Abstract
Vascular/parenchymal crosstalk is increasingly recognized as important in the development and maintenance of healthy vascularized tissues. The retina is an excellent model in which to study the role of cell type-specific contributions to the process of blood vessel and neuronal growth. During retinal vascular development, glial cells such as astrocytes provide the template over which endothelial cells migrate to form the retinal vascular network, and hypoxia-regulated vascular endothelial growth factor (VEGF) has been demonstrated to play a critical role in this process as well as pathological neovascularization. To investigate the nature of cell-specific contributions to this process, we deleted VEGF and its upstream regulators, the hypoxia-inducible transcription factors HIF-1α and HIF-2α, and the negative regulator of HIFα, von Hippel-Lindau protein (VHL), in astrocytes. We found that loss of hypoxic response and VEGF production in astrocytes does not impair normal development of retinal vasculature, indicating that astrocyte-derived VEGF is not essential for this process. In contrast, using a model of oxygen-induced ischemic retinopathy, we show that astrocyte-derived VEGF is essential for hypoxia-induced neovascularization. Thus, we demonstrate that astrocytes in the retina have highly divergent roles during developmental, physiological angiogenesis and ischemia-driven, pathological neovascularization.
Introduction
Angiogenesis in the retina occurs as part of normal development as well as proliferative vascular diseases such as diabetic retinopathy, age-related macular degeneration, retinal vein occlusion, and retinopathy of prematurity. These conditions collectively account for about two thirds of legal blindness in developed nations (Resnikoff et al. 2004). Vascular endothelial growth factor (VEGF) has been shown to play a fundamental role in both developmental angiogenesis (Alon et al. 1995) and pathological retinal neovascularization (Aiello et al. 1995). VEGF is a hypoxia-inducible gene which is directly regulated by the hypoxia-inducible transcription factors HIF (Liu et al. 1995). HIFs are heterodimeric proteins which consist of a constitutive subunit HIF-1β and one of either α-subunits, HIF-1α or HIF-2α. HIFs are the master regulators of the hypoxic transcriptional response, which control expression of a broad range of genes involved in angiogenesis, metabolism, and erythropoiesis (Kaelin and Ratcliffe 2008; Weidemann and Johnson 2008). HIF-α subunits are rapidly degraded in normoxia but highly inducible by hypoxia. The oxygen-dependent degradation of the HIF-α subunits in normoxia is initiated by the hydroxylation of two conserved prolyl residues in the HIFα-proteins. HIF-α hydroxylation under normoxia regulates the interaction with the von Hippel-Lindau tumor suppressor protein (pVHL), which targets HIF-α for proteolysis by the ubiquitin-proteasome pathway (Maxwell et al. 1999). The involvement of both HIFα isoforms and the hypoxic response in the regulation of retinal physiology and pathology has been well documented (Arjamaa and Nikinmaa 2006; Ding et al. 2005; Dioum et al. 2008; Grimm et al. 2005).
In retinal vascular development, astrocytes form a template that provides guidance for the developing vascular network and may also serve as an important source of VEGF (Dorrell et al. 2002; Stone et al. 1995). It appears likely that astrocytes also play a central role in retinal revascularization following ischemic injury (Dorrell et al. 2010). However, cell type-specific functions of VEGF and HIFs in development and homeostasis of the retinal vasculature have so far not been completely identified. In order to clarify this issue, we generated mice with conditional deletion of the genes encoding VEGF, HIF-1α, HIF-2α, and the negative regulator of HIFα, von Hippel-Lindau protein (VHL), in astrocytes to specifically investigate the contribution of astrocytes to developmental and pathologic angiogenesis in the retina. We demonstrate that loss of VEGF or the HIFα isoforms in astrocytes does not impair the normal development of the murine retinal vasculature. In contrast, in a model of oxygen-induced retinopathy (OIR), astrocyte-derived VEGF, driven by HIF-2α but not by HIF-1α, is a key mediator of in hypoxia-driven vascular proliferation. Thus, we provide genetic evidence that the glial compartment in the retina has distinct functions in developmental and pathological angiogenesis.
Material and methods
Transgenic mice
All procedures involving animals were approved by the UCSD Animal Care Committee, which serves to ensure that all federal guidelines concerning animal experimentation are met. Generation of mice carrying the loxP-flanked conditional alleles of Hif1a, Epas1, Vegfa and Vhlh was described previously (Gerber et al. 1999; Gruber et al. 2007; Haase et al. 2001; Ryan et al. 1998). Astrocyte-specific inactivation was achieved by cross-breeding those mice to GFAP-Cre transgenic mice (Bajenaru et al. 2002); generously provided by D. Guttman) in a C57BL/6 background or the respective double knockouts (VHL/HIF-1α, VHL/HIF-2α and VHL/VEGF) in a mixed background. Cre-negative homozygous littermates for the conditional alleles were used as controls. Animals were 10 weeks old or at the indicated ages at the time of the experiments. Investigated mouse strains were screened for morphological signs of inherited retinal degeneration and tested for the Pde6brd1 mutation using a published genotyping protocol (Jackson Laboratory genotyping protocol 974, available at http://jaxmice.jax.org/pub-cgi/protocols/protocols.sh?objtype=protocol&protocol_id=974; accessed: June 27, 2009). Results are presented as supporting information.
Oxygen-induced retinopathy
The mouse model of oxygen-induced retinopathy (OIR) was induced as described earlier (Smith et al. 1994). Briefly, P7 pups and their mothers were exposed to 75% oxygen for 5 days until P12 in a hyperoxic chamber (Biospherix), followed by a return to room air. At P17, retinal flat-mounts were obtained and areas of vaso-obliteration and preretinal neovascular tuft formation analyzed as in (Banin et al. 2006). Gene expression analysis upon return to normoxia was performed 24 h after hyperoxia, on P13.
Hypoxia exposure
Adult 10 week-old animals were exposed to 7.5% oxygen for 10 h in a hypoxic animal chamber (Biospherix Ltd.) and sacrificed immediately thereafter. Eyes were swiftly removed and the extracted retina was frozen in liquid nitrogen for further analysis.
Retinal dissection, staining, and analysis
Retinal preparation and analysis largely followed previously published protocols (Dorrell et al. 2002). A detailed description is presented as supporting information.
DNA recombination analysis
DNA from retinae isolated at P3 was extracted with DNeasy kit (Qiagen) according to the manufacturer’s recommendations. To determine relative gene deletion by cre-mediated excision in retinae, copies of the floxed allele quantified by real-time PCR were compared to a wildtype allele using the delta-ct method, and 25 ng of genomic DNA was used in the PCR reaction. Primer sequences are presented as supporting information.
RNA analysis by reverse transcription and real-time quantitative PCR
Total RNA was isolated from each retina using RNEasy kit (Qiagen) according to the manufacturer’s protocol. First-strand synthesis was performed with 300 ng of total RNA by the SuperScript System (Invitrogen) according to the manufacturer’s recommendations. For real-time PCR analyses, cDNAs were diluted to a final concentration of 0.3 ng/μl and amplified in SYBR Green or TaqMan Universal Master Mix (Applied Biosystems) with an ABI Prism 7700 sequence detection system (Applied Biosystems). Expression levels were related to 18s using the delta-ct method. Primer sequences are presented as supporting information.
Statistical analyses
All statistic analyses were performed using Prism software (GraphPad Software, La Jolla, CA). Unless otherwise noted, a two-sided unpaired Student’s t-test was used for the analysis of differences in mean values between groups. As for most of our experiments we used only one retina per animal, the given n-values represent both the number of animals and the number of single eyes, unless stated otherwise.
Results
Generation of mice with targeted deletion of VEGF and HIFα isoforms in astrocytes
The concept of endothelial cell guidance by angiogenic factors produced by hypoxic cells in the avascular retina is generally thought to be the underlying mechanism of retinal vascular development (Dorrell and Friedlander 2006). However, the individual contribution of specific cytokines and cell types to retinal angiogenesis is still poorly defined. To address the role of astrocytes in retinal angiogenesis in vivo, we conditionally deleted the gene encoding VEGF and the upstream factors of the hypoxia response pathway in astrocytes. To this end, GFAPcre-IRES-LacZ mice, which express Cre-recombinase in an astrocyte-specific manner (Bajenaru et al. 2002), were crossed with mice homozygous for floxed alleles of Vegfa, Hif1a, Epas1 (encoding HIF-2α), and Vhlh (e.g. resulting in GFAPcre+/VEGF+f/+f mice). Transgene expression in retinal wholemounts was analyzed by means of LacZ reporter gene expression during early retinal vascular development at postnatal day 3 (P3): Robust gene activation is detected spreading from the center to the periphery of the retina of GFAPcre-positive mice (Fig. 1A), well ahead of the developing vascular front (Fig. 1B). At P14 when the retinal vasculature covers the entire retina, strong LacZ expression is observed throughout the flatmounted retina (Fig. 1C-D). Analysis of retinal sections confirms that expression is confined to the inner surface of the retina, consistent with the retinal location of astrocytes, but is absent from all other retinal layers (Fig. 1E). These results are in accordance with our previous finding that in brains of these mice, GFAPcre expression is restricted to astrocytes and not detectable in neuronal cells (Weidemann et al. 2009) as well as with another study reporting the same finding in optic nerve tissue (Zhu et al. 2005). Moreover, we demonstrate by quantitative PCR using total retinal DNA that significant deletion of floxed genes (here VHL) occurs as a result of successful recombination on P3 (Fig. 1F).
Figure 1. Tissue specific deletion of floxed genes in astrocytes in the developing retina using GFAPcre-expressing mice.

(A) LacZ reporter gene expression was analyzed in retinal flatmounts by X-gal staining (blue). LacZ expression in GFAPcre-negative animals (left) is absent. In GFAPcre-positive mice (right) at P3, the transgene expression is most pronounced in the central retina but is also detectable already in the periphery. (B) At the same age the growing vascular front is still limited to the immediate surroundings of the optic nerve and well within the area of LacZ expression. (C) At P14, strong LacZ expression is now observed throughout the retinal flatmounts. (D) Retinal vascular development at this age has significantly advanced, already covering the entire retina. (E) In retinal sections, LacZ expression is confined to the inner surface of the retina, consistent with the localization of astrocytes. (F) Copy numbers of a floxed allele (VHL) was quantified in retinal DNA relative to an unfloxed allele (VEGF). In GFAPcre-positive mice at P3, relative abundance of the floxed allele is significantly reduced compared to GFAPcre-negative animals, thus indicating successful recombination.
Mice with conditional deletion of VEGF, HIF-1α or HIF-2α in astrocytes exhibit normal retinal vascular development
The contribution of the hypoxic response of astrocytes to the retinal vascular development was analyzed in GFAPcre+/VEGF+f/+f, GFAPcre+/HIF-1α+f/+f, and GFAPcre+/HIF-2α+f/+f pups. Retina wholemounts at P7 were stained to visualize the developing vascular network. Intriguingly, deletion of Vegfa, Hif1a or Epas1 (Hif2a) in astrocytes does not affect the normal retinal vascular development in mice (Fig. 2A and Fig. S2A): the superficial vascular plexus does not exhibit any apparent morphological alterations as compared to wildtype (GFAPcre-) littermate controls. Moreover, no delay of vascular outgrowth occurs, as measured by the distance between optic nerve head and peripheral vascular front (Fig. 2B, Fig. S2B). In GFAPcre+/VEGF+f/+f mice, we further examined the timing of vascular development by quantifying the number of vascular sprouts that start developing at P7 from the superficial vascular plexus to subsequently branch down to form the deep vascular plexus. Here again, we did not detect a significant difference compared to wildtype littermates (Fig S2C). Likewise, the vasculature of the adult retina of all three conditional knockout strains is morphologically normal and without apparent differences compared to wildtype controls (Fig. 2A and Fig. S2A).
Figure 2. Conditional deletion of VEGF and HIFα-isoforms in astrocytes does not impair normal retinal vascular development in mice.
(A) Respresentative picture of wildtype (left) and astrocyte specific VEGF knockout littermates (right) on P7 (upper panel) and at adult age (both >4). Knockout of VEGF does not result in apparent morphological alterations in the developing vasculature. (B) Retinal vascular development is not delayed in animals which lack VEGF, HIF-1α or HIF-2α in astrocytes as measured by distance of the growing vascular front from the optic nerve head compared to their wildtype littermates (data represents mean ± S.D.). (C,D) Total VEGF mRNA expression (D) and EPO mRNA expression (E) in retinae of P7 pups with the indicated conditional knockout in astrocytes is not significantly different compared to wildtype expression levels (data represent mean ± S.E.M.).
The expression of angiogenic growth factors such as VEGF is considered to be crucial for normal retinal vessel growth (Dorrell et al. 2002; Stalmans et al. 2002). Additionally, erythropoietin (EPO) has been implicated in retinal vascular development (Pierce et al. 1995). We therefore investigated the expression of both factors in the developing retina of GFAPcre+/VEGF+f/+f, GFAPcre+/HIF-1α+f/+f and GFAPcre+/HIF-2α+f/+f pups. Consistent with the absence of phenotypical changes in vessel development, mRNA levels of VEGF and EPO are also unchanged in retina of P7 old pups (Fig. 2C,D). Moreover, expression levels of the alternatively transcribed VEGF isoforms (VEGF120, VEGF164, and VEGF188), which have differential roles in retinal vascular development (Stalmans et al. 2002), are not significantly altered (Fig. S2D). Other relevant angiogenic factors such as SDF-1 (Butler et al. 2005), PDGF-A, PDGF-B (Mori et al. 2002) and extracellular matrix genes such as TIMP-1 and -2 are also unchanged in the developing retina of conditional knockout mice compared to wildtype littermates (data not shown). Moreover, we do not observe increased macrophage infiltration in those retinae as assessed by F4/80 expression (data not shown).
Taken together, these data demonstrate that VEGF or HIFα-isoforms in astrocytes are not essential for the physiological vessel development in the retina. This is in contrast to the current paradigm that VEGF produced by hypoxic astrocytes is essential for proper vessel formation. The unchanged expression levels of VEGF and other angiogenic cytokines in retinal tissue suggest that other, non-astrocytic cell compartments in the retina are capable of producing these factors, either as part of the developmental process or compensatory to ensure normal angiogenesis.
Loss of VHL in astrocytes induces severe retinal vascular changes
To further elucidate the role of the hypoxia response in retinal astrocytes we generated gain-of-function mutations of HIFα by deleting the negative regulator of HIFα, the murine orthologue of the von Hippel-Lindau gene (Vhlh). Loss of Vhlh strongly induces both HIFα isoforms in normoxia and induces HIFα target genes (Boutin et al. 2008; Weidemann et al. 2009). The retinae of GFAPcre+/VHL+f/+f mice show extensive hypervascularity already during retinal development at P7 which persists into adulthood (Fig. 3A). Expression of VEGF mRNA in the retina is significantly increased compared to GFAPcre-negative littermates (2.0 ± 0.28-fold vs. 1.1 ± 0.2-fold; Fig. 3B). Interestingly, the amplitude of VEGF upregulation is comparable to that observed in severely hypoxic wildtype retinae (2.2 ± 0.41-fold; Fig. S3A), which indicates that deletion of Vhlh in astrocytes induces retinal VEGF production to almost maximal levels.
Figure 3. Developmental vascular defects in retinae of mice with conditional deletion of VHL in astrocytes.
(A) During retinal vascular development at P7, loss of VHL in astrocytes induces extensive retinal hypervascularity which persists into adulthood (representative picture of retina flatmounts; n=3). (B) VEGF mRNA expression levels are significantly elevated in retinal tissue of GFAPcre+/VHL+f/+f mice compared to wildtype littermates (data represents mean ± S.E.M.). (C) The excessive vessels form convoluted bundles of vessels that are ensheathed by astrocytes (scalebars 100 μm). (D) In histological sections, the abnormal vessel convolutes are located in the level of the superficial vascular plexus, locally extending into the intermediated plexus. The immunoreactivity for GFAP is increased, indicating an increased number of astrocytes that accompany the hypervascular abnormalities (scalebars: 200 μm). (E) Confocal images of the different vascular plexi in flat-mounted retinae confirm that the hypervascular changes occur predominantly in the superficial and partially in the intermediate vascular plexus, resulting in gross alterations of the vascular structure of these two plexus. In contrast, structure of the deep vascular plexus is largely preserved or rather decreased with no apparent hypervascularity (scalebars: 200 μm).
Morphological analysis demonstrates that the outermost developing front of the retinal vasculature at P7 exhibits only minor alterations while more centrally, behind these peripheral developing vessels, continuing angiogenesis results in the formation of extensive hypervascularity (Fig. S3B). On closer examination, vasculature of affected retinae contains extensive vessel convolutes that each consists of bundles of multiple vessels with largely normal individual diameter, and these vessel bundles are surrounded by sheaths of astrocytes (Fig. 3C). In adult animals, these hypervascular changes eventually cover the entire retina (Fig. 3A), which suggests that an uncontrolled VEGF expression in astrocytes leads to hypervascularity in the retinal superficial plexus.
In histological sections, the normal neural retinal layers are preserved in adult mice with conditional VHL knockout (Fig. 3D). Large vascular convolutes are present in the superficial vascular plexus and focally extend downwards into the intermediate vascular plexus. In contrast, the deep vascular plexus does not appear to be affected by the hypervascular changes but is indeed rather rarified. Immunoreactivity for the astrocyte marker GFAP is markedly increased in the nerve fiber layer of affected retinae, indicating increased astrocyte cell number associated with the hypervascular phenotype. Consistent with the histological findings, confocal imaging of the three vascular plexuses in retinal wholemounts revealed extensive hypervascularity and convoluted vessels in the superficial plexus, extending into the intermediate plexus (Fig. 3E). The deep plexus appears largely normal in structure but hypovascular compared to wildtype littermate controls (Fig. S3C). This is consistent with the idea that astrocytes mainly control the formation of the superficial plexus. Interestingly, this also suggests that the deep plexus forms by other mechanisms, with its density based on need.
In summary, loss of VHL in astrocytes increases retinal VEGF expression and induces a severe proliferative retinopathy dominated by extensive hypervascularity in the superficial vascular plexus.
Retinal vascular pathology in GFAPcre+/VHL+f/+f mice is caused by HIF-2α-dependent VEGF overexpression in astrocytes
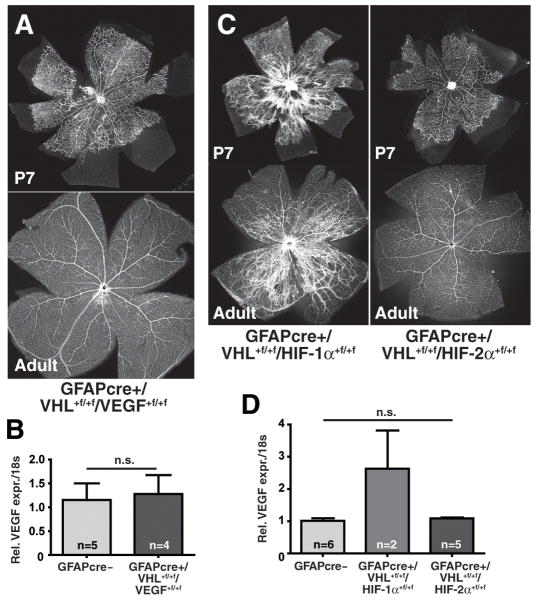
To determine whether VEGF is the key factor for hypervascularity in GFAPcre+/VHL+f/+f mice, we conditionally deleted both, VHL and VEGF, in astrocytes (GFAPcre+/VHL+f/+f/VEGF+f/+f). Simultaneous loss of Vhlh and Vegfa rescues the vascular pathology of the GFAPcre+/VHL+f/+f mice (Fig. 4A) and also normalizes VEGF mRNA levels (Fig. 4B). To investigate which HIFα isoform is controlling the VEGF overexpression in the background of Vhlh deletion, we generated double knockout animals of VHL and HIF-1α (GFAPcre+/VHL+f/+f/HIF-1α+f/+f) as well as VHL and HIF-2α (GFAPcre+/VHL+f/+f/HIF-2α+f/+f). Overexpression of HIF-2α but not of HIF-1α was found to be responsible for the phenotype of VHL deletion in astrocytes since GFAPcre+/VHL+f/+f/HIF-2α+f/+f mice exhibit a normal vasculature both at P7 and in adults (Fig. 4C) whereas GFAPcre+/VHL+f/+f/HIF-1α+f/+f mice demonstrated retinal abnormalities similar to those described above for GFAPcre+/VHL+f/+f mice. Consistent with these morphological results, VEGF mRNA expression is reduced to normal levels in GFAPcre+/VHL+f/+f/HIF-2α+f/+f but not in GFAPcre+/VHL+f/+f/HIF-1α+f/+f mice (Fig. 4D). EPO mRNA levels, also significantly elevated in GFAPcre+/VHL+f/+f retinae, are similarily regulated by HIF-2α (Fig. S3D). Interestingly, loss of both, VHL and VEGF does not reduce those elevated EPO mRNA levels but results in a normal vascular phenotype which indicates that EPO alone does not act as a strong angiogenic factor in the retina.
Figure 4. Pathological neovascularization in GFAPcre+/VHL+f/+f mice is driven by HIF-2α-mediated VEGF overexpression.
(A) Double knockout of VHL and VEGF in astrocytes completely rescues the hypervascular phenotype observed with Vhlh-deletion (representative picture of retina flatmounts; n=3). (B) VEGF expression levels are normal in GFAPcre+/VHL+f/+f/VEGF+f/+f mice (data represents mean ± S.E.M.). (C) Double knockout of VHL and HIF-1α in astrocytes (left) does not change the developmental hypervascularity in the retina, and the phenotype closely resembles the one of GFAPcre+/VHL+f/+f mice. In contrast, double deletion of VHL and HIF-2α (right) results in a normal development of the vasculature into adulthood (representative picture of retina flatmounts; n=3). (D) VEGF mRNA expression levels are reduced to wildtype levels by double deletion of VHL and HIF-2α, but not by VHL and HIF-1α (data represents mean ± S.E.M.).
Taken together, these data provide evidence that VEGF overexpression determines the pathology observed in the retina of GFAPcre+/VHL+f/+f mice. We demonstrate genetically that overexpression of VEGF and EPO in the background of VHL-deletion is dependent on HIF-2α, and not HIF-1α, in astrocytes. Thus, HIF-2α is the dominant HIFα-isoform in VHL-null astrocytes.
VEGF and HIF-2α in astrocytes are essential for pathological neovascular tuft formation in a model of oxygen induced retinopathy
Loss of VEGF in astrocytes or loss of the master regulators of the hypoxic response, HIF-1α and HIF-2α, does not impair the physiological vascular development in the retina. However, we observe a VEGF-driven pathology after loss of VHL in astrocytes, which is determined by constitutive activation of HIF-2α. This finding led us to speculate whether astrocyte VEGF and/or HIFα play a role in pathological neovascularization. To test for this, we utilized a murine model of oxygen-induced retinopathy (OIR) and employed it in GFAPcre+/VEGF+f/+f, GFAPcre+/HIF-1α+f/+f and GFAPcre+/HIF-2α+f/+f mice. To achieve OIR, pups were exposed to 75% oxygen for 5 days from P7 until P12 and were subsequently returned to room air for an additional 5 days before analysis on P17 (Smith et al. 1994). In this model, retinal hyperoxia causes extensive vaso-obliteration of the already formed vessels in the central retina. Following return to a normoxic environment, hypoxia of the vaso-obliterated retinal tissue results in both physiological revascularization of the central retina and pathological neovascularization with formation of preretinal vascular tufts.
One day after hyperoxia treatment at P13, the area of the central vaso-obliteration is not affected by conditional knockout of VEGF, Hif-1α and HIF-2α in astrocytes, indicating that the astrocytic hypoxic response is not involved in the hyperoxic regression of vessels in this model (Fig. 5A, Fig. S4A). As we have previously demonstrated that OIR-treatment can induce GFAP expression in Müller cells that is most pronounced at P14 (Dorrell et al. 2010), we examined whether Müller cells, in addition to astrocytes, express the GFAPcre transgene in OIR-treated GFAPcre-positive mice. Surprisingly, expression of the LacZ reporter gene in retinal sections is unchanged after OIR and is still confined to the inner surface of the retina, thus excluding OIR-induced Müller cell expression of the GFAPcre transgene (Fig. S4B). Hypoxia-responsive genes such as VEGF, EPO and the different VEGF-isoforms are upregulated at the onset of retinal hypoxia on P13 compared to age-matched controls, but we find no genotype-specific difference in the gene expression profile (Fig. 5B,C, Fig. S4C-E). Comparison of EPO expression indicates, however, that the amplitude of hypoxic induction achieved in the OIR model is only moderate compared to other models such as hypoxia-treated adult animals, in which the hypoxic induction is clearly HIF-2α dependent (Fig. S4F).
Figure 5. Astrocyte-derived VEGF, controlled by HIF-2α, is a key mediator for neovascular tuft formation in OIR.
(A) In a murine model of oxygen-induced retinopathy (OIR), the area of vaso-obliteration induced by hyperoxia was quantified 24 hours after oxygen treatment (P13): there are no significant differences between GFAPcre+/VEGF+f/+f, GFAPcre+/HIF-1α+f/+f and GFAPcre+HIF-2α+f/+f animals and their respective wildtype littermates (data represents mean ± S.D.). (B,C) Gene expression analysis of VEGF (B) and EPO (C) in P13 retinae after hyperoxia shows that both genes are upregulated, indicating retinal hypoxia. However no genotype-specific difference of expression levels is observed between mice with conditional knockout of VEGF, HIF-1α and HIF-2α in astrocytes. (D-M) Vaso-obliteration and neovascular tuft formation in OIR was assessed at P17, 5 days after the end of the hyperoxic phase. (D-F) Loss of VEGF in astrocytes does not change vaso-obliteration but significantly reduces neovascularization. (G-I) Loss of HIF-1α in astrocytes does not affect vaso-obliteration or neovascularization in this OIR model, whereas (K-M) conditional deletion of HIF-2α significantly reduces vaso-obliteration and neovascularization relative to wildtype littermate controls (D,G,K: images show representative retina flatmounts (n>4 for all); E,F,H,I,L,M: data represents mean area relative to respective wildtype littermate controls ± S.D.).
Following hyperoxia treatment in OIR, physiological revascularization progressively reduces the vaso-obliterated retinal area. At P17, revascularization is not significantly impacted by astrocyte-specific knockout of VEGF (Fig. 5D,E) and Hif-1α (Fig. 5G,H). In contrast, GFAPcre+/HIF-2α+f/+f mice exhibit a significantly reduced area of vaso-obliteration on P17 (0.787 ± 0.07; Fig. 5K,L) which indicates accelerated revascularization, possibly mediated by suppression of HIF-2α-controlled cytokines other than VEGF. More importantly, the preretinal neovascular tuft formation is significantly reduced on P17 by astrocyte-specific knockout of VEGF (to 0.538 ± 0.48; Fig. 5D,F) and Hif-2α (to 0.583 ± 0.19; Fig. 5K,M) but not of Hif-1α (Fig. 5G,I). This clearly demonstrates that the hypoxic response in astrocytes, specifically HIF-2α and its downstream target VEGF, are centrally involved in the vascular pathology observed in OIR.
Taken together, we provide genetic evidence that HIF-2α signaling in astrocytes and, furthermore, the induction of astrocyte-derived VEGF are critical factors for the development of vascular pathologies in OIR. This finding is in contrast to the observed redundant role of the astrocytes’ hypoxic response during retinal development. Thus, our data highlights the diverse roles and different contributions of the astrocyte compartment in the retina in development and disease.
Discussion
Developmental angiogenesis and pathological neovascularization in the retina share certain characteristics: angiogenic factors such as VEGF are intimately involved in both processes, and the hypoxic response, orchestrated by the hypoxia-inducible transcription factor HIF, may be responsible for their induction. However, basic molecular mechanisms regulating these processes are still unclear. The normal vasculature of the retina is characterized by a highly organized structure consisting of three distinct capillary plexuses that are generated during retinal development in a well-orchestrated series of events. In contrast, pathological angiogenesis exhibits unorganized, leaky, and tortuous vessels prone to exudation, hemorrhage, and fibrosis that can lead to blinding neovascular eye disease.
Retinal vascularization occurs mainly by angiogenic sprouting (Gariano 2003), and VEGF has been shown to be essential for normal development (Chan-Ling et al. 1995; Ozaki et al. 2000; Stalmans et al. 2002; Stone et al. 1995). It has been suggested that VEGF production by astrocytes may precede vascular outgrowth and that a VEGF gradient from the avascular, and thus presumably hypoxic, peripheral retina towards the center provides guidance for invading endothelial cells (Dorrell et al. 2002; Gerhardt et al. 2003). Thus, it is surprising that normal vessel development is observed in mice where VEGF has been conditionally deleted in astrocytes. Moreover, our data do not indicate that other factors compensate for the loss of astrocytes-produced VEGF; overall VEGF mRNA expression levels in the developing retina at P7 are not changed between wildtype and conditional knockout animals, and we do not see altered expression profiles of other angiogenic cytokines.
The evidence for a hypoxia-induced VEGF production in the retina by astrocytes has been indirect so far (Chan-Ling et al. 1995), and VEGF expression is not restricted to the leading edge of retinal vascularization (Gariano et al. 2006). Thus, it is difficult to determine the exact site of VEGF production in the retina. Two possible explanations for the observed phenotype result in the following questions that remain to be tested in further studies: (i) whether other retinal cells upregulate VEGF expression to compensate for the loss of astrocyte-specific VEGF production or (ii) whether normal vascular development in the retina is indeed entirely independent of astrocyte-derived VEGF.
In addition to astrocytes, Müller cells have been suggested as a source of VEGF (Pierce et al. 1995) and have been implicated in the formation of the deep vascular plexus during retinal development (Sandercoe et al. 2003; Stone et al. 1995). In our model, however, the normal organization of the different vascular plexuses and, in particular, the unaffected temporal sequence of their development argues against a substantial compensatory VEGF upregulation in Müller cells in response to loss of astrocyte-specific VEGF. Rather, the observed normal formation of the superficial vascular plexus at P7 with normal guidance of vessels in the inner retinal surface suggests that cell types within this layer, such as ganglion cells, pericytes, or endothelial cells may serve as sources of VEGF. Indeed, hypoxia-driven VEGF expression has been demonstrated for each of these retinal cell types (Darland et al. 2003; Sandercoe et al. 2003) and ganglion cells in particular were found to be capable of compensating for loss of VEGF expression in astrocytes (Stone et al. 1996).
Myeloid cells such as macrophages and microglia cells may provide another possible source for VEGF in the retina. However, conditional deletion of VEGF in myeloid cells (Stockmann et al. 2008) does result in any overt developmental abnormalities of the retinal vasculature (unpublished observations, A.W. and T.U.K). Given the important role of microglia cells in retinal angiogenesis (Ritter et al. 2006) and the cross-talk of astrocytes and microglia being essential for this process (Dorrell et al. 2010; Stevenson et al. 2010) it would nevertheless be conceivable that disruption of this cellular interplay in the conditional knockout mice contributes to our observed effects.
Hypoxia has been well documented as a driving factor in the OIR model and human diseases in which pathological retinal neovascularization is observed. Utilizing this model with our conditional knockout mice, we do see that astrocytes function as key mediators for the pathological neovascularization: conditional deletion of VEGF and the upstream regulator HIF-2α in astrocytes significantly reduces neovascularization. Thus, by eliminating those two factors, the vascular overdrive stimulated by hypoxic astrocytes is blunted.
Interestingly, we observe that deletion of HIF-2α, but not VEGF, in astrocytes accelerates revascularization of the vaso-obliterated retina following hyperoxia. This result may be explained by suppression of EPO induction in HIF-2α conditional knockout animals and would be in accordance with previous findings that siRNA-mediated EPO knockdown in the retina accelerates re-vascularization in OIR (Chen et al. 2009). Consistent with these findings, independent roles for VEGF and EPO in retinal vascular disease have been demonstrated before (Watanabe et al. 2005). Another intriguing finding of our study is that EPO levels are still highly elevated in VHL/VEGF double knockout animals. As those mice exhibt normal vasculature, this indicates that EPO overexpression alone is not sufficient to drive retinal angiogenesis. Further studies are clearly needed to precisely define the role of EPO for the retinal vasculature.
Despite the observed effects of the conditional knockouts in the OIR model, overall retinal tissue levels of VEGF were not found to be altered, suggesting that the cellular origin of VEGF can be more important than overall VEGF tissue concentrations. This is in accordance with a previous study by our group in a tumor model of angiogenesis which demonstrates that conditional deletion of VEGF in myeloid cells does not affect VEGF mRNA levels in tumor tissue but increases vascular normalization within the tumor and accelerates tumorigenesis (Stockmann et al. 2008). These data highlight the important effects of cell type-specific expression of VEGF.
One possible explanation for the observed differential effect observed in our knockout model in normal vascular development and pathological neovascularization is that the latter is mediated by astrocyte-derived VEGF while the former is not. Alternatively, the differences may relate to a different extent of tissue hypoxia in the developing retina and OIR (Saito et al. 2008). A lower degree of tissue hypoxia in retinal development may result in comparatively moderate VEGF secretion by astrocytes that can still be compensated for by other retinal cell types in our knockout model. In contrast, the excessive retinal ischemia in OIR may cause a significantly higher astrocyte secretion of VEGF that cannot be compensated for and thus results in the observed effects in conditional knockout mice.
Taken together, we provide genetic evidence that astrocyte-derived VEGF or, indeed the entire hypoxic response pathway, in retinal astrocytes is not required for normal retinal vascularization. Overexpression of HIF-2α, but not HIF-1α, in astrocytes directly induces VEGF and EPO and results in a severely pathological hypervascular retinal phenotype. In a model of OIR, the loss of VEGF or HIF-2α, but not HIF-1α, reduces neovascularization. Thus, vascular pathology, and not physiological development, in the retina is regulated to a large extent by the astrocyte compartment, with HIF-2α playing a central role. A better understanding of cell-specific functions in retinal physiology and disease is crucial for the development of therapeutic concepts useful for developing drugs to inhibit pathological neovascularization in the eye.
Supplementary Material
Acknowledgments
We thank Michelle Salcedo, Jeung Yeun Choi, Logan Stark, Zachary Quiza-Magase, Stacey Moreno, Jeffrey Friedlander, and Matthew Wang for excellent technical assistance, Helene Rundqvist for providing LysMcre/VEGF+f/+f mice for pilot studies, and Jakob Weidemann and Mathilda Krohne for initiating the collaboration that resulted in this study.
This research was supported by grants from the NIH (EY11254 to MF) and the MacTel Foundation (to MF). AW and TUK are supported by the German Research Foundation (DFG; grants WE 4275/1-1 and KR 2863/6-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92(23):10457–61. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1(10):1024–8. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res. 2006;83(3):473–83. doi: 10.1016/j.exer.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Bajenaru ML, Zhu Y, Hedrick NM, Donahoe J, Parada LF, Gutmann DH. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol Cell Biol. 2002;22(14):5100–13. doi: 10.1128/MCB.22.14.5100-5113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin E, Dorrell MI, Aguilar E, Ritter MR, Aderman CM, Smith AC, Friedlander J, Friedlander M. T2-TrpRS inhibits preretinal neovascularization and enhances physiological vascular regrowth in OIR as assessed by a new method of quantification. Invest Ophthalmol Vis Sci. 2006;47(5):2125–34. doi: 10.1167/iovs.05-1096. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Johnson LV. Vascular atrophy in the retinal degenerative rd mouse. J Comp Neurol. 1986;254(4):543–53. doi: 10.1002/cne.902540407. [DOI] [PubMed] [Google Scholar]
- Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, Wiesener M, Eckardt KU, Koch CJ, Ellies LG, et al. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell. 2008;133(2):223–34. doi: 10.1016/j.cell.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Guthrie SM, Koc M, Afzal A, Caballero S, Brooks HL, Mames RN, Segal MS, Grant MB, Scott EW. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115(1):86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division. Evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36(7):1201–14. [PubMed] [Google Scholar]
- Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, Smith LE. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2009;50(3):1329–35. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264(1):275–88. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Ding K, Scortegagna M, Seaman R, Birch DG, Garcia JA. Retinal disease in mice lacking hypoxia-inducible transcription factor-2alpha. Invest Ophthalmol Vis Sci. 2005;46(3):1010–6. doi: 10.1167/iovs.04-0788. [DOI] [PubMed] [Google Scholar]
- Dioum EM, Clarke SL, Ding K, Repa JJ, Garcia JA. HIF-2alpha-haploinsufficient mice have blunted retinal neovascularization due to impaired expression of a proangiogenic gene battery. Invest Ophthalmol Vis Sci. 2008;49(6):2714–20. doi: 10.1167/iovs.07-1469. [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43(11):3500–10. [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Jacobson R, Trauger SA, Friedlander J, Siuzdak G, Friedlander M. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia. 2010;58(1):43–54. doi: 10.1002/glia.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res. 2006;25(3):277–95. doi: 10.1016/j.preteyeres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Gariano RF. Cellular mechanisms in retinal vascular development. Prog Retin Eye Res. 2003;22(3):295–306. doi: 10.1016/s1350-9462(02)00062-9. [DOI] [PubMed] [Google Scholar]
- Gariano RF, Hu D, Helms J. Expression of angiogenesis-related genes during retinal development. Gene Expr Patterns. 2006;6(2):187–92. doi: 10.1016/j.modgep.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126(6):1149–59. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Hermann DM, Bogdanova A, Hotop S, Kilic U, Wenzel A, Kilic E, Gassmann M. Neuroprotection by hypoxic preconditioning: HIF-1 and erythropoietin protect from retinal degeneration. Semin Cell Dev Biol. 2005;16(4-5):531–8. doi: 10.1016/j.semcdb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A. 2007;104(7):2301–6. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;98(4):1583–8. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5’ enhancer. Circ Res. 1995;77(3):638–43. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Mori K, Gehlbach P, Ando A, Dyer G, Lipinsky E, Chaudhry AG, Hackett SF, Campochiaro PA. Retina-specific expression of PDGF-B versus PDGF-A: vascular versus nonvascular proliferative retinopathy. Invest Ophthalmol Vis Sci. 2002;43(6):2001–6. [PubMed] [Google Scholar]
- Otani A, Dorrell MI, Kinder K, Moreno SK, Nusinowitz S, Banin E, Heckenlively J, Friedlander M. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest. 2004;114(6):765–74. doi: 10.1172/JCI21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Seo MS, Ozaki K, Yamada H, Yamada E, Okamoto N, Hofmann F, Wood JM, Campochiaro PA. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000;156(2):697–707. doi: 10.1016/S0002-9440(10)64773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92(3):905–9. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–51. [PMC free article] [PubMed] [Google Scholar]
- Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J Clin Invest. 2006;116(12):3266–76. doi: 10.1172/JCI29683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. Embo J. 1998;17(11):3005–15. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Invest Ophthalmol Vis Sci. 2008;49(4):1591–8. doi: 10.1167/iovs.07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandercoe TM, Geller SF, Hendrickson AE, Stone J, Provis JM. VEGF expression by ganglion cells in central retina before formation of the foveal depression in monkey retina: evidence of developmental hypoxia. J Comp Neurol. 2003;462(1):42–54. doi: 10.1002/cne.10705. [DOI] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–11. [PubMed] [Google Scholar]
- Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109(3):327–36. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson L, Matesanz N, Colhoun L, Edgar K, Devine A, Gardiner TA, McDonald DM. Reduced Nitrooxidative Stress and Neural Cell Death Suggests a Protective Role for Microglial Cells in TNF{alpha}-/- Mice in Ischemic Retinopathy. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.09-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456(7223):814–8. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Chan-Ling T, Pe’er J, Itin A, Gnessin H, Keshet E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1996;37(2):290–9. [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15(7 Pt 1):4738–47. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, Suzuma I, Ohashi H, Ojima T, Murakami T, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353(8):782–92. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15(4):621–7. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Kerdiles YM, Knaup KX, Rafie CA, Boutin AT, Stockmann C, Takeda N, Scadeng M, Shih AY, Haase VH, et al. The glial cell response is an essential component of hypoxia-induced erythropoiesis in mice. J Clin Invest. 2009;119(11):3373–83. doi: 10.1172/JCI39378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Harada T, Liu L, Lush ME, Guignard F, Harada C, Burns DK, Bajenaru ML, Gutmann DH, Parada LF. Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development. 2005;132(24):5577–88. doi: 10.1242/dev.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.