Abstract
The endoplasmic reticulum (ER) is abundant in the acinar cells of the exocrine pancreas. To test the role of ER homeostasis in acute pancreatitis, we manipulated GRP78 levels, a major ER chaperone, in mice. Grp78+/+ and +/− littermates were fed either a regular diet (RD) or a high-fat diet. Acinar cells were examined for ER structure by electron microscopy, and ER chaperone levels were assessed by immunoblotting. Pancreatitis was induced by cerulein injection, and multiple pathological parameters were analyzed. Grp78+/− mice showed decreased GRP78 expression in acinar cells. Exocrine pancreata of RD-fed Grp78+/− mice in an outbred C57BL/6 × 129/sv genetic background exhibited ER lumen dilation, a reduction in chaperones calnexin (CNX) and calreticulin (CRT), and exacerbated pancreatitis associated with high CHOP induction. With the high-fat diet regimen, Grp78 heterozygosity triggered GRP94 up-regulation and restoration of GRP78, CNX, and CRT to wild-type levels, corresponding with mitigated pancreatitis on cerulein insult. Interestingly, after backcrossing into the C57BL/6 background, RD-fed Grp78+/− mice exhibited an increase in GRP94 and levels of CNX and CRT equivalent to wild type, associated with decreased experimental pancreatitis severity. Administration of a chemical chaperone, 4-phenolbutyrate, was protective against cerulein-induced death. Thus, in exocrine pancreata, Grp78 heterozygosity regulates ER chaperone balance, in dietary- and genetic background–dependent manners, and improved ER protein folding capacity might be protective against pancreatitis.
The exocrine pancreas is highly specialized for production and secretion of digestive enzymes. In response to meal stimulation, acinar cells of the exocrine pancreas exhibit the highest protein synthesis rate among human tissues.1 Consistent with this cellular function, acinar cells are morphologically characterized by abundant endoplasmic reticulum (ER). The normal function of exocrine pancreas depends on balance between protein load and folding capacity of the ER.2,3 Protein folding in ER is mediated by multiple chaperoning systems. The 78-kilodalton glucose regulated protein (GRP78), also known as BiP (immunoglobulin heavy chain binding protein), is a general chaperone recognizing the unfolded proteins by its hydrophobic residues.3,4,5 GRP94, another major ER chaperone, forms a large complex with GRP78 and other chaperones to process nascent peptides.5,6,7 Calnexin (CNX) and calreticulin (CRT) are lectin chaperones, composing a system specifically targeting proteins with monoglucosylated N-glycan for subsequent assembly.5,8
Acute pancreatitis is pathologically characterized by inflammation, edema, and cell necrosis of exocrine pancreas. ATP depletion and premature activation of digestive enzymes contribute to necrosis of pancreatic acinar cells.9 To study the pathogenesis of pancreatitis, multiple experimental models for pancreatitis have been established in rodent. Administration of cerulein, a cholecystokinin analogue, leads to activation of digestive proenzymes, severe acinar cell necrosis, and inflammation in mouse.9 Recently, activation of ER stress signaling was observed in pancreas of rats treated with secretagogues or following arginine-induced acute pancreatitis.10,11 ER stress caused by misfolding of mutant digestive zymogens has been linked to hereditary chronic pancreatitis in human.12 However, the pathophysiological role of ER stress in the pathogenesis of acute pancreatitis remains unknown.
Not only is GRP78 a major molecular chaperone, it is also a quencher of ER stress signaling transducers under non-stress status.3 GRP78 also maintains ER homeostasis by targeting misfolded proteins to ER-associated protein degradation, and ER Ca2+ storage by serving as a Ca2+ binding protein.13 In a Grp78 heterozygous mouse model with C57BL/6 × 129/sv genetic background, we observed abnormal ER morphology and down-regulated ER chaperones in exocrine pancreas, associated with an exacerbated experimental pancreatitis response. Interestingly, after high-fat diet (HFD) regimen, ER structure as well as chaperone levels were restored in Grp78+/− acinar cells, which correlated with improvement in experimental pancreatitis. Fortuitously, we discovered that Grp78+/− mice backcrossed into the C57BL/6 background also exhibited improved ER chaperone profile in pancreas, and no greater severity of experimental pancreatitis compared to wild-type littermates. Furthermore, we showed that administration of 4-phenolbutyrate (4-PBA), a chemical chaperone assisting protein folding,14 protected against cerulein-induced acinar cell death. Taken together, our studies suggest a protective role of ER chaperone balance against pancreatitis.
Materials and Methods
Animals
The Grp78+/− mice used in this study were generated as previously described15 and maintained in C57BL/6 × 129/sv background through sibling mating, unless indicated. Mice were maintained under a 12-hour light–dark cycle with ad libitum access to water and food. Mice were fed on regular diet (RD) (11% fat by calories, Harlan Teklad, Indianapolis, IN) continuously after weaning (at about 3 weeks old), or changed to HFD (45% fat by calories, Research Diets, New Brunswick, NJ) at 10 weeks old. Only male mice were used in this study. Mouse body weight was measured after overnight fasting. Food intake was analyzed by daily food mass measurement for five successive days, during the 10th week of HFD regimen. Mouse stool was collected during the 20th week of HFD regimen and processed to Oil Red O staining for lipids as described.16 The Grp78+/− mouse strain was also backcrossed into C57BL/6 genetic background for seven generations and analyzed for ER chaperone levels in pancreas and severity of experimental pancreatitis. All protocols for animal use and euthanasia were reviewed and approved by the University of Southern California Institutional Animal Care and Use Committee.
Tissue Processing
After sacrifice by cervical dislocation following CO2 anesthesia, mouse tissues were immediately frozen in liquid nitrogen and stored at −80°C for immunoblotting or fixed in 10% formalin for paraffin sections. For GRP78 immunofluorescence, pancreas was fixed for 2 hours with 4% paraformaldehyde, and tissue pieces were equilibrated for 2 hours at 4°C in a 15% sucrose-phosphate buffer solution and then embedded in OCT compound (Miles, Elkhart, IN).
Islet Isolation
After sacrifice by cervical dislocation following CO2 anesthesia, mouse pancreas was digested by injection of Liberase RI (Roche, Indianapolis, IN) and DNase (Roche) via the pancreatic duct,17 followed by incubation at 37°C for 25 minutes. Islets of Langerhans were hand-picked under a light microscope.
Immunoblotting Analysis
Tissues were homogenized in ice-cold radio-immunoprecipitation assay (RIPA) buffer18 containing cocktails of proteinase inhibitors (Roche) and phosphatase inhibitors (Roche) with a Dounce homogenizer (Wheaton, Millville, NJ), followed by centrifugation at 13000 × g at 4°C for 15 minutes. The Western blotting was performed as described previously.19 Antibodies used included GRP78 (C20), CHOP, GADD34, XBP-1, EDEM, GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA); KDEL, GRP94, calnexin, calreticulin (Stressgen, Ann Arbor, MI); pSer51-eIF2α, eIF2α (Cell Signaling, Danvers, MA); amylase (Calbiochem, Whitehouse Station, NJ); trypsin (Chemicon, Temecula, CA); and β-actin (Sigma-Aldrich, St. Louis, MO). For each experimental condition, tissue samples from three or more animals were examined. The Western blotting procedure was repeated two to six times.
Histological Analysis, Immunohistochemistry, Immunofluorescence, and TUNEL Assay
Formalin-fixed pancreas sections were embedded in paraffin. Five-micrometer sections were stained with hematoxylin and eosin (H&E) for assessment of general morphology. PCNA (PC10) and GADD153 (B-3) primary antibodies (Santa Cruz Biotechnology) were used for PCNA immunofluorescence and CHOP immunohistochemistry, respectively. Insulin and glucagon primary antibodies (Signet Labs, Dedham, MA) were also used for immunohistochemistry. Staining procedure was as previously described.20 For GRP78 immunofluorescence analysis, 5-μm frozen pancreatic tissue sections were fixed in 0.1 M phosphate buffer containing 4% paraformaldehyde, washed several times in phosphate-buffered saline (PBS) supplemented with 0.5% saponin, 0.2% Tween-20, and blocked with 2% donkey serum. Tissue sections were then incubated overnight with GRP78 (C-20) primary antibody (Santa Cruz Biotechnology) followed by incubation with secondary antibody conjugated with FITC (Jackson ImmunoResearch Laboratories, West Grove, PA). Images were visualized using the Zeiss LSM510 laser scanning confocal microscope with a ×63 objective. TUNEL assay was performed on paraffin sections using the cell death detection kit (Roche), and counted in an average of 25 fields by the Quantity One software (Bio-Rad, Hercules, CA).
Transmission Electron Microscopy
The procedure was similar with that previously described.20 Pancreas tissues were fixed in half-strength Karnovsky’s fixative (final concentrations: 2% paraformaldehyde, 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4) overnight at 4°C, then post fixed in 1% osmium tetroxide for 2 hours on ice. Samples were dehydrated in a graded ethanol series and infiltrated with Eponate resin (Ted Pella Inc., Redding, CA). Ultrathin sections were cut at 70 nm and stained with uranyl acetate and lead citrate. Sections were examined on a JEM 2100 electron microscope (JEOL, Peabody, MA) and photographed with the Orius SC1000B digital camera (Gatan, Pleasanton, CA).
Experimental Pancreatitis
Mice were subjected to seven hourly intraperitoneal (IP) injections of cerulein (Sigma-Aldrich) at a dosage of 50 μg/kg body weight per injection. Mice subjected to comparable injections of PBS served as controls. One hour after the last injection, mice were sacrificed and collected for tissues.
Quantitation of Neutrophil Infiltration, Edema, and Necrosis
H&E-stained pancreas sections were examined by Nikon Eclipse TE2000-S inverted phase microscope (Nikon Corp., Tokyo, Japan). Neutrophils were identified by their histological characteristics as described previously.21 The number of infiltrating neutrophils was obtained by counting the neutrophils at ×40 magnification in an average of 50 fields covering at least 1000 acinar cells. For each animal, neutrophil numbers were expressed as a percentage of acinar cells. For quantitation of edema, the percentage area of non-parenchymal space was analyzed in an average of 50 fields, using the ImageJ software (National Institutes of Health, Bethesda, MD). Quantification of necrosis was performed as described previously.22 Cells with swollen cytoplasm, loss of plasma membrane integrity and leakage of organelles into interstitium were considered necrotic. A total of at least 1000 acinar cells were counted on pancreatic tissue sections from each animal.
Serum Amylase and Lipase
Serum amylase and lipase levels were measured in a Hitachi 707 analyzer (Antech Diagnostics, Irvine, CA), as described previously.22
Cell Culture and Treatment Conditions
NIH3T3 cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and antibiotics. To induce ER stress, cells were treated with 300 nmol/L thapsigargin (Sigma-Aldrich) for 16 hours. Rat exocrine pancreas cell line AR42J was cultured in F-12K medium supplied with 20% fetal bovine serum and antibiotics. After administration with cerulein (Sigma-Aldrich) and/or sodium 4-phenolbutyrate (Calbiochem), cells were subjected to trypan blue exclusion assay for cell viability.23
Statistical Analysis
Two-tailed Student’s t-test was applied for all pairwise comparisons.
Results
ER Lumen Dilation and Chaperone Reduction in Grp78+/− Exocrine Pancreas
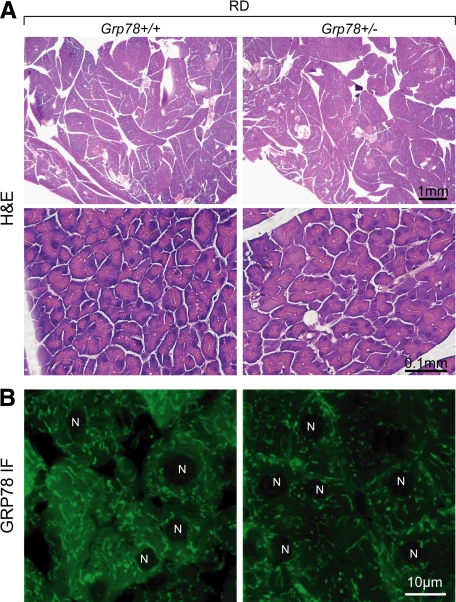
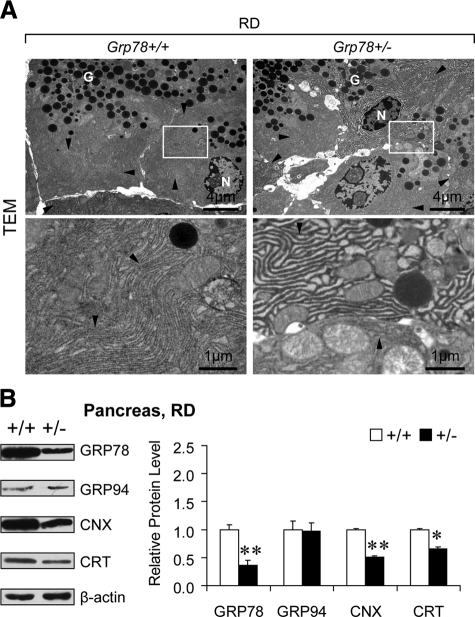
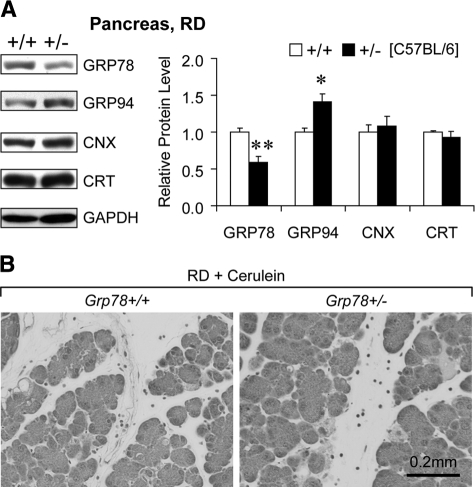
Consistent with the normal growth and organ development in the heterozygous Grp78 (Grp78+/−) mice reported previously,24 their general pancreatic morphology was comparable with that of the wild-type (Grp78+/+) mice, as revealed by H&E staining (Figure 1A). Confocal microscopy of immunofluorescence staining revealed the reduction of GRP78 in the exocrine pancreas (Figure 1B). As professional cells in protein production and secretion,25 pancreatic acinar cells might have specific demands on GRP78, the major chaperone and master regulator of ER homeostasis.3 To investigate whether decreased level of GRP78 would lead to changes in subcellular organelles in pancreatic acinar cells, transmission electron microscopy (TEM) was used to examine the organelle structure and organization (Figure 2A). In contrast to the tightly packed ER in wild-type (0 with dilated ER out of 35 cells from 10 random fields), notable dilation of ER lumen was observed in ∼35% (12 out of 34 cells from 10 random fields) of Grp78+/− acinar cells, suggesting GRP78 mediates maintenance of normal ER structure. There was no apparent difference in number or appearance of secretory granules between the two genotypes.
Figure 1.
Reduction of GRP78 protein levels in pancreatic acinar cells of adult Grp78+/− mice. A: Representative H&E staining of pancreatic sections from 7-month-old Grp78+/+ and +/− mice fed regular diet (RD, n ≥ 3 for each genotype). B: Confocal microscopy of GRP78 (green) immunofluorescence (IF) on pancreatic acinar cells. N, nucleus. n = 2 (+/+) or 4 (+/−).
Figure 2.
Dilated ER lumen and ER chaperone reduction in Grp78+/− exocrine pancreas. A: Representative transmission electron micrographs (TEM) of pancreatic acinar cells of 6-month-old Grp78+/+ and +/− mice fed RD (n = 2 for each genotype). Lower panels demonstrate higher magnification of the boxed area of the corresponding upper panels. Arrowheads indicate endoplasmic reticulum (ER). N, nucleus; G, secretory granules. B: Protein levels of ER chaperones GRP78, GRP94, calnexin (CNX), and calreticulin (CRT) were examined in pancreas from 7-month-old Grp78+/+ and +/− mice fed RD (n ≥ 3 for each genotype) by Western blotting. Left panel: representative blots. Right panel: quantitative levels normalized against β-actin. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01.
To address whether the abnormal ER structure associates with changes in ER homeostasis, we measured protein levels of ER chaperones, including GRP78, GRP94, calnexin (CNX), and calreticulin (CRT), in pancreas (Figure 2B). Grp78 heterozygosity led to notably reduction of not only GRP78 (by 63%, P = 0.008), but also CNX (by 49%, P = 0.004) and CRT (by 34%, P = 0.011). GRP94 level in Grp78+/− and +/+ pancreas was comparable (P = 0.91). Considering that endocrine islet cells represent only a few percentage of total pancreatic cells, these data inferred reduction of specific ER chaperones GRP78, CNX, and CRT in exocrine pancreas, which resulted from Grp78 heterozygosity. The depleted chaperones might correlate with the abnormal ER structure, representing disturbance in ER homeostasis.
Recovery of ER Structure and Chaperone Levels in Grp78+/− Exocrine Pancreas After High-Fat Diet
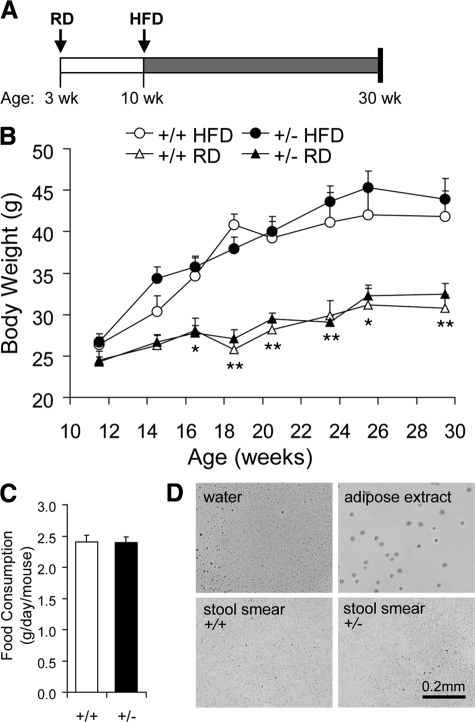
Previous studies revealed that high-fat diet (HFD) enhances the synthesis and secretion of digestive enzymes from exocrine pancreas.26,27 With reduced GRP78 level in the Grp78+/− mice, the increased demand on digestive enzyme synthesis in the pancreatic acinar cells might lead to mild chronic ER stress, which is known to trigger adaptive up-regulation of ER chaperones.28 To test this, Grp78+/+ and +/− male littermates were fed HFD (45% fat by calories) from 10 weeks old, for 20 weeks (Figure 3A). HFD regimen elevated body weight in both genotypes at a similar level (Figure 3B). Food intake of Grp78+/− mice was approximate to that of Grp78+/+ mice (Figure 3C). There was no evidence of fat malabsorption in these mice, as determined by Oil Red O staining of stool smears (Figure 3D).
Figure 3.
HFD-fed Grp78+/− mice gained similar weight as wild types. A: Scheme of high-fat diet (HFD) feeding. Cohorts of Grp78+/+ and +/− male littermates were fed a RD after weaned at 3 weeks of age, and switched to HFD feeding from 10 weeks of age. B: Fasting body weight. n ≥ 5 mice per condition. *P < 0.05, **P < 0.01 for HFD versus RD. C: Food intake measurement during the 10th week of HFD. n = 9 mice for each genotype. Data are presented as the mean ± SEM. D: Oil Red O staining of stool smear from mice during the 20th week of HFD. Negative control, dH2O; positive control, white adipose extract. n = 3 (+/+) or 4 (+/−).
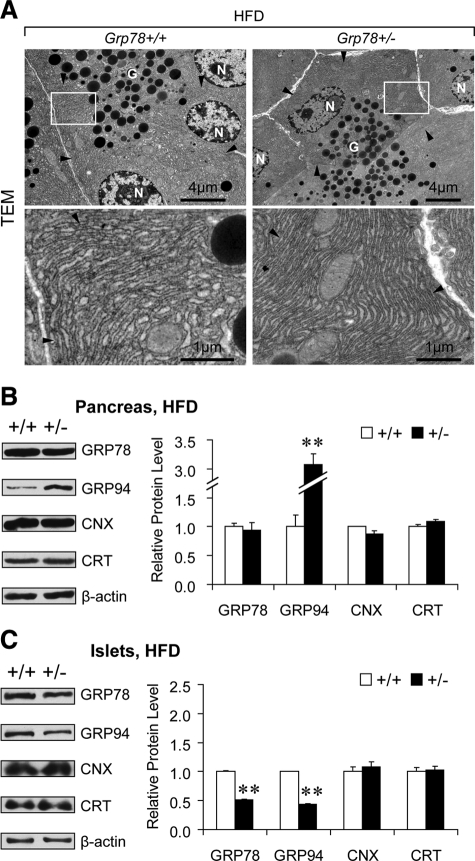
Strikingly, after HFD regimen, Grp78+/− mice exhibited well-organized, tightly-packed ER in pancreatic acinar cells, as revealed by TEM (Figure 4A). Noting the association between dilated ER lumen (Figure 2A) and reduced chaperones (Figure 2B) in exocrine pancreas of Grp78+/− mice fed RD (11% fat by calories), the ER chaperone levels in pancreas of the HFD-fed mice were examined (Figure 4B). Interestingly, despite the haploinsufficiency of Grp78 gene, GRP78 protein level in Grp78+/− pancreas was comparable with that in the Grp78+/+ mice (94%, P = 0.69), suggesting that HFD triggers recovery of GRP78 level in the pancreas of the heterozygous mice. In contrast to the unaltered GRP94, reduced CNX and CRT in the RD-fed mice (Figure 2B), HFD regimen led to up-regulation of GRP94 (3.1-fold, P = 0.002), as well as recovery of CNX (87%, P = 0.15) and CRT (110%, P = 0.15) in Grp78+/− pancreas in the levels comparable to the Grp78+/+ mice (Figure 4B). The restoration of the GRP78 level and up-regulation of GRP94 responses were mainly contributed by the exocrine pancreas, because the Grp78+/− islets showed reduced GRP78 (52%, P = 0.002) and GRP94 (43%, P = 0.001) but comparable levels of CNX (108%, P = 0.50) and CRT (102%, P = 0.82) with that of Grp78+/+ mice (Figure 4C). Collectively, these studies showed that GRP78 protein level is restored in Grp78+/− pancreas after chronic HFD, which is accompanied by the reinforcement of other ER chaperones and relative normal ER structure in pancreatic acinar cells. These findings further support the link between ER chaperone balance and ER structure maintenance.
Figure 4.
Recovery of ER morphology and chaperone levels in exocrine pancreas of Grp78+/− mice after HFD. A: Representative TEM of pancreatic acinar cells from mice after 12 weeks of HFD (n = 2 for each genotype). Lower panels demonstrate higher magnification of the boxed area of the corresponding upper panels. Arrowheads indicate ER. N, nucleus; G, secretory granules. B and C: Protein levels of ER chaperones GRP78, GRP94, calnexin (CNX), and calreticulin (CRT) were examined in whole pancreas (B) and isolated islets of Langerhans (C) from Grp78+/+ and +/− mice after 20 weeks of HFD (n ≥ 3 for each genotype) by Western blotting. Left panels: representative blots. Right panels: quantitative levels normalized against β-actin. Data are presented as the mean ± SEM. **P < 0.01.
Differential Response to Experimental Pancreatitis in Grp78+/− Mice Is Associated with ER Chaperone Balance in Pancreas
Toward further understanding of the role of ER homeostasis in pancreatitis, we used cerulein-induced acute pancreatitis as an experimental model. After seven hourly intraperitoneal (IP) injections of cerulein (50 μg/kg body weight), Grp78+/+ and +/− mice continuously fed RD, or after 20 weeks of HFD, were sacrificed for analyses (Figure 5A). Corresponding to the abnormal ER structure (Figure 2A) and decreased chaperone levels (Figure 2B) under physiological conditions, the exocrine pancreas from Grp78+/− mice showed greater morphological changes of pancreatitis compared to wild-type. There was conspicuous augment of cell necrosis, edema, and inflammation observed pancreas from Grp78+/− mice compared to wild-type mice (Figure 5B, left panels). Chaperone profile was improved (Figure 4B) and ER structure was reinstated (Figure 4A) in the pancreas of Grp78+/− mice after HFD regimen. Consistently, there was much less severe experimental pancreatitis observed in the HFD-fed Grp78+/− mice (Figure 5B, right panels) than in the RD-fed Grp78+/− mice.
Figure 5.
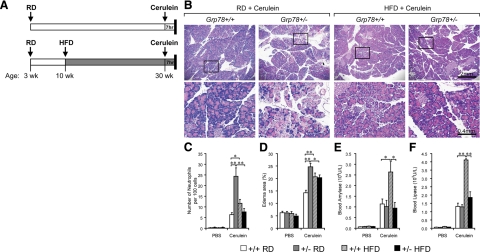
Diet-induced differential response to experimental pancreatitis in Grp78+/− mice. A: Scheme of cerulein-induced acute pancreatitis. Thirty-week-old Grp78+/+ and +/− male littermates, fed RD or HFD, were subjected to seven hourly IP injections of cerulein (50 μg/kg body weight), followed by sacrifice and analysis. B: Representative H&E staining of pancreatic sections from mice after cerulein injection (n ≥ 3 for each condition). Lower panels demonstrate higher magnification of the boxed area of the corresponding upper panels. C and D: H&E-stained pancreas sections were counted for neutrophils (C) or quantitated for area of edema represented by non-parenchymal space (D). E and F: Serum samples were assayed for amylase (E) and lipase (F). Data are presented as the mean ± SEM. n ≥ 3 mice for each condition. *P < 0.05, **P < 0.01.
To further characterize the severity of acute pancreatitis in cerulein-treated animals, infiltrated neutrophil counting (Figure 5C) was used as a key quantitative index of inflammation.9 Consistent with the morphological abnormalities (Figure 5B, left panels), there was a 3.8-fold increase (24 ± 4 vs. 6 ± 1) in neutrophilic infiltration observed in the pancreas of RD-fed Grp78+/− mice, in comparison to that from Grp78+/+ mice. After HFD, the pancreas from Grp78+/− mice showed fewer neutrophils than that from Grp78+/+ mice (8 ± 2 vs. 12 ± 2). When edema area was quantitated (Figure 5D), RD-fed Grp78+/− mice displayed a 73% increase compared with Grp78+/+ mice. After HFD, the edema area in the pancreas from Grp78+/− mice was comparable with that of Grp78+/+ mice, and 18% lower than that of the RD-fed Grp78+/− mice (P = 0.02). In the RD-fed group, blood amylase (Figure 5E) and lipase (Figure 5F) levels were similar between the two genotypes. In the HFD-fed group, amylase and lipase levels increased by 2.5- and fourfold respectively in the Grp78+/+ mice (P = 0.03 and 0.00001 respectively); whereas Grp78+/− mice displayed levels comparable to the RD-fed mice.
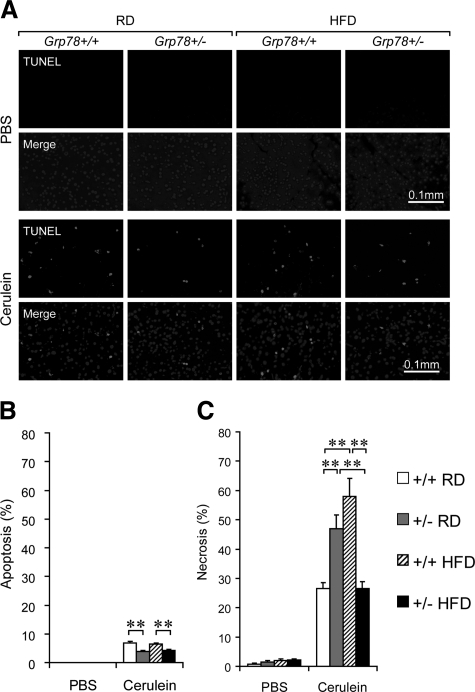
Next we determined the extent of cell death contributed by apoptosis and necrosis. TUNEL assays used for detection of apoptosis revealed that no apoptotic signal in PBS-treated mice, whereas after cerulein treatment, about 7% of Grp78+/+ acinar cells were apoptotic, in either RD-fed or HFD-fed mice, compared with about 4% apoptotic acinar cells in Grp78+/− mice, either RD-fed or HFD-fed (Figure 6, A and B). Histological examination was performed to detect necrosis. While less than 3% of the acinar cells were necrotic in PBS-treated mice, after cerulein treatment, about 27% of Grp78+/+ acinar cells, compared with 47% of the Grp78+/− mice (1.7-fold increase, P = 0.004), underwent necrosis (Figure 6C). After HFD, about 58% of Grp78+/+ acinar cells, compared to 27% of the Grp78+/− mice (2.1-fold decrease, P = 0.001) were necrotic (Figure 6C). PCNA immunofluorescence assay showed no apparent cell proliferation in the pancreas of all of the experimental groups (see Supplemental Figure S1, A and B at http://ajp.amjpathol.org). Furthermore, no difference in the level of pancreatic digestive enzymes (amylase and trypsinogen) was detected between the two genotypes (see Supplemental Figure S2A at http://ajp.amjpathol.org), and the endocrine production of insulin and glucagon was also similar (see Supplemental Figure S2B at http://ajp.amjpathol.org). Taken together, Grp78+/− mice exhibited exacerbated necrotizing pancreatitis, which was improved after HFD regimen, associating with recovery of chaperone levels (Figure 4B).
Figure 6.
Differential cell death in exocrine pancreas of Grp78+/− mice with experimental pancreatitis. The Grp78+/+ and +/− mice were fed with either RD or HFD and treated with either PBS or cerulein as indicated. A: TUNEL assay on pancreas sections. Representative fields showing TUNEL signal alone (upper panels) or merged with DAPI (lower panels). B: Apoptotic cells (TUNEL merged with DAPI) were counted as percentage of total cells (DAPI). C: Necrotic cells were counted on H&E-stained pancreas sections as percentage of total cells. Data are presented as the mean ± SEM. n ≥ 3 mice for each condition. **P < 0.01.
Serendipitously, we examined the chaperone levels in pancreas from RD-fed Grp78+/− mice backcrossed into pure C57BL/6 genetic background for seven generations. Although decreased GRP78 (59%, P = 0.002) was observed in these Grp78+/− mice, GRP94 was up-regulated (1.4-fold, P = 0.02) while CNX (108%, P = 0.7) and CRT (93%, P = 0.3) were comparable to those in the wild-type littermates (Figure 7A). Cerulein administration led to similar severity of pancreatitis between the two genotypes in C57BL/6 background mice (Figure 7B). These fortuitous findings suggest that the general ER chaperone balance, rather than GRP78 alone, contributes to the protection against experimental pancreatitis.
Figure 7.
Specific regulation of pancreatic ER chaperones and response to experimental pancreatitis in Grp78+/− mice backcrossed into a C57BL/6 background. A: Protein levels of ER chaperones were examined in pancreas from RD-fed 7-month-old Grp78+/+ and +/− littermates backcrossed into C57BL/6 background for seven generations (n ≥ 3 for each genotype) by Western blotting. Left panel: representative blots. Right panel: quantitative levels normalized against GAPDH. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01. B: Representative H&E staining of pancreatic sections from mice after cerulein injection (n ≥ 3 for each genotype).
Modulation of ER Stress Response by Cerulein and Diet in Pancreas of Grp78+/− Mice
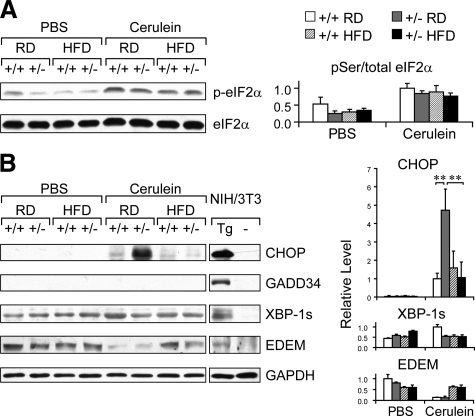
To evaluate the effect of cerulein treatment and diet on ER stress, pancreas lysates from Grp78+/+ and +/− mice (C57BL/6 × 129/sv background), fed RD or HFD, were prepared and subjected to immunoblot analysis of ER stress markers. First we examined the phosphorylation status of the translational initiation factor eIF2α. Compared to mice injected with PBS, cerulein treatment induced eIF2α phosphorylation, in both RD- and HFD-fed mice of both genotypes (Figure 8A). Cerulein treatment also induced the nuclear transcriptional factor CHOP and the spliced form of X-box binding protein (XBP-1s) in RD-fed Grp78+/+ mice (Figure 8B). Interestingly, in the cerulein-treated group, we observed a 4.7-fold increase (P = 0.00002) in CHOP level in the RD-fed Grp78+/− mice, compared with Grp78+/+ mice. After HFD, CHOP levels in both genotypes were reduced to that comparable to the RD-fed Grp78+/+ mice (Figure 8B). The differential levels of CHOP induction were further confirmed by immunostaining of pancreas sections (see Supplemental Figure S3 at http://ajp.amjpathol.org). On the other hand, the growth arrest and DNA damage inducible 34 protein (GADD34) was not detectable in mice of all of the experimental groups, although GADD34 induction was readily observed in thapsigargin-treated mouse fibroblast NIH3T3 cells (Figure 8B). For the ER degradation-enhancing mannosidase-like protein (EDEM), similar levels were observed for PBS-treated mice of both genotypes; after cerulein treatment, reduction in EDEM level was observed in the RD group but not in the HFD group (Figure 8B). Collectively, these results show that cerulein treatment induces ER stress in mouse pancreas, and that cerulein and diet exert differential effects on selective ER stress markers in the pancreas of Grp78+/− mice.
Figure 8.
Modulation of ER stress response by cerulein and diet in pancreas of Grp78+/− mice. Whole cell lysates of pancreas from Grp78+/+ and +/− mice, fed RD or HFD, after seven hourly PBS or cerulein injections (n ≥ 3 for each condition), were subjected to immunoblotting of the indicated ER stress response proteins: (A) pSer51- and total eIF2α, (B) CHOP, GADD34, spliced form of XBP-1 (XBP-1s), and EDEM, with GAPDH serving as loading control. NIH3T3 cells, either nontreated (−) or treated with 300 nmol/L thapsigargin for 16 hours (Tg) served as negative and positive controls, respectively. Left panels: representative Western blots. Lanes for the NIH3T3 samples were run on the same gel as the tissue samples but were noncontiguous. Right panels: quantitation of relative protein levels after normalization against GADPH levels. Data are presented as the mean ± SD. **P < 0.01.
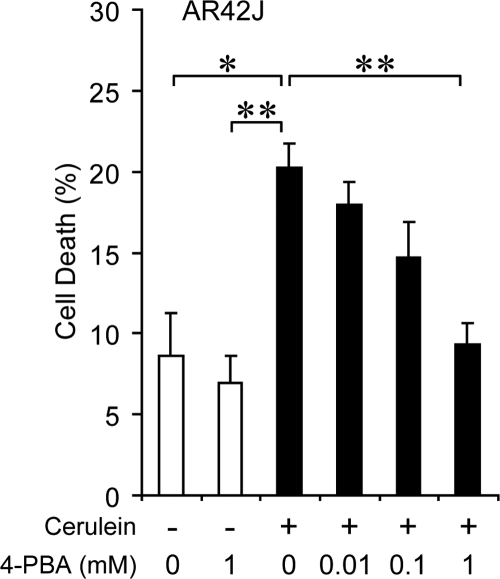
Protection Against Cerulein-Induced Cell Death by 4-PBA
Previous work has established that 4-PBA, a chemical chaperone, can assist protein folding and alleviate ER stress.14,29 To test whether improvement in protein folding capacity can attenuate the severity of acute pancreatitis, increasing doses of 4-PBA was administrated to AR42J, a differentiated rat pancreatic acinar cell line producing digestive enzymes and a well-established in vitro model for pancreatitis studies.30,31 The AR42J cells were treated with cerulein to induce acute pancreatitis. Treatment of 4-PBA alone did not affect cell viability, and as expected, cerulein treatment significantly induced cell death, which was suppressed by 4-PBA in a dose-dependent manner (Figure 9). These results directly support the protective role of ER protein folding capacity in cerulein-induced cell death in acinar cells.
Figure 9.
4-PBA protected against cerulein-induced AR42J cell death. Trypan blue exclusion assay was performed on AR42J cells after 72-hour treatment of 4-PBA at the indicated doses, with (+) or without (−) simultaneous treatment of 1 μmol/L cerulein during the last 24 hours. 120–500 cells were counted for each sample. Percentage of dead cells is presented as the mean ± SEM from triplicate samples. *P < 0.05, **P < 0.01.
Discussion
Although molecular signaling in the pathogenesis of acute pancreatitis remains incomplete, ER homeostasis is emerging as a mediator of pancreatic acinar cell function under both physiological and pathological conditions.32 Using a targeted mutant mouse model of GRP78, we discovered the potential protective role of ER chaperones against experimental pancreatitis. Reduction of multiple ER chaperones, along with partial ER lumen dilation, occurred in Grp78+/− mice with C57BL/6 × 129/sv background, when fed RD. These physiological changes were associated with an increased severity of cerulein-induced experimental pancreatitis. Further, in RD-fed wild-type mice, cerulein treatment induced ER stress response, including phosphorylation of eIF2α and induction of CHOP and XBP-1s, as previously reported for the arginine-induced acute pancreatitis in rats.11 Noticeably, CHOP level was significantly elevated in pancreas of cerulein-treated, RD-fed Grp78+/− mice, as compared to wild-type control. Nonetheless, CHOP elevation in the pancreas of the Grp78+/− mice did not result in GADD34 induction or increase in apoptosis. Rather, we observed decrease in apoptosis in these mice, associating with increase in necrosis, neutrophil infiltration, and pancreatitis severity. This implies that CHOP-mediated ER stress apoptotic pathway was not activated after seven hours of cerulein treatment.
More mechanistic insights are provided from our discovery that after chronic HFD regimen, the chaperone levels (including GRP78) and morphology of ER in pancreas from Grp78+/− mice were restored, thus physiologically mimicking a gain-of-function of ER chaperones. This is consistent with our hypothesis that partial depletion of GRP78 in the exocrine pancreas of the Grp78+/− mice leads to adaptive increase in ER protein folding capacity when challenged with HFD. Accordingly, these mice showed improvement in acute pancreatitis. Despite the use of blood digestive enzymes as a measure of severity in clinical pancreatitis, blood amylase levels do not always correlate with clinical severity of the disease, rather the inflammatory and necrosis scores show a much stronger correlation.33,34,35 This was also observed in our mouse model after cerulein treatment. In direct support of our mechanistic explanation, Grp78+/− mice backcrossed to C57BL/6 genetic background did not exhibit general decrease in ER chaperone levels, and exacerbated acute pancreatitis was not observed in these mice. Furthermore, 4-PBA treatment rescued pancreatic acinar cells from cerulein-induced cell death in a dose-dependent manner. Taken together, the findings from our mouse models suggest that ER chaperone balance in exocrine pancreas protects against severity of acute pancreatitis.
The impact of Grp78 insufficiency on ER homeostasis and cellular function could be dose-dependent and tissue-specific. Homozygous knockout of Grp78 suppresses proliferation of mouse embryonic blastocysts and leads to apoptosis of the inner cell mass, resulting in embryonic lethality at day 3.5,15 but does not affect prostate development when the targeted mutation is restricted to the postnatal prostate epithelium.36 Nonetheless, knockout of GRP78 in these same cells blocks prostate cancer progression.36 Correspondingly, Grp78 heterozygosity did not change superficial phenotypes in mice, but significantly impedes the growth of MMTVPyVT transgene-induced mammary tumor progression and tumor angiogenesis,24 implying that GRP78 function is particularly critical for pathophysiological conditions such as cancer.37 Knockdown of Grp78 by siRNA results in massive ER lumen expansion and disorganization in mammalian cell lines HEK293 and HeLa.38 Here we report that moderate dilation of ER lumen was observed in pancreatic acinar cells with Grp78 heterozygosity. Compensatory up-regulation of ER chaperones is observed in liver and mouse embryonic fibroblasts,15 but not in pancreas. Our discovery of different ER chaperone profiles in pancreas from RD-fed Grp78+/− mice in different genetic backgrounds also suggests a polygenic response to Grp78 heterozygosity, which awaits further investigation.
There are several mechanisms that could potentially explain the effect we observed of ER chaperones on the severity of acute pancreatitis. As pancreatic acinar cells are major professional cells in protein production and secretion, in the C57BL/6 × 129/sv genetic background, ER chaperone reduction resulting from Grp78 heterozygosity could reduce protein folding capacity and ER-associated protein degradation, leading to accumulation of malfolded proteins, thereby exacerbate pancreatic acinar cell necrosis. In human hereditary chronic pancreatitis, mutation-induced misfolding of chymotrypsinogen C causes ER stress and cell death.12,39 Additionally, ER Ca2+ homeostasis may also be affected. Reduction of both CRT and GRP78, the major Ca2+-binding chaperones in ER,5,40,41 might promote ER Ca2+ depletion in Grp78+/− acinar cells on pathological insults. The abnormal elevation of cytosolic Ca2+, in turn, will lead to mitochondria dysfunction, ATP depletion, and autoactivation of digestive enzymes, resulting in necrotic cell death.42
ER stress could also exacerbate acute pancreatitis through signaling inflammation. Different signaling pathways initiated by ER stress have been reported to induce inflammatory responses.43 For example, a liver-specific ER membrane–anchored transcription factor CREBH is activated by site-1 and site-2 protease-mediated cleavage in response to ER stress and subsequently initiates an acute, systemic inflammatory response.44 In intestinal epithelia cells, inhibition of ER stress contributes to the antiinflammatory mechanisms of interleukin-10.45 During the pathogenesis of type 2 diabetes, ER stress activates inflammation signaling via IKKβ/NF-κB and JNK pathways, which contribute to insulin resistance in liver and white adipose.46,47 In the acute pancreatitis, although molecular signaling between ER stress and inflammatory response awaits future investigation, our results reveal a correlation between the ER stress marker CHOP induction and neutrophil infiltration.
Our studies suggest improving ER protein folding capacity as a potential target for prevention and therapy of acute pancreatitis. Administration of chemical chaperones, including 4-PBA, has been shown to attenuate ER stress and confer improvement in a variety of diseases, including Parkinson’s disease,29,48 leptin resistance,49 insulin resistance,50 and autosomal dominant familial isolated hypoparathyroidism,51 as well as adipose tissue differentiation and maturation,52 and palmitate-mediated hepatocyte cell death.53 Here we report the protective effect of 4-PBA on pancreatic acinar cell death induced by cerulein. Enhancement of ER protein folding capacity might inhibit the vicious signaling leading to severe inflammation and cell necrosis, thus reducing the high mortality rate in patients with necrotizing pancreatitis. A number of studies now demonstrate that obesity is associated with a more severe course and a greater mortality rate in patients with acute pancreatitis.54 The mechanisms underlying the more severe course are not known, but hypotheses have been expressed relating the more severe disease to an exacerbated inflammatory response and/or contribution of fat necrosis.54 Although our study was not designed to address the mechanisms of obesity-regulated severity, there are potentially important findings presented in this work that may provide insights with further investigation. First, the measures of pancreatitis were worse in the HFD-fed wild-type mice compared with the RD-fed ones, consistent with the human reports. Second, although HFD does impose stress on exocrine pancreas and enhance acute pancreatitis in the wild-type mice, an adaptive response to the chronic ER stress on HFD is triggered by Grp78 heterozygosity, resulting in up-regulation of GRP94 level and recovery of other ER chaperone levels including GRP78. Future studies creating new mouse models with gain of chaperone function will provide direct proof of our hypothesis which has significant therapeutic implications for acute pancreatitis.
Acknowledgments
We thank Dr. Robert Chow’s laboratory for helpful discussions and assistance with pancreatic islet isolation.
Footnotes
Address reprint requests to Amy S. Lee, Ph.D., Department of Biochemistry and Molecular Biology, University of Southern California Keck School of Medicine, USC Norris Comprehensive Cancer Center, 1441 Eastlake Avenue, Los Angeles, CA 90089-9176. E-mail: amylee@ccnt.usc.edu.
Supported in part by University of Southern California Center for Liver Disease grant DK048522, National Institute of Health grants CA027607 and DK070582 (A.S.L.), and the Department of Veterans Affairs and by National Institute of Health Grants AA11999 and AA16010 (S.J.P.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Case RM. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc. 1978;53:211–354. doi: 10.1111/j.1469-185x.1978.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Iida K, Li Y, McGrath BC, Frank A, Cavener DR. PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol. 2007;8:38. doi: 10.1186/1471-2121-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Hendershot LM. The ER function BiP is a master regulator of ER function. Mt Sinai J Med. 2004;71:289–297. [PubMed] [Google Scholar]
- Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollins DE, Warren JJ, Immormino RM, Gewirth DT. Structures of GRP94-nucleotide complexes reveal mechanistic differences between the hsp90 chaperones. Mol Cell. 2007;28:41–56. doi: 10.1016/j.molcel.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little E, Lee AS. Generation of a mammalian cell line deficient in glucose-regulated protein stress induction through targeted ribozyme driven by a stress-inducible promoter. J Biol Chem. 1995;270:9526–9534. [PubMed] [Google Scholar]
- Caramelo JJ, Parodi AJ. Getting in and out from calnexin/calreticulin cycles. J Biol Chem. 2008;283:10221–10225. doi: 10.1074/jbc.R700048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Kubisch CH, Logsdon CD. Secretagogues differentially activate endoplasmic reticulum stress responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1804–G1812. doi: 10.1152/ajpgi.00078.2007. [DOI] [PubMed] [Google Scholar]
- Kubisch CH, Sans MD, Arumugam T, Ernst SA, Williams JA, Logsdon CD. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G238–G245. doi: 10.1152/ajpgi.00471.2005. [DOI] [PubMed] [Google Scholar]
- Kereszturi E, Szmola R, Kukor Z, Simon P, Weiss FU, Lerch MM, Sahin-Toth M. Hereditary pancreatitis caused by mutation-induced misfolding of human cationic trypsinogen: a novel disease mechanism. Hum Mutat. 2009;30:575–582. doi: 10.1002/humu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer and neurological disorders. Antioxid Redox Signal. 2009;11:2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- Michael DJ, Geng X, Cawley NX, Loh YP, Rhodes CJ, Drain P, Chow RH. Fluorescent cargo proteins in pancreatic beta-cells: design determines secretion kinetics at exocytosis. Biophys J. 2004;87:L03–L05. doi: 10.1529/biophysj.104.052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Baumeister P, Roy B, Phan T, Foti D, Luo S, Lee AS. ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol Cell Biol. 2000;20:5096–5106. doi: 10.1128/mcb.20.14.5096-5106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Lee AS. Requirement of the p38 MAPK signaling pathway for the induction of Grp78/BiP by azetidine stress: ATF6 as a target for stress-induced phosphorylation. Biochem J. 2002;366:787–795. doi: 10.1042/BJ20011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ye R, Barron E, Baumeister P, Mao C, Luo S, Fu Y, Luo B, Dubeau L, Hinton DR, Lee AS. Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ. 2010;17:488–498. doi: 10.1038/cdd.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval D, Gukovskaya A, Reavey P, Gukovsky S, Sisk A, Braquet P, Pandol SJ, Poucell-Hatton S. The role of neutrophils and platelet-activating factor in mediating experimental pancreatitis. Gastroenterology. 1996;111:1081–1091. doi: 10.1016/s0016-5085(96)70077-x. [DOI] [PubMed] [Google Scholar]
- Mareninova OA, Sung KF, Hong P, Lugea A, Pandol SJ, Gukovsky I, Gukovskaya AS. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem. 2006;281:3370–3381. doi: 10.1074/jbc.M511276200. [DOI] [PubMed] [Google Scholar]
- Dong D, Ko B, Baumeister P, Swenson S, Costa F, Markland F, Stiles C, Patterson JB, Bates SE, Lee AS. Vascular targeting and antiangiogenesis agents induce drug resistance effector GRP78 within the tumor microenvironment. Cancer Res. 2005;65:5785–5791. doi: 10.1158/0008-5472.CAN-05-0754. [DOI] [PubMed] [Google Scholar]
- Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, Mao C, Ye R, Wang M, Pen L, Dubeau L, Groshen S, Hofman FM, Lee AS. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Spannagel AW, Nakano I, Tawil T, Chey WY, Liddle RA, Green GM. Adaptation to fat markedly increases pancreatic secretory response to intraduodenal fat in rats. Am J Physiol. 1996;270:G128–G135. doi: 10.1152/ajpgi.1996.270.1.G128. [DOI] [PubMed] [Google Scholar]
- Chowdhury P, Nishikawa M, Blevins GW, Jr, Rayford PL. Response of rat exocrine pancreas to high-fat and high-carbohydrate diets. Proc Soc Exp Biol Med. 2000;223:310–315. doi: 10.1046/j.1525-1373.2000.22344.x. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K, Niinuma Y, Kaneko M, Okuma Y, Sugai M, Omura T, Uesugi M, Uehara T, Hosoi T, Nomura Y. Suppressive effects of 4-phenylbutyrate on the aggregation of Pael receptors and endoplasmic reticulum stress. J Neurochem. 2006;97:1259–1268. doi: 10.1111/j.1471-4159.2006.03782.x. [DOI] [PubMed] [Google Scholar]
- Jessop NW, Hay RJ. Characteristics of two rat pancreatic exocrine cell lines derived from transplantable tumours. In Vitro. 1980;16:212. [Google Scholar]
- Yuan J, Lugea A, Zheng L, Gukovsky I, Edderkaoui M, Rozengurt E, Pandol SJ. Protein kinase D1 mediates NF-kappaB activation induced by cholecystokinin and cholinergic signaling in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1190–G1201. doi: 10.1152/ajpgi.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol SJ. Acute pancreatitis. Curr Opin Gastroenterol. 2006;22:481–486. doi: 10.1097/01.mog.0000239861.89209.5f. [DOI] [PubMed] [Google Scholar]
- Al-Bahrani AZ, Ammori BJ. Clinical laboratory assessment of acute pancreatitis. Clin Chim Acta. 2005;362:26–48. doi: 10.1016/j.cccn.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Winslet M, Hall C, London NJ, Neoptolemos JP. Relation of diagnostic serum amylase levels to aetiology and severity of acute pancreatitis. Gut. 1992;33:982–986. doi: 10.1136/gut.33.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DB, Robinson AW, Pettit RJ. Acute pancreatitis with normal amylase. Dig Dis Sci. 1986;31:779–780. doi: 10.1007/BF01296459. [DOI] [PubMed] [Google Scholar]
- Fu Y, Wey S, Wang M, Ye R, Liao CP, Roy-Burman P, Lee AS. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of the stress response chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci USA. 2008;105:19443–19448. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–1471. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmola R, Sahin-Toth M. Pancreatitis-associated chymotrypsinogen C (CTRC) mutant elicits endoplasmic reticulum stress in pancreatic acinar cells. Gut. 2010;59:365–372. doi: 10.1136/gut.2009.198903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievremont JP, Rizzuto R, Hendershot L, Meldolesi J. BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+. J Biol Chem. 1997;272:30873–30879. doi: 10.1074/jbc.272.49.30873. [DOI] [PubMed] [Google Scholar]
- Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417:651–666. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- Criddle DN, Gerasimenko JV, Baumgartner HK, Jaffar M, Voronina S, Sutton R, Petersen OH, Gerasimenko OV. Calcium signalling and pancreatic cell death: apoptosis or necrosis? Cell Death Differ. 2007;14:1285–1294. doi: 10.1038/sj.cdd.4402150. [DOI] [PubMed] [Google Scholar]
- Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Shkoda A, Ruiz PA, Daniel H, Kim SC, Rogler G, Sartor RB, Haller D. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132:190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Ikemoto M, Kawarabayashi T, Ikeda M, Nishinakagawa T, Hosokawa M, Shoji M, Takahashi M, Nakashima M. A chemical chaperone, sodium 4-phenylbutyric acid, attenuates the pathogenic potency in human alpha-synuclein A30P + A53T transgenic mice. Parkinsonism Relat Disord. 2009;15:649–654. doi: 10.1016/j.parkreldis.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R, Waheed A, Shah GN, Sly WS. Signal sequence mutation in autosomal dominant form of hypoparathyroidism induces apoptosis that is corrected by a chemical chaperone. Proc Natl Acad Sci USA. 2007;104:19989–19994. doi: 10.1073/pnas.0708725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basseri S, Lhotak S, Sharma AM, Austin RC. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J Lipid Res. 2009;50:2486–2501. doi: 10.1194/jlr.M900216-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 2010;298:E1027–1035. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frossard JL, Lescuyer P, Pastor CM. Experimental evidence of obesity as a risk factor for severe acute pancreatitis. World J Gastroenterol. 2009;15:5260–5265. doi: 10.3748/wjg.15.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]