Abstract
Pemphigus vulgaris is a blistering disease associated with autoantibodies to the desmosomal adhesion protein, desmoglein 3. Genetic deficiency of desmoglein 3 in mice mimics autoimmunity to desmoglein 3 in pemphigus vulgaris, with mucosal-dominant blistering in the suprabasal layer of the epidermis. Mice with an epidermal-specific deletion of desmocollin 3, the other major desmosomal cadherin isoform expressed in the basal epidermis, develop suprabasal blisters in skin that are histologically identical to those observed in pemphigus vulgaris, suggesting that desmocollin 3 might be a target of autoantibodies in some pemphigus vulgaris patients. We now demonstrate that desmocollin 3 is an autoantigen in pemphigus vulgaris, illustrated in a patient with mucosal-dominant blistering. Six of 38 pemphigus vulgaris and one of 85 normal serum samples immunoprecipitate desmocollin 3 (P = 0.003). Incubation of patient IgG with human keratinocytes causes loss of intercellular adhesion, and adsorption with recombinant desmocollin 3 specifically prevents this pathogenic effect. Additionally, anti-desmocollin 3 sera cause loss of keratinocyte cell surface desmocollin 3, but not desmoglein 3 by immunofluorescence, indicating distinct cellular pathogenic effects in anti-desmocollin and anti-desmoglein pemphigus, despite their identical clinical presentations. These data demonstrate that desmocollin 3 is a pathogenic autoantigen in pemphigus vulgaris and suggest that pemphigus vulgaris is a histological reaction pattern that may result from autoimmunity to desmoglein 3, desmocollin 3, or both desmosomal cadherins.
Pemphigus vulgaris (PV) is a potentially fatal autoimmune disease, classically associated with autoantibodies against the desmosomal cadherin, desmoglein (Dsg) 3. Seven desmosomal cadherins, desmogleins 1–4 and desmocollins (Dsc) 1–3, have been described.1 Dsg3 and Dsc3 are the predominant isoforms expressed in the basal epidermis, which is the site of blister formation in PV. Dsg1, Dsg4, and Dsc1 are expressed in an inverted pattern, predominantly in the superficial epidermis with low to undetectable levels in the basal layers. Dsg2 and Dsc2 have weak to undetectable expression levels in the basal skin epidermis. Homophilic interactions between the extracellular domains of Dsg3 confer adhesion in cell aggregation assays2; both cell aggregation assays and functional data using adhesion-blocking peptides support the relevance of heterophilic interactions between desmogleins and desmocollins in desmosomal adhesion.3,4,5 However, the relevant in vivo interactions of the desmosomal cadherins remain poorly understood.
The desmoglein compensation theory proposes that Dsg1 can compensate for the functional loss of Dsg3, and vice versa, regarding desmosomal cell adhesion, which in part explains the clinical and microscopic localization of blisters in PV.6,7 Enzyme-linked immunosorbent assay (ELISA) studies have shown that patients with mucosal-dominant PV react mainly against Dsg3,8,9,10 causing blisters in the basal layer of the mucous membranes where Dsg1 expression is minimal. In mucosal-dominant PV, Dsg1 in the basal layer of the skin epidermis is thought to compensate for the functional loss of Dsg3, thereby preventing cutaneous blistering. In support of this theory, PV patients who progress from mucosal dominant to mucocutaneous disease often develop anti-Dsg1 in addition to anti-Dsg3 autoantibodies.11
Genetic deficiency of Dsg3 in mice leads to suprabasal blistering of the mucosa and skin at sites of trauma, similar to findings in mucosal-dominant PV patients.12 Subsequent studies have shown that pemphigus autoantibodies cause endocytosis of cell surface Dsg3, leading to its depletion from desmosomes13,14,15 and supporting the hypothesis that autoantibody binding causes loss of desmoglein function. Recently, K14-Cre mediated deletion of Dsc3 in mouse epidermis was shown to cause suprabasal blisters in the skin that were histologically identical to those observed in PV patients.16 These studies raised the question of whether Dsc3 might also be a target autoantigen in PV.
We have identified a mucosal PV patient who demonstrates pathogenic autoantibodies to Dsc3. Patient serum causes loss of cell surface Dsc3 but not Dsg3, in contrast to anti-Dsg3 PV serum, which causes internalization of Dsg3 but not Dsc3. Testing of additional sera confirms that Dsc3 is a significant autoantigen in PV.
Materials and Methods
All studies were performed under research protocols approved by the relevant institutional review boards.
Production and Purification of Recombinant Desmoglein and Desmocollin Proteins
Baculoviral vectors pET Dsg1E-3E and pET Dsc3E, encoding the extracellular domains of desmogleins 1–3 and desmocollins 2–3 expressing an E and 6× histidine tag,9,17,18 were transfected into Sf9 cells using the BaculoGold expression system (BD Bioscience, San Diego, CA). Recombinant baculovirus was amplified for four passages in Sf9 cells, followed by infection of Hi5 cells for recombinant protein expression. Expression of proteins was confirmed by immunoblot with horseradish peroxidase-labeled anti-E tag antibody (Abcam, Cambridge, MA) and chemiluminescence detection (GE Health care, Uppsala, Sweden). In some experiments, recombinant proteins were purified by Talon cobalt-affinity chromatography (Clontech, Mountain View, CA) with imidazole elution, followed by buffer exchange into PBS containing 1 mmol/L CaCl2.
Cell Culture
Primary human keratinocytes were isolated from neonatal foreskin by the Penn Skin Disease Research Core facility. Cells from passages 4 to 5 were cultured in defined keratinocyte serum free media (DK-SFM) (Invitrogen, Carlsbad, CA) supplemented with penicillin/streptomycin. Hi5 and Sf9 insect cells were cultured at 27°C in Express Five SFM or Sf-900 medium (Gibco, Carlsbad, CA), respectively, supplemented with Antibiotic-Antimycotic (Gibco) and l-glutamine.
Human Sera and Antibodies
The index patient developed mucosal erosions at age 49. Her past medical and family histories were unremarkable, and age-appropriate screening has shown no evidence of malignancy. Previously, her disease was successfully controlled with 50 mg azathioprine twice daily (1.6 mg/kg/day) and 60 mg prednisone daily (1 mg/kg/day). She also had a history of response to intramuscular gold and intravenous immunoglobulin in the past. The patient presented to clinic with a disease flare off-therapy. She was on no medications at the time of serum collection and responded well to systemic therapy with prednisone; she currently remains in clinical remission on 5 mg prednisone daily.
PV (n = 38) and normal (n = 78) sera were obtained from the University of Pennsylvania clinical laboratory. The diagnosis of PV was confirmed by clinical presentation, suprabasal blistering on histology, and positive direct and/or indirect immunofluorescence testing. Normal sera were derived from citrated human plasma samples by incubating with 120 mmol/L CaCl2 for 2 hours at 37°C, followed by overnight incubation at 4°C and centrifugation to remove the resulting clot. IgG was purified from human serum samples using Melon Gel IgG spin purification systems (Pierce, Rockford, IL).
Additional normal (n = 7) and cell surface immunofluorescence-positive (n = 18) sera were obtained from the University of Utah clinical immunodermatology laboratory; of the latter, seven were Dsg3 ELISA-negative and 11 were Dsg3 ELISA-positive.
Antibodies used in this study include: mouse monoclonal anti-Dsg3 (5G11, Invitrogen), mouse monoclonal anti-Dsc3 (U114, Meridian Life Science Inc., Saco, ME), horseradish peroxidase-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Lab, Inc., West Grove, PA); horseradish peroxidase-conjugated goat anti-E (Abcam); Alexa 488 or 594-conjugated goat anti-human IgG, and Alexa 488 or 594-conjugated donkey anti-mouse IgG (Molecular Probes, Carlsbad, CA).
Immunofluorescence Studies
Frozen sections (8 to 10 μm) of normal human skin were blocked in PBS containing 2% bovine serum albumin, 1 mmol/L CaCl2, followed by incubation with primary antibody or human sera. Alexa 488- or 594-conjugated secondary antibodies were used to visualize binding of primary antibodies. For immuno-adsorption assays, human sera were diluted into 100 μl desmocollin or desmoglein baculoviral supernatant (containing approximately 500 ng recombinant protein) before incubation with human skin sections.
For immunofluorescence localization experiments, primary human keratinocytes were treated with antibodies in DK-SFM containing 1.2 mmol/L calcium for 4 hours at 37°C, then fixed for immunofluorescence as previously described.15 Nuclear staining was performed with 4,6-diamidino-2-phenylindole (Sigma, St. Louis, MO; or Molecular Probes, Invitrogen). Immunofluorescence images were acquired using a Hamamatsu Orca ER camera on an Olympus BX61 microscope with Slidebook 4.2 software. Nearest neighbor deconvolution was performed on the acquired images.
Immunoprecipitation
For recombinant proteins, 10 μl human sera were incubated with 100 μl baculoviral supernatant and 20 μl protein A or G beads (Invitrogen) overnight at 4°C. Primary keratinocyte lysates were obtained as previously described.15 Triton X-100 soluble lysate (200 μl) was precleared with protein G beads, then incubated with 10 μl normal human or patient serum, or 5 μl Dsc3 mAb (U114) overnight at 4°C, followed by incubation with 20 μl protein G beads. Bound proteins were eluted with Laemmli sample buffer (BioRad, Hercules, CA) containing β-mercaptoethanol for 5 minutes at 100°C, separated by SDS-polyacrylamide gel electrophoresis, and detected by immunoblot using anti-Dsc3 (U114) or anti-E tag antibodies.
ELISA
Sera were tested for IgG Dsg3, Dsg1, BP180, and BP230 antibody reactivity by ELISA (MBL International, Woburn, MA) according to manufacturer’s protocols, using a cutoff value of 20.0 and 9.0 for the desmoglein and BP180/230 ELISA, respectively. Anti-Dsg3 IgA was tested using horseradish peroxidase-anti-IgA secondary antibody (Dako, Carpinteria, CA).
Keratinocyte Dissociation Assay
Patient and normal human serum IgG (175 to 200 μg/ml) were evaluated for pathogenicity using a primary human keratinocyte dissociation assay as previously described.19,20 Recombinant exfoliative toxin A produced in Staphylococcus aureus [1 μg/ml, generously provided by John Stanley21] was added to all human IgG dissociation experiments to inactivate Dsg1. For adsorption studies, patient IgG (100 μg) was pre-incubated with 25 μg purified recombinant Dsc3 or Dsg3 for 4 hours at 4°C. P1 pathogenic PV mAb (25 μg/ml) was used as a positive control.15,20
Results
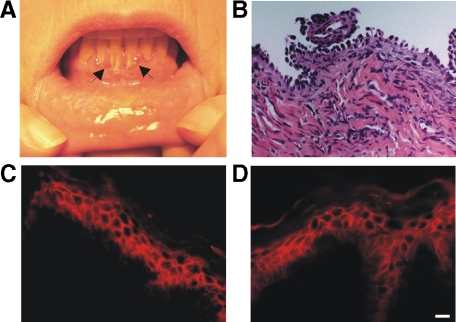
A 55-year-old woman presented with gingival erosions (Figure 1A). The diagnosis of PV was confirmed by a maxillary gingival biopsy, demonstrating suprabasal acantholysis (Figure 1B) and indirect immunofluorescence on monkey esophagus indicating a high titer (1:2048) of circulating cell surface autoantibodies in the patient’s serum. Unexpectedly, clinical testing for Dsg3 autoantibodies by ELISA was repeatedly negative. Additional ELISA testing indicated that the patient’s serum was also negative for IgG Dsg1, BP180, and BP230 autoantibodies, as well as IgA Dsg3 autoantibodies (data not shown).
Figure 1.
Clinical, histological, and immunochemical findings in a case of anti-desmocollin 3 (Dsc3) pemphigus. A: Characteristic findings of desquamative gingivitis (arrows) in a patient with mucosal pemphigus vulgaris (PV). B: H&E staining (×40 magnification) of a gingival biopsy, demonstrating suprabasal acantholysis. C: Indirect immunofluorescence (IIF) of patient serum on normal human skin, demonstrating IgG cell surface autoantibodies. D: Anti-Dsc3 mAb immunofluorescence on normal human skin, indicating a similar staining pattern compared to patient serum. Scale bar = 20 μm.
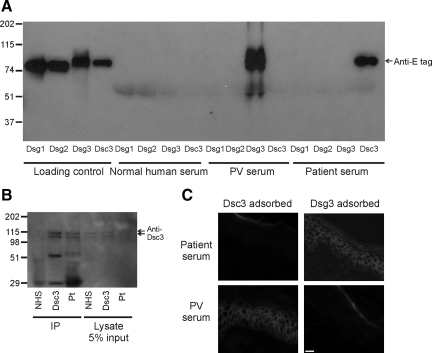
We hypothesized that the patient may have autoantibodies to Dsc3, the other major desmosomal cadherin isoform expressed in the basal epidermis. Indirect immunofluorescence of normal human skin using patient serum and a Dsc3 monoclonal antibody indicated a similar cell surface staining pattern, confirming the diagnosis of PV (Figure 1, C and D). To evaluate whether patient serum antibodies specifically bind Dsc3, we tested patient serum, normal human serum, and a known anti-Dsg3 PV serum for the ability to immunoprecipitate the recombinant extracellular domains of Dsc3 and Dsgs 1, 2, and 3 produced in baculovirus. Patient serum immunoprecipitated the recombinant extracellular domain of human Dsc3, but not Dsgs 1–3 (Figure 2A), while anti-Dsg3 pemphigus serum bound Dsg3 only, and normal serum did not react with any of the desmosomal cadherins. Additionally, patient serum did not bind Dsc1 or Dsc2 (data not shown). Patient serum, but not normal serum, also immunoprecipitated Dsc3 endogenously expressed in human keratinocytes (Figure 2B). Recombinant extracellular Dsc3 but not Dsg3 adsorbed all cell surface binding from the patient serum by immunofluorescence (Figure 2C), suggesting that Dsc3 is the primary target of serum cell surface autoantibodies.
Figure 2.
A: Patient serum immunoprecipitates recombinant Dsc3. Recombinant extracellular domains of desmogleins (Dsg) 1–3 and Dsc3 bearing an E tag were produced in a baculovirus expression system. Culture supernatants containing recombinant proteins were immunoprecipitated with normal human serum, anti-Dsg3 PV serum, or patient serum followed by immunoblotting using an anti-E tag detection antibody. PV serum bound Dsg3, while patient serum immunoprecipitated Dsc3, and normal serum did not react with any recombinant proteins. B: Patient serum recognizes Dsc3 endogenously expressed in human keratinocytes. Primary human keratinocyte lysates (200 ml) were immunoprecipitated using normal human serum (NHS), Dsc3 mAb, or the patient’s serum, followed by immunoblotting with Dsc3 mAb. Endogenously expressed Dsc3 is detected as a doublet (corresponding to the “a” and “b” splice isoforms). Lower bands in lane 2 correspond to murine mAb heavy and light chains. Five percent input (10 ml) for each immunoprecipitation is shown in the right three lanes. C: Dsc3 is the primary target of cell surface autoantibodies. Patient serum or anti-Dsg3 PV serum was adsorbed with supernatants containing either Dsc3 or Dsg3 before immunostaining of human skin cryosections. Alexa-594 anti-human IgG secondary antibody was used to detect binding of serum autoantibodies to skin sections. Scale bar = 20 μm.
To determine the prevalence of Dsc3 autoantibodies in PV, we tested 85 normal and 37 additional PV sera by immunoprecipitation using the recombinant extracellular domain of Dsc3. Six of 38 PV (15.8%) and one of 85 normal sera (1.2%) immunoprecipitated Dsc3, indicating a significant association of Dsc3 autoantibodies with PV (Table 1, P = 0.003 by Fisher’s exact test). Three of 38 PV sera (7.9%) were positive by indirect immunofluorescence but negative for Dsg1 and Dsg3 autoantibodies by ELISA; of these samples, one (33.3%, the index patient) was positive for Dsc3 autoantibodies. To further examine immunofluorescence-positive, Dsg-negative sera, we obtained 18 additional cell surface immunofluorescence-positive sera from the University of Utah clinical immunodermatology laboratory (seven Dsg3 negative and 11 Dsg3 positive by ELISA). Two of seven Dsg3-negative sera (28.6%) and three of 11 Dsg3-positive sera (27.2%) immunoprecipitated Dsc3. Of the 11 total Dsc3-reactive sera identified, eight reacted with both Dsc3 and Dsg3, while three reacted with Dsc3 only. A total of 10 Dsg3-negative sera were identified; three of these (30%) were positive for Dsc3. A summary of the serology and clinical phenotype of the six anti-desmocollin 3 PV patients identified at our clinical center appears in Table 2.
Table 1.
Prevalence of Dsc3 Autoantibodies in Pemphigus Vulgaris and Normal Human Sera
| Desmocollin 3 positive
|
Desmocollin 3 negative
|
Total | |||
|---|---|---|---|---|---|
| Dsg3− | Dsg3+ | Dsg3+ | Dsg3− | ||
| Pemphigus vulgaris | |||||
| Penn | 1* | 5 | 30 | 2 | 38 |
| Utah | 2 | 3 | 8 | 5 | 18 |
| Total | 3 | 8 | 38 | 7 | 56 |
| Normal | 1 | 84 | 85 | ||
Index patient.
Table 2.
Correlation of Clinical Phenotype and Serology in Anti-Dsc3 PV
| Patient | Dsc3 | Dsg3 | Dsg1 | Clinical phenotype |
|---|---|---|---|---|
| 1* | + | Mucosal | ||
| 2 | + | + | Mucocutaneous | |
| 3 | + | + | Mucosal | |
| 4 | + | + | + | Mucocutaneous |
| 5 | + | + | + | Mucocutaneous |
| 6 | + | + | + | Mucocutaneous |
Index patient.
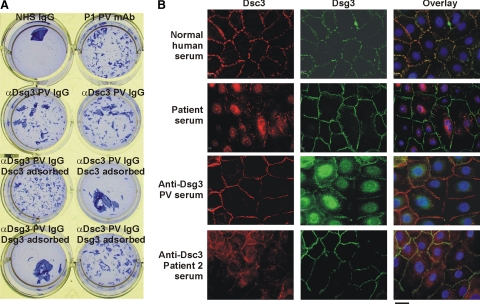
Anti-Dsc3 PV IgG caused the loss of cell adhesion in a primary human keratinocyte cell dissociation assay (Figure 3A). Adsorption of anti-Dsc3 PV IgG with the recombinant extracellular domain of Dsc3, but not Dsg3, prevented this pathogenic effect. Pathogenic anti-Dsg3 PV IgG and mAbs have previously been shown in human keratinocytes to cause internalization of Dsg3 into early endosomes, which are subsequently targeted to lysosomes.13,14,15 We sought to determine whether patient autoantibodies have similar pathogenic effects on Dsc3. Incubation of the index patient serum with human keratinocytes caused loss of cell surface Dsc3 but not Dsg3 by immunofluorescence, the opposite of what is observed with anti-Dsg3 PV serum (Figure 3B). A second anti-Dsc3 patient serum showed similar results, with loss of cell surface Dsc3 but not Dsg3.
Figure 3.
A: Anti-desmocollin 3 IgG causes loss of cell adhesion. Primary keratinocytes were incubated with anti-Dsg3 PV IgG or anti-Dsc3 PV IgG that had been pre-adsorbed with recombinant Dsg3 or Dsc3. Dsc3, but not Dsg3, adsorbed pathogenic activity from anti-Dsc3 PV IgG. NHS = normal human serum, P1 = positive control PV mAb. B: Distinct cellular phenotypes for anti-Dsc3 and anti-Dsg3 pemphigus. Primary human keratinocytes were treated with normal human serum, patient serum, anti-Dsg3 PV serum, or a second anti-Dsc3 patient serum for 4 hours during calcium-induced desmosome assembly. Cells were fixed and stained with a primary antibody specific to Dsc3 or Dsg3, followed by a secondary antibody conjugated with Alexa-594 (red) or Alexa-488 (green). Cell nuclei were stained with 4,6-diamidino-2-phenylindole. Anti-desmocollin pemphigus sera both demonstrate loss of cell surface Dsc3 but not Dsg3, while anti-desmoglein pemphigus serum causes loss of cell surface Dsg3 but not Dsc3. Scale bar = 10 μm.
Discussion
Pemphigus vulgaris is a disease of desmosomal cell adhesion, classically associated with autoantibodies against Dsg3. We present a case of anti-Dsc3 PV, illustrated in a patient with typical mucosal disease. Previously, atypical or paraneoplastic pemphigus patients with human Dsc3 immunoreactivity have been described,17,22,23,24 although autoantibodies in these patients reacted with multiple skin antigens, and pathogenicity against Dsc3 was not demonstrated, raising the possibility that the Dsc3 autoantibodies may not have caused disease, but instead arose secondarily as a result of epidermal damage. Figure 2A and unpublished data indicate that our PV patient has autoantibodies only to Dsc3, and not Dsgs 1–3 or Dscs 1–2, which include all known desmosomal cadherin isoforms expressed in the basal epidermis. Affinity adsorption experiments (Figure 2C) further support that Dsc3 is the only keratinocyte cell surface antigen targeted by serum autoantibodies in this patient. Finally, pathogenicity studies indicate that patient IgG causes the loss of human keratinocyte cell adhesion through loss of cell surface Dsc3 but not Dsg3 (Figure 3). The effects on Dsc3, shown for two different anti-Dsc3 sera, precisely parallel the effects of anti-Dsg3 PV IgG and mAbs in regard to Dsg3 internalization, which has been shown to correlate with pathogenic activity.13,14,15 Taken together, these data suggest distinct pathogenic mechanisms for anti-Dsg3 and anti-Dsc3 pemphigus, leading to the same histological presentation of suprabasal blistering.
To date, no human genetic blistering diseases have been documented in association with either Dsg3 or Dsc3. Germline deletion of Dsc3 in mice leads to pre-implantation lethality before desmosomes are formed,25 suggesting that Dsc3 deficiency may be incompatible with normal embryonic development, independent of its desmosomal adhesive function. Recently, a mutation in one family with hereditary hypotrichosis was associated with a nonsense mutation in Dsc3, predicted to occur at the junction of the transmembrane and cytoplasmic domains.26 However, there was no histological evidence of skin blistering, and it is unknown whether truncated Dsc3 protein is expressed in these patients.27 The finding of anti-Dsc3 autoantibodies in PV patients provides in vivo evidence from humans on the critical role of Dsc3 in epithelial cell adhesion.
Compelling evidence in mice also indicates that epidermal integrity requires both Dsg3 and Dsc3. Genetic loss of Dsg3 in mice causes suprabasal blisters similar to those in anti-Dsg3 PV patients, who demonstrate mucosal dominant disease.12 Our anti-Dsc3 PV patient, like anti-Dsg3 PV patients, demonstrated mucosal but not skin disease. It is possible that other desmosomal cadherin isoforms such as Dsg1 and Dsg3 may compensate for the functional loss of Dsc3-mediated adhesion in skin, a hypothesis that has been experimentally validated for human Dsgs 1 and 3.6,28
Of the six anti-Dsc3 PV patients identified at our center, one (the index patient) demonstrated Dsc3 autoantibodies only, two had both Dsc3 and Dsg3 autoantibodies, and three had Dsc3, Dsg3, and Dsg1 autoantibodies (Table 2). All three patients with Dsc3, Dsg3, and Dsg1 autoantibodies had mucocutaneous disease. The index patient with Dsc3 autoantibodies demonstrated mucosal disease. Of the two patients with both Dsc3 and Dsg3 autoantibodies, one had mucocutaneous PV, and the other had mucosal only disease, although it is unknown whether the anti-Dsc3 autoantibodies were pathogenic in these patients. Extending the desmoglein compensation theory to include the desmocollins would predict that patients with Dsg3 and Dsc3 autoantibodies would be more likely to have skin blisters, since Dsg1-mediated adhesion alone may be insufficient for the maintenance of skin integrity in the basal epidermis. Future studies will be necessary to elucidate the clinical-serological correlation in anti-desmocollin and anti-desmoglein PV.
Targeted deletion of Dsc3 in mouse epidermis results in skin but not mucosal blistering,16 which is distinct from the phenotype of our anti-Dsc3 PV patient. There could be multiple reasons for the discrepancy between the human autoimmune disease and mouse gene deletion model. Previous studies have shown that K14 transgenes may not be well expressed in oral mucosa.29 Deletion of Dsc3 was only verified in mouse skin keratinocytes, raising the possibility that mucous membrane lesions were not observed in the K14-Cre;Dsc3 fl/fl mouse model due to retained Dsc3 expression in the oral mucosa. The discrepancy may also reflect variations in desmosomal cadherin isoform expression between humans and mice. Additionally, autoimmunity to Dsc3 has different mechanisms of pathogenicity as compared to genetic deficiency of Dsc3. In the autoimmune disease, pathogenic autoantibodies cause internalization of newly synthesized Dsg3, leading to Dsg3-depleted desmosomes that lose intercellular adhesion.13,14,15,30 Genetic loss of Dsg3 would thus be predicted to have a more severe phenotype than autoimmunity to Dsg3, since skin keratinocytes would have no cell surface Dsg3 compared to a reduction in the steady-state level of cell surface Dsg3. Consistent with this finding, Dsg3-deficient mice demonstrate both mucosal erosions and skin erosions at sites of trauma, whereas passive transfer of pathogenic anti-Dsg3 autoantibodies does not cause skin blisters even after mechanical shear stress,6,20 and anti-Dsg3 PV patients typically demonstrate mucosal disease only.10,31
In testing other normal (n = 85) and PV (n = 38) sera, we found a significant association of Dsc3 autoantibodies with PV (Table 1). To our knowledge this is the first systematic association of Dsc3 with human genetic or acquired blistering disease. Previous studies have not identified significant Dsc3 autoreactivity in the sera of PV patients. However, many of these studies used ELISA, which has been shown to be suboptimal for detecting autoreactive antibodies to desmocollin 1 in cases of IgA pemphigus,32 potentially due to destruction or masking of epitopes. We have similarly found that autoreactive Dsc3 epitopes are not significantly detected by ELISA testing of pemphigus sera, in part due to the sensitivity of these epitopes to freezing and thawing (data not shown).
Interestingly, three of 38 PV sera (7.9%) in our initial screens were negative for Dsg3 autoantibodies by ELISA. This concurs with prior sensitivity data indicating that 85.2–97.5% of PV patients demonstrate serum Dsg3 autoantibodies by ELISA, depending on the cutoff value used for the assay.9,33 We subsequently obtained seven additional Dsg3-negative, indirect immunofluorescence-positive sera from a clinical immunodermatology laboratory. Of the total combined 10 Dsg3-negative sera, three (30%) immunoprecipitated Dsc3. It is possible that the seven remaining Dsc and Dsg-negative sera may have an undetectably low titer of Dsg3 or Dsc3-reactive autoantibodies, as many of these samples had lower immunofluorescence titers. However, these findings also suggest that autoantigens other than Dsc3 and Dsg3 may exist in PV, as has been proposed.34,35,36
In summary, our findings suggest that PV is a histological reaction pattern that may result from autoimmunity to Dsg3, Dsc3, or both desmosomal cadherin isoforms, which may explain the site of blister formation in the basal epidermis where the expression patterns of these desmosomal cadherins overlap. Desmosomal cadherins are thought to engage primarily in heterophilic interactions between desmogleins and desmocollins, with weaker homophilic binding.2,3,37 Recently, this theory has been challenged by the finding that Dsc3 and Dsg3 do not interact in a cell-free system.38 The finding of similar clinical and histological phenotypes in anti-Dsc3 and anti-Dsg3 pemphigus supports a role for the biological interaction of these two desmosomal cadherins in the basal layers of the epidermis. Further studies are necessary to determine the relevant in vivo interactions of the human desmosomal cadherins.
Acknowledgments
We thank John Stanley, Teruki Dainichi and Takahiro Hamada for reagents and helpful discussions.
Footnotes
Address reprint requests to Aimee S. Payne, M.D., Ph.D., 217A Clinical Research Building, 415 Curie Boulevard, Philadelphia, PA 19104. E-mail: paynea@mail.med.upenn.edu.
Supported by NIH grants K08-AR053505, P30-AR057217, and the Edwin and Fannie Hall Gray Center for Human Appearance (A.S.P.); a Veterans Administration Merit Review grant (J.J.Z.); and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (M.A.).
References
- Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- Amagai M, Karpati S, Klaus-Kovtun V, Udey MC, Stanley JR. The extracellular domain of pemphigus vulgaris antigen (desmoglein 3) mediates weak homophilic adhesion. J Invest Dermatol. 1994;102:402–408. doi: 10.1111/1523-1747.ep12372164. [DOI] [PubMed] [Google Scholar]
- Chitaev NA, Troyanovsky SM. Direct Ca2+-dependent heterophilic interaction between desmosomal cadherins, desmoglein and desmocollin, contributes to cell-cell adhesion. J Cell Biol. 1997;138:193–201. doi: 10.1083/jcb.138.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runswick SK, O'Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat Cell Biol. 2001;3:823–830. doi: 10.1038/ncb0901-823. [DOI] [PubMed] [Google Scholar]
- Tselepis C, Chidgey M, North A, Garrod D. Desmosomal adhesion inhibits invasive behavior. Proc Natl Acad Sci USA. 1998;95:8064–8069. doi: 10.1073/pnas.95.14.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney MG, Wang Z, Rothenberger K, Koch PJ, Amagai M, Stanley JR. Explanation for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103:461–468. doi: 10.1172/JCI5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AS, Hanakawa Y, Amagai M, Stanley JR. Desmosomes and disease: pemphigus and bullous impetigo. Curr Opin Cell Biol. 2004;16:536–543. doi: 10.1016/j.ceb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Ding X, Diaz LA, Fairley JA, Giudice GJ, Liu Z. The anti-desmoglein 1 autoantibodies in pemphigus vulgaris sera are pathogenic. J Invest Dermatol. 1999;112:739–743. doi: 10.1046/j.1523-1747.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- Ishii K, Amagai M, Hall RP, Hashimoto T, Takayanagi A, Gamou S, Shimizu N, Nishikawa T. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010–2017. [PubMed] [Google Scholar]
- Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40:167–170. doi: 10.1016/s0190-9622(99)70183-0. [DOI] [PubMed] [Google Scholar]
- Miyagawa S, Amagai M, Iida T, Yamamoto Y, Nishikawa T, Shirai T. Late development of antidesmoglein 1 antibodies in pemphigus vulgaris: correlation with disease progression. Br J Dermatol. 1999;141:1084–1087. doi: 10.1046/j.1365-2133.1999.03209.x. [DOI] [PubMed] [Google Scholar]
- Koch PJ, Mahoney MG, Ishikawa H, Pulkkinen L, Uitto J, Shultz L, Murphy GF, Whitaker-Menezes D, Stanley JR. Targeted disruption of the pemphigus vulgaris antigen (desmoglein 3) gene in mice causes loss of keratinocyte cell adhesion with a phenotype similar to pemphigus vulgaris. J Cell Biol. 1997;137:1091–1102. doi: 10.1083/jcb.137.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama Y, Kitajima Y. Pemphigus vulgaris-IgG causes a rapid depletion of desmoglein 3 (Dsg3) from the Triton X-100 soluble pools, leading to the formation of Dsg3-depleted desmosomes in a human squamous carcinoma cell line, DJM-1 cells. J Invest Dermatol. 1999;112:67–71. doi: 10.1046/j.1523-1747.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- Calkins CC, Setzer SV, Jennings JM, Summers S, Tsunoda K, Amagai M, Kowalczyk AP. Desmoglein endocytosis and desmosome disassembly are coordinated responses to pemphigus autoantibodies. J Biol Chem. 2006;281:7623–7634. doi: 10.1074/jbc.M512447200. [DOI] [PubMed] [Google Scholar]
- Mao X, Choi EJ, Payne AS. Disruption of desmosome assembly by monovalent human pemphigus vulgaris monoclonal antibodies. J Invest Dermatol. 2009;129:908–918. doi: 10.1038/jid.2008.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Den ZN, Koch PJ. Loss of desmocollin 3 in mice leads to epidermal blistering. J Cell Sci. 2008;121:2844–2849. doi: 10.1242/jcs.031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamatsu Y, Amagai M, Garrod DR, Kanzaki T, Hashimoto T. The detection of IgG and IgA autoantibodies to desmocollins 1–3 by enzyme-linked immunosorbent assays using baculovirus-expressed proteins, in atypical pemphigus but not in typical pemphigus. Br J Dermatol. 2004;151:73–83. doi: 10.1111/j.1365-2133.2004.05995.x. [DOI] [PubMed] [Google Scholar]
- Ota T, Amagai M, Watanabe M, Nishikawa T. No involvement of IgG autoantibodies against extracellular domains of desmoglein 2 in paraneoplastic pemphigus or inflammatory bowel diseases. J Dermatol Sci. 2003;32:137–141. doi: 10.1016/s0923-1811(03)00072-0. [DOI] [PubMed] [Google Scholar]
- Ishii K, Harada R, Matsuo I, Shirakata Y, Hashimoto K, Amagai M. In vitro keratinocyte dissociation assay for evaluation of the pathogenicity of anti-desmoglein 3 IgG autoantibodies in pemphigus vulgaris. J Invest Dermatol. 2005;124:939–946. doi: 10.1111/j.0022-202X.2005.23714.x. [DOI] [PubMed] [Google Scholar]
- Payne AS, Ishii K, Kacir S, Lin C, Li H, Hanakawa Y, Tsunoda K, Amagai M, Stanley JR, Siegel DL. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115:888–899. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa Y, Schechter N, Lin C, Garza L, Li H, Yamaguchi T, Fudaba Y, Nishifuji K, Sugai M, Amagai M, Stanley JR. Molecular mechanisms of blister formation in bullous impetigo and staphylococcal scalded skin syndrome. J Clin Invest. 2002;110:53–60. doi: 10.1172/JCI15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling MC, Mekkes JR, Goldschmidt WFM, van Noesel CJM, Jonkman MF, Pas HH. Acquired palmoplantar keratoderma and immunobullous disease associated with antibodies to desmocollin 3. Br J Dermatol. 2007;157:168–173. doi: 10.1111/j.1365-2133.2007.07920.x. [DOI] [PubMed] [Google Scholar]
- Kozlowska A, Hashimoto T, Jarzabek-Chorzelska M, Amagai A, Nagata Y, Strasz Z, Jablonska S. Pemphigus herpetiformis with IgA and IgG antibodies to desmoglein 1 and IgG antibodies to desmocollin 3. J Am Acad Dermatol. 2003;48:117–122. doi: 10.1067/mjd.2003.23. [DOI] [PubMed] [Google Scholar]
- Müller R, Heber B, Hashimoto T, Messer G, Müllegger R, Niedermeier A, Hertl M. Autoantibodies against desmocollins in European patients with pemphigus. Clin Exp Dermatol. 2009;34:898–903. doi: 10.1111/j.1365-2230.2009.03241.x. [DOI] [PubMed] [Google Scholar]
- Den ZN, Cheng X, Merchad-Sauvage M, Koch PJ. Desmocollin 3 is required for pre-implantation development of the mouse embryo. J Cell Sci. 2006;119:482–489. doi: 10.1242/jcs.02769. [DOI] [PubMed] [Google Scholar]
- Ayub M, Basit S, Jelani M, Ur Rehman R, Iqbal M, Yasinzai M, Ahmad W. A homozygous nonsense mutation in the human desmocollin-3 (DSC3) gene underlies hereditary hypotrichosis and recurrent skin vesicles. Am J Hum Genet. 2009;85:1–6. doi: 10.1016/j.ajhg.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AS. No evidence of skin blisters with human desmocollin-3 gene mutation. Am J Hum Genet. 2010;86:292. doi: 10.1016/j.ajhg.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wang ZH, Yan A, Lyle S, Fakharzadeh S, Wahl JK, Wheelock MJ, Ishikawa H, Uitto J, Amagai M, Stanley JR. Protection of neonates against pemphigus foliaceus by desmoglein 3. N Engl J Med. 2000;343:31–35. doi: 10.1056/NEJM200007063430105. [DOI] [PubMed] [Google Scholar]
- Hanakawa Y, Matsuyoshi N, Stanley JR. Expression of desmoglein 1 compensates for genetic loss of desmoglein 3 in keratinocyte adhesion. J Invest Dermatol. 2002;119:27–31. doi: 10.1046/j.1523-1747.2002.01780.x. [DOI] [PubMed] [Google Scholar]
- Sato M, Aoyama Y, Kitajima Y. Assembly pathway of desmoglein 3 to desmosomes and its perturbation by pemphigus vulgaris-IgG in cultured keratinocytes, as revealed by time-lapsed labeling immunoelectron microscopy. Lab Invest. 2000;80:1583–1592. doi: 10.1038/labinvest.3780168. [DOI] [PubMed] [Google Scholar]
- Ding X, Aoki V, Mascaro JM, Jr, Lopez-Swiderski A, Diaz LA, Fairley JA. Mucosal and mucocutaneous (generalized) pemphigus vulgaris show distinct autoantibody profiles. J Invest Dermatol. 1997;109:592–596. doi: 10.1111/1523-1747.ep12337524. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Kiyokawa C, Mori O, Miyasato M, Chidgey MA, Garrod DR, Kobayashi Y, Komori K, Ishii K, Amagai M, Nishikawa T. Human desmocollin 1 (Dsc1) is an autoantigen for the subcorneal pustular dermatosis type of IgA pemphigus. J Invest Dermatol. 1997;109:127–131. doi: 10.1111/1523-1747.ep12319025. [DOI] [PubMed] [Google Scholar]
- Amagai M, Komai A, Hashimoto T, Shirakata Y, Hashimoto K, Yamada T, Kitajima Y, Ohya K, Iwanami H, Nishikawa T. Usefulness of enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3 for serodiagnosis of pemphigus. Br J Dermatol. 1999;140:351–357. doi: 10.1046/j.1365-2133.1999.02752.x. [DOI] [PubMed] [Google Scholar]
- Morioka S, Lazarus GS, Jensen PJ. Involvement of urokinase-type plasminogen activator in acantholysis induced by pemphigus IgG. J Invest Dermatol. 1987;89:474–477. doi: 10.1111/1523-1747.ep12460937. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Ndoye A, Grando SA. Pemphigus vulgaris antibody identifies pemphaxin. J Biol Chem. 2000;275:29466–29476. doi: 10.1074/jbc.M003174200. [DOI] [PubMed] [Google Scholar]
- Marques MR, Ihrie RA, Horner JS, Attardi LD. The requirement for Perp in postnatal viability and epithelial integrity reflects an intrinsic role in stratified epithelia. J Invest Dermatol. 2006;126:69–73. doi: 10.1038/sj.jid.5700032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidgey MA, Clarke JP, Garrod DR. Expression of full-length desmosomal glycoproteins (desmocollins) is not sufficient to confer strong adhesion on transfected L929 cells. J Invest Dermatol. 1996;106:689–695. doi: 10.1111/1523-1747.ep12345525. [DOI] [PubMed] [Google Scholar]
- Spindler V, Moritz Heupel W, Efthymiadis A, Schmidt E, Eming R, Rankl C, Hinterdorfer P, Müller T, Drenckhahn D, Waschke J. Desmocollin 3-mediated binding is crucial for keratinocyte cohesion and is impaired in pemphigus. J Biol Chem. 2009;284:30556–30564. doi: 10.1074/jbc.M109.024810. [DOI] [PMC free article] [PubMed] [Google Scholar]