Abstract
The overexpression of insulin-like growth factor receptor-I (IGF-IR) and the activation of its signaling pathways both play critical roles in the development and progression of gastric cancer. Dopamine (DA), a major enteric neurotransmitter, has been reported to have a wide variety of physiological functions in the gastrointestinal tract, including the stomach. We have previously reported that both DA and tyrosine hydroxylase, the rate-limiting enzyme required for the synthesis of DA, are lost in malignant gastric tissues. The effect of this loss of DA on IGF-IR-induced growth of gastric cancer has not yet been elucidated; we therefore investigated the role of DA, if any, on IGF-IR-induced proliferation of malignant gastric cells. There was a significant increase in the expression of phosphorylated IGF-IR and its downstream signaling molecule AKT in human malignant gastric tissues compared with normal nonmalignant tissues. Furthermore, to determine whether this loss of DA has any effect on the activation of IGF-IR signaling pathways in malignant gastric tumors, in vitro experiments were undertaken, using AGS gastric cancer cells. Our results demonstrated that DA acting through its D2 receptor, inhibits IGF-I-induced proliferation of AGS cells by up-regulating KLF4, a negative regulator of the cell cycle through down regulation of IGF-IR and AKT phosphorylation. Our results suggest that DA is an important regulator of IGF-IR function in malignant gastric cancer cells.
Among the different growth factors influencing the initiation, progression, and metastasis of gastric cancer, the insulin-like growth factor (IGF)/insulin-like growth factor receptor- I (IGF-IR) axis plays a critical role in the stimulation of gastric cancer cell proliferation, survival, angiogenesis, and resistance to apoptosis.1,2,3 The phosphatidylinositol 3-kinase (PI3K)/AKT pathway is one of the major intracellular pathways in the IGF system that is frequently activated in cancer cells.4,5,6,7,8 Clinically, overexpression of IGF-IR has been reported in several human cancers, including stomach.9,10,11,12,13
Dopamine (DA) as a neurotransmitter controls a wide variety of physiological functions in the central nervous system. Evidence also indicates that a considerable amount of DA is synthesized in the stomach.14,15 DA in the gastrointestinal tract stimulates exocrine secretions, inhibits gut motility, modulates sodium absorption and mucosal blood flow, and is protective against gastroduodenal ulcer disease.16,17,18,19,20 DA acts on target cells through its different receptor subtypes, like D1 (D1 and D5) and D2 (D2, D3, D4) that are expressed throughout the gastrointestinal tract.21,22,23 Our previous reports have shown that in human malignant gastric tumor tissues, concentrations of DA and its rate- limiting synthesizing enzyme tyrosine hydroxylase (TH) were significantly reduced or absent in comparison to normal gastric tissues.24 However, to date, no information is available regarding the effect of this neurotransmitter on IGF-IR signaling through the PI3K/AKT pathway in relation to the regulation of human gastric adenocarcinoma cell proliferation.
Our results indicate the overexpression of phosphorylated IGF-IR and its downstream signaling molecule AKT in human gastric adenocarcinoma tissues. Therefore, to correlate this present observation with our previous finding that DA and its synthesizing enzyme TH was lost in human gastric tumor tissues, in vitro experiments were carried out in a human gastric adenocarcinoma cell line, AGS, which expresses the DA D2 receptor and IGF-IR. Stimulation of D2 with its specific D2 receptor agonist, quinpirole, inhibited IGF-I-induced AGS cell proliferation by up-regulating KLF4, a negative regulator of the cell cycle,25 through the down-regulation of IGF-IR and downstream AKT phosphorylation.
Materials and Methods
Patient Characteristics
Fifty gastric cancer patients of both sexes (male = 43; female = 7) with a median age of 50 (30–79) years were selected. Malignant gastric tumor tissues were taken during surgery from different cancer patients. Additionally, nontumorous tissues were collected from biopsy specimens that were free of tumors. All tissue samples were confirmed histologically. The protocol to obtain tissue samples for this study was approved by the Institutional Review Board, and the diagnosis of all samples was confirmed histologically.2
Immunohistochemistry
In brief, 4-μm-thick sections were deparaffinized in xylene and rehydrated in a graded ethanol series; sections were then subjected to heat-induced epitope retrieval by immersion in a 0.01 M citrate buffer (pH 6). Endogenous peroxidise activity was blocked for 15 minutes in 3% hydrogen peroxide in methanol. Nonspecific binding was blocked by treatment with 3% bovine serum albumin for 30 minutes at room temperature. The slides were then incubated overnight with primary antibody at 4°C in a humidified chamber. In the present study, rabbit polyclonal anti-phospho IGF-IR antibody and anti-pAKT antibody (Ser473) (Santa Cruz Biotechnology, Santa Cruz, CA) were used. All primary antibodies were diluted to a concentration of 1:50. Sections were incubated with secondary antibody conjugated with biotin (Santa Cruz Biotechnology) for 30 minutes. Immunodetection was performed using the avidin-biotin complex kit (Vector Laboratories, Burlingame, CA) as per the manufacturer’s protocol.26,27,28 Immunohistochemical results were quantified using ImageJ software (National Institues of Health, Bethesda, MD).
Cell Lines and Culture Conditions
The AGS cell line was obtained from the American Type Culture Collection (Manassas, VA). AGS cells were grown in F12K medium supplemented with 1500 mg/L NaHCO3, 2 mmol/L l-glutamine, 10% (v/v) fetal bovine serum, 100 μg/ml streptomycin, and 100 IU/ml penicillin at 37°C with saturating humidity and 5% CO2.25
Immunoprecipitation and Immunoblotting
Protein extracts from cells of various experimental groups were immunoprecipitated with different antibodies, and immunoprecipitates were captured on protein A-agarose beads. The immunocomplexes were subjected to SDS-PAGE and then transferred to polyvinyl difluoride membranes (Millipore, Bedford, MA) and immunoblotted. Primary antibodies used were anti-IGF-IR (Cell Signaling Technology, Beverly, MA), anti-AKT1/2/3, anti-p-AKT, and anti-phospho IGF-IR (Santa Cruz Biotechnology). Antibody-reactive bands were detected by enzyme-linked chemiluminescence. (Pierce ECL, SuperSignal, Pierce Biotechnology, Rockford, IL).29,30
ELISA
Tissue sample and reagent preparation and assays were performed following the instruction manual for STAR phospho IGF-IR ELISA Kit (Upstate Biotechnology, Lake Placid, NY).31
RT-PCR
Total RNA was extracted by RNA isolation kit following the manufacturer’s protocol (Ambion, Austin, TX). Reverse transcription followed by PCR was carried out in a DNA thermal cycler (Gene Amp-9700; Applied Biosystems, Foster City, CA) with KLF4 receptor primers. The sequence for primers for KLF4 was described previously25 as follows: KLF4 forward: 5′-GGCGCTGGACCCCCTCTC-3′; and KLF4 reverse: 5′-GCAGCCCGCGTAATCACAAGT-3′.
Cell Proliferation Assay
Thymidine incorporation assay was performed to determine the optimum dose for inhibition of AGS cell proliferation following D2 stimulation by quinpirole and the concentration of IGF-I that induces maximum proliferation of AGS. Cells were plated in 96-well plates and, 24 hours later, were exposed to a range of drug concentrations. After 48 hours at 37°C, cells were pulsed with 4 μCi/ml [3H]thymidine. After 8 hours, cells were trypsinized, harvested onto UniFilter-96 GF/B plates (Perkin-Elmer, Boston, MA), and counted on a 1209 RACK BETA liquid scintillation counter. [3H]Thymidine uptake by the proliferating cells was proportional to counts per minute.30
Cell Cycle Analysis
AGS cells were harvested by trypsinization and washed three times with PBS. The cells were suspended in supplemented F-12K medium containing 4 mg/ml DNase (type I bovine pancreatic DNase; Sigma-Aldrich, St. Louis, MO) and incubated at 37°C for 10 minutes, followed by washes in PBS and resuspended in 200 ml PBS. An addition of 2 ml of ice-cold 70% ethanol (30% distilled water) was performed and the samples left on ice for at least 30 minutes. The cells were then harvested by centrifugation at 300 × g for 5 minutes and resuspended in 400 ml of PBS (pH 7.3). One hundred microliters of RNase I (1 mg/ml) and 100 μl propidium iodide (400 mg/ml; Sigma-Aldrich) solutions were added and incubated at 37°C for 30 minutes in a water bath. Samples were immediately measured by flow cytometry using (FACSCalibur; BD Biosciences, San Jose, CA).32
Small Interfering RNA Transfection
AGS cells were plated in 6-well plates at a density of 2 × 105 cells/well in 2 ml of antibiotic-free normal growth medium supplemented with FBS and allowed to attach overnight. The cells were then transfected with AKT 1 small interfering (si)RNA (h). Transfection was performed using siRNA transfection medium and siRNA transfection reagent (Santa Cruz Biotechnology) as per the manufacturer’s instruction. Following transfection, the cells were left to grow for 48 hours and analyzed by Western blot and immunofluorescence staining using appropriate protocols.33
Statistical Analysis
Data are means of at least six different experiments ± SD Student’s t-test was used to compare the result. Correlations between quantitative variables were established through Pearson Correlation coefficient. A P value <0.05 was considered statistically significant.34
Results
Overexpression of Phosphorylated IGF-IR in Human Malignant Gastric Tissues
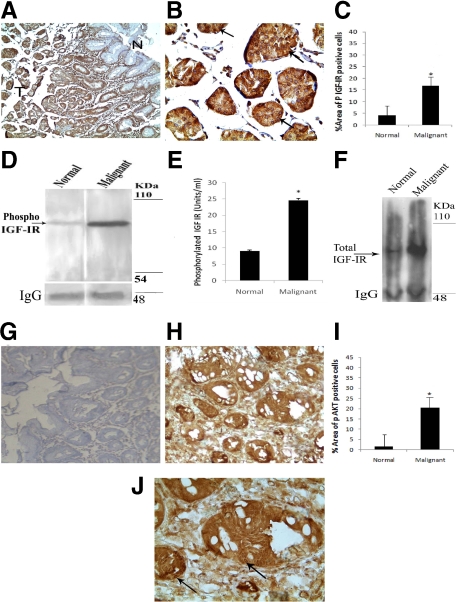
Status of phosphorylated IGF-IR has been determined in malignant gastric and normal nonmalignant tissues by immunohistochemistry, Western blot, and ELISA. Immunohistochemical analysis showed significantly higher expression of phosphorylated IGF-IR in primary gastric adenocarcinoma tissues compared with adjacent nontumorous areas (Figure 1A). Phospho-IGF-IR expression was localized in the cytoplasm and membrane of the malignant glands (Figure 1B). Quantitative analysis confirmed significant higher percentage area of phospho IGF-IR-positive cells in gastric tumor tissues compared with normal gastric tissues (Figure 1C). In addition to these immunohistochemical studies, immunoprecipitation followed by immunoblot (Figure 1D), and quantitative ELISA (Figure 1E) also indicated significantly higher levels of phospho-IGF-IR in gastric tumor samples in comparison to normal samples from the same patients. Total IGF-IR level was also significantly high in gastric adenocarcinoma tissues compared with normal gastric tissues (Figure 1F).
Figure 1.
Overexpression of phospho-IGF-IR and phospho-AKT in malignant gastric tissue. A: Immunohistochemical staining showing overexpression of phospho-IGF-IR in the majority of the tumor cells in the invasive malignant glands in the deeper region of the mucosa as indicated by (T) compared with the adjacent superficial nonmalignant glands (N). Original magnification, ×40. B: Tumor cells are strongly positive for phospho-IGF-IR antibody showing intense membrane and cytoplasmic staining indicated by arrows as shown in higher magnification (×300). D and F: Immunoprecipitation followed by immunoblot shows phospho-IGF-IR and total IGF-IR expression levels were significantly higher in gastric cancer tissues compared with matched normal tissue preparations from the same patients. Results are representative of six separate experiments. E: The concentration of phospho-IGF-IR in gastric cancer tissues as determined by ELISA was significantly higher (24.64 ± 0.1315 U/ml, P < 0.001) compared with that in normal tissues (8.994 ± 0.0904 U/ml). The bar graph is representative of six separate experiments. H: Representative example of mucosa from patients with gastric cancer showing higher expression of phospho-AKT than normal mucosa (×100) (G). J: Intense positive nuclear staining for phospho-AKT as indicated by arrows in the mucosal epithelial cells of the malignant glands (×300). C and I: Bar graphs represent percentage area of phospho-IGF-IR and phospho-AKT-positive cells, respectively *P < 0.05.
Overexpression of Phosphorylated AKT in Human Malignant Gastric Tissues
In the present investigation, since significant higher phosphorylation of IGF-IR was detected in malignant gastric tissues compared with their histologically proven normal nonmalignant tissues, we therefore investigated whether IGF-IR/AKT signaling pathway is functional in these tissues. Results indicated that in addition to increased expression of phosphorylated IGF-IR, there was also increased expression of phosphorylated AKT in the gastric malignant tumor tissues in comparison to normal nonmalignant gastric tissues. Image analysis also showed significantly higher percentage area of phospho AKT-positive cells in primary gastric tumor tissues than normal gastric tissues (Figure 1G–J).
In addition, phosphorylation of IGF-IR also had significant positive correlation with its downstream signaling molecule AKT (Pearson, P = 0.505; P < 0.01) in malignant gastric tissues when compared with normal nonmalignant tissues.
In Vitro Activation of DA D2 Receptors Inhibited IGF-I-Mediated Human Gastric Adenocarcinoma, AGS Cell Proliferation
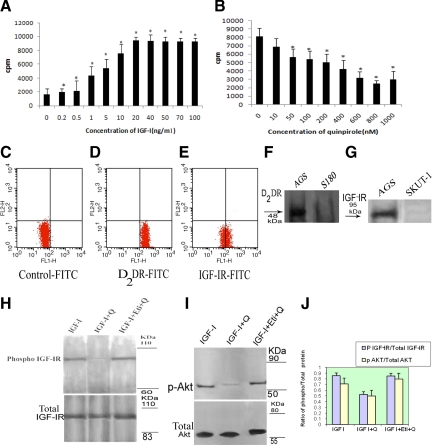
To correlate our observation on overexpression of phosphorylated IGF-IR in human gastric adenocarcinoma tissues with our previous findings that DA and its rate-limiting synthesizing enzyme TH was lost in human gastric tumor tissues,24 in vitro experiments were then undertaken in the human gastric adenocarcinoma cell line AGS. When these cells were stimulated with IGF-I in serum-free medium, the proliferation of AGS significantly increased in a dose-dependent manner, reaching a peak at 20 ng/ml (Figure 2A). However, following pretreatment of these tumor cells with different (D1–D5) DA receptor agonists, only the DA D2 receptor agonist, quinpirole, significantly inhibited IGF-I (20 ng/ml)-induced AGS cell proliferation, and the maximum inhibition was observed at the 800 nmol/L concentration (Figure 2B). Other classes of DA receptor agonists did not elicit any significant effect on IGF-I-induced AGS cell proliferation (data not shown). To further confirm the specificity of DA D2 receptor-mediated inhibition of IGF-I-induced AGS cell proliferation, these cells were pretreated with a specific DA D2 receptor antagonist, eticlopride (80 μmol/L), followed by DA D2 receptor agonist quinpirole (800 nmol/L); the inhibitory effect of this agonist was abrogated (data not shown). Furthermore, FACS analysis (Figure 2C–E) and Western blot analysis (Figure 2, F and G) confirmed the presence of DA D2 receptors and IGF-I receptors in these tumor cells.
Figure 2.
A: Effects of various concentrations of IGF-I on proliferation of AGS cells in vitro by [3H]thymidine incorporation assay. Maximum proliferation was observed when cells were treated with a 20 ng/ml dose of IGF-I. Results are the mean ± SEM; *P < 0.05. B: Effects of various concentrations of Q on IGF-I (20 ng/ml)-induced proliferation of AGS cells in vitro. Q at a concentration of 800 nmol/L was found to cause maximum inhibition in IGF-I-mediated proliferation of AGS cells. Flow cytometric analysis of unstained AGS cells (C) as controls for D2 DR (D) and IGF-IR-stained (E) AGS cells. Percentages 99 and 50.92% of the gated cells as evident from the lower right quadrant are D2 DR- and IGF-IR-positive cells, respectively. F and G: Western blot analysis of D2 DR and IGF-IR in AGS cells. D2 DR is absent in negative control sarcoma 180 (S180) tumor cells. IGF-IR is absent in negative control leiomyosarcoma cells (SKUT-1). Western blot showing Phospho-IGF-IR (H) and phosphor-AKT (upper blots) (I). Lane 1: Cells stimulated with IGF-I (20 ng/ml). Lane 2: Cells pretreated with Q (800 nmol/L) before being exposed to IGF-I (20 ng/ml). Lane 3: Cells treated with Eti (80 μmol/L) followed by Q and IGF-I. Activation of D2 DA receptor by Q significantly down-regulates IGF-IR and AKT phosphorylation compared with IGF-I-treated control whereas pretreatment with Eti (80 μmol/L), abrogated Q-induced down-regulation of IGF-IR and AKT phosphorylation. Protein loading was verified by stripping and reprobing the membrane with anti-IGF-IR and anti-AKT, which showed that total IGF-IR and total AKT expression was equal (lower blots). J: The phospho/total protein ratio data are expressed as mean ± SE of six separate experiments. Q, quinpirole; Eti, eticlopride.
Stimulation of DA D2 Receptors in AGS Cells Inhibited Phosphorylation of IGF-IR and Its Downstream Signaling Molecule AKT
IGF-IR phosphorylation induces downstream signals leading to survival and proliferation of tumor cells. Among these pathways, the PI3K/AKT pathway plays a crucial role. Since activation of the DA D2 receptor inhibits AGS cell proliferation and, as recent reports indicate, DA through its D2 receptor down-regulates AKT in the corpus striatum,35 we therefore examined whether the molecular mechanisms of DA D2 receptor-mediated inhibition of AGS cell proliferation is due to its effect on the phosphorylation of IGF-IR and its subsequent downstream signaling molecule AKT. Interestingly, when AGS cells were treated with quinpirole, the inhibition of IGF-I-induced cell proliferation was associated with a significant down-regulation of IGF-IR phosphorylation (Figure 2H) and AKT phosphorylation (Figure 2I), respectively. However, total IGF-IR and total AKT levels remained unchanged (Figure 2, H and I).
DA D2 Receptor-Mediated IGF-I-Induced AGS Cell Proliferation Inhibition was Associated with Up-Regulation of KLF4
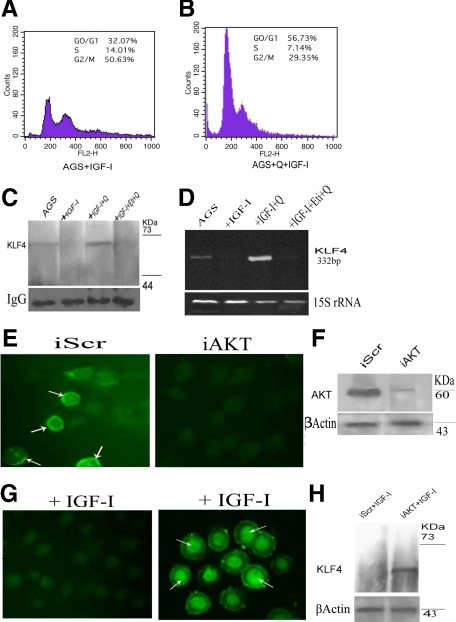
IGF-I is a well-established mitogen that stimulates G1-S phase progression through the activation of the phosphoinositide 3-kinase/AKT pathway.3,36 Since activation of the DA D2 receptor by quinpirole inhibited IGF-I-induced AGS cell proliferation, we therefore investigated whether this inhibition of DA D2 receptor agonist-induced AGS cell proliferation is due to arrest of cells at the G1 phase of cell cycle. As expected, our results showed increased number of AGS cells arrested in G1 cell cycle phase following activation of DA D2 receptors by its specific agonist quinpirole (800 nmol/L) compared with IGF-I (20 ng/ml)-treated cells alone (Figure 3, A and B), leading to decreased cell proliferation.
Figure 3.
A and B: Flow cytometric analysis showing significant increased numbers of cells in G1 phase of cell cycle in Q (800 nmol/L) + IGF-I (20 ng/ml)-treated group than IGF-I (20 ng/ml)-treated alone. Similar results were obtained in at least six independent experiments. C: Immunoprecipitation followed by immunoblot. Lane 1: Unstimulated AGS cells showing low expression of KLF4. Lane 2: Cells stimulated with IGF-I (20 ng/ml) did not express KLF4. Lane 3: Cells pretreated with Q (800 nmol/L) following IGF-I treatment showed significant expression of KLF4. Lane 4: Cells treated with Eti (80 μmol/L), followed by Q and IGF-I abrogated the effects of D2 receptor-mediated stimulation of KLF4. D: KLF4 mRNA levels determined by semiquantitative RT-PCR corroborates well with Western blot data. 15S rRNA expression is positive control. Results are representative of six separate experiments ± SEM. Q, quinpirole; Eti, eticlopride. E: Immunofluorescence staining of methanol-fixed control AGS transfected with scrambled sequence (iScr) and AKT-silenced AGS (iAKT). iAKT AGS cells showing diminished staining compared to iScr AGS cells that shows distinct membrane and cytoplasmic staining as indicated by arrows, when probed with anti-AKT antibody. F: Western blotting shows significant reduction in AKT expression after 48 hours of transfection. AGS cells were transfected with nonspecific iScr or iAKT as described, and 48 hours after transfection, cells were stimulated with 20 ng/ml IGF-I; subsequently, cells were fixed in methanol and KLF4 expression was analyzed by immunofluorescence staining (G) and Western Blotting (H). Marked nuclear staining is noted showing induction of KLF4 protein in AKT-silenced cells even after IGF-I treatment. Western blot analysis corroborates well with the immunofluorescence pattern of KLF4 expression. The pattern of protein expression was similar in triplicate experiments. β-Actin served as loading control.
GKLF/KLF4 is an epithelial-specific transcription factor known as an inhibitor of the cell cycle at G1 phase, and its induction is associated with growth arrest in gastrointestinal epithelial cells.25,37 KLF4 expression is significantly decreased or lost in gastric cancer.38 We therefore further examined whether DA D2 receptor-mediated inhibition of AGS cell proliferation is due to the arrest of cell growth at the G1 phase of cell cycle and whether it is also correlated with KLF4 expression. Our data showed that in untreated AGS cells, KLF4 RNA and proteins were expressed at very low levels (Figure 3C). This corroborates well with results from other laboratories.38 We also observed that following IGF-I treatment, there was loss of KLF4 expression in these cells (Figure 3C), and this loss in turn correlated well with increased cell proliferation (Figure 2A). Furthermore, immunoprecipitation followed by immunoblot (Figure 3C) and semiquantitative RT-PCR (Figure 3D) showed that when these cells were pretreated with quinpirole (800 nmol/L), followed by IGF-I (20 ng/ml) treatment, the expression of KLF4 was significantly higher in comparison to untreated cells. In contrast, the DA D2 receptor antagonist, eticlopride (80 μmol/L) abrogated this effect of DA D2 receptor agonist-mediated stimulation of KLF4 expression (Figure 3, C and D).
Since the phosphorylation of IGF-IR and its downstream molecule AKT in IGF-I-stimulated AGS cells is associated with a loss of KLF4 expression, it can be assumed that there exists a unique association between IGF-IR-AKT signaling and expression of KLF4. Thus, to further confirm that the quinpirole-induced down-regulation of AKT phosphorylation is associated with the stimulation of KLF4 expression, AKT was silenced with siRNA. Concentrations of siRNA were optimized to ensure that it did not affect cell viability (data not shown). After 48 hours, both AKT siRNA-transfected cells (iAKT) and cells transfected with control siRNA containing a scrambled sequence (iScr) were stimulated with IGF-I (20 ng/ml). Our results indicated that even after IGF-I treatment, AKT-silenced cells showed marked expression of KLF4, whereas control cells in which scrambled RNA was transfected showed no expression of KLF4 following IGF-I treatment (Figure 3E–H).
Discussion
Several reports have indicated a significant association and role of IGF-IR in different human malignant tumors, including gastric cancer.3,9,39,40,41,42 In the present investigation, a significantly higher phosphorylation level of IGF-IR was observed in gastric malignant tumor tissues in comparison to normal gastric tissues. This indicates a loss of negative regulators of IGF-IR signaling in malignant gastric tissues.
DA, a major enteric neurotransmitter, plays important roles in the central nervous system as well as in the gastrointestinal tract, especially in the stomach.16,17,18,19,20 Interestingly, it is to be noted here that in our early experiments, we demonstrated that there is a complete absence of DA and its rate-limiting synthesizing enzyme, TH, in malignant gastric tumor tissues.24 In addition, our previous results also showed that exogenous administration of DA significantly inhibited growth of gastric cancer in animals by suppressing vascular endothelial growth factor A receptor-2 phosphorylation acting through its D2 receptors present in the tumor endothelial cells.24 Therefore, our present results showing increased phosphorylation of IGF-IR and loss of TH and DA in human gastric malignant tissues as reported in our previous study indicate the important role of DA as an enteric neurotransmitter in the IGF-IR pathway for the development of gastric cancer.
The PI3K/AKT pathway is one of the major intracellular pathways in the IGF system that is frequently activated in cancer.4 In the present investigation, we observed an association of increased expression of phosphorylated IGF-IR and its downstream signaling molecule AKT in human gastric malignant tissues. Thereafter, to correlate these present findings with our previous findings that DA is lost in human gastric tumor tissues, in vitro experiments were carried out in the human gastric adenocarcinoma cell line, AGS, which expresses both the DA D2 receptor and IGF-IR, simulating the in situ condition. It was observed that activation of DA D2 receptors inhibited IGF-IR and AKT phosphorylation, thereby inhibiting IGF-I-induced AGS proliferation. The mechanism of DA action was attributed to the arrest of a significant number of tumor cells in the G1 phase of the cell cycle following stimulation of its D2 receptor.
KLF4 activates promoters of the negative cell cycle regulator, cyclin-dependent kinase inhibitor p21WAF1/cip1 gene in a p53-dependent manner.43,44 On the other hand, it represses promoters of the positive cell cycle regulator gene cyclin D1 and induces cell cycle arrest at the G1-S boundary.45,46 As KLF4 is an important regulator of the cell cycle in the gastric epithelial cells,25 we therefore investigated whether DA D2-mediated cell growth arrest has any correlation with the expression of KLF4, which is frequently down-regulated in gastric cancer tissues.38 Under serum-starved conditions, following IGF-I treatment, expression of KLF4 was lost in these cells. However, the cells pretreated with the DA D2 receptor specific agonist, quinpirole, followed by IGF-I treatment, showed stimulation of KLF4 expression higher than untreated cells. In contrast, DA D2 receptor antagonist, eticlopride, abrogated the DA D2 receptor agonist-mediated re-expression of KLF4 in these tumor cells, thereby indicating the mechanism to be DA D2 receptor-specific. Also in AKT-silenced cells, marked expression of KLF4 was observed, whereas in control cells transfected with scrambled RNA, no expression of KLF4 following IGF-I treatment was seen, thus suggesting a novel IGF-IR/AKT pathway that regulates the expression of KLF4 in these gastric tumor cells.
Among numerous growth factors, the IGF-I/IGF-IR axis plays a critical role in gastric cancer.1,2,3 Therefore from the present investigation, it is evident for the first time that DA through its D2 receptor present in human gastric tumor cells cross talks with the IGF-IR/AKT pathway and thereby, regulates tumor cell proliferation through the upregulation of KLF4, an important inhibitor of the cell cycle in gastric cancer.
Because the growth, survival, and metastasis of tumor cells are dependent on many factors, targeting multiple growth factors and their signaling pathways in the tumor microenvironment is now being considered an important therapeutic strategy instead of targeting a single molecule.47,48 Furthermore, since the DA D2 dopamine receptor agonist was previously reported by us to inhibit the actions of vascular endothelial growth factor-A on endothelial cells24 and it was also found to inhibit IGF-IR/AKT pathway-induced gastric tumor cell proliferation, the D2 receptor agonist may be a rational choice for the treatment of gastric cancer. Most importantly, these drugs are being used in the clinics at present for other diseases; therefore, they may be tried in clinical trials for the treatment of gastric cancer.
Acknowledgments
We thank Dr. Rathindranath Baral (Chittaranjan National Cancer Institute, Kolkata, India) for FACS.
Footnotes
Address reprint requests to Partha Sarathi Dasgupta, Ph.D., Signal Transduction and Biogenic Amines Department, Chittaranjan National Cancer Institute, 37 S.P. Mukherjee Road, Kolkata 700026, India; or Sujit Basu, M.D., Ph.D., Department of Pathology and Arthur G. James Cancer Center, 166 Hamilton Hall, 1645 Neil Avenue, Columbus, OH 43210. E-mail: partha42002@yahoo.com or sujit.basu@osumc.edu.
Supported by the Department of Science and Technology grant (SR/SO/HS-23/2004), Government of India grant (P.S.D.G. and S.B.), National Institutes of Health grants CA118265 (S.B.) and CA124763 (S.B.), Department of Defense grant W81XWH-07-1-0051 (S.B.), and an award from the Prevent Cancer Foundation. This work was also supported by CSIR, Government of India Fellowship 9/30(54)/2009-EMR-1(S.S.).
References
- Yi HK, Hwang PH, Yang DH, Kang CW, Lee DY. Expression of the insulin-like growth factors (IGFs) and the IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur J Cancer. 2001;37:2257–2263. doi: 10.1016/s0959-8049(01)00269-6. [DOI] [PubMed] [Google Scholar]
- Matsubara J, Yamada Y, Hirashima Y, Takahari D, Okita NT, Kato K, Hamaguchi T, Shirao K, Shimada Y, Shimoda T. Impact of insulin like growth factor type 1 receptor, epidermal growth factor receptor and HER2 expressions on outcomes of patients with gastric cancer. Clin Cancer Res. 2008;14:3022–3029. doi: 10.1158/1078-0432.CCR-07-1898. [DOI] [PubMed] [Google Scholar]
- Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- Hwang PH, Kim SY, Lee JC, Kim SJ, Yi HK, Lee DY. PTEN/MMAC1 enhances the growth inhibition by anticancer drugs with downregulation of IGF-II expression in gastric cancer cells. Exp Mol Med. 2005;37:391–398. doi: 10.1038/emm.2005.49. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hailey J, Williams D, Wang Y, Lipari P, Malkowski M, Wang X, Xie L, Li G, Saha D, Ling WL, Cannon-Carlson S, Greenberg R, Ramos RA, Shields R, Presta L, Brams P, Bishop WR, Pachter JA. Inhibition of insulin-like growth factor-I receptor (IGF-IR) signaling and tumor cell growth by a fully human neutralizing anti-IGF-IR antibody. Mol Cancer Ther. 2005;4:1214–1221. doi: 10.1158/1535-7163.MCT-05-0048. [DOI] [PubMed] [Google Scholar]
- Liu TJ, LaFortune T, Honda T, Ohmori O, Hatakeyama S, Meyer T, Jackson D, de Groot J, Yung WK. Inhibition of both focal adhesion kinase and insulin-like growth factor-I receptor kinase suppresses glioma proliferation in vitro and in vivo. Mol Cancer Ther. 2007;6:1357–1367. doi: 10.1158/1535-7163.MCT-06-0476. [DOI] [PubMed] [Google Scholar]
- Ryan PD, Goss PE. The emerging role of the insulin-like growth factor pathway as a therapeutic target in cancer. Oncologist. 2008;13:16–24. doi: 10.1634/theoncologist.2007-0199. [DOI] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Adachi Y, Yamamoto H, Imsumran A, Oka T, Oki M, Nosho K, Min Y, Shinomura Y, Lee C-T, Carbone DP, Imai K. Insulin-like growth factor-I receptor as a candidate for a novel molecular target in gastrointestinal cancers. Dig Endosc. 2006;18:245–251. [Google Scholar]
- Gee JM, Robertson JF, Gutteridge E, Ellis IO, Pinder SE, Rubini M, Nicholson RI. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer. 2005;12:S99–S111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- Chitnis MM, Yuen JSP, Protheroe AS, Pollak M, Macaulay VM. The type1insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- Lopez T, Hanahan D. Elevated levels of IGF-I receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339–353. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Jones RA, Campbell CI, Gunther EJ, Chodosh LA, Petrik JJ, Khokha R, Moorehead RA. Transgenic overexpression of IGF-IR disrupts mammary ductal morphogenesis and induces tumor formation. Oncogene. 2007;26:1636–1644. doi: 10.1038/sj.onc.1209955. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Åneman A, Friberg P, Hooper D, Fåndriks L, Lonroth H, Hunyady B, Mezey E. Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab. 1997;82:3864–3871. doi: 10.1210/jcem.82.11.4339. [DOI] [PubMed] [Google Scholar]
- Mezey É, Eisenhofer G, Hansson S, Hunyady B, Hoffman BJ. Dopamine produced by the stomach may act as a paracrine/autocrine hormone in the rat. Neuroendocrinology. 1998;67:336–348. doi: 10.1159/000054332. [DOI] [PubMed] [Google Scholar]
- Flemstrom G, Safsten B, Jedstedt G. Stimulation of mucosal alkaline secretion in rat duodenum by dopamine and dopaminergic compounds. Gastroenterology. 1993;104:825–833. doi: 10.1016/0016-5085(93)91019-e. [DOI] [PubMed] [Google Scholar]
- Finkel Y, Eklof AC, Granquist L, Soares-da-Silva P, Bertorello AM. Endogenous dopamine modulates jejunal sodium absorption during high-salt diet in young but not in adult rats. Gastroenterology. 1994;107:675–679. doi: 10.1016/0016-5085(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Glavin GB, Szabo S. Dopamine in gastrointestinal disease. Dig Dis Sci. 1990;35:1153–1161. doi: 10.1007/BF01537589. [DOI] [PubMed] [Google Scholar]
- Haskel Y, Hanani M. Inhibition of gastrointestinal motility by MPTP via adrenergic and dopaminergic mechanisms. Dig Dis Sci. 1994;39:2364–2367. doi: 10.1007/BF02087652. [DOI] [PubMed] [Google Scholar]
- Glavin GB, Hall AM. Central and peripheral dopamine D1/DA1 receptor modulation of gastric secretion and experimental gastric mucosal injury. Gen Pharmacol. 1995;26:1277–1279. doi: 10.1016/0306-3623(95)00009-p. [DOI] [PubMed] [Google Scholar]
- Willems JL, Buylaert WA, Lefebvre RA, Bogaert MG. Neuronal dopamine receptors on autonomic ganglia and sympathetic nerves and dopamine receptors in the gastrointestinal system. Pharmacol Rev. 1985;37:165–216. [PubMed] [Google Scholar]
- Hernandez DE, Mason GA, Walker CH, Valenzuela JE. Dopamine receptors in human gastrointestinal mucosa. Life Sci. 1987;41:2717–2723. doi: 10.1016/0024-3205(87)90464-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Iaccarino C, Saiardi A, Heidt V, Bozzi Y, Picetti R, Vitale C, Westphal H, Drago J, Borrelli E. Simultaneous absence of dopamine D1 and D2 receptor-mediated signaling is lethal in mice. Proc Natl Acad Sci USA. 2004;101:11465–11470. doi: 10.1073/pnas.0402028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakroborty D, Sarkar C, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res. 2004;10:4349–4356. doi: 10.1158/1078-0432.CCR-04-0059. [DOI] [PubMed] [Google Scholar]
- Kang W, Nielsen O, Fenger C, Leslie G, Holmskov U, Reid KB. Induction of DMBT1 expression by reduced ERK activity during a gastric mucosa differentiation-like process and its association with human gastric cancer. Carcinogenesis. 2005;26:1129–1137. doi: 10.1093/carcin/bgi045. [DOI] [PubMed] [Google Scholar]
- Tarn C, Rink L, Merkel E, Flieder D, Pathak H, Koumbi D, Testa JR, Eisenberg B, Mehren MV, Godwin AK. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci USA. 2008;105:8387–8392. doi: 10.1073/pnas.0803383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S, Tsuda H, Sato K, Takeuchi H, Shigekawa T, Matsubara O, Hiraide H, Mochizuki H. Alternative tyrosine phosphorylation of signaling kinases according to hormone receptor status in breast cancer overexpressing the insulin-like growth factor receptor type 1. Cancer Sci. 2006;97:597–604. doi: 10.1111/j.1349-7006.2006.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima E, Tomimori K, Kawakami H, Ishikawa C, Sawada S, Tomita M, Senba M, Kinjo F, Mimuro H, Sasakawa C, Fujita J, Mori N. NF-κB activation by Helicobacter pylori requires Akt-mediated phosphorylation of p65. BMC Microbiol. 2009;9:36. doi: 10.1186/1471-2180-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kwon MJ, Nam TJ. Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines. Life Sci. 2006;79:1956–1962. doi: 10.1016/j.lfs.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Haluska P, Carboni JM, Loegering DA, Lee FY, Wittman M, Saulnier MG, Frennesson DB, Kalli KR, Conover CA, Attar RM, Kaufmann SH, Gottardis M, Erlichman C. In vitro and in vivo antitumor effects of the dual insulin-like growth factor-I/insulin receptor inhibitor, BMS-554417. Cancer Res. 2006;66:362–371. doi: 10.1158/0008-5472.CAN-05-1107. [DOI] [PubMed] [Google Scholar]
- Ubagai T, Kikuchi T, Fukusato T, Ono Y. Aflatoxin B1 modulates the insulin-like growth factor-2 dependent signaling axis. Toxicol In Vitro. 2010;24:783–789. doi: 10.1016/j.tiv.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Jiang XH, Wong BC, Yuen ST, Jiang SH, Cho CH, Lai KC, Lin MC, Kung HF, Lam SK. Arsenic trioxide induces apoptosis in human gastric cancer cells through up-regulation of p53 and activation of caspase-3. Int J Cancer. 2001;91:173–179. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1039>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Xin M, Deng X. Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J Biol Chem. 2005;280:10781–10789. doi: 10.1074/jbc.M500084200. [DOI] [PubMed] [Google Scholar]
- Pallares J, Llobet D, Santacana M, Eritja N, Velasco A, Cuevas D, Lopez S, Palomar-Asenjo V, Yeramian A, Dolcet X, Matias-Guiu X. CK2β is expressed in endometrial carcinoma and has a role in apoptosis resistance and cell proliferation. Am J Pathol. 2009;174:287–296. doi: 10.2353/ajpath.2009.080552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Gao N, Zhang Z, Jiang BH, Shi X. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem Biophys Res Commun. 2003;310:1124–1132. doi: 10.1016/j.bbrc.2003.09.132. [DOI] [PubMed] [Google Scholar]
- Mahatan CS, Kaestner KH, Geiman DE, Yang VW. Characterization of the structure and regulation of the murine gene encoding gut-enriched Krüppel-like factor (Krüppel-like factor 4). Nucleic Acids Res. 1999;27:4562–4569. doi: 10.1093/nar/27.23.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Gong W, Kanai M, Schlunk C, Wang L, Yao JC, Wu TT, Huang S, Xie K. Drastic down-regulation of Krüppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 2005;65:2746–2754. doi: 10.1158/0008-5472.CAN-04-3619. [DOI] [PubMed] [Google Scholar]
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades N. Treatment of hematologic malignancies and solid tumors by inhibiting IGF receptor signaling. Expert Rev Anticancer Ther. 2005;5:487–499. doi: 10.1586/14737140.5.3.487. [DOI] [PubMed] [Google Scholar]
- Larsson O, Girnita A, Girnita L. Role of insulin-like growth factor 1 receptor signaling in cancer. Br J Cancer. 2005;92:2097–2101. doi: 10.1038/sj.bjc.6602627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wang L, Gong W, Wei D, Le Xiangdong, Yao J, Ajani J, Abbruzzese JL, Huang S, Xie K. A high expression level of Insulin-like growth factor 1 receptor is associated with increased expression of transcription factor sp1 and regional lymph node metastasis in human gastric cancer. Clin Exp Metastasis. 2004;21:755–764. doi: 10.1007/s10585-005-1198-2. [DOI] [PubMed] [Google Scholar]
- Zhang W, Geiman DE, Shields JM, Dang DT, Mahatan CS, Kaestner KH, Biggs JR, Kraft AS, Yang VW. The gut-enriched Krüppel-like factor (Krüppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J Biol chem. 2000;275:18391–18398. doi: 10.1074/jbc.C000062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Johns DC, Geiman DE, Marban E, Dang DT, Hamlin G, Sun R, Yang VW. Krüppel- like factor 4 (gut-enriched Krüppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J Biol Chem. 2001;276:30423–30428. doi: 10.1074/jbc.M101194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shie SL, Chen ZY, Fiu M, Pestell RG, Tsing CC. Gut enriched Kruppel like factor represses cyclin D1 promoter activity through sp1 motif. Nucleic acid Res. 2000;28:2969–2976. doi: 10.1093/nar/28.15.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HS, Chen X, Yang VW. Kruppel like factor 4 mediates p53 dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem. 2003;278:2101–2105. doi: 10.1074/jbc.M211027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleespies A, Jauch KW, Bruns CJ. Tyrosine kinase inhibitors and gemcitabine: new treatment options in pancreatic cancer? Drug Resist Updat. 2006;9:1–18. doi: 10.1016/j.drup.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Pytel D, Sliwinski T, Poplawski T, Ferriola D, Majsterek I. Tyrosine kinase blockers: new hope for successful cancer therapy. Anticancer Agents Med Chem. 2009;9:66–76. doi: 10.2174/187152009787047752. [DOI] [PubMed] [Google Scholar]