Abstract
Much interest is currently focused on the emerging role of tumor-stroma interactions essential for supporting tumor progression. Carcinoma-associated fibroblasts (CAFs), frequently present in the stroma of human breast carcinomas, include a large number of myofibroblasts, a hallmark of activated fibroblasts. These fibroblasts have an ability to substantially promote tumorigenesis. However, the precise cellular origins of CAFs and the molecular mechanisms by which these cells evolve into tumor-promoting myofibroblasts remain unclear. Using a coimplantation breast tumor xenograft model, we show that resident human mammary fibroblasts progressively convert into CAF myofibroblasts during the course of tumor progression. These cells increasingly acquire two autocrine signaling loops, mediated by TGF-β and SDF-1 cytokines, which both act in autostimulatory and cross-communicating fashions. These autocrine-signaling loops initiate and maintain the differentiation of fibroblasts into myofibroblasts and the concurrent tumor-promoting phenotype. Collectively, these findings indicate that the establishment of the self-sustaining TGF-β and SDF-1 autocrine signaling gives rise to tumor-promoting CAF myofibroblasts during tumor progression. This autocrine-signaling mechanism may prove to be an attractive therapeutic target to block the evolution of tumor-promoting CAFs.
Keywords: CXCR4, Smad, tumor microenvironment, alpha-smooth muscle actin
Myofibroblasts are often observed in the stroma of various human carcinomas that include those of the breast (1). The presence of these cells in large numbers is also associated with higher-grade malignancy and poor prognosis in patients (2–4). Myofibroblasts express α-smooth muscle actin (α-SMA) that distinguishes these cells from fibroblasts and represents a hallmark of activated fibroblasts (5–10). The activated myofibroblast state of stromal fibroblasts also correlates with their ability to promote tumor growth (11–14). Although different types of mesenchymal cells and epithelial cells are proposed to be precursors of the myofibroblasts present in tumors (15–20), their precise cellular origins and functional contributions to tumor growth still remain unclear.
In recent years, the tumor-promoting roles of stromal fibroblasts and α-SMA-positive myofibroblasts, collectively termed carcinoma-associated fibroblasts (CAFs), have been studied (21). CAFs, when inoculated with carcinoma cells, have potently promoted the in vivo proliferation of carcinoma cells and tumor growth in mouse xenograft models (14, 21–25). We previously demonstrated that CAFs, prepared directly from invasive human mammary carcinomas, contain substantial numbers of myofibroblasts that secrete elevated levels of the proangiogenic chemokine, stromal cell-derived factor-1 (SDF-1, also called CXCL12) (14). SDF-1 signaling via its cognate receptor CXCR4, expressed on the surface of carcinoma cells, directly boosts the proliferation of these cells and can stimulate neoangiogenesis by recruiting circulating endothelial progenitor cells (EPCs) into the tumor stroma (14, 26).
Previous research has shed little light on the molecular mechanisms that mediate formation of the myofibroblastic state and the associated tumor-promoting capability of CAFs. The similarities between tumor-associated myofibroblasts and those present in sites of wound healing and chronic fibrosis have long been recognized (5, 27, 28). Though TGF-β-Smad autocrine signaling is apparently responsible for the activated state of myofibroblasts in fibrosis (29–31), it is not known if myofibroblastic CAFs also depend on TGF-β autocrine signaling and/or additional signaling to maintain their unique phenotypes.
We therefore investigated the biochemical alteration(s) underlying the tumor-promoting myofibroblastic phenotype of CAFs and the cellular origins of these cells. Our findings show that the establishment of two autocrine signaling loops, mediated by TGF-β and SDF-1 cytokines, endows resident fibroblasts with the tumor-promoting myofibroblastic phenotype, thereby driving their differentiation into CAF myofibroblasts.
Results
Experimental Generation of CAFs from Human Mammary Fibroblasts.
Myofibroblasts have been generated in vitro from fibroblasts through their transdifferentiation following exposure to TGF-β (32, 33). Moreover, the CAF populations in tumor-associated stroma are known to include both fibroblasts and myofibroblasts (14, 34). For these reasons, we speculated that preexisting normal stromal fibroblasts could potentially convert into myofibroblasts in vivo, specifically during the course of tumor progression. Such conversion has not previously been demonstrated.
To test this hypothesis, primary normal human mammary fibroblasts were isolated from reduction mammoplasty tissue and immortalized with hTERT, the catalytic subunit of the telomerase holoenzyme (35). Retroviral constructs encoding GFP and puromycin-resistance protein were also introduced into these fibroblasts. The resulting fibroblasts were then mingled with MCF-7-ras human breast carcinoma cells expressing an activated ras oncogene (36), and the mixtures were injected s.c. into immunodeficient nude mice.
As described in Fig. 1A, the tumor xenografts were resected at 42, 70, and 200 d after implantation and dissociated into single-cell suspensions. These cells were then cultured in vitro in the presence of puromycin for 5 d to eliminate any contaminating carcinoma cells and host stromal cells. The resulting puromycin-resistant cells were termed experimentally generated CAF1 (exp-CAF1) cells. These cells, resected 42 d after implantation, were once again mixed with MCF-7-ras cells and implanted s.c. into host mice as before. The resulting second group of tumors, which were allowed to grow for an additional period of 200 d, were once again dissected, dissociated, and cultured in the presence of puromycin. The isolated puromycin-resistant cells were termed experimentally generated CAF2 (exp-CAF2) cells (242 d old).
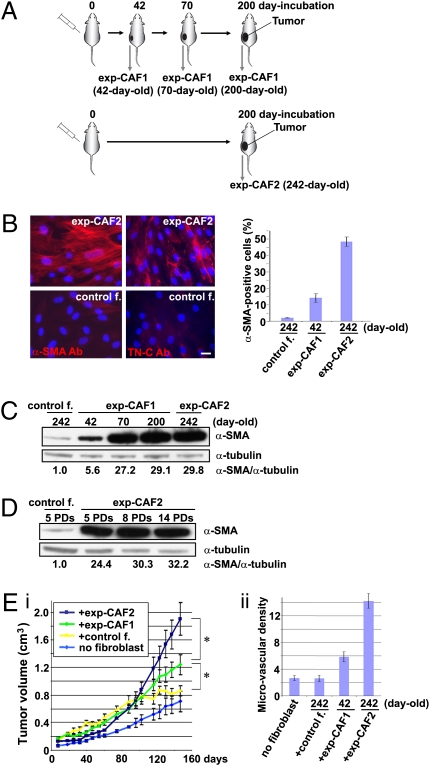
Fig. 1.
exp-CAFs mimic the tumor-promoting behavior of CAFs prepared from breast cancer patients. (A) Isolation of exp-CAFs. Normal GFP-labeled, puromycin-resistant, immortalized human mammary fibroblasts were coinjected with MCF-7-ras breast cancer cells s.c. into nude mice. Tumors were dissected at the indicated days and dissociated. The injected human fibroblasts were isolated under puromycin selection in culture and were termed exp-CAFs. See result for details. (B) Immunofluorescence of exp-CAF2 cells and control fibroblast-2 cells (control f.) to detect α-SMA (red) and tenascin-C (TN-C, red). Cell nuclei were stained with 4′,6-diamino-2-phenylindole (DAPI, blue). (Scale bar, 50 μm.) Error bars indicate SEM. (C and D) Western blotting of fibroblasts using an anti-α-SMA antibody. The membranes were also probed by an anti-α-tubulin antibody. The ratios of the signal intensity of α-SMA relative to α-tubulin are indicated. PDs, population doublings. (E) MCF-7-ras breast carcinoma cells were injected alone (n = 12) or coinjected with control fibroblast-2 cells (control f.; n = 10), 42-d-old exp-CAF1 cells (n = 12), or 242-d-old exp-CAF2 cells (n = 10) s.c. into nude mice. Tumor volume (i) was measured at the indicated days and microvascular density (ii) was quantified in tumors admixed with various fibroblasts 150 d after injection. *P < 0.05. Error bars, SE.
As a control, normal GFP-labeled, puromycin-resistant, immortalized human mammary stromal fibroblasts were injected s.c. into nude mice as pure cultures without MCF-7-ras cells. The fibroblasts that survived at the site of injection for the same period as the exp-CAF-2 cells were isolated in the same way and termed control fibroblast-2 cells (242 d old; Fig. S1A). The mesenchymal nature and human origin of exp-CAFs and the control fibroblasts were confirmed by immunofluorescence analysis (Fig. S1B).
Conversion of Resident Fibroblasts into Tumor-Promoting CAF Myofibroblasts Within the Tumor.
To determine whether the initially admixed normal human fibroblasts had converted into a myofibroblast-rich population during the course of tumor growth, we performed immunofluorescence using an anti-α-SMA antibody. We observed that ∼48% of the total cell population of 242-d-old exp-CAF2 cells stained positive for α-SMA, a far greater number than the ∼14% of myofibroblasts present in the 42-d-old exp-CAF1 and the ∼2.5% present in the 242-d-old control fibroblast-2 cell populations (Fig. 1B). The expression level of the extracellular matrix glycoprotein tenascin-C, another marker of myofibroblasts (11, 37), was also dramatically elevated in exp-CAF2 cells relative to the control fibroblast-2 cells (Fig. 1B), providing additional evidence to support for the myofibroblastic state of these cells.
Western blot analysis further confirmed progressive up-regulation of α-SMA expression in 42- (5.6-fold), 70- (27.2-fold), 200- (29.1-fold) day-old exp-CAF1 cells, and exp-CAF2 cells (29.8-fold) relative to the control fibroblast-2 cells (Fig. 1C). Exp-CAF2 cells also retained their increased α-SMA expression, when propagated as pure populations for periods of 14 population doublings (PDs) in vitro following their extraction from tumors (Fig. 1D). Moreover, exp-CAF2 cell populations extracted from four different tumor xenografts also showed similar increased levels of α-SMA protein expression (Fig. S1C).
We wished to determine whether the myofibroblast differentiation was due to the transfer of the ras oncogene from the admixed MCF-7-ras cells. We therefore checked for the presence of oncogenic ras in the exp-CAFs by PCR and Western blot analyses. No oncogenic ras gene and protein expression could be detected in these cells (Fig. S1D). Collectively, these observations indicate that myofibroblast differentiation is progressively increased during tumor progression and, once established, the differentiated state of the resulting cell populations is stably maintained in a cell-autonomous manner.
To determine the ability of exp-CAFs to promote tumor growth, we performed a tumor xenograft assay. In accordance with previous observations (14), tumors from MCF-7-ras cells admixed with 42-d-old exp-CAF1 or exp-CAF2 cells showed increased growth kinetics and larger volumes by 1.4- or 2.2-fold, respectively, at 147 d after injection compared with tumors containing control fibroblast-2 cells (Fig. 1E, i). Moreover, significant numbers of GFP-positive exp-CAF2 and control fibroblast-2 cells were still present in 150-d-old tumors, as determined by immunofluorescence (Fig. S1E, e and f). Tumors containing 42-d-old exp-CAF1 and exp-CAF2 cells also showed an increase in microvessel density by 2.2- and 5.5-fold, respectively, compared with the control fibroblast-containing tumors (Fig. 1E, ii and Fig. S1E). Taken together, these various observations demonstrate that exp-CAFs closely mimic the tumor-promoting behavior of CAFs extracted from actual human invasive breast carcinomas (14).
Role of TGF-β Autocrine Signaling Responsible for Myofibroblast Differentiation in exp-CAFs.
As TGF-β autocrine signaling regulates myofibrogenesis during fibrosis, we wondered if this was also the case for the myofibroblast differentiation observed in CAFs. We therefore measured TGF-β mRNA expression in these cells by real-time PCR analysis. Levels of TGF-β1 and -β2 expression progressively increased in 42- (1.6- and 1.6-fold, respectively), 70- (2.0- and 2.2-fold), and 200- (2.4- and 2.4-fold) day-old exp-CAF1 cells and exp-CAF2 cells (2.7- and 4.4-fold) compared with the control fibroblast-2 cells (Fig. 2A, i). TGF-β3 expression, however, remained unchanged (Fig. S2A). We also observed increased TGF-β bioactivity in culture medium conditioned by 42-d-old exp-CAF1 (2.7-fold) and exp-CAF2 (4.5-fold) cells compared with control fibrobroblast-2 cells (Fig. 2A, ii).
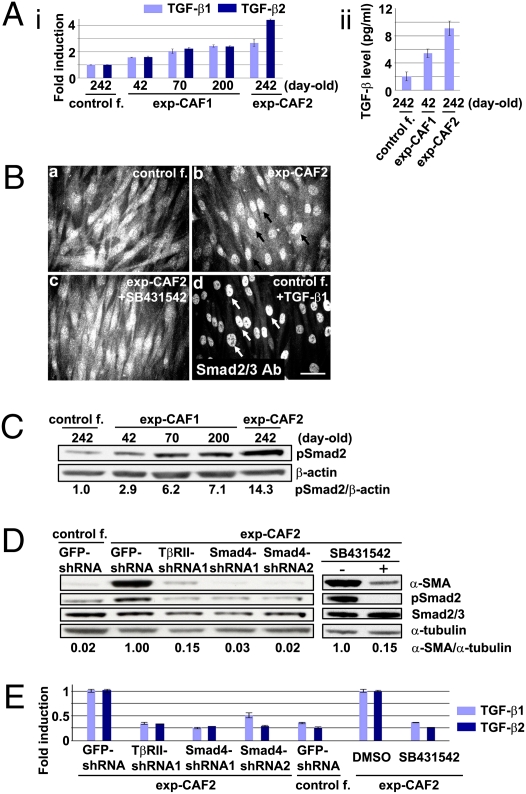
Fig. 2.
TGF-β autocrine signaling suffices to induce and maintain myofibroblast differentiation in exp-CAFs. (A, i) TGF-β1 and TGF-β2 mRNA expression in fibroblasts measured by real-time PCR. (ii) Active TGF-β concentrations in the media conditioned by fibroblasts measured by a luciferase assay. Error bars, SE. (B) Immunofluorescence for Smad2/3. Fibroblasts were incubated with control DMSO (a and b), SB431542, an inhibitor of the TGF-β type I receptor kinase (c), or recombinant TGF-β1 (d). Arrows (b, black; d, white) indicate nuclear Smad2/3 staining. (Scale bar, 100 μm.) (C) Western blotting of fibroblasts using anti-pSmad2 and anti-β-actin antibodies. The ratios of the signal intensity of pSmad2 relative to β-actin are indicated. (D) Western blotting of fibroblasts treated with the indicated shRNAs or SB431542 (10 μM). The membrane stripped was probed using different antibodies to detect α-SMA, pSmad2, Smad2/3, and α-tubulin. (E) Real-time PCR analysis of the fibroblasts described above. Error bars, SE.
Given the increases in TGF-β expression and bioactivity observed in exp-CAF myofibroblasts, we speculated that TGF-β ligands may induce Smad signaling via their receptor in an autocrine fashion, thereby contributing to the myofibroblastic state of these cells. Indeed, immunofluorescence using an antibody against Smad2/3 revealed intense nuclear staining in exp-CAF2 cells (Fig. 2B, b) and in normal fibroblasts treated with TGF-β1 (10 ng/mL) for 1 h (Fig. 2B, d). In contrast, the nuclear Smad2/3 staining was rarely observed in control fibroblast-2 cells (Fig. 2B, a). Moreover, exp-CAF2 cells treated with SB431542 (10 μM), an inhibitor of the TGF-β type I receptor (TβRI) kinase, for 24 h significantly reduced the level of nuclear staining (Fig. 2B, c), indicating constitutive induction of Smad signaling via activation of the TβRI in these cells. Western blot analysis further confirmed progressive elevation of phosphorylated Smad2 expression in 42- (2.9-fold), 70- (6.2-fold), and 200- (7.1-fold) day-old exp-CAF1 cells and exp-CAF2 cells (14.3-fold) relative to the control fibroblast-2 cells (Fig. 2C). Taken together, these observations indicate that exp-CAF-secreted TGF-β activates Smad2/3 signaling in these cells in an autocrine fashion, ostensibly through the TGF-β I/II receptor.
To determine if TGF-β autocrine signaling contributes to maintaining the myofibroblastic state and up-regulation of TGF-β synthesis in exp-CAFs, we generated shRNAs to suppress significantly either TGF-β type II receptor (TβRII) or Smad4 expression (Fig. S2B). Inhibition of Smad signaling by treatment with the TβRII-shRNA, Smad4-shRNA, or SB431542 (10 μM) for 5 d attenuated expression levels of α-SMA (by 85%, 99%, or 85%, respectively), TGF-β1 (by 68%, 48–74%, or 65%), and TGF-β2 (by 66%, 71%, or 75%) in exp-CAF2 cells, compared with the relevant controls (Fig. 2 D and E). Furthermore, activation of Smad signaling by expressing a constitutively active TGF-β1 construct (38), or by adding recombinant TGF-β1, induced α-SMA and TGF-β expression in normal mammary fibroblasts (Fig. S2C), consistent with previous literature (39). Taken together, these findings suggest that activation of TβR-Smad signaling in exp-CAFs induces and maintains their myofibroblast differentiation and TGF-β synthesis, thereby generating a positive feedback TGF-β signaling loop.
Role of Self-Stimulating SDF-1-CXCR4 Autocrine Signaling in Myofibroblast Differentiation.
Elevated expression levels of SDF-1 have previously been observed in CAF myofibroblast populations in vitro and in vivo (14, 40–42). Consistently, real-time PCR analysis showed progressive up-regulation of SDF-1 expression in the 42- (5.9-fold), 70- (10.3-fold), and 200- (38-fold) day-old exp-CAF1 cells and exp-CAF2 cells (34-fold) relative to the control fibroblast-2 cells (Fig. 3A). An ELISA also showed increased levels of SDF-1 released by 42-d-old exp-CAF1 (2.3-fold) and exp-CAF2 (20-fold) cells (Fig. S3A). Moreover, Western blot and immunofluorescence analyses revealed a fivefold increase in expression of the SDF-1 cognate receptor, CXCR4, in exp-CAF2 cells (∼14 PDs) relative to the control fibroblasts (Fig. 3B), consistent with previous literature (43). We therefore speculated that the released SDF-1 may act via CXCR4 upon these cells in an autocrine fashion, thus contributing to their myofibroblastic phenotype.
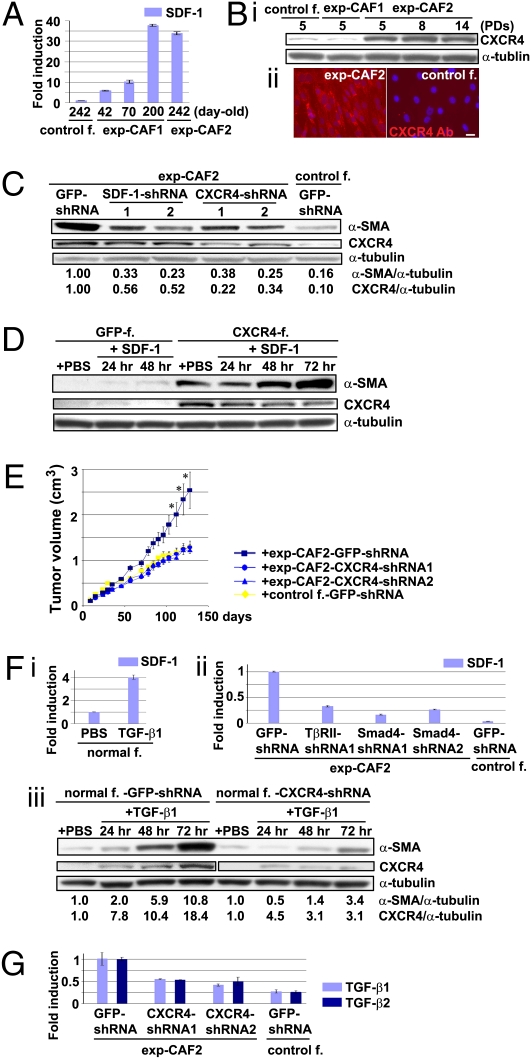
Fig. 3.
SDF-1 autocrine signaling crosstalks with the TGF-β signaling to further boost myofibroblastic differentiation in exp-CAFs. (A) SDF-1 mRNA expression in fibroblasts measured by real-time PCR. Error bars, SE (B, i) Immunoblotting of fibroblasts using anti-CXCR4 and anti-α-tubulin antibodies. (ii) Immunofluorescence using an anti-CXCR4 antibody (red). Cell nuclei are stained with DAPI (blue). (Scale bar, 50 μm.) (C) Western blotting of fibroblasts expressing the indicated shRNAs to detect CXCR4. The membrane was also probed using antibodies against α-SMA and α-tubulin. The ratios of the signal intensity of either α-SMA or CXCR4 relative to α-tubulin are indicated. (D) Immunoblotting of human mammary fibroblasts expressing GFP or CXCR4 treated with or without SDF-1. (E) Exp-CAF2 (n = 12) or control fibroblast-2 (n = 10) cells expressing CXCR4- or GFP-shRNAs were injected with MCF-7-ras cells s.c. into nude mice. *P < 0.05. Error bars, SE. (F, i) Real-time PCR analysis of normal mammary fibroblasts treated with TGF-β1. (ii) Real-time PCR of fibroblasts treated with the indicated shRNAs. Error bars, SE. (iii) Western blotting of mammary fibroblasts expressing GFP- or CXCR4-shRNA treated with or without TGF-β1. The membrane stripped was probed with different antibodies to detect α-SMA, CXCR4, and α-tubulin. The observed CXCR4 signal in CXCR4-shRNA-expressing cells could only be detected using an enhanced chemiluminescence substrate. (G) Real-time PCR analysis of fibroblasts expressing CXCR4- or GFP-shRNA. Error bars, SE.
We examined this possibility using two different shRNA constructs against SDF-1 or CXCR4. The SDF-1 and CXCR4 protein expression levels were significantly inhibited by 72–76% and by 66–78%, respectively, in exp-CAF2 cells compared with the effect of control GFP-shRNA (Fig. S3B and Fig. 3C). Immunoblot and immunofluorescence analyses also demonstrated attenuated levels of α-SMA expression in exp-CAF2 cells expressing SDF-1-shRNA (by 67–77%), CXCR4-shRNA (by 62–75%), or treated with AMD3100, a CXCR4-specific inhibitor (44) for 6 d (Fig. 3C and Fig. S3C). These data suggest that SDF-1-CXCR4 autocrine signaling is required for maintaining the myofibroblastic phenotype of exp-CAF2 cells.
To determine whether triggering the SDF-1-CXCR4 signaling induces the myofibroblastic phenotype, a retroviral construct encoding CXCR4 cDNA (45) was introduced into human mammary fibroblasts (Fig. S3D). We observed that α-SMA expression was increased by ∼100-fold in these CXCR4-expressing cells when exposed to SDF-1 (200 ng/mL) for 72 h compared with the control GFP-expressing fibroblasts (Fig. 3D). The ligand-induced activation of CXCR4 could also suffice to induce and continuously maintain elevated levels of SDF-1 expression in exp-CAFs (Fig. S3E). Taken together, these findings suggest that, like the described TGF-β autocrine signaling, the SDF-1 autocrine signaling pathway also operates in a self-stimulating fashion and contributes to the myofibroblastic differentiation in exp-CAFs.
Importantly, MCF-7-ras tumors with admixed exp-CAF2 cells expressing either CXCR4-shRNA exhibited a reduction in tumor volume by 49% or 52%, respectively, and in neoangiogenesis by 45% at 128 d after injection compared with tumors containing control GFP-shRNA-expressing cells (Fig. 3E and Fig. S3F, a). In contrast, MCF-7-ras tumors that grew in the presence of CXCR4-expressing fibroblasts exhibited a 56% increase in tumor volume at 116 d after injection relative to tumors containing GFP-expressing fibroblasts (Fig. S3F, b). Collectively, these data suggest that SDF-1-CXCR4 autocrine signaling is responsible for the induction and maintenance of the ability of these mesenchymal cells to accelerate tumor growth in vivo.
Crosstalk Between TGF-β and SDF-1 Autocrine Signaling Loops in exp-CAFs.
As both the TGF-β and SDF-1 autocrine signaling pathways are required for myofibroblast differentiation of exp-CAFs, we reasoned that these pathways may cross-communicate and activate one another to further boost the myofibroblastic phenotype. Indeed, PCR analysis showed that exposure of mammary fibroblasts to TGF-β1 (10 ng/mL) for 24 h up-regulated SDF-1 (fourfold) and CXCR4 (2.5-fold) expression (Fig. 3F, i and Fig. S4A). Inhibition of TβR-Smad signaling by TβRII- (by 70%) or Smad4- (by 62–70%) shRNA attenuated the induction of SDF-1 expression by TGF-β1 in these cells compared with the control GFP-shRNA (Fig. S4B). SDF-1 mRNA expression was also decreased in exp-CAF2 cells expressing TβRII- or Smad4-shRNA by 67% or 74–83%, respectively (Fig. 3F, ii), indicating that SDF-1 expression is mediated by TβR-Smad signaling in these cells. In contrast, inhibition of Smad signaling by Smad4-shRNA failed to suppress CXCR4 expression induced by TGF-β1 (10 ng/mL) for 24 h in mammary fibroblasts (Fig. S4C). Collectively, these data suggest that TGF-β signaling induces and maintains SDF-1-CXCR4 autocrine signaling by elevating SDF-1 expression through a Smad-dependent pathway and by increasing CXCR4 expression through a Smad-independent pathway in mammary fibroblasts.
We further demonstrated that this TGF-β–induced SDF-1-CXCR4 signaling mediates the myofibroblast formation induced by TGF-β. Inhibition of CXCR4 expression by CXCR4-shRNA in mammary fibroblasts incubated with TGF-β1 (10 ng/mL) for 72 h resulted in attenuated induction of α-SMA expression by 3.4-fold compared with the 10.8-fold induction observed in control GFP-shRNA–expressing cells (Fig. 3F, iii). These data indicate the requirement of CXCR4 signaling for the TGF-β-induced myofibroblastic state. We therefore conclude that TGF-β autocrine signaling boosts myofibroblast differentiation in exp-CAFs, not only directly through TβR-Smad signaling pathway, but also through the subsequently induced SDF-1-CXCR4 autocrine signaling pathway.
We also observed that activation of SDF-1-CXCR4 signaling induces and helps to maintain elevation of TGF-β expression in exp-CAFs. Inhibition of CXCR4 expression by CXCR4-shRNA resulted in attenuation of TGF-β1 (by 46–59%) and TGF-β2 (by 47–51%) mRNA expression in exp-CAF2 cells compared with the control GFP-shRNA, indicating some dependence of TGF-β expression on SDF-1-CXCR4 autocrine signaling (Fig. 3G). In contrast, exposure of CXCR4-expressing fibroblasts to recombinant SDF-1 protein (100 ng/mL) for 24 h resulted in up-regulation of endogenous TGF-β1 (4.4-fold) and TGF-β2 (4.2-fold) mRNA expression compared with control GFP-expressing fibroblasts cultured without SDF-1 (Fig. S4D). Taken together, these findings strongly suggest that TGF-β and SDF-1 autocrine signaling loops converge to stimulate each other within exp-CAFs.
SDF-1 and TGF-β Autocrine Signaling Loops Exist in Myofibroblasts in Invasive Human Breast Cancers.
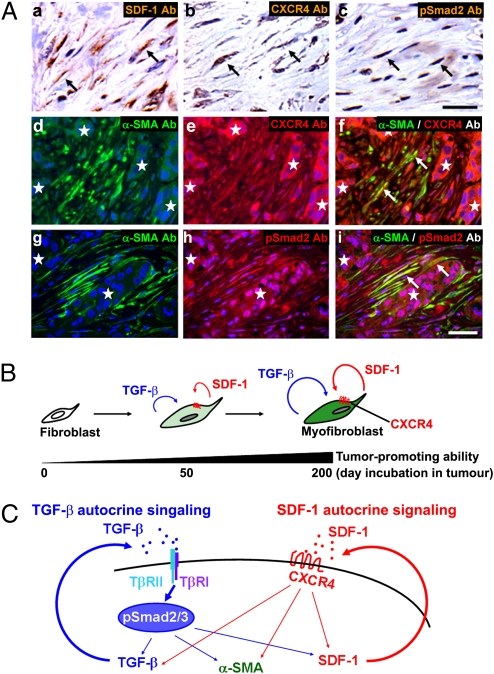
To determine whether both SDF-1 and TGF-β autocrine signaling loops operate within actual human breast carcinomas, we performed immunostaining of tumor sections prepared from two invasive ductal breast cancers that displayed a nontriple negative phenotype. SDF-1, CXCR4, and phosphorylated Smad2 proteins were readily detected in tumor-associated, α-SMA–positive myofibroblasts, but not stromal fibroblasts in normal human breast tissue (Fig. 4A and Fig. S5). Importantly, we also observed that primary CAF myofibroblasts extracted from three different breast carcinoma patients showed elevation of SDF-1 and TGF-β mRNA expression and increased TGF-β bioactivity (Fig. S6A). We further confirmed the significance of cross-communicating, self-stimulating SDF-1 and TGF-β autocrine signaling pathways in maintaining the myofibroblast state of these cells using shRNAs (Fig. S6B). Taken together, we conclude that the stromal myofibroblasts present within invasive human mammary carcinomas require both SDF-1 and TGF-β autocrine signaling loops in self-stimulating and cross-communicating fashions to maintain myofibroblast differentiation.
Fig. 4.
TGF-β and SDF-1 autocrine signaling operates in CAFs in invasive human breast carcinomas. (A) Immunohistochemistry of sections prepared from invasive human breast carcinomas using antibodies against SDF-1 (a, brown), CXCR4 (b, brown; e and f, red), pSmad2 (c, brown; h and i, red), and α-SMA (d, f, g, and i, green). The sections were also stained with hematoxylin (a–c, pale blue) or DAPI (d–i, blue). Cells staining positive are highlighted by arrows. A group of carcinoma cells is indicated by an asterisk. (Scale bar, 50 μm.) (B) During tumor progression, resident stromal fibroblasts within the tumor increasingly acquire two autocrine signaling loops involving TGF-β and SDF-1 that mediate transdifferentiation into tumor-promoting CAF myofibroblasts. (C) Schematic illustration describing two self-stimulating and cross-communicating signaling loops mediated by TGF-β and SDF-1 in CAF myofibroblasts. CAF-secreted TGF-β and SDF-1 ligands act upon TβR and CXCR4, respectively, in an autocrine fashion. The subsequent activation of TβR-Smad2/3 and CXCR4 signaling pathways drives myofibroblast differentiation and endogenous TGF-β and SDF-1 expression, thereby generating self-stimulating autocrine signaling loops that act in a feed-forward manner. Importantly, the TβR-Smad2/3 signaling also increases SDF-1 expression, thereby boosting SDF-1-CXCR4 autocrine signaling. This in turn elevates endogenous TGF-β expression. Cross-talk between these autocrine signaling loops therefore stimulates one another and further boosts myofibroblast differentiation in CAFs. A thick straight arrow indicates direct stimulatory modification, and thin straight arrows depict transcriptional contribution.
Discussion
CAFs, myofibroblast-rich cell populations, extracted from human carcinomas maintain an ability to promote tumorigenesis. These cells, passaged for 10 PDs in vitro without ongoing interaction with carcinoma cells, retained their ability to promote tumor growth when coinjected with carcinoma cells into immunodeficient mice (14, 46). However, the molecular mechanisms underlying their tumor-promoting ability are poorly understood. Some have reported the importance of somatic genetic alterations in forming the tumor-promoting stroma, yet their existence remains controversial (47–49). The cellular origins of tumor-associated myofibroblasts and the molecular processes that regulate their myofibroblastic state also remain unclear.
In the present experiments, we show that mammary fibroblasts present within a tumor mass can be substantially converted into tumor-promoting CAFs, presumably through their myofibroblast differentiation. We cannot formally exclude the possibility that a small population of α-SMA-positive cells preexisting in normal mammary fibroblasts served as precursors of the tumor-associated myofibroblasts. This alternative origin is rendered less likely, however, by the fact that such myofibroblastic conversion can be efficiently induced in stromal fibroblasts exposed to media conditioned by carcinoma cells (50, 51).
During the course of tumor progression, preexisting stromal fibroblasts acquire progressively enhanced TGF-β and SDF-1 autocrine signaling loops in a self-sustaining fashion that mediate their myofibroblast differentiation and the associated tumor-promoting capability (Fig. 4 B and C). Such autostimulating signaling may fulfill the prerequisites of an epigenetic mechanism that can stably maintain a cellular phenotype—in the present case the myofibroblast differentiation state. We note, in passing, that positive feedback loops are used, for example, to maintain the undifferentiated state of embryonic stem cells and hematopoietic progenitor cells (52, 53).
We imagine that during the course of tumor progression, the autocrine signaling is initially triggered by TGF-β released in significant quantities by carcinoma cells (54–57). TGF-β can elicit enhanced endogenous TGF-β and SDF-1 production via TβR-Smad signaling and induce CXCR4 expression in stromal fibroblasts, thereby facilitating the generation of two autocrine signaling loops, mediated by TGF-β and SDF-1, acting in a positive feedback manner. Such autocrine signaling loops self-stimulate and cross-communicate with each other to maintain the myofibroblastic phenotype.
We cannot exclude the possibility that genetic alterations, acquired in the initially present normal fibroblasts during their incubation within the tumor, influenced the observed autocrine signaling and myofibroblast differentiation. However, we have found that two types of CAFs—those prepared from human invasive breast carcinomas and those extracted from experimental xenografted tumors—exhibited a normal karyotype and were, when implanted on their own, nontumorigenic (14). We also indicated that wild-type p53 continues to be expressed in both types of CAFs (Fig. S7), strongly suggesting that alteration in p53 signaling is not responsible for the induction or maintenance of such autocrine signaling in tumor-promoting myofibroblasts.
The present observations show that tumor-promoting CAF myofibroblasts can originate from preexisting stromal fibroblasts by establishing TGF-β and SDF-1 autocrine signaling in a cell autonomous fashion during tumor progression. Pharmacological approaches to target and disrupt such autocrine signaling preventing the formation and maintenance of tumor-promoting myofibroblasts may prove to be a useful antitumor therapeutic strategy in the future.
Materials and Methods
Primary stromal fibroblasts were extracted from healthy human breast tissue, as described previously (14). The retroviral pMIG (MSCV-IRES-GFP) vector, expressing both hTERT and GFP, and a pBabe-puro vector encoding a puromycin resistance gene, were infected into these fibroblasts before coimplantation with breast carcinoma cells into nude mice to generate exp-CAFs.
Cell culture, immunoblotting, immunostaining, real-time PCR, flow cytometry, ELISA, virus infection, tumorigenicity assay, and evaluation of angiogenesis are performed according to standard procedures. Details are given in SI Materials and Methods. Antibodies, chemicals, and DNA constructs used are also described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Joseph Sodroski, Daniel Rifkin, Lalage Wakefield, and Luciano Zardi for reagents; Garry Ashton, Dr. Tsukasa Shibue, and Paul Chantry for technical assistance; Dr. David Sabatini for useful discussion; Dr. Nic Jones for critical reading of this manuscript; and members of R.A.W.'s and A.O.’s laboratories. This work was conducted using the core facility at the Paterson Institute of Cancer Research and the W. M. Keck Foundation Biological Imaging Facility at the Whitehead Institute. This work was supported by National Institutes of Health/National Cancer Institute Grant R21CA87081-02 (to R.A.W.), the German Academic Exchange Service (C.S.), National Institutes of Health Grants P01 CA080111 and R01 CA078461 (to R.A.W.), the Breast Cancer Research Foundation (R.A.W.), the Massachusetts Institute of Technology/Ludwig Fund for Cancer Research (to R.A.W.), and Cancer Research UK Grant C147/A6058 (to A.O.). R.A.W. is an American Cancer Society Research Professor and a Daniel K. Ludwig Cancer Research Professor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013805107/-/DCSupplemental.
References
- 1.Sappino AP, Skalli O, Jackson B, Schürch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer. 1988;41:707–712. doi: 10.1002/ijc.2910410512. [DOI] [PubMed] [Google Scholar]
- 2.Kellermann MG, et al. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: Induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008;44:509–517. doi: 10.1016/j.oraloncology.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Cardone A, Tolino A, Zarcone R, Borruto Caracciolo G, Tartaglia E. Prognostic value of desmoplastic reaction and lymphocytic infiltration in the management of breast cancer. Panminerva Med. 1997;39:174–177. [PubMed] [Google Scholar]
- 4.Maeshima AM, et al. Modified scar grade: A prognostic indicator in small peripheral lung adenocarcinoma. Cancer. 2002;95:2546–2554. doi: 10.1002/cncr.11006. [DOI] [PubMed] [Google Scholar]
- 5.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- 7.Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21:19–25. doi: 10.1016/j.semcdb.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 9.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 10.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Wever O, et al. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004;18:1016–1018. doi: 10.1096/fj.03-1110fje. [DOI] [PubMed] [Google Scholar]
- 12.Desmoulière A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: A key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 13.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 14.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Jeon ES, et al. Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells. Stem Cells. 2008;26:789–797. doi: 10.1634/stemcells.2007-0742. [DOI] [PubMed] [Google Scholar]
- 16.Mishra PJ, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii G, et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun. 2003;309:232–240. doi: 10.1016/s0006-291x(03)01544-4. [DOI] [PubMed] [Google Scholar]
- 18.Direkze NC, et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 19.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 20.Petersen OW, et al. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olumi AF, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietras K, Östman A. Hallmarks of cancer: Interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 23.Hu M, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang G, et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci USA. 2006;103:16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 26.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 27.Schäfer M, Werner S. Cancer as an overhealing wound: An old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 28.Dvorak HF. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 29.Kim KK, et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varga J. Scleroderma and Smads: Dysfunctional Smad family dynamics culminating in fibrosis. Arthritis Rheum. 2002;46:1703–1713. doi: 10.1002/art.10413. [DOI] [PubMed] [Google Scholar]
- 31.Mori Y, Chen SJ, Varga J. Expression and regulation of intracellular SMAD signaling in scleroderma skin fibroblasts. Arthritis Rheum. 2003;48:1964–1978. doi: 10.1002/art.11157. [DOI] [PubMed] [Google Scholar]
- 32.Rønnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696–707. [PubMed] [Google Scholar]
- 33.Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rønnov-Jessen L, Van Deurs B, Nielsen M, Petersen OW. Identification, paracrine generation, and possible function of human breast carcinoma myofibroblasts in culture. In Vitro Cell Dev Biol. 1992;28A:273–283. doi: 10.1007/BF02634244. [DOI] [PubMed] [Google Scholar]
- 35.Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 36.Kasid A, Lippman ME, Papageorge AG, Lowy DR, Gelmann EP. Transfection of v-rasH DNA into MCF-7 human breast cancer cells bypasses dependence on estrogen for tumorigenicity. Science. 1985;228:725–728. doi: 10.1126/science.4039465. [DOI] [PubMed] [Google Scholar]
- 37.Mackie EJ, et al. Tenascin is a stromal marker for epithelial malignancy in the mammary gland. Proc Natl Acad Sci USA. 1987;84:4621–4625. doi: 10.1073/pnas.84.13.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakefield LM, Kondaiah P, Hollands RS, Winokur TS, Sporn MB. Addition of a C-terminal extension sequence to transforming growth factor-beta 1 interferes with biosynthetic processing and abolishes biological activity. Growth Factors. 1991;5:243–253. doi: 10.3109/08977199109000288. [DOI] [PubMed] [Google Scholar]
- 39.Barnard JA, Beauchamp RD, Coffey RJ, Moses HL. Regulation of intestinal epithelial cell growth by transforming growth factor type beta. Proc Natl Acad Sci USA. 1989;86:1578–1582. doi: 10.1073/pnas.86.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tait LR, et al. Dynamic stromal-epithelial interactions during progression of MCF10DCIS.com xenografts. Int J Cancer. 2007;120:2127–2134. doi: 10.1002/ijc.22572. [DOI] [PubMed] [Google Scholar]
- 41.Ao M, et al. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67:4244–4253. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 42.Allinen M, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Eck SM, Côté AL, Winkelman WD, Brinckerhoff CE. CXCR4 and matrix metalloproteinase-1 are elevated in breast carcinoma-associated fibroblasts and in normal mammary fibroblasts exposed to factors secreted by breast cancer cells. Mol Cancer Res. 2009;7:1033–1044. doi: 10.1158/1541-7786.MCR-09-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 45.Babcock GJ, Farzan M, Sodroski J. Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J Biol Chem. 2003;278:3378–3385. doi: 10.1074/jbc.M210140200. [DOI] [PubMed] [Google Scholar]
- 46.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 47.Haviv I, Polyak K, Qiu W, Hu M, Campbell I. Origin of carcinoma associated fibroblasts. Cell Cycle. 2009;8:589–595. doi: 10.4161/cc.8.4.7669. [DOI] [PubMed] [Google Scholar]
- 48.Weinberg RA. Coevolution in the tumor microenvironment. Nat Genet. 2008;40:494–495. doi: 10.1038/ng0508-494. [DOI] [PubMed] [Google Scholar]
- 49.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem. 2008;283:19864–19871. doi: 10.1074/jbc.M800798200. [DOI] [PubMed] [Google Scholar]
- 51.Noma K, et al. The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology. 2008;134:1981–1993. doi: 10.1053/j.gastro.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grass JA, et al. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc Natl Acad Sci USA. 2003;100:8811–8816. doi: 10.1073/pnas.1432147100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyer LA, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jakowlew SB. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 2006;25:435–457. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- 55.Gold LI. The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit Rev Oncog. 1999;10:303–360. [PubMed] [Google Scholar]
- 56.Nørgaard P, Hougaard S, Poulsen HS, Spang-Thomsen M. Transforming growth factor beta and cancer. Cancer Treat Rev. 1995;21:367–403. doi: 10.1016/0305-7372(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 57.Gorsch SM, Memoli VA, Stukel TA, Gold LI, Arrick BA. Immunohistochemical staining for transforming growth factor beta 1 associates with disease progression in human breast cancer. Cancer Res. 1992;52:6949–6952. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.