proteins during wound repair, how these extracellular factors modulate the integrin-mediated signaling pathway that culminates in the appropriate cellular responses remain less well understood.9
Integrins on the cell surface are well suited to function as biosensors to constantly interrogate the wound environment and modulate cell responses accordingly. The binding of an integrin to its cognate matrix proteins activates intracellular signaling pathways to modulate a broad range of cellular processes, including cell migration.10 Ligand-activated integrins are continuously internalized from the plasma membrane into the endosomal compartments and recycled back to the cell surface.11 It is well established that integrin recycling contributes to the motility of rapidly migrating cells, such as wound keratinocytes, and permits constant monitoring of the wound cellular environment. The recycling process is apparently selective, with certain integrin heterodimers being cycled rapidly while others remain at the plasma membrane. However, the extracellular factors and mechanisms that provide such selectivity remain unclear.
Adipose tissue produces and secretes a variety of bioactive molecules called adipocytokines that are involved in energy homeostasis. Emerging evidence shows that certain adipocytokines, including leptin and plasminogen activator inhibitor type-1, also have a profound local impact on wound healing.12,13 The angiopoietin-like 4 (ANGPTL4) protein is an adipocytokine that plays important roles in lipid and glucose metabolism.14 Its expression is up-regulated by the nuclear hormone receptor peroxisome proliferator-activated receptor15 and by hypoxia.16 Its plasma abundance is increased by fasting and decreased by chronic high-fat feeding. ANGPTL4 decreases blood glucose and improves glucose tolerance in mice.17 ANGPTL4 is also implicated in breast cancer metastasis via the regulation of vascular integrity.18,19 The native ANGPTL4 is proteolytically cleaved, giving rise to the N-terminal coiled-coil fragment (nANGPTL4) and the C-terminal fibrinogen-like domain (cANGPTL4). The former assembles into multimeric structures and inhibits the activity of lipoprotein lipase.20 cANGPTL4 exists as a monomer, whose function is still relatively unclear, but it has been implicated in the maintenance of vascular endothelial integrity.21 Despite its multiple functions, the significance of the different cleaved fragments of ANGPTL4 is only beginning to be understood. Importantly, how ANGPTL4 relays its action from the cell surface and initiates intracellular signaling cascade remain unknown, which limits our understanding of the mechanisms by which ANGPTL4 contributes to wound healing and cancer metastasis.
Here we show that ANGPTL4 interacts with wound integrins β1 and β5. This interaction activates integrin-mediated intracellular signaling and allows for selective integrin recycling enhancing cell migration. ANGPTL4-deficient cells showed impaired cell migration and diminished FAK-Src-PAK1 activation. This defect was observed in vivo as delayed reepithelialization in ANGPTL4-knockout mice. Our results reveal a novel role of ANGPTL4 in modulating integrin-mediated signaling during wound healing. Considering the importance of cell migration to numerous pathophysiological processes, our findings fill crucial gaps in the understanding of integrin-mediated cell migration.
Materials and Methods
Wounding Experiment
Wounding and treatment were performed as described.22 Wounds were topically treated daily with 50 μg of either preimmune IgG, anti-cANGPTL4 antibodies, or 10 μg recombinant ANGPTL4. The wounds were kept moist using occlusive Tegaderm (3M, USA) dressing. Treatments were rotated to avoid site bias. At the indicated days postinjury, wounds were excised for analysis. Pure-bred ANGPTL4+/+ and ANGPTL4−/− mice on a C57Bl/6 background were used.23 Animal experiments were approved by the University Institutional Animal Care and Use Committee (ARF-SBS/NIE-A-0093, −0078, and −004). Hematoxylin and eosin (H&E) stained images and histomorphometric measurements were taken using a MIRAX MIDI with Plan-Apochromatic ×20/0.8 objective and MIRAX Scan software (Carl Zeiss). Polyclonal antibodies against human (amino acids 186-406) and mouse (190-410) cANGPTL4 were produced in-house.
Knockdown of ANGPTL4 and Real-Time PCR
siRNA against human ANGPTL4 and a scrambled sequence as control were subcloned into the pFIV-H1/U6-puro pFIV/siRNA lentivirus system. Pseudovirus purification and transduction were performed as described.22 Endogenous ANGPTL4 in human keratinocytes was transiently suppressed using either siGLO control or ON-TARGETplus SMARTpool ANGPTL4 siRNA (Dharmacon; L-007807-00) by means of DharmaFECT1. The knockdown efficiency and relative expression level of indicated genes were determined by qPCR using the KAPA FAST qPCR kit (KAPA Biosystems). All oligonucleotides and TaqMan probe sequences are provided in Table 1. The Interferon Response Detection Kit was from System Biosciences.
Table 1.
Oligonucleotide Sequences of siRNA and Real-Time PCR Primers Used in this Work
| Oligonucleotide | Sequence |
|---|---|
| siRNA | |
| ANGPTL4 siRNA | |
| Sense | 5′-AAAGCTGCAAGATGACCTCAGATGGAGGCTG-3′ |
| Anti-sense | 5′-AAAAGGCTTAAGAAGGGAATCTTCTGGAAGAC-3′ |
| Control siRNA | |
| Sense | 5′-AAAGCTGTCTTCAAGATTGATATCGAAGACTA-3′ |
| Anti-sense | 5′-AAAATAGTCTTCGATATCAAGCTTGAAGACA-3′ |
| Real-time qPCR* | |
| Human ANGPTL4 | |
| Forward | 5′-CTCCCGTTAGCCCCTGAGAG-3′ |
| Reverse | 5′-AGGTGCTGCTTCTCCAGGTG-3′ |
| Taqman probe | 5′-(6-FAM)ACCCTGAGGTCCTTCACAGCCTGC(TAMRA)-3′ |
| Mouse ANGPTL4 | |
| Forward | 5′-GCTTTGCATCCTGGGACGAG-3′ |
| Reverse | 5′-CCCTGACAAGCGTTACCACAG-3′ |
| Taqman probe | 5′-(6-FAM)ACTTGCTGGCTCACGGGCTGCTAC(TAMRA)-3′ |
| L27 | |
| Forward | 5′-CTGGTGGCTGGAATTGACCGCTA-3′ |
| Reverse | 5′-CAAGGGGATATCCACAGAGTACCTTG-3′ |
| Taqman probe | 5′-(HEX)CTGCCATGGGCAAGAAGAAGATCGCC(BHQ1)-3′ |
Melting curve analysis was performed to assure that only one PCR product was formed. Primers were designed to generate a PCR amplification product of 100 to 250 bp. Only primer pairs yielding unique amplification products without primer dimer formation were subsequently used for real-time PCR assays.
Recombinant ANGPTL4 Expression and Purification
The cDNAs encoding various domains of human ANGPTL4 were isolated by PCR, subcloned into pET30a vector and transformed into E. coli Rosetta-gami bacteria (Novagen). Protein expression was induced by 0.5 mmol/L IPTG and purified either by affinity nickel-Sepharose, size-exclusion or anion-exchange chormatographies according to standard procedures (Supplemental Figure 1, A and B at http://ajp.amjpathol.org). Drosophila S2 cells stably expressing either human integrin β1, β5, or β3 were maintained as previously described.24 S2 cells were routinely cultured in serum-free medium. Cell membranes were first isolated using the ProteoExtract Native Protein Extraction Kit (Calbiochem) and were enriched by step sucrose gradient ultracentrifugation.25
In Vitro Scratch-Wound Assay
Scratch-wound assays were performed as described.26 Images were taken at 2-minute intervals over 6 hours using a temperature-controlled, 5% CO2-chambered Axiovert 200M microscope (Carl Zeiss) with a Plan-Neofluar ×10/0.3 or ×20/0.5 objective, CoolSNAP HQ2 camera (Photometrics), and MetaMorph software (Molecular Devices). Preimmune IgG or anti-cANGPTL4 antibodies were used at 2 μg/ml, recombinant ANGPTL4 at 6 μg/ml.
Surface Plasmon Resonance
Surface plasmon resonance was used to determine the dissociation constants of the interactions of integrins β1, β5 with ANGPTL4 immobilized onto CM5 chip. Anti-cANGPTL4 antibodies against the immobilized ANGPTL4 determined the Rmax value of 251.8 resonance units (RU). Six concentrations (0.16, 0.32, 0.63, 1.25, 2.50, and 5.0 μmol/L) of various matrix proteins or integrins were used. Global fitting of the data to a Langmuir 1:1 model was used to determine the dissociation constant (KD) using kinetic analysis calculated with the BiaEvaluation software (BIAcore, version 3.1). The experimental Rmax values of integrins β1 and β5 for ANGPTL4 were 261.1 RU and 229.3 RU, respectively. Values are mean ± SD of five independent preparations of recombinant proteins. Various anti-integrin α5β1 and αvβ5 antibodies from R&D Systems and Abnova.
Affinity Coprecipitation Assay
In vivo coimmunoprecipitation was performed using indicated antibodies as previously described.27 The samples were lysed and coimmunoprecipitation was performed using resin immobilized with either anti-cANGPTL4, antiintegrin β1, or preimmune IgG. Immunoprecipitates were released by Laemmli’s buffer and probed with the indicated antibodies. For specificity of coimmunoprecipitation, immunodetection of cytoplasmic ERK, which does not interact directly with integrins β1 and β5, was performed.
Rho GTPase Assay
Active GTP-bound Rac1 was quantified as previously described.26 Briefly, 500 μg of cell lysates were incubated for 1 hour at 4°C with GST-p21 binding domain of PAK coupled to glutathione Sepharose beads. Bound proteins were solubilized in Laemmli’s buffer, resolved by SDS-PAGE, and immunoblotted using the corresponding antibodies against Rac1. Total Rac1was detected using total cell lysate. Anti-Rac1 antibodies were from Cytoskeleton, Inc.
In Situ Proximity Ligation Assay (PLA)
Wound biopsies were frozen in Tissue-Tek OCT compound medium (Sakura). Keratinocytes subcultured onto glass chamber slides (Lab-Tek), or wound sections were fixed with 4% paraformaldehyde for 15 minutes. The slides were washed twice with PBS, blocked for 1 hour at 25°C with 2% BSA in PBS containing 0.1% Triton-X, followed by incubation overnight at 4°C with indicated antibody pairs. The slides were washed as described above. DUOlink in situ PLA was performed as recommended by the manufacturer (OLink Biosciences). The negative control was performed without primary antibody. Images were taken using an LSM710 META confocal laser scanning microscope with a Plan-Apochromat ×63/1.40 Oil objective and ZEN 2008 software (Carl Zeiss).
Integrin Internalization and FACS Analysis
Surface labeling of membrane receptors was performed on adherent cells as described,28 with minor modifications. Surface proteins were directly labeled at 4°C with 0.2 mg/ml NHS-SS-biotin (Thermo) in PBS for 30 minutes. Labeled cells were washed twice with cold PBS and transferred to serum-free DMEM at 37°C to eliminate exogenous ANGPTL4 and to permit internalization. After removing biotin from all remaining surface proteins using 20 mmol/L MesNa for 15 minutes followed by 20 mmol/L IAA for 10 minutes, cells were lysed. Supernatants were corrected to equivalent protein concentrations, and biotinylated proteins were captured overnight by NeutrAvidin agarose resins (Thermo) at 4°C. Immobilized proteins were released using Laemmli’s buffer and resolved by 10% SDS-PAGE, followed by immunoblot with the indicated antiintegrin antibodies. Cell surface expression of integrin at the indicated time was evaluated as previously described.29
Statistical Analysis
Statistical analysis was determined using the two-tailed Mann–Whitney test using SPSS software. P < 0.05 was considered statistically significant.
Results
Elevated ANGPTL4 Expression in Skin Wounds
We found that during the healing of a full-thickness excisional wound in mouse skin, ANGPTL4 mRNA peaked at day 3–5 postwounding, as shown by quantitative PCR (qPCR) and immunodetection (Figure 1A). Using polyclonal antibodies that recognize either the N- or C-terminal region of ANGPTL4, only the native ANGPTL4 and cANGPTL4 were detected in wound biopsies (Figure 1B). Immunoblot showed the specificity of anti-cANGPTL4 (Supplemental Figure 1C at http://ajp.amjpathol.org). Dual immunofluorescence staining revealed that the expression of ANGPTL4 increased progressively in both the wound epithelia and wound bed, coinciding with an increase in Ki-67–positive proliferating keratinocytes (Figure 1C). ANGPTL4 was detected only at basal levels in unwounded skin (Supplemental Figure 1D at http://ajp.amjpathol.org). A retrospective examination of human skin ulcers, which reflects a situation of impaired healing, also revealed higher ANGPTL4 expression in ulcers compared with normal skin (Figure 1D and Supplemental Figure 1E at http://ajp.amjpathol.org). Although ulcers are different from acute wounds, their examination can provide clues on ANGPTL4 expression in human wounds, as it was not possible for us to obtain equivalent biopsies from healthy volunteers. These observations suggest an important role of ANGPTL4 during wound healing.
Figure 1.
ANGPTL4 expression is elevated in wound biopsies. Expression profiles of ANGPTL4 (A) mRNA and (B) protein during wound healing determined by qPCR and immunoblotting, respectively. Ribosomal protein L27 was used as a normalizing reference gene. Polyclonal antibodies that recognized the N (anti-nANGPTL4) and C termini (anti-cANGPTL4) of ANGPTL4 were used. β-tubulin was used as loading and transfer control. Values at each time point are mean ± SEM of 15 mice. C: Immunofluorescence staining of ANGPTL4 in wound biopsies. Mouse skin wound biopsies at indicated days of postwounding were cryosectioned, stained for cANGPTL4 and Ki-67, and counterstained with DAPI. Representative pictures from the wound edge and adjacent wound bed are shown. Arrow denotes the wound edge at day 0. Dotted white line represents epidermal-dermal junction. Scale bar = 40 μm. D: Relative expression levels of ANGPTL4 mRNA in normal human skin biopsies and ulcers determined by qPCR. Ribosomal protein L27 was used as a reference gene. Each circle shows the mean values of three different paraffin sections from an individual sample; horizontal bars show average values obtained from human skin biopsy or ulcers.
ANGPTL4 Deficiency Delays Wound Reepithelialization
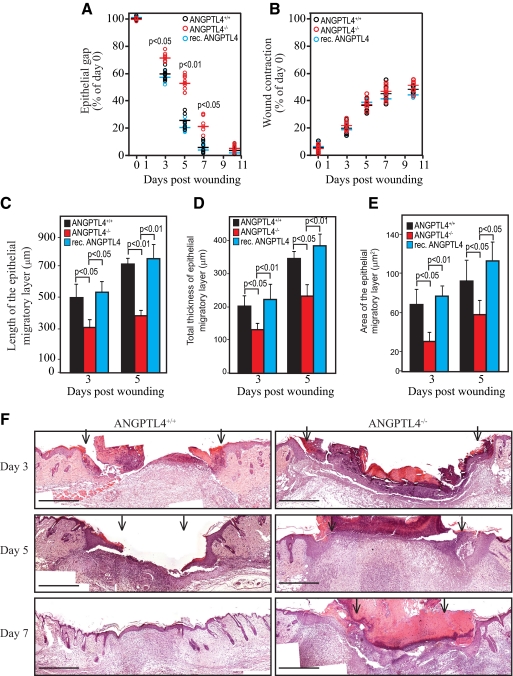
We examined the healing of full-thickness skin wound in wild type (ANGPTL4+/+) and ANGPTL4-knockout (ANGPTL4−/−) mice. Histomorphometric analysis of day 3–10 wound biopsies showed delayed reepithelialization of ANGPTL4−/− wounds when compared with ANGPTL4+/+ wounds (Figure 2A). No difference in wound contraction was observed (Figure 2B). The length, thickness, and area of the epithelial tongue were reduced in ANGPTL4−/− wounds at days 3–5 postinjury (Figure 2, C–E). The ANGPTL4+/+ wounds were completely reepithelialized by day 7, in contrast to ANGPTL4−/− wounds (Figure 2, A and F). The topical application of recombinant ANGPTL4 onto ANGPTL4−/− wounds resulted in complete reepithelialization at day 7 postapplication, in contrast to untreated ANGPTL4−/− wounds (Figure 2A). To eliminate a potential systemic effect of ANGPTL4 on wound closure, we examined the effect of topically applied anti-cANGPTL4 antibody on wound reepithelialization. We reasoned that the antibody might interfere with the action of ANGPTL4, and thus recapitulate ANGPTL4−/− wounds. Our analysis revealed impaired reepithelialization, reduced length and thickness of the epithelial tongue in wounds treated with anti-cANGPTL4 as compared to preimmune IgG-treated wounds (Supplemental Figure 2, A and B at http://ajp.amjpathol.org). No significant difference in wound contraction was observed (Supplemental Figure 2A at http://ajp.amjpathol.org). Images of serial sections encompassing complete wounds at days 3–10 postinjury showed that the impaired reepithelialization of the epidermis was not a local random alteration; rather it was distributed over the entire healing wound edge. Altogether, these results indicate ANGPTL4 is important for efficient wound healing.
Figure 2.
ANGPTL4 is important for efficient wound reepithelialization. Quantification of (A) epithelial gap, (B) wound contraction, (C) length, (D) thickness, and (E) area of wound epithelia in ANGPTL4+/+, ANGPTL4−/−, and recombinant ANGPTL4-treated ANGPTL4−/− wounds (rec. ANGPTL4). Each circle shows the mean values of 10 centrally dissected sections obtained from individual mouse; horizontal bars show average values obtained for each genotype or treatment. Values in C to D were mean of left and right wound epithelia measured using day-3 and day-5 wound biopsies from 10 mice. Epithelial gap and wound contraction are defined as the distance between the advancing edges of clear multiple layer neoepidermis and between the first hair follicle on both of the wound edge, respectively. The length of the wound epidermis measured from the first hair follicle to the tip of the wound epithelial tongue is used as an indicator of keratinocyte migration. F: Hematoxylin and eosin (H&E) pictures of postinjury wound edges from ANGPTL4+/+ and ANGPTL4−/− mice. Scale bar = 500 μm. Arrows point to the epithelial wound edge. Representative pictures of centrally dissected wound sections are shown.
ANGPTL4 Deficiency Impairs Cell Adhesion and Migration
To better understand the role of ANGPTL4, we examined the effect of ANGPTL4 on cell adhesion and migration using primary human keratinocytes. We suppressed endogenous ANGPTL4 expression by RNA interference. Keratinocytes were either transduced with a lentivirus-mediated ANGPTL4 siRNA (Figure 3A) or transiently transfected with ON-TARGETplus SMARTpool siRNAs (Supplemental Figure 2C at http://ajp.amjpathol.org). Lentivirus-mediated control scrambled siRNA and siGLO siRNA served as corresponding controls. The ANGPTL4 expression level in ANGPTL4-knockdown keratinocytes (KANGPTL4) was reduced by 90% compared with control-siRNA keratinocytes (KCTRL) (Figure 3A). The induction of interferon responses has been reported as a challenge to the specificity of some RNA interference approaches.30 Therefore, we measured the expression of key interferon response genes by qPCR, which showed no induction in KANGPTL4 when compared with either wild-type nontransduced cells or KCTRL (Figure 3B), suggesting no off-target effect. KANGPTL4 did not undergo spontaneous apoptosis in standard growth conditions, as determined by FACS analysis (Figure 3C). Next, we performed a cell adhesion assay on KCTRL using serum-free medium. The results showed that cells attached more rapidly onto ANGPTL4-coated surfaces compared to control uncoated surfaces. The attachment rate was delayed in the presence of anti-cANGPTL4 antibody compared with preimmune IgG (Figure 3D), suggesting that ANGPTL4 facilitated cell attachment. ANGPTL4−/− mouse primary keratinocytes adhered poorly to the culture surface and underwent apoptosis, so we were unable to culture sufficient cells for experiments.
Figure 3.
ANGPTL4 is important for cell adhesion. A: mRNA and/or protein levels of ANGPTL4 and ANGPTL3 in keratinocytes transduced with either control (KCTRL) or ANGPTL4 siRNA (KANGPTL4). Values below each band represent the mean fold differences in protein expression level compared with control from five independent experiments. Coomassie-stained blot showed equal loading. B: qPCR of interferon response genes in KANGPTL4 compared with KCTRL. 2′,5′-oligoadenylate synthetase isoforms 1 and 2 (OAS1 and OAS2), interferon-induced myxovirus resistance 1 (MX1), interferon-inducible trans-membrane protein (IFITM), and interferon-stimulated transcription factor 3γ (ISGF3γ). Ribosomal protein L27 was used as a normalizing reference gene. C: FACS analysis of KCTRL and KANGPTL4 stained with annexin V-FITC/PI. The percentage of apoptotic cells (lower right quadrant) is indicated in bold. D: Cell adhesion of KCTRL onto cANGPTL4-coated surface in the presence of either preimmune IgG or anti-cANGPTL4.
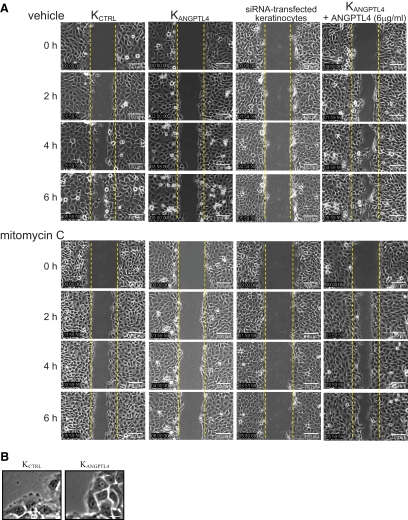
We examined the impact of ANGPTL4 on keratinocyte migration. In an in vitro scratch-wound assay, KCTRL closed the wound by 6 hours, whereas KANGPTL4 took 18 hours, indicating impaired keratinocyte migration (KCTRL vs. KANGPTL4:11.92 ± 0.31 vs. 6.66 ± 0.12 μm/h, P < 0.05) (Figure 4A). Similar observations were also made in transiently siRNA-transfected keratinocytes (5.87 ± 0.15 μm/h), indicating that the impaired migration was not due to an adaptation to the reduced ANGPTL4 level (Figure 4A). Similar experiments in the presence of mitomycin C showed that KCTRL closed the wound by 8 hours, whereas KANGPLT4 and siRNA-transfected keratinocytes failed to close the wound even after 24 hours (Figure 4A). Importantly, the application of recombinant ANGPTL4 rescued the impaired migration of KANGPTL4 (12.03 ± 0.42 μm/h), regardless of mitomycin C treatment (9.24 ± 0.37 μm/h) (Figure 4A). Conversely, the presence of anti-cANGPTL4 antibody delayed KCTRL migration (preimmune versus anti-cANGPTL4: 13.09 ± 0.23 vs. 6.80 ± 0.17 μm/h) (Supplemental Figure 2D at http://ajp.amjpathol.org). KANGPTL4 or anti-cANGPTL4-treated KCTRL did not display pronounced lamellipodia at the leading edge of migrating cells (Figure 4B).
Figure 4.
ANGPTL4 modulates cell migration. A: Representative time-lapsed images of wounded cultures of KCTRL, KANGPTL4, and ANGPTL4 siRNA-transfected keratinocytes and recombinant cANGPTL4 (rec. cANGPTL4)-treated KANGPTL4 in the absence or presence of mitomycin C (2 μg/ml). Yellow dotted lines represent the scratch gap at the time of wounding. Scale bar = 100 μm. B: Phase-contrast images showing lamellipodia of KCTRL and KANGPTL4 during migration. Scale bar = 20 μm.
ANGPTL4 Interacts with Integrin β1 and β5
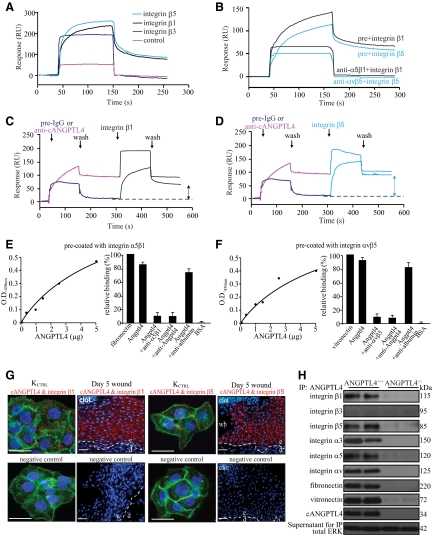
How ANGPTL4 mediates its action remains a central question in our understanding of ANGPTL4 in cell migration. Cell migration is an integrin-dependent process, and ANGPTL3, a close relative of ANGPTL4, binds to integrin αvβ3.31 This prompted us to inquire whether ANGPTL4 interacts with integrins, particularly integrins β1 and β5, which are essential for keratinocyte migration and whose expression is increased during wound healing.32 We first bacterially expressed and purified the various domains of ANGPTL4 (Supplemental Figure 1B at http://ajp.amjpathol.org). Next, we ectopically expressed and purified human integrins β1, β3, and β5 in Drosophila S2 cells cultured in serum-free medium (Supplemental Figure 3A at http://ajp.amjpathol.org). Membrane extract enriched in either integrin β1, β3, or β5 was used for interaction studies with cANGPTL4 by surface plasmon resonance. Integrins β1 and β5, but not β3, interacted with cANGPTL4 with KD of ∼10−8 mol/L (Figure 5A). The interaction between ANGPTL4 and integrin β1 or β5 was specific, as it was reciprocally blocked with neutralizing antibodies raised against either integrins (α5β1 or αvβ5), or cANGTPL4 (Figure 5B-D). Thus, ANGPTL4 can directly interact with specific integrins, in the absence of cognate matrix proteins. We further confirmed this interaction by ELISA (Figure 5, E and F). In situ PLA performed using various antibody pairs on KCTRL and day-5 wound sections confirmed that cANGPTL4 interacted with integrins β1 and β5 in vivo (Figure 5G). The PLA signal from each detected interacting protein pair is visualized as an individual red dot.33 Double immunostaining performed using anti-vinculin and anti-cANGPTL4 on KCTRL of an “in vitro” scratch wound revealed strong ANGPTL4 expression near focal contact regions, which was further confirmed using PLA (ANGPTL4 & integrin β1) and immunofluorescence (vinculin), underscoring the role of ANGPTL4 in keratinocyte migration (Supplemental Figure 3B at http://ajp.amjpathol.org). Immunoblot analysis of anti-cANGPTL4 and specific anti-integrin immunoprecipitates of ANGPTL4+/+ and ANGPTL4−/− wound biopsy homogenates showed that the integrin β1 and β5 were present, as well as α3, α5, and αv subunits (Figure 5H, and Supplemental Figure 3C at http://ajp.amjpathol.org). Interaction of integrin β1 and β5 with ANGPTL4 did not compete with the binding of integrins to their natural cognate ligands, but rather they appeared to strengthen the integrin–matrix interactions. Consistent with the above findings, integrin β3 was not detected in anti-cANGPTL4 immunoprecipitates. Next, we performed cell adhesion assays and in vitro wound assays on fibronectin- and vitronectin-coated surfaces. The results showed that KANGPTL4 adhered more slowly to both coated surfaces than KCTRL (Supplemental Figure 3, D and E at http://ajp.amjpathol.org). Cell migration assays were also performed on coated surfaces using KCTRL and KANGPTL4 treated with mitomycin C, to exclude any effects of proliferation (Supplemental Figure 3F at http://ajp.amjpathol.org). The repopulation of the in vitro wound by KCTRL and KANGPTL4 was faster on both matrix protein-coated surfaces compared with the cognate controls on uncoated surface (KCTRL on coated versus uncoated: 6 hours vs. 8 hours; KANGPTL4 on coated versus uncoated: 19 hours vs. ≥24 hours). Notably, the application of recombinant ANGPTL4 accelerated the migration and closure of the in vitro wound by KANGPTL4 (compare Figure 4A and Supplemental Figure 3F at http://ajp.amjpathol.org). Taken together, these data indicate that integrins β1 and β5, but not β3, are novel interacting protein partners of ANGPTL4. This interaction aided cell migration.
Figure 5.
ANGPTL4 interacts with integrins β1 and β5. Representative sensorgrams showing binding profiles between immobilized cANGPTL4 and S2-membrane extracts containing either control, integrin β1, β3, or β5 (A), or integrin β1 or β5 preincubated with either preimmune IgG (pre) or cognate anti-integrin antibody (B). Integrin β1 (C) or integrin β5 (D) after preblocked with either preimmune IgG or anti-cANGPTL4 antibody. Each sensorgram was corrected by subtracting a sensorgram obtained from a reference flow cell with no immobilized protein. Anti-cANGPTL4 antibodies against the immobilized cANGPTL4 determined the Rmax value to be 138.2 resonance units (RU). Five independent experiments were performed. Dose-dependent ANGPTL4 binding to immobilized (E) integrin α5β1 or (F) integrin αvβ5, which was specifically blocked by anti-cANGPTL4, as determined by ELISA. Detection of (G) the ANGPTL4-integrin β1 (left panel) and ANGPTL4-integrin β5 (right panel) complexes in KCTRL and in day-5 ANGPTL4+/+ wound biopsies using DUOlink PLA. PLA signals (red) and Hoechst dye for nuclei (blue). For KCTRL, the cells were counterstained with Alexa 488-phallodin for actin stress fibers. The nuclear image was acquired in one z-plane using a LSM510 META confocal laser-scanning microscope (Carl Zeiss). Dotted white line represents epidermal-dermal junction. Negative control was performed without primary antibodies. Representative pictures from wound sections with epidermis (e), dermis (d), wound bed (wb), and KCTRL from six independent experiments or sections from three mice are shown. Scale bar = 40 μm. H: Immunodetection of indicated proteins from anti-cANGPTL4 immunoprecipitates of ANGPTL4+/+ and ANGPTL4−/− wound biopsy homogenates. Total ERK from supernatant were used to verify equal loading.
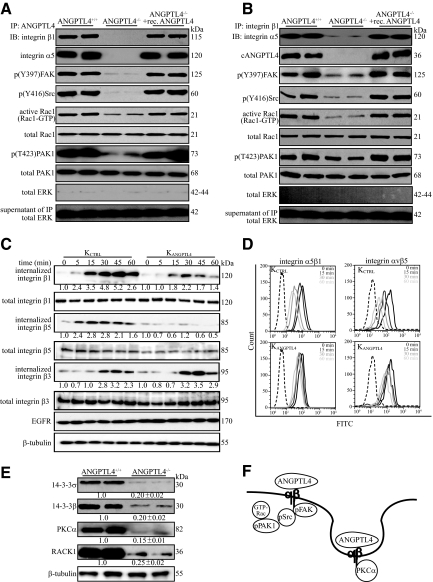
ANGPTL4 Modulates Integrin-Mediated Signaling and Internalization
To gain insight into the intracellular signaling pathway, we performed in vivo coimmunoprecipitation using either anti-cANGPTL4 or anti-integrin β1 antibodies, followed by immunodetection of specific mediators of integrin-mediated signaling. ANGPTL4 bound to integrin β1 recruited more FAK-Src complex with more active Rac1-GTP and phosphorylated PAK1 in the membrane fraction of ANGPTL4+/+ and ANGPTL4-treated ANGPTL4−/− wounds than their cognate controls (Figure 6, A and B). We detected cytoplasmic ERK, which does not interact directly with integrin, only in the supernatant of the immunoprecipitates, indicating the specificity of the affinity coimmunoprecipitation (Figure 6, A and B). Neither integrin β1 nor ANGPTL4 was immunoprecipitated with preimmune IgG (Supplemental Figure 4A at http://ajp.amjpathol.org). Similar observations were also made in KCTRL (Supplemental Figure 4, B and C at http://ajp.amjpathol.org), confirming that ANGPTL4 potentiates integrin-mediated signaling. Integrin recycling contributes to the motility of rapidly migrating cells and permits constant monitoring of the wound cellular environment.34 We observed a selective internalization of cell-surface biotin-labeled integrins β1 and β5, but not integrin β3. Importantly, the rapid internalization of integrins β1 and β5 was reduced in ANGPTL4 deficiency (Figure 6C). The internalization of integrin β3 was similar under all examined conditions (Figure 6C). These observations were further corroborated by FACS analysis of the cell-surface expression of integrins (Figure 6D and Supplemental Figure 4D at http://ajp.amjpathol.org). Similar results were also observed in KCTRL treated with anti-cANGPTL4 antibody (Supplemental Figure 4E at http://ajp.amjpathol.org).
Figure 6.
ANGPTL4 modulates integrin-mediated signaling and internalization. Immunodetection of indicated proteins in anti-cANGPTL4 (A) or anti-integrin β1 immunoprecipitates (B) (top panel); of Rac1-GTP and phosphorylated PAK1 (p(T423)PAK1) (lower panels) from membrane extract of indicated wound biopsies. Total PAK from total cell lysate were used to verify equal loading. Specificity of immunoprecipitation was verified by immunodetection of ERK in the immunoprecipitates and its supernatant. C: Kinetics of integrin internalization. Internalized biotinylated-integrins were detected using corresponding antibodies after immunoprecipitation with NeutrAvidin agarose resins. The level of total integrins β1, β3, and β5 were determined using total cell lysate before immunoprecipitation. EGFR and β-tubulin from total cell lysate were used to verify equal loading. Values denote mean fold change of three independent experiments compared to KCTRL at time 0. D: Cell-surface expression of the integrins α5β1 and αvβ5 in KCTRL and KANGPTL4 at the indicated time was determined by FACS. The negative control (only secondary antibody) is indicated by the dotted graph. E: Immunoblot analysis of membrane extracts from day-5 ANGPTL4+/+ and ANGPTL4−/− wound biopsies for indicated proteins. Values below the band represent the mean fold differences in protein expression levels relative to ANGPTL4+/+ from eight wound biopsies for each genotype. β-tubulin was used as loading and transfer control. F: Schematic illustration showing ANGPTL4 interacting with integrin, activating FAK-Src-PAK1 signaling and facilitating integrin internalization, which involves PKCα and 14-3-3σ/β, to aid cell migration.
Integrins β1 and β5 are internalized with the aid of adaptor protein 14-3-3σ and PKCα, which binds directly to integrin cytoplasmic tails.35,36 Prompted by our above observations, we examined the membrane expression of these proteins in ANGPTL4-deficient keratinocytes and wound biopsies. Immunodetection showed that the expression of 14-3-3σ, β and PKCα was significantly reduced in ANGPTL4−/− wound biopsies when compared with their cognate controls (Figure 6E). Besides reduced expression of 14-3-3σ, β and PKCα, the ANGPTL4−/− wounds also exhibited decreased expression of RACK1,37 indicating attenuated PKC-mediated signal transduction (Figure 6E). Similar findings were obtained in keratinocytes transiently transfected with ANGPTL4-siRNA, suggesting that the reduced levels of total signaling proteins observed is not an adaptation to the reduction in ANGPTL4 level (Supplemental Figure 4F at http://ajp.amjpathol.org). To examine whether ANGPTL4 has a direct effect on the expression of these signaling proteins, we examined their mRNA levels in KANGPTL4 treated with recombinant ANGPTL4 in the presence of either actinomycin D or cycloheximide. The increased mRNA levels of 14-3-3σ, β, and PKCα induced by ANGPTL4 was abolished in actinomycin D– but not cycloheximide-treated cells, suggesting a transcriptional regulatory mechanism (Supplemental Figure 4G at http://ajp.amjpathol.org). Thus, our results show that more activated FAK-Src complexes were formed when ANGPTL4 was bound to integrin β1, indicating that ANGPTL4 mediates its action at least partially via the FAK-Src-PAK1 axis. ANGPTL4 deficiency dysregulated 14-3-3σ and its effector PKCα expression, which would influence integrin internalization and thus keratinocyte migration. Altogether, our results reveal a novel function of ANGPTL4 in promoting keratinocyte migration during wound healing by activating integrin-mediated signaling and internalization.
Discussion
Wound healing is a complex process that involves a cascade of overlapping events, including inflammation, reepithelialization, and remodeling, all directed at the restoration of the epidermal barrier. Throughout the healing process, cellular interactions with ECM components coordinate the individual events, enabling temporal and spatial control. Reepithelialization is accomplished by increased keratinocyte proliferation and guided migration of the keratinocytes over the wound ECM. This process requires orderly changes in keratinocyte behavior and phenotype in which integrin-mediated signaling plays a crucial role. We reveal a newly discovered role of ANGPTL4 in cell migration via direct interaction with integrin β1 and β5 to modulate integrin-mediated FAK-Src-PAK1 signaling and internalization (Figure 6F).
Adipocytokines secreted by adipose tissue play important roles in energy homeostasis.38 Emerging evidence points to additional nonmetabolic roles, such as wound healing, of some adipocytokines. We show that the expression of the adipocytokine ANGPTL4, while only weakly detectable in normal intact skin, was markedly elevated during the reepithelialization phase of wound healing. ANGPTL4 deficiency had a dramatic impact on cell migration in vitro and in vivo. Thus, in addition to its well-established role in energy homeostasis, we revealed an unsuspected role of ANGPTL4 as a matricellular protein in wound repair. Multiple functions of other matricellular proteins have been described, including SPARC, which is implicated in adipose tissue hyperplasia and adipogenesis.39 ANGPTL4 undergoes proteolytic cleavage after secretion, to release the N-terminal coiled-coil domain (nANGPTL4) and a C-terminal fibrinogen-like domain (cANGPTL4). nANGPTL4 binds lipoprotein lipase and inhibits its activity,20 but little is known about the role of cANGPTL4. Therefore, how ANGPTL4 triggers intracellular signaling to propagate its effect remains a central question in relation to its functions. Although ANGPTL4 is related to angiopoietins, it does not bind to the Tie receptors.40 Using various methods, we identify integrin β1 and β5, but not β3, as novel interacting protein partners of ANGPTL4. We further show that the fibrinogen-like domain of ANGPTL4 associates with heterodimeric integrins, via the β1/β5 subunits and modulates integrin-mediated signaling, revealing crucial insight into its mechanism of action. This interaction modulates the FAK-Src-PAK1 signaling cascade, which is essential for keratinocyte migration.2
Integrins on cell surface are well suited to function as biosensors to constantly monitor changes in the wound microenvironment. They consist of α and β subunits that associate in various combinations to form at least 25 receptors. Each αβ combination possesses specific binding and signaling properties. During wound healing, migrating keratinocytes enlarge their integrin repertoire concomitantly with changes in the extracellular matrix composition, suggesting a close interplay of these two groups of molecules during reepithelialization. ANGPTL4 interacts with the β subunits of α5β1, αvβ5, and α3β1 to enhance integrin-mediated signalings, integrin internalization, and keratinocyte migration. Consistent with the role of α5β1and αvβ5 in facilitating cell migration and adhesion, their expression was increased in wound keratinocytes and their deficiencies have been associated with impaired cell migration, adhesion, or wound healing.32,41,42 The role of integrin α3β1 in cell migration is controversial,43 depending on the complex of the various matrices present at the wound bed and, more importantly, on the context in which the intact matrix protein is presented to the cells. Small soluble matrix protein fragments generated by the action of proteases during reepithelialization can compete with substrate-anchored matrix proteins for integrin and impair cell migration.44,45 We also showed that ANGPTL4 binding to specific integrins does not interfere with the association of integrins and their cognate matrix ligands. Although not studied herein, it is tempting to speculate that ANGPTL4 may also interact with specific matrix proteins and form a ternary complex with its cognate integrin receptor to further fine-tune cell-matrix communication which is crucial for cell migration.
During wound healing, migrating cells must display appropriate cellular behavior in response to the changing wound environment to enable effective wound closure. Interestingly, the deficiency in ANGPTL4 resulted in decreased expression of 14-3-3σ, β and PKCα, which also modulates cell migration via integrin internalization. The underlying mechanism by which ANGPTL4 regulates their expression remains to be determined. Thus, ANGPTL4 is a novel matricellular protein that modulates keratinocyte migration on at least two fronts. First, ANGPTL4 potentiates integrin-mediated signaling to facilitate cell migration. ANGPTL4 binding to specific integrins does not interfere with the association of integrins and their cognate matrix ligands. Second, ANGPTL4-bound integrins provide a novel means by which selective integrin signaling cascades can be activated, depending on the local context of the ECM. ANGPTL4 regulates 14-3-3σ and its effector PKCα expression, which would influence integrin internalization. This allows migrating wound keratinocytes to better scrutinize the changes in the wound ECM and fine-tune their cellular behavior.
Metastasis and wound repair share numerous characteristics during cell migration, so it is not surprising that ANGPTL4 has been implicated in cancer metastasis. Previous studies have reported that ANGPTL4 prevents metastasis by inhibiting vascular leakiness.46,47 In contrast, recent work has revealed that one of the genes most highly associated with breast cancer metastasis to lung is ANGPTL4.18 Tumor-derived ANGPTL4 was proposed to disrupt endothelial cell–cell contacts to aid the extravasation and metastasis of tumor cells.48 Therefore, whether ANGPTL4 promotes or inhibits vascular leakiness and thus cancer metastasis remains controversial. Although this question is not directly addressed in this study, our data clearly showed that ANGPTL4 binds to integrin β1, and it has been shown that neutralizing antibody against integrin α5β1 increases paracellular endothelial permeability.49 Altogether, our finding that ANGPTL4 interacts with integrins β1 and β5 to modulate integrin-FAK-Src-PAK1 signaling and integrin internalization provides valuable mechanistic insight into its roles in cancer metastasis.
Acknowledgments
We thank Dr. Samuel Ko and Anna Teo (Carl Zeiss, Singapore Pte Ltd.) for their expertise in image acquisition using LSM710 confocal microscope and MIRAX MIDI.
Footnotes
Address reprint requests to Nguan Soon Tan, Ph.D., School of Biological Science, 60 Nanyang Drive, Singapore 637551. E-mail: nstan@ntu.edu.sg.
Supported by A*STAR BMRC grant (05/1/22/19/377), Ministry of Education, Singapore (ARC 18/08), Nanyang Technological University, Singapore (RGD 127/05, 158/06). M.P., L.P. and P.C.Z. are recipients of the Nanyang Research Scholarship. C.K.T. is recipient of ARC18/08 grant Scholarship.
Y.Y.G., M.P., and H.C.C. contributed equally to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Mercurio AM, Rabinovitz I, Shaw LM. The alpha 6 beta 4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001;13:541–545. doi: 10.1016/s0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- Dans M, Gagnoux-Palacios L, Blaikie P, Klein S, Mariotti A, Giancotti FG. Tyrosine phosphorylation of the beta 4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J Biol Chem. 2001;276:1494–1502. doi: 10.1074/jbc.M008663200. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617–647. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- Dellambra E, Patrone M, Sparatore B, Negri A, Ceciliani F, Bondanza S, Molina F, Cancedda FD, De Luca M. Stratifin, a keratinocyte specific 14-3-3 protein, harbors a pleckstrin homology (PH) domain and enhances protein kinase C activity. J Cell Sci. 1995;108 (Pt 11):3569–3579. doi: 10.1242/jcs.108.11.3569. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36:1031–1037. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic. 2006;7:14–21. doi: 10.1111/j.1600-0854.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- Caswell P, Norman J. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 2008;18:257–263. doi: 10.1016/j.tcb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Frank S, Stallmeyer B, Kämpfer H, Kolb N, Pfeilschifter J. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J Clin Invest. 2000;106:501–509. doi: 10.1172/JCI9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, Tokunaga K, Matsuzawa Y. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med. 1996;2:800–803. doi: 10.1038/nm0796-800. [DOI] [PubMed] [Google Scholar]
- Oike Y, Akao M, Kubota Y, Suda T. Angiopoietin-like proteins: potential new targets for metabolic syndrome therapy. Trends Mol Med. 2005;11:473–479. doi: 10.1016/j.molmed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Mandard S, Zandbergen F, Tan NS, Escher P, Patsouris D, Koenig W, Kleemann R, Bakker A, Veenman F, Wahli W, Müller M, Kersten S. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem. 2004;279:34411–34420. doi: 10.1074/jbc.M403058200. [DOI] [PubMed] [Google Scholar]
- Belanger AJ, Lu H, Date T, Liu LX, Vincent KA, Akita GY, Cheng SH, Gregory RJ, Jiang C. Hypoxia up-regulates expression of peroxisome proliferator-activated receptor gamma angiopoietin-related gene (PGAR) in cardiomyocytes: role of hypoxia inducible factor 1alpha. J Mol Cell Cardiol. 2002;34:765–774. doi: 10.1006/jmcc.2002.2021. [DOI] [PubMed] [Google Scholar]
- Xu A, Lam MC, Chan KW, Wang Y, Zhang J, Hoo RLC, Xu JY, Chen B, Chow WS, Tso AWK, Lam KSL. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc Natl Acad Sci USA. 2005;102:6086–6091. doi: 10.1073/pnas.0408452102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Crawford PA, O'Donnell D, Gordon JI. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc Natl Acad Sci USA. 2007;104:606–611. doi: 10.1073/pnas.0605957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci USA. 2006;103:17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YH, Wang Y, Lam KSL, Yau MH, Cheng KKY, Zhang J, Zhu W, Wu D, Xu A. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol. 2008;28:835–840. doi: 10.1161/ATVBAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- Chong HC, Tan MJ, Philippe V, Tan SH, Tan CK, Ku CW, Goh YY, Wahli W, Michalik L, Tan NS. Regulation of epithelial-mesenchymal IL-1 signaling by PPARbeta/delta is essential for skin homeostasis and wound healing. J Cell Biol. 2009;184:817–831. doi: 10.1083/jcb.200809028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster A, Chao YB, Mosior M, Ford A, Gonzalez-DeWhitt PA, Hale JE, Li D, Qiu Y, Fraser CC, Yang DD, Heuer JG, Jaskunas SR, Eacho P. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- Tan NS, Ho B, Ding JL. High-affinity LPS binding domain(s) in recombinant factor C of a horseshoe crab neutralizes LPS-induced lethality. FASEB J. 2000;14:859–870. doi: 10.1096/fasebj.14.7.859. [DOI] [PubMed] [Google Scholar]
- Tang VW. Proteomic and bioinformatic analysis of epithelial tight junction reveals an unexpected cluster of synaptic molecules. Biol Direct. 2006;1:37. doi: 10.1186/1745-6150-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SH, Pal M, Tan MJ, Wong MHL, Tam FU, Teo JWT, Chong HC, Tan CK, Goh YY, Tang MBY, Cheung PCF, Tan NS. Regulation of Cell Proliferation and Migration by TAK1 via Transcriptional Control of von Hippel-Lindau Tumor Suppressor. J Biol Chem. 2009;284:18047–18058. doi: 10.1074/jbc.M109.002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IJpenberg A, Tan NS, Gelman L, Kersten S, Seydoux J, Xu J, Metzger D, Canaple L, Chambon P, Wahli W, Desvergne B. In vivo activation of PPAR target genes by RXR homodimers. EMBO J. 2004;23:2083–2091. doi: 10.1038/sj.emboj.7600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M, Barry S, Woods A, van der Sluijs P, Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol. 2001;11:1392–1402. doi: 10.1016/s0960-9822(01)00442-0. [DOI] [PubMed] [Google Scholar]
- Sterk LM, Geuijen CA, Oomen LC, Calafat J, Janssen H, Sonnenberg A. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin alpha6beta4 and may regulate the spatial organization of hemidesmosomes. J Cell Biol. 2000;149:969–982. doi: 10.1083/jcb.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge AJ, Pebernard S, Ducraux A, Nicoulaz AL, Iggo R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat Genet. 2003;34:263–264. doi: 10.1038/ng1173. [DOI] [PubMed] [Google Scholar]
- Camenisch G, Pisabarro MT, Sherman D, Kowalski J, Nagel M, Hass P, Xie MH, Gurney A, Bodary S, Liang XH, Clark K, Beresini M, Ferrara N, Gerber HP. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J Biol Chem. 2002;277:17281–17290. doi: 10.1074/jbc.M109768200. [DOI] [PubMed] [Google Scholar]
- Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fässler R, Brakebusch C, Werner S. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development. 2002;129:2303–2315. doi: 10.1242/dev.129.9.2303. [DOI] [PubMed] [Google Scholar]
- Söderberg O, Leuchowius KJ, Gullberg M, Jarvius M, Weibrecht I, Larsson LG, Landegren U. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods. 2008;45:227–232. doi: 10.1016/j.ymeth.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Jones M, PT JC. Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol. 2006;18:549–557. doi: 10.1016/j.ceb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Han DC, Rodriguez LG, Guan JL. Identification of a novel interaction between integrin beta1 and 14-3-3beta. Oncogene. 2001;20:346–357. doi: 10.1038/sj.onc.1204068. [DOI] [PubMed] [Google Scholar]
- Ng T, Shima D, Squire A, Bastiaens PI, Gschmeissner S, Humphries MJ, Parker PJ. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 1999;18:3909–3923. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panetti TS, Wilcox SA, Horzempa C, McKeown-Longo PJ. Alpha v beta 5 integrin receptor-mediated endocytosis of vitronectin is protein kinase C-dependent. J Biol Chem. 1995;270:18593–18597. doi: 10.1074/jbc.270.31.18593. [DOI] [PubMed] [Google Scholar]
- Bulcão C, Ferreira SRG, Giuffrida FMA, Ribeiro-Filho FF. The new adipose tissue and adipocytokines. Curr Diabetes Rev. 2006;2:19–28. doi: 10.2174/157339906775473617. [DOI] [PubMed] [Google Scholar]
- Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, Lee ZH, Koh GY. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J. 2000;346 Pt 3:603–610. [PMC free article] [PubMed] [Google Scholar]
- Bata-Csorgo Z, Cooper KD, Ting KM, Voorhees JJ, Hammerberg C. Fibronectin and alpha5 integrin regulate keratinocyte cell cycling. A mechanism for increased fibronectin potentiation of T cell lymphokine-driven keratinocyte hyperproliferation in psoriasis. J Clin Invest. 1998;101:1509–1518. doi: 10.1172/JCI171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Griffiths M, Wu J, Farese RV, Sheppard D. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol Cell Biol. 2000;20:755–759. doi: 10.1128/mcb.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen T, Zhang Z, Yu Y, Qu H, Koch M, Aumailley M. Integrin alpha3 subunit regulates events linked to epithelial repair, including keratinocyte migration and protein expression. Wound Repair Regen. 2010;18:325–334. doi: 10.1111/j.1524-475X.2010.00590.x. [DOI] [PubMed] [Google Scholar]
- Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–223. doi: 10.1016/j.tcb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–516. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Ito Y, Oike Y, Yasunaga K, Hamada K, Miyata K, Matsumoto SI, Sugano S, Tanihara H, Masuho Y, Suda T. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res. 2003;63:6651–6657. [PubMed] [Google Scholar]
- Galaup A, Cazes A, Le Jan S, Philippe J, Connault E, Le Coz E, Mekid H, Mir LM, Opolon P, Corvol P, Monnot C, Germain S. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc Natl Acad Sci USA. 2006;103:18721–18726. doi: 10.1073/pnas.0609025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua D, Zhang XHF, Wang Q, Nadal C, Gerald WL, Gomis RR, Massagué J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Resnati M, Dejana E, Marchisio PC. The role of integrins in the maintenance of endothelial monolayer integrity. J Cell Biol. 1991;112:479–490. doi: 10.1083/jcb.112.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]