Abstract
Alcohol addiction is a chronically relapsing disorder that includes certain maladaptive learning and memory. The serine and threonine kinase complex, mammalian target of rapamycin complex 1 (mTORC1), has been implicated in synaptic plasticity, learning, and memory by controlling protein translation. Here we show that administration of alcohol and excessive voluntary consumption of alcohol induce the activation of the mTORC1-mediated signaling pathway in the nucleus accumbens (NAc) of rodents. We further show that the protein expression levels of GluR1 and Homer, two synaptic proteins whose translation has been shown to be modulated by mTORC1, are up-regulated in the NAc of rodents with a history of excessive alcohol consumption. In addition, our results document that the Food and Drug Administration-approved inhibitor of mTORC1, rapamycin, decreases expression of alcohol-induced locomotor sensitization and place preference, as well as excessive alcohol intake and seeking in preclinical rodent models of alcohol abuse. Together, our results suggest that mTORC1 within the NAc is a contributor to molecular mechanisms underlying alcohol-drinking behaviors. Furthermore, despite its massive health and socioeconomic impact worldwide, pharmacotherapies for alcohol abuse and addiction remain limited. Our data therefore put forward the possibility that targeting the mTORC1 signaling cascade is an innovative and valuable strategy for the treatment of alcohol use and abuse disorders.
Keywords: addiction, mTOR, ethanol, reward, nucleus accumbens
Addiction is a chronically relapsing and devastating disorder characterized by the compulsion to seek and consume drugs, loss of control, and emergence of a negative emotional state when access to the drug is withdrawn (1). Although the consequences of alcohol abuse disorders are major health and socioeconomic problems (2), available medications are still very limited. Furthermore, because alcohol does not have a well defined pharmacological site of action (2), the molecular mechanisms underlying long-lasting adaptations that result in adverse phenotypes such as excessive uncontrolled alcohol consumption remain elusive.
Addiction is thought to be a pathological usurpation of mechanisms underlying learning and memory (3). This is particularly the case in the nucleus accumbens (NAc), where plasticity induced by drugs of abuse is documented (4, 5). Long-lasting forms of synaptic plasticity and memory depend on new protein synthesis (6), specifically at dendrites (7), and numerous studies have shown that the serine-threonine kinase complex mammalian target of rapamycin (mTOR) complex 1 (mTORC1) plays an essential role in initiating local synaptic protein translation (8, 9). mTORC1 is sensitive to the acute treatment of the inhibitor rapamycin (10), and is best characterized for its ability to control the translation initiation of mRNAs via the phosphorylation and activation of the p70 ribosomal S6 kinase (S6K), and the phosphorylation of the eukaryotic translation initiation factor-4E binding protein (4E-BP) (6, 11, 12). Importantly, S6K and 4E-BP are localized at the synapse (6, 13, 14), where mTORC1 was shown to be important for stabilizing plastic changes (15, 16). The mTORC1-mediated pathway was also reported to control soma and dendritic morphology (17), long-term potentiation, and long-term depression (6, 18). Accordingly, the mTORC1-mediated signaling pathway has been shown to contribute to memory formation, consolidation, and reconsolidation (6, 19), as well as memory deficits (20).
Because mTORC1 plays a major role in the molecular mechanisms underlying long-term plasticity and its maintenance, we hypothesized that this complex contributes to the persistence of neuroadaptations that result in alcohol-related behaviors, including excessive alcohol intake. To address this question, we focused our studies on the NAc, a key component of the reward system (21) that plays a critical role in the development of drug and alcohol addiction (1, 4).
Results
Alcohol Administration Activates the mTORC1-Mediated Signaling Pathway in the NAc of Mice.
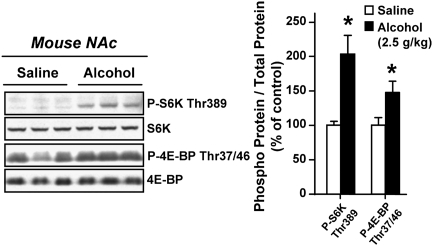
First, we examined whether administration of alcohol alters the mTORC1-mediated signaling pathway in the NAc. To do so, C57BL/6J and DBA/2J mice were systemically treated (i.p.) with a nonhypnotic dose of alcohol, and the phosphorylation levels of the mTORC1 substrates, S6K and 4E-BP, were measured 30 min after treatment. We found that acute in vivo exposure of both strains of mice to alcohol significantly increased the phosphorylation level of S6K and 4E-BP proteins (Fig. 1 and Fig. S1A). These results indicate that acute alcohol treatment triggers the activation of the mTORC1 signaling pathway in the NAc.
Fig. 1.
Alcohol administration activates the mTORC1-mediated signaling pathway in the NAc of mice. C57BL/6J mice were systemically treated (i.p.) with 2.5 g/kg of alcohol or saline solution and the NAc was removed 30 min later. The levels of S6K and 4E-BP phosphorylation were determined by Western blot analyses (n = 6 per group). Data are presented as mean ± SEM and expressed as percentage of control; *P < 0.05, two-tailed unpaired t test.
Systemic Administration of Rapamycin Inhibits the Expression of Alcohol-Induced Locomotor Sensitization.
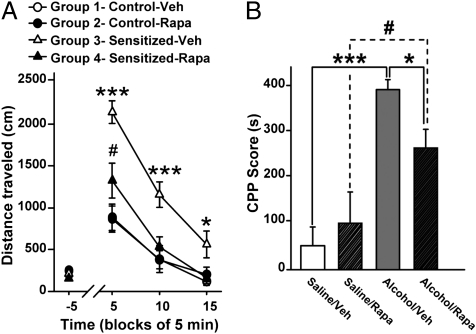
Repeated exposure of rodents to drugs of abuse and alcohol promotes a progressive enhancement of responsiveness to the drug. This phenomenon of sensitization is thought to underlie certain aspects of addiction, and has been related in part to plasticity induced by drugs of abuse and alcohol within the NAc (22, 23). Therefore, we tested whether the mTORC1 inhibitor rapamycin could attenuate alcohol-induced locomotor sensitization in mice. We found that a single systemic administration of 10 mg/kg of rapamycin diminished the expression of locomotor sensitization promoted by repeated exposure to alcohol (Fig. 2A and Fig. S2). This result suggests that mTORC1 signaling contributes to the maintenance of neuroadaptations implicated in alcohol-induced locomotor sensitization.
Fig. 2.
Systemic administration of rapamycin reduces the expression of alcohol-induced locomotor sensitization and CPP in mice. (A) Locomotor sensitization experiment. DBA/2J mice were administered daily with saline solution (groups 1 and 2) or with 2 g/kg of alcohol (groups 3 and 4) for 10 successive days. On day 11, mice were treated (i.p.) with vehicle (Veh) or rapamycin (Rapa, 10 mg/kg). Three hours later, all mice received alcohol (2 g/kg, i.p.). Locomotor activity was recorded 5 min before and after alcohol injection for 15 min (n = 15–17 per group). Data are represented as mean ± SEM. Two-way ANOVA with repeated measures showed an interaction between treatment and time [F(3,180) = 10.67, P < 0.001] (Newman-Keuls post hoc test), #P < 0.05 (group 4 vs. group 1 and group 2), ***P < 0.001 (group 3 vs. group 1, group 2, and group 4), and *P < 0.05 (group 4 vs. group 3). (B) CPP experiment. During the conditioning phase (6 d), DBA/2J mice were daily administered (i.p.) alcohol (1.8 g/kg) or saline solution and were then confined in the drug- or non-drug-paired compartment. One day after the sixth session, saline solution- and alcohol-conditioned mice were treated (i.p.) with vehicle (saline/veh, alcohol/veh) or 10 mg/kg of rapamycin (saline/Rapa, alcohol/Rapa). Three hours later, a 15-min postconditioning test was conducted (n = 11 per group). Data are represented as mean ± SEM. Two-way ANOVA showed an interaction between drug (saline solution or alcohol) and treatment (Veh or Rapa) factors [F(1,40) = 4.20, P < 0.05]. *P < 0.05, #P < 0.05, and ***P < 0.001 (Newman-Keuls post hoc test).
Systemic Administration of Rapamycin Attenuates Conditioned Place Preference to Alcohol.
The NAc plays a major role in mechanisms that underlie the reinforcing properties of drugs of abuse including alcohol (4). We therefore tested whether inhibition of mTORC1 in mice affects the expression of alcohol-induced conditioned place preference (CPP), a measure of alcohol reward-seeking behavior (24). We found that a single administration of rapamycin attenuates the expression of CPP to alcohol (Fig. 2B). Together with the data outlined earlier, these results suggest that mTORC1 signaling is implicated in neuroadaptations underlying alcohol-related behaviors.
Excessive Alcohol Consumption Activates the mTORC1-Mediated Signaling Pathway in the NAc of Mice and Rats.
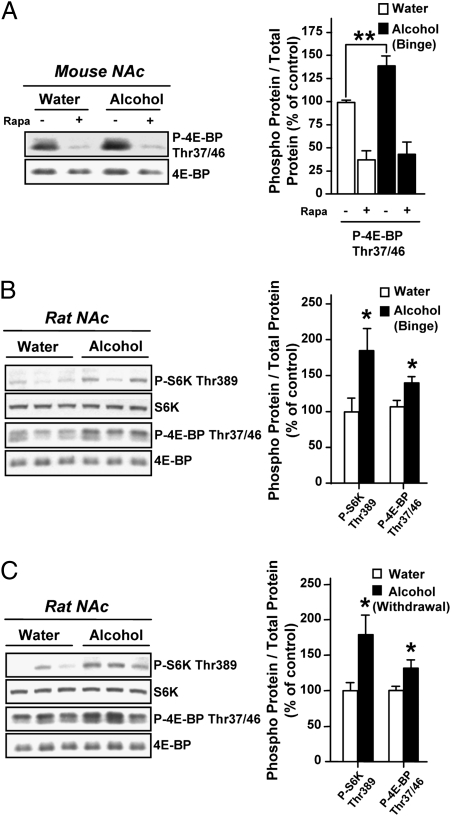
Recurring alcohol intake and withdrawal periods trigger seeking and excessive drinking behaviors in humans (2, 25). As we found that the mTORC1 pathway plays a role in alcohol reward-seeking behavior, we set out to determine whether the mTORC1 pathway in the NAc contributes to neuroadaptations that underlie alcohol consumption. Thus, we first assessed whether the mTORC1 pathway was activated in the NAc in rodent models of excessive alcohol consumption. Mice and rats were trained to drink large amounts of alcohol in a 4-h intermittent-limited access or a 24-h intermittent two-bottle choice access procedure, respectively. These paradigms result in voluntary alcohol consumption that generates a blood alcohol concentration (BAC) of 97 ± 9 mg% in mice (SI Materials and Methods), and 81 ± 7 mg% in rats (26), 4 h or 30 min after the beginning of an alcohol-drinking session, respectively. The BAC values correspond to human binge drinking as defined by the National Institute on Alcohol Abuse and Alcoholism (27). We found that binge drinking of alcohol results in the activation of the mTORC1-mediated signaling cascade in the NAc as reflected by a higher level of phosphorylation of the mTORC1 substrate, 4E-BP, in mice (Fig. 3A and Fig. S1B) and rats (Fig. 3B), as well as increased levels of the phosphorylation of the mTORC1 substrate S6K in rats (Fig. 3B) compared with controls. Importantly, we found that a single administration of rapamycin efficiently blocked the 4E-BP phosphorylation resulting from alcohol binge drinking in mice (Fig. 3A). Next, we determined whether the activation of the mTORC1 signaling pathway is observed after alcohol withdrawal in rats and found that the phosphorylation levels of both mTORC1 substrates, 4E-BP and S6K, in the NAc were increased even after 24 h of alcohol deprivation (Fig. 3C). These data suggest that repeated cycles of excessive alcohol consumption and withdrawal result in a prolonged activation of the mTORC1-mediated signaling pathway.
Fig. 3.
Excessive alcohol consumption activates the mTORC1-mediated signaling pathway in the NAc of rodents. (A) C57BL/6J mice had access to a 20% solution of alcohol for 4 h every other day for 3 wk. Three hours before the tenth 4-h alcohol-drinking session, mice were treated (i.p.) with vehicle or 20 mg/kg of rapamycin (Rapa), and the NAc were removed immediately after the alcohol-drinking session (n = 8 per group). Two-way ANOVA showed a significant main effect of the fluid [water or alcohol; F(1,28) = 5.27, P < 0.05] and treatment [Veh or rapamycin; F(1,28) = 63.26, P < 0.001] but no interaction [F(1,28) = 2.86, P = 0.10]. Subsequent analysis using the method of contrasts detected a significant difference between water and alcohol within the vehicle group (**P < 0.01) but not within the rapamycin group. The level of 4E-BP phosphorylation was determined by Western blot (the level of S6K phosphorylation was too low to be accurately quantified). (B and C) Rats experienced at least 3 mo of intermittent-access 20% alcohol two-bottle choice drinking sessions. Control animals underwent the same paradigms but did not have access to alcohol. (B) After the last 24 h of alcohol deprivation, rats had access to a 20% solution of alcohol for 30 min, leading to an average intake of 1.16 ± 0.06 g/kg, and the NAc were immediately removed. (C) The NAc were removed after the last 24 h of alcohol deprivation session (withdrawal). The levels of S6K and 4E-BP phosphorylation were determined by Western blot (n = 9 per group in B and C). Data are presented as mean ± SEM and expressed as percentage of control; *P < 0.05, two-tailed unpaired t test.
Synaptic Proteins GluR1 and Homer Are Up-Regulated in the NAc of Rodents with a History of Excessive Alcohol Consumption.
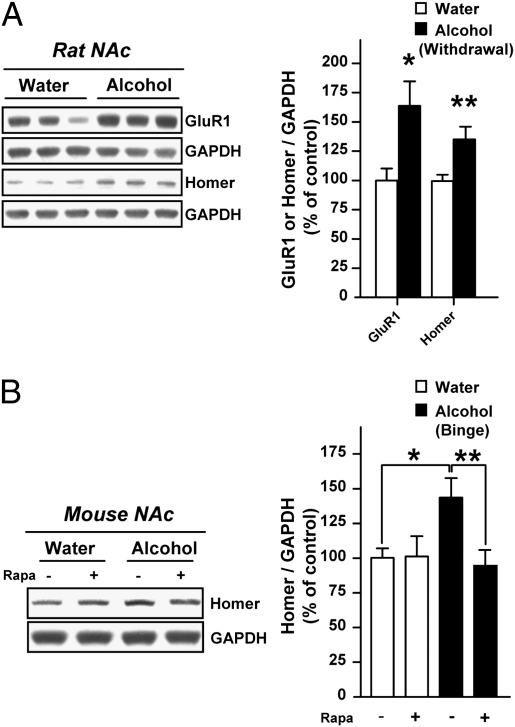
Because mTORC1 is known to regulate synaptic protein translation in the brain through 4E-BP as well as S6K (6, 8, 9), we hypothesized that alcohol-induced mTORC1 activation may change the expression level of synaptic proteins in the NAc. Among the synaptic proteins whose translation has been shown to be regulated by mTORC1 are the GluR1 subunit of the AMPA receptor and the scaffolding protein Homer (13, 28). Both proteins have been implicated in mechanisms that underlie synaptic plasticity induced by drugs of abuse in the NAc (29, 30). We therefore tested whether GluR1 and Homer are up-regulated in the NAc of rats with a history of excessive alcohol consumption. As shown in Fig. 4A, we found that the protein levels of GluR1 and Homer were increased in the NAc of rats after 24 h of alcohol withdrawal, a time point at which the mTORC1 signaling pathway is still activated (Fig. 3C). Importantly, we further found that the up-regulation of Homer protein following alcohol binge drinking in mice was blocked by rapamycin administration (Fig. 4B). Together, these data suggest that mTORC1 activation in the NAc of rodents that experienced recurring exposure to alcohol results in changes in the abundance of synaptic proteins, which, in turn, may alter synaptic function.
Fig. 4.
The synaptic proteins GluR1 and Homer are up-regulated in the NAc of rodents with a history of excessive alcohol drinking. (A) Rats experienced the same procedure as Fig. 3C (n = 12–13 per group); *P < 0.05 and **P < 0.01, two-tailed unpaired t test. (B) C57BL/6J mice experienced the same procedure as Fig. 3A (n = 10 per group). Two-way ANOVA detected an interaction between the fluid (water or alcohol) and the treatment (Veh or Rapa) [F(1,36) = 4.34, P < 0.05]; *P < 0.05 and **P < 0.01 by Newman-Keuls post hoc test. The levels of GluR1 and Homer proteins were determined by Western blot analyses using pan antibodies. Data are presented as mean ± SEM and expressed as percentage of control.
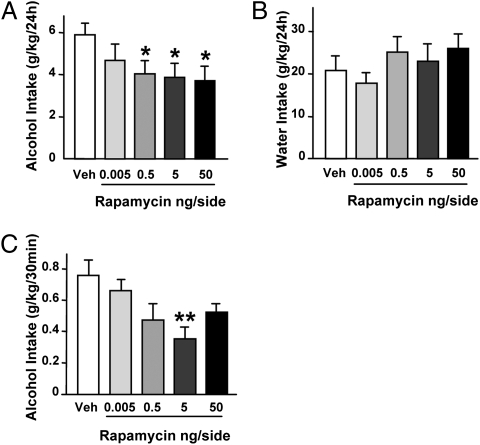
Intra-NAc Infusion of Rapamycin Decreases Binge Drinking and Sustained Consumption of Alcohol in Rats.
Molecular neuroadaptations promoted by recurring alcohol exposure are thought to underlie the mechanisms that lead to detrimental behaviors such as excessive consumption of alcohol (2). Therefore, we examined whether the activation of the mTORC1 signaling in the NAc contributes to mechanisms that underlie excessive alcohol intake. To do so, rats that have been trained to consume large quantities of alcohol were infused with rapamycin or vehicle into the NAc 3 h before the drinking session, and alcohol and water consumption were monitored (Fig. S3). We found that intra-NAc infusion of rapamycin significantly decreased alcohol (Fig. 5A) but not water (Fig. 5B) intake over a period of 24 h access. Notably, we also observed that inhibiting mTORC1 in the NAc was effective in reducing binge drinking occurring during the first 30 min of the session (Fig. 5C), as well as alcohol intake during the rest of the drinking period (i.e., 30 min to 24 h; Fig. S4). These data show that the mTORC1 pathway in the NAc contributes to both the first bout and sustained consumption of alcohol.
Fig. 5.
Intra-NAc infusion of rapamycin reduces alcohol drinking in rats. Vehicle (Veh) or 0.005, 0.5, 5, or 50 ng rapamycin per side was infused into the NAc 3 h before the beginning of the 24-h alcohol-drinking session in rats trained to consume a high amount of a 20% solution of alcohol in a two-bottle choice paradigm. (A) Alcohol intake after 24 h. One-way ANOVA with repeated measures showed significant effects of treatment [F(4,32) = 3.58, P < 0.05]. (B) Water intake at the end of the 24-h session. (C) Alcohol intake after the first 30 min of the session. One-way ANOVA with repeated measures showed significant effects of treatment [F(4,32) = 4.11, P < 0.01]. Alcohol and water consumptions are expressed in g/kg body weight (n = 9 per group in A–C). Data are presented as mean ± SEM; *P < 0.05 and **P < 0.01 compared with vehicle (Newman-Keuls post hoc test).
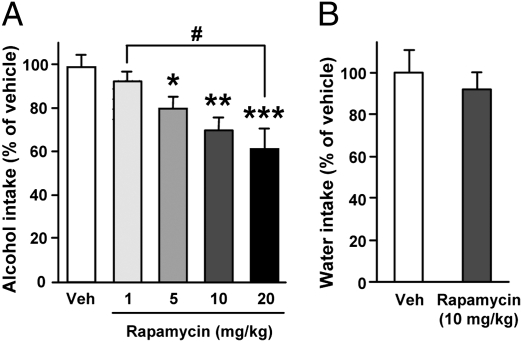
Systemic Administration of Rapamycin Decreases Binge Drinking in Mice.
To determine whether rapamycin can be used systemically, and if its actions to reduce excessive alcohol consumption can be generalized across species, rapamycin was administered i.p. to binge-drinking mice 3 h before the beginning of an alcohol-intake session in a limited alcohol access paradigm (Fig. S5A). We found that rapamycin treatment dose-dependently reduced excessive alcohol drinking (Fig. 6A and Fig. S5 B–D) without affecting water intake (Fig. 6B and Fig. S5E). Importantly, systemic administration of rapamycin did not induce place preference or aversion (Fig. S6), and did not affect taste palatability (Fig. S7) or motor coordination in the absence or presence of alcohol in mice (Fig. S8). Furthermore, mice locomotor activity and anxiety-like behavior were previously shown to be unaltered by rapamycin treatment (19). Therefore, the decrease of alcohol consumption following rapamycin administration is unlikely to be caused by nonspecific alterations in behavior but is likely to be caused by a selective effect of mTORC1 inhibition on binge drinking of alcohol.
Fig. 6.
Systemic administration of rapamycin in mice dose-dependently reduces alcohol intake. (A) C57BL/6J mice had access to a 20% solution of alcohol for 4 h every other day for 3 wk. Three hours before the tenth 4-h alcohol-drinking session, mice were treated (i.p.) with vehicle (Veh) or 1, 5, 10, or 20 mg/kg of rapamycin. Alcohol intake was measured at the end of the 4-h drinking session. One-way ANOVA showed significant effects of the treatment [F(4,68) = 6.95, P < 0.001; n = 26 (vehicle) and n = 11–12 per rapamycin group]. (B) After 2 wk without access to alcohol, mice were systemically treated with 10 mg/kg of rapamycin or vehicle 3 h before the beginning of a water-drinking session. Water intake was measured 4 h later (n = 11–12 per group). Data are presented as mean ± SEM and expressed as percentage of vehicle. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the vehicle group and #P < 0.05 (Newman-Keuls post hoc test).
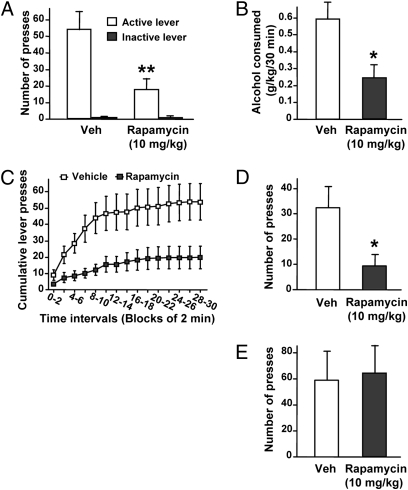
Systemic Administration of Rapamycin Decreases Alcohol Self-Administration and Seeking.
To gain insight into the behavioral processes underlying the decrease in alcohol intake by rapamycin, we examined the effect of the drug on the motivation of rats to consume alcohol. For this purpose, rats with a history of excessive alcohol consumption were trained to self-administer alcohol in an operant procedure (31). Consistent with the results described earlier, a single rapamycin administration (i.p.) significantly reduced operant responding for alcohol (Fig. 7A), which was accompanied by a large decrease (>50%) in the amount of alcohol consumed by the rats during the 30-min session (Fig. 7B). This effect of rapamycin on operant alcohol self-administration cannot be attributed to a nonspecific alteration in locomotion because acute administration of rapamycin did not affect rats’ locomotor activity (Fig. S9). Importantly, we observed that rapamycin reduced the high rate of lever presses occurring at the beginning of the self-administration session (Fig. 7C), suggesting that the drug decreases the motivation to seek alcohol. To test this possibility, instrumental performance was analyzed during an extinction session in which alcohol was not delivered upon lever-pressing. As shown in Fig. 7D, rapamycin administration decreased alcohol-seeking in rats as reflected by a reduction in the number of presses on the alcohol lever during an extinction session, revealing a potential mechanism underlying the inhibitory effect of rapamycin on alcohol intake.
Fig. 7.
Systemic administration of rapamycin selectively decreases operant alcohol self-administration-related behaviors. (A–D) Rats with a history of high levels of alcohol consumption were trained to self-administer a solution of 20% alcohol. Three hours before the beginning of a 30-min session, rats were systemically administered (i.p.) 10 mg/kg of rapamycin or vehicle (Veh). (A) Number of lever presses during the 30 min operant alcohol self-administration session. Two-way ANOVA with repeated measures showed main effects of lever [F(1,6) = 23.81, P < 0.01]; treatment [F(1,6) = 6.12, P < 0.05]; and a significant interaction [F(1,6) = 7.40, P < 0.05]. (B) Amount of alcohol consumed during the session. One-way ANOVA with repeated measures showed significant effect of treatment [F(1,6) = 7.92, P < 0.05]. (C) Cumulative mean presses in bins of 2 min, indicative of the rate of presses for alcohol during the session. Two-way ANOVA with repeated measures showed a significant main effect of time [F(14,84) = 15.86, P < 0.001] and treatment [F(1,84) = 7.49, P < 0.05]. Subsequent analysis using the method of contrasts detected a significant difference between vehicle and rapamycin treatments for all time intervals (all P values < 0.05). (D) Number of presses on the alcohol lever during a 30-min extinction session. One-way ANOVA with repeated measures showed significant effect of treatment [F(1,6) = 6.02, P < 0.05]. (E) Three hours before the beginning of a 30-min session, rats were systemically treated (i.p.) with 10 mg/kg of rapamycin or vehicle (Veh), and the number of presses on the sucrose lever in rats trained to self-administer a solution of 1.5% sucrose was recorded (n = 7 per group in A–E). Data are presented as mean ± SEM; *P < 0.05 and **P < 0.01 compared with vehicle (Newman-Keuls post hoc test).
Systemic Administration of Rapamycin Does Not Decrease Sucrose Self-Administration.
To determine whether the effect of rapamycin was specific to alcohol or could be generalized to other reinforcing substances, we examined the ability of rapamycin to modulate the self-administration of sucrose, a nondrug reinforcer. We observed that systemic rapamycin administration did not alter lever-press responding for sucrose (Fig. 7E). This result further confirms that the decrease in alcohol self-administration induced by rapamycin was not a result of a deficit in locomotor activity. It also indicates that the effect of rapamycin on operant self-administration is selective for alcohol and does not reflect a general attenuation of motivation to obtain a reward. Taken together, our data suggest that rapamycin selectively decreases alcohol intake by reducing motivation to seek alcohol.
Discussion
Here we show that the mTORC1-mediated signaling pathway contributes to mechanisms that underlie alcohol-mediated behaviors such as the expression of locomotor sensitization and CPP to alcohol, as well as excessive alcohol consumption. Our results further imply that mTORC1 is an important contributor to mechanisms underlying alcohol-seeking behaviors. This conclusion stems from the finding that a single administration of rapamycin reduces the expression of CPP, a measure of seeking for alcohol reward, and attenuates seeking during rat operant alcohol self-administration.
We observed that both mTORC1 substrates, S6K and 4E-BP, are phosphorylated in the NAc of rodents in response to a single exposure to alcohol, in response to voluntary alcohol consumption, and after 24 h of alcohol deprivation. S6K and 4E-BP are key regulators of the translation initiation, the major rate-limiting step in mRNA translation into proteins (6, 11). For example, phosphorylation of S6K and 4E-BP by mTORC1 is thought to result in the translation initiation of mRNAs displaying a 5′ cap structure (11, 32). Importantly, the mTORC1 pathway plays an important role in local translation at synapses (8, 9), and local cap-dependent translation of mRNAs has been implicated in various forms of synaptic plasticity (33). We show that the protein levels of the AMPA receptor GluR1 and Homer, whose translation is mediated by mTORC1 (13, 28), were up-regulated in the NAc of rats with a history of excessive alcohol consumption. We further show that rapamycin blocked alcohol-mediated increase in Homer level in the NAc of mice. Our results are in line with Cozzoli et al., showing that Homer expression is elevated in the NAc of mice that experienced alcohol binge drinking (34). Homer plays a key role in the expression of adverse phenotypes associated with exposure to alcohol by modulating glutamate neurotransmission (35). Together, these findings raise the possibility that the mTORC1-mediated translation of proteins such as GluR1 and Homer that regulate synaptic functions contribute to the molecular mechanisms that underlie the development, maintenance, and/or expression of excessive alcohol consumption. Interestingly, the level of several other synaptic proteins, including the scaffolding protein postsynaptic density protein 95 (PSD-95) and the activity-regulated cytoskeleton-associated protein (Arc), have been linked to the mTORC1 pathway (36, 37). The possibility that the activation of the mTORC1 pathway in response to alcohol exposure results in the translation of these and other synaptic proteins is intriguing and merits further investigation.
Rapamycin is a Food and Drug Administration-approved drug used for the prevention of host rejection of organ transplants (38). Our results document that rapamycin not only decreases the expression of alcohol-induced locomotor sensitization and place preference, but also attenuates excessive alcohol intake as well as the motivation to seek alcohol in preclinical rodent models of alcohol use and abuse. It is unlikely that rapamycin blocks the effects of alcohol per se, as it does not alter the acute increase in locomotor activity or the latency to fall off the Rotarod following a single administration of alcohol. Furthermore, systemic rapamycin treatment does not alter the ability of rats to discriminate between the lever delivering alcohol and the inactive lever, and does not affect the responding for sucrose in the same paradigm. Therefore, the inhibitory effect of rapamycin on alcohol-related behaviors is unlikely to be a result of the mere impairment of learning and memory processes. It is plausible, however, that rapamycin, by inhibiting mTORC1, selectively reverses certain maladaptive neuroadaptations associated with alcohol disorder, such as the up-regulation of Homer in the NAc (35).
mTOR signals through two multiprotein complexes, mTORC1 and mTOR complex 2 (mTORC2), which phosphorylate different substrates and regulate different cellular functions (10, 11). As mTORC1, but not mTORC2, is sensitive to acute rapamycin treatment (10), the inhibitory actions of rapamycin on alcohol-related behaviors are likely to result from mTORC1 blockade only. In addition, the inhibition of alcohol operant self-administration by rapamycin is specific for alcohol and does not affect natural reward, which is a critical point from a therapeutic perspective (39). Notably, we found that rapamycin attenuates “binge-like” alcohol drinking behavior. This last finding is of particular interest because binge drinking is an increasingly prevalent problem during adolescence and young adulthood, and is a strong predictor of future alcohol-related problems (40, 41). Our findings are also relevant for the treatment of alcoholism, in which the pattern of alcohol consumption is often characterized by successive cycles of daily episodes of binge drinking and withdrawal (25).
Rapamycin treatment was recently reported to delay aging processes and increase longevity and lifespan in mice (42), to reverse neurodegenerative progression in a mouse model of Alzheimer disease (43, 44), and to reduce high fat diet-induced obesity in mice (45). Our data therefore put forward the possibility of yet another beneficial action of novel rapamycin-like compounds that are currently being developed (10). Importantly, clinical studies indicate that mTORC1 inhibitor therapies in humans are tolerated with manageable side effects and no cognitive impairments have been reported (46–48). Therefore, our work suggests that targeting the mTORC1 signaling cascade may be an innovative and valuable strategy for the treatment of alcohol use and abuse disorders.
Materials and Methods
All animal procedures in this report were approved by the Gallo Center Institutional Animal Care and Use Committee and were conducted in agreement with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). Information on reagents, animals, preparation of solutions, Western blot analysis; mouse experiments including systemic administration of alcohol, sensitization, CPP, BAC measurements, intermittent limited-access 20% alcohol drinking paradigm, quinine intake, and Rotarod test; rat experiments including intermittent 20% drinking paradigm, surgery, and intra-NAc administration of rapamycin; and operant self administration of alcohol and sucrose, locomotor activity, histology and data analysis are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Jerome Jeanblanc for technical assistance and helpful discussions. We thank Jana Lim for technical assistance and Drs. Antonello Bonci, Robert Messing, Patricia Janak, and Ray White for critical review of the manuscript. This work was supported by National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism Grant P50 AA017072 (D.R.) and the State of California for Medical Research on Alcohol and Substance Abuse through the University of California, San Francisco (D.R.)
Footnotes
This article is a PNAS Direct Submission.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005554107/-/DCSupplemental.
References
- 1.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spanagel R. Alcoholism: A systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- 3.Hyman SE. Addiction: A disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 4.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 5.Kelley AE. Memory and addiction: Shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Hoeffer CA, Klann E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang DO, Martin KC, Zukin RS. Spatially restricting gene expression by local translation at synapses. Trends Neurosci. 2010;33:173–182. doi: 10.1016/j.tins.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: Lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804:433–439. doi: 10.1016/j.bbapap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 12.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang SJ, et al. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casadio A, et al. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- 16.Tsokas P, et al. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci. 2005;25:5833–5843. doi: 10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- 18.Mameli M, Balland B, Luján R, Lüscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- 19.Blundell J, Kouser M, Powell CM. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem. 2008;90:28–35. doi: 10.1016/j.nlm.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puighermanal E, et al. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- 21.Carelli RM. The nucleus accumbens and reward: Neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev. 2002;1:281–296. doi: 10.1177/1534582302238338. [DOI] [PubMed] [Google Scholar]
- 22.Carlezon WA, Jr, Nestler EJ. Elevated levels of GluR1 in the midbrain: A trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- 23.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 24.Gremel CM, Cunningham CL. Involvement of amygdala dopamine and nucleus accumbens NMDA receptors in ethanol-seeking behavior in mice. Neuropsychopharmacology. 2009;34:1443–1453. doi: 10.1038/npp.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 26.Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institute on Alcohol Abuse and Alcoholism Council approves definition of binge drinking. NIAAA Newsletter. 2004;3:3. [Google Scholar]
- 28.Slipczuk L, et al. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS ONE. 2009;4:e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szumlinski KK, Kalivas PW, Worley PF. Homer proteins: Implications for neuropsychiatric disorders. Curr Opin Neurobiol. 2006;16:251–257. doi: 10.1016/j.conb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci USA. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Banko JL, Klann E. Cap-dependent translation initiation and memory. Prog Brain Res. 2008;169:59–80. doi: 10.1016/S0079-6123(07)00004-0. [DOI] [PubMed] [Google Scholar]
- 34.Cozzoli DK, et al. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: Functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: Implications for addiction. Biochem Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CC, Huang CC, Wu MY, Hsu KS. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J Biol Chem. 2005;280:18543–18550. doi: 10.1074/jbc.M414112200. [DOI] [PubMed] [Google Scholar]
- 37.Takei N, et al. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartford CM, Ratain MJ. Rapamycin: Something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82:381–388. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- 39.Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- 40.Bloomfield K, Stockwell T, Gmel G, Rehn N. International comparisons of alcohol consumption. Alcohol Res Health. 2003;27:95–109. [PMC free article] [PubMed] [Google Scholar]
- 41.Grant KA, et al. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mTOR, A{beta} and tau: Effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spilman P, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS ONE. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang GR, et al. Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. J Pharmacol Sci. 2009;109:496–503. doi: 10.1254/jphs.08215fp. [DOI] [PubMed] [Google Scholar]
- 46.Bhatia S, Thompson JA. Temsirolimus in patients with advanced renal cell carcinoma: An overview. Adv Ther. 2009;26:55–67. doi: 10.1007/s12325-008-0138-3. [DOI] [PubMed] [Google Scholar]
- 47.Bissler JJ, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang UE, et al. Immunosuppression using the mammalian target of rapamycin (mTOR) inhibitor everolimus: Pilot study shows significant cognitive and affective improvement. Transplant Proc. 2009;41:4285–4288. doi: 10.1016/j.transproceed.2009.08.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.