Abstract
Uveitis is a major and common cause of visual disability. Recent studies have shown that Th17 cells are implicated in the pathogenesis of this serious intraocular disorder. Activated T cells express an inducible costimulatory molecule called OX40, and OX40 in turn promotes the activation and proliferation of these lymphocytes. Nevertheless, it is unclear whether OX40 plays a vital role in enhancing the effector function of Th17 cells as well as the severity of uveitis. In this study, we demonstrated an increase of OX40 transcription in ovalbumin-induced uveitis, whereas anti-OX40L antibody substantially inhibited the antigen-specific ocular inflammation. Next, results from flow cytometry showed that activated Th17 cells expressed OX40, and OX40-activating antibody significantly augmented the production of Th17 cytokines in vitro. To validate the impact of OX40 in vivo, we stimulated ovalbumin-specific T cells with the OX40-activating antibody. Compared to donor cells without OX40 activation, adoptive transfer of OX40-stimulated lymphocytes elicited more severe ocular inflammation. Furthermore, an interleukin-17-neutralizing antibody attenuated OX40-mediated uveitis. In conclusion, our findings suggest that activation of OX40 augments Th17 cell function and thereby contributes to ocular inflammation. This study thus enhances our knowledge of costimulatory molecule-mediated immunopathological mechanisms of uveitis and suggests a future therapeutic strategy to treat uveitis by the targeting of OX40.
Uveitis is a common and serious ophthalmologic disorder. It is comparable to diabetes as a major cause of years of visual loss.1 Many systemic diseases including sarcoidosis, ankylosing spondylitis, and juvenile idiopathic arthritis are frequently associated with uveitis. The histological changes associated with uveitis include infiltration of the intraocular space with leukocytes.2,3
Although the etiology of uveitis is complex and multifactorial, T lymphocytes play an important role in the development of most forms of uveitis.4,5 T cell dominant infiltration is well documented in the ocular tissue and aqueous humor in many types of uveitis, especially the ones associated with systemic inflammatory diseases.6 In parallel with the observation in human patients, CD4+ and other lymphocytes mediate many animal models of uveitis such as experimental autoimmune uveitis (EAU) and the antigen-specific DO11.10 uveitis model.7,8,9 These models greatly advance our understanding of the adaptive immune response in ocular inflammation.
Under a proper cytokine milieu, naïve T cells differentiate to unique T helper (Th) subsets. Th17 cells are a recently discovered CD4+ T lymphocyte subpopulation distinct from Th1 and Th2 cells.10,11,12 This unique Th subset produces a repertoire of signature cytokines including interleukin (IL)-17A/F, IL-21, IL-22, and CCL20.13 The importance of Th17 cells is underscored by their emerging role in various autoimmune diseases (eg, inflammatory bowel disease, multiple sclerosis, psoriasis, rheumatoid arthritis, and uveitis) as well as host defense against extracellular microbes.13,14,15,16,17,18,19 Recent studies show that certain combinations of cytokines, namely transforming growth factor-β, IL-6, and/or IL-1β, are essential for the induction of Th17 cells from naïve CD4+ lymphocytes.11,12 Moreover, IL-21 and IL-23 receptor (IL-23R) play an important role in the proliferation and expansion of Th17 cells.11,12
Despite recent progress, the activation mechanism of Th17 cells has not been fully defined, especially in the context of uveitis. However, one contributing event is the engagement of various costimulatory molecules at the time that the T cell receptor interacts with antigens presented by antigen-presenting cells. These costimulatory molecules provide a second signal that is crucial to the amplification and optimization of the T cell response.20,21
OX40 (CD134) is a costimulatory molecule that belongs to the tumor necrosis factor receptor superfamily. It is not constitutively expressed on resting naïve T cells,22,23,24,25,26 and OX40 expression becomes evident at 24 to 72 hours following specific T cell activation. Engagement between OX40 and OX40 ligand (OX40L) on antigen-presenting cells has a critical role in the maintenance of an immune response following antigen stimulation due to its ability to enhance T cell survival and effector function.25 Although OX40 plays an important role in the activation of Th1 and Th2 cells,21 it is unclear whether ligation of OX40 could propagate Th17-mediated effects. According to a recent study, IL-17 does not induce OX40 expression.26 Nevertheless, activation of T cells through OX40 ligation enhances IL-17 production.26 This indicates that the expression of OX40 by Th17 cells and signaling through OX40 also contributes to Th17 response. Lastly, it is well documented that OX40 is implicated in many T cell-mediated diseases.27,28,29,30 Nevertheless, little is known of OX40 during the activation of uveitogenic lymphocytes and the development of uveitis.
In this study, we aimed to examine the impact of OX40 activation on Th17-related effects and the role of OX40 in uveitis. Here, we demonstrate an up-regulation of OX40 in ovalbumin (OVA)-induced uveitis, and blocking OX40 signaling by anti-OX40L antibody inhibited the ocular inflammation. In addition, activation of OX40 augmented the expression of IL-17, IL-21, and IL-23R. Furthermore, IL-17 neutralizing antibody attenuated OX40-enhanced uveitis. Collectively, these results indicate that the OX40-mediated costimulatory signal is an important step in the activation of Th17 cells as well as the development of ocular inflammation.
Materials and Methods
Mice
Six- to 8-week-old BALB/c, DO11.10 mice on a BALB/c background, and B10.RIII mice (Jackson Laboratory, Bar Harbor, ME) were used for the experiments. The animal experimental protocols are in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and have been approved by our institutional animal care and use committee.
BALB/c mice that express transgenic dsRedII under the control of the CD4 promoter were kindly provided by Dr. Ulrich H. von Andrian (Harvard University). For OVA-induced uveitis experiment, these mice were further crossed to DO11.10 mice for 10 generations such that the mice express dsRedII on a CD4 promoter but carry OVA-specific CD4+ T cells.
Cell Culture, Isolation, and Stimulation
After DO11.10 mice were sacrificed, their spleens were removed. Single cell suspensions were prepared by passing the tissue through a 70-μm cell strainer (BD Biosciences, Mountain View, CA). Red blood cells were lysed with 1X red blood cell lysis buffer (Sigma, St. Louis, MO) at room temperature for 5 minutes. The cell suspension was washed twice with RPMI 1640 and then cultured in RPMI 1640 with 10% fetal bovine serum in an atmosphere of 95% air and 5% CO2 at 37°C.
Sorted naïve CD4+ T cells (2 × 105/200 μl) were cultured in the presence of OVA323-339 peptide (Ile-Ser-Gln-Ala-Val-His-Ala-Ala-His-Ala-Glu-Ile-Asn-Glu-Ala-Gly-Arg) (AnaSpec, Fremont, CA) with irradiated (3300 rad) BALB/c splenocytes (2 × 106/200 μl) as antigen-presenting cells. We used irradiated “feeder” cells from naïve nontransgenic BALB/c mice instead of DO11.10 mice to eliminate the possible contamination of any radiation-surviving OVA-reactive T cells.
Some DO11.10 cells were further stimulated with and without OX40-activating antibody (clone OX86). The antibody was produced in Dr. Weinberg’s laboratory from hybridomas and affinity purified over protein G columns. For adoptive transfer, the lymphocytes were further purified using Lympholyte-M (Cedar Lane Laboratories, Burlington, NC) according to the manufacturer’s instructions. After the purification, 85 to 90% of DO11.10 lymphocytes were CD4+ T cells.
Uveitis Models
To generate uveitis in direct response to antigen stimulation, DO11.10 mice were injected with 100 μg of OVA (Sigma) in 2 μl of PBS or irrelevant antigen bovine serum albumin into the vitreous chamber of each eye. The injections were performed with ultrathin, pulled borosilicate glass needles (outer diameter about 50 μm) and Hamilton syringes under direct visualization through a surgical microscope. Some mice also simultaneously received intravenous (10 μg per mouse) and intravitreal (1 μg per eye) anti-OX40 ligand (OX40L) antibody (clone 18260, R&D Systems, Minneapolis, MN) during intravitreal OVA challenge.
For the adoptive transfer model of uveitis, OVA-activated DO11.10 lymphocytes with and without OX40-activating antibody priming were injected into naive BALB/c mice (1.5 × 107 cells/animal) via the tail vein. Then, these mice were challenged intravitreally with 250 ng of Escherichia coli strain 055:B5 lipopolysaccharide (Sigma) plus 100 μg of OVA in PBS. Twenty-four or 48 hours after the OVA challenge, uveitis was evaluated by intravital microscopy.
For EAU, B10.RIII mice received subcutaneous immunization (near the base of the tail) of 50 μg of interphotoreceptor retinoid-binding protein (IRBP)161-180 peptide (Ser-Gly-Ile-Pro-Tyr-Ile-Ile-Ser-Tyr-Leu-His-Pro-Gly-Asn-Thr- Ile-Leu-His-Val-Asp) (AnaSpec) in 200 μl of complete Freund’s adjuvant (Sigma) with Mycobacterium tuberculosis strain H37RA. Some B10.RIII mice were also treated with anti-OX40L antibody (10 μg per mouse) via tail vein injection on days 0, 3, 7, and 14 after IRBP immunization. On day 21, the eyes were harvested, and the severity of EAU was examined by histology and graded on a four-point scale based on inflammatory cell infiltration, retinal folding, and destruction.31
Intravital Microscopy
For DO11.10 mice that did not express fluorescent protein under the CD4 promoter, 150 μl of rhodamine (0.2% in PBS) was administered intraperitoneally into the mice to label intravascular leukocytes right before intravital microscopy as we have previously described.32,33 Labeled inflammatory cells in the iris and ciliary/limbal region were observed by intravital epifluorescence videomicroscopy. This imaging system comprised of a modified DM-LFS microscope (Leica, Bannockburn, IL) and a CF 84/NIR B&W camera from Kappa (Gleichen, Germany), or a color Optronics DEI-750CE camera (Optronics International, Chelmsford, MA). This technique has been reported in detail previously.32,33 Real-time videos were recorded in NTSC format for 10 seconds each. Both rolling and adherent leukocytes in the iris vessels were identified as a marker for anterior chamber uveitis.32,33 These cells were quantified to assess the severity of the ocular inflammation.32,33 For further histological evaluation, the eyes were fixed in 3% paraformaldehyde. Then, the tissues were embedded in paraffin, sectioned, and stained with H&E. Ocular inflammation was assessed by light microscopy.
Differentiation of Th17 Cells
Naïve DO11.10 CD4+ T cells (2 × 105/200 μl) were co-cultured with the irradiated BALB/c splenocytes (2 × 106/200 μl) in the presence of 1 μg/ml OVA323-339 peptide. Th17-polarizing conditions were 1 ng/ml transforming growth factor-β, 30 ng/ml IL-6, 10 ng/ml IL-1β, 10 ng/ml tumor necrosis factor-α, 20 ng/ml IL-23, 20 μg/ml anti-interferon-γ and anti-IL-4 antibodies. After 4 days of incubation, Th17 polarizing media were replaced with regular RPMI containing 10% fetal bovine serum for 12 hours. This allowed differentiated lymphocytes to rest before further real-time PCR analysis and intracellular staining of IL-17.
Flow Cytometry
DO11.10 splenocytes were suspended in PBS containing 2% fetal bovine serum and 0.1% sodium azide. Anti-CD4 (clone RM4-5) and anti-OX40 antibodies conjugated with different fluorescent colors were used to label these cell surface markers. For IL-17 staining, the cells were stimulated with phorbol myristate acetate (50 ng/ml) and ionomycin (1 μg/ml) for 5 hours. Then, brefeldin A (1:1000) was added for 2 hours. The cells were collected and stained with fluorescein isothiocyanate-labeled anti-mouse CD4 antibody for 30 minute. After PBS wash, the cells were fixed and permeabilized overnight with 1X fixation/permeabilization solution (eBioscience, San Diego, CA) at 4°C. Then these cells were stained intracellularly with allophycocyanin-conjugated monoclonal antibody against IL-17 (clone eBio17B7) (eBioscience) for 1 hour at 4°C. Data acquisition was performed on a FACSCalibur flow cytometer, and data were analyzed using CellQuest software.
Enzyme-Linked Immunosorbent Assay
The culture media of DO11.10 splenocytes and naïve CD4+ T cells from various experimental groups were collected for enzyme-linked immunosorbent assay to measure the IL-17 and IL-21 levels according to the manufacturer’s protocols (R&D Systems).
Western Blot
DO11.10 lymphocytes treated with or without OX40-activating antibody were collected in 1X LDS lysis buffer (Invitrogen) on ice. The lysates were then centrifuged at 12,000 × g for 10 minutes. Thirty microliters of total protein from each group were separated by electrophoresis through a 4 to 12% gradient Tris-glycine SDS gel and then transferred to nitrocellulose membrane using an Xcell SureLock Mini Cell (Novex, San Diego, CA). After milk blocking, the nitrocellulose membrane was incubated with polyclonal antibody against IL-23R (R&D Systems) or β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), followed by horseradish peroxidase-conjugated secondary antibody. The signals of IL-23R and β-actin were detected by enhanced chemiluminescence luminol reagent.
Real-Time PCR
Total RNA from cultured CD4+ cells was isolated with RNAeasy Mini Kit (Qiagen, Valencia, CA). First-strand cDNA synthesis was accomplished with an oligo(dT)-primed Omniscript reverse transcriptase kit (Qiagen, Valencia, CA). Gene-specific cDNA was amplified by PCR using mouse specific primer pairs (IL-17A sense: 5′-GTGGCG GCTACAGTGAAGGCA-3′ and IL-17A antisense: 5′-GACAATCGAGGCCACGCAGGT-3′; IL-21 sense: 5′-ACCAGACCAAGGCCCTGTC-3′ and IL-21 anti-sense: 5′-TGGGCTCTTGTTGAGTTGAGATT-3′; IL-22 sense: 5′-TCAGACAGGTTCCAGCC-3′ and IL-22 antisense: 5′-TCCAGTTCCCCAATCGCC-3′; RORγt sense: 5′-ACCTCTTTTCACGGGAGGA-3′ and RORγt antisense: 5′-TCCCACATCTCCCACATTG-3′; β-actin sense, 5′-ATGCCAACACAGTGCTGTCT-3′, and β-actin antisense, 5′-AAGCACTTGCGGTGCACGAT-3′). OX40 primers were commercially purchased from SABiosciences (Frederick, MD). The real-time PCR was performed using a RT2 Realtime PCR Master Mix (SABiosciences) and running for 40 cycles at 95°C for 15 seconds and 55°C for 40 seconds. The mRNA levels of Th17-related genes in each sample was normalized to β-actin mRNA and quantified using a formula: 2 [(Ct/β-actin – Ct/gene of testing gene)]. The result was expressed as fold difference in the cells stimulated with both OVA and OX40-activating antibody compared to the group treated with OVA alone.
Statistics
Data are expressed as the average ± SEM, and a representative experiment is shown for each figure. For EAU scoring, median difference between control and experimental groups was compared using exact Wilcoxon two-sample test. Other statistical probabilities were evaluated by Student’s t-test, with a value of P < 0.05 considered significant.
Results
Up-Regulation of OX40 in OVA-Induced Uveitis
Recently, we have generated a novel uveitis model by direct administration of OVA into the vitreous chamber of DO11.10 mice. These mice develop a rapid onset anterior uveitis due to the specific T cell receptor response to OVA. The OVA-induced uveitis is CD4+ T cell dependent and mainly mediated by IL-17.9 Since activated T cells express OX40, we investigated OX40 induction in this T cell-dependent uveitis model. DO11.10 mice were intravitreally challenged with 100 μg OVA or bovine serum albumin as a control. Twenty-four hours later, the eyes were harvested to examine OX40 expression. Real-time PCR revealed a mean 3.3-fold increase (SD = 0.8) of OX40 transcripts in the ocular samples after intravitreal OVA challenge compared to non-antigenic bovine serum albumin injection.
OX40L Neutralizing Antibody Inhibits Experimental Uveitis
To further confirm the role of OX40 signaling in uveitis, we compared the severity of ocular inflammation between the mice treated with anti-OX40L monoclonal antibody and rat IgG2a as an isotype control. We first used DO11.10 mice that express transgenic dsRedII under the control of the CD4 promoter.34 Anti-OX40L antibody was administered simultaneously along with OVA on the induction of uveitis. DsRedII-labeled T cell infiltration in the eye was monitored by intravital microscopy at 48 hours after the intraocular antigen challenge.
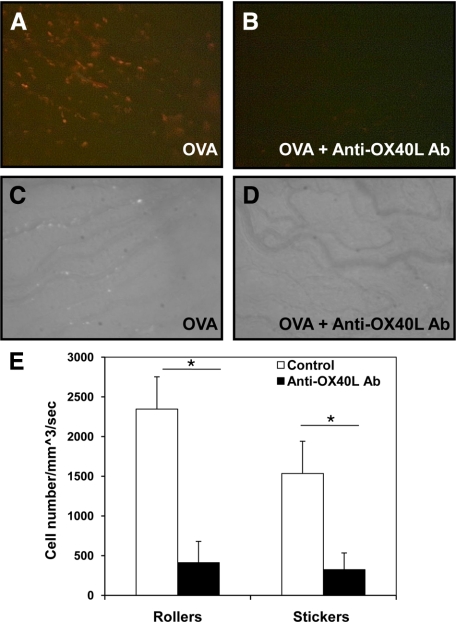
As shown in Figure 1A, a marked infiltration of red T cells was observed in the extravascular area of the iris. No infiltrating T cells were observed in the eyes at 0 hour before OVA injection or in the control animals that received bovine serum albumin (data not shown). Compared to OVA stimulation alone, anti-OX40L monoclonal antibody significantly inhibited the lymphocyte infiltration in the iris (Figure 1, A and B). To examine inflammatory cell migration in the eye, we administered OVA intravitreally to regular DO11.10 mice. Forty-eight hours later, the circulating leukocytes in these mice were labeled with rhodamine by intraperitoneal injection, and uveitis was monitored by intravital microscopy. The intravitreal challenge of OVA resulted in a marked ocular influx of leukocytes, whereas anti-OX40L antibody substantially reduced rolling and adherent leukocytes in the vasculature of the iris (Figure 1, C–E).
Figure 1.
Blocking OX40 signaling by anti-OX40L antibody attenuates OVA-induced uveitis. OVA was administered intravitreally into the DO11.10 mice, which have transgenic dsRed fluorescent protein under the control of CD4 promoter. In addition, some mice were simultaneously treated with anti-OX40L antibody during intravitreal OVA challenge. Ocular-infiltrating cells were assessed by intravital microscopy at 48 hours. Representative image of a single frame from intravital microscopy videos taken of the iris during the course of inflammation induced by OVA (n = 3 mice per group). Of note, intravitreal OVA challenge elicited a marked infiltration of red fluorescent T cells in the extravascular area of the iris (A), which was substantially inhibited by anti-OX40L antibody (B). In addition, intravascular leukocytes of regular DO11.10 mice were labeled with rhodamine at 48 hours after intravitreal OVA stimulation, and ocular inflammatory cells were assessed by intravital microscopy. Of note, compared to OVA challenge alone (C), anti-OX40L antibody inhibited OVA-induced inflammatory cell infiltration in the eye (D). E: Quantitation of rolling and adherent cells in the vasculature of the iris treated with and without anti-OX40L antibody. *P < 0.05.
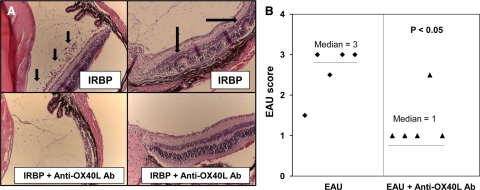
OVA-induced uveitis is ideal for visualizing the ocular immune response, and it uniquely mimics human anterior uveitis. However, this transgenic model is elicited by non-ocular antigen. To validate the role of OX40 in a self-antigen-mediated model, we used IRBP-induced EAU. Compared to the control group, the anti-OX40L antibody treatment reduced the severity of EAU as evidenced by less retinal folding and cellular infiltrates (Figure 2, A and B). These data suggest that OX40 signaling plays a key role in the development of antigen-induced uveitis.
Figure 2.
Anti-OX40L antibody ameliorates EAU. EAU was induced in B10.RIII mice by subcutaneous injection of IRBP161-180 peptide in complete Freund’s adjuvant with M. tuberculosis. OX40L-blocking antibody (10 μg per mouse) was administered intravenously on days 0, 3, 7, and 14 after IRBP immunization. On day 21, the mice were euthanized. Eyes were harvested for histology. A: Representative sections of EAU. Of note, OX40L antibody markedly attenuated ocular leukocyte infiltration (small arrows) and retina folding (large arrows) (n = 5/group). B: EAU scoring of control and anti-OX40L antibody-treated groups.
Activation of OX40 Enhances Th17 Cytokine and IL-23 Receptor Expression
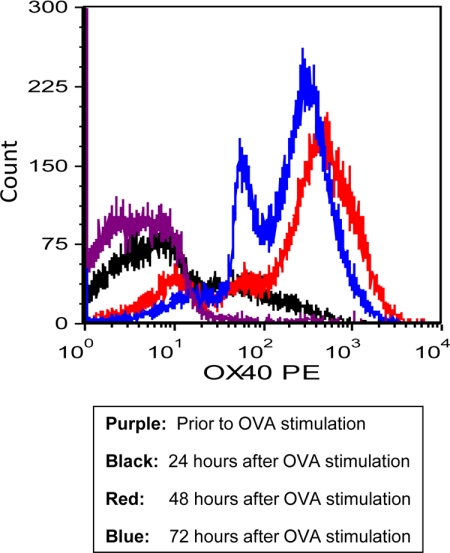
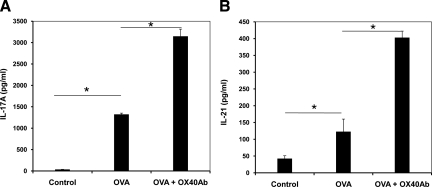
Previously, we and others demonstrated that the OVA-induced uveitis and EAU are mediated by IL-17.35,36 To test if OX40 is implicated in Th17 cell activation during the ocular inflammatory process, we first isolated naïve CD4+ T cells from the DO11.10 splenocytes using MagCellect Mouse Naïve CD4+ T Cell Isolation Kit (R&D Systems). Next, we stimulated the CD4+ T cells with OVA323-339 peptide (2 μg/ml) in the presence of “feeder” antigen-presenting cells for up to 96 hours. The surface expression of OX40 on CD4+ cells after OVA323-339 peptide treatment was assessed by flow cytometry. In the absence of OVA stimulation, very few CD4+ cells co-expressed OX40 (Figure 3). However, OVA stimulation caused marked OX40 induction after 24-hour antigen challenge, and the OX40 expression reached a more steady level at 72 hours (Figure 3). Furthermore, as measured by enzyme-linked immunosorbent assay, OVA stimulation induced a substantial production of IL-17 (Figure 4A). To further examine the effect of OX40 on IL-17-producing T cells, we stimulated DO11.10 CD4+ cells with 5 μg/ml OX40-activating antibody (OX86) in the presence of OVA323-339 peptide. A recent study showed that the OX40-activating antibody promotes a T cell response in wild-type mice but not in OX40 knockout animals, suggesting that this agonistic antibody specifically activates OX40.37 Compared to OVA treatment alone, activation of OX40 by the agonistic antibody significantly augmented IL-17 production in response to the antigen challenge (Figure 4A).
Figure 3.
OVA induces OX40 expression in CD4+ T cells from DO11.10 mice. DO11.10 lymphocytes were stimulated with OVA323-339 peptide in vitro for up to 72 hours. Cell surface CD4 and OX40 expression were analyzed by flow cytometry. Representative plot of OX40 expression in gated CD4+ lymphocytes from two independent studies.
Figure 4.
Activation of OX40 enhances OVA-induced IL-17 and IL-21 production in naïve DO11.10 CD4+ T cells. The CD4+ lymphocytes (2 × 105/200 μl) co-cultured with irradiated antigen-presenting cells (2 × 106/200 μl) were cultured with OVA323-339 (5 μg/ml) in the presence or absence of OX40-activating antibody (4 μg/ml) for 72 hours. The cell culture media of the naïve CD4+ T cells were collected for enzyme-linked immunosorbent assay of IL-17 (A) and IL-21 (B). The data represent the mean of triplicates of two independent experiments. *P < 0.05.
In light of above finding, we further examined whether OX40 up-regulated Th17-related cytokine IL-21. Recent studies show that IL-21 plays an important role in promoting Th17 lineage in an autocrine fashion. In addition, it primes CD4+ lymphocytes to become IL-23R-expressing Th17 cells.38,39,40 We measured the IL-21 level in the culture media of sorted naïve DO11.10 CD4+ cells after 3-day stimulation with the OX40-activating antibody. As shown in Figure 4B, the stimulation of OX40 significantly enhanced OVA-induced IL-21 secretion. Thus, in addition to our previous findings, OX40 stimulation also plays an important role in IL-21 induction, which putatively contributes to the expansion of activated Th17 cells.
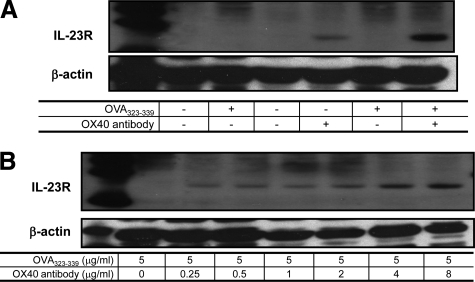
Next, we focused on the role of OX40 in the expression of IL-23R for the following reasons. It is well documented that IL-23R is expressed predominantly in differentiated Th17 cells, leading to further commitment to effector Th17 lineage.41,42,43 Thus, we asked whether activation of OX40 is also associated with IL-23R induction. For this experiment, DO11.10 lymphocytes were divided into four groups: control; OVA stimulation alone; OX40-activating antibody treatment alone; and costimulation with OVA and OX40-activating antibody. IL-23R expression in CD4+ cells was analyzed by Western blot. IL-23R was not evident in control or OVA323-339 peptide-treated cells (Figure 5A). However, OX40 agonistic antibody induced IL-23R expression in OVA-activated DO11.10 T cells in a dose-dependent manner (Figure 5, A and B).
Figure 5.
Activation of OX40 augments OVA-induced IL-23R expression in CD4+ lymphocytes. DO11.10 lymphocytes were treated with OVA323-339 peptide (5 μg/ml) and OX40-activating antibody (4 μg/ml) for 72 hours. The expression of IL-23R in CD4+ cells was analyzed by Western blot analysis. A: OX40 induced IL-23R in response to OVA stimulation. B: Dose-response effect of OX40-activating antibody on IL-23R expression. Representative plot of IL-23R from three independent studies.
Activated Th17 Cells Express OX40 and Stimulation of OX40 Enhanced Th17 Gene Expression
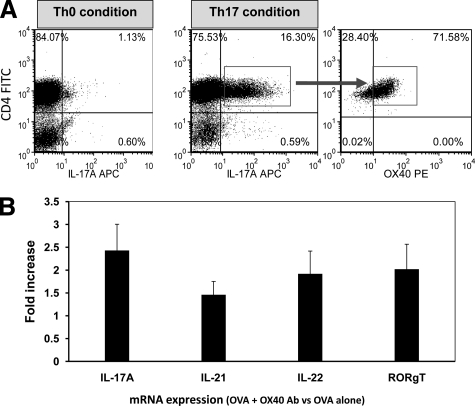
Since we showed that activation of OX40 up-regulated Th17 cytokine and receptor in activated CD4+ lymphocytes, we then assessed whether Th17 cells can directly express OX40. DO11.10 lymphocytes were cultured with 2 μg/ml OVA323-339 peptide in vitro under Th0 or Th17 condition for 4 days. After overnight resting in regular culture medium, these cells were stimulated with phorbol myristate acetate and ionomycin for 5 hours. IL-17 was detected by intracellular staining to correlate OX40 expression with Th17 cells. Compared to Th0 culture, the proportion of CD4+IL-17+ cells was markedly elevated under Th17-differentiating condition (Figure 6A). Moreover, the majority of activated Th17 cells expressed surface OX40 (Figure 6A).
Figure 6.
Activated Th 17 cells express OX40. DO11.10 CD4+ splenocytes were cultured under Th0 or Th17-polarizing conditions with OVA323-339 peptide (1 μg/ml). A: CD4+IL-17+ cells were determined by flow cytometry. Then, gated Th17 cells were further analyzed for the expression of OX40. Of note, the majority of activated IL-17+ cells expressed OX40. Arrow indicates determined OX40 expression in gated Th17 cells. B: Expression of IL-17A, IL-21, IL-22, and RORγt transcripts in OVA peptide-treated CD4+ cells after 4 days of Th17 differentiation in the presence of OX40-activating antibody (average of two independent studies). The level of Th17-related mRNA was normalized to β-actin, and the relative quantity was further compared with Th17 cells only stimulated with OVA.
To further characterize the effect of OX40 activation on Th17 cells, OVA-activated CD4+ T cells under Th17 polarizing condition were stimulated with OX40-activating antibody (4 μg/ml) for 4 days. Then, real-time PCR was performed to probe the transcriptional changes of Th17-related genes. Compared to the cells treated with OVA alone, OX40-activating antibody augmented the expression of transcripts for IL-17A, IL-21, IL-22, and RORγt (Figure 6B). Thus, this result implicates OX40 in the activation of Th17 cells.
Stimulation of OX40 Augments OVA-Induced Uveitis and IL-17 Mediates OX40-Enhanced Ocular Inflammation
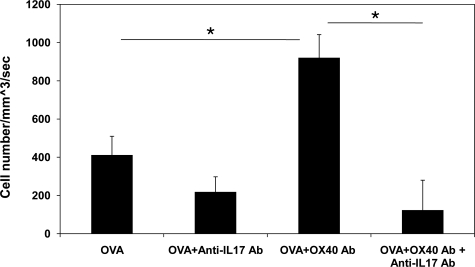
Next, we examined the in vivo effect of OX40 activation on effector lymphocyte function using an adoptive transfer uveitis model developed in our laboratory.31 The OVA323-339 peptide specific T cells were harvested from the spleen of DO11.10 transgenic mice, and stimulated in vitro with 5 μg/ml OVA in the presence or absence of 4 μg/ml OX40-activating antibody for 72 hours. Then, these cells were further purified using Lympholyte-M (Cedar Lane Laboratories). The activated DO11.10 lymphocytes (1.5 × 107 per mouse) were adoptively transferred to syngeneic BALB/c host mice. Uveitis was induced by intravitreal injection of 100 μg of OVA and 250 ng of lipopolysaccharide to increase the permeability of ocular vasculature. Although lipopolysaccharide alone can rapidly elicit a transient uveitis with a peak inflammation at 6 hours, this acute inflammatory response is not mediated by T cells. Indeed, we are unable to detect IL-17 transcription and protein expression in the eyes treated with lipopolysaccharide alone (data not shown). Twenty-four hours after the induction of uveitis in the recipient animals, the mice received intraperitoneal injection of rhodamine to label circulating leukocytes, and uveitis was assessed as the infiltration of fluorescent leukocytes within the anterior uvea.31 As shown in Figure 7, intravitreal administration of OVA resulted in an influx of leukocytes in the eyes of BALB/c mice that received activated DO11.10 lymphocytes. However, adoptive transfer of OX40-activating antibody-primed DO11.10 T cells significantly increased the number of adhering leukocytes in the iris vasculature in response to OVA stimulation (Figure 7). To further confirm that IL-17 mediates OX40-enhanced uveitis, we intravenously treated the recipient mice with 100 μg of anti-mouse IL-17 polyclonal antibody (R&D Systems) on the induction of uveitis. Compared to isotype control (goat IgG), the IL-17 neutralizing antibody significantly reduced adherent leukocytes in the vasculature of the eyes (Figure 7). These findings suggest that OX40 activation potentiates the effector function of Th17 cells, thereby exaggerating uveitis.
Figure 7.
Activation of OX40 enhances OVA-induced ocular inflammation in OVA-induced uveitis. OVA-activated DO11.10 cells were primed with and without OX40-activating antibody in vitro for 3 days. Then, these cells were transferred to BALB/c mice through tail vein injection, followed by intravitreal administration of 100 μg of OVA and 250 ng of lipopolysaccharide into the eyes of recipient mice (n = 3). In addition, some recipient mice received intravenous injection of 100 μg of anti-mouse IL-17 antibody. Twenty-four hours later, ocular inflammatory cells were assessed by intravital microscopy. Quantification of adherent cells in the vasculature of the iris with and without further OX40 activation. Of note, OX40-activating antibody priming augmented OVA-induced inflammatory cell infiltration in the eye, whereas anti-IL-17 antibody inhibited OX40-enhanced uveitis. *P < 0.05.
Discussion
In the present study, we have demonstrated that up-regulation of OX40 enhanced effector function of Th17 lymphocytes, and adoptive transfer of OX40-activated T cells significantly augmented OVA-induced ocular inflammation. Th17 cells are a unique effector lymphocyte subset involved in the pathogenesis of many autoimmune and inflammatory diseases. However, the mechanism of Th17 cell activation remains to be fully elucidated. T cell activation and differentiation requires a dual signaling process. The first signal is mediated by the T cell receptor interacting with an antigen fragment presented by the major histocompatibility complex on the antigen-presenting cell. Subsequently, an array of costimulatory molecules provides a second signal that is crucial to the amplification and optimization of the T cell response. Without further ligation of costimulatory molecules with their corresponding partners, stimulation of the T cell receptor alone leads to T cell anergy. It has been shown that the costimulatory molecules CD28 and ICOS participate in the induction of Th17 differentiation.44,45 Although OX40 has been implicated in Th17-mediated diseases such as EAE and multiple sclerosis,29,30,46,47 little is known about OX40 during the differentiation of Th17 cells. In the current study, we have shown that the activation of Th17 cells is influenced by the expression and subsequent ligation of OX40.
OX40, a member of tumor necrosis factor receptor superfamily, is mainly expressed by activated effector T cells.22,23 OX40 signals through TRAF adaptor molecules and phosphatidyl inositol 3 kinase, which further trigger the nuclear factor κB pathway.48,49 Activation of nuclear factor κB by OX40 provides a crucial costimulatory signal for T cell activation, proliferation, and survival.24 Unlike constitutively expressed CD28 that is responsible for initial T cell activation, OX40 is preferentially up-regulated in activated CD4+ T cells. This suggests that OX40 contributes to the enhancement of the T cell function and expansion instead of initiation of T cell activation. This is consistent with our observation that the marked effect of OX40 agonistic antibody mainly occurs after antigen stimulation.
OX40 activation requires the interaction with OX40L. OX40L is expressed in antigen-presenting cells such as dendritic cells.50,51 Initial engagement of these two special binding partners establishes a reciprocal communication between T cells and antigen-presenting cells. Such interaction allows antigen-presenting cells to further optimize the T cell response by providing a second wave of stimulatory signals such as IL-1β, IL-6, and transforming growth factor-β. It is well characterized that the combination of transforming growth factor-β, IL-6, and/or IL-1β primes Th17 differentiation. Our previous study showed that OVA stimulation up-regulated IL-1β and IL-6 transcription in the DO11.10 mice.9 This may explain how OVA challenge alone can induce IL-17 production in DO11.10 lymphocytes as observed in the current study.
In this study, we found that antigen activation specifically induced the expression of OX40 in the CD4+ cells. When additional stimulation was provided by the OX40 agonistic antibody, increased expression of IL-23R was noted within the cell populations also stimulated with antigen. This finding strongly indicates a role for OX40 ligation in the activation and further development of Th17 cells. Recent studies showed that Th17 cells characteristically produce IL-21, and IL-21 in turn promotes the expansion of the Th17 lineage.52 This is further supported by the report that IL-21 knockout mice display a deficiency of memory Th17 cells.52 Thus, analogous to the role of interferon-γ and IL-4 in the differentiation of Th1 and Th2 cells, respectively, IL-21 plays an essential role in the commitment and amplification of the Th17 population. In the present study, we found that the production of IL-17 was enhanced by OX40-activating antibody. More interestingly, activation of OX40 augmented antigen-induced IL-21 and IL-23R expression. These findings suggest that OX40 and its subsequent ligation play an important role in the expansion and maintenance of the Th17 subset. In light of the fact that OX40 is pivotal to T cell survival and proliferation, it is conceivable that OX40 is implicated in the proliferation of Th17 cells by up-regulating IL-21 and subsequently IL-23R.
Recently, several lines of evidence support the premise that OX40 amplifies Th17 activity. In EAE, a central nervous system demyelinating disease model that is mainly mediated by Th17 cells, OX40 and OX40L are found to be critical for the induction and priming of antigen specific CD4+ T cells.14,53 Furthermore, neutralization of OX40L by monoclonal antibody ameliorates the development of EAE.27 Lastly, Nakae and colleagues54 showed Th17 cells express OX40. All these data highlight the need to further study the role of OX40 in Th17 cell activation in clinically related settings.
Although OX40 has been implicated in many T cell-mediated systemic diseases which are often associated with uveitis, little is known of the effect of OX40 in the process of ocular inflammation. Studying the role of OX40 and other costimulatory molecules is important for understanding the mechanism of lymphocyte activation during uveitis. Using the adoptive transfer model, we clearly demonstrate that neutralization of IL-17 by the blocking antibody attenuated OX40-mediated uveitis.
In summary, we have demonstrated that up-regulation of OX40 exerts a greater inflammatory response in antigen-induced uveitis, whereas inhibition of OX40L reduced ocular inflammation. Moreover, we have presented strong data here that activation of Th17 cells entails the participation of OX40. Thus, further validation of the role of OX40 in Th17 cell activation and uveitis development has an important implication for understanding lymphocyte biology as well as developing immunotherapy for uveitis.
Acknowledgments
We thank Dr. Grazyna Adamus for technical assistance of EAU scoring. We also thank Judie McDonald for editorial assistance.
Footnotes
Address reprint requests to Zili Zhang, M.D., Ph.D., or James Rosenbaum, M.D., Oregon Health & Science University, 707 Gaines St., Mail Code CDRCP, Portland, OR 97239. E-mail: zhangzi@ohsu.edu or rosenbaj@ohsu.edu.
Supported by National Institutes of Health grants EY016788 (Z.Z.), EY013093 (J.T.R.), and EY006484 (J.T.R.); and a CDHNF Young Investigator Award (Z.Z.). Funds from the Stan and Madelle Rosenfeld Family Trust, the William and Mary Bauman Foundation, Research to Prevent Blindness and William C. Kuzell Foundation also supported this work.
References
- Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- Becker MD, Adamus G, Davey MP, Rosenbaum JT. The role of T cells in autoimmune uveitis. Ocul Immunol Inflamm. 2000;8:93–100. [PubMed] [Google Scholar]
- Martin TM, Smith JR, Rosenbaum JT. Anterior uveitis: current concepts of pathogenesis and interactions with the spondyloarthropathies. Curr Opin Rheumatol. 2002;14:337–341. doi: 10.1097/00002281-200207000-00001. [DOI] [PubMed] [Google Scholar]
- Atalla L, Linker-Israeli M, Steinman L, Rao NA. Inhibition of autoimmune uveitis by anti-CD4 antibody. Invest Ophthalmol Vis Sci. 1990;31:1264–1270. [PubMed] [Google Scholar]
- Kilmartin DJ, Fletcher ZJ, Almeida JA, Liversidge J, Forrester JV, Dick AD. CD69 expression on peripheral CD4+ T cells parallels disease activity and is reduced by mycophenolate mofetil therapy in uveitis. Invest Ophthalmol Vis Sci. 2001;42:1285–1292. [PubMed] [Google Scholar]
- Rothova A, Buitenhuis HJ, Meenken C, Brinkman CJ, Linssen A, Alberts C, Luyendijk L, Kijlstra A. Uveitis and systemic disease. Br J Ophthalmol. 1992;76:137–141. doi: 10.1136/bjo.76.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Sonoda KH, Ohguro N, Ohsugi Y, Ishibashi T, Cua DJ, Kobayashi T, Yoshida H, Yoshimura A. Involvement of Th17 cells and the effect of anti-IL-6 therapy in autoimmune uveitis. Rheumatol. 2009;48:347–354. doi: 10.1093/rheumatology/ken489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lee YS, Yu CR, Egwuagu CE. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J Immunol. 2008;180:6070–6076. doi: 10.4049/jimmunol.180.9.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhong W, Spencer D, Chen H, Lu H, Kawaguchi T, Rosenbaum JT. Interleukin-17 causes neutrophil mediated inflammation in ovalbumin-induced uveitis in DO11.10 mice. Cytokine. 2009;46:79–91. doi: 10.1016/j.cyto.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- Redmond WL, Ruby CE, Weinberg AD. The role of OX40-mediated co-stimulation in T-cell activation and survival. Crit Rev Immunol. 2009;29:187–201. doi: 10.1615/critrevimmunol.v29.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4:420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- Weinberg AD. OX40: targeted immunotherapy–implications for tempering autoimmunity and enhancing vaccines. Trends Immunol. 2002;23:102–109. doi: 10.1016/s1471-4906(01)02127-5. [DOI] [PubMed] [Google Scholar]
- Lane P. Role of OX40 signals in coordinating CD4 T cell selection, migration, and cytokine differentiation in T helper (Th)1 and Th2 cells. J Exp Med. 2000;191:201–206. doi: 10.1084/jem.191.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229:173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MC, Liao JJ, Bonasera S, Longo DL, Goetzl EJ. Nuclear factor-kappaB-dependent reversal of aging-induced alterations in T cell cytokines. FASEB J. 2008;22:2142–2150. doi: 10.1096/fj.07-103721. [DOI] [PubMed] [Google Scholar]
- Weinberg AD, Bourdette DN, Sullivan TJ, Lemon M, Wallin JJ, Maziarz R, Davey M, Palida F, Godfrey W, Engleman E, Fulton RJ, Offner H, Vandenbark AA. Selective depletion of myelin-reactive T cells with the anti-OX-40 antibody ameliorates autoimmune encephalomyelitis. Nat Med. 1996;2:183–189. doi: 10.1038/nm0296-183. [DOI] [PubMed] [Google Scholar]
- Nohara C, Akiba H, Nakajima A, Inoue A, Koh CS, Ohshima H, Yagita H, Mizuno Y, Okumura K. Amelioration of experimental autoimmune encephalomyelitis with anti-OX40 ligand monoclonal antibody: a critical role for OX40 ligand in migration, but not development, of pathogenic T cells. J Immunol. 2001;166:2108–2115. doi: 10.4049/jimmunol.166.3.2108. [DOI] [PubMed] [Google Scholar]
- Caspi RR, Roberge FG, Chan CC, Wiggert B, Chader GJ, Rozenszajn LA, Lando Z, Nussenblatt RB. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490–1495. [PubMed] [Google Scholar]
- Dullforce PA, Seitz GW, Garman KL, Michael JA, Crespo SM, Fleischman RJ, Planck SR, Parker DC, Rosenbaum JT. Antigen-specific accumulation of naive, memory and effector CD4 T cells during anterior uveitis monitored by intravital microscopy. Cell Immunol. 2006;239:49–60. doi: 10.1016/j.cellimm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Becker MD, Crespo S, Martin TM, Planck SR, Naramura M, Rosenbaum JT. Intraocular in vivo imaging of activated T-lymphocytes expressing green-fluorescent protein after stimulation with endotoxin. Graefes Arch Clin Exp Ophthalmol. 2001;239:609–612. doi: 10.1007/s004170100320. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Peng Y, Han G, Shao H, Wang Y, Kaplan HJ, Sun D. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2007;48:4153–4161. doi: 10.1167/iovs.07-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Qian J, Guo J, Yuan YF, Xue K. Suppression of experimental autoimmune uveoretinitis by Anti-IL-17 antibody. Curr Eye Res. 2009;34:297–303. doi: 10.1080/02713680902741696. [DOI] [PubMed] [Google Scholar]
- Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68:5206–5215. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- Frucht DM. IL-23: a cytokine that acts on memory T cells. Sci Signal. 2002;114:PE1. doi: 10.1126/stke.2002.114.pe1. [DOI] [PubMed] [Google Scholar]
- Huber M, Brüstle A, Reinhard K, Guralnik A, Walter G, Mahiny A, von Löw E, Lohoff M. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci USA. 2008;105:20846–20851. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Cong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Treuting P, Duong J, Flavell RA, Dong C. Inducible costimulator is essential for collagen-induced arthritis. J Clin Invest. 2003;111:701–706. doi: 10.1172/JCI17321. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ndhlovu LC, Ishii N, Murata K, Sato T, Sugamura K. Critical involvement of OX40 ligand signals in the T cell priming events during experimental autoimmune encephalomyelitis. J Immunol. 2001;167:2991–2999. doi: 10.4049/jimmunol.167.5.2991. [DOI] [PubMed] [Google Scholar]
- Carboni S, Aboul-Enein F, Waltzinger C, Killeen N, Lassmann H, Peña-Rossi C. CD134 plays a crucial role in the pathogenesis of EAE and is upregulated in the CNS of patients with multiple sclerosis. J Neuroimmunol. 2003;145:1–11. doi: 10.1016/j.jneuroim.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Arch RH, Thompson CB. 4–1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol Cell Biol. 1998;18:558–565. doi: 10.1128/mcb.18.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata S, Hori T, Imura A, Takaori-Kondo A, Uchiyama T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J Biol Chem. 1998;273:5808–5814. doi: 10.1074/jbc.273.10.5808. [DOI] [PubMed] [Google Scholar]
- Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- Chen AI, McAdam AJ, Buhlmann JE, Scott S, Lupher ML, Jr, Greenfield EA, Baum PR, Fanslow WC, Calderhead DM, Freeman GJ, Sharpe AH. OX40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity. 1999;11:689–698. doi: 10.1016/s1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J Leukoc Biol. 2007;81:1258–1268. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]