Abstract
Neurofibromatosis type 1 (NF1) is a common genetic disorder and is characterized by both malignant and nonmalignant neurofibromas, which are composed of Schwann cells, degranulating mast cells, fibroblasts, and extracellular matrix. We and others have previously shown that hyperactivation of the c-Kit pathway in an Nf1 haploinsufficient microenvironment is required for both tumor formation and progression. Mast cells play a key role in both tumorigenesis and neoangiogenesis via the production of matrix metalloproteinases, heparin, and a range of different growth factors. In the present study, we show that tumorigenic Schwann cells derived from Nf1−/− embryos promote increased degranulation of Nf1+/− mast cells compared with wild-type mast cells via the secretion of the Kit ligand. Furthermore, we used genetic intercrosses as well as pharmacological agents to link the hyperactivation of the p21Ras-phosphatidylinositol 3-kinase (PI3K) pathway to the increased degranulation of Nf1+/− mast cells both in vitro and in vivo. These studies identify the p21Ras-PI3K pathway as a major regulator of the gain in Nf1+/− mast cell degranulation in neurofibromas. Collectively, these studies identify both c-Kit and PI3K as molecular targets that modulate mast cell functions in cases of NF1.
Mutations in the Nf1 tumor suppressor gene cause neurofibromatosis type 1 (NF1), a common autosomal dominant genetic disorder (with an incidence of 1:3500), which is characterized by cutaneous and plexiform neurofibroma formation. Neurofibromin, the protein product of Nf1, functions as a GTPase activating protein (GAP) for p21Ras (Ras) by accelerating the hydrolysis of active Ras-GTP to inactive Ras-GDP.1,2 Neurofibromas are pathognomonic for NF1 and constitute a major source of morbidity in NF1 patients. These complex tumors are composed of high concentrations of Schwann cells, fibroblasts, and degranulating mast cells found in close physical association with each other and within a dense extracellular matrix.
Inflammation and alterations associated with the tumor microenvironment are increasingly recognized as critical components of tumor initiation and progression.3,4,5 Studies using genetically engineered mice demonstrated that nullizygosity of Nf1 in Schwann cells was necessary but not sufficient for neurofibroma formation and that haploinsufficiency of Nf1 (Nf1+/−) in non-neuronal lineages of the tumor microenvironment was an additional requirement for tumor progression.6 Recently, we refined this finding using an Nf1 conditional knockout model demonstrating that haploinsufficient loss of Nf1 in the hematopoietic compartment of the microenvironment specifically was required for in vivo tumor formation.7 We further found that c-Kit pathway signaling is critical for the tumor progression.
Mast cells release heparin, histamine, tumor necrosis factor-α, transforming growth factor-β, and metalloproteinases. These mediators alter the extracellular matrix, modulate growth factor presentation to cells within the growing tumor, promote fibroblast proliferation and collagen synthesis, and provide a scaffold for the invasion of blood vessels. However, evaluation of the specific mediators that promote release of these factors from mast cells in the context of neurofibroma development and detailed studies to examine the biochemical pathways that promote this increase in function have not been described. Identification of these degranulation-promoting factors and the biochemical pathways that they activate is important for understanding the pathogenesis of neurofibroma progression and identifying potential molecular targets for treating existing tumors and/or preventing tumor formation.
Previous studies in human neurofibromas have found that Nf1−/− Schwann cell conditioned medium contains sevenfold higher concentrations of c-Kit ligand (Kit-L) as well as other growth factors that may enhance mast cell degranulation.8,9 Studies in human and animal neurofibromas have also observed higher concentrations of Kit-L transcripts within neurofibromas.10 Here we provide genetic and biochemical evidence that Nf1−/− Schwann cell conditioned medium (SCCM) contains a potent degranulation stimulus, Kit-L, that is responsible for the elevated Nf1+/− mast cell degranulation. Further, we demonstrate that genetic disruption of the p85α regulatory subunit of the class1A PI3K or the addition of a PI3K inhibitor, Ly294002, is sufficient to abrogate this cellular and biochemical gain of function both in vitro and in vivo.
Materials and Methods
Animals
Nf1+/− mice were obtained from Dr. Tyler Jacks at the Massachusetts Institute of Technology (Cambridge, MA) in a C57BL/6J.129 background and backcrossed for 13 generations into a C57BL/6J strain.11 The Nf1 allele was genotyped as described previously.9,12,13,14 C57BL/6J W41/W41 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The W41 genotyping was inferred from the characteristic mottled white coat color in W41/W41 mice and a white abdominal spot on W41/+ mice, as described previously.15,16 p85α+/− and p85α−/− mice were obtained in a mixed C57BL/6J.129 background from Dr. Lewis Cantley at Harvard University (Boston, MA) and were backcrossed for 12 generations into a C57BL/6J genetic strain. The p85α alleles were genotyped by PCR as described previously.17 All studies were conducted with a protocol approved by the Indiana University Animal Care and Use Committee.
Culture of Bone Marrow-Derived Mast Cells
Bone marrow-derived mast cells (BMMCs) were generated from 6-week-old mice as described previously.18 In brief, mononuclear bone marrow cells were cultured for 4 weeks in RPMI 1640 containing 1% glutamine (BioWhittaker, Walkersville, ME), 1.5% HEPES (BioWhittaker), 2% penicillin/streptomycin (BioWhittaker), 10% fetal bovine serum (HyClone Laboratories, Logan, UT), and 10 ng/ml recombinant murine interleukin-3 (PeproTech, Rocky Hill, NJ) in a 37°C, 5% CO2 humidified incubator. The homogeneity of BMMCs was determined by Alcian Blue-Safranin O staining.9 Furthermore, fluorescence-activated cytometric analysis (BD Biosciences, San Jose, CA) revealed similar forward and side light scatter characteristics and the same percentage of c-Kit+ expression in BMMCs for all murine experimental genotypes (data not shown).
Schwann Cell Culture and Generation of Schwann Cell Conditioned Media
Murine Schwann cells were isolated from WT and Nf1−/− mutant mouse embryo dorsal root ganglia at embryonic day 13.5 as described previously.19 In brief, dorsal root ganglia of the embryos were enzymatically dissociated, and cells from single embryos were plated in a single well of a 12-well tissue culture plate in Dulbecco’s modified Eagle’s medium (Invitrogen, Grand Island, NY) containing10% fetal bovine serum supplemented with 250 ng/ml nerve growth factor (Harlan Bioproducts for Science Inc., Indianapolis, IN). The medium was changed to serum-free defined N2 medium (Invitrogen) containing 250 ng/ml nerve growth factor and penicillin/streptomycin (1 mM) (BioWhittaker) on the next day. After 5 to 6 days, Schwann cells and neurons were separated from fibroblasts by lifting up the Schwann cell-neuron layers from the dish, leaving the fibroblasts behind. Cells from the same genotype were pooled, and Schwann cells were enzymatically dissociated from the neurons in 0.01% collagenase (Sigma-Aldrich, St. Louis, MO). Cells were centrifuged, resuspended in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, and plated on poly-l-lysine-coated 100-mm diameter cell culture plates (BD, Franklin Lakes, NJ) at a density of approximately 106 cells/plate. These cells were considered passage 0. Cells were changed on the next day to Schwann cell growth medium containing 10 ng/ml recombinant human glial growth factor 2 (β-heregulin epidermal growth factor-like domain peptide, Sigma-Aldrich), and 1 mg/ml ***penicillin/streptomycin, with 2 mmol/L forskolin (EMD Biosciences Inc., San Diego, CA) added to promote Schwann cell mitogenesis and suppress fibroblast growth. After 1 week, cells were trypsinized and replated at 106 cells/plate (passage 1). Cultures were stained with an antibody directed against S-100 (Sigma-Aldrich), an acidic, calcium-binding protein present in Schwann cells and not fibroblasts, to verify the purity of Schwann cell populations.
β-Hexosaminidase Release Assay
To test whether Nf1−/− SCCM affects mast cell function, mast cell degranulation was evaluated by the β-hexosaminidase release assay.20 In brief, BMMCs were sensitized at 1 × 106/ml in the presence or absence of 1:1000 dilution anti-Ack antibody (eBiosciences, San Diego, CA) in complete RPMI 1640 without cytokines along with 1.5 μg/ml anti-dinitrophenyl (DNP) IgE (clone SPE-7, Sigma-Aldrich) for 2 hours at 37°C in 5% CO2. Cells were then washed once in Tyrode’s buffer (130 mmol/L NaCl, 10 mmol/L HEPES, 1 mmol/L MgCl2, 5 mmol/L KCl, 1.4 mmol/L CaCl2, 5.6 mmol/L glucose, and 0.05% bovine serum albumin, pH 7.4) and resuspended at 2 × 106/ml in Tyrode’s buffer. Cells were then stimulated with either wild-type SCCM, Nf1−/− SCCM, or DNP-human serum albumin (HSA) (50 ng/ml, Sigma-Aldrich) and Kit-L (10 ng/ml, recombinant murine stem cell factor, PeproTech) for 5 minutes at 37°C. After the cells were spun down, 30 μl of supernatant was transferred to a 96-well flat-bottom plate. Then 30 μl of 1 mmol/L p-nitrophenyl-N-acetyl–d-glucosamide was added to each supernatant and mixed before incubation for 1 hour at 37°C. The reaction was terminated by the addition of 200 μl of 0.1 M Na2CO3-NaHCO3 buffer, and optical density was read on a plate reader at a wavelength of 405 nm.
Western Blot
WT or Nf1+/− mast cells were stimulated with Kit-L (10 ng/ml, PeproTech), WT SCCM, or Nf1−/− SCCM for 5 minutes. Cells were then washed with cold PBS, and whole cell lysates were prepared by adding 1× cell lysis buffer (10 mmol/L K2HPO4, 1 mmol/L EDTA, 5 mmol/L EGTA, 10 mmol/L MgCl2, 1 mmol/L Na3VO4, 50 mmol/L sodium β-glycerophosphate, 10 μg of aprotinin/ml, 10 μg leupeptin/ml, and 1 μg of pepstatin A/ml, pH 7.2). Immunoblot analyses were performed with rabbit antibodies for phospho-Akt (Ser-473) (1:1000, New England Biolab, Ipswich, MA) and total Akt (1:2000, New England Biolabs).
Histological and Immunohistological Analysis
To examine mast cell numbers in vivo, mast cell frequency in ear and peritoneal lavage was determined. In brief, mice were sacrificed by CO2 inhalation. Ears were removed, fixed in buffered formalin, and processed in paraffin-embedded sections. Specimens were stained with Alcian Blue to identify mast cells. Mast cell numbers per 1 mm2 were quantitated in a blinded fashion. Peritoneal lavage was performed as described previously,21 with 10 ml of peritoneal lavage fluid concentrated by centrifugation and stained with toluidine blue to quantify total number of mast cells per 10 ml of lavage.
In Vivo Anaphylaxis Assay
To evaluate mast cell function in vivo, we used a previously described anaphylaxis assay.9 In brief, WT, Nf1+/−, p85α−/−, and Nf1+/−;p85α−/− mice received an intradermal injection of 20 μl of 1:44 dilution of stock monoclonal anti-DNP IgE (clone SPE-7, Sigma-Aldrich) and 50 ng of Kit-L in PBS in the right ear and a PBS injection in the left ear. Twenty hours later, they received a tail vein injection of DNP-HSA (300 μl of a 10 mg/ml DNP-HSA [Sigma-Aldrich]), and 1% Evans blue dye. Thirty minutes later, the mice were sacrificed, and tissue samples were acquired and imaged using an Epson Perfection 4990 photo scanner. Dye was extracted from a 5-mm punch biopsy taken from the sensitization site. These samples were treated with 1 N KOH overnight at 37°C. The next day 900 μl of extraction buffer (85% H3PO4, acetone, and H2O) was added to digested ear, followed by sample agitation and centrifugation. Samples were read at 620 nm with a spectrophotometer.
Statistical Analysis
All P values were generated using analysis of variance and post-analysis of variance t-test.
Results
Nf1−/− SCCM Induces Elevated Mast Cell Degranulation in Nf1+/− Bone Marrow-Derived Mast Cells
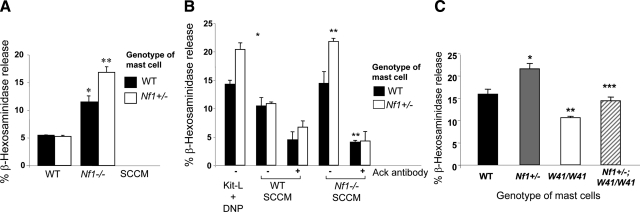
An increasingly recognized paradigm of tumorigenic cells is their ability to co-opt the normal functions of nonmalignant cells by increasing secretion of growth factors. To test whether paracrine factors produced by Schwann cells promote degranulation, Nf1−/− SCCM was used to examine the release of β-hexosaminidase, a preformed enzyme in granules of mast cells that is commonly used as a measure of mast cell degranulation. In four independent experiments, an equivalently low level of β-hexosaminidase release was observed in WT and Nf1+/− mast cells in response to WT SCCM (Figure 1A). Both WT and Nf1+/− mast cells demonstrated a significant increase in degranulation after stimulation with Nf1−/− SCCM compared with stimulation with WT SCCM. However, Nf1+/− mast cells demonstrated a 33% higher concentration of β-hexosaminidase release compared with WT mast cells.
Figure 1.
Nf1−/− SCCM significantly promotes Nf1+/− mast cell degranulation. Degranulation of mast cells was assessed by the release of β-hexosaminidase. β-Hexosaminidase activity is measured in the supernatant and the extent of degranulation is reported as a percentage of total cellular β-hexosaminidase activity. A: Degranulation of WT and Nf1+/− BMMCs was assessed by the release of β-hexosaminidase after SCCM stimulation. *P < 0.01 for WT versus Nf1−/− SCCM. **P < 0.01 for Nf1+/− versus WT mast cells stimulated with Nf1−/− SCCM. Data are means ± SEM from triplicate samples in four independent experiments. B: β-Hexosaminidase release was measured after stimulation with either Kit-L plus DNP or SCCM (WT or Nf1−/−) with or without the addition of Ack. *P < 0.01 for WT versus Nf1+/− mast cells stimulated with Kit-L/DNP. **P < 0.01 for Nf1+/− versus WT mast cells stimulated with Nf1−/− SCCM and Nf1+/− treated with Ack versus Nf1+/− treated with vehicle. Data are means ± SEM from triplicate samples in four independent experiments. C: The Nf1−/− SCCM promotes mast cell degranulation via the c-Kit receptor. Degranulation of BMMCs derived from WT, Nf1+/−, W41/W41, and Nf1+/−;W41/W41 mice was assessed by the release of β-hexosaminidase after stimulation with Nf1−/− Schwann cell-conditioned medium. *P < 0.01 for WT versus Nf1+/− mast cells stimulated with Nf1−/− SCCM. **P < 0.01 for WT versus W41/W41 mast cells stimulated with Nf1−/− SCCM. ***P < 0.01 for Nf1+/− versus Nf1+/−;W41/W41 mast cells stimulated with Nf1−/− SCCM. Data are means ± SEM from triplicate samples in four independent experiments.
Neutralizing Antibody to c-Kit or Genetic Disruption of c-Kit Is Sufficient to Inhibit Mast Cell Degranulation
We have previously reported that Nf1−/− Schwann cells secrete greater amounts of Kit-L than WT Schwann cells.22,23,24 In addition, we have shown that Nf1+/− mast cells have increased degranulation in response stimulation with Kit-L in conjunction with allergen-induced cross-linking of FcεRI by DNP-HSA.25 To determine whether the increased secretion of Kit-L by Nf1−/− Schwann cells was responsible for the increase in degranulation of Nf1+/− mast cells after Nf1−/− SCCM, we added a c-Kit neutralizing antibody (anti-Ack) to the SCCM before evaluation of mast cell degranulation. Mast cells stimulated with Kit-L/DNP alone were used as a positive control. Kit-L induced a significantly increased amount of β-hexosaminidase release in Nf1+/− mast cells compared with that for WT mast cells (Figure 1B). Of importance, the addition of the c-Kit neutralizing antibody to Nf1−/− SCCM was sufficient to significantly inhibit degranulation of Nf1+/− mast cells to basal levels (Figure 1B), implying that hypersecretion of Kit-L by Nf1−/− Schwann cells is responsible for the increased degranulation of Nf1+/− mast cells after exposure to Nf1−/− SCCM.
To genetically verify that Kit-L is mediating mast cell degranulation in response to SCCM, Nf1+/− mice were intercrossed with mice containing an inactivating mutation in the c-Kit receptor (W41/W41). After generation of mast cells from these intercrossed mice, cells were stimulated with Nf1−/− SCCM, and degranulation was assessed. Consistent with the above data, mast cells from the Nf1+/− mice had significant elevation of degranulation compared with WT mast cells. However, mast cells isolated from mice containing mutations at both the Nf1 and W loci had a reduction in degranulation compared with Nf1+/− mast cells, to a level that was comparable to that of WT mice (Figure 1C), providing genetic confirmation that hyperactivation of the Kit-L/c-Kit pathway in Nf1+/− mast cells mediates the excessive degranulation in response to Nf1−/− SCCM.
Pharmacological Inhibition of the PI3K Signaling Pathway Reduces Kit-L-Mediated Nf1+/− Mast Cell Gain of Function
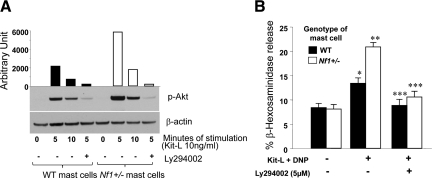
The PI3K pathway is recognized as an important downstream amplifier of both FcεR1 signals and a transducer of c-Kit degranulation signals.20 To verify that Kit-L stimulation causes hyperactivation of PI3K in Nf1+/− mast cells, AKT phosphorylation at Ser-473 was evaluated as a measure of PI3K activity. As shown in Figure 2A, Nf1+/− mast cells have increased AKT Ser-473 phosphorylation at 5 minutes after Kit-L stimulation compared with WT cells. Furthermore, mast cells treated with the PI3K inhibitor Ly294002 before Kit-L stimulation had reduced AKT Ser-473 phosphorylation. As an initial test to examine whether the c-Kit-mediated increase in degranulation by Nf1+/− mast cells is PI3K-dependent, the cells were incubated with Ly294002 in a similar manner and stimulated with Kit-L/DNP to induce degranulation as above. The addition of the PI3K inhibitor was sufficient to produce significant inhibition of degranulation in WT and Nf1+/− mast cells after c-Kit stimulation, demonstrating a crucial role for PI3K in mediating this phenotype (Figure 2B).
Figure 2.
Effect of a pharmacological PI3K inhibitor on Nf1+/− mast cell degranulation. A: WT or Nf1+/− mast cells were incubated with or without Ly294002 for the time indicated and then were stimulated with Kit-L. AKT phosphorylation at Ser-473 was examined by Western blot. Data are representative of one of three independent experiments using different primary cell lines. B: WT and Nf1+/− mast cells were incubated in the absence or presence of Ly294002 and stimulated with Kit-L/DNP for 15 minutes. β-Hexosaminidase release was examined to determine degranulation. Results are representative of one of four independent experiments performed in triplicate. *P < 0.01 for Kit-L-induced WT mast cell degranulation versus basal level. **P < 0.01 for Nf1+/− versus WT mast cell degranulation induced by Kit-L. ***P < 0.01 for WT or Nf1+/− mast cell degranulation mediated by Kit-L with versus without Ly294002 inhibition.
Genetic Disruption of PI3K Signaling Pathway Reduces Kit-L-Mediated Gain of Function in Nf1+/− Mast Cells
Given the above observations with pharmacological inhibitors, we next sought to independently validate these results using mice containing a disruption in the regulatory subunit of class 1A PI3K (p85α−/−), resulting in the deletion of the p85α subunit but leaving the p55α and p50α isoforms intact,26 which leads to loss of PI3K signaling. Bone marrow-derived mast cells were generated from the F2 progeny of the intercross of these p85α−/− and Nf1+/− mice, and Kit-L-mediated AKT phosphorylation was measured. Analogous to the data of Figure 2A, abrogation of p85α reduces the hyperactivation of AKT at Ser-473 observed in Nf1+/− mast cells to below WT levels (Figure 3A). In a similar manner, genetic disruption of p85α in the context of Nf1 haploinsufficiency significantly decreased degranulation after stimulation with Kit-L/DNP, normalizing β-hexosaminidase release to WT levels (Figure 3B). Taken together, these data supply genetic evidence that PI3K activity is critical in mediating the increase in degranulation of Nf1+/− mast cells in response to Kit-L/DNP.
Figure 3.
Genetic disruption of p85α restores Kit-L-mediated gain of function in Nf1+/− mast cells to WT levels. A: Mast cells derived from WT, Nf1+/−, p85α−/−, and Nf1+/−;p85α−/− mice were stimulated with Kit-L, and Akt phosphorylation was examined by Western blot. Data are representative of one of three independent experiments using different primary cell lines. B: Mast cells derived from WT, Nf1+/−, p85α−/−, and Nf1+/−;p85α−/− mice were stimulated with Kit-L/DNP for 15 minutes, and mast cell degranulation was examined. Results are representative of one of four independent experiments performed in triplicate. *P < 0.01 for Nf1+/− versus WT mast cell degranulation induced by Kit-L. **P < 0.01 for p85α−/− versus WT mast cell degranulation induced by Kit-L. ***P < 0.01 for Nf1+/− versus Nf1+/−;p85α−/− mast cell degranulation induced by Kit-L.
Genetic Disruption of PI3K Signaling Restored the Gain in Nf1+/− Mast Cell Functions in Vivo to WT Levels
We have previously demonstrated that Nf1+/− mice have increased numbers of cutaneous mast cells and increased numbers of mast cells in the peritoneal lavage fluid at baseline and in response to Kit-L administration.20 To determine whether genetic disruption of class1A PI3K results in corrections of Kit-L-mediated mast cell gains in function in vivo, the frequency of mast cells in ear and peritoneal lavage fluid was evaluated using Nf1+/− and Nf1+/−;p85α−/− mice.
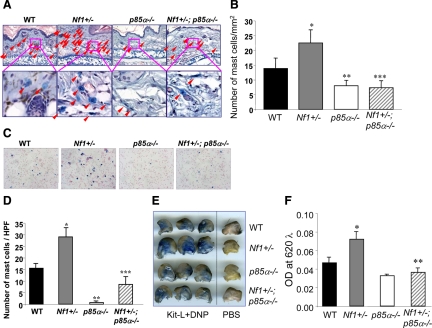
Representative Alcian Blue-stained histological sections from ears of four genotypes of mice are shown in Figure 4A. Consistent with previous studies, there was a 1.8-fold increase in the number of tissue mast cells in ears from Nf1+/− mice compared with that in WT controls (Figure 4B). A significant reduction in the number of mast cells in the ears of p85α−/− mice was observed compared with that in the ears of WT mice (Figure 4B). Furthermore, loss of PI3K signaling in Nf1+/−;p85α−/− mice dramatically reduced the number of mast cells in the ears of these animals (Figure 4B).
Figure 4.
Effect of genetic inactivation of p85α on mast cell numbers in vivo. A and B: Ear sections were stained with Alcian Blue, and mast cells were quantitated in a blinded fashion by counting 1-mm2 sections. Mast cells are identified by arrowheads. *P < 0.01 comparing Nf1+/− versus WT mice. **P < 0.01 for p85α−/− versus WT mice. ***P < 0.01 for Nf1+/− versus Nf1+/−;p85α−/− mice. C and D: Representative cytospins from peritoneal lavages stained for mast cells from individual mice of the four Nf1 and p85α genotypes. Peritoneal cells were stained with toluidine blue to quantify the total number of mast cells per peritoneal lavage. *P < 0.01 for Nf1+/− versus WT mice. **P < 0.01 for p85α−/− versus WT mice. ***P < 0.01 for Nf1+/− versus Nf1+/−;p85α−/− mice. E and F: Genetic disruption of p85α diminishes PCA in vivo. WT, Nf1+/−, p85α−/−, and Nf1+/−;p85α−/− mice were sensitized by intradermal injection of anti-DNP IgE (1:44 dilution, 1 μg/ml) into the right ear (20 μl/injection) and PBS (20 μl/injection) into the left ear. After 20 hours, mice were challenged by intravenous injection of antigen (DNP-HSA) and Kit-L along with Evans blue injection. Photographs of representative IgE-primed (left) and control (right) ears 20 minutes after antigen/Kit-L challenge are shown qualitatively. From each ear, Evans blue was extracted, and the intensity of the dye was measured by absorption at 620 nm. *P < 0.01 comparing Nf1+/− versus WT mice. **P < 0.01 for Nf1+/− versus Nf1+/−;p85α−/− mice.
Furthermore, a similar result was found in peritoneal lavage as shown in Figure 4, C and D, in which an increased number of mast cells was observed in the peritoneum of Nf1+/− mice compared with that in WT mice, consistent with previous studies.21 However, we found that loss of p85α corrects this phenotype by significantly reducing the number of peritoneal mast cells in Nf1+/−;p85α−/− mice (Figure 4D).
To determine whether our in vitro degranulation findings are relevant in a more physiological system, we used a previously described passive cutaneous anaphylaxis model8 to investigate the role of PI3K in regulating Kit-L-dependent mast cell functions. passive cutaneous anaphylaxis produces a profound localized allergic reaction triggered by administration of Kit-L in conjunction with allergen-induced cross-linking of FcεRI. The ears of the mice are first sensitized by intradermal injection of monoclonal anti-DNP IgE. Twenty hours after cutaneous sensitization, degranulation was induced by systemic injection of Kit-L and DNP with Evans blue dye. After 20 minutes, the degranulation response was quantified by measuring extravasation of Evans blue dye into the tissue. This extravasation process is reflective of increased local vascular permeability, a process dependent on mast cell release of histamine and serotonin after degranulation. Representative photographs from treated and untreated ears 20 minutes after stimulation are shown in Figure 4E to illustrate the extravasation of Evans blue caused by Kit-L and DNP. A 1.5-fold increase in extravasation was observed in the ears of Nf1+/− mice after Kit-L/DNP treatment compared with that in WT mice (Figure 4F), indicating increased mast cell degranulation in these animals. Of importance, Nf1+/−;p85α−/− mice had significant reductions in Evans blue extravasation compared with Nf1+/− mice, providing in vivo support to the hypothesis that Kit-L-mediated hyperactivation of PI3K has a key role in modulating the excessive degranulation in Nf1+/− mast cells.
Discussion
Degranulating mast cells are found at a 10-fold increase in concentration in neurofibromas compared with adjacent areas of unaffected skin27,28,29 and are found in close association with Schwann cells, fibroblasts, and blood vessels. The physical proximity of mast cells with these tumor components, together with observations that many factors found in mast cell granules are angiogenic or can alter the extracellular matrix, has led to the hypothesis that mast cell degranulation may promote tumor progression.30,31,32 This hypothesis is supported by reports that describe neurofibromas as being highly neoangiogenic with a large amount of extracellular matrix.9
Previously, we have demonstrated that Nf1−/− Schwann cells secrete increased amounts of proteins that have been linked to the elevated level of Nf1+/− mast cell degranulation compared with WT Schwann cells, including Kit-L.9,33 Further, both primary murine Nf1−/− Schwann cells and Schwann cell lines from patients with NF1 secrete high concentrations of Kit-L.8 Despite the diversity of proteins secreted by Nf1−/− Schwann cells, we provide here genetic and pharmacological lines of evidence to demonstrate that Kit-L is the predominant growth factor in these conditioned media that promotes degranulation of Nf1+/− mast cells. These in vitro studies are intriguing, given previous studies demonstrating that Kit-L transcripts are increased in neurofibromas34 and Kit-L is found in increased concentrations in serum from patients with NF1 9.
Having identified Kit-L as the major paracrine mediator of mast cell degranulation secreted by Nf1−/− Schwann cells, we next designed experiments to identify the signaling networks downstream of c-Kit responsible for increased degranulation of Nf1+/− mast cells. An important priority in studying Nf1+/− cells is the identification of specific Ras effector pathways, which are responsible for functional aberrations. Although we have previously shown that multiple Ras effector pathways are altered in Nf1+/− mast cells in response to Kit-L,9,35,36 in this report we provide biochemical, pharmacological, and genetic evidence that c-Kit-mediated hyperactivation of the class 1A PI3K pathway is specifically responsible for the increased degranulation of Nf1+/− mast cells. We show that disruption of the p85 subunit of PI3K results in corrections in mast cell accumulation in tissue and used the passive cutaneous anaphylaxis assay as a model of in vivo degranulation to validate the fact that the c-Kit/PI3K pathway regulates this phenotype. This is an important observation because we have previously shown that increased activation of this signaling pathway is also responsible for the increased proliferation and survival of Nf1+/− mast cells in vivo.35 Thus, the recruitment, expansion, and now degranulation of mast cells within neurofibromas seem to be mediated via a common cytokine (Kit-L) and a specific Ras effector (PI3K).
In previous studies we found that Nf1+/− mast cell progenitors have increased survival, proliferation, and migration in response to recombinant Kit-L.7 We have recently published studies demonstrating that Nf1+/−- and c-Kit-dependent bone marrow is necessary for formation and progression of plexiform neurofibromas in the context of Nf1−/− Schwann cells, which have previously been shown to be the tumorigenic cells. Nf1−/− Schwann cells do not form tumors in the context of WT bone marrow or mast cell-depleted Nf1+/−;Wv/Wv bone marrow, but do form tumors in the context of heterozygous Nf1 bone marrow, highlighting the contribution of mast cells in tumor formation. Further, we have demonstrated that treatment with imatinib mesylate, a known inhibitor of Kit-L/c-Kit signaling, reduced the size and metabolic activity of tumors in an in vivo murine model. In the current study, we have identified increased mast cell degranulation resulting from c-Kit-PI3K hyperactivation as a potential mechanism by which imatinib mesylate exerts its tumor-suppressive activity. The novel observation described here that the increased release of tumor-modulating mediators is due to c-Kit-PI3K hyperactivation provides additional rationale for focusing on this pathway as a target for other rational therapeutic agents. Collectively, these studies show that the increase in Nf1+/− mast cell degranulation in response to Kit-L is mediated by class 1A PI3K, and this signaling network is deserving of future studies as a target for neurofibroma therapies. Based on the findings reported here, we are developing murine models using conditional Nf1 knockout mice with additional disruption at the p85α locus to test whether genetic ablation of the c-Kit/PI3K axis is sufficient to prevent the development of plexiform neurofibromas.
Acknowledgments
We thank Susan Stanley for administrative support.
Footnotes
Address reprint requests to Feng-Chun Yang, M.D., Ph.D., Indiana University School of Medicine, Cancer Research Institute, 1044 W. Walnut St., R4/427, Indianapolis, IN 46202. E-mail: fyang@iupui.edu.
Supported by the Department of Defense (NF043032 and NF073112 to F.-C.Y.) and National Institutes of Health (grant R01-CA74177-06 to D.W.C.).
S.C. and S.B. contributed equally to this work.
The authors declare no competing interests.
Current address of W.K.: Vanderbilt University Department of Medicine, Nashville, TN.
References
- Viskochil D, Buchberg AM, Xu G, Cawthon RM, Stevens J, Wolff RK, Culver M, Carey JC, Copeland NG, Jenkins NA. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187–192. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, Saulino AM, Fountain JW, Brereton A, Nicholson J, Mitchell AL. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Shapiro SD, Soloway PD, Werb Z. Models for gain-of-function and loss-of-function of MMPs. Transgenic and gene targeted mice. Methods Mol Biol. 2001;151:149–179. doi: 10.1385/1-59259-046-2:149. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FC, Ingram DA, Chen S, Zhu Y, Yuan J, Li X, Yang X, Knowles S, Horn W, Li Y, Zhang S, Yang Y, Vakili ST, Yu M, Burns D, Robertson K, Hutchins G, Parada LF, Clapp DW. Nf1-dependent tumors require a microenvironment containing Nf1+/− and c-kit-dependent bone marrow. Cell. 2008;135:437–448. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S, Nomura S, Asada H, Ito A, Morii E, Kitamura Y. Possible involvement of c-kit receptor and its ligand in increase of mast cells in neurofibroma tissues. Arch Pathol Lab Med. 1993;117:996–999. [PubMed] [Google Scholar]
- Yang FC, Ingram DA, Chen S, Hingtgen CM, Ratner N, Monk KR, Clegg T, White H, Mead L, Wenning MJ, Williams DA, Kapur R, Atkinson SJ, Clapp DW. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for Nf1+/− mast cells. J Clin Invest. 2003;112:1851–1861. doi: 10.1172/JCI19195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nat Genet. 1994;7:353–361. doi: 10.1038/ng0794-353. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Vik TA, Ryder JW, Srour EF, Jacks T, Shannon K, Clapp DW. Nf1 regulates hematopoietic progenitor cell growth and ras signaling in response to multiple cytokines. J Exp Med. 1998;187:1893–902. doi: 10.1084/jem.187.11.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneline LS, White H, Yang FC, Chen S, Orschell C, Kapur R, Ingram DA. Genetic reduction of class IA PI-3 kinase activity alters fetal hematopoiesis and competitive repopulating ability of hematopoietic stem cells in vivo. Blood. 2006;107:1375–1382. doi: 10.1182/blood-2005-05-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munugalavadla V, Borneo J, Ingram DA, Kapur R. p85α subunit of class IA PI-3 kinase is crucial for macrophage growth and migration. Blood. 2005;106:103–109. doi: 10.1182/blood-2004-10-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf WF, White H, Wenning MJ, Orazi A, Kapur R, Ingram DA. K-Ras is essential for normal fetal liver erythropoiesis. Blood. 2005;105:3538–3541. doi: 10.1182/blood-2004-05-2021. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Snapper SB, Yballe CM, Alt FW, Cantley LC. Phosphoinositide 3-kinase knockout mice: role of p85α in B cell development and proliferation. Biochem Soc Trans. 1999;27:624–629. doi: 10.1042/bst0270624. [DOI] [PubMed] [Google Scholar]
- Ueki K, Fruman DA, Yballe CM, Fasshauer M, Klein J, Asano T, Cantley LC, Kahn CR. Positive and negative roles of p85α and p85β regulatory subunits of phosphoinositide 3-kinase in insulin signaling. J Biol Chem. 2003;278:48453–48466. doi: 10.1074/jbc.M305602200. [DOI] [PubMed] [Google Scholar]
- Yang FC, Kapur R, King AJ, Tao W, Kim C, Borneo J, Breese R, Marshall M, Dinauer MC, Williams DA. Rac2 stimulates Akt activation affecting BAD/Bcl-XL expression while mediating survival and actin function in primary mast cells. Immunity. 2000;12:557–568. doi: 10.1016/s1074-7613(00)80207-1. [DOI] [PubMed] [Google Scholar]
- Valchanov KP, Proctor GB. Enzyme histochemistry of tryptase in stomach mucosal mast cells of the mouse. J Histochem Cytochem. 1999;47:617–622. doi: 10.1177/002215549904700504. [DOI] [PubMed] [Google Scholar]
- Wu JN, Jordan MS, Silverman MA, Peterson EJ, Koretzky GA. Differential requirement for adapter proteins Src homology 2 domain-containing leukocyte phosphoprotein of 76 kDa and adhesion- and degranulation-promoting adapter protein in FcεRI signaling and mast cell function. J Immunol. 2004;172:6768–6774. doi: 10.4049/jimmunol.172.11.6768. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Yang FC, Travers JB, Wenning MJ, Hiatt K, New S, Hood A, Shannon K, Williams DA, Clapp DW. Genetic and biochemical evidence that haploinsufficiency of the Nf1 tumor suppressor gene modulates melanocyte and mast cell fates in vivo. J Exp Med. 2000;191:181–188. doi: 10.1084/jem.191.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JD, Jaffer ZM, Park SJ, Burgin S, Hofmann C, Sells MA, Chen S, Derr-Yellin E, Michels EG, McDaniel A, Bessler WK, Ingram DA, Atkinson SJ, Travers JB, Chernoff J, Clapp DW. p21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics. Blood. 2009;113:2695–2705. doi: 10.1182/blood-2008-06-160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker SA, Caldwell KK, Hall A, Martinez AM, Pfeiffer JR, Oliver JM, Wilson BS. Wortmannin blocks lipid and protein kinase activities associated with PI 3-kinase and inhibits a subset of responses induced by FcεR1 cross-linking. Mol Biol Cell. 1995;6:1145–1158. doi: 10.1091/mbc.6.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosseller K, Stella G, Yee NS, Besmer P. c-kit receptor signaling through its phosphatidylinositide-3′-kinase-binding site and protein kinase C: role in mast cell enhancement of degranulation, adhesion, and membrane ruffling. Mol Biol Cell. 1997;8:909–922. doi: 10.1091/mbc.8.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendl GG, Prieschl EE, Thumb W, Harrer NE, Auer M, Baumruker T. Effects of phosphatidylinositol-3-kinase inhibitors on degranulation and gene induction in allergically triggered mouse mast cells. Int Arch Allergy Immunol. 1997;112:392–399. doi: 10.1159/000237486. [DOI] [PubMed] [Google Scholar]
- Khalaf WF, Yang FC, Chen S, White H, Bessler W, Ingram DA, Clapp DW. K-ras is critical for modulating multiple c-kit-mediated cellular functions in wild-type and Nf1+/− mast cells. J Immunol. 2007;178:2527–2534. doi: 10.4049/jimmunol.178.4.2527. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Terauchi Y, Fujiwara M, Aizawa S, Yazaki Y, Kadowaki T, Koyasu S. Xid-like immunodeficiency in mice with disruption of the p85α subunit of phosphoinositide 3-kinase. Science. 1999;283:390–392. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammatory cells and cancer: think different! J Exp Med. 2001;193:F23–F26. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashkin P, Razin E, Eldor A, Vlodavsky I. Degranulating mast cells secrete an endoglycosidase that degrades heparan sulfate in subendothelial extracellular matrix. Blood. 1990;75:2204–2212. [PubMed] [Google Scholar]
- Clinton M, Long WF, Williamson FB, Duncan JI, Thompson WD. Effect of the mast cell activator compound 48/80 and heparin on angiogenesis in the chick chorioallantoic membrane. Int Microcirc Clin Exp. 1988;7:315–326. [PubMed] [Google Scholar]
- Ribatti D, Finato N, Crivellato E, Marzullo A, Mangieri D, Nico B, Vacca J, Beltrami CA A. Neovascularization and mast cells with tryptase activity increase simultaneously with pathologic progression in human endometrial cancer. Am J Obstet Gynecol. 2005;193:1961–1965. doi: 10.1016/j.ajog.2005.04.055. [DOI] [PubMed] [Google Scholar]
- Dang I, Nelson JK, DeVries GH. c-Kit receptor expression in normal human Schwann cells and Schwann cell lines derived from neurofibromatosis type 1 tumors. J Neurosci Res. 2005;82:465–471. doi: 10.1002/jnr.20648. [DOI] [PubMed] [Google Scholar]
- Mashour GA, Driever PH, Hartmann M, Drissel SN, Zhang T, Scharf B, Felderhoff-Muser U, Sakuma S, Friedrich RE, Martuza RL, Mautner VF, Kurtz A. Circulating growth factor levels are associated with tumorigenesis in neurofibromatosis type 1. Clin Cancer Res. 2004;10:5677–5683. doi: 10.1158/1078-0432.CCR-03-0769. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Hiatt K, King AJ, Fisher L, Shivakumar R, Derstine C, Wenning MJ, Diaz B, Travers JB, Hood A, Marshall M, Williams DA, Clapp DW. Hyperactivation of p21ras and the hematopoietic-specific Rho GTPase. Rac2, cooperate to alter the proliferation of neurofibromin-deficient mast cells in vivo and in vitro. J Exp Med. 2001;194:57–69. doi: 10.1084/jem.194.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel AS, Allen JD, Park SJ, Jaffer ZM, Michels EG, Burgin SJ, Chen S, Bessler WK, Hofmann C, Ingram DA, Chernoff J, Clapp DW. (2008). Pak1 regulates multiple c-Kit mediated Ras-MAPK gain-in-function phenotypes in Nf1+/− mast cells. Blood. 2008;112:4646–4654. doi: 10.1182/blood-2008-04-155085. [DOI] [PMC free article] [PubMed] [Google Scholar]