Abstract
Context
Tourette disorder is a chronic and typically impairing childhood-onset neurological condition. Antipsychotic medications, the first-line treatments for moderate to severe tics, are often associated with adverse effects. Behavioral interventions, although promising, have not been evaluated in large-scale controlled trials.
Objective
To determine the efficacy of a comprehensive behavioral intervention for reducing tic severity in children and adolescents.
Design, Setting, Participants
Randomized, observer-blind, controlled trial of 126 youngsters recruited from December, 2004 through May, 2007 and aged 9–17 years with impairing Tourette or chronic tic disorder as primary diagnosis randomized to 8 sessions over 10 weeks of behavior therapy (n=61) or a control treatment consisting of supportive therapy and education (n=65). Responders received 3 monthly treatment booster sessions and were reassessed at 3- and 6-months post-treatment.
Intervention
Comprehensive behavioral intervention.
Main Outcome Measures
Yale Global Tic Severity Scale (range 0–40, score >15 indicating clinically significant tics), Clinical Global Impression-Improvement Scale (range 1-very much improved to 8-very much worse).
Results
Behavioral intervention led to a significantly greater decrease on the Yale Global Tic Severity Scale (24.7; CI:23.1,26.3) to 17.1 CI:15.1,19.1) from baseline to endpoint compared to the control treatment (24.6 CI:23.2,26.0) to 21.1 CI:19.2,23.0) (P<.001; 95% CI for difference between groups: 6.2, 2.0); (effect size=0.68). Compared to children in control treatment, significantly more children receiving behavioral intervention were rated as “very much” or “much improved” on the Clinical Global Impression-Improvement scale (52.5% to 18.5%, respectively; P<0.001; number-needed-to-treat=3). Attrition was low (12/126 or 9.5%); tic worsening was reported by 4% of children (5/126). Treatment gains were durable with 87% of available responders to behavior therapy showing continued benefit 6 months post-treatment.
Conclusions
A comprehensive behavioral intervention, compared with supportive therapy and education, resulted in greater improvement in symptom severity among children with Tourette and chronic tic disorder.
Tourette disorder is a chronic neurological disorder, characterized by motor and vocal tics. Prevalence estimates in school-age children range from 1–10/1000 with a rate of 6 per 1000 replicated in several countries.1,2 Tics are usually brief, rapid movements (e.g., blinking, facial grimacing), or vocalizations (e.g., throat clearing, grunting) but can include more complex movements and vocalizations. Tics begin in childhood; severity peaks in early adolescence and often declines in young adulthood.3 Epidemiologic and clinical data indicate that Tourette disorder can be associated with considerable impairment2 and social isolation4 in school age children. Tics are commonly preceded by premonitory urges or sensations that are experienced as noxious and relieved upon completion of the tic.5,6 The most effective treatments for reducing tic severity are antipsychotic medications such as haloperidol, pimozide, and risperidone, although these medications rarely eliminate tics and are often associated with unacceptable sedation, weight gain, cognitive dulling, and motor side effects.7 In addition, nearly all previous randomized medication trials targeting tics in children with Tourette disorder have been brief ranging from 4–8 weeks in duration and included less than 50 participants.7 Few trials have provided controlled (or even open maintenance) data beyond acute treatment. Thus, data on long-term outcomes of medication for tics are limited.
The most promising behavioral intervention for reducing tic severity is habit reversal training.8 Habit reversal acknowledges the neurological basis of tics, but proposes that situational factors, including the reaction of others to the tics as well as the internal experience of premonitory urges, play an important and ongoing role in tic expression.9,10,11 Establishing the effectiveness of behavioral treatments for reducing tic severity in children would advance public health by broadening treatment options and expanding the types of clinicians who can effectively treat tic disorders. This trial was designed to evaluate the efficacy of a Comprehensive Behavioral Intervention for Tics (or CBIT)12, based on habit reversal training, for reducing tics and tic-related impairment in a large sample of youth with Tourette disorder.
Methods
Design
This was a two-phase, multicenter, randomized controlled trial for children and adolescents with Tourette or chronic tic disorder.13 Phase 1 was a 10-week acute comparison of the behavioral intervention to a structured control condition consisting of supportive therapy and education about tics. The control treatment was selected to control for time and attention. In addition, we presumed that it would be acceptable to children and families. Phase 2 was a 6-month, naturalistic observation period for participants showing a positive response to either study intervention. The assessments at 3 and 6 months post-treatment, provided an estimate of the durability of treatment response. Children who did not show a positive response to either intervention in the randomized trial were not assessed after completing Phase 1. The study was implemented at three sites: Johns Hopkins School of Medicine, the University of California at Los Angeles, and the University of Wisconsin–Milwaukee. Collaborating investigators provided training of clinical raters, data management and analysis (Yale University), therapist supervision (Massachusetts General Hospital/Harvard Medical School), and coding of secondary outcome measures (University of Texas Health Science Center at San Antonio). The Tourette Syndrome Association, Inc. provided grant management and recruitment support. An independent data and safety monitoring board provided regular oversight. The trial was approved by the institutional review boards at each site and was registered on clinicaltrials.gov (NCT00218777). Prior to enrollment, study personnel provided a detailed description of study procedures, risks, and benefits to interested families, following which interested parents/guardians provided informed consent and children provided informed assent.
Objectives
The primary study aim was to evaluate whether the Comprehensive Behavioral Intervention for Tics (CBIT)12 would prove superior to supportive therapy and education for reducing tics and tic-related impairment in youth with a chronic tic disorder. We were also interested in evaluating the impact of the behavioral intervention on children taking stable medication for tics.
Participants
Eligible participants were 9–17 years old with Tourette or chronic tic disorder of moderate or greater severity as measured by a Yale Global Tic Severity Scale14 Total score>13 (>9 for children with motor or vocal tics only), English-fluency and IQ>80. Co-occurring attention deficit–hyperactivity disorder, obsessive–compulsive disorder, other anxiety disorders, depressive disorders, or oppositional-defiant disorder were allowed unless the disorder required immediate treatment or change in current treatment. Children on psychotropic medications for tics or allowed psychiatric disorders were eligible if the dose was stable for six weeks with no planned changes during study participation. The lack of data regarding pre-medication tic severity did not allow us to establish the degree of prior symptom reduction in medicated children. Exclusion criteria included an unstable medical condition, current diagnosis of substance abuse/dependence, lifetime diagnosis of pervasive developmental disorder, mania or psychosis, or four or more prior sessions of habit reversal training.
Treatments
The primary component of CBIT12 is habit reversal training. The primary components of habit reversal are tic-awareness and competing-response training.15 Awareness training entails self-monitoring of current tics, focusing on the premonitory urge or other early signs that a tic is about to occur. Competing-response training is based on the observation that performance of a tic results in a decrease in the premonitory urge. Over time, the reduction in the urge after completion of the tic reinforces repetition of the tic (i.e. a negative reinforcement cycle).9 Competing response training involves engagement in a voluntary behavior physically incompatible with the tic, contingent on the premonitory urge or other signs of impending tic occurrence. Competing-response training is distinct from deliberate tic suppression in that it teaches the patient to initiate a voluntary behavior to manage the premonitory urge (and disrupt the negative reinforcement cycle) rather than simply suppressing the tic. Initially, patient and therapist create a tic hierarchy and rank tics from most to least distressing with more distressing tics addressed earlier in treatment. Awareness training and competing response training are then implemented and practiced in session one tic at a time. For example, a child with a neck-jerking tic may be taught to look forward with his chin slightly down, while gently tensing neck muscles for 1 minute or until the urge goes away. As noted, the competing response can be initiated when the patient notices a tic is about to occur, during the tic, or after the tic has occurred. For vocal tics, slow rhythmic diaphragmatic breathing is the most common competing response. Patients are encouraged to use their competing responses throughout the day. Optimally, competing responses are compatible with maintaining participation in ongoing activities, but incompatible with execution of the tic. With practice, patients are able to complete the competing response without disengaging from routine activities.
In addition to habit reversal training, CBIT also included relaxation training and a functional intervention to address situations that sustained or worsened tics. The functional intervention first identified situational antecedents and consequences influencing tic severity, and second, developed individualized behavioral strategies to reduce the impact of these factors.16,17 For example, parents were taught to manage tic increases often occurring when their child returned home from school by encouraging and praising the youngster for practicing behavioral intervention techniques. Parents were also taught to manage their own reactions to the tics and to prevent the tics from exerting undue influence on family life.
The control treatment, supportive psychotherapy and education, provided information about tic disorders and was designed to mimic recommended adjunctive components of psychopharmacologic treatment.18 Children and their parents were allowed to discuss tics and related issues, but therapists were prohibited from providing direct instructions about tic management.19
Both treatments were delivered in eight sessions over 10 weeks and matched for session length and duration. The first two sessions were 90 minutes to facilitate rapport building and information gathering. The remaining sessions were 60 minutes in length. The first six sessions occurred weekly; the remaining two biweekly. Although both interventions focused on the child, parents were included for all or part of a session depending on session content. Following systematic training and certification, therapists with masters-level or higher education implemented both interventions according to detailed treatment manuals. Therapists also received weekly site-level and cross-site supervision with an emphasis on maintaining the integrity of both interventions. Independent raters completed treatment integrity ratings on a random 13% sample of video-recorded therapy sessions using a detailed checklist outlining the required and prohibited elements of each treatment session in both treatment conditions. Overall, 88% of behavioral intervention sessions and 98% of control treatment sessions were rated as good or better.
Randomization and Blinding
Eligible children were randomly assigned to treatment in a 1:1 ratio by the data center using a computer algorithm. The randomization was done within site and stratified by medication status to ensure that equal numbers of participants on tic medication would be in each treatment group. Children, their parents, and therapists were aware of assigned treatment condition. Independent outcome evaluators were masked to treatment assignment. Several methods were used to maintain the treatment blind, including segregation of assessment and treatment staff and instruction to children and parents to avoid discussion of treatment assignment with the independent evaluators.
Outcome Measures
Demographics, symptom severity, psychiatric diagnoses and psychosocial functioning were obtained via self-report and clinical interview from children and their parents at screening and baseline. Children’s racial/ethnic status was collected to provide comparability with similar studies and designated by parents on a parent-report questionnaire. Diagnostic eligibility was established using a DSM-IV-based, semi-structured clinical interview20 administered separately to parent and child and modified to cover Tourette and other tic disorders.21 Outcome assessments were repeated at weeks 5 and 10. The primary outcome measures were the Yale Global Tic Severity Scale Total Tic Score14 and the Clinical Global Impression–Improvement scale.22 The Yale Global Tic Severity Scale is a clinician-rated measure that begins with the completion of a checklist of all tics present in the past week. Current motor and vocal tics are then rated on five dimensions (number, frequency, intensity, complexity, and interference; range 0–5 each) which are summed to yield separate Motor and Vocal Tic scores (range 0–25) and a combined Total Tic score (range 0–50). An associated Impairment scale (range 0–50) assesses tic-related disability over the past week. Functional status was also assessed using the clinician-rated Children’s Global Assessment Scale (score 0–100).23 Scores of 60 or lower indicate a need for treatment; scores above 70 reflect normal functioning. The Clinical Global Impression–Improvement scale was used to assess overall treatment response. Lower scores indicate improvement and higher scores indicate worsening. By convention, scores of Very Much (1) or Much Improved (2) define positive response.23
Parents completed the Parent Tic Questionnaire,24 which lists 28 motor and vocal tics to be marked as present or absent during the past week and rated on a 1–4 severity scale (range=0–112). Parents in both treatment groups also rated how much they expected their child's assigned treatment to be beneficial at the end of their first therapy session using a 3-item scale (total score from 3–15).
Tic outcomes were rated by independent evaluators masked to treatment condition who were masters-level or higher clinicians and trained to reliability. Following didactic training and demonstration of reliability on three video-taped assessments, evaluators received ongoing supervision within-site and via biweekly cross-site teleconference. All study interviews were recorded and 13% were randomly-selected over the course of the study and independently rated for quality on a 7-item checklist using a 0–3 scale with higher scores reflecting better quality. The mean item score of 2.33 reflects high quality and uniformity in the study outcome assessments; there were no site differences.
Adverse Events
Adverse events were monitored at each therapy session. Therapists asked about recent health complaints, behavioral changes, visits for medical/mental health care, need for concomitant medications, change in ongoing medications, and hospitalizations and offered the opportunity for spontaneous report of any other problem. Affirmative responses prompted further inquiry concerning the onset, severity, measures taken, and outcome of the adverse event. Tic worsening was rated as an adverse event when child or parent spontaneously reported worsening inconsistent with the child's usual waxing and waning pattern.
Statistical Analysis
Our sample size calculation was based on examination of recently completed placebo-controlled medication trials for tics in children with TS. These trials report mean baseline YGTSS Total Tic scores between 24 to 28 with standard deviations of 6 to 8 points. Change scores with medications superior to placebo range from 7 to 9 points compared to 2 to 4 points for placebo yielding effect sizes of .9 to 1.0. 25,26,27 In this study, we planned to enroll medication-free children and those on tic-suppressing medication, which would predictably result in greater variability at baseline. In addition, we predicted that the supportive treatment condition would provide greater benefit than typically observed for pill placebo in medication trials. Therefore, we proposed a minimally significant effect size of .55 resulting in a sample size of 60 per group given 10% attrition, significance level of 5% and power of 80%.28
Baseline characteristics were compared between groups using t-tests for continuous variables and chi-square tests for categorical variables. Outcome data are presented as least squares means from a mixed model repeated measures analysis29 which assumes that missing data are missing at random and is more robust than other alternatives such as analysis of completers only or using last-observation-carried-forward.30 The effect of treatment on the primary outcome, Yale Global Tic Severity Scale Total Score, as well as the secondary outcomes was tested with mixed-model repeated-measures analyses adjusted for baseline scores.31 These efficacy analyses were conducted on the modified intent-to-treat population (i.e. all participants with at least one post-randomization visit), with all participants analyzed in their assigned treatment condition. The models included fixed effects for treatment (2 levels), time (5 and 10 weeks), site, time-by-treatment interaction and a random effect for participant. Treatment-by-site interactions were not significant for any of the outcome variables and were excluded from the models. Comparison of least squares means at Week 10 were conducted using orthogonal contrasts. Sensitivity analyses were conducted using the last observation carried forward which resulted in the same conclusions and are therefore not presented. Separate analyses examined modification of treatment effect by presence of tic medication at baseline by examining the two- and three-way interactions of treatment with time and medication status. Effect sizes were estimated by subtracting the 10-week baseline-adjusted least squares mean in the control group from the mean change in the treatment group and dividing by the pooled standard deviation for the entire study sample (N=126) at baseline. The proportion of positive responses on the Clinical Global Impressions-Improvement Scale was compared across time using Mantel-Haenszel Chi-square to adjust for site. Comparisons of adverse event rates were made using Fisher’s exact tests. Data regarding treatment durability were examined using all participants showing positive response at Week 10 and participants who returned for follow up assessment. Because of low power, between group comparisons of CGI-I positive response rates and YGTSS scores over the follow-up period were not made. All analyses were performed with SAS Version 9.1 (Cary, NC) at the two-sided 0.05 level of significance. There was no adjustment for multiple comparisons for testing secondary outcomes.
Results
Baseline Characteristics
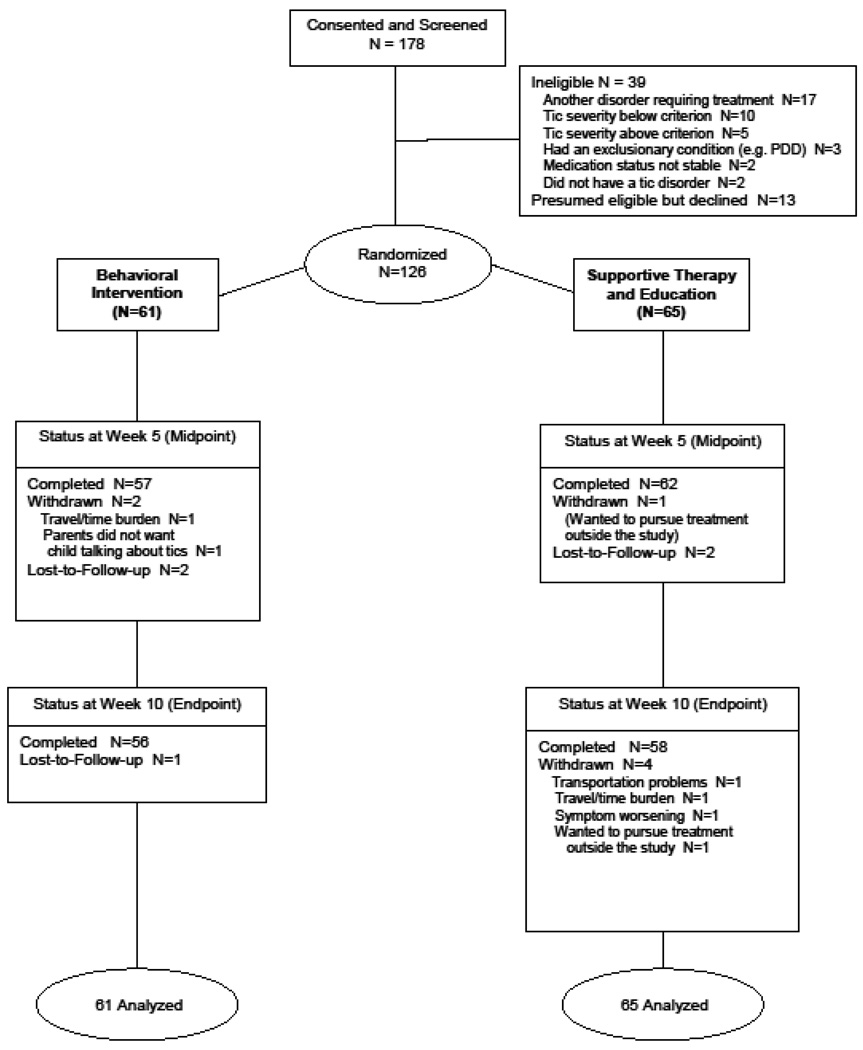
Over the 30-month period from December, 2004 to May, 2007, 178 youths were screened and 126 randomly assigned to one of the two treatment conditions (Figure 1). Enrollment across sites was similar (Table 1). Participants ranged in age from 9–17 years (mean=11.7, SD=2.3 years); 99 (78.5%) were boys, 106 (84.1%) were Caucasian, 93.5% (118/126) met criteria forTourette disorder. Overall, 36.5% of children entered the trial on stable anti-tic medication. There were no significant between-group differences in baseline demographic or clinical characteristics, including tic medication status. Attrition in the behavioral intervention group was 10% (6/61) versus 11% for the control treatment (7/65). Children in the behavioral intervention attended 94.1% of scheduled sessions compared to 93.7% for the control condition. Two (3.3%) of participants in the behavioral intervention and four (6.2%) in the control group reported a change in their tic medication type or dose during acute treatment. In light of the low frequency of medication changes, no adjustments were made in the analysis.
Figure 1.
Enrollment and Outcomes
Table 1.
Baseline demographic and clinical characteristics by treatment group ‡
| Characteristic | Behavioral Intervention (N=61) |
Education and Support (N=65) |
||
|---|---|---|---|---|
| Study Center (N, %) | ||||
| Johns Hopkins University | 20 | (32.8) | 21 | (32.3) |
| University of California, Los Angeles | 21 | (34.4) | 24 | (36.9) |
| University of Wisconsin, Milwaukee | 20 | (32.8) | 20 | (30.8) |
| Demographics | ||||
| Age (mean, SD) | 11.6 | (2.34) | 11.7 | (2.32) |
| WASI IQ (mean, SD) | 111.7 | (13.5) | 108.6 | (14.0) |
| Male Gender (N, %) | 46 | (75.4) | 53 | (81.5) |
| On Tic Meds at Entry (N, %) | 23 | (37.7) | 23 | (35.4) |
| Two Parent Family (N, %) | 50 | (82) | 57 | (87.7) |
| Parent Occupation (N, %) * | ||||
| Laborer/Homemaker/Clerical | 4 | (6.6) | 2 | (3.1) |
| Craftsperson/Artist | 1 | (1.6) | 3 | (4.6) |
| Technician/Skilled Laborer | 5 | (8.2) | 9 | (13.8) |
| Professional | 51 | (83.6) | 50 | (76.9) |
| Parent Education (N, %) * | ||||
| High School | 4 | (6.6) | 1 | (1.5) |
| Technical School/Some College | 7 | 11.5) | 13 | (20) |
| College Graduate | 21 | (34.4) | 17 | (26.2) |
| Graduate or Professional School | 29 | (47.5) | 34 | (52.3) |
| Race or Ethnicity (N, %) | ||||
| White (Non-Hispanic) | 51 | (83.6) | 56 | (86.2) |
| White (Hispanic) | 6 | (9.8) | 3 | (4.6) |
| Black | 1 | (1.6) | 3 | (4.6) |
| Asian/Pacific Islander | 2 | (3.3) | 2 | (3.1) |
| Other | 1 | (1.6) | 1 | (1.5) |
| Tic Disorder (N, %) | ||||
| Tourette disorder | 56 | (91.8) | 62 | (95.4) |
| Chronic Motor Tic | 4 | (6.6) | 3 | (4.6) |
| Chronic Vocal Tic | 1 | (1.6) | 0 | (0.0) |
| Other Diagnoses (N, %)† | ||||
| Attention Deficit Hyperactivity Disorder | 20 | (32.8) | 13 | (20.0) |
| Obsessive-compulsive Disorder | 8 | (13.1) | 16 | (24.6) |
| Generalized Anxiety | 10 | (16.4) | 15 | (23.1) |
| Separation Anxiety | 6 | (9.8) | 5 | (7.7) |
| Social Anxiety | 13 | (21.3) | 14 | (21.5) |
| Medication Status (N, %)# | ||||
| No Medication | 38 | (62.3) | 42 | (64.6) |
| Antipsychotic | 8 | (13.1) | 3 | (4.6) |
| Alpha Agonist | 11 | (18.0) | 14 | (21.5) |
| Anticonvulsant | 1 | (1.6) | 1 | (1.5) |
| Benzodiazapine | 0 | (0.0) | 1 | (1.5) |
| Alpha Agonist+Antipsychotic ^ | 3 | (4.9) | 2 | (3.1) |
| Alpha Agonist+Levetiracetam | 0 | (0.0) | 1 | (1.5) |
| Antipsychotic+Donepezil | 0 | (0.0) | 1 | (1.5) |
| Yale Global Tic Severity Scale | ||||
| Total Score (mean, SD) | 24.7 | (6.2) | 24.6 | (6.0) |
| Total Motor (mean, SD) | 14.6 | (4.4) | 14.6 | (3.2) |
| Total Vocal (mean, SD) | 10.1 | (4.5) | 10.0 | (4.7) |
There were no significant between-group differences for any of the listed variables
Parent Occupation and Education classifications were based on the parent with the highest level in two-parent homes or parent of primary resident in single-parent homes.
Some participants had more than one coexisting diagnosis.
Antipsychotics: haloperidol, pimozide, risperidone, aripiprazole, olanzapine; Alpha Agonist: guanfacine, clonidine; Anticonvulsants: valproate, levetiracetam; Benzodiazepines: clonazepam.
One child was on an alpha agonist and two antipsychotic medications.
Outcomes
After 10 weeks of treatment, the Yale Global Tic Severity Scale Total Tic score was significantly reduced in the behavioral intervention group as compared to control treatment (P<0.001). Behavior therapy was associated with a 7.6 point decrease in YGTSS Total Tic Score compared to a 3.5 point decline in the control treatment. This 4 point difference between groups is similar to placebo-controlled medication trials and was clinically meaningful as suggested by an effect size of 0.68.32 Moreover, the rate of positive treatment response as measured by a rating of 1 (very much improved) or 2 (much improved) on the Clinical Global Impressions–Improvement Scale was significantly higher for the behavioral (52.5%; 32/61) versus control (18.5%; 12/65) intervention (P<0.001). For behavior therapy this difference reflects a number needed to treat (NNT) of 3 and an absolute risk reduction (ARR) of 34%.
Table 2 displays mean scores, effect sizes and confidence intervals on the difference between CBIT and the control condition for all study outcomes. Positive results for CBIT relative to control treatment were again evident on motor tics, phonic tics, and tic-related impairment. Children randomized to behavioral intervention showed a 51% drop (25.0 to 12.2) on YGTSS Impairment scale from baseline to Week 10 compared to a 30% decline (23.4 to 16.4) for the control treatment (P<0.01, effect size=0.57). Both groups showed improvement on the clinician-rated Child Global Assessment Scale (CGAS). However, youngsters in the CBIT group showed greater improvement on the CGAS compared to PST (59.0 to 69.4 versus 59.3 to 64.1, respectively; p <.001, effect size=.64). There were no treatment differences across sites. In addition, neither the presence of tic-suppressing medication nor tic severity at baseline significantly moderated treatment outcome.
Table 2.
Baseline, Week 5 and Week 10 Scores on key outcome measures ‡
| Behavioral Intervention (N=61) |
Education and Support (N=65) |
Group Difference at Week 10 |
||||
|---|---|---|---|---|---|---|
| Mean | 95%CI | Mean | 95%CI | 95%CI | Effect Size* | |
| Yale Global Tic Severity Scale | ||||||
| Total Tic Score | ||||||
| Baseline | 24.7 | (23.1,26.3) | 24.6 | (23.2,26.0) | ||
| Week 5 | 19.7 | (17.6,21.7) | 22.8 | (20.7,24.9) | 3.3 (5.4,1.2) | 0.54 |
| Week 10 | 17.1 | (15.1,19.1) | 21.1 | (19.2,23.0) | 4.1 (6.2,2.0) | 0.68 |
| Total Motor | ||||||
| Baseline | 14.6 | (13.5,15.7) | 14.6 | (13.8,15.4) | ||
| Week 5 | 12.2 | (10.8,13.6) | 13.6 | (12.4,14.7) | 1.3 (2.7,0.1) | 0.34 |
| Week 10 | 10.7 | (9.3,12.1) | 12.5 | (11.5,13.5) | 1.9 (3.3,0.4) | 0.49 |
| Total Vocal | ||||||
| Baseline | 10.1 | (9.0,11.2) | 10.0 | (8.9,11.1) | ||
| Week 5 | 7.4 | (6.2,8.6) | 9.3 | (7.9,10.6) | 2.0 (3.3,0.7) | 0.43 |
| Week 10 | 6.5 | (5.4,7.6) | 8.6 | (7.4,9.8) | 2.2 (3.6,0.9) | 0.50 |
| Impairment | ||||||
| Baseline | 25.0 | (22.6,27.4) | 23.4 | (21.6,25.2) | ||
| Week 5 | 16.8 | (14.0,19.5) | 20.1 | (17.6,22.7) | 3.8 (6.8,0.7) | 0.47 |
| Week 10 | 12.2 | (9.8,14.6) | 16.4 | (13.8,19.0) | 4.7 (7.8,1.6) | 0.57 |
| Parent Tic Questionnaire Total Score | ||||||
| Baseline | 34.2 | (29.5,38.9) | 35.7 | (30.3,41.1) | ||
| Week 5 | 25.8 | (21.5,30.1) | 33.7 | (27.8,39.6) | 7.3 (13.1,1.5) | 0.28 |
| Week 10 | 20.0 | (16.3,23.7) | 27.6 | (23.0,32.2) | 7.8 (13.8,1.9) | 0.30 |
| Children’s Global Assessment Scale * | ||||||
| Baseline | 59.0 | (57.1,60.9) | 59.3 | (57.3,61.3) | ||
| Week 10 | 69.4 | (66.9,71.9) | 64.1 | (59.5,68.7) | 5.8 (2.8,8.8) | 0.64 |
Data are presented as least square mean values and 95% confidence intervals for each assessment point. Group differences with 95% confidence intervals are also presented
Effect sizes were calculated as follows: change from baseline in behavior therapy minus change in the control group divided by the pooled standard deviation for the entire study sample at baseline.
Children’s Global Assessment Scale only administered at Baseline and Week 10
(Behavioral: Mean=11.3, 95%CI 10.8,11.8) Comparison: Mean=10.4, 95%CI 9.9,10.9). Although statistically significant (P=.02), this small difference was not clinically meaningful.
Adverse Events
Two hundred adverse events were reported during the 10-week Phase 1 trial. Of these, 193 were rated as mild or moderate and 7 as severe (broken bones, n=3; concussion, n=1; neck pain, n=1; neck injury, n=1; nausea and vomiting, n=1); none of the severe events was considered study-related. There were no serious adverse events. Tic worsening above and beyond usual fluctuation was spontaneously reported by 1 participant (1.6%) receiving behavioral intervention and by 4 participants (6.2%) in the control treatment. (Table 3).
Table 3.
Count and percentage of Adverse Events by treatment group
| Adverse Event | Behavioral Intervention (N=61) |
Education and Support (N=65) |
|||
|---|---|---|---|---|---|
| N | %, 95% CI | N | %, 95% CI | P- value† | |
| Upper Respiratory Infection | 21 | 34.4(22.7,47.7) | 27 | 41.5(29.4,54.4) | 0.47 |
| Irritability and explosive behavior | 10 | 16.4( 8.2,28.0) | 10 | 15.4(7.6,26.5) | 0.99 |
| Headache | 10 | 16.4(8.2,28.0) | 14 | 21.5(12.3,33.5) | 0.50 |
| Muscle or joint pain | 9 | 14.8(7.0,26.2) | 13 | 20.0(11.1,31.8) | 0.49 |
| Accidental injury ‡ | 7 | 11.5(4.7,22.2) | 19 | 29.2(18.6,41.8) | 0.02 |
| Anxiety and nervousness | 4 | 6.6(1.8,15.9) | 3 | 4.6(0.9,12.9) | 0.71 |
| Disruptive behavior § | 4 | 6.6(1.8,15.9) | 4 | 6.2(1.7,15.0) | 0.99 |
| Sore Throat | 4 | 6.6(1.8,15.9) | 7 | 10.8(4.4,20.9) | 0.53 |
| Nausea, vomiting | 2 | 3.3(0.4,11.3) | 5 | 7.7(2.5,17.0) | 0.44 |
| Stomach Discomfort | 2 | 3.3(0.4,11.3) | 9 | 13.8(6.5,24.7) | 0.06 |
| Dermatological problems ¶ | 1 | 1.6(0.04,8.8) | 5 | 7.7(2.5,17.0) | 0.21 |
| Tic worsening | 1 | 1.6(0.04,8.8) | 4 | 6.2(1.7,15.0) | 0.37 |
| Tiredness, fatigue | 1 | 1.6(0.04,8.8) | 4 | 6.2(1.7,15.0) | 0.37 |
Defined as Mild (new event that did not interfere with activities of daily living); Moderate (new event that posed some interference or required intervention to prevent interference) or Severe (new event that posed interference and required intervention)
Fisher’s Exact test (two-sided);
Falls, athletic injuries;
Increase in impulsive and oppositional behavior;
Skin rash, dermatitis, sunburn
Treatment Durability
Acute phase positive responders received three monthly booster treatment sessions and were re-evaluated three and six months post-treatment. Of the 32 children classified as positive responders to CBIT at Week 10, 28 returned for assessment at 3 months and 23 returned at 6 months post-treatment. In the PST group, all 12 classified as positive responders at Week 10 returned for assessment at 3 months and 8 returned at 6 months post-treatment. As shown in Table 4, Table 20 of 32 (62.5%) positive responders to CBIT continued to show benefit (9 children were lost to follow up). In the PST group, 6 of 12 children (50%) showed continued benefit (6 were lost to follow up). Considering only those with complete data in the CBIT group (n=23) the mean score on the YGTSS was 13.7 (95 %CI=10.5–16.9) at Week 10; 13.9 (95% CI=10.4–17.3) and 13.3 (95% CI=9.8–16.8) at Months 3 and 6, respectively. For PST, 6 children with complete data had a mean score on the YGTSS of 13.0 (95% CI=9.3–16.7) at Week 10; 9.9 (95% CI=2.1–15.7) and 10.4 (95% CI=2.6–18.2) at Months 3 and 6 respectively.
Table 4.
Children showing continued positive response on the CGI-I at three-months and six-months post-treatment
| Available Participants | All Acute Phase Responders |
|||
|---|---|---|---|---|
| Follow-up Period | N † | (%) | N | (%) |
| Behavioral Treatment | ||||
| Three Months | 24/28 | (85.7%) | 24/32 | (75.0%) |
| Six months | 20/23 | (86.9%) | 20/32 | (62.5%) |
| Control Treatment | ||||
| Three Months | 11/12 | (91.7%) | 11/12 | (91.7%) |
| Six Months | 6/8 | (75.0%) | 6/12 | (50.0%) |
28 of 32 children showing positive response to behavioral treatment at Week 10 were available at the three month follow-up;23 of the 32 were available at six months. All 12 children showing positive response to the control intervention were available for the three month follow-up; 8 of the 12 were available at six months. The proportion of contined positive response is expressed over available participants as well all acute phase responders. Study participants who were lost to follow up were not counted as positive responders.
Discussion
A comprehensive behavioral intervention based on habit reversal training was effective in reducing tics and tic-related impairment in youth with Tourette or chronic tic disorder of moderate or greater severity. Benefits of the behavioral intervention were observed in independent masked-clinician and parent ratings, regardless of tic-medication status and were durable over a six-month follow-up interval for children who showed a positive response to acute treatment. The findings of this trial validate several smaller studies.8,33 Given the more active control treatment in this trial, the magnitude of response in this study is comparable to results of controlled trials with antipsychotic medications for Tourette disorder.25,27 The absolute decrease of 7.6 points (31% from baseline) on the Total Tic score of the YGTSS in the CBIT group is only slightly lower than the effects of antipsychotic medications in children with Tourette’s disorder. Recent placebo-controlled trials reported a decrease of 8.6 points (35%) and 9.7 points (36%) after eight weeks of treatment with ziprasidone27 and risperidone25, respectively. In addition, the number needed to treat (NNT) of 3 found for behavior therapy in the present study compares favorably with that found in recent trials for other child psychiatric disorders.21,34,35,36
The sample included children with a range of tics and associated impairment as well as co-occurring psychiatric conditions, suggesting that the study results are applicable to clinical populations of children with moderate to severe Tourette disorder. The generalizability of our findings is further supported by the fact that, unlike prior psychopharmacologic trials for Tourette disorder which excluded children on medication37, 38% of children in our study were taking stable tic-suppressing medication at study entry. The lack of data on premedication tic severity in this subgroup is a study limitation. Parents reported high expectation for positive outcome for both treatments, and the attrition rate was low for both interventions suggesting each was acceptable and well tolerated by children and families. The relative absence of tic worsening in the behavioral intervention should reassure clinicians, patients, and families who might be concerned that behavioral strategies to reduce tic severity are inadvisable or contraindicated.38 The low attrition rate in the supportive therapy and education condition suggests that children and families also found this intervention meaningful.18 Although it did not have a significant impact on tic severity, the control treatment was associated with a 31% decrease in tic-related impairment. Thus, it is unlikely that the superiority of active treatment was mediated by differences in parental expectancy or treatment acceptability.
Our results have several clinical implications. First, the efficacy of the behavioral intervention expands available treatment options for tic disorders. Second, by emphasizing the development of skills that promote autonomy and mastery, this intervention offers patients and their families an active role in treatment. Third, the dissemination of the behavioral intervention may improve public health by increasing access to care through expanding the range of practitioners who can treat children with Tourette and chronic tic disorder. Published treatment manuals and existing educational outreach funded by the Center for Disease Control (CDC U38 DD000343) will aide dissemination to trained behavioral therapists.
The 10 week duration of the acute efficacy phase compares favorably to recent randomized medication trials targeting tic severity in children, all of which ranged from 4–8 weeks in duration.(e.g., 25,27,39,40) While the behavioral intervention demonstrated efficacy in this trial, a sizeable number of children did not benefit. In addition, although neither baseline tic severity nor medication status moderated treatment outcome, future analyses may provide guidance on patient selection and future research may provide insight on the underlying mechanism of this intervention.
The durability of treatment is an important consideration in treatment choice, but to date has been poorly studied for chronic tic disorder.41 Although our study design did not include evaluation of all children post-treatment, resulting in a loss of randomization, findings provide preliminary support for the durability of response to behavioral intervention.
The observation in the 1960s that haloperidol was effective in reducing tic severity led to a fundamental reconceptualization of Tourette disorder as a neurotransmitter-based neurological disorder42,43 and stimulated a generation of neurobiological research. The results of this study may prompt a similar reconceptualization of tic disorders and provide a new platform for future research. However, acknowledging that behavioral and learning processes play a role in tic severity does not imply that tics have a purely psychological etiology or that patients can suppress tics by force of will. Rather our study lends clinical support to advances in basic science that emphasize the role of both cortical44,45,46 and basal ganglia47,48,49 circuitry on motor function and habit formation.
Acknowledgements
Funding/Support: This work was supported by grant R01MH070802 from the National Institute of Mental Health to Dr. Piacentini with subcontracts to Drs. Walkup, Woods, Scahill, Wilhelm, and Peterson. Also Drs. Scahill & Dziura receive support from the Yale University Clinical and Translational Sciences Award: grant UL1 RR024139 from the National Center for Research Resources (NCRR), National Institutes of Health (NIH)
Role of the Sponsor: The funding organization was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Trial Registration clinicaltrials.gov Identifier: NCT00218777
Author Contributions: Dr Piacentini had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Piacentini, Woods, Scahill, Wilhelm, Peterson, Chang, Ginsburg, Deckersbach, Dzuira, Walkup.
Acquisition of data: Piacentini, Woods, Scahill, Wilhelm, Peterson, Chang, Ginsburg, Deckersbach, Walkup.
Statistical Analysis: Dzuira
Interpretation of data: Piacentini, Woods, Scahill, Wilhelm, Peterson, Chang, Ginsburg, Deckersbach, Dzuira, Walkup
Drafting of the manuscript: Piacentini, Woods, Scahill, Wilhelm, Peterson, Dzuira, Walkup.
Critical revision of the manuscript for important intellectual content: Piacentini, Woods, Scahill, Wilhelm, Peterson, Chang, Ginsburg, Deckersbach, Dzuira, Levi-Pearl, Walkup.
Obtained funding: Piacentini.
Administrative, technical, or material support: Levi-Pearl.
Financial Disclosures: Drs. Piacentini, Woods, Scahill, Wilhelm, Peterson, Chang, Ginsburg, Deckersbach, and Walkup all report receiving royalties from Oxford University Press for treatment manuals on tic disorders. Drs. Piacentini, Woods, Scahill, Wilhelm, Peterson, Chang, and Walkup all report receiving honoraria for CME presentations from the Tourette Syndrome Association. Drs. Piacentini, Woods, and Walkup also receive royalties from Guilford Press for a book on Tourette disorder. Dr. Piacentini also reports receiving grant support in the form of free medication and matching placebo from Eli Lilly for clinical trials funded by the NIMH, speaking honoraria from Janssen Cilag, royalties from Oxford University Press for treatment manuals on child obsessive-compulsive disorder and APA Books for other books on child mental health, receiving speaking honoraria from Janssen-Cilag, and support in the form of free medication and matching placebo from Pfizer for clinical trials funded by NIMH. Dr. Woods also reports receiving book royalties from New Harbinger and Springer Publications and receives speaking honoraria from the Tourette Syndrome Association. Dr. Scahill also reports receiving royalties from Oxford University Press for a textbook on pediatric psychopharmacology and has served as a consultant for Janssen Pharmaceutic, Bristol-Myers Squibb, Neuropharm, Supernus and Shire Pharmaceuticals; has received research support from Seaside Therapeutics and Shire Pharmaceuticals and support in the form of free medication and matching placebo from Janssen and Shire for clinical trials funded by NIMH. Dr. Wilhelm also reports receiving support in the form of free medication and matching placebo from Forest Laboratories for clinical trials funded by NIH, and receives book royalties from Guilford Publications, New Harbinger Publications, and Oxford University Press, and speaking honoraria from PRIMEDIA Healthcare, a publicly traded company working as a logistics collaborator for the MGH Psychiatry Academy (The education programs conducted by the MGH Psychiatry Academy were supported through Independent Medical Education grants from pharmaceutical companies co-supporting the overall program along with participant tuition). Dr. Deckersbach also reports receiving consulting fees from the Constella Group for serving as a reviewer for the Congressionally Directed Medical Research Program. Dr. Walkup has received consulting fees from Eli Lilly and JAZZ Pharmaceuticals and lecture fees from CMP Media, Medical Education Reviews, McMahon Group, DiMedix, and the Tourette Syndrome Association. He has received free drug and matching placebo fromm Pfizer and Lilly, and free drug from Abbott for NIMH-funded clinical trials. He has received fees for consultation with defense counsel and submission of written reports in litigation involving GlaxoSmithKline. No other potential conflicts of interest relevant to this article were reported.
Comprehensive Behavioral Intervention for Tics (CBITS) Study Team: The following individuals participated and were compensated for participation in this study: Site Principal Investigators: John Piacentini, Ph.D. (UCLA), John Walkup. M.D. (JHU), Douglas Woods, Ph.D. (UWM), Lawrence Scahill, M.S.N., Ph.D. (Yale), Sabine Wilhelm, Ph.D. (MGH/Harvard Medical School), Alan Peterson, Ph.D. (UTHSCSA). Study Co-Investigators: Susanna Chang, Ph.D. (UCLA), Golda Ginsburg, Ph.D. (JHU) Thilo Deckersbach, Ph.D. (MGH/Harvard Medical School), Sue Levi-Pearl (TSA). Study Coordinators/Research Assistants: Diane Findley, Ph.D. (Yale, supervising coordinator), Brian Buzzella, B.A., Melody Keller, B.A., Amanda Pearlman, B.A., Michele Rozenman, B.A. (UCLA); ); Amanda Adcock, M.S., Christine Conelea, M.S., Michael Himle, Ph.D., Michael Walther, M.S. (UWM); Catherine Gaze, Ph.D., Hayden Kepley, Ph.D., Luke Mason, B.A., Matthew Specht, Ph.D. (JHU); Dieu -My Phan, M.S.W., L.C.S.W., Shana Franklin, B.A. (MGH), Trisha Benson, M.S., Elizabeth Cedillos, B.A., Cindy Luethcke, B.A., Annette Martinez, B.A. (UTHSCSA); Joseph McGuire, B.A., Ethan Schilling, B.A., (Yale). Cognitive Behavior Therapists: Melody Keller, B.A., Eunice Kim, Ph.D., Joyce Lee, Ph.D., Tara Peris, Ph.D., Lesley Stahl, Ph.D. (UCLA); Christopher Flessner, M.S., Amy Sato, M.S. (UWM), Kelly Drake, Ph.D., Hayden Kepley, Ph.D., Julie Newman Kingery, Ph.D., Courtney Pierce-Keeton, Ph.D., Jessica Samson, Psy.D., Matthew Specht, Ph.D. (JHU). Independent Evaluators: Janice Keener, M.A., Katharina Kircanski, M.A., Audra Langley, Ph.D., Tami Roblek, Ph.D. (UCLA); Michael Gaffrey, M.S., Frank Gallo, M.S., Brook Marcks, M.S. (UWM); Mary Beth Beaudry, M.S.N., M.P.H., Margaret Schlossberg, Ph.D. (JHU). Data Center: (Yale) Lawrence Scahill, M.S.N., Ph.D., James Dziura, Ph.D., Lily Katsovich, M.S., M.B.A., Haibei Liu, M.P.H., Stephanie Argraves, M.S., Allison Gavaletz, B.A., Denis Sukhodolsky, Ph.D. Grant Management: Judit Ungar, M.S.W., Sue Levi-Pearl, Heather Cowley, Julie Noulas (TSA).
Additional Contributions: We thank the children and their families who made this study possible; and Judit Ungar, Heather Cowley, Julie Noulas (TSA), Joel Sherrill, Ph.D. (NIMH) and Gerald Golden, M.D. and Kevin Black, M.D. (Data and Safety Monitoring Board).
References
- 1.Khalifa N, von Knorring A. Prevalence of tic disorders and Tourette syndrome in a Swedish school population. Dev Med Child Neurol. 2003;45:315–319. doi: 10.1017/s0012162203000598. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prevalence of Diagnosed Tourette Syndrome in Persons Aged 6--17 Years --- United States, 2007. Morbidity and Mortality Weekly Report. 2009;58:581–585. [PubMed] [Google Scholar]
- 3.Leckman JF, Bloch MH, King RA, Scahill L. Phenomenology of tics and natural history of tic disorders. In: Walkup JT, Mink JW, Hollenbeck PJ, editors. Advances in Neurology: Tourette Syndrome. Vol. 99. Philadelphia, PA, US: Lippincott Williams & Wilkins Publishers; 2006. pp. 1–16. [Google Scholar]
- 4.Woods D, Marcks B, Flessner C. Management of social and occupational difficulties in persons with Tourette syndrom. In: Woods D, Piacentini J, Walkup JT, editors. Treating Tourette Syndrome and Tic Disorders. NY: Guilford Press; 2007. pp. 265–278. [Google Scholar]
- 5.Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette’s syndrome. Am J Psychiatry. 1993;150:98–102. doi: 10.1176/ajp.150.1.98. [DOI] [PubMed] [Google Scholar]
- 6.Jankovic J. Tourette’s Syndrome. NEJM. 2001;345:1184–1192. doi: 10.1056/NEJMra010032. [DOI] [PubMed] [Google Scholar]
- 7.Scahill L, Erenberg G, Berlin C, et al. Contemporary Assessment and Pharmacotherapy of Tourette Syndrome (2006) NeuroRx: J Am Soc Experimental NeuroTherapeutics. 2006;3:192–206. doi: 10.1016/j.nurx.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook C, Blacher J. Evidence-based psychosocial treatments for tic disorders. Clin Psychol: Sci Practice. 2007;14:252–267. [Google Scholar]
- 9.Evers R, Van De Wetering B. A treatment model for motor tics based on a specific tension reduction technique. J BehTher Exp Psychiat. 1994;25:255–260. doi: 10.1016/0005-7916(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 10.Miguel EC, Coffey BJ, Baer L, Savage CR, et al. Phenomenology of intentional repetitive behaviors in obsessive-compulsive disorder and Tourette's disorder. J Clin Psychiatry. 1995;56:246–255. [PubMed] [Google Scholar]
- 11.Conelea CA, Woods DW. The role of contextual factors in tic expression. J Psychosom Res. 2008;65:487–496. doi: 10.1016/j.jpsychores.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Woods DW, Piacentini JC, Chang S, et al. New York: Oxford University Press; 2008. Managing Tourette’s Syndrome: A Behavioral Intervention for Children and Adults. [Google Scholar]
- 13.Diagnostic and statistical manual of mental disorders, 4th ed., text rev.: DSMIV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 14.Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adol Psychiat. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Peterson A. Psychosocial management of tics and intentional repetitive behaviors associated with Tourette syndrome. In: Woods DW, Piacentini JC, Walkup JT, editors. Treating Tourette Syndrome and Tic Disorders: A Guide for Practitioners. New York: The Guilford Press; 2007. pp. 154–184. [Google Scholar]
- 16.Hanley G, Iwata B, McCord B. Functional analysis of problem behavior: A review. Journal of Applied Behavior Analysis. 2003;36:147–185. doi: 10.1901/jaba.2003.36-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson TS, Dufrene B, Weaver A, Butler T, Meeks C. Brief Antecedent Assessment and Treatment of tics in the general education classroom. Beh Modif. 2005;29:839–857. doi: 10.1177/0145445505279252. [DOI] [PubMed] [Google Scholar]
- 18.Goetz C, Horn S. Handbook of Tourette’s Syndrome and Related Tic and Behavioral Disorders. In: Kurlan R, editor. The treatment of tics. 2. New York: Marcel Dekker; 2005. pp. 411–426. [Google Scholar]
- 19.Wilhelm S, Deckersbach T, Coffey BJ, Bohne A, Peterson AL, Baer L. Habit reversal versus supportive psychotherapy for Tourette’s disorder: A randomized controlled trial. Am J Psychiat. 2003;160:1175–1177. doi: 10.1176/appi.ajp.160.6.1175. [DOI] [PubMed] [Google Scholar]
- 20.Albano AM, Silverman WK. The anxiety disorders interview schedule for DSM-IV, child version: clinician manual. New York: Oxford University Press; 1996. [Google Scholar]
- 21.Walkup J, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. NEJM. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guy W, Bonato R. CGI: Clinical Global Impressions. Chevy Chase, MD: National Institute of Mental Health. 1970 [Google Scholar]
- 23.Shaffer D, Gould MS, Brasic J, et al. A Children ’s Global Assessment Scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 24.Chang S, Himle M, Woods D, Tucker B, Piacentini J. Initial Psychometric Properties of a Brief Parent-Report Instrument for Assessing Tic Severity in Children with Chronic Tic Disorders. Child Fam Beh Ther. 2009;31:181–191. [Google Scholar]
- 25.Scahill L, Leckman J, Schultz R, Katsovich L, Peterson B. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60:1130–1135. doi: 10.1212/01.wnl.0000055434.39968.67. [DOI] [PubMed] [Google Scholar]
- 26.Jankovic J, Jimenez-Shahed J, Brown LW. A randomised, double-blind, placebo-controlled study of topiramate in the treatment of Tourette syndrome. J Neurology, Neurosurgery & Psychiatry. 2010;81(1):70–73. doi: 10.1136/jnnp.2009.185348. [DOI] [PubMed] [Google Scholar]
- 27.Sallee FR, Kurlan R, Goetz CG, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39:292–299. doi: 10.1097/00004583-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Borenstein M, Rothstein H, Cohen J. Power and Precision. Teaneck, NJ: Biostat; 1997. [Google Scholar]
- 29.Carpenter JR, Kenward MG. Birmingham: National Institute for Health Research, Publication RM03/JH17/MK; Missing data in randomised controlled trials - a practical guide. 2008 Available at http://www.pcpoh.bham.ac.uk/publichealth/methodology/projects/RM03_JH17_MK.shtml.
- 30.Allison PD. Sage. Thousand Oaks, CA: University Papers Series on Quantitative Applications in the Social Sciences; 2001. Missing Data. [Google Scholar]
- 31.Brown H, Prescott R. Applied Mixed Models in Medicine. Chichester, Eng: John Wiley & Sons, Ltd; 1999. [Google Scholar]
- 32.Samsa G, Edelman D, Rothman ML, et al. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics. 1999;15:141–155. doi: 10.2165/00019053-199915020-00003. [DOI] [PubMed] [Google Scholar]
- 33.Himle MB, Woods DW, Piacentini JC, Walkup J. Brief review of habit reversal training for Tourette's syndrome. J Child Neurol. 2006;21:719–725. doi: 10.1177/08830738060210080101. [DOI] [PubMed] [Google Scholar]
- 34.Swanson JM, Kraemer HC, Hinshaw SP, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40:168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Research Units on Pediatric Psychopharmacology Autism Network: Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- 36.Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292:1969–1976. doi: 10.1001/jama.292.16.1969. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert DL, Buncher CR. Assessment of scientific and ethical issues in two randomized clinical trial designs for patients with Tourette's syndrome: A model for studies of multiple neuropsychiatric diagnoses. J Neuropsychiatry Clin Neurosci. 2005;17:324–332. doi: 10.1176/jnp.17.3.324. [DOI] [PubMed] [Google Scholar]
- 38.Burd L, Kerbeshian J. Treatment-generated problems associated with behavior modification in Tourette disorder. Dev Med Child Neurol. 1987;29:831–833. [PubMed] [Google Scholar]
- 39.Gaffney GR, Perry PJ, Lund BC, Bever-Stille KA, Arndt S, Kuperman S. Risperidone versus clonidine in the treatment of children and adolescents with Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 2002;41:330–336. doi: 10.1097/00004583-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert DL, Batterson JR, Sethuraman G, Sallee FR. Tic reduction with risperidone versus pimozide in a randomized, doubleblind, crossover trial. J Am Acad Child Adolesc Psychiatry. 2004;43:206–214. doi: 10.1097/00004583-200402000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Ross MS, Moldofsky H. Comparison of pimozide and haloperidol in the treatment of Gilles de la Tourette syndrome. Am J Psychiatry. 1978;135:585–587. doi: 10.1176/ajp.135.5.585. [DOI] [PubMed] [Google Scholar]
- 42.Kushner HA. Cursing brain? The histories of Tourette syndrome. Cambridge: Harvard University Press; 1999. [Google Scholar]
- 43.Stevens J, Blachly H. Successful treatment of the maladie des tics in Gilles de la Tourette's syndrome. Am J Dis Children. 1966;112:541–545. doi: 10.1001/archpedi.1966.02090150085006. [DOI] [PubMed] [Google Scholar]
- 44.Baym CL, Corbett BA, Wright SB, Bunge SA. Neural correlates of tic severity and cognitive control in children with tourette syndrome. Brain: A Journal of Neurology. 2008;131:165–179. doi: 10.1093/brain/awm278. [DOI] [PubMed] [Google Scholar]
- 45.Church JA, Fair DA, Dosenbach NUF, et al. Control networks in paediatric tourette syndrome show immature and anomalous patterns of functional connectivity. Brain: A Journal of Neurology. 2009;132:225–238. doi: 10.1093/brain/awn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry. 2009;166:664–674. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graybiel A, Canales J. The neurobiology of repetitive behaviors: Clues to the neurobiology of Tourette syndrome. In: Cohen DJ, Jankovic J, Goetz C, editors. Advances in Neurology:Tourette syndrome. Vol. 99. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 123–132. [PubMed] [Google Scholar]
- 48.Kalanithi PSA, Zheng W, Kataoka Y, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci USA. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mink JW. Neurobiology of basal ganglia and Tourette syndrome: Basal ganglia circuits and thalamocortical outputs. In: Walkup JT, Mink JW, Hollenbeck PJ, editors. Advances in Neurology: Tourette syndrome. Vol. 99. Philadelphia: Lippincott Williams & Wilkins Publishers; 2006. pp. 89–98. [PubMed] [Google Scholar]