Abstract
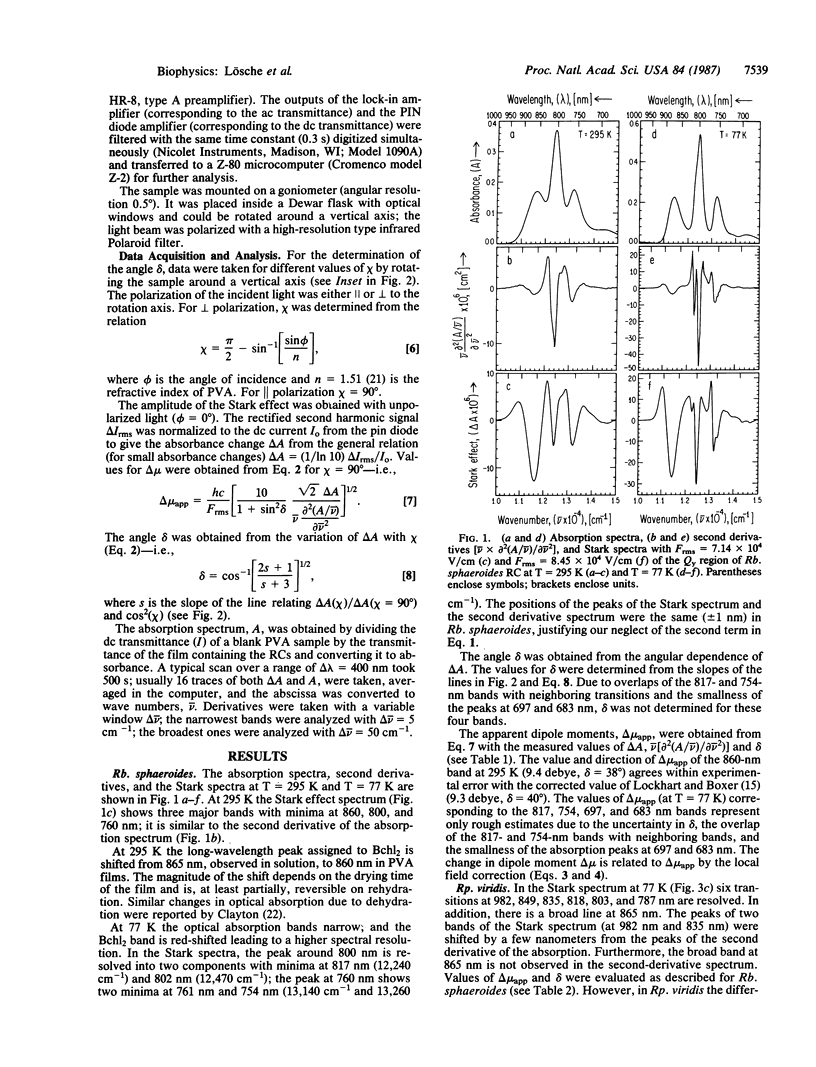
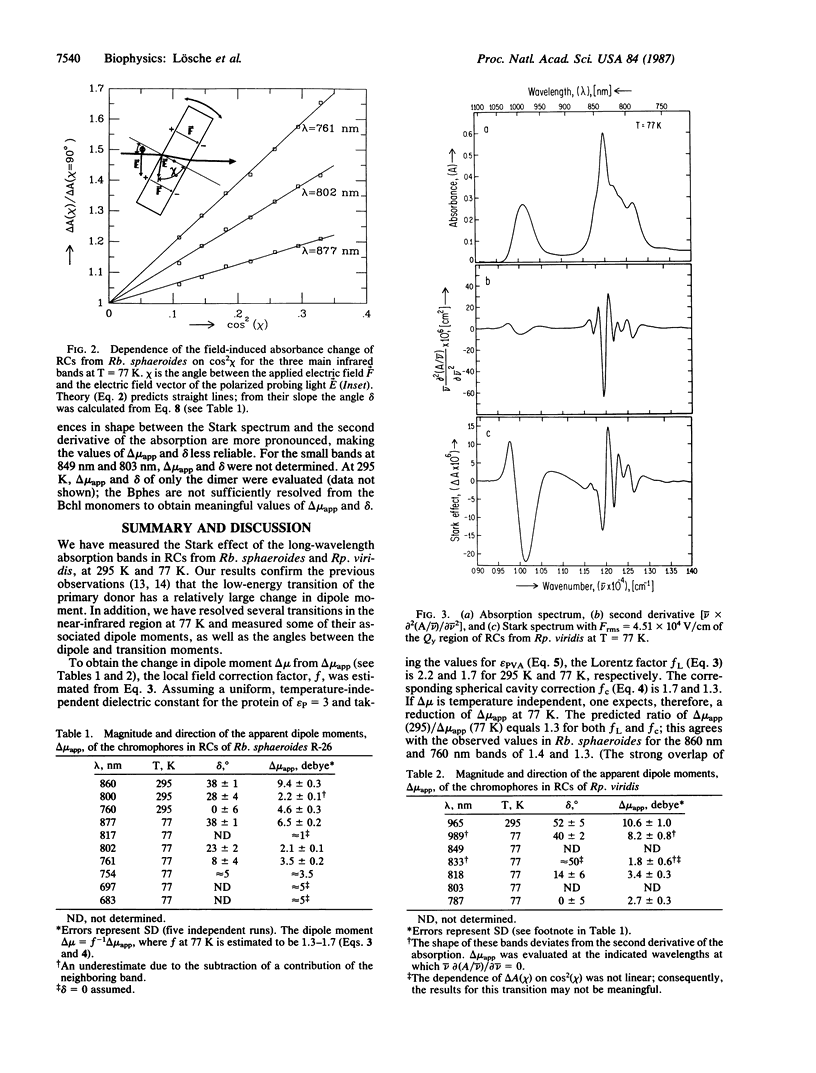
The effect of an electric field on the optical absorption (Stark effect) of reaction centers (RCs) from Rhodobacter sphaeroides and Rhodopseudomonas viridis embedded in films of poly(vinyl alcohol) was measured. The infrared bands were investigated at 295 K and 77 K. In RCs from Rp. viridis at 77 K six peaks (at 982, 849, 835, 818, 803, and 787 nm), associated with the Qy transitions of the six pigments, were resolved; in addition, a small broad band at 865 nm was resolved. In RCs from Rb. sphaeroides only five bands (at 877, 817, 802, 761, and 754 nm) assigned to the Qy transitions were resolved; in addition, two small bands at 697 and 683 nm were observed. The additional bands have been tentatively assigned to vibrational side bands, although the contribution from charge-transfer states cannot be excluded. The Stark spectra had line shapes similar to the second derivative of the absorption spectra and were interpreted in terms of the interaction between the applied electric field and the dipole moments of the ground and excited states. Analyses of the spectra yielded the apparent change in dipole moment delta mu app = f delta mu (where the factor f corrects for the difference between the local field and the applied field) and delta, the angle between delta mu---- and the transition moment mu trans. At 77 K the values of delta mu----app and delta for the peaks at 877, 802, and 761 nm in Rb. sphaeroides were 6.5 debye, 38 degrees; 2.1 debye, 23 degrees; and 3.5 debye, 8 degrees. In Rp. viridis the debye values for the peaks at 982, 835, 818, and 787 were 8.2, 40 degrees; 1.8, 50 degrees; 3.4, 14 degrees; and 2.7, 0 degrees. The large values of delta mu app associated with the long-wavelength peak of the bacteriochlorophyll dimers are consistent with a significant charge-transfer contribution to the excited state of the primary donor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the cofactors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5730–5734. doi: 10.1073/pnas.84.16.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K. Effects of dehydration on reaction centers from Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1978 Nov 9;504(2):255–264. doi: 10.1016/0005-2728(78)90174-3. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Feher G. Some chemical and physical properties of a bacterial reaction center particle and its primary photochemical reactants. Photochem Photobiol. 1971 Sep;14(3):373–387. doi: 10.1111/j.1751-1097.1971.tb06180.x. [DOI] [PubMed] [Google Scholar]
- Knapp E. W., Fischer S. F., Zinth W., Sander M., Kaiser W., Deisenhofer J., Michel H. Analysis of optical spectra from single crystals of Rhodopseudomonas viridis reaction centers. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8463–8467. doi: 10.1073/pnas.82.24.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H. Three-dimensional crystals of a membrane protein complex. The photosynthetic reaction centre from Rhodopseudomonas viridis. J Mol Biol. 1982 Jul 5;158(3):567–572. doi: 10.1016/0022-2836(82)90216-9. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Cogdell R. J., Seftor R. E., Webster G. D. Further studies on the composition and spectral properties of the photochemical reaction centers of bacteriochlorophyll b-containing bacteria. Biochim Biophys Acta. 1980 Nov 5;593(1):60–75. doi: 10.1016/0005-2728(80)90008-0. [DOI] [PubMed] [Google Scholar]



