Abstract
Rationale
PPARs (α, γ and δ/β) are nuclear hormone receptors and ligand-activated transcription factors that serve as key determinants of myocardial fatty acid metabolism. Long-term cardiomyocyte-restricted PPARδ deficiency in mice leads to depressed myocardial fatty acid oxidation (FAO), bioenergetics and premature death with lipotoxic cardiomyopathy.
Objective
To explore the essential role of PPARδ in the adult heart.
Methods and Results
We investigating the consequences of inducible short-term PPARδ knockout in the adult mouse heart. In addition to a substantial transcriptional downregulation of lipid metabolic proteins, short-term PPARδ knockout in the adult mouse heart attenuated cardiac expression of both Cu/Zn superoxide dismutase (SOD1) and manganese superoxide dismutase (SOD2), leading to increased oxidative damage to the heart. Moreover, expression of key mitochondrial biogenesis determinants such as PPARγ coactivator-1 were substantially decreased in the short-term PPARδ deficient heart, concomitant with decreased mitochondrial DNA copy number. Rates of palmitate and glucose oxidation were markedly depressed in cardiomyocytes of PPARδ knockout hearts. Consequently, PPARδ deficiency in the adult heart led to depressed cardiac performance and cardiac hypertrophy.
Conclusions
PPARδ is an essential regulator of cardiac mitochondrial protection and biogenesis and PPARδ activation can be a potential therapeutic target for cardiac disorders.
Keywords: PPARδ, anti-oxidants, mitochondrial biogenesis, cardiac function
Introduction
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated nuclear receptors with well documented roles in the transcriptional regulation of lipid and carbohydrate metabolisms in various tissues. In the heart, the three subtypes of PPARs, PPARα, γand δ are expressed at various levels and play important roles in myocardial lipid and glucose metabolisms1–7. We have demonstrated that PPARδ is required for normal transcript expression of series essential enzymes of fatty acid oxidation (FAO) in the heart and that cardiomyocyte-restricted PPARδ knockout resulted in depressed myocardial FAO and bioenergetics, cardiac hypertrophy and heart failure8, 9. Furthermore, there is emerging evidence demonstrating that PPARs may play a role in regulating transcriptional expression of antioxidants, such as Cu/Zn-Superoxide dismutase (SOD1) 10, manganese superoxide dismutase (SOD2)11, 12, and catalase13–15. In a recent study we demonstrated that PPARγ is essential for the full expression of SOD2 transcript in the heart14. Recent evidence has been emerging that PPARδ may contribute to transcriptional regulation of mitochondrial biogenesis in skeletal muscle16, 17. However, it remains unknown whether PPARδ regulates transcriptional expression of important anti-oxidants and key determinants of mitochondrial biogenesis to maintain cardiac mitochondrial biogenesis and function.
Mitochondria are the powerhouses for the cell and are vulnerable targets of oxidative damage. The maintenance of redox homeostasis is critical for normal cellular function and is particularly important for the heart with high energy demand. Impaired mitochondria structure/function as a result of increased oxidative stress is an important contributor to the pathogenesis of heart disease. Even under normal physiological conditions, the utilization of oxygen for energy generation in the mitochondria results in the production of reactive oxygen species (ROS). Typically redox homeostasis is maintained by a balance between ROS formation and endogenous antioxidant defenses. It has been well established that multiple endogenous antioxidants such as SOD110, SOD211, 12 and catalase18, 19 are all crucial in maintaining cellular redox balance. However, if this balance is disturbed, oxidative stress increases, leading to damage of essential cellular components, including the mitochondria. On the other hand, mitochondrial biogenesis is a continuous renewal process marked by regular mitochondrial turnover with a defined half-life. The molecular mechanisms underlying its regulation, especially its response to pathophysiological stimuli, including oxidative stress, remain puzzling. The roles of PPAR coactivator 1 (PGC-1α and β)20–24, nuclear regulatory factor 1 (NRF-1) and NRF2s (NRF2a and b)24, 25 in mitochondrial biogenesis have been documented. It is also well documented that PGC-1α is an important coactivator for a series of nuclear receptors, including PPARδ and estrogen related receptor-α (ERRα)26, 27. PPARδ can directly regulate the expression of PGC-1α and may contribute to transcriptional regulation of mitochondrial biogenesis in skeletal muscle17, 26. However, it remains unclear whether PPARδ is essential in the transcriptional regulation of mitochondrial biogenesis in the heart.
In the present study, we used an inducible gene targeting approach that enables short-term inactivation of PPARδ in the adult heart. We utilized this approach to examine the role of PPARδ in the adult heart not only in the transcriptional regulation of myocardial fatty acid oxidation (FAO) but also its potential role in regulating endogenous antioxidants as well as mitochondrial biogenesis. We demonstrate for the first time that PPARδ not only regulates the expression of both SOD1 and SOD2, but also is an essential determinant of mitochondrial biogenesis in the heart. These findings will help provide insights into the role of PPARδ in governing metabolic homeostasis of the heart and in the development of novel therapeutic target for cardiac disorders.
Methods and Materials
Tamoxifen-inducible cardiomyocyte-restricted PPARδ knockout line (TMPD)
Homozygous TMPD mice (C57/B6) were generated by crossing a transgenic line with α-MyHC driven Mer-Cre-Mer (MCM) overexpression28 and the PPARδflox/flox line29. Cardiomyocyte-restricted PPARδ knockout was induced in 2–3 month-old mice by 5 days of Tamoxifen treatment (2 mg/200 µ l in sunflower oil, intraperitoneal injection). All experimental procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals of the National Institutes of Health, and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham (UAB).
Glucose uptake, glucose oxidation and palmitate oxidation rates in cultured adult mouse cardiomyocytes
Adult cardiomyocytes were isolated from 9~13 week old mice according to a published protocol30. Glucose uptake and glucose and palmitate oxidation rates were measured as described in the Online material.
Transcript analyses
Total RNA samples were extracted from left ventricles and analyzed by Quantitative real-time RT-PCR (QPCR) as described in the Online material.
Protein analysis
Protein samples were prepared and analyzed using Western blots as described in the Online material.
Analysis of Mitochondrial DNA copies
Mitochondrial DNA copy number was evaluated as described in the Online material.
Myocardial lipid profile
Quantification of TG content and individual species was measured by the Analytical Resources Core of the National Mouse Metabolic Phenotyping Center at Vanderbilt University as described in the Online material.
Echocardiography measurement
Echocardiographic measurement with a high resolution echocardiograph system (Visualsonic VEVO 770 System) was used to detect cardiac structure alterations and cardiac function in vivo by using echocardiogram with a 35 MHz probe. Details were described in the Online material.
Mitochondrial oxidative stress
Methods for analyses of oxidative stress of cardiac mitochondria were described in the Online material.
Statistics analysis
Data for two group comparison were analyzed using Student's t-test; otherwise the data were analyzed by one factor or mixed, two-factor analysis of Variance (ANOVA) using GraphPad Prism software (GraphPad Software Inc.). Values of quantitative results were expressed as mean±SEM. Differences between groups and treatments were regarded as significant at the P<0.05 probability level, denoted as * p<0.05, ** p<0.01, *** p<0.001 in the charts and tables, unless indicated.
Results
Induced cardiomyocyte-restricted PPARδ inactivation in adult mice down-regulates cardiac expression of lipid metabolic protein
To investigate the potential roles of PPARδ in the adult heart, we studied a mouse line with tamoxifen induced cardiomyocyte-restricted PPARδ knockout. QPCR revealed that PPARδ transcript was markedly decreased in TMPD compared with TMCM hearts 5 days after the end of tamoxifen treatment (Figure 1A). Western blot analysis revealed that PPARδ protein contents in left ventricles from TMPD were about 20% of those from TMCM hearts from the above treatment groups (Figure 1B). On the other hand, the expression of the other two PPARs was not altered in the TMPD heart (Online Figure I). QPCR further revealed that transcript levels fatty acid uptake and FAO genes, such as heart type fatty acid binding protein (FABP), fatty acid transport protein 1 (FATP-1), palmitoyltransferase Ib (CPTIb) and palmitoyl acyl-CoA oxidase 1 (ACOX1) were respectively decreased by ~40, ~39, ~37, and ~61% in TMPD mice compared with TMCM controls (Figure 1C). Correspondingly, protein contents of FABP, FATP-1 and CPTIb in TMPD hearts were also decreased relative to those in TMCM mice, (Figure 1D). Interestingly, transcript expression of Glut1, but not Glut4, was decreased in the TMPD heart (Figure 1E). In protein level, Glut1expression was decreased, whereas Glut4 expression was upregulated (Figure 1F). These results confirmed that PPARδ plays essential role in transcriptional regulation of myocardial lipid and glucose metabolism.
Figure 1. Temporal PPARδ knockout in adult mouse heart (5 days after the end of tamoxifen treatment) and substrate metabolic gene expression (14 days after the end of tamoxifen treatment).
A) QPCR results of PPARδ mRNA levels on RNA samples extracted from ventricular tissues of TMCM and TMPD mice (n=4~6, ** p<0.01 vs TMCM). B) Western blotting results of PPARδ protein levels in nuclear proteins from ventricular tissues of TMCM and TMPD mice (n=6, ***p<0.001 vs TMCM). C) Transcript level of FABP, FATP-1, CPT1b and ACOX1 in samples from ventricular tissues of TMCM and TMPD mice (n=4~6, * p<0.05, **p<0.01 vs TMCM). D) Protein levels of FABP, FATP-1, and CPT-Ib in samples from ventricular tissues of TMCM and TMPD mice (n=4~6, *p<0.05, ***p<0.001 vs TMCM). E) Transcript levels of Glut1 and Glut4 in samples from ventricular tissues of TMCM and TMPD mice (n=4~6, *p<0.05 vs TMCM). F) Protein levels of Glut1 and Glut4 in samples from ventricular tissues of TMCM and TMPD mice 14 (n=4~6, *p<0.05 vs TMCM).
Short-term induction of PPARδ deficiency in adult hearts leads to downregulation of SOD1 and SOD2, leading to increased oxidative stress
We further investigate whether PPARδ regulates transcriptional expression of antioxidants. QPCR revealed that transcripts of SOD1 and SOD2 were substantially attenuated in TMPD relative to TMCM hearts (Figure 2A). Western blotting analysis showed that the protein levels of SOD1 and SOD2 in TMPD hearts were decreased by ~43% and ~15% of those of control hearts (Figure 2B). However, both transcript and protein levels of catalase were unaltered in the TMPD relative to TMCM hearts (Figure 2A and 2B). We further evaluated cardiac mitochondrial oxidative stress on freshly isolated mitochondria samples from cardiac ventricular tissue. Cytochrome C reduction assays revealed that superoxide production in the TMPD hearts was increased by ~33% compared with those of controls (Figure 3A). As a result, the TMPD hearts exhibited substantially suppressed mitochondrial aconitase activities (Figure 3B). Moreover, mitochondrial membrane potential estimated by JC-1 uptake on samples isolated from TMPD hearts was declined by ~30% compared with those of TMCM hearts (Figure 3C). MTT assay revealed that the activity of mitochondrial dehydrogenases was ~25% lower in mitochondria from TMPD group than those of control (Figure 3D). Taken together, these results indicate that short-term PPARδ deletion leads to augmentation of reactive oxidative stress, supporting an essential role of PPARδ in anti-oxidant defense of the heart.
Figure 2. Cardiac expression of endogenous anti-oxidants in mice 14 days after tamoxifen treatment.
A) QPCR analyses of transcript expression of SOD1, SOD2 and catalase on samples extracted from ventricular tissue of TMPD and TMCM mice (n=4, *p<0.05, **p<0.01). B) Western blotting analyses of protein level of SOD1, SOD2 and Catalase (n=6~12, **p<0.01, ***p<0.001).
Figure 3. Assessments of cardiac mitochondrial oxidative stress in mice 14 days after tamoxifen treatment.
A) Superoxide release from mitochondria was evaluated by using cytochrome C reduction assays on cardiac mitochondria freshly isolated from TMPD and TMCM mice (n=10~16, *p<0.05). B) Measurement of aconitase activity on samples isolated from TMPD and TMCM mice (n=6~9, ***p<0.001). C) Mitochondria membrane potential was accessed JC-1 uptake in mitochondria isolated from TMPD and TMCM hearts (n=4, ***p<0.001). D) Mitochondrial dehydrogenase activities were assessed by MTT assay on samples from TMPD and TMCM hearts (n=18, ***p<0.001).
Short-term induction of PPARδ deficiency in adult hearts leads to depressed expression of transcriptional regulators of mitochondrial biogenesis, concomitant with mitochondrial depletion
We further investigate whether PPARδ is a key determinant of mitochondrial biogenesis. QPCR revealed that the expression of mitochondrial biogenesis determinants, such as PPARγ co-activator 1 (PGC -1α and -1β), nuclear respiratory factor (NRF) 1, and NRF2 (α and β subunit), was substantially declined in TMPD compared to TMCM hearts (Figure 4A). Western blotting analysis on samples of nuclear proteins extracted from ventricular tissues also confirmed that protein levels of PGC-1α, PGC-1β, NRF-1, NRF2a and NRF2b in TMPD hearts were markedly decreased compared with those of the TMCM (Figure 4B, respectively).
Figure 4. Expression of key determinants of mitochondrial biogenesis in mice 14 days after tamoxifen treatment.
A) QPCR measurement of transcript levels of NRF-1, NRF2 (a and b subunits), and PGC -1α and -1β on samples from TMPD and TMCM hearts (n=4~5, ** p<0.01, ***P<0.001). B) Western blotting analyses on relative protein levels of PGC-1α, PGC-1β, NRF-1, NRF-2a and NRF2-b on samples of nuclear proteins extracted from ventricular tissues of TMPD and TMCM mice (n=4~6, * p<0.05, ** P<0.01, ***P<0.001).
TEM assessment of heart sections from TMPD mice revealed striking mitochondrial abnormalities (Figure 5A), with a 45% decrease of mitochondria volume (Figure 5B) and apparent increase of vacuolated mitochondria compared with those of controls (Figure 5A). TEM also showed the widening of the I-band in TMPD heart sections (Figure 5A). The mtDNA copy number was decreased by ~1/3 at 14 days after tamoxifen treatment in the TMPD relative to TMCM hearts (Figure 5C). Transcript expression of mitochondrial proteins such as mitofusin 1 (Mfn1), mitofusin 2 (mfn2), Cytochrome b5 (Cyto b), Cytochrome c oxidase subunit II and Cytochrome c oxidase subunit 7c (Cox7c) was also decreased in TMPD hearts (Figure 5D). Western blot analysis revealed that Cyt b, Mfn2, and mitochondrial fission 1 (Drp1) were decreased, whereas mitochondrial fission 1 protein (fis1) remain unchanged in the TMPD compared to TMCM hearts (Figure 5E). Therefore, these results point to a novel function of PPARδ in the heart as a key regulator of mitochondrial biogenesis, and may also be involved in the regulation of mitochondrial fission and fusion.
Figure 5. Effect of PPARδ deficiency (14 days after the end of tamoxifen treatment) on mitochondrial depletion.
A) Representative TEM micrographs from hearts section of TMCM and TMPD hearts. B) Volume of mitochondria analyzed on electron micrographs (2700×) of TMPD and TMCM heart sections. The volume of mitochondria was expressed as a percentage of the total area in the TEM micrographs. Data are from 7 randomly selected images (*** p<0.001). C) QPCR measurement of Mitochondrial DNA to nuclear DNA copy number in cardiac tissues from TMPD and TMCM mice. The TMCM value was arbitrary set as 100 (n=4, ** p<0.01). D) QPCR measurement of transcript levels of Mfn1, mfn2, Cyto b, Cytochrome c oxidase subunit II and Cytochrome c oxidase subunit 7c (Cox7c) on samples from TMPD and TMCM hearts (n=4~5, * p<0.05, ** p<0.01, *** p<0.01). E) Western blot analysis of the protein levels of mitochondrial proteins, Cyt b, DRP1, fis1 and Mfn2 in TMPD vs TMCM mice (n=4~7, * P<0.05, ** p<0.01).
Short-term PPARδ deficiency in the adult heart leads to depressed rates of both palmitate and glucose oxidation in cardiomyocytes
To determine the consequence of the depressed lipid metabolic gene expression and mitochondrial depletion on energy metabolism, rates of palmitate and glucose oxidation were evaluated in cultured cardiomyocytes from the short-term PPARδ deficient hearts. 14C palmitate oxidation rate in cultured cardiomyocytes isolated from adult TMPD hearts (14 days after the end of Tamoxifen treatment) was attenuated by ~40% compared with those of TMCM hearts (Figure 6A). The rate of glucose oxidation was also repressed in TMPD cardiomyocytes compared with controls (Figure 6B), which was in consistent with the glucose uptake activity (Figure 6C). Lipid profiles revealed that phospholipids, triglyceride and cholesterol in the ventricular tissues remained at similar levels (Figure 6F). However, the percentage of triglyceride with palmitic (16:00) was increased by about 20%, whereas percentage of linoleic (18:02) was slightly declined (Figure 6G). The phospholipids profile displayed little change with only the proportion of arachidonic (20:04) was modestly increased (Figure 6H). These results further confirm the essential role of PPARδ in mitochondrial substrate metabolism in the adult heart.
Figure 6. Rates of palmitate and glucose oxidation and lipid profile in TMPD mice 14 days after the end Tamoxifen treatment.
A) Rate of palmitate oxidation in cultured cardiomyocytes from TMPD and TMCM mice were measured using 14C labeled palmitate (n=6~9, ***p<0.001). B) Rate of glucose oxidation in cardiomyocytes from TMPD and TMCM mice was measured using 14C labeled glucose (n=6~9, **p<0.01). C) Activity of glucose uptake measured on cultured cardiomyocytes from TMPD and TMCM mice 14 days after the end of Tamoxifen treatment (n=9~14, ** p<0.01). D) Lipid profiles of myocardial phospholipid, triglyceride, and Cholesterol in TMCM and TMPD mouse heart 14 days after the end of Tamoxifen treatment (n=5~7, *p<0.05). E) The fatty acyl composition of triglyceride in TMPD and TMCM hearts 14 days after the end of Tamoxifen treatment (n=6~7, *p<0.05). F) The fatty acyl composition of phospholipids in TMPD and TMCM hearts (n=5~7, *p<0.05).
PPARδ deficiency in adult heart leads to cardiac dysfunction and cardiac hypertrophy
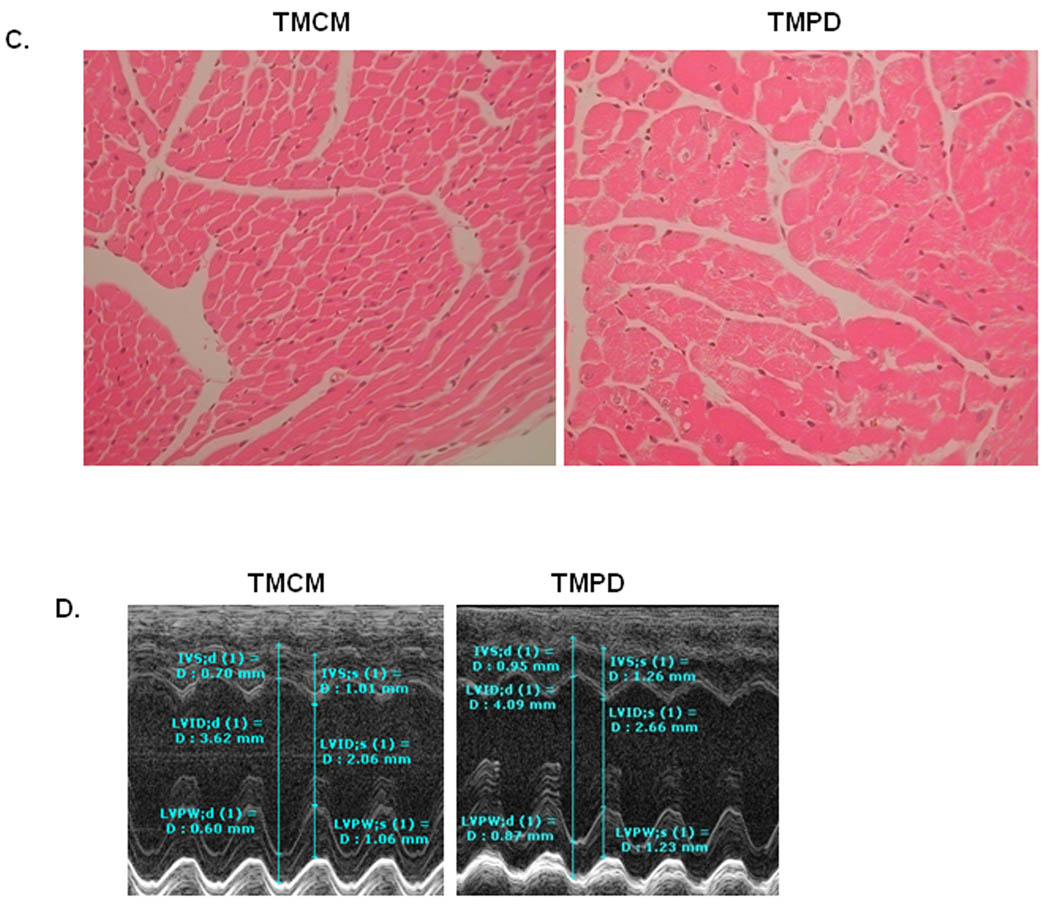
With defects of myocardial FAO and mitochondrial depletion, the TMPD mice with PPARδ deficiency in a short period (14 days after tamoxifen treatment) in their hearts exhibited cardiac hypertrophy and cardiac dysfunction, and became more severe with longer duration (7 months after the end of tamoxifen treatment). Heart weight /body weight ratio were increased about 14% in TMPD mice after 14 days of induced PPARδ knockout compared to TMCM mice (Figure 7A). The transcript expression level of a hypertrophic marker, β Myosin heavy-chain (MYH7B), was increased (Figure 7B). Histological images of H&E staining heart sections demonstrated cardiomyocyte hypertrophy with slight disarray (Figure 7C). Echocardiographic assessment (Figure 7D) revealed that the prolonged PPARδ deficient hearts were enlarged, with dilated chambers and thickening septum and posterior wall of left ventricles (Online Table II). Cardiac hypertrophy in the TMPD mice was further confirmed by the increased LV mass to body weight ratio compared with that of the TMCM mice (Online Table II). As a result, ejection fraction (EF) was declined by ~12% and fractional shortening (FS %) was declined by ~16.5 % in the TMPD hearts compared with TMCM hearts (Online Table II). Mitral valve inflow velocity with relatively prolonged deceleration rate and time was also noted in TMPD compared with TMCM hearts (Online Table II). Cardiac function were further impaired in TMPD relative to TMCM mice 7 months after the end of tamoxifen treatment (Online Table III). EF was declined by ~22% and FS % was declined by ~28.9% in the TMPD hearts compared with TMCM hearts (Online Table III).
Figure 7. Cardiac hypertrophy and heart function in mice 14 days after tamoxifen treatment.
A) Measurement of heart weight /body weight ratio in TMPD and TMCM mice (n=4~6, * p<0.05). B). QPCR measurement of transcript expression of β-MyHC (MYH7b) on samples from TMPD and TMCM hearts. (n=4, **p<0.01). B) Images of heart sections stained with H&E from TMPD and TMCM mice (objective 20×). Note the enlarged cardiomyocytes in the TMPD heart relative to TMCM heart. C) Representative images of Echocardiograph in TMPD and TMCM mice. The photographs show enlargement of the heart, with increase of left ventricular chamber and thickness of septum and posterior wall (for further details, see Online Tables II and III).
Discussion
The goal of this study was to examine the role of PPARδ in the adult heart using a temporal, tissue-specific PPARδ knockout approach in mice. Our findings indicate that short-term PPARδ deficiency in the adult mouse heart suppresses cardiac expression of key lipid metabolic proteins, essential endogenous anti-oxidants and transcriptional regulators of mitochondrial biogenesis, resulting in diminished fatty acid and glucose metabolism, mitochondrial depletion and abnormalities, cardiac dysfunction and hypertrophy.
We previously demonstrated that PPARδ is required for normal transcript expression of series essential enzymes that govern myocardial FAO in the long-term cardiomyocyte-restricted PPARδ knockout (CR-PPARδ−/−) mouse model3, These mice exhibited depressed myocardial FAO and bioenergetics, eventually leading to dilated cardiomyopathy with myocardial lipid accumulation in adult and older mice3, 9. The gene knockout event in the CR-PPARδ−/− mice, mediated by α-MyHC-driven expression of Cre recombinase, is expressed in ventricles during early development and robustly from new born stage. The heart mainly utilizes glucose as substrate to generate ATP in a new born animal and switches to utilize long chain fatty acids after weaning, which is concomitant with transcriptional upregulation of FAO genes31. Consequently, the early deficiency of fatty acid metabolism due to PPARδ inactivation may impact the normal developmental transition on the switch of substrate utilization, causing confounding phenotypes. Subsequent compensatory adaptive responses could obscure some of the primary regulatory effects of PPARδ in the heart. The temporally controlled, cardiomyocyte-restricted PPARδ deletion has the advantage in uncovering PPARδ’s function in an adult heart. More important regulation roles of PPARδ could therefore be detected. In the current model, we uncovered additional functions of PPARδ, such as glucose metabolism, anti-oxidants and mitochondrial biogenesis, in the adult heart. Recently, Koitabashi et al have reported that tamoxifen-mediated MerCreMer nuclear translocation can induce severe transient dilated cardiomyopathy and dramatic increase of mortality in mice with or without loxP transgene32. However, we did not observed similar phenotypic changes at various time points using this approach (Online Figure I). Most importantly, our study used TMCM mice as control to account for confounding effects of Tamoxifen induced Cre expression in the heart. While the CR-PPARδ−/− hearts manifested progressive cardiac hypertrophy and heart failure, it is noteworthy that the long-term effect of PPARδ deficiency initiated in an adult heart was relatively modest compared with those of CR-PPARδ−/− hearts at similar adult ages. We observed more severe cardiac hypertrophy and cardiac dysfunction in TMPD mice but with no substantial decline of survival 7 months after the end of tamoxifen treatment. The consequences of early cardiac PPARδ deficiency likely contribute to the severe phenotypic changes in the CR-PPARδ−/− mice. It appears that the adult heart might have greater compensatory capacity in response to disturbances in myocardial homeostasis. Noticeably, the declined expression of both anti-oxidants and key factors in mitochondrial biogenesis was reversed (data not shown), possibly by other transcriptional regulators. Additional stimulation can augment the compensatory effect33. Therefore, the current findings are only possible with the use of the temporal conditional gene targeting approach, defining an important role of PPARδ in the adult heart.
In the current study we report for the first time that PPARδ is a key transcription regulator of anti-oxidant defense and mitochondrial biogenesis in the adult heart. Transcriptional regulation of antioxidants has been extensively investigated. Furthermore, SOD1 was reported to be activated partly through the PPAR response element (PPRE) in its promoter13. We have also demonstrated that SOD2 is a direct target gene of PPARγ with a functioning PPRE consensus sequence in SOD2 promoter14. Our current study indicates that both SOD1 and SOD2 may be constitutively regulated by PPARδ. Despite the potential compensatory upregulation of these proteins in response to hypertrophic stimuli34, 35, the expression of these proteins is remarkably decreased in the PPARδ deficient heart. In addition, the increased superoxide leads to augmented oxidative stress with marked mitochondrial oxidative injuries. Therefore, these observations provide insights into the novel aspects that endogenous antioxidants are transcriptional regulating targets of PPARs.
Mitochondrial biogenesis, which is the growth and division of pre-existing mitochondria, is essential for the cell to cope with changes in energy demand25. The link between mitochondrial biogenesis and cardiac pathologies such as the hypertrophied or failing heart has increased the interest of the scientific community in this process and its regulations36. Regulation of mitochondrial biogenesis and function has been extensively studied, in particular with regard to the role of the PGC-1 family of transcriptional coactivators20, 24. A potential role of PPARδ in determining mitochondrial biogenesis in skeletal muscle has been suggested16. However, it remains unclear whether PPARδ directly mediates the transcriptional regulation of mitochondrial biogenesis in the adult heart37, 38. We have recently demonstrated that PPARδ directly regulates the expression of Mfn 2, an important mitochondrial protein in mitochondrial fusion and hence biogenesis39. In the current study, we found that PPARδ deficiency led to a remarkable decreased transcript and protein expression of both PGC-1α and PGC-1-β, the well documented transcriptional determinants of mitochondrial biogenesis20. It has been known that disruption of normal mitochondrial function is an important stimulus for mitochondrial biogenesis 25. With increased oxidative stress due to the diminished expression of key anti-oxidants, the depressed mitochondrial biogenesis in the TMPD heart is not likely a secondary response. Furthermore, it has been shown that PPARδ regulates PGC-1α gene expression by binding to the PPRE consensus sequence in the PGC-1α promoter17, 26. Since PGC-1α could induce NRF-1 and NRF-2 expression40, 41, it is plausible that the reduced NRF-1 and NRF2 expression is directly related to decrease expression of PGC-1α in the PPARδ deficient heart. Because PGC-1α functions as a coactivator that boosts PPARδ activation by direct protein–protein interaction42, 43, stimulation of mitochondrial biogenesis by PGC-1α could at least in part be PPARδ-mediated and could be relevant for the downstream metabolic responses. Therefore, it appears that PPARδ directed key mitochondrial protein expression may represent part of PPARδ’s role in governing mitochondrial biogenesis. The downregulation of Glut1 expression could be the results of depressed PGC-1α37. In addition, the mitochondrial deficiency due to the depressed mitochondrial biogenesis and function should further contribute to the decrease of both palmitate and glucose oxidation in the TMPD heart.
With the obvious advantage of the tamoxifen inducible, cardiomyocyte-restricted gene knockout approach, we have found for the first time that PPARδ plays a crucial role not only in regulating myocardial lipid and glucose metabolism, but also in maintaining mitochondrial biogenesis and function via controlling expression of important endogenous anti-oxidants and key transcription regulators of mitochondrial protein expression. Therefore, we conclude that PPARδ not only regulates mitochondrial gene subgroup with roles in FAO, but also control other gene subgroups with roles in mitochondrial biogenesis and function.
Novelty and Significance
What is Known?
PPARδ plays an essential role in regulating myocardial fatty acid metabolism.
Long-term PPARδ deficiency in the heart leads to a myocardial bioenergetic defect and lipotoxic cardiomyopathy.
Cardiac-specific transgenic overexpression of PPARδ enhances glucose utilization and protects the heart from ischemia/reperfusion damage.
What New Information Does This Article Contribute?
PPARδ deficiency in the adult heart impairs expression of important proteins in myocardial fatty acid and glucose metabolism, endogenous anti-oxidants and mitochondrial biogenesis.
PPARδ is an essential determinant of substrate metabolism and mitochondrial capacity in the adult heart.
The study is the first to show that PPARδ is essential in the transcriptional regulation of mitochondrial defense and biogenesis in the adult heart, in addition to its important roles in regulating fatty acid and glucose utilization. The heart as a pump requires continuous supply of energy. Mitochondria are the powerhouse in generating energy through oxidative phosphorylation. In a failing heart, myocardial energy homeostasis is perturbed, with oxidative damage, excessive reactive oxygen species (ROS), and mitochondrial depletion. Therefore, the ability of PPARδ to optimize myocardial substrate utilization, remove excessive ROS, and maintain mitochondrial biogenesis makes it a potentially effective therapeutic intervention for heart failure.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by grants from National Institute of Health (1R01HL085499, 1R01HL084456 and R21 AT003734).
Non-standard Abbreviations and Acronyms
- FAO
fatty acid oxidation
- NRF
nuclear respiratory factor
- PGC-1
PPARγco-activator 1
- ERRα
estrogen related receptor-α
- ACOX1
palmitoyl acyl-CoA oxidase 1
- CPTIb
carnitine palmitoyltransferase Ib
- FATP-1
fatty acid transport protein-1
- h-FABP
heart type fatty acid binding protein
- Mfn1
mitofusin 1
- Mfn2
mitofusin 2
- Cyto b
Cytochrome b5
- Cox2
Cytochrome c oxidase subunit 2
- Cox7c
Cytochrome c oxidase subunit 7c
- DRP1
mitochondrial fission 1
- Fis1
mitochondrial fission 1 protein
- MYH7B
β Myosin heavy-chain
- AMPK
AMP-activated protein kinase
- rcan1
regulator of calcineurin 1
- TEM
transmission electron microscopy
- LVID;s and LVID;d
left ventricular dimension at systole and diastole
- LVPW;s and LVPW;d
posterior wall thickness at systole and diastole
- IVS;s and IVS; d
interventricular septal wall thickness (diastole and systole)
- EF%
Ejection fraction
- FS%
fractional shortening
- volume;s and volume;d
left ventricle volume at systole and diastole
- SV
left ventricle stroke volume
- CO
cardiac output
- MV E
E wave velocity of mitral valve inflow
- MV A
A wave velocity of mitral valve inflow
- MV Decel rate
mitral valve deceleration rate
- MV Decel Time
Mitral valve deceleration time
- MV E/A
ratio of E and A wave velocity of mitral valve inflow
- BPM
beats per minute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
None
References
- 1.Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ, Kelly DP. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator- activated receptor alpha- deficient mice. The Journal of clinical investigation. 1998;102:1083–1091. doi: 10.1172/JCI3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilde AJ, van der Lee KA, Willemsen PH, Chinetti G, van der Leij FR, van der Vusse GJ, Staels B, van Bilsen M. Peroxisome proliferator-activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circulation research. 2003;92:518–524. doi: 10.1161/01.RES.0000060700.55247.7C. [DOI] [PubMed] [Google Scholar]
- 3.Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, Chen YE, Yang Q. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–1250. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Cheng LH. Molecular regulation of lipotoxicity in the heart. Drug Discovery Today. 2005;2:101–107. [Google Scholar]
- 5.Yang Q, Li Y. Roles of PPARs on regulating myocardial energy and lipid homeostasis. Journal of molecular medicine (Berlin, Germany) 2007;85:697–706. doi: 10.1007/s00109-007-0170-9. [DOI] [PubMed] [Google Scholar]
- 6.Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. The Journal of clinical investigation. 2007;117:3930–3939. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. The Journal of clinical investigation. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng L, Ding G, Qin Q, Xiao Y, Woods D, Chen YE, Yang Q. Peroxisome proliferator-activated receptor delta activates fatty acid oxidation in cultured neonatal and adult cardiomyocytes. Biochem Biophys Res Commun. 2004;313:277–286. doi: 10.1016/j.bbrc.2003.11.127. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Hu Q, Nakamura Y, Wang X, Zhang X, Zhu X, Chen W, Yang Q, Zhang J. Open-chest 31P magnetic resonance spectroscopy of mouse heart at 4.7 Tesla. J Magn Reson Imaging. 2006;24:1269–1276. doi: 10.1002/jmri.20766. [DOI] [PubMed] [Google Scholar]
- 10.Francke U, Taggart RT. Assignment of the gene for cytoplasmic superoxide dismutase (Sod-1) to a region of chromosome 16 and of Hprt to a region of the X chromosome in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:5230–5233. doi: 10.1073/pnas.76.10.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Patel VV, Kostetskii I, Xiong Y, Chu AF, Jacobson JT, Yu C, Morley GE, Molkentin JD, Radice GL. Cardiac-specific loss of N-cadherin leads to alteration in connexins with conduction slowing and arrhythmogenesis. Circulation research. 2005;97:474–481. doi: 10.1161/01.RES.0000181132.11393.18. [DOI] [PubMed] [Google Scholar]
- 12.Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo HY, Chang MS, Rho HM. Induction of the rat Cu/Zn superoxide dismutase gene through the peroxisome proliferator-responsive element by arachidonic acid. Gene. 1999;234:87–91. doi: 10.1016/s0378-1119(99)00176-6. [DOI] [PubMed] [Google Scholar]
- 14.Ding G, Fu M, Qin Q, Lewis W, Kim HW, Fukai T, Bacanamwo M, Chen YE, Schneider MD, Mangelsdorf DJ, Evans RM, Yang Q. Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc Res. 2007;76:269–279. doi: 10.1016/j.cardiores.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Girnun GD, Domann FE, Moore SA, Robbins ME. Identification of a functional peroxisome proliferator-activated receptor response element in the rat catalase promoter. Mol Endocrinol. 2002;16:2793–2801. doi: 10.1210/me.2002-0020. [DOI] [PubMed] [Google Scholar]
- 16.Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuler M, Ali F, Chambon C, Duteil D, Bornert JM, Tardivel A, Desvergne B, Wahli W, Chambon P, Metzger D. PGC1alpha expression is controlled in skeletal muscles by PPARbeta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell metabolism. 2006;4:407–414. doi: 10.1016/j.cmet.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 19.Kunau WH, Dommes V, Schulz H. beta-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog Lipid Res. 1995;34:267–342. doi: 10.1016/0163-7827(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 20.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. The Journal of clinical investigation. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell metabolism. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 25.Hock MB, Kralli A. Transcriptional Control of Mitochondrial Biogenesis and Fucntion. Anuu. Rev. Physiol. 2009;71:177–203. doi: 10.1146/annurev.physiol.010908.163119. [DOI] [PubMed] [Google Scholar]
- 26.Hondares E, Pineda-Torra I, Iglesias R, Staels B, Villarroya F, Giralt M. PPARdelta, but not PPARalpha, activates PGC-1alpha gene transcription in muscle. Biochem Biophys Res Commun. 2007;354:1021–1027. doi: 10.1016/j.bbrc.2007.01.092. [DOI] [PubMed] [Google Scholar]
- 27.Huss JM, Torra IP, Staels B, Giguere V, Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Molecular and cellular biology. 2004;24:9079–9091. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circulation research. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 29.Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 31.Nagao M, Parimoo B, Tanaka K. Developmental, nutritional, and hormonal regulation of tissue-specific expression of the genes encoding various acyl-CoA dehydrogenases and alpha-subunit of electron transfer flavoprotein in rat. The Journal of biological chemistry. 1993;268:24114–24124. [PubMed] [Google Scholar]
- 32.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circulation research. 2009;105:12–15. doi: 10.1161/CIRCRESAHA.109.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Cheng L, Qin Q, Liu J, Lo WK, Brako LA, Yang Q. High-fat feeding in cardiomyocyte-restricted PPARdelta knockout mice leads to cardiac overexpression of lipid metabolic genes but fails to rescue cardiac phenotypes. Journal of molecular and cellular cardiology. 2009;47:536–543. doi: 10.1016/j.yjmcc.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Kiningham KK, Devalaraja MN, Yeh CC, Majima H, Kasarskis EJ, St Clair DK. An intronic NF-kappaB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-alpha and interleukin-1beta. DNA Cell Biol. 1999;18:709–722. doi: 10.1089/104454999314999. [DOI] [PubMed] [Google Scholar]
- 35.Sam F, Kerstetter DL, Pimental DR, Mulukutla S, Tabaee A, Bristow MR, Colucci WS, Sawyer DB. Increased reactive oxygen species production and functional alterations in antioxidant enzymes in human failing myocardium. J Card Fail. 2005;11:473–480. doi: 10.1016/j.cardfail.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 37.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7815–7820. doi: 10.1073/pnas.0802057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraegen EW, Cooney GJ, Turner N. Muscle insulin resistance: a case of fat overconsumption, not mitochondrial dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7627–7628. doi: 10.1073/pnas.0803901105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Yin R, Liu J, Wang P, Wu S, Luo J, Zhelyabovska O, Yang Q. Peroxisome proliferator-activated receptor delta regulates mitofusin 2 expression in the heart. Journal of molecular and cellular cardiology. 2009;46:876–882. doi: 10.1016/j.yjmcc.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 41.Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, Willy PJ, Schulman IG, Heyman RA, Lander ES, Spiegelman BM. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol. 2003;17:2477–2493. doi: 10.1210/me.2003-0151. [DOI] [PubMed] [Google Scholar]
- 43.Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-Proliferator-Activated Receptor delta Activates Fat Metabolism to Prevent Obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.