Abstract
Coiled coils are extensively and successfully used nowadays to rationally design multistranded structures for applications, including basic research, biotechnology, nanotechnology, materials science, and medicine. The wide range of applications as well as the important functions these structures play in almost all biological processes highlight the need for a detailed understanding of the factors that control coiled-coil folding and oligomerization. Here, we address the important and unresolved question why the presence of particular oligomerization-state determinants within a coiled coil does frequently not correlate with its topology. We found an unexpected, general link between coiled-coil oligomerization-state specificity and trigger sequences, elements that are indispensable for coiled-coil formation. By using the archetype coiled-coil domain of the yeast transcriptional activator GCN4 as a model system, we show that well-established trimer-specific oligomerization-state determinants switch the peptide’s topology from a dimer to a trimer only when inserted into the trigger sequence. We successfully confirmed our results in two other, unrelated coiled-coil dimers, ATF1 and cortexillin-1. We furthermore show that multiple topology determinants can coexist in the same trigger sequence, revealing a delicate balance of the resulting oligomerization state by position-dependent forces. Our experimental results should significantly improve the prediction of the oligomerization state of coiled coils. They therefore should have major implications for the rational design of coiled coils and consequently many applications using these popular oligomerization domains.
Keywords: protein folding, protein structure, protein–protein interactions
Because of its simplicity, the α-helical coiled coil has been used traditionally in a vast number of studies aimed at understanding the fundamental principles of protein stability, folding, and oligomerization (reviewed in refs. 1 and 2). As a result, coiled coils are exploited nowadays as multipurpose tools in a steadily increasing number of applications ranging from basic research to medicine (2). For example, designed two- and three-stranded coiled coils were successfully used as lead molecules to target the adenomatous polyposis coli tumor-suppressor protein that is implicated in colorectal cancers (3) or to inhibit HIV infection (4), respectively. For these and many other applications, knowledge of the factors that control the folding and oligomerization state appears essential to rationally engineer specific coiled-coil structures with the desired properties.
It is well established that so-called “trigger sequences,” short, distinct amino acid sequences, play an important role in controlling coiled-coil formation (reviewed in ref. 1). A characteristic feature of many trigger sequences is that they fold into reasonably stable monomeric helices before the coiled-coil structure forms (5–7). We recently solved the NMR structure of a peptide spanning the GCN4 trigger sequence and showed that its structure is stabilized by a network of hydrogen bonds and electrostatic interactions (7). The helical conformation of the trigger sequence provides an effective local structural scaffold for the interaction of key residues of colliding chains. Accordingly, preformed and folding competent helices form a nucleation site, which promotes productive in-register chain association. Interacting helices then “zip up” along the molecule to direct formation of a stable coiled-coil structure. Trigger sequences of different proteins show considerable diversity, indicating that a consensus sequence is unlikely to exist (7). As a result, the prediction of trigger sequences on the basis of sequence information alone remains a challenge and still requires experimental verification.
With respect to coiled-coil topology, several determinants that control the oligomerization state of coiled coils have been identified and studied in detail (reviewed in refs. 1 and 2). It is generally acknowledged, for example, that the distribution of hydrophobic core residues, in particular isoleucine and leucine, correlates well with the oligomerization state of coiled coils. The occurrence of isoleucine and leucine residues at the heptad repeat a and d core positions, respectively, favors the formation of dimers whereas the reverse arrangement results in tetrameric structures. In contrast, a more even distribution of isoleucine at both the a and d positions facilitates trimer formation. Buried polar residues in hydrophobic interfaces also play an important role in determining the number of strands in coiled coils. Many two-stranded coiled-coil domains of transcription regulators, for example, frequently contain at least one conserved asparagine or lysine residue at an a position toward the center of the sequence (2).
Interhelical interactions between side chains of residues at the e and g positions as well as the packing of these amino acids against the hydrophobic a and d core residues have also been shown to contribute significantly to oligomerization-state specificity of coiled coils (reviewed in refs. 1 and 2). This is exemplified by the trimerization motif Arg(g)-h(a)-x-x-h(d)-Glu(e) (denoted R-hxxhE where h(a) = Ile, Leu, Val, Met; h(d) = Leu, Ile, Val; x = any amino acid residue) that specifies a three-stranded, parallel topology of coiled-coil domains (8). The trimerization driving force of the motif can be explained by optimal side chain–side chain interactions whereby the strictly conserved arginine and glutamate residues form a distinct bifurcated interhelical salt-bridge network and participate in the formation of the hydrophobic core by establishing tight packing interactions to the neighbouring residues at the a and d positions through their aliphatic moieties. Thus, similar to contacts stabilizing thermostable proteins, the trimerization motif encompasses both networks of surface salt bridges and optimal internal hydrophobic packing interactions (8).
An open issue is that the presence of a specific oligomerization-state determinant does frequently not correlate with the corresponding coiled-coil topology. Although the trimerization motif R-hxxhE is predominantly found in many diverse protein families harboring parallel three-stranded coiled-coil domains, it is also present in some dimers and antiparallel trimers, such as the transcriptional activators GCN4 (9) and ATF1 (10) or the actin-bundling protein cortexillin-1 from Dictyostelium discoideum (6). To understand the molecular basis of this discrepancy, we have carried out a detailed biophysical and structural study to systematically address how the topology of these two-stranded coiled-coil model systems is affected when particular oligomerization-state determinants are placed at different coiled-coil positions. We thus address the fundamental and still unresolved question how the position of specific motifs within a coiled coil defines its topology.
Results
The Topology of GCN4-p1 Variants Depends on the Position of the Trimerization Motif R-hxxhE.
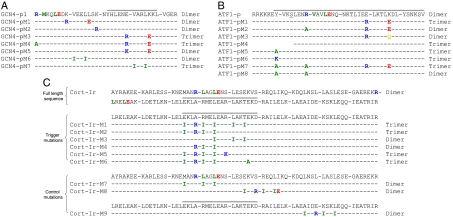
To test the importance of sequence context for oligomerization-state specificity, three mutant peptides of the GCN4 coiled coil (denoted GCN4-p1) were produced in which the trimerization motif R-hxxhE was inserted at different positions along the sequence (GCN4-pM1, GCN4-pM2, and GCN4-pM3; Fig. 1A). Asn16 at the central a position was not mutated because this residue is known to play an important role for specific dimer formation of GCN4-p1 (9). To assess their properties in solution, the structures of the GCN4-p1 variants were investigated and compared to the wild-type coiled coil by CD spectroscopy and analytical ultracentrifugation (AUC) or multiangle light scattering (MALS).
Fig. 1.
Rational design of GCN4-p1 (A), ATF1-p (B), and Cort-Ir (C) coiled-coil variants. Heptad repeats are shown as blocks of seven residues. Experimentally determined trigger sequences are indicated with a gray bar on top of the respective protein sequences. Arginine and glutamate residues of the native and generated trimerization motifs as well as the different substitutions are highlighted in color according to the amino acids’ physicochemical properties: blue, positively charged; red, negatively charged; green, hydrophobic. To prevent complications in the interpretation of results due to disulfide-bridge formation, Cys10 of ATF1-p was mutated to serine (underlined in B). The 18 heptad repeats of Cort-Ir are shown in C, Top. For simplicity, only the respective halves of the Cort-Ir sequences are depicted for the trigger and control mutations. The oligomerization state of the peptides is indicated on the right of each alignment. For molecular weight values, see Table 1.
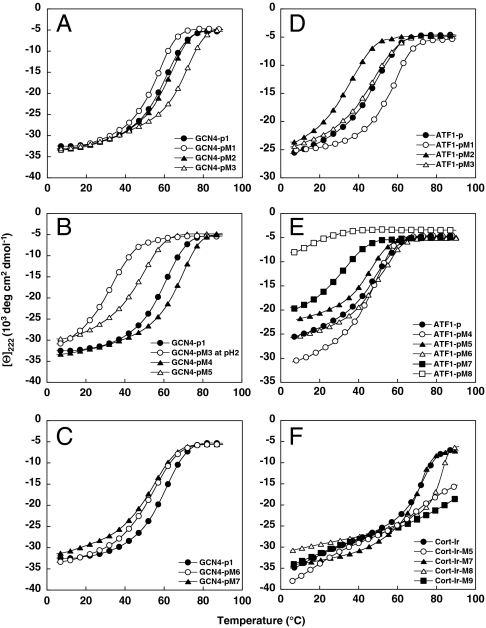
The far-UV CD spectra recorded from GCN4-p1 and the three mutants at 5 °C were characteristic of proteins with a high α-helical content (Table 1). The stabilities of the peptides were assessed by thermal unfolding profiles recorded by CD at 222 nm (Fig. 2A and Table 1). Consistent with native-like coiled-coil structures, all peptides revealed reversible sigmoid-shaped unfolding profiles. Sedimentation equilibrium analysis of GCN4-pM1 and GCN4-pM2 revealed the formation of dimers with lower or similar stabilities, respectively, than the wild-type peptide. In marked contrast, GCN4-pM3 yielded an average molecular mass consistent with the formation of a trimeric structure despite the presence of Asn16 at a heptad repeat position a and showed a significantly higher melting temperature than GCN4-p1. Notably, the mutations introduced into this particular GCN4-p1 variant fall into the trigger sequence (5, 7).
Table 1.
CD, sedimentation equilibrium AUC, and MALS data of GCN4-p1, ATF1-p, and Cort-Ir variants
| Peptide | [Θ]222103 deg cm2 dmol-1 | Tm, °C * | Mw, kDa † |
| GCN4-p1 | −32.4 | 61 | 8.8 |
| GCN4-pM1 | −33.4 | 53 | 9.0 ‡ |
| GCN4-pM2 | −33.3 | 61 | 8.7 ‡ |
| GCN4-pM3 | −33.1 | 70 (35) § | 11.9 (8.5) ¶ |
| GCN4-pM4 | −33.4 | 69 | 11.3 |
| GCN4-pM5 | −29.7 | 49 | 8.6 |
| GCN4-pM6 | −33.4 | 55 | 7.8 |
| GCN4-pM7 | −31.4 | 54 | 11.9 |
| ATF1-p | −25.6 | 47 | 11.3 |
| ATF1-pM1 | −25.0 | 57 | 14.0 |
| ATF1-pM2 | −23.6 | 36 | 10.0 |
| ATF1-pM3 | −24.5 | 48 | 10.4 |
| ATF1-pM4 | −30.8 | 51 | 11.7 |
| ATF1-pM5 | −21.9 | 47 | 15.0 |
| ATF1-pM6 | −25.6 | 49 | 13.9 |
| ATF1-pM7 | −19.6 | 32 | 13.8 |
| ATF1-pM8 | −8.1 | 15 | 8.5 |
| Cort-Ir | −35.0 | 73 | 28 |
| Cort-Ir-M1 | −33.5 | ND | 43 |
| Cort-Ir-M2 | −31.5 | ND | 44 |
| Cort-Ir-M3 | −36.6 | ND | 28 |
| Cort-Ir-M4 | −34.1 | 72 | 27 |
| Cort-Ir-M5 | −38.3 | ND | 43 |
| Cort-Ir-M6 | −35.1 | ND | 41 |
| Cort-Ir-M7 | −34.3 | 71 | 26 |
| Cort-Ir-M8 | −30.8 | 83 | 29 |
| Cort-Ir-M9 | −34.1 | ND | 30 |
*Tm determined at peptide concentrations (monomer) of 35 μM. Estimated error: ± 1 °C. ND, not determined because protein does not unfold completely within the temperature range investigated.
†Average molecular masses determined by sedimentation equilibrium AUC. Estimated error: ± 10%. The calculated molecular masses of the GCN4-p1, ATF1-p, ATF1-pM4, and Cort-Ir monomers are 4.2 kDa, 5.1 kDa, 3.8 kDa, and 15.0 kDa, respectively.
‡Average molecular masses determined by MALS. Error: < 5%.
§Tm determined at pH 2 is given in brackets.
¶The average molecular mass value determined at pH 2 is given in brackets.
Fig. 2.
CD analysis of GCN4-p1 (A–C), ATF1-p (D and E), and Cort-Ir (F) varaints. Thermal unfolding profiles recorded by CD at 222 nm are shown. The experiments were carried out at 35-μM peptide concentration (monomer) in PBS (pH 7.4) or 5-mM sodium phosphate (pH 2).
As outlined in the introduction, a canonical trimerization motif R-hxxhE is also present at the N terminus of GCN4-p1 (Fig. 1A). To exclude the possibility that this motif collaborates with the generated trimerizer in the observed switch of the peptides oligomerization state, we replaced Arg1 of GCN4-pM3 by alanine (GCN4-pM4; Fig. 1A). The substitution did not restore the dimeric structure of the mutant peptide, which showed a similar melting temperature like GCN4-pM3, indicating that the N-terminal native trimerization motif does not contribute substantially to the thermal stability of the coiled coil (Fig. 2B and Table 1).
These findings suggest that the observed switch in oligomerization state from a dimer to a trimer can be attributed to a network of interhelical surface salt bridges and optimal hydrophobic packing interactions within the GCN4-pM3 trigger sequence, which is the hallmark of the trimerization motif (8). To test this hypothesis, a control peptide was generated in which Arg22 of GCN4-pM3 was substituted by lysine (GCN4-pM5; Fig. 1A). As a result of its different side chain geometry, lysine is not anticipated to substitute for arginine within the trimerization motif. The mutation did not compromise coiled-coil formation, with the peptide variant being more than 90% helical. As expected, GCN4-pM5 formed a two-stranded coiled-coil structure (Fig. 2B and Table 1), demonstrating that the characteristic bifurcated Arg(g)-to-Glu(e′) (where the prime denotes a residue of a neighboring chain) salt bridge of the trimerization motif is a key element for trimer formation (8). This conclusion is further supported by CD thermal unfolding and sedimentation equilibrium experiments of GCN4-pM3 carried out at pH 2 where glutamate residues are protonated. Disruption of the network of surface salt bridges of the motif at low pH not only restored the oligomerization state of GCN4-pM3 back to a dimer but also resulted in a significant decrease of the Tm to 35 °C (Fig. 2B and Table 1).
Taken together, these findings demonstrate that the position of the R-hxxhE motif within the GCN4-p1 coiled-coil domain is crucial for capitalizing on its trimerization potential. Only for the mutant in which the trimerization motif was incorporated into the trigger sequence a switch of the oligomerization state to the trimer was observed, whereas the other variants were dimeric.
A General Link Between Trigger Sequence Function and Coiled-Coil Oligomerization-State Specificity.
The observation that incorporation of the trimerization motif into the trigger sequence results in a switch of the oligomerization state offers the prospect that such topological changes can also be generated by other interchain interactions. To confirm this hypothesis, we extended our GCN4-p1 studies to the hydrophobic core. It is well established that the distribution of hydrophobic core residues has an impact on the oligomerization state (see the introduction). However, in the seminal studies by Alber, Kim, and co-workers (11, 12), all hydrophobic core positions of GCN4-p1 were simultaneously substituted, and it is therefore unknown how the position of individual hydrophobic core residues affects the oligomerization state of a coiled coil.
To address this issue, we generated a GCN4-p1 variant with the potential to fold into a trimer by substituting the central hydrophobic core residues of the trigger sequence, Val23(a) and Leu26(d), by isoleucine (GCN4-pM7; Fig. 1A). To verify that a conformational switch occurs only when mutations are introduced into the trigger sequence, we also generated a control peptide in which we made identical replacements [Val9(a) and Leu12(d)] in the second heptad repeat of the coiled coil (GCN4-pM6; Fig. 1A). Consistent with our previous findings (see above), introduction of isoleucine residues in the trigger sequence resulted in the formation of a trimer, whereas the control peptide was dimeric (Table 1). The thermal stabilities of GCN4-pM6 and GCN4-pM7 are slightly decreased when compared with the wild-type coiled coil (Fig. 2C and Table 1), which can be explained by an unfavorable rotamer conformation of Ile9(a) in the GCN4-pM6 dimer and by the presence of Asn16(a) in both GCN4-p1 variants. In this context, it is interesting to note that the trigger sequence of the trimeric coiled-coil domain of the human macrophage scavenger receptor (173-IDEISKS) shows the signature of a heptad repeat characteristic of trimers (173-IDEISKS) with isoleucine residues (underlined) at both the a and d positions (13), suggesting that these residues are involved in specifying the domain’s three-stranded oligomerization state.
Our results on GCN4-p1 demonstrate that the trigger sequence, apart from its essential role in coiled-coil folding, also plays an important function in controlling the protein’s oligomerization state. To generalize the concept of a link between trigger sequence function and oligomerization-state specificity, we investigated the coiled-coil domains of the human transcriptional activator ATF1 and the actin-bundling protein cortexillin-1 from D. discoideum. These two-stranded, parallel coiled coils were selected because they also contain trimerization motifs and because for both domains, referred to as ATF1-p and Cort-Ir, high-resolution crystal structures are available (10, 14).
To determine the unknown ATF1-p trigger sequence, an analogous chimeric approach was used as previously described for GCN4-p1 (5). In these experiments, the N- and C-terminal halves of ATF1-p were fused to the N-terminal two heptad repeats of GCN4-p1 (Fig. S1A) and trigger sequences identified on the basis of productive coiled-coil formation. Both chimera folded into stable coiled-coil structures (Fig. S1 B and C), indicating that the entire ATF1-p coiled coil can act as a trigger sequence. Consistent with this hypothesis, introduction of a second trimerization motif into the third and fourth heptad repeat of the ATF1-p sequence (ATF1-pM1; Fig. 1B) yielded a trimeric coiled coil with a significantly increased thermal stability when compared to the wild-type peptide (Fig. 2D and Table 1). However, one trimerization motif is not sufficient to switch the oligomerization state of ATF1-p from a dimer to a trimer. This is evident in the wild-type peptide or the ATF1-pM2 and ATF1-pM3 mutants (Fig. 1B) in which the native or the introduced trimerization motif of ATF1-pM1, respectively, are apparently overruled (Fig. 2D and Table 1) by the presence of putatively strong dimerization determinants.
To identify determinants that could be responsible for specific dimer formation of ATF1-p apart from Asn22 at the central heptad repeat a position, we analyzed the protein’s crystal structure (10). The most prominent feature seen in the structure is an interhelical hydrogen bond between Tyr7 and Glu12 at heptad repeat positions g and e′, respectively. To elucidate whether this distinct structural feature is responsible for dimer formation of ATF1-p, we produced a shortened coiled-coil domain starting at Leu11 that lacks the hydrogen bond (ATF1-pM4; Fig. 1B). Molecular weight and CD measurements indicated that ATF1-pM4 forms a stable trimer (Fig. 2E and Table 1). Identical results were obtained after mutation of Tyr7 in the wild-type sequence (ATF1-pM5 or ATF1-pM6) or in ATF1-pM2 (ATF1-pM7) (Fig. 1B). These results indicate that the distinct hydrogen bond between Tyr7(g) and Glu12(e′) within the trigger sequence controls dimerization of ATF1-p (Fig. 2E and Table 1). Notably, this interaction is conserved in the ATF1 family of transcription factors and contributes to ATF1 stability [Fig. 2E; (10)]. A corresponding Tyr(g)-to-Glu(e′) hydrogen bond is also conserved in the coiled-coil neck of fungal kinesins where it is required for stabilization of the dimer (15). Accordingly, mutation of both the Tyr(g)-to-Glu(e′) hydrogen bond and the native trimerization motif of ATF1-p resulted in the formation of a significantly destabilized dimer (ATF1-pM8; Fig. 2E and Table 1). Dimerization of ATF1-pM8 can be explained by the presence of Asn22 at a heptad repeat a position.
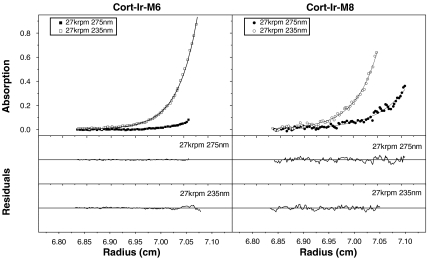
Next we performed corresponding experiments with the two-stranded Cort-Ir coiled-coil domain. This coiled coil has been studied extensively and its trigger sequence is well characterized (6, 14). Because of its substantial size (18 continuous heptad repeats), we used a combination of both trimer-specific determinants for the design of the mutants. Accordingly, we rationally substituted residues to complement two ideal trimerizer motifs of the type R-IxxIE and to introduce one hydrophobic core isoleucine residue in the trigger sequence of the Cort-Ir coiled coil (Cort-Ir-M1; Fig. 1C). Notably these substitutions were designed to span the entire trigger sequence. Consistent with our results on GCN4-p1 and ATF1-p, the trigger sequence mutant folded into a stable three-stranded coiled-coil structure (Table 1 and Fig. S2). To determine the minimal number of mutations required to switch the peptide’s oligomerization state from a dimer to a trimer, we produced two mutants, Cort-Ir-M2 and Cort-Ir-M3 (Fig. 1C), which each contain one ideal R-IxxIE trimerization motif and one isoleucine substitution. Molecular weight measurements demonstrated that Cort-Ir-M2 formed a trimer, whereas Cort-Ir-M3 was dimeric (Table 1 and Fig. S2). To exclude that the putative salt bridge between Arg91(g) and Glu96(e′) in the trigger sequence of Cort-Ir contributes to trimer formation, it was mutated (Cort-Ir-M5 and Cort-Ir-M6; Fig. 1C). AUC analysis demonstrated that the introduced ideal trimerization motif is sufficient to switch the dimer to a trimer (Table 1, Fig. 3, and Figs. S2 and S3). However, mutation of Glu81 to isoleucine (compare Cort-Ir-M5/M6 with Cort-Ir-M4; Fig. 1C) is required for the switch of the oligomerization state (Table 1 and Fig. S2). This finding suggests that the interhelical salt bridge between Glu81(d) and Arg85(a′) seen in the crystal structure of Cort-Ir (14) is a strong determinant of specific dimer formation of the coiled coil that can overrule the presence of one ideal trimerizer.
Fig. 3.
Analysis of the oligomerization state of Cort-Ir variants by sedimentation equilibrium AUC. The sequences of Cort-Ir-M6 and Cort-Ir-M8 are shown in Fig. 1C. (Upper) UV absorbance gradients as a function of the radial position (data points) and fits according to a single ideal species model (lines). (Lower) Residuals showing the difference between the experimental data and the theoretical model. The fitted values are listed in Table 1.
As in the experiments described for GCN4-p1, Cort-Ir controls were prepared in which identical replacements were made outside of the trigger sequence (Cort-Ir-M7, Cort-Ir-M8, and Cort-Ir-M9; Fig. 1C). These controls were designed to cover a spectrum of different mutations and notably in Cort-Ir-M8 the same a and d residues as in Cort-Ir-M2 were replaced by isoleucine. In agreement with our results on GCN4-p1, all controls were dimeric like Cort-Ir (Table 1, Figs. 2F and 3, and Fig. S3). The observation that C-terminal Cort-Ir mutants do not completely unfold up to 90 °C whereas N-terminal control peptides are less stable indicates an important role of the C terminus in coiled-coil stability (Table 1, Fig. 2F, and Fig. S2).
Taken together, these results suggest that oligomerization-state determinants specify the topology of a coiled coil only when present in the trigger sequence. They therefore establish a general link between trigger sequence function and oligomerization-state specificity. Our data further uncovered two previously undescribed dimerization motifs, the Tyr(g)-Glu(e′) interhelical hydrogen bond and the Glu(d)-Arg(a′) interhelical salt bridge, within the trigger sequences of ATF1-p and Cort-Ir, respectively. The observation that multiple motifs can coexist in the same trigger sequence reveals a delicate balance of position-dependent forces that control a specific coiled-coil topology. Furthermore, we found that the trigger sequence of ATF1-p extends over four rather than the approximately two heptad repeats characteristic of GCN4-p1 and Cort-Ir. This observation indicates that trigger sequences can not only vary with respect to sequence (7) but also in terms of length.
Structural Basis of Trimer Formation of GCN4-p1 Variants.
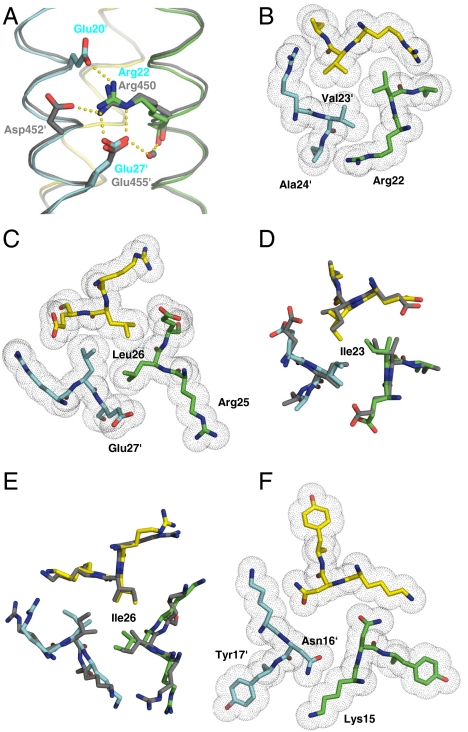
To understand the molecular basis of how the trimerization motif R-hxxhE or isoleucine substitutions at hydrophobic core positions in the trigger sequence of the GCN4-p1 result in a switch of conformation from dimer to trimer and how these mutations overrule the dimerization potential of Asn16, we determined the crystal structures of GCN4-pM3 and GCN4-pM7 at 1.9- and 2.0-Å resolution, respectively. The overall structures of both trimers display all characteristics of left-handed, parallel, and in-register coiled coils (Fig. S4).
The most prominent feature seen in the crystal structure of GCN4-pM3 is a network of surface salt bridges and internal hydrophobic packing interactions in the vicinity of the trimerization motif, which are similar to those described for the parallel three-stranded coiled-coil domain of coronin-1 (Fig. 4 A–C) (8). Accordingly, the conformations of side chains and the position of the water molecule between Arg22 and Glu27′ can be almost perfectly superimposed to the trimerization motif of the coronin-1 coiled coil, demonstrating structural conservation of the motif. In addition to the characteristic bifurcated contact between Arg22∶Nε, Nη2 at position g of one chain and Glu27′∶Oε1, Oε2 at position e′ of the neighboring chain, an interaction between Arg22∶Nη1 and Glu20′∶Oε1 at position e′ extends the network of surface salt bridges (Fig. 4A and Fig. S4).
Fig. 4.
Packing of side chains within X-ray crystal structures of GCN4-pM3 and GCN4-pM7. (A) Side view of an overlay of the salt-bridge network (indicated by yellow dots) of the trimerization motifs seen in GCN4-pM3 and the coiled-coil domain of coronin-1 (8) (dark gray ribbons). (B) Axial view of the a4 layer of GCN4-pM3 showing the shielding of the Val23′ residues from solvent by the aliphatic side-chain moieties of Arg22. (C) Axial view of the d4 layer of GCN4-pM3 showing the hydrophobic packing of Ile26 residues and the aliphatic side-chain moieties of the Glu27′. (D and E) Axial views of an overlay of the acute packing of isoleucine residues at the a4 and d4 layers seen in GCN4-pM7 and GCN4-pII (dark gray) (12). (F) Axial view of the a3 layer showing the conformation of the Asn16 residues and the unfavorable packing of their polar moieties against the aliphatic portion of Lys15′ as well as the formation of a large cavity in the hydrophobic core of GCN4-pM3. A similar conformation is seen in the crystal structure of GCN4-pM7. Side chains of residues are shown as stick representation (A–F) and van der Waals spheres (B, C, and F). Monomers are shown as Cα traces and the bound water molecules as a small red (GCN4-pM3) or gray (coiled-coil domain of coronin-1) sphere (A). Oxygen and nitrogen atoms are colored in red and blue, and carbon atoms are shown in green, cyan, and yellow for GCN4-pM3 and GCN4-pM7 monomers A, B, and C, respectively. The prime indicates a residue of chain B.
The interior packing of GCN4-pM7 at layers a23 and d26 provides a structural basis for the switch of the oligomerization state from a dimer to a trimer (Fig. 4 D and E). In the structure the two introduced isoleucine residues can be accommodated in the most preferred rotamer at the hydrophobic core positions. Accordingly, the acute packing geometries of Ile23 and Ile26 are very similar to those seen in the crystal structure of the GCN4-pII variant (Fig. 4 D and E) (12).
In contrast to the favorable interactions seen in the two structures, the polar side chain of Asn16 at the central a position points out of the hydrophobic core, generating both an energetically unfavorable cavity and compromised packing contacts against the hydrophobic moiety of Lys15 at the preceding g′ position (Fig. 4F). In contrast, in dimers, asparagine residues point into the hydrophobic interface and form a distinctive hydrogen bond (9). These findings therefore provide a structural explanation why an asparagine at core a positions favors dimer over trimer formation.
Discussion
Detailed knowledge of the oligomerization state of coiled coils is crucial for exploiting their potential in a wide range of applications and understanding their functions in a large number of biological processes. Over the years, several oligomerization-state determinants have been identified and studied in detail; however, their presence does frequently not correlate with coiled-coil topology. Here, using three diverse coiled-coil model systems we addressed this discrepancy and demonstrated that oligomerization-state determinants specify the topology of a coiled coil when present in the trigger sequence. Furthermore, we show that multiple motifs can coexisit in the same trigger sequence and the resulting oligomerization state is controlled by the strength of the determinants relative to each other. The contribution of the relative strength of individual motifs to final coiled-coil topology remains to be elucidated.
Our findings on a unique and general link between oligomerization-state specificity and trigger sequence function explain some puzzling results reported in the literature. We previously showed that a chimera in which the trigger sequence of GCN4 was replaced by the one of cortexillin-1 (GCN4p-Cort T/L; note that Thr95 of Cort-Ir has been replaced by leucine to result by chance in a canonical trimerization motif) folds into a three-stranded coiled-coil structure despite the presence of Asn16(a) (5). This finding can now be rationalized by the presence of a trimerization motif (R-LAKLE) in the GCN4p-Cort T/L trigger sequence. Further, the coiled-coil domain of the human dystrophia myotonica kinase contains a motif (509-R-NRDLE), which is very similar to the R-hxxhE consensus. Although it is currently not known how mutations of the hydrophobic residues of the trimerization motif influence its function, the motif of the human dystrophia myotonica kinase is a good candidate to determine trimerization of the coiled coil by overruling the strong dimerization driving force of two asparagine residues at heptad a positions in the sequence (16). Another example is the coiled-coil domain of the transcription factor HY5 from Arabidopsis thaliana, which was reported to harbor two putative and overlapping consensus trigger sequences (6-SELENR-VKDLENK and 13-KDLENK-NSELEER) containing a trimerization motif (underlined in the first sequence stretch) and an asparagine residue at a heptad repeat a position (underlined in the second sequence stretch), respectively (17). A second asparagine residue, Asn33, is present at heptad repeat a position outside the predicted trigger sequences. The wild-type peptide is dimeric but substitution of one or both asparagine by leucine or valine resulted in a switch of the oligomerization state from a dimer to a trimer (17). A similar context-dependent effect of the trimerization motif (591-R-LNRVE) was recently also demonstrated for the K(V)7.1 (KCNQ1) A-domain tail coiled coil, where C-terminal truncation resulted in a switch of the oligomerization state from a tetramer to a trimer (18).
In conclusion, our study represents an important complementation and substantial extension of the sequence-to-structure rules outlined in the pioneering work of Alber, Kim, and colleagues (11, 12). These rules together with the characteristics of the heptad repeat have allowed the development of a variety of statistics- and pattern-based methods that predict the occurrence of coiled coils with a high degree of confidence (reviewed in ref. 19). However, despite the numerous efforts during the last three decades, a reliable prediction of the specific oligomerization state of many native coiled coils remains difficult (1). This highlights the need for improving existing algorithms by implementation of experimentally verified sequence-to-structure rules like the ones based on the present study. The inclusion of these parameters as well as the detailed dataset obtained in our study should therefore significantly improve and extend the kit toward predicting and engineering the structure and function of coiled-coil proteins, which should have major implications for all applications using these popular oligomerization domains.
Methods
Peptide Preparations and Crystal Structure Determination.
Standard recombinant peptide preparation and crystal structure determination is described in SI Text.
AUC, MALS, and CD Spectroscopy.
All solution studies were performed in 5 mM sodium phosphate, pH 7.4, supplemented with 150 mM PBS.
AUC was carried out on an Optima XLA analytical ultracentrifuge (Beckman) equipped with an adsorption optical system and an An-60Ti (GCN4-p1, ATF-p) or an An-50Ti (Cort-Ir) rotor. Sedimentation equilibrium data were collected at three different protein concentrations (0.14, 0.27, and 0.55 mg/mL for GCN4-p1 variants and 0.15, 0.3, and 0.6 mg/mL for ATF1-p variants) and at rotor speeds of 28,000, 35,000, and 41,000 rpm. The MW reported in Table 1 is calculated at the highest sample concentration. Sedimentation equilibrium data of Cort-Ir were recorded at three different protein concentrations (0.25, 0.5, and 1.0 mg/mL) and at rotor speeds of 27,000, 31,000, and 35,000 rpm. The sedimentation profiles were monitored at two wavelengths (275 nm for tyrosine and 235 nm for peptide backbone absorption). The data were fitted globally using a single ideal species model. The partial specific volumes of the synthetic peptides were calculated from their amino acid sequence composition. Solvent density for PBS was taken as 1.004 g/mL.
Size exclusion chromatography coupled to MALS was performed on a DAWN EOS 18-angle detector connected to an Optilab Rex refractometer (Wyatt). Peptide solutions (100 μL of 3–5 mg/mL) were injected on a Superdex 75 10/30 size exclusion chromatography column equilibrated with PBS. Molecular weights were calculated by using the Wyatt ASTRA version 4.90.08 software package.
Far-UV CD spectroscopy was carried out on Jasco J-810 (Jasco Inc.; GCN4-p1 and ATF-p variants) or Chirascan-plus (Applied Photophysics; Cort-Ir variants) spectropolarimeters equipped with temperature-controlled quartz cells of 0.1-cm path length. A ramping rate of 1 °C per min was used to record the thermal unfolding profiles. Midpoints of the transitions, Tms, were taken as the maximum of the derivative d[θ]222/dT.
Supplementary Material
Acknowledgments.
We are indebted to Ariel Lustig for performing initial AUC measurements. We thank Edward McKenzie, Sanjai Patel, Karolina Zielinska, and the Biomolecular Analysis and Crystallization Core Facilities (Faculty of Life Sciences, University of Manchester) for excellent technical assistance. M.O.S. and R.A.K. acknowledge support by grants from the Swiss National Science Foundation and the Wellcome Trust, respectively.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3GJP and 2O7H).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008502107/-/DCSupplemental.
References
- 1.Lupas AN, Gruber M. The structure of alpha-helical coiled coils. Adv Protein Chem. 2005;70:37–38. doi: 10.1016/S0065-3233(05)70003-6. [DOI] [PubMed] [Google Scholar]
- 2.Woolfson DN. The design of coiled-coil structures and assemblies. Adv Protein Chem. 2005;70:79–112. doi: 10.1016/S0065-3233(05)70004-8. [DOI] [PubMed] [Google Scholar]
- 3.Sharma VA, Logan J, King DS, White R, Alber T. Sequence-based design of a peptide probe for the APC tumor suppressor protein. Curr Biol. 1998;8:823–830. doi: 10.1016/s0960-9822(98)70324-0. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi E, et al. Covalent stabilization of coiled coils of the HIV gp41 N region yields extremely potent and broad inhibitors of viral infection. Proc Natl Acad Sci USA. 2005;102:12903–12908. doi: 10.1073/pnas.0502449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kammerer RA, et al. An autonomous folding unit mediates the assembly of two-stranded coiled coils. Proc Natl Acad Sci USA. 1998;95:13419–13424. doi: 10.1073/pnas.95.23.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinmetz MO, et al. A distinct 14 residue site triggers coiled-coil formation in cortexillin I. EMBO J. 1998;17:1883–1891. doi: 10.1093/emboj/17.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinmetz MO, et al. Molecular basis of coiled-coil formation. Proc Natl Acad Sci USA. 2007;104:7062–7067. doi: 10.1073/pnas.0700321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kammerer RA, et al. A conserved trimerization motif controls the topology of short coiled coils. Proc Natl Acad Sci USA. 2005;102:13891–13896. doi: 10.1073/pnas.0502390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Shea EK, Klemm JD, Kim PS, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 10.Schumacher MA, Goodman RH, Brennan RG. The structure of a CREB bZIP.somatostatin CRE complex reveals the basis for selective dimerization and divalent cation-enhanced DNA binding. J Biol Chem. 2000;275:35242–35247. doi: 10.1074/jbc.M007293200. [DOI] [PubMed] [Google Scholar]
- 11.Harbury PB, Zhang T, Kim PS, Alber T. A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science. 1993;262:1401–1407. doi: 10.1126/science.8248779. [DOI] [PubMed] [Google Scholar]
- 12.Harbury PB, Kim PS, Alber T. Crystal structure of an isoleucine-zipper trimer. Nature. 1994;371:80–83. doi: 10.1038/371080a0. [DOI] [PubMed] [Google Scholar]
- 13.Frank S, Lustig A, Schulthess T, Engel J, Kammerer RA. A distinct seven-residue trigger sequence is indispensable for proper coiled-coil formation of the human macrophage scavenger receptor oligomerization domain. J Biol Chem. 2000;275:11672–11677. doi: 10.1074/jbc.275.16.11672. [DOI] [PubMed] [Google Scholar]
- 14.Burkhard P, Kammerer RA, Steinmetz MO, Bourenkov GP, Aebi U. The coiled-coil trigger site of the rod domain of cortexillin I unveils a distinct network of interhelical and intrahelical salt bridges. Struct Fold Des. 2000;8:223–230. doi: 10.1016/s0969-2126(00)00100-3. [DOI] [PubMed] [Google Scholar]
- 15.Schafer F, et al. A conserved tyrosine in the neck of a fungal kinesin regulates the catalytic motor core. EMBO J. 2003;22:450–458. doi: 10.1093/emboj/cdg036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia P, et al. Molecular insights into the self-assembly mechanism of dystrophia myotonica kinase. FASEB J. 2006;20:1142–1151. doi: 10.1096/fj.05-5262com. [DOI] [PubMed] [Google Scholar]
- 17.Yoon MK, Kim HM, Choi G, Lee JO, Choi BS. Structural basis for the conformational integrity of the Arabidopsis thaliana HY5 leucine zipper homodimer. J Biol Chem. 2007;282:12989–13002. doi: 10.1074/jbc.M611465200. [DOI] [PubMed] [Google Scholar]
- 18.Xu Q, Minor DL., Jr Crystal structure of a trimeric form of the K(V)7.1 (KCNQ1) A-domain tail coiled-coil reveals structural plasticity and context dependent changes in a putative coiled-coil trimerization motif. Protein Sci. 2009;18:2100–2114. doi: 10.1002/pro.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber M, Soding J, Lupas AN. Comparative analysis of coiled-coil prediction methods. J Struct Biol. 2006;155:140–145. doi: 10.1016/j.jsb.2006.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.