Abstract
Deregulation of both ErbB-2 signaling and matriptase activity has been associated with human prostate cancer (PCa) progression. In this communication, we investigated the roles of both ErbB-2 signaling in matriptase zymogen activation and matriptase in ErbB-2-induced PCa malignancy. In a human PCa cell progression model, we observed that advanced PCa C-81 LNCaP cells exhibited an aggressive phenotype with increased cell migration and invasion capacity; these cells concurrently showed both enhanced ErbB-2 phosphorylation and increased matriptase zymogen activation compared with parental C-33 LNCaP cells. Moreover, ErbB2 activation, both ligand-dependent (eg, epidermal growth factor treatment) and ligand-independent (eg, overexpression), was able to induce matriptase zymogen activation in this cell line. Inhibition of ErbB-2 activity by either the specific inhibitor, AG825, in epidermal growth factor-treated C-33 LNCaP cells or ErbB-2 knockdown in C-81 LNCaP cells, reduced matriptase activation. These observations were confirmed by similar studies using both DU145 and PC3 cells. Together, these data suggest that ErbB-2 signaling plays an important role in matriptase zymogen activation. ErbB-2-enhanced matriptase activation was suppressed by a phosphatidylinositol 3-kinase inhibitor (ie, LY294002) but not by a MEK inhibitor (ie, PD98059). Suppression of matriptase expression by small hairpin RNA knockdown in ErbB-2-overexpressing LNCaP cells dramatically suppressed cancer cell invasion. In summary, our data indicate that ErbB-2 signaling via the phosphatidylinositol 3-kinase pathway results in up-regulated matriptase zymogen activity, which contributes to PCa cell invasion.
Prostate cancer (PCa) is one of the most common malignancies among men in Western countries and the incidence is progressively rising within low-risk populations including China, Japan, and Taiwan.1,2 The death of patients with PCa is mainly due to hormone refractory and metastatic disease,3 and metastatic ability is believed to be due to cancer cells gaining the ability to degrade the extracellular matrix.4 Overexpression of ErbB-2 has been reported and is believed to participate in oncogenic signaling in metastatic prostate cancer.5,6,7,8,9 ErbB-2 signaling has been shown to induce several phenotypic changes associated with more aggressive disease such as increased cell motility,10 cell proliferation,11 and/or invasive ability.12 Although no direct ligand for ErbB-2 has yet been identified, ErbB-2 activation is known to occur in both a ligand-independent and ligand-dependent manner.13,14,15,16 Ligand-dependent ErbB-2 activation involves heterodimerization of the receptor with another ligand-activated ErbB receptor (epidermal growth factor [EGF] receptor/ErbB-1, ErbB-3, and ErbB-4).17,18 For example, in prostate cancer cells, the ErbB ligand EGF induces the formation of both ErbB-1/ErbB-1 homodimers and ErbB-1/ErbB-2 heterodimers.19 Ligand-independent ErbB-2 activation is largely thought to be due to ErbB-2 overexpression, resulting in spontaneous homodimerization and autoactivation.20,21 Clinical data show that ErbB-2 protein levels are elevated in a subset of patients with clinically significant prostate cancer, especially in the recurrent, hormone-refractory phase of the disease.6,8,22 ErbB-2 overexpression promotes the survival and growth of prostate cancer cells through the MAPK and/or phosphatidylinositol 3-kinase (PI3K)/AKT pathway in the absence of androgens,8,23 and abnormal ErbB-2 activation due to overexpression may serve as one of the key factors leading to prostate cancer recurrence during hormone ablation therapy, and/or to promote prostate tumors with a highly metastatic potential.8,24 Thus, ErbB-2 overexpression in patients with prostate cancer has been associated with poor prognosis.25 The exact nature of the contribution made by elevated ErbB-2 protein levels to prostate cancer cell invasion during the cancer progression is, however, still not well-characterized.
Cell surface proteolysis by secreted or plasma membrane-anchored proteases has been strongly implicated in cancer cell invasion and metastasis due to their roles in the degradation of the Extracellular matrix, cell growth, adhesion, and migration.26 Recently, several lines of evidence have shown that deregulation of type II transmembrane serine proteases may play a role in many diseases and, in particular, may enhance tumor growth, invasion, and metastasis.27,28,29,30,31 One of the best characterized type II transmembrane serine proteases is the enzyme matriptase.32,33,34,35,36 In human cancer, matriptase is expressed in a variety of epithelial-derived tumors including prostate, breast, colon, stomach, and ovarian carcinomas.35,36,37,38 In the prostate, matriptase expression is increased in primary prostate cancers and in metastatic lesions.39 Immunohistochemical analysis has shown that increased matriptase protein level is correlated with tumor grade and poor prognosis.40 Moreover, a role for matriptase in carcinogenesis is further supported by data from animal models. Overexpression of matriptase in the skin of transgenic mice, driven by a keratin-5 promoter, induces ras-independent multistage carcinogenesis and promotes ras-mediated, carcinogen-induced tumor formation.41 Recently, matriptase has been suggested to be involved in the activation process of the pro-oncogenic and prometastatic factors pro-uPA and pro-HGF/SF.42,43,44,45 Despite these varied observations, little is known about the role of oncogenic signaling in matriptase activation or how this might be involved in enhanced cancer malignancy during the progression of prostate cancer.
In this study, we explore the possible role that ErbB-2 signaling may play in the deregulation of cell-surface proteolysis through modulation of matriptase zymogen activation, and how the ErbB-2-matriptase axis may promote an invasive phenotype in prostate cancer cells.
Materials and Methods
Materials
Lipofectamine 2000 transfection reagent and penicillin-streptomycin were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium, and RPMI 1640 culture media were obtained from Hyclone (Logan, UT). Protein assay kits were from Bio-Rad (Hercules, CA). Protein molecular weight standard markers and an anti-Myc epitope antibody (Ab) were obtained from Bioman Scientific Company, Ltd (Taipei, Taiwan). Polyclonal anti-ErbB-2 Ab (C-18) for immunoblotting and horseradish peroxidase-conjugated anti-rabbit IgG Ab were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-phosphotyrosine (pY100), anti-phospho-p44/p42 ERK1/2 (Thr202/Tyr204), rabbit polyclonal anti-phospho-ErbB-2 (Y877), rabbit polyclonal anti-phospho-ErbB-2 (Y1221/Y1222), rabbit polyclonal anti-phospho-ErbB-2 (Y1248), anti-phospho Akt, and anti-Akt Abs were obtained from Cell Signaling Technology (Beverly, MA), and a polyclonal anti-Erk1/2 Ab was purchased from Millipore (Millipore, CA). The mouse monoclonal anti-phosphotyrosine (4G10) Ab was purchased from Upstate Biotechnology (Lake Placid, NY). The Enhanced Chemiluminescence (ECL) detection system was purchased from Pierce (Rockford, IL). AG825, LY294002, and PD98059 were from Calbiochem (San Diego, CA). EGF, heregulin-β1, and all other chemicals and reagents were purchased from Sigma (St. Louis, MO), unless otherwise noted.
Cell Culture
The human prostate carcinoma cell lines DU145, C-33, and C-81 LNCaP cells were gifts from Dr. Ming-Fong Lin at the Department of Biochemistry and Molecular Biology, University of Nebraska Medical Center (Omaha, NE). C-33 cells, C-81 cells, and DU 145 cells were maintained in a regular medium consisting of RPMI 1640 supplemented with 5% FBS, 1% glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. HEK 293T cells were used for lentiviral production. HEK 293T cells and PC-3 cells were gifts from Dr. Pei-Wen Hsiao, Institute of Agricultural Biotechnology Research Center, Academia Sinica, Taiwan, and cultured in Dulbecco’s modified Eagle’s medium with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Transient Transfection with ErbB-2, Constitutively Active MEK, and Akt (Myr-Akt) cDNA Constructs
Cells were seeded in regular culture medium at a density of 4 × 105 cells for C-81 LNCaP cells and 6 × 105 cells for C-33 LNCaP cells per 60-mm dish. One day after plating, transient transfection with wild-type ErbB-2, constitutively active MEK, or Akt plasmids was performed by Lipofectamine 2000 reagent in Optimum-Minimum Essential Medium (Invitrogen, Carlsbad, CA) medium, according to the manufacturer’s instruction. The transfected cells were harvested 48 hours after transfection. The ErbB-2 and a constitutively active MEK expression constructs were gifts from Dr. Ming-Fong Lin.46,47 The Myr-Akt plasmid was a gift from Dr. Ruey-Hwa Chen, Institute of Biological Chemistry, Academia Sinica, Taiwan.
Immunoprecipitation
LNCaP cells (1 × 107 cells) were washed with PBS and a lysate was prepared by adding 1 ml of immunoprecipitation assay buffer (50 mmol/L Tris–HCl, pH 7.8, 150 mmol/L NaCl, 5 mmol/L EDTA, 0.5% Triton X-100, 0.5% Nonidet P-40, 0.1% deoxycholate, and 10 μg/ml each of leupeptin, aprotinin, and phenylmethylsulfonyl fluoride) to the cells. The lysate was then centrifuged by using a microcentrifuge at 10,000 × g for 20 minutes. The supernatant was added to an anti-ErbB-2 Ab at 4°C for 1 hour. Protein-A/G-agarose beads (30 μl; Calbiochem) were added to the lysate, and the mixture was incubated with shaking for 1 hour at 4°C. The beads were finally collected by centrifugation and washed three times with immunoprecipitation assay buffer. Proteins binding to the beads were eluted by adding 20 μl of 2× electrophoresis sample buffer and analyzed by immunoblotting with an anti-phosphotyrosine Ab (4G10). After analysis the membranes were stripped and reblotted with an anti-ErbB-2 Ab to detect total protein levels.
Establishment of Stable ErbB-2-Overexpressing LNCaP Transfectants
Two days after transient transfection with the ErbB-2 plasmid or parent vector alone, the cells were treated with 400 μg/ml Geneticin/G418 to select for stable integration of the plasmids. Stable pools of ErbB-2 or control transfected cells were maintained in a regular medium supplemented with 400 μg/ml of Geneticin.
Lentiviral Particle Preparation and Infection for Small Hairpin RNA Delivery
Small hairpin RNAs (shRNA) in a lentiviral infection system were obtained from the National RNAi Core Facility of Academia Sinica, Nankang, Taiwan. shRNAs for the knockdown of ErbB-2 (shErbB-2, clone ID: TRCN 0000039879; shErbB-2u, clone ID: TRCN 0000303527), Akt1 (shAkt1-1, clone ID: TRCN 0000039795; Akt1-2, clone ID: TRCN 0000010174), Akt2 (shAkt2-1, clone ID: TRCN 0000265834; Akt2-2, clone ID: TRCN 0000255915), matriptase (shMTX, clone ID: TRCN 0000038053), or a negative control shRNA for knockdown of luciferase (shLuc) were all in the pLKO.1-puro vector and were packaged into lentiviral particles by using HEK 293T cells by co-transfection with pCMVΔR8.91 and pMD.G, using Lipofectamine 2000, according to the recommended protocol. Conditioned medium from the transfected cells containing lentiviral particles was collected 24 and 48 hours after the addition of fresh medium. For lentiviral infection, cells were seeded at up to 70% confluence and cultured for 24 hours. Lentiviral infection was performed by adding 10% (v/v) of lentivirus-containing medium to the cell culture. Twenty-four hours after infection, transduced cells were selected by exposure to 1 μg/ml puromycin for 3 days.
Western Blot
After washing twice with cold PBS (50 mmol/L potassium phosphate and 150 mmol/L NaCl, pH 7.2), the cells were lyzed in a standard lysis buffer (1% Triton X-100 in PBS) or a lysis buffer containing phosphatase and protease inhibitors (20 mmol/L HEPES [pH = 7.0], 0.5% NP-40, 2 mmol/L sodium vanadate, and 1× proteinase inhibitor cocktail; Roche Diagnostics GmbH, Mannheim, Germany). The lysates were centrifuged at 12,000 rpm for 10 minutes at 4°C, and the supernatants were collected. Protein concentrations in the cell lysates were determined by Protein Assay solution (BioRad) with reference to a bovine serum albumin standard curve. Equal amounts of total protein were then mixed with Laemmli sample buffer for electrophoresis. Generally, 5% β-mercaptoethanol was added to the samples, which were then boiled for 10 minutes before loading on the gels; however, when blotting for matriptase or HAI-1, this was not done, because boiling disrupts matriptase-HAI-1 complexes, and reducing agents destroy the epitopes recognized by the monoclonal antibodies (mAbs).48 Fifty micrograms of proteins per well were loaded and separated by SDS-polyacrylamide gel electrophoresis, and then transferred to nitrocellulose membranes with a transfer buffer consisting of 25 mmol/L Tris-base, 192 mmol/L glycine, and 20% methanol. After protein transfer and between antibody incubations, the membranes were washed with Tris-Buffered Saline Tween-20 (TBST) wash buffer (20 mmol/L Tris-HCl [pH 7.5], 0.87% [w/v] NaCl, and 0.1% Tween 20). The membranes were blocked with 5% skim milk in TBST, and the specific proteins were recognized by primary antibodies and subsequently by secondary antibodies conjugated with Horseradish peroxidase (Jackson, PA) in 5% skim milk. The target proteins were revealed by enhanced chemiluminescent reagents (Pierce) and detected by X-ray films or a luminescent image analyzer with a CCD camera (LAS-4000; Fujifilm, Tokyo, Japan).
Wound Healing Assay
Cells for wound healing assays were cultured for 5 to 6 days in 60-mm Petri dishes until fully confluent. Wound gaps were made by scraping cell monolayers with a 10-μl pipette tip, after which the cells were washed with PBS twice and fed with fresh culture medium. Images of wound gaps were taken by a light microscope (TS100; Nikon, Taipei, Taiwan) at 100× and the cells were incubated in a humidified, 37°C, 5% CO2 incubator until subsequent images were taken. Widths of the wound and migratory distances were measured and calculated by using NIS-Elements D software (Nikon). Each observation was carried out in triplicate.
Transwell Invasion/Migration Assay
Transwell assays were performed according to previously described procedures with slight modification.49 In brief, cell culture inserts (Millipore) were placed in the wells of 24-well plates (Corning Incorporated, Corning, NY). For an invasion assay, 10 μg of Matrigel (BD Biosciences, San Jose, CA) diluted in 100 μl distilled water was added to the filer of each insert, which was then air-dried overnight. The dried Matrigel was reconstituted with 100 μl serum-free RPMI 1640 medium for 1 hour before usage. No Matrigel was coated on filters for migration assay. Cells were serum-starved for 24 hours and then seeded in the upper chambers of transwell with 200 μl serum-free RPMI 1640 medium. The lower chambers were filled with 600 μl RPMI 1640 medium containing 10% FBS as a chemoattractant. After incubation for 48 hours, cells that had penetrated to the external surface of the filters were fixed in methanol for 10 minutes and stained with 1% crystal violet for 10 minutes. After washing with distilled water, the nonpenetrating cells on the inner surfaces of the upper chambers were wiped off by using cotton swabs. The cells that had penetrated were photographed (100×) and counted by light microscope (eight random 200× fields of each filter were observed). Each assay was carried out in triplicate.
Statistical Analysis
A mean ± SE was calculated from three repeated groups in all experiments. A statistical significance between groups was determined by Student’s t-test. A P value below 0.05 was considered as a significant difference between two groups.
Results
Androgen Refractory C-81 LNCaP Prostate Cancer Cells Exhibit Increased Motility and Invasive Behavior, and Have Higher Levels of ErbB2 and Matriptase Activation than the C-31 Prenatal Control Cells
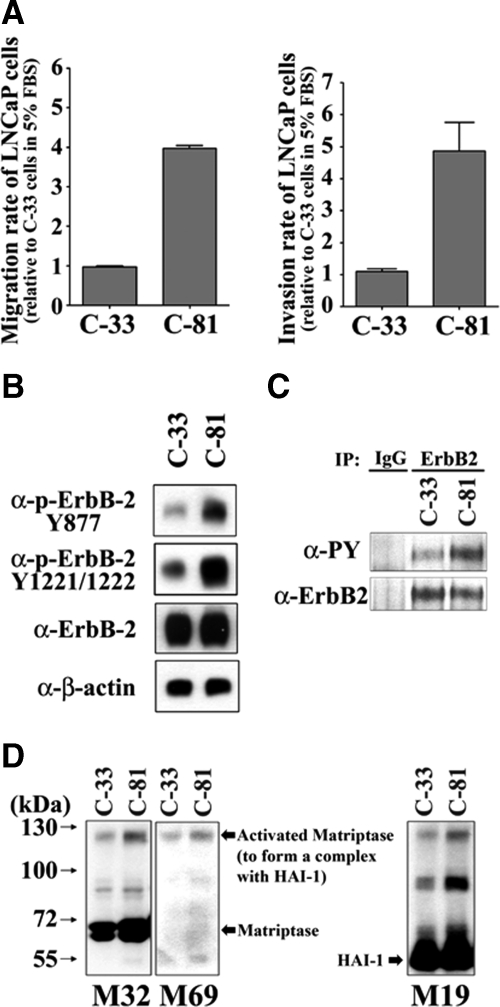
Increased levels of both ErbB-2 and matriptase are correlated with more advanced human prostate cancer.6,8,40,50 This observation led us to wonder if increased ErbB-2 signaling might be mechanistically linked with some aspects of tumor progression by modulation of matriptase activity. We therefore set out to find a suitable experimental system that might allow us to evaluate this hypothesis. We started by evaluating the properties of a cell model based on the well known LNCaP prostate cancer cell line. Dr. Ming-Fong Lin at the Department of Biochemistry and Molecular Biology, University of Nebraska Medical Center has developed two LNCaP sublines (C-33 and C-81 LNCaP cells) that have been shown to model prostate cancer tumor progression with increased ErbB-2 activity and prostate-specific antigen (PSA) secretion, and decreased PAcP expression and androgen dependence.51,52 We first examined the migratory/invasive abilities of the C-33 and C-81 LNCaP cells using modified Boyden chamber (transwell) assays, using uncoated or matrigel coated membranes. As shown in Figure 1A, C-81 LNCaP cells exhibited strong migration and invasion capability, compared with the parental LNCaP (C-33) cells. C-81 LNCaP cells therefore also model advanced human prostate cancer with respect to high cell migration and invasion capacity, compared with the C-33 LNCaP cells, which model early-stage cancer with low rates of cell migration and invasion. We then examined the state of ErbB-2 and matriptase activation in the two sublines by immunoblot analysis. The activation of ErbB-2 was assessed by determining the degree of protein phosphorylation at specific tyrosine residues by using anti-phospho-ErbB-2 Tyr877 and anti-phospho-ErbB-2 Tyr-1221/Tyr-1222 Abs. The data generated showed that although both LNCaP sublines expressed similar levels of total ErbB-2 protein, the C-81 cells exhibited a significantly higher level of ErbB-2 activation compared with the C-33 cells (Figure 1B). The data were confirmed by studies in which ErbB-2 was immunoprecipitated from the cells and then immunoblotted with an anti-phospho-Tyr antibody (Figure 1C).
Figure 1.
Migratory and invasive capabilities of different LNCaP cells and the activated levels of ErbB-2 and matriptase in those cells. A: Migratory and invasive capabilities of C-33 and C-81 LNCaP cells. After trypsinization, 4 × 105 of the cells were seeded in the upper chamber of each transwell coated without or with matrigel (30 μg/cm2) in serum-free RPMI 1640 medium, and the lower chambers were filled with 5% FBS RPMI medium. Transwell migration assay was carried out for 24 hours. Migratory and invasive cells were fixed in methanol and stained with 0.25% crystal violet. Each assay was performed in triplicate for calculation of means ± SE. B: Analysis of ErbB-2 expression and phosphorylation levels by immunoblotting assays. Cell lysates were collected with 0.5% NP-40 in HEPES buffer. The ErbB-2 tyrosine phosphorylation levels were detected by using anti-phospho-ErbB-2 Tyr877 and anti-phospho-ErbB-2 Tyr-1221/Tyr-1222 antibodies (Abs). Total ErbB-2 protein level was determined by using an anti-ErbB-2 Ab (C18). C: Whole cell lysates were immunoprecipitated with an anti-ErbB-2 antibody followed by immunoblotting with anti-pTyr (4G10) and anti-ErbB-2 Abs. D: Analysis of the levels of total and activated matriptase and HAI-1 in different LNCaP cells by immunoblotting assay. LNCaP cells were lysed in 1% Triton X-100 PBS and then collected under nonboiling and nonreducing conditions. The immunoblots for total matriptase, activated matriptase, and HAI-1 were conducted by using anti-total matriptase (M32), anti-activated matriptase (M69), and anti-HAI-1 (M19) mAbs.
To evaluate the levels of matriptase and its activated status within the LNCaP sublines, cell extracts were prepared and blotted with monoclonal antibodies that respectively recognize total matriptase (M32), the activated form of the enzyme (M69), and the cognate matriptase inhibitor HAI-1 (M19). The development and characterization of these antibodies has been described extensively in our previous publications,32,53,54 and their use allows simple assessment of overall levels of the enzyme and the degree of zymogen activation. The M32 antibody recognized the third LDLR domain of matriptase and detects levels of total matriptase as a band at 70 kDa, representing the latent zymogen, and a band at 120 kDa, representing the active enzyme in a complex with the inhibitor HAI-1. The M69 antibody is specific for the two-chain, active form of the enzyme, which is principally detected in the 120-kDa HAI-1-matriptase complex. The M19 antibody is specific to a region between the two kunitz domains of HAI-1 and detects this protein as a 50-kDa band representing free, uncomplexed HAI-1 and in the 120-kDa matriptase-HAI-1 complex. The data shown in Figure 1D demonstrate that although the overall level of matriptase is similar in the two LNCaP sublines, the level of matriptase activation appears to be significantly higher in the C-81 cells, compared with C-33 cells with the presence of a significantly more intense band at 120 kDa being detected by all three antibodies (Figure 1D). These data demonstrate that the C-81 cells are significantly more motile and invasive than the C-33 cells, and that both ErbB-2 and matriptase activation was also increased in the C-81 cells. This observation prompted us to explore the possible relationship between ErbB-2 activation, matriptase activation, and prostate cancer cell migration and invasiveness.
EGF-Induced ErbB2 Activation Stimulates Matriptase Zymogen Activation in C-33 LNCaP Prostate Cancer Cells
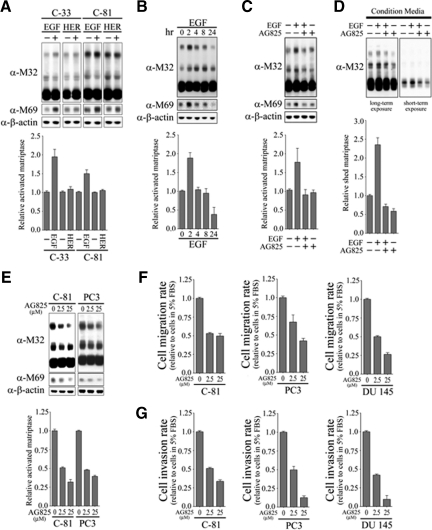
To begin to evaluate the possible effect of ErbB2 signaling on matriptase zymogen activation, we tested the ability of ErbB ligands to induce matriptase zymogen activation in PCa cells. LNCaP C-33 and C-81 cells were stimulated with one of two different Erb family ligands (EGF or heregulin-β1), and their effects on matriptase activation were assessed by immuno-blot studies. As shown in Figure 2A, treatment with EGF but not heregulin-β1 was able to dramatically induce matriptase zymogen activation in both cells with a reduced fold stimulation in C-81 LNCaP cells. The lower fold stimulation in C-81 cells may be due to a high basal level of activated matriptase in the cells. Thus, C-33 LNCaP cells were more sensitive to EGF stimulation on matriptase zymogen activation than C-81 LNCaP cells. To further examine the effect of EGF on matripase zymogen activation and the role of ErbB-2 in this biological event, C-33 LNCaP cells were treated with EGF for different times. As shown in Figure 2B, after EGF administration, the activated level of matriptase (indicated by the formation of 120-kDa matriptase/HAI-1 complexes) started to increase at 2 hours and decline after 24 hours. To confirm that this was an ErbB-2 mediated effect, cells were then treated with EGF for 2 hours in the presence or absence of a specific ErbB-2 inhibitor AG825. EGF-induced matriptase zymogen activation was dramatically reduced in the presence of the ErbB-2 inhibitor (Figure 2C), suggesting that ErbB-2 is indeed the receptor responsible for mediating EGF-inducing matriptase zymogen activation. Since shedding of matriptase into the extracellular environment is known to occur rapidly after activation, we determined if the decline in matriptase levels after prolonged EGF treatment was due to shedding of the activated enzyme. Levels of matriptase in the conditioned medium were assayed after 24-hour EGF treatment in the presence or absence of AG825. The data show that matriptase shedding was significantly increased after EGF-induced matriptase activation and that treatment with AG825 was able to inhibit EGF-induced matriptase shedding (Figure 2D). These data strongly suggest that ErbB-2 is involved in EGF stimulated matriptase zymogen activation in PCa cells. To further examine the role of ErbB-2 in matriptase zymogen activation of human PCa cells, C-81 LNCaP cells and PC-3 cells with high ErbB-2 activity (Figure 1, B and C, and Supplemental Figure S1 at http://ajp.amjpathol.org) were also treated with specific ErbB-2 inhibitor AG825. As shown in Figure 2E, treatment with AG825 efficiently inhibited matriptase zymogen activation in a dose-dependent manner. We then further addressed the role of ErbB-2 in prostate cancer cell migration and invasion. LNCaP C-81, PC3, and DU145 cells were treated with different concentrations of AG825 and cell migration and invasion assays were performed. The data presented in Figure 2, F and G, show that inhibition of ErbB-2 resulted in a reduction in the cell migration and invasion of these three PCa cells in a dose-response manner. These data suggest that ErbB-2 is involved in matriptase zymogen activation of PCa cells, as well as in their cell migration and invasion.
Figure 2.
Effects of EGF and heregulin-β1 on matriptase zymogen activation and shedding in C-33 LNCaP cells. A: Effects of EGF and heregulin-β1 on matriptase zymogen activation in C-33 LNCaP cells. LNCaP C-33 cells were seeded at a density of 1 × 106 per well in each 6-cm dish. Two days after plating, cells were starved with serum-free medium for 36 hours. After treatment with 20 ng/ml EGF or 40 ng/ml heregulin-β1 for 2 hours, cell lysates were collected under nonboiling and nonreducing conditions and immunoblotted for matriptase and activated matriptase with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. B: Kinetics of EGF-stimulated matriptase activation in C-33 LNCaP cells. Starved cells were treated with 20 ng/ml EGF for 0, 2, 4, 8, or 24 hours. Cell lysates were then prepared and used to assay total and activated matriptase with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. C: Serum-starved cells were treated with or without 20 ng/ml EGF in the presence or absence of 2.5 μmol/L AG825 for 2 hours. The immunoblots were performed as described in B. D: Conditioned media were collected from the cells treated with or without 20 ng/ml EGF in the presence or absence of 2.5 μmol/L AG825 for 24 hours. The immunoblots were performed as described in B. The levels of secreted matriptase were quantitated by using a densitometer and normalized to their respective cell numbers. E: The effect of AG825 on matriptase activation in C-81 LNCaP cells (left panel) and PC-3 cells (right panel). Starved cells were treated with AG825 at 0, 2.5, and 25 μmol/L for 24 hours. The immunoblots were performed as described in B. The effects of AG825 on cell migration (F) and invasion (G). After cell seeding into transwells, LNCaP C-81, PC3, and DU145 cells were treated with the indicated concentrations of AG825. Cell migration and invasion assays were performed, according to the protocol, as described in Figure 1A.
Enhanced ErbB-2 Activation Resulting from Overexpression of the Receptor Results in Enhanced Matriptase Zymogen Activation
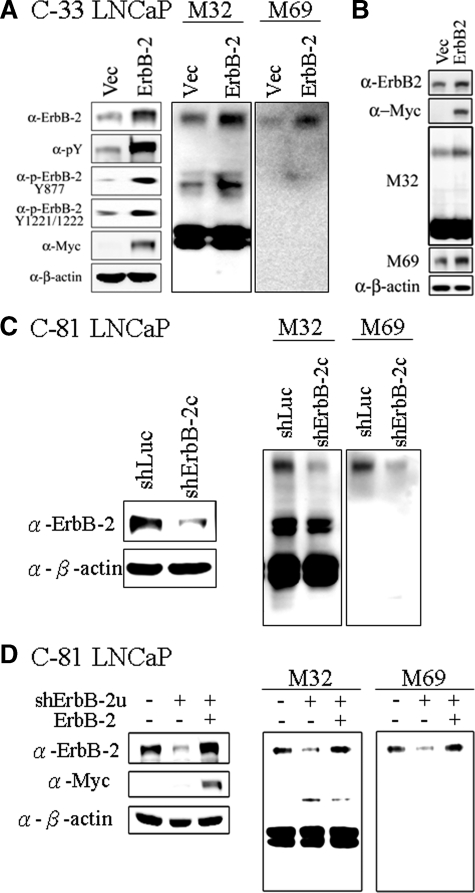
To more directly explore the role of ErbB-2 in matriptase zymogen activation, we set out to test the effect of increased ErbB-2 expression on matriptase activation. LNCaP C-33 cells were transiently transfected with a wild-type ErbB-2 expression vector or with the vector alone as a control. Overexpression of ErbB-2 resulted in increased ErbB-2 activity, as indicated by receptor tyrosine phosphorylation levels at the specific residues Tyr877 and Tyr-1221/Tyr-1222. Interestingly, this increase in receptor activation was also associated with a concomitant increase in matriptase zymogen activation, with enhanced formation of the 120 kDa matriptase/HAI-1 complex (Figure 3A). A similar result was also obtained by using DU145 cells demonstrating that overexpression of ErbB-2 further promotes matriptase zymogen activation (Figure 3B). Thus, enhanced ErbB-2 activity caused by enforced overexpression of the receptor resulted in increased matriptase zymogen activation in PCa cells. These data raise the possibility that the enhanced ErbB-2 activation resulting from ErbB-2 overexpression, which has been shown to occur in a subgroup of human PCa patients, may lead to deregulation of matriptase activation, which in turn may participate in cancer progression and enhanced malignancy.
Figure 3.
Effects of ErbB-2 level and activity on matriptase zymogen activation in PCa cells. A: Effects of ErbB-2 overexpression on matriptase activation in C-33 LNCaP cells. LNCaP C-33 cells were transiently transfected with ErbB-2 cDNA by Lipofectamine 2000 reagents, and control cells were transiently transfected with vector alone. Two days after transfection, cells were harvested for Western blot analysis. The separated proteins were transferred to a NC membrane and detected by immunoblotting with anti-pTyr (PY100), anti-ErbB-2 (C18), anti-c-Myc, and anti-β-actin (AC-15) Abs. For analyses of matriptase and HAI-1, nonreduced and nonboiled cell lysates were collected with Triton 1% X-100 in PBS, and immunoblotted with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. B: Effects of ErbB-2 overexpression on matriptase activation in DU 145 cells. DU 145 cells were transiently transfected with ErbB-2 cDNA, and the immunoblots were performed as described in A. C: Effects of ErbB-2 knockdown on activation of matriptase in C-81 LNCaP cells. Cells were seeded at a density of 1.2 × 106 per well in 6-cm dishes. One day after plating, cells were infected with lentiviral particles containing ErbB-2 shRNA for 24 hours. Control cells were infected with lentiviral particles containing luciferase shRNA. Three days after selection with 1 μg/ml puromycin, cells were harvested for Western blot analysis. For analyses of the p-Tyr and protein levels of ErbB-2, cell lysates were collected and detected by anti-pTyr (PY100) and anti-ErbB-2 (C18) Abs. For analyses of matriptase and HAI-1, nonreduced and nonboiled cell lysates were collected with Triton 1% X-100 in PBS. Matriptase and HAI-1 were detected by immunoblotting with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. Equal protein loading was verified by blotting the membranes with an anti-β-actin Ab (AC-15). D: Effects of ErbB-2 re-expression on matriptase activation in ErbB-2-knockdown cells. C-81 LNCaP cells were infected with viral particles with control luciferase shRNA or ErbB-2u shRNA for 3 days. The ErbB-2u shRNA was designed to target a specific sequence located in the 3′ UTR of ErbB-2. The ErbB-2- knockdown cells were transfected with control vector or ErbB-2 cDNA and cultured for 3 days. Cell lysates were collected and assayed by using an anti-ErbB-2 Ab (C18). For analyses of matriptase, cell lysates were collected as described in Figure 1D to detect the levels of total and activated matriptase by immunoblotting with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. Equal protein loading was verified by blotting the membranes with an anti-β-actin Ab (AC-15).
Suppression of ErbB-2 Protein Levels by shRNA Mediated Knockdown Results in Suppressed Matriptase Activation
To further establish a causal relationship between ErbB-2 levels and matriptase zymogen activation by using an independent approach, we tested the effect of suppressing ErbB2 protein levels on matriptase activation in the LNCaP C-81 cells. As we demonstrated in Figure 1, ErbB2 activity is much higher in these cells compared with the C-33 subline, and matriptase zymogen activation is also very high in these cells. Transduction of the C-81 cells with an ErbB-2 targeting lentiviral vector resulted in a greater than 70% reduction in ErbB-2 protein levels compared with cells transduced with a control virus (Figure 3C, left panel). Concomitant with ErbB-2 knockdown, matriptase activation was also significantly suppressed, as shown by a reduction in the level of the 120 kDa matriptase/HAI-1 complex (Figure 3C, right panel). To control for potential off-target effect of the ErbB-2 shRNA and to further confirm the role of ErbB-2 in inducing matriptase zymogen activation, we confirmed our observation through the use of another independent shRNA construct (shErbB-2u), which is designed to target a specific ErbB-2 sequence located in the 3′ UTR of ErbB-2, and through the use of a “rescue” strategy in which the levels or ErbB-2 expression were increased by transfection with an shRNA insensitive expression construct that does not contain the sequences targeted by shErbB-2u. As shown in Figure 3D, the reduction of ErbB-2 expression induced by shErbB-2u resulted in the inhibition of matriptase zymogen activation. This reduced matriptase activation was rescued by stable ErbB2 overexpression with the shErbB-2u-resistant construct (Figure 3D). Taken together, these data strongly suggest that ErbB-2 is involved in the regulation of matriptase zymogen activation in PCa cells.
Signaling Through the ERK1/2 Pathway Does Not Appear to Be Involved in ErbB-2-Induced Matriptase Activation
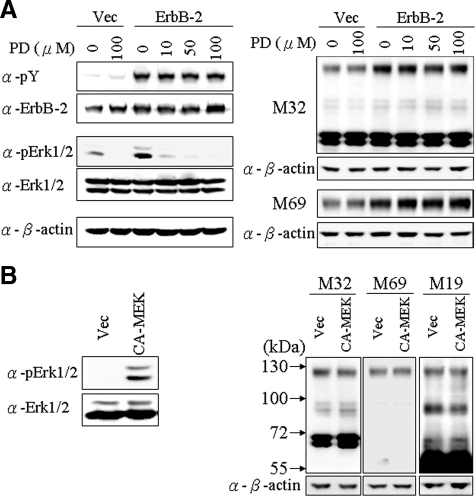
We were next interested in trying to define the down-stream signaling pathways responsible for ErbB-2 induced matriptase activation. The ERK1/2 pathway is known to be important in mediating downstream signaling from ErbB-2 in human prostate cancer cells.47 To explore the role of the ERK1/2 pathway in ErbB-2-induced matriptase activation, we established stable pools of ErbB-2-overexpressing C-33 LNCaP cells. ErbB-2 activity in the transfectants was dramatically increased, as indicated by receptor tyrosine phosphorylation (Figure 4A). Consistent with our results from the transient transfection experiments described above (Figure 3A), this increased ErbB-2 activity was accompanied by enhanced matriptase activation. Using these cells, we tested the effect of various concentrations of the MEK inhibitor PD98059 on ERK1/2 activity and on matriptase zymogen activation. Interestingly, although the MEK inhibitor efficiently suppressed ErbB-2-induced ERK1/2 activity in a dose-response manner (Figure 4A, left panel), the compound had no significant effect on the level of matriptase activation (Figure 4A, right panel). To further analyze the possible role of ERK1/2 in matriptase zymogen activation in PCa cells, we transfected C-33 LNCaP cells with a constitutively activated MEK expression construct. As shown in Figure 4B, although expression of constitutively activated MEK resulted in abundant ERK1/2 activation, indicated by immunoblotting analysis with an anti-phosphoMAP kinase Ab, the increased ERK1/2 activity had no effect on matriptase activation. Together with the inhibitor studies shown in Figure 4A, these data strongly suggest that signaling through the ERK1/2 pathway is not responsible for ErbB-2-induced matriptase zymogen activation in PCa cells.
Figure 4.
Role of MEK/ERKs in ErbB-2-induced matriptase activation in C-33 LNCaP cells. A: Stable ErbB-2-overexpressing C-33 LNCaP cells were seeded at a density of 6 × 105 per well in a 6-well plate. Two days after plating, cells were treated with PD98059 at concentrations of 0, 10, 50, and 100 μmol/L; control transfected cells were treated with concentrations of 0 and 100 μmol/L PD98059. Treatment was carried out for 24 hours, and then cells were harvested for Western blot analysis. Cell lysates were collected with 0.5% NP-40 in HEPES buffer. The p-Tyr and protein levels of ErbB-2 were analyzed by immunoblotting with anti-pTyr (PY100) and anti-ErbB-2 (C18) Abs. The phosphorylation and protein levels of Erk1/2 were detected by anti-phosphoErk (Thr202 and Tyr204 of Erk1; Thr185 and Tyr187 of Erk2) and anti-Erk1/2 Abs. Nonreduced and nonboiled cell lysates were used for immunoblots to detect the levels of total and activated matriptase with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. Equal loading was evaluated with an anti-β-actin Ab. B: Effects of a constitutively active MEK on matriptase activation in C-33 LNCaP cells. Cells were seeded at a density of 1.2 × 106 per well in 6-cm dishes. Two days after plating, cells were transiently transfected with a constitutively active MEK (CA-MEK) cDNA and harvested 48 hours after transfection; control cells were transfected with vector alone. Cell lysates were collected with 0.5% NP-40 in HEPES buffer. The phosphorylation and protein levels of Erk1/2 were analyzed by immunoblotting with an anti-phosphoErk (Thr202 and Tyr204 of Erk1; Thr185 and Tyr187 of Erk2) and anti-Erk1/2 Abs. Nonreduced and nonboiled cell lysates were used to assay the activation status and total matriptase levels with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. An anti-β-actin (AC-15) Ab was used to evaluate protein loading.
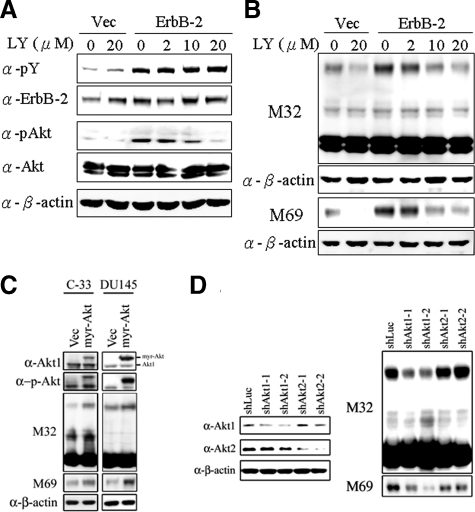
ErbB-2 Stimulation of Matriptase Activation Is Dependent on Phosphatidylinositol 3 Kinase Signaling
In addition to the MAPK pathway, ErbB-2 is known to signal through the PI3 kinase pathway and so we next set out to determine whether PI3 kinase signaling is responsible for ErbB-2 stimulated matriptase zymogen activation in PCa cells. To do this we again made use of the stable ErbB-2-overexpressing C-33 LNCaP cells, treating them with different concentrations of the PI3 kinase-specific inhibitor LY294002. As shown in Figure 5A, treatment with LY294002 efficiently inhibited ErbB-2-induced PI3 kinase activity, as indicated by the suppression of the phosphorylation of its downstream target Akt. Suppression of PI3K activity also simultaneously blocked ErbB-2-induced matriptase zymogen activation in a dose-dependent manner (Figure 5B). To further examine the possible role of Akt in matriptase zymogen activation in PCa cells, we transiently transfected C-33 LNCaP and DU145 cells with a constitutively active Akt1 (Myr-Akt) and control plasmids. As shown in Figure 5C, the expression of the constitutively active Akt enhanced the level of activated matriptase. Since several Akt isoforms are known to play distinct roles in tumor cell growth, invasion, and migration,55,56 and Akt1 and Akt2 are the forms predominantly expressed in PCa cells,57,58 we set out to identify which Akt isoform was involved in regulating matriptase zymogen activation by using small-interfering RNA technology to specifically knock down Akt1 and Akt2 and examining the effect on matriptase zymogen activation. As shown in Figure 5D, Akt1 but not Akt2 appears to be important for matriptase zymogen activation in PCa cells. These data indicated that signaling through the PI3 kinase/Akt1 pathway is responsible for ErbB-2-induced matriptase zymogen activation in these cancer cells.
Figure 5.
Role of PI 3 kinases/Akt in ErbB-2-induced matriptase activation in C-33 LNCaP cells. Stable ErbB-2-overexpressing C-33 LNCaP cells were seeded at a density of 6 × 105 per well in a 6-well plate. Two days after plating, cells were treated with LY294002 at concentrations of 0, 2, 10, and 20 μmol/L; control cells were treated with concentrations of 0 and 20 μmol/L PD98059. Treatment was carried out for 24 hours, and then cells were harvested for Western blot analysis. A: Cell lysates were collected with 0.5% NP-40 in HEPES buffer and evaluated by immunoblots for p-Tyr and protein levels of ErbB-2, performed as described previously. The phosphorylation and protein levels of Akt were detected by anti-phosphoAkt (Ser473) and anti-Akt1/2 Abs. B: Nonreduced and nonboiled cell lysates were used for immunoblotting to detect the activation status and total matriptase with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. An anti-β-actin (AC-15) Ab was used to evaluate protein loading. C: Role of a constitutively activated Akt (Myr-Akt) in matriptase zymogen activation in C-33 LNCaP cells and DU 145 cells. LNCaP C-33 cells and DU 145 cells were transiently transfected with Myr-Akt and control plasmids by using Lipofectamine 2000. Two days after transfection, cell lysates were collected and analyzed by immunoblotting with anti-phosphoAkt (Ser473), anti-Akt1/2, anti-total matriptase (M32), and anti-activated matriptase (M69) Abs, respectively. D: Effect of Akt1 or Akt2 knockdown on activation of matriptase in LNCaP C-81 cells. Cells were seeded at a density of 1.2 × 106 per well in 6-cm dishes. One day after plating, cells were transfect with Akt1 shRNAs (shAkt1-1 and shAkt1-2), Akt2 shRNAs (shAkt2-1 and shAkt2-2), and control luciferase shRNA with Lipofectamine 2000. Cell lysates were collected for analysis of the levels of Akt1 and Akt2 protein by immunoblot with anti-Akt1 and anti-Akt2 Abs. For analyses of matriptase, cell lysates were collected and immunoblotted with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. Equal protein loading was assessed with an anti-β-actin Ab (AC-15).
Potential Role of Matriptase in ErbB-2-Induced Prostate Cancer Progression
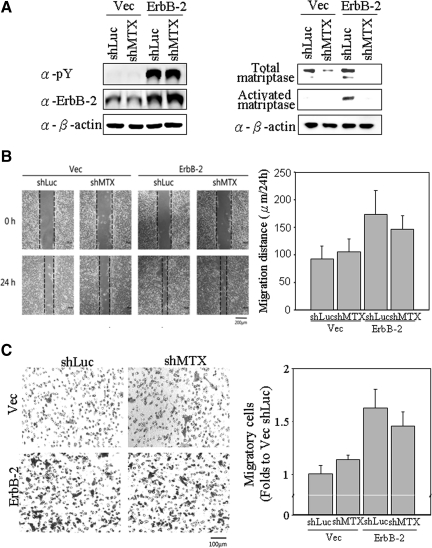
Several studies have recently shown that ErbB-2 overexpression is associated clinically with metastatic potential in prostate cancer,50 and that matriptase is a potential oncoprotein related to prostate cancer progression.40 In the light of our data demonstrating the relationship between ErbB-2 activity and matriptase activation, we wondered if matriptase might play a role in ErbB-2-induced prostate cancer malignancy. To begin to explore this possibility, we decided to evaluate the role of matriptase in various aspects of the pathogenic behavior of control or ErbB-2 overexpressing C-33 LNCaP cells through the use of lentiviral-shRNA mediated matriptase knockdown. As shown in Figure 6A, transduction of the control (Vec) or ErbB-2 overexpressing (ErbB-2) C-33 LNCaP cells with the matriptase-targeting (shMTX) lentiviral constructs suppressed matriptase levels to less than 20% of the level in cells transduced with a control lentivirus (shLuc). As seen before, the cells overexpressing ErbB-2 exhibit high levels of activated matriptase. Transduction with the matriptase targeting shRNA construct also dramatically suppressed the levels of activated matriptase in these cells. The effect of this matriptase knockdown on ErbB-2-inducing cell motility, migration, and invasion was then evaluated as shown in the following experiments.
Figure 6.
Effects of matriptase knockdown on ErbB-2-promoted cell migration of prostate cancer cells. Cells were seeded at a density of 1.2 × 106 per well in 6-cm dishes. Cells were infected by lentiviral particles with shRNAs specific to matriptase for 24 hours, selected by 1 μg/ml puromycin for 72 hours and then harvested for Western blot analysis. A: Cell lysates were collected with 0.5% NP-40 in HEPES buffer. The p-Tyr and protein levels of ErbB-2 were detected by anti-pTyr (PY100) and anti-ErbB-2 (C18) Abs. Nonreduced and nonboiled cell lysates were used for immunoblotting to detect the activation status and total matriptase with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. Loading was analyzed with an anti-β-actin mAb (AC-15). B: Effects of matriptase knockdown on ErbB-2-promoting cell motility by wound-healing assays. Wounds with widths of approximately 250 μm were made by scraping by using 10-μl pipette tips. Cells were incubated for 24 hours for wound-healing assay. Images were captured by a light microscopy with a magnification of 100×. The dotted lines define the edges of the wounds. Migratory distances (widths at 0 hours to widths at 24 hours) were analyzed by a NIS-Elements D software (Nikon) and are represented as means ± SE calculated from triplicates; a statistically significant difference (*P < 0.05) was observed between Vec/shLuc and ErbB-2/shLuc. C: Effects of matriptase knockdown on ErbB-2-promoting cell motility by transmigration assays. After trypsinization, 1 × 105 cells were seeded with serum-free RPMI 1640 medium in each of the upper chambers, and the lower chambers were filled with 10% FBS RPMI 1640 medium. Transwell migration assay was carried out for 48 hours. Migratory cells were fixed in methanol and stained with 1% crystal violet, and images were captured by a light microscopy (original magnification, ×100). Amounts of migratory cells on each filter were counted from eight random fields (original magnification, ×200). Each assay was performed in triplicate for calculation of means ± SE; a statistically significant difference, *P < 0.05 was observed between Vec shLuc and ErbB-2 shLuc.
Matriptase Does Not Contribute to ErbB-2-Induced Cell Motility of Prostate Cancer Cells
To determine whether matriptase plays a role in ErbB-2-induced cell motility, we performed wound healing and transwell migration assays by using the control and ErbB-2-overexpressing LNCaP cells. Results from the wound healing assays showed that the ErbB-2-overexpressing LNCaP cells exhibited greater motility than control cells (Figure 6B, Vec shLuc versus ErbB-2 shLuc), consistent with the notion that ErbB-2 overexpression is clinically associated with malignant progression in prostate cancer.50 Reduction in matriptase activity, however, had no significant affect on the motility of the ErbB-2-overexpressing LNCaP cells (Figure 6B, ErbB-2 shLuc versus ErbB-2 shMTX). Similarly, results from the transwell migration assays failed to demonstrate any effect of reduced matriptase levels/activity on ErbB-2-promoted transmigration of prostate cancer cells (Figure 6C, ErbB-2 shLuc versus ErbB-2 shMTX). Taken together, these data suggest that matriptase is most likely not involved in ErbB-2-induced cell motility in prostate cancer.
Matriptase Is Involved in ErbB-2-Induced of Prostate Cancer Cell Invasion
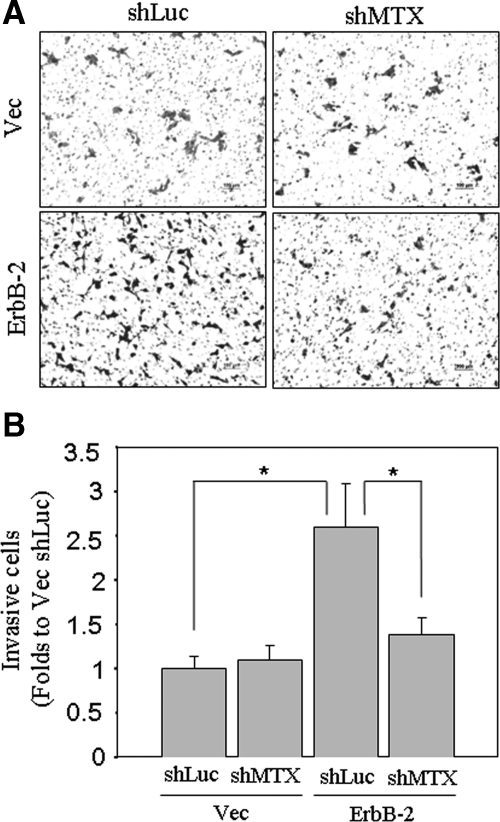
The invasive ability of cancer cells allows them to penetrate through the ECM, which is a determinant step for metastasis.30 To examine the role of matriptase in ErbB-2-induced cancer cell invasion, we made use of the widely used matrigel-coated Boyden chamber invasion assay to assess the invasive properties of the cells. ErbB-2 overexpression greatly enhanced the invasive ability of the C-33 LNCaP cells (Figure 7, A and B, Vec shLuc versus ErbB-2 shLuc). Interestingly, shRNA-mediated matriptase knockdown almost completely abrogated the increased invasive potential of the ErbB-2 overexpressing cells (Figure 7, A and B, ErbB-2 shLuc versus ErbB-2 shMTX), suggesting that matriptase plays an important role in ErbB-2 stimulated invasiveness of human prostate cancer cells.
Figure 7.
Invasion assay of ErbB-2-overexpressing LNCaP cells with or without matriptase knockdown. Matriptase knockdown was performed as described in Figure 6A. For cell invasion assays, each filter insert was coated with 30 μg/cm2 matrigel. After trypsinization, 1 × 105 cells were seeded with serum-free RPMI 1640 medium in each insert chamber, and lower chambers were filled with 10% FBS RPMI 1640 medium. A: Transwell invasion assays were carried out for 48 hours. Invasive cells were fixed in methanol and stained with 1% crystal violet. Images were captured by a light microscopy (original magnification, ×100). B: Numbers of invasive cells on each filter were counted from eight random fields (original magnification, ×200). Each assay was performed in triplicate for calculation of means ± SE; statistically significant differences, *P < 0.05 were observed between Vec/shLuc and ErbB-2/shLuc, as well as ErbB-2/shLuc and ErbB-2/shMTX.
Discussion
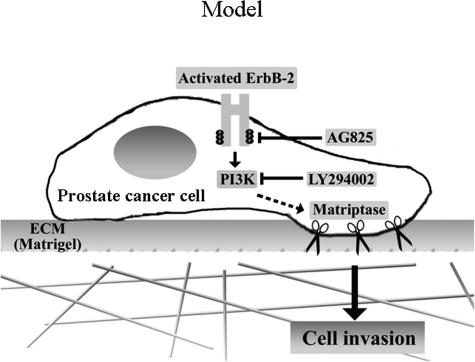
Cancer metastasis is the driving cause of poor prognosis in prostate cancer. In this report, hyperactivation of ErbB-2 was shown to result in prostate cancer cells having increased invasive and migratory phenotypes. Activation of ErbB-2 by treatment with EGF or by overexpression also resulted in the up-regulated matriptase zymogen activation, mediated at least in part through the PI3 kinase pathway. Furthermore, we found that suppression of matriptase levels/activation was sufficient to block ErbB-2-induced prostate cancer cell invasion, while not affecting cancer cell motility. These observations suggest a mechanism by which dysregulation of these two proteins might interact to drive malignant progression in prostate cancer. A tentative model for this interaction is presented in Figure 8. Enhanced ErbB-2 signaling resulting from receptor overexpression and/or increase levels of ErbB ligands leads to enhanced down-stream signaling through the PI3 kinase pathway, resulting in increased matriptase zymogen activation and increased PCa cell invasion (Figure 8). Our data suggest that aberrant ErbB-2 activation or overexpression promotes prostate cancer progression, at least in part due to up-regulation of matriptase activation promoting cell invasion, consistent with the clinical observations that dysregulation of ErbB-2 by EGF-related signaling or overexpression, as well as dysregulation of matriptase activity all contribute to prostate cancer progression to an invasive phenotype.
Figure 8.
A model for the role of matriptase in ErbB-2-driven prostate cancer cell invasion. ErbB-2 hyperactivation by EGF stimulation or receptor overexpression results in the up-regulation of matriptase zymogen activation, at least in part via PI 3 kinase, leading to enhanced prostate cancer cell invasion.
Although ErbB-2 has been shown to be overexpressed in several carcinomas, including breast and ovarian cancers, the assessment of ErbB-2 overexpression in prostate cancer is controversial with published frequencies for ErbB-2 overexpression in primary carcinomas ranging from 0% to 100%.59,60,61,62,63 The discrepancy in reports of ErbB-2 expression in prostate cancer may be due to tissue selection and preparation, antibodies, methods, and the various scoring systems used. In 2000, Signoretti et al8 used the standardized Food and Drug Administration-approved Dako immunohistochemical assay to stain prostate cancer tissues and used the absolute scoring system to define the ErbB-2 protein overexpression as samples with ≥10% of tumor cells with complete membrane staining. Their results showed that elevated expression of ErbB-2 protein was observed in 25% of untreated primary tumors, 59% of localized tumors after total androgen ablation therapy, and 78% of metastatic tumors with bone metastasis. A similar result was also found by Osman et al.6 Together, this suggests that the protein level of ErbB-2 could be elevated in a subgroup of recurrent, hormone-refractory prostate cancer. In prostate tumor tissue, the results from the chromogenic in situ hybridization showed that ErbB-2 gene is not amplified, as reported by several groups.8,63,64,65,66 Thus, it is clear that ErbB-2 gene is not amplified in prostate carcinomas although the protein level may be elevated in a subgroup of patients, especially with metastasis.6,8 During the progression of human prostate cancer cells, ErbB-2 activity increases with no significant protein alteration.51 In this study, our data showed that increased ErbB-2 activity resulting from EGF stimulation or receptor overexpression induced matriptase zymogen activation, leading to prostate cancer cell invasion.
Several lines of evidence support the hypothesis that ErbB-2 and matriptase are both related to cancer metastasis and malignancy.44,67,68 The expression level of ErbB-2 has been reported to be associated with the progression of prostate cancer to a hormone-refractory state after androgen deprivation therapy in patients,6,7 and clinically, prostate tissues with ErbB-2 overexpression exhibit high metastatic potential.5,6,8,9,50,69 These findings are further supported by the fact that ErbB-2 signaling can induce cell proliferation and PSA secretion in prostate cancer cells,46,47 and can accelerate progression to androgen independence in castrated animals.70 In this study, we also show, using a model system of prostate cancer progression (Figure 1A; the C-33 cells compared with the C-81 cells), that progression is associated with increased ErbB-2 and matriptase activities (Figure 1, B–D). This observation is consistent with the finding that tissue levels of ErbB-2 and matriptase are elevated following the progression of prostate cancer in patients.6,7,40 Thus, the LNCaP cell model also imitates the progression of human prostate cancer from a low to high capability for cell migration and invasion.
The activation of ErbB-2 can occur in a ligand-dependent manner or a ligand-independent manner. ErbB-2 possesses a functional tyrosine kinase domain, but no direct acting ligand for the receptor has been identified to date, and it is believed that ErbB-2 needs to form a heterodimer with another ligand-activated ErbB receptor to be activated in a ligand-dependent fashion.13,14,15,16 In prostate cancer cells, we found that EGF but not heregulin-β1 was able to induce matriptase zymogen activation and that ErbB-2 kinase activity was important for EGF-induced matriptase activation and shedding. This is the first report, therefore, to show that matriptase activation is induced by EGF and that this activation is most likely mediated through epidermal growth factor receptor/ErbB2 heterodimers in prostate cancer cells. It is not clear why heregulin-β1 had no significant effect on matriptase activation (Figure 2A). One possible explanation may relate to the physiological function of heregulin-β1 as a paracrine differentiation factor, which is able to promote differentiation of hormone-sensitive PCa cells, to inhibit cell growth,71,72,73 or to induce PCa cell apoptosis, although it can increase the formation of ErbB-2/ErbB-3 heterodimers.74 Recent reports have also shown that heregulin is mainly present in normal human adult prostate and benign prostatic hyperplasia with little expression in advanced PCa cells.72,73 The data suggest that ErbB-2 mediates signaling from a growth-promoting factor, EGF, but not a differentiation-induced factor, heregulin-β1, to enhance matriptase zymogen activation. On ligand stimulation, ErbB-2 exhibits differentially physiological roles dependent on what type of ligand stimulation.
It has been reported that ligand-independent ErbB-2 activation is principally due to overexpression in prostate cancer, which is associated with the cancer progression. Our data demonstrate that enhanced ErbB-2 activation resulting from overexpression leads to increased matriptase activation, via a PI3 kinase mediated pathway, thereby increasing the proteolytic capacity and invasive potential of the cells, and so providing a possible mechanism for the enhanced pathogenesis associated with ErbB-2 overexpression (Figures 5 and 7). This observation provides a molecular linkage between two oncogenic proteins, ErbB-2 and matriptase, which contributes to cancer cell invasion. ErbB-2-induced matriptase activation may directly increase digestion of extracellular matrix proteins, such as fibronectin and laminin,75 and activate uPA, matrix metalloproteinase (MMP)-3, PAR-2, and proHGF,45,76 all culminating in enhanced cancer cell invasion and cancer metastasis. Thus, matriptase may represent a good candidate for targeted therapy to prevent cancer cells from invading adjacent tissues and metastatic dissemination, particularly in the context of ErbB-2- or EGF-promoting cancer malignancy.
It has been shown that PI3 kinase signaling, a signaling pathway activated by ErbB-2, is involved in the stimulation of PCa cell invasion,77 through the up-regulation of several MMPs, including membrane type 1 matrix metalloproteinase, MMP-2, and MMP-9.78,79 In this study, our data demonstrate that matriptase activity was up-regulated by via ErbB-2/PI3 kinase/Akt1 signaling (Figures 3, 5, and 6), and that this was important for ErbB-2-promoted cell invasion (Figure 7). These findings suggest that matriptase may be an important recipient of signaling from ErbB-2/PI3 kinase/Akt1 resulting in an increase in proteolytic activity, which promotes prostate cancer cell invasion. This is a particularly appealing possibility since, based on other evidence, matriptase is believed to be a key regulatory protease, responsible for initiating a cascade of proteolytic activity, increasing the activity of several down-stream enzymes.45,48,80
In addition to acting through the PI3 kinase pathway, ErbB-2 is known to signal through the MAP kinase pathway, and both PI3 kinase and Erk1/2 mediating ErbB-2 signaling are believed to participate in the transformation of prostate cancer from an androgen-sensitive phenotype to a hormone-refractory state.23,47,81 However, no involvement of Erk1/2 was detected in ErbB-2-induced matriptase activation (Figure 4), leading us to conclude that PI3 kinase, and not Erk1/2, is an important downstream mediator of ErbB-2 induced matriptase zymogen activation and prostate cancer cell invasion.
Matriptase zymogen activation requires two proteolytic processes.54 The first proteolytic cleavage occurs at Gly149 of the SEA domain and is required to generate the 70-kDa latent form of matriptase. The second proteolytic cleavage at Arg614 occurs in the protease domain of latent matriptase and is required to initiate serine protease activity. The second cleavage is believed to occur through an autoactivation process and is a determinant step for matriptase activation.54 It is an intriguing possibility that ErbB-2/PI3 kinase signaling may promote matriptase autoactivation by posttranslational modification, such as phosphorylation, of the protease or some other protein.82,83 The ErbB-2-induced PI3 kinase pathway may directly phosphorylate serine/threonine residues on the intracellular regions of matriptase and/or HAI-1, which then initiate the autoactivation process. Analysis of the matriptase and HAI-1 sequences for potential Akt-phosphorylation sites using the NetPhosK prediction engine84 failed to find any consensus sites (RXRXXS/T) within the intracellular regions of the proteins. Akt has, however, been reported to phosphorylate nonconsensus sequences,85,86,87 and so it is possible that Akt can modulate the proteolytic activity through action on noncanonical sites on either matriptase or HAI-1. Another possibility is via indirect downstream kinase signaling of Akt to phosphorylate the Ser3 or Ser24 residue of matriptase and up-regulate the protease activity. Alternatively, ErbB-2/PI3 kinase signaling may cause the recruitment of matriptase and HAI-1, and/or activate other regulatory proteins resulting in the formation of activation foci, the locus of zymogen activation.88 Thus, ErbB-2/PI3 kinase signaling may serve as an organizer to coordinate cell surface proteases for tissue remodeling or to abnormally up-regulate them to promote cancer cell invasion. More experiments are needed to clarify the molecular mechanisms for matriptase zymogen activation.
In conclusion, our results indicate that ErbB-2 activation by EGF or overexpression can stimulate matriptase zymogen activation, which contributes to prostate cancer cell invasion. The results may explain, in part, the observation that ErbB-2 overexpression or increased matriptase activity during PCa progression is clinically associated with a high metastatic potential and poor prognosis. These data provide compelling evidence for a functional linkage from ErbB-2 signaling to matriptase activity in cell invasion and strongly suggests that matriptase could be a valuable target for the development of novel therapeutic approaches to prevent or suppress prostate cancer cell invasion and metastasis.
Acknowledgments
We thank Dr. Ming-Fong Lin (University of Nebraska Medical Center) for his gifts of prostate cancer cell lines and cDNA plasmids expressing ErbB-2 and constitutively active MEK; Dr. Ruey-Hwa Chen (Institute of Biological Chemistry, Academia Sinica, Taiwan) for her gift of Myr-Akt plasmids; Dr. Pei-Wen Hsiao (Institute of Agricultural Biotechnology Research Center, Academia Sinica, Taiwan) for his gifts of prostate cancer cells; and Dr. Ren-Jang Lin (Beckman Research Institute of the City of Hope, Duarte, California) for his critical suggestions and revision.
Footnotes
Address reprint requests to Ming-Shyue Lee, Ph.D., Department of Biochemistry and Molecular Biology, College of Medicine, National Taiwan University, R817, 8F, No. 1, Section 1, Jen-Ai Rd, Taipei, Taiwan. E-mail: mslee2006@ntu.edu.tw.
Supported by Taiwan National Science Council grants NSC 96-2320-B-002-078 and NSC 97-2320-B-002-052-MY3 (M.S.L.), The Cooperative Research Program of National Taiwan University College of Medicine and China Medical University College of Medicine (M.S.L. and W.C.C.), and the Postdoctoral Fellowship from the Aim For Top University Program, National Taiwan University (T.S.C.).
S.-R.W. and T.-S.C. contributed equally to this article.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Chen CJ, You SL, Lin LH, Hsu WL, Yang YW. Cancer epidemiology and control in Taiwan: a brief review. Jpn J Clin Oncol. 2002;32 Suppl:S66–S81. doi: 10.1093/jjco/hye138. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Sporn MB. The war on cancer. Lancet. 1996;347:1377–1381. doi: 10.1016/s0140-6736(96)91015-6. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Carles J, Lloreta J, Salido M, Font A, Suarez M, Baena V, Nogue M, Domenech M, Fabregat X. Her-2/neu expression in prostate cancer: a dynamic process? Clin Cancer Res. 2004;10:4742–4745. doi: 10.1158/1078-0432.CCR-04-0115. [DOI] [PubMed] [Google Scholar]
- Osman I, Scher HI, Drobnjak M, Verbel D, Morris M, Agus D, Ross JS, Cordon-Cardo C. HER-2/neu (p185neu) protein expression in the natural or treated history of prostate cancer. Clin Cancer Res. 2001;7:2643–2647. [PubMed] [Google Scholar]
- Shi Y, Brands FH, Chatterjee S, Feng AC, Groshen S, Schewe J, Lieskovsky G, Cote RJ. Her-2/neu expression in prostate cancer: high level of expression associated with exposure to hormone therapy and androgen independent disease. J Urol. 2001;166:1514–1519. doi: 10.1016/s0022-5347(05)65822-3. [DOI] [PubMed] [Google Scholar]
- Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, Balk S, Thomas G, Kaplan I, Hlatky L, Hahnfeldt P, Kantoff P, Loda M. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92:1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- Zellweger T, Ninck C, Bloch M, Mirlacher M, Koivisto PA, Helin HJ, Mihatsch MJ, Gasser TC, Bubendorf L. Expression patterns of potential therapeutic targets in prostate cancer. Int J Cancer. 2005;113:619–628. doi: 10.1002/ijc.20615. [DOI] [PubMed] [Google Scholar]
- Grothey A, Hashizume R, Ji H, Tubb BE, Patrick CW, Jr, Yu D, Mooney EE, McCrea PD. C-erbB-2/ HER-2 upregulates fascin, an actin-bundling protein associated with cell motility, in human breast cancer cell lines. Oncogene. 2000;19:4864–4875. doi: 10.1038/sj.onc.1203838. [DOI] [PubMed] [Google Scholar]
- Engelsen IB, Stefansson IM, Beroukhim R, Sellers WR, Meyerson M, Akslen LA, Salvesen HB. HER-2/neu expression is associated with high tumor cell proliferation and aggressive phenotype in a population based patient series of endometrial carcinomas. Int J Oncol. 2008;32:307–316. [PubMed] [Google Scholar]
- Spencer KS, Graus-Porta D, Leng J, Hynes NE, Klemke RL. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J Cell Biol. 2000;148:385–397. doi: 10.1083/jcb.148.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson JP, Bu Z, Lemmon MA. Ligand-induced structural transitions in ErbB receptor extracellular domains. Structure. 2007;15:942–954. doi: 10.1016/j.str.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Kofler M, Jorissen RN, Nice EC, Burgess AW, Ward CW. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell. 2003;11:495–505. doi: 10.1016/s1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- Lemmon MA. Ligand-induced ErbB receptor dimerization. Exp Cell Res. 2009;315:638–648. doi: 10.1016/j.yexcr.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese DJ, 2nd, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- El Sheikh SS, Domin J, Abel P, Stamp G, Lalani el N. Phosphorylation of both EGFR and ErbB2 is a reliable predictor of prostate cancer cell proliferation in response to EGF. Neoplasia. 2004;6:846–853. doi: 10.1593/neo.04379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- Montironi R, Mazzucchelli R, Barbisan F, Stramazzotti D, Santinelli A, Scarpelli M, Lopez Beltran A. HER2 expression and gene amplification in pT2a Gleason score 6 prostate cancer incidentally detected in cystoprostatectomies: comparison with clinically detected androgen-dependent and androgen-independent cancer. Hum Pathol. 2006;37:1137–1144. doi: 10.1016/j.humpath.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- Scher HI. HER2 in prostate cancer: a viable target or innocent bystander? J Natl Cancer Inst. 2000;92:1866–1868. doi: 10.1093/jnci/92.23.1866. [DOI] [PubMed] [Google Scholar]
- Hernes E, Fossa SD, Berner A, Otnes B, Nesland JM. Expression of the epidermal growth factor receptor family in prostate carcinoma before and during androgen-independence. Br J Cancer. 2004;90:449–454. doi: 10.1038/sj.bjc.6601536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Netzel-Arnett S, Hooper JD, Szabo R, Madison EL, Quigley JP, Bugge TH, Antalis TM. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 2003;22:237–258. doi: 10.1023/a:1023003616848. [DOI] [PubMed] [Google Scholar]
- Dano K, Romer J, Nielsen BS, Bjorn S, Pyke C, Rygaard J, Lund LR. Cancer invasion and tissue remodeling: cooperation of protease systems and cell types. Apmis. 1999;107:120–127. doi: 10.1111/j.1699-0463.1999.tb01534.x. [DOI] [PubMed] [Google Scholar]
- Johnsen M, Lund LR, Romer J, Almholt K, Dano K. Cancer invasion and tissue remodeling: common themes in proteolytic matrix degradation. Curr Opin Cell Biol. 1998;10:667–671. doi: 10.1016/s0955-0674(98)80044-6. [DOI] [PubMed] [Google Scholar]
- Kohn EC, Liotta LA. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995;55:1856–1862. [PubMed] [Google Scholar]
- Chen WT. Proteases associated with invadopodia, and their role in degradation of extracellular matrix. Enzyme Protein. 1996;49:59–71. doi: 10.1159/000468616. [DOI] [PubMed] [Google Scholar]
- Lin CY, Wang JK, Torri J, Dou L, Sang QA, Dickson RB. Characterization of a novel, membrane-bound, 80-kDa matrix-degrading protease from human breast cancer cells: monoclonal antibody production, isolation, and localization. J Biol Chem. 1997;272:9147–9152. [PubMed] [Google Scholar]
- Cao J, Cai X, Zheng L, Geng L, Shi Z, Pao CC, Zheng S. Characterization of colorectal-cancer-related cDNA clones obtained by subtractive hybridization screening. J Cancer Res Clin Oncol. 1997;123:447–451. doi: 10.1007/BF01372549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MG, Chen C, Lyu MS, Cho EG, Park D, Kozak C, Schwartz RH. Cloning and chromosomal mapping of a gene isolated from thymic stromal cells encoding a new mouse type II membrane serine protease, epithin, containing four LDL receptor modules and two CUB domains. Immunogenetics. 1999;49:420–428. doi: 10.1007/s002510050515. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Shuman MA, Craik CS. Reverse biochemistry: use of macromolecular protease inhibitors to dissect complex biological processes and identify a membrane-type serine protease in epithelial cancer and normal tissue. Proc Natl Acad Sci USA. 1999;96:11054–11061. doi: 10.1073/pnas.96.20.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto H, Underwood LJ, Wang Y, Shigemasa K, Parmley TH, O'Brien TJ. Ovarian tumor cells express a transmembrane serine protease: a potential candidate for early diagnosis and therapeutic intervention. Tumour Biol. 2001;22:104–114. doi: 10.1159/000050604. [DOI] [PubMed] [Google Scholar]
- Oberst M, Anders J, Xie B, Singh B, Ossandon M, Johnson M, Dickson RB, Lin CY. Matriptase and HAI-1 are expressed by normal and malignant epithelial cells in vitro and in vivo. Am J Pathol. 2001;158:1301–1311. doi: 10.1016/S0002-9440(10)64081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santin AD, Cane S, Bellone S, Bignotti E, Palmieri M, De Las Casas LE, Anfossi S, Roman JJ, O'Brien T, Pecorelli S. The novel serine protease tumor-associated differentially expressed gene-15 (matriptase/MT-SP1) is highly overexpressed in cervical carcinoma. Cancer. 2003;98:1898–1904. doi: 10.1002/cncr.11753. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- Saleem M, Adhami VM, Zhong W, Longley BJ, Lin CY, Dickson RB, Reagan-Shaw S, Jarrard DF, Mukhtar H. A novel biomarker for staging human prostate adenocarcinoma: overexpression of matriptase with concomitant loss of its inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer Epidemiol Biomarkers Prev. 2006;15:217–227. doi: 10.1158/1055-9965.EPI-05-0737. [DOI] [PubMed] [Google Scholar]
- List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T, Burke B, Nielsen BS, Gutkind JS, Bugge TH. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev. 2005;19:1934–1950. doi: 10.1101/gad.1300705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbs D, Thiel S, Stella MC, Sturzebecher A, Schweinitz A, Steinmetzer T, Sturzebecher J, Uhland K. In vitro inhibition of matriptase prevents invasive growth of cell lines of prostate and colon carcinoma. Int J Oncol. 2005;27:1061–1070. [PubMed] [Google Scholar]
- Kang JY, Dolled-Filhart M, Ocal IT, Singh B, Lin CY, Dickson RB, Rimm DL, Camp RL. Tissue microarray analysis of hepatocyte growth factor/Met pathway components reveals a role for Met, matriptase, and hepatocyte growth factor activator inhibitor 1 in the progression of node-negative breast cancer. Cancer Res. 2003;63:1101–1105. [PubMed] [Google Scholar]
- Suzuki M, Kobayashi H, Kanayama N, Saga Y, Suzuki M, Lin CY, Dickson RB, Terao T. Inhibition of tumor invasion by genomic down-regulation of matriptase through suppression of activation of receptor-bound pro-urokinase. J Biol Chem. 2004;279:14899–14908. doi: 10.1074/jbc.M313130200. [DOI] [PubMed] [Google Scholar]
- Lee SL, Dickson RB, Lin CY. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem. 2000;275:36720–36725. doi: 10.1074/jbc.M007802200. [DOI] [PubMed] [Google Scholar]
- Meng TC, Lee MS, Lin MF. Interaction between protein tyrosine phosphatase and protein tyrosine kinase is involved in androgen-promoted growth of human prostate cancer cells. Oncogene. 2000;19:2664–2677. doi: 10.1038/sj.onc.1203576. [DOI] [PubMed] [Google Scholar]
- Lee MS, Igawa T, Yuan TC, Zhang XQ, Lin FF, Lin MF. ErbB-2 signaling is involved in regulating PSA secretion in androgen-independent human prostate cancer LNCaP C-81 cells. Oncogene. 2003;22:781–796. doi: 10.1038/sj.onc.1206066. [DOI] [PubMed] [Google Scholar]
- Oberst MD, Williams CA, Dickson RB, Johnson MD, Lin CY. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J Biol Chem. 2003;278:26773–26779. doi: 10.1074/jbc.M304282200. [DOI] [PubMed] [Google Scholar]
- Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- Ricciardelli C, Jackson MW, Choong CS, Stahl J, Marshall VR, Horsfall DJ, Tilley WD. Elevated levels of HER-2/neu and androgen receptor in clinically localized prostate cancer identifies metastatic potential. Prostate. 2008;68:830–838. doi: 10.1002/pros.20747. [DOI] [PubMed] [Google Scholar]
- Igawa T, Lin FF, Lee MS, Karan D, Batra SK, Lin MF. Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. Prostate. 2002;50:222–235. doi: 10.1002/pros.10054. [DOI] [PubMed] [Google Scholar]
- Lin MF, Meng TC, Rao PS, Chang C, Schonthal AH, Lin FF. Expression of human prostatic acid phosphatase correlates with androgen-stimulated cell proliferation in prostate cancer cell lines. J Biol Chem. 1998;273:5939–5947. doi: 10.1074/jbc.273.10.5939. [DOI] [PubMed] [Google Scholar]
- Lee MS, Tseng IC, Wang Y, Kiyomiya K, Johnson MD, Dickson RB, Lin CY. Autoactivation of matriptase in vitro: requirement for biomembrane and LDL receptor domain. Am J Physiol Cell Physiol. 2007;293:C95–C105. doi: 10.1152/ajpcell.00611.2006. [DOI] [PubMed] [Google Scholar]
- Lin CY, Tseng IC, Chou FP, Su SF, Chen YW, Johnson MD, Dickson RB. Zymogen activation, inhibition, and ectodomain shedding of matriptase. Front Biosci. 2008;13:621–635. doi: 10.2741/2707. [DOI] [PubMed] [Google Scholar]
- Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 2006;18:2262–2271. doi: 10.1016/j.cellsig.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Woodgett JR. Functional distinctions of protein kinase B/Akt isoforms defined by their influence on cell migration. Trends Cell Biol. 2006;16:461–466. doi: 10.1016/j.tcb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Estrada AC, Syrovets T, Pitterle K, Lunov O, Buchele B, Schimana-Pfeifer J, Schmidt T, Morad SA, Simmet T. Tirucallic acids are novel pleckstrin homology domain-dependent Akt inhibitors inducing apoptosis in prostate cancer cells. Mol Pharmacol. 2010;77:378–387. doi: 10.1124/mol.109.060475. [DOI] [PubMed] [Google Scholar]
- Le Page C, Koumakpayi IH, Alam-Fahmy M, Mes-Masson AM, Saad F. Expression and localisation of Akt-1, Akt-2 and Akt-3 correlate with clinical outcome of prostate cancer patients. Br J Cancer. 2006;94:1906–1912. doi: 10.1038/sj.bjc.6603184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivan R, Morgan R, Jennings S, Austenfeld M, Van Veldhuizen P, Stephens R, Noble M. Overexpression of Her-2/neu may be an indicator of poor prognosis in prostate cancer. J Urol. 1993;150:126–131. doi: 10.1016/s0022-5347(17)35413-7. [DOI] [PubMed] [Google Scholar]
- Myers RB, Srivastava S, Oelschlager DK, Grizzle WE. Expression of p160erbB-3 and p185erbB-2 in prostatic intraepithelial neoplasia and prostatic adenocarcinoma. J Natl Cancer Inst. 1994;86:1140–1145. doi: 10.1093/jnci/86.15.1140. [DOI] [PubMed] [Google Scholar]
- Gu K, Mes-Masson AM, Gauthier J, Saad F. Overexpression of her-2/neu in human prostate cancer and benign hyperplasia. Cancer Lett. 1996;99:185–189. doi: 10.1016/0304-3835(95)04061-7. [DOI] [PubMed] [Google Scholar]
- McCann A, Dervan PA, Johnston PA, Gullick WJ, Carney DN. c-erbB-2 oncoprotein expression in primary human tumors. Cancer. 1990;65:88–92. doi: 10.1002/1097-0142(19900101)65:1<88::aid-cncr2820650119>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Savinainen KJ, Saramaki OR, Linja MJ, Bratt O, Tammela TL, Isola JJ, Visakorpi T. Expression and gene copy number analysis of ERBB2 oncogene in prostate cancer. Am J Pathol. 2002;160:339–345. doi: 10.1016/S0002-9440(10)64377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn EJ, Kurnot RA, Sesterhenn IA, Chang EH, Moul JW. Expression of the c-erbB-2 (HER-2/neu) oncoprotein in human prostatic carcinoma. J Urol. 1993;150:1427–1433. doi: 10.1016/s0022-5347(17)35799-3. [DOI] [PubMed] [Google Scholar]
- Mark HF, Feldman D, Das S, Kye H, Mark S, Sun CL, Samy M. Fluorescence in situ hybridization study of HER-2/neu oncogene amplification in prostate cancer. Exp Mol Pathol. 1999;66:170–178. doi: 10.1006/exmp.1999.2242. [DOI] [PubMed] [Google Scholar]
- Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser TC, Willi N, Mihatsch MJ, Sauter G, Kallioniemi OP. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999;59:803–806. [PubMed] [Google Scholar]
- Oberst MD, Johnson MD, Dickson RB, Lin CY, Singh B, Stewart M, Williams A, al-Nafussi A, Smyth JF, Gabra H, Sellar GC. Expression of the serine protease matriptase and its inhibitor HAI-1 in epithelial ovarian cancer: correlation with clinical outcome and tumor clinicopathological parameters. Clin Cancer Res. 2002;8:1101–1107. [PubMed] [Google Scholar]
- Eccles SA. The role of c-erbB-2/HER2/neu in breast cancer progression and metastasis. J Mammary Gland Biol Neoplasia. 2001;6:393–406. doi: 10.1023/a:1014730829872. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Yamada Y, Kokubo H, Nakamura K, Aoki S, Taki T, Honda N, Nakagawa A, Saga S, Hara K. Prognostic significance of immunohistochemical expression of the HER-2/neu oncoprotein in bone metastatic prostate cancer. Urology. 2006;68:110–115. doi: 10.1016/j.urology.2006.01.060. [DOI] [PubMed] [Google Scholar]
- Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- Bacus SS, Yarden Y, Oren M, Chin DM, Lyass L, Zelnick CR, Kazarov A, Toyofuku W, Gray-Bablin J, Beerli RR, Hynes NE, Nikiforov M, Haffner R, Gudkov A, Keyomarsi K. Neu differentiation factor (Heregulin) activates a p53-dependent pathway in cancer cells. Oncogene. 1996;12:2535–2547. [PubMed] [Google Scholar]
- Grasso AW, Wen D, Miller CM, Rhim JS, Pretlow TG, Kung HJ. ErbB kinases and NDF signaling in human prostate cancer cells. Oncogene. 1997;15:2705–2716. doi: 10.1038/sj.onc.1201447. [DOI] [PubMed] [Google Scholar]
- Lyne JC, Melhem MF, Finley GG, Wen D, Liu N, Deng DH, Salup R. Tissue expression of neu differentiation factor/heregulin and its receptor complex in prostate cancer and its biologic effects on prostate cancer cells in vitro. Cancer J Sci Am. 1997;3:21–30. [PubMed] [Google Scholar]
- Tal-Or P, Di-Segni A, Lupowitz Z, Pinkas-Kramarski R. Neuregulin promotes autophagic cell death of prostate cancer cells. Prostate. 2003;55:147–157. doi: 10.1002/pros.10200. [DOI] [PubMed] [Google Scholar]
- Satomi S, Yamasaki Y, Tsuzuki S, Hitomi Y, Iwanaga T, Fushiki T. A role for membrane-type serine protease (MT-SP1) in intestinal epithelial turnover. Biochem Biophys Res Commun. 2001;287:995–1002. doi: 10.1006/bbrc.2001.5686. [DOI] [PubMed] [Google Scholar]
- Jin X, Yagi M, Akiyama N, Hirosaki T, Higashi S, Lin CY, Dickson RB, Kitamura H, Miyazaki K. Matriptase activates stromelysin (MMP-3) and promotes tumor growth and angiogenesis. Cancer Sci. 2006;97:1327–1334. doi: 10.1111/j.1349-7006.2006.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorsi L, Marchiani S, Muratori M, Forti G, Baldi E. Gefitinib (‘IRESSA’. ZD1839) inhibits EGF-induced invasion in prostate cancer cells by suppressing PI3 K/AKT activation. J Cancer Res Clin Oncol. 2004;130:604–614. doi: 10.1007/s00432-004-0581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim S, Koh H, Yoon SO, Chung AS, Cho KS, Chung J. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001;15:1953–1962. doi: 10.1096/fj.01-0198com. [DOI] [PubMed] [Google Scholar]
- Zhang D, Brodt P. Type 1 insulin-like growth factor regulates MT1-MMP synthesis and tumor invasion via PI 3-kinase/Akt signaling. Oncogene. 2003;22:974–982. doi: 10.1038/sj.onc.1206197. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem. 2000;275:26333–26342. doi: 10.1074/jbc.M002941200. [DOI] [PubMed] [Google Scholar]
- Shi XB, Ma AH, Tepper CG, Xia L, Gregg JP, Gandour-Edwards R, Mack PC, Kung HJ, DeVere White RW. Molecular alterations associated with LNCaP cell progression to androgen independence. Prostate. 2004;60:257–271. doi: 10.1002/pros.20039. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Weigel NL, Moore NL. Kinases and protein phosphorylation as regulators of steroid hormone action. Nucl Recept Signal. 2007;5:e005. doi: 10.1621/nrs.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14–3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- Hoyal CR, Gutierrez A, Young BM, Catz SD, Lin JH, Tsichlis PN, Babior BM. Modulation of p47PHOX activity by site-specific phosphorylation: Akt-dependent activation of the NADPH oxidase. Proc Natl Acad Sci USA. 2003;100:5130–5135. doi: 10.1073/pnas.1031526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Pacquelet S, Lane WS, Eam B, Catz SD. Akt regulates the subcellular localization of the Rab27a-binding protein JFC1 by phosphorylation. Traffic. 2005;6:667–681. doi: 10.1111/j.1600-0854.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kiyomiya K, Benaud C, Dickson RB, Lin CY. Simultaneous activation and hepatocyte growth factor activator inhibitor 1-mediated inhibition of matriptase induced at activation foci in human mammary epithelial cells. Am J Physiol Cell Physiol. 2005;288:C932–C941. doi: 10.1152/ajpcell.00497.2004. [DOI] [PubMed] [Google Scholar]